Abstract
Desmoplasia is a hallmark of intrahepatic cholangiocarcinoma (ICC), which constitutes a barrier to infiltration of lymphocyte, but not myeloid cells. Given that dense desmoplastic stroma has been reported to be a barrier to infiltration of lymphocyte, but not myeloid cells. We here investigated whether fibroblastic FAP influenced ICC progression via non-T cell-related immune mechanisms. We demonstrated fibroblastic FAP expression was critical for STAT3 activation and CCL2 production, and ICC-CAFs were the primary source of CCL2 in human ICC microenvironment by using ICC-Fbs from six ICC patients. Fibroblastic knockdown of FAP significantly impaired the ability of ICC-CAFs to promote ICC growth, MDSCs infiltration and angiogenesis, which was restored by adding exogenous CCL2. Furthermore, interestingly, the tumor-promoting effect of fibroblastic FAP is dependent on MDSCs via secretion of CCL2, as depletion of Gr-1+ cells reversed the restoring effects of exogenous CCL2 on tumor growth and angiogenesis. In vitro migration assay confirmed that exogenous CCL2 could rescue the impaired ability of ICC-Fbs to attract Gr-1+ cells caused by fibroblastic FAP knockdown. In contrast, fibroblastic FAP knockdown had no effect on ICC cell proliferation and apoptotic resistance. Depletion MDSCs by anti-Gr-1 monoclonal antibody in subcutaneous transplanted tumor model abrogated tumor promotion by ICC-CAFs suggested that the pro-tumor function of Fibroblastic FAP relied on MDSCs. Mechanical, flow cytometry and chamber migration assay were conducted to find Fibroblastic FAP was required by the ability of ICC-CAFs to promote MDSC migration directly. Moreover, fibroblastic FAP knockdown had no effect on cell proliferation and apoptotic resistance. Here, we revealed the T-cell independent mechanisms underlying the ICC-promoting effect of fibroblastic FAP by attracting MDSCs via CCL2, which was mainly attributed to the ability of FAP to attract MDSCs and suggests that specific targeting fibroblastic FAP may represent a promising therapeutic strategy against ICC.
Abbreviations: CAFs, cancer-associated fibroblasts; EMT, epithelial–mesenchymal transition; FAP, fibroblast activation protein; MDSCs, myeloid derived suppression cells; Fbs, fibroblasts; ICC, intrahepatic cholangiocarcinoma; MVD, microvessel density; TAMs, tumor-associated macrophages
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common type of primary liver cancer with a very poor prognosis [1]. The incidence and mortality rates of ICC are recently increasing worldwide, however, the cellular and molecular pathogenesis of ICC is poorly understood [2], [3]. Dense desmoplastic stroma has long been considered to be a hallmark histological feature of ICC, although ICC is heterogeneous in many aspects such as cellular and molecular phenotypes and genomic differences [4], [5], [6]. The desmoplastic stroma of ICC is characterized by the enrichment of activated myofibroblasts that express α-SMA, which is also a common marker for cancer-associated fibroblasts (CAFs) [4], [5]. Clinical studies suggest that desmoplastic stroma is associated with ICC prognosis [2], [7]. For example, abundance in the fibrous stroma and the higher levels of α-SMA in ICC patients with resected tumor were reported to negatively correlate with the clinic prognosis of ICC [7]. Moreover, increasing evidence demonstrates that fibroblasts contribute to the progression of ICC by influencing tumor cell biology [8], [9], [10], [11].
α-SMA+CAFs have been considered to be heterogeneous with phenotypically distinct subpopulations, for example, ICC patients with CAFs expressing a mucin-like transmembrane glycoprotein have increased lymph node metastasis and worse clinic outcome [12], [13]. Fibroblast activation protein (FAP), a serine protease, is selectively expressed on CAFs in many human solid tumor [14], [15]. Accumulating data demonstrate that targeting FAP genetically, or with vaccines or pharmacological agents including inhibitor and CAR-T cells [16], [17], [18], as well as targeting FAP-expressing cells [19], [20], could impair tumor progression in several mouse models via different mechanisms. Our previous study using tumor model of immunocompetent mouse demonstrated that FAP is responsible for the inflammatory phenotype of CAFs by activating STAT3-CCL2 signaling, which mediates tumor recruitment of myeloid-derived suppressive cells (MDSCs), thereby antagonizing anti-tumor T cell immunity, leading to tumor growth [21]. Importantly, we demonstrated the adverse predictive role of stromal FAP levels in human ICC by immunohistochemical staining of ICC tissue microarrays, suggesting that FAP+CAFs may contribute to ICC growth [21].
Infiltration of immature myeloid cells is one of the hallmarks of cancer [22]. Tumor-associated macrophages (TAMs) and MDSCs are two major myeloid cell types found in solid tumors [22], [23]. CD163+ TAMs with a M2 phenotype have been implicated in ICC progression, predicting a poor clinical outcome [24]. In addition to their immunosuppressive function, TAMs and MDSC aggravate tumor progression via many other mechanisms including the promotion of angiogenesis and epithelial-mesenchymal transition (EMT) [25], [26], [27]. Thus, the understanding of their functions of myeloid cells in addition to their immunosuppressive function would be helpful to develop a more specific anticancer strategy targeting the patients with weak immune system.
In the present study, we aimed to examine whether and how FAP expression in ICC patients derived fibroblasts promoted ICC tumor growth in immunodeficient situations by using nude mice. We confirmed the FAP was necessary for inflammatory phenotype of ICC-CAFs. On the mechanism, we found that fibroblastic FAP contributed to CAF-mediated tumor growth by promoting tumor cell proliferation and angiogenesis, which relay on CCL2-MDSCs axis.
Materials and methods
Mice and cell lines
Male nude mice were purchased from the Chinese Academy of Sciences (Shanghai, China). All mice were kept and bred in a specific pathogen-free environment in the animal facility of Shanghai Medical College, Fudan University, and all animal experiments were approved by the Animal Care and Use Committee at Fudan University, Shanghai, China. ICC cell lines of QBC939, HCCC-9810, and RBE were obtained from Liver Cancer Institute, Zhongshan Hospital, Fudan University [28]. All cell lines were tested to exclude mycoplasma contamination before experiments.
Human samples
Freshly resected tumor, non-cancerous liver tissues and blood samples from ICC patients were obtained from Liver Cancer Institute, Zhongshan Hospital between July 2014 and March 2016. The study protocol was approved by the Institute Review Board of Zhongshan Hospital, Fudan University.
The isolation of human fibroblasts
Fibroblasts were isolated as previously described [21]. Briefly, tumor or non-cancerous liver tissues resected from ICC patients were digested in 1 mg/ml collagenase IV (Sigma) at 37 °C for 1 h. Fibroblasts were enriched by anti-human Fibroblast Microbeads (#130-050-601, Miltenyi Biotec) and cultured in complete DMEM.
Lentivirus vector preparation and fibroblast transduction
The sequences for siRNA targeting fap (fap1, GCTTCAAATTACGGCTTAT, fap2, GCTCTCTGGTGGTCTC CTA, fap3, GGTGGATTCTTTGTTTCAA) were designed and synthesized by GenePharma (GenePharma). Western blotting data showed that siRNA fap3 sequence was most efficient in inhibiting FAP expression. Thus, siRNA fap3 was used for FAP knockdown. Short-hairpin RNA (shRNA) specifically targeting human FAP was constructed in lentiviral vector pSIH1-H1-copGFP vector (System Biosciences). Short-hairpin RNA (shRNA) specifically targeting scramble siRNA (GGTGCATTCTATGTATCAA) was constructed in lentiviral vector pSIH1-H1-copGFP vector as the control vector. Lentivirus was generated and transduced vectors into ICC-CAFs according to the manufacturer's protocol to generate Control CAF or FAPkdCAF.
Coimmunoprecipitation and Western blotting
For coimmunoprecipitation (Co-IP), fap gene (NM_007986.2) was cloned into pCMV-Tag2B-flag vector, and Plaur gene (NM_011113.3) into PCDNA3.1-HA vector. Primary antibodies against uPAR (R&D Systems), FAP (Abgent), HA (Santa Cruz Biotechnology) were used. The rabbit normal IgG antibodies (Santa Cruz Biotechnology) were added as a control. Anti-FLAG M2 Affinity Gel (Sigma) was used for immunoprecipitation. For Western blotting, Protein concentration was measured by using BCA protein assay kit (Pierce). 20 μg protein samples were used and transferred onto polyvinylidene fluoride (PVDF) transfer membrane (Millipore). The membrane was blocked with 5% blotting grade milk powder in TBST (50 mM Tris-HCl, 0.15 M NaCl, 0.1% Tween-20, pH: 7.4). The Western blotting was performed as previously described [21]. The antibodies were shown in Supporting Table S1.
Gr-1+ cells depletion
Gr-1+ Cells were depleted by 100 μg anti-Gr-1 monoclonal antibody (αGr-1, clone RB6-8C5) (Bioxcell) via intravenous injection 24 hr before tumor cells challenge for every 3 days.
RNA isolation and quantitative real-time PCR (qRT-PCR)
RNA was isolated by TRIzol (Invitrogen) and reverse-transcribed into cDNA by PrimeScript RT Master Mix (TaKaRa). qRT-PCR was performed by Applied Biosystems 7500 using Power SYBR Green Master kit (TaKaRa). The relative expression of target gene was calculated using the 2ΔC(t) method. Fold induction of target gene expression were calculated by normalization to control group. The primer sequences of all genes for PCR are shown in Supporting Table S3.
Enzyme-linked immunosorbent assay (ELISA)
ICC-CAFs or FAPkd-ICC-CAFs were cultured for 24 h, and the supernatants were collected. CCL2 concentrations were measured using a human CCL2 ELISA Kit (R&D Systems).
Subcutaneous transplanted tumor model
Fibroblasts derived from ICC tissues (ICC-CAFs) or FAPkd-ICC-CAFs (in which FAP was knockdown) (2X105 each) were subcutaneously co-injected with QBC939 cells (1 × 106) into nude mice. The tumor volume was calculated by the following formula: V = π/6X (larger diameter) × (smaller diameter)2. CCL2 protein (5 μg/mouse, Peprotech) or PBS as control were administered around the site of the tumor every other day starting from day 7.
Immunohistochemistry (IHC)
IHC staining of tumor tissue sections was performed using the avidin-biotin-peroxidase complex method. Antibodies against CD31 (1:200, sc-1506, Santa Cruz) or Ki67 (1:100, AF7689, R&D Systems) were used. The proliferation index was quantified according to the mean numbers of ki67+ cells in five high power fields (HPF) [17]. For the evaluation of microvessel density (MVD), brown-stained endothelial cell or endothelial cell cluster that clearly separated from tumor cells or stromal cells were counted as one microvessel [29].
Chemotaxis assay
Blood samples from ICC patients were obtained from Liver Cancer Institute, Zhongshan Hospital. CD14+ cells were sorted by CD14+ beads (Miltenyi Biotec). Ten thousand sorted CD14+ cells were added to the upper chambers of Transwell (5 μm, 24-well format; Corning, USA). The supernatants from various fibroblasts were added to the lower chambers with or without CCL2 (100 ng/ml). Cells migrated to the lower chambers were counted by CyAn (Beckman Coulter) after 4 h incubation.
Flow cytometry
The tumor tissue was cut into pieces and digested by collagenase IV (1 mg/ml) for 1 hour at 37 °C. Resuspended cells were blocked with Fc antibody first and then stained with antibody. Ly6G+CD11b+F4/80− cells were considered as PMN-MDSCs. Ly6C+CD11b+F4/80− cells were considered as Mo-MDSCs. F4/80+CD11b+ cells were considered as tumor-associated macrophages. Sorted CD14+ myeloid cells from peripheral blood of ICC patients were further confirmed by staining with antibodies as CD33+CD14+CD11b+ cells. The following antibodies were shown in Supporting Table S2. Samples were acquired by FACS Cyan instrument and analyzed with Summit (Beckman Coulter).
Statistical analysis
The comparisons between two groups were performed by two-tailed Student's t-tests. Multiple-group comparisons were performed by two-way ANOVA followed by a Bonferroni correction to compare each group. The χ2 test or Fisher’s exact test was used for qualitative variables comparison. Statistical analyses were performed by Graph Prism V5.2. p < 0.05 was considered statistically significant.
Results
Fibroblasts derived from tumor tissues of ICC patients express FAP, which promotes STAT3 activation
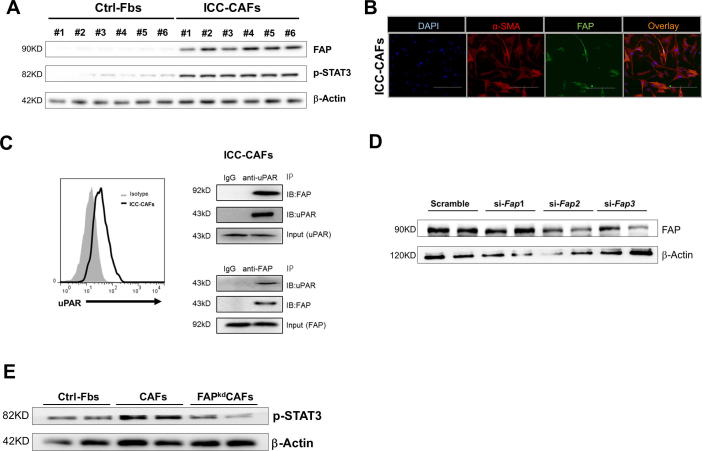
We first attempted to confirm the FAP expression in fibroblasts derived from ICC tissues (ICC-CAFs). Fibroblasts isolated from paired non-cancerous liver tissues were used as control (Ctrl-Fbs). ICC-CAFs from six ICC patients each expressed FAP, while Ctrl-Fbs expressed very little, if any, FAP by western blotting (Figure 1A). Immunofluorescence staining revealed extensive expression of α-SMA in ICC-CAFs, and only a subset of α-SMA+CAFs expressed FAP (Figure 1B), indicating that FAP+CAFs may represent a phenotypically distinct subpopulation. Although FAP has a short intracellular domain, our previous study suggested that it interacts with uPAR in mouse hepatocellular cancer (HCC) CAFs [21]. Firstly, we examined that ICC-CAFs expressed uPAR. Moreover, Co-IP results showed that FAP interacts with uPAR in ICC-CAFs, which suggesting that FAP may induce the intercellular signal in ICC-CAFs through uPAR (Figure 1C). STAT3 was an important transcription factor for inducing inflammatory signals in CAFs [30]. To investigate whether FAP effected on the activation of STAT3 in CAFs, we specifically knocked down FAP in ICC-CAFs by using FAP specific shRNA adenovirus (FAPkdCAF). ICC-CAFs transfected with vector contained scramble shRNA as control CAFs (CAF). The efficiency of FAP specific siRNA was confirmed by Western blotting (Figure 1D). Compared with Ctrl-Fbs, ICC-CAFs had marked increases in p-STAT3 protein levels, which was significantly reduced by knockdown of FAP (Figure 1A and E). Collectively, these data demonstrate that fibroblasts isolated from ICC patients express FAP, which is important for the activation of fibroblastic STAT3 inflammatory signaling.
Figure 1.
Fibroblasts derived from tumor tissues of ICC patients express FAP, which promotes STAT3 activation. (A) Western blots showing the protein levels of FAP and phosphorylated STAT3 (p-STAT3) in fibroblasts derived from ICC tissues (CAFs) and paired non-cancerous liver tissues (Ctrl-Fbs). (B) Representative images of immunofluorescence staining of cultured CAFs with α-SMA (red) and FAP (green) antibodies and counterstained with DAPI (blue) (400X magnification). The scale bars represent 200 μM. (C) Representative flow cytometry analysis of uPAR expression and CoIP assays to analyze the interaction between FAP and uPAR in ICC tissue-derived fibroblasts. Normal rabbit IgG antibodies were served as a negative control. (D) Western blots showing the efficiency of FAP knockdown. (E) Western blots showing the protein levels of p-STAT3. Results are representative of at least three independent experiments.
Knockdown of FAP in ICC-CAFs inhibits tumor growth in a xenograft mouse model of human ICC
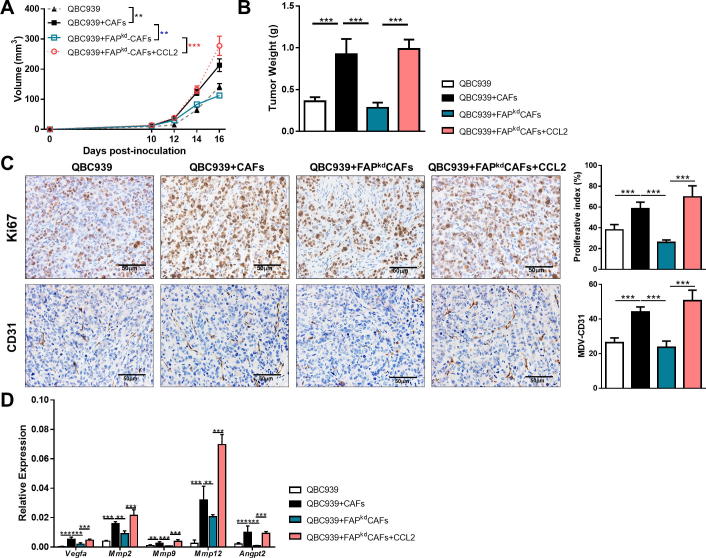
To further investigate whether FAP contributed to the ability of ICC-CAFs to promote ICC growth in vivo, we established a xenograft model of human ICC by subcutaneously injecting QBC939 ICC cells alone or with ICC-CAFs or FAPkd-ICC-CAFs in the flanks of nude mice. Co-injection with ICC-CAFs significantly promoted the volume and the weight of tumors, which was markedly reduced by specifically knockdown of FAP in ICC-CAFs (Figure 2A and B). To evaluate the ICC cell proliferation rate, tumor sections were stained with anti-Ki67 antibodies. There were significantly less Ki67-positive cells in tumors co-injected with FAPkd-ICC-CAFs than those with CAFs (Figure 2C), indicating that fibroblastic FAP is required for promoting tumor cell proliferation in vivo. We therefore analyzed MVD by staining CD31 and gene expression of several pro-angiogenic factors in tumors co-injected with CAFs or FAPkdCAFs. FAP knockdown caused an obvious decrease in CD31-positive staining, suggesting a less MVD in tumors (Figure 2C). Consistently, significantly decreased gene expression of angiogenic factors was detected in tumors co-injected with FAPkd-ICC-CAFs (Figure 2D). Take together, FAP knockdown in ICC-CAFs still could suppress tumor growth in nude mice that are deficient in T cells prompted us to propose that FAP may promote tumor growth via non-T cell-related mechanism.
Figure 2.
Knockdown of FAP in ICC-CAFs inhibits tumor growth in a xenograft mouse model of human ICC. QBC939 cells were injected alone or co-injected with various fibroblasts subcutaneously into nude mice with or without CCL2, respectively. (A) Tumor growth was measured at the indicated time points. (B) Tumor weight (C) representative photomicrographs of IHC-staining of Ki67 and CD31 of tumor sections and proliferation index calculated by surface quantification of Ki67 positive and CD31 positive cells. Original magnification, 200X; scale bar, 50 μm. (D) qRT-PCR analysis of the expression of proangiogenic in tumor tissues. Data are represented as mean ± SEM, n = 5–7. Results are representative of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Fibroblastic FAP does not directly promote ICC cells proliferation and apoptosis
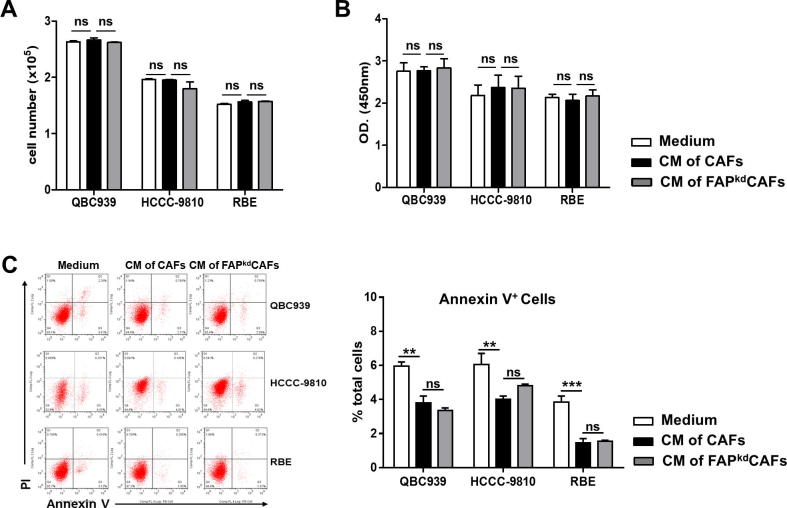
Accumulating data showed that CAFs play a crucial role in accelerating the progression of ICC via the direct effect on the biology of tumor cells [5]. Our in vivo study suggested that fibroblastic FAP induced tumor cells proliferation. We then asked whether FAP contributed to the effect of ICC-CAFs on the biology of ICC cells including proliferation and apoptosis directly. To this end, we incubated three ICC cell lines, QBC939, HCCC-9810 and RBE, with supernatants from ICC-CAFs or FAPkd-ICC-CAFs. Incubation with supernatants from ICC-CAFs or FAPkd-ICC-CAFs did not affect the proliferation of all three ICC cell lines as determined by CCK8 and BrdU assay (Figure 3A and B). In contrast, incubation with supernatants from ICC-CAFs significantly decreased the percentages of Annexin V+ apoptotic cells in the culture of three ICC cell lines, which, however, was not affected by FAP knockdown (Figure 3C). These data suggest that ICC-CAFs have no effect on the proliferation of ICC cells, but could promote apoptosis resistance of ICC cells in a FAP-independent manner.
Figure 3.
Fibroblastic FAP does not directly promote ICC cells proliferation and apoptosis. ICC cell line QBC939, HCCC9810 and RBE were treated with supernatants of various fibroblasts. (A and B) CCK8 and BrdU analysis of tumor cell number and proliferation. (C) Representative flow cytometry data of AnnexinV/PI double staining and averaged percentages of Annexin V+ cells. Data represented as mean ± SEM, n = 5–7. Results are representative of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
CCL2 mediates ICC-promoting effect of fibroblastic FAP
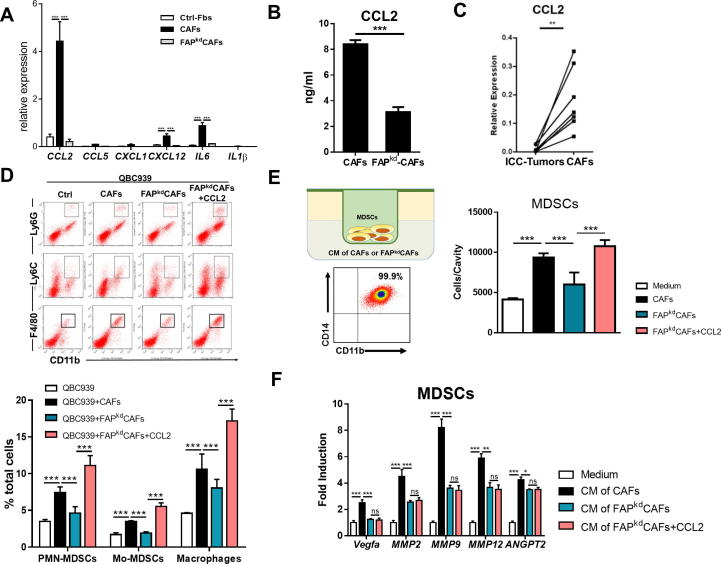
As inflammatory CAFs in tumor microenvironment was important for tumor cell proliferation and angiogenesis, we examined the inflammatory associated genes regulated by STAT3 in ICC-CAFs. Compared with Ctrl-Fbs, ICC-CAFs had marked increases in gene expression of STAT3 regulating inflammatory cytokines, particularly CCL2 (Figure 4A). Consistently, FAP knockdown significantly reduced the secretion of CCL2 production by ICC-CAFs (Figure 4B). Moreover, we found that CAFs were the major source for CCL2 in ICC microenvironment, as evidenced by much higher levels of CCL2 gene expression in CAFs derived from 7 patients than those in tumor tissues from the same patients (Figure 4C). To determine whether CCL2 mediated the tumor-promoting effect of FAP in ICC-CAFs, exogenous CCL2 was injected into the tumor co-injected with FAPkd-ICC-CAFs. Addition of CCL2 could restore the reduction in tumor growth accompanied by increased tumor cell proliferation and angiogenesis (Figure 2A–D). These results together suggest that ICC-promoting effect of fibroblastic FAP is mediated by CCL2. In addition to their immunosuppressive functions, MDSCs are reported to promote tumor growth and metastasis by promoting angiogenesis [25], [26], and MDSCs are reported to be the important source for angiogenic factors in the tumor microenvironment [26], [31]. Flow cytometry results showed significantly increased frequencies of two subsets of MDSCs, PMN-MDSCs and M-MDSCs, as well as macrophages, were observed in tumors co-injected with ICC-CAFs which was significantly reduced by specifically knockdown of fibroblastic FAP (Figure 4D). We then investigated how FAP expression in ICC-CAFs influenced MDSCs. We first examined the chemotaxis of sorted myeloid cells from peripheral blood of ICC patients toward supernatants from ICC-CAFs or FAPkd-ICC-CAFs by transwell assay. FAP knockdown significantly impaired the ability of ICC-CAFs to attract myeloid cells, which was rescued by addition of CCL2 (Figure 4E). In contrast, compared with supernatants from ICC-CAFs, those from FAPkd-ICC-CAFs had a significantly impaired ability to enhance angiogenic gene expression, which could not be restored by addition of CCL2 (Figure 4F). These results together suggest that FAP is required for the ability of ICC-CAFs to mediate the migration in a CCL2-dependent way, and angiogenic function in a CCL2-independent way.
Figure 4.
CCL2 mediates ICC-promoting effect of fibroblastic FAP. (A) qRT-PCR analysis of STAT3 regulating inflammatory cytokines particularly CCL2 expression of Ctrl-Fbs, CAFs or FAPkdCAFs. n = 3. (B) ELISA analysis of CCL2 concentrations in the culture of CAFs treated with negative control or fap siRNA. n = 3. (C) qRT-PCR analysis of CCL2 concentrations in the culture of ICC tumor tissues or CAFs. n = 7. (D) Representative flow cytometry analysis of PMN-MDSCs (Ly6G+CD11b+F4/80−), Mo-MDSCs (Ly6C+CD11b+F4/80−) and tumor-associated macrophages (F4/80+CD11b+). (E) Representative flow cytometry analysis of sorted myeloid cells from peripheral blood of ICC patients (CD33+CD14+CD11b+). Transwell assays of MDSC chemotaxis toward supernatants of various fibroblasts with or without CCL2 protein. (F) qRT-PCR analysis of gene expression of proangiogenic factor in MDSCs treated by supernatants of various fibroblasts with or without CCL2. Values are mean ± SEM of three replicate wells from a representative of three experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
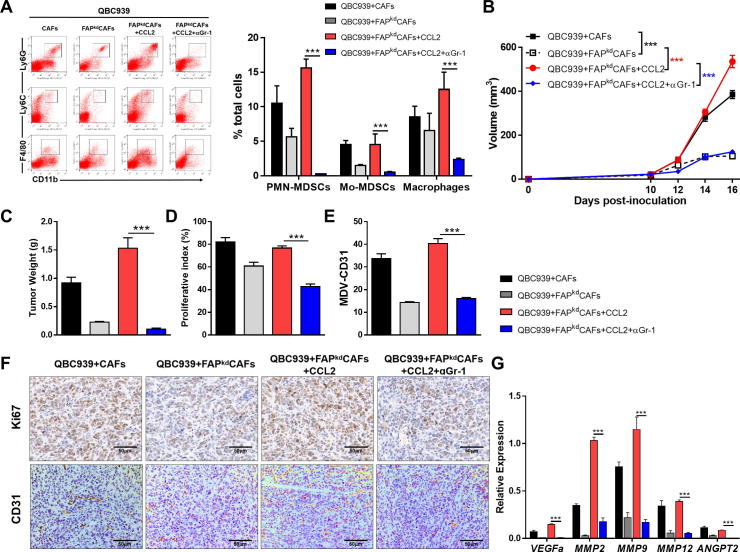
ICC-promoting effect of fibroblastic FAP is dependent on Gr-1+ MDSCs
Given that exogenous CCL2 restored infiltrating MDSCs in tumors co-injected with FAPkd-ICC-CAFs, we then investigated whether ICC-promoting effect of fibroblastic FAP was dependent on Gr-1+ MDSCs via CCL2. To this end, we depleted Gr-1+ MDSCs by i.v. injecting αGr-1 neutralizing antibodies into nude mice which were s.c co-injected QBC939 ICC cells with FAPkd-ICC-CAFs followed by CCL2 treatment. The depletion deficiency was confirmed by flow cytometric analysis of tumor tissues showing no detectable Gr-1+ MDSCs (Figure 5A). Depletion of Gr-1+ MDSCs abrogated the restoring effects of CCL2 in tumors co-injected with FAPkd-ICC-CAFs, as evidenced by marked reduction in tumor volume and weight of tumors (Figure 5B and C). These findings were further supported by IHC staining showing significant less Ki67 positive cells observed in tumor sections after Gr-1+ MDSCs Depleted (Figure 5D and E). Consistently, the number of CD31+ cells and the expression of angiogenesis associated genes also significantly decreased in MDSCs Depleted group (Figure 5E and F). These data together demonstrate that tumor-infiltrating Gr-1+ MDSCs are required for the ability of fibroblastic FAP to promote ICC growth.
Figure 5.
ICC-promoting effect of fibroblastic FAP is dependent on Gr-1 + MDSCs. QBC939 cells were injected alone or co-injected with various fibroblasts subcutaneously into nude mice with or without CCL2 and Gr-1 antibody, respectively. (A) Representative flow cytometry data and averaged percentages of PMN-MDSCs, M-MDSCs, and macrophages. (B) Tumor growth was measured at the indicated time points. (C) Tumor weight. (D–F). Representative photomicrographs of IHC-staining of Ki67 and CD31 (F) of tumor sections and proliferation index calculated by surface quantification of Ki67 positive (D) and CD31 positive cells (E). Original magnification, 200×; scale bar, 50 μm. (G) qRT-PCR analysis of the expression of proangiogenic in tumor tissues. Data are represented as mean ± SEM, n = 5–7. Results are representative of at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Despite accumulating evidence demonstrating that fibroblasts play a crucial role in ICC development and FAP is expressed in CAFs from many tumor types, the direct evidence that FAP is expressed in fibroblasts derived from ICC is still lacking and how fibroblastic FAP contributes to ICC-promoting effect of fibroblasts remains unknown. First, this study provides the direct evidence that FAP was expressed in fibroblasts isolated from 6 individual surgical samples of ICC patients by western blotting and immunofluorescence staining. Moreover, we here demonstrate that fibroblasts derived from tumors of ICC patients express FAP have higher STAT3 activation and CCL2 production, which was greatly reduced by FAP knockdown. Furthermore, fibroblastic FAP is required for tumor growth and MDSC infiltration in a CCL2-dependent manner by using a xenograft mouse model of human ICC. Moreover, in vitro studies reveal that fibroblastic FAP is critical for ICC-CAFs to mediate migration of MDSCs via CCL2.
Lots of studies have suggested that there were various fibroblast subsets in the tumor microenvironment and inflammatory diseases, and only part fibroblast subsets contribute to inflammation, tumor progression and cancer therapy resistance [21], [32], [33], [34]. A recent study found that it was FAP+ fibroblasts contributing to inflammation in arthritis by using single-cell RNA sequencing [32]. Our previous study showed that FAP+CAFs were a subset of inflammatory CAFs in HCC [21]. Although CAFs isolated from ICC patients were α-SMA positive fibroblasts, we found that FAP only expressed on a subset of α-SMA+CAFs In the ICC tumor microenvironment. Moreover, our data demonstrated that FAP knockdown in ICC-CAFs has lower STAT3 activation and inflammatory cytokines expression, suggesting that FAP was a functional marker of activated CAFs, more than a by-product marker. MDSCs are a crucial immune component in the tumor microenvironment, facilitating all stages of tumor development via various mechanisms [22], [35]. Despite accumulating data about MDSCs in tumor-bearing mouse model [21], [35], the information of MDSCs is relatively limited in human cancers. FAP was previously demonstrated to induce immunosuppression in the tumor microenvironment by antagonizing anti-tumor T cell immunity [19], [20], [21]. Considering limited infiltration of T cells, but not myeloid cells, in cancer with dense desmoplastic stroma [36], [37], we speculated that fibroblastic FAP may also facilitate ICC development via non-T cell-dependent mechanisms. Using a tumor model by co-injecting fibroblasts and tumor cells into immunodeficient mice, we demonstrated that ICC-CAFs promoted QBC939 ICC cell growth in vivo accompanied by increases in tumor infiltration of MDSCs and macrophages, and gene expression of angiogenic factors. Importantly, FAP knockdown completely impaired the tumor-promoting effect of ICC-CAFs, which was reversed by adding exogenous CCL2. Altogether, these data emphasize a non-T cell-dependent mechanism underlying ICC growth inhibition by targeting fibroblastic FAP. Otherwise, the previous study showed that FAP expressed on the surface of tumor-associated macrophage in human breast cancer [38]. However, we found that the TAM of ICC did not express FAP (data not shown). Therefore, whether FAP expressed on TAM may rely on its tumor microenvironment and tumor types.
We further clarified the possible non-T cell-dependent mechanisms by which fibroblastic FAP promoted ICC growth. FAP can promote tumor growth via its enzyme activity or non-enzyme activity to shape the pro-tumorigenic functions of CAFs [39], [40]. Ours and other studies have demonstrated that FAP regulated the expression of CCL2. Moreover, FAP did not cleave CCL2 spite FAP cleavage sites being present in CCL2 [39]. Taking together, these results suggested that pro-tumorigenic functions of FAP in ICC via CCL2 were dependent on its intro-cellular activation of STAT3 signal in CAFs. Moreover, we demonstrated that fibroblastic FAP mediated tumor recruitment of MDSCs in CCL2-dependent manner. In addition to mediating immunosuppression, MDSCs could promote tumor progression by enhancing angiogenesis in a paracrine pathway [26]. We demonstrated that ICC-CAFs enhanced the ability of MDSCs to express various angiogenic factors, particularly MMP9 that was reported to be primarily produced by MDSCs [26], in a FAP-dependent manner, however, considering that ICC is a hypovascular cancer [41], the enhancement of tumor angiogenesis may not be the major attribution to the ICC-promoting effect of fibroblastic FAP. CCL2 production regulated the vicious cycle between tumor cells and macrophages that promotes the progression of tumors [42]. Fibroblast-derived CCL2 was recently shown to promote breast cancer progression by regulating NOTCH activation and macrophage infiltration [43], [44]. Consistent with this study, we showed that fibroblasts were the major source of CCL2 in human ICC microenvironment. However, co-culture with supernatants from ICC-CAFs failed to enhance cell proliferation. Thus, FAP and CCL2-dependent increases in ICC cell proliferation in vivo could not result from the direct effect of ICC-CAFs on ICC cells. Given that myeloid cells including MDSCs and macrophages are implicated in promoting cancer growth [25], [26], [35], it is likely that CCL2 derived from FAP+ ICC-CAFs mediates migration of MDSCs to tumor sites, where they support cancer cell proliferation.
Most studies focused on the cross-talk between fibroblasts and cholangiocarcinoma cells, demonstrating that fibroblasts directly promote cancer cell apoptotic resistance, migration and/or EMT by secreting a variety of mediators [8], [9], [10], [11], [13]. We demonstrated that ICC-CAFs directly reduced the frequencies of apoptotic cells in the cultures of three ICC cell lines, but not dependent on FAP. However, the previous study showed that overexpression FAP in LX-2, a liver fibroblast cell line, did not increase tumor cells apoptosis directly, but can enhance staurosporine streptomyces-induced tumor cells apoptosis [45]. As the CAFs play an important role in the chemotherapy resistance, therefore, we did not exclude that FAP may play a synergetic effect on apoptosis resistance under some chemotherapeutic treatment.
Conclusion
In summary, our study provides the first evidence that FAP is expressed by ICC-derived fibroblasts, which is the main source of CCL2 in the ICC microenvironment. We further demonstrate that fibroblastic FAP promotes ICC growth indirectly by attracting MDSCs to tumor sites via CCL2 specific targeting fibroblastic FAP may represent a more effective and safer strategy against ICC.
Ethic approval and consent to participate
The current study was approved by Ethical Committee of Zhongshan Hospital and the Institutional Animal Care and Use Committee of Fudan University.
Consent for publication
All authors reached an agreement to publish the study in this journal.
Availability of data and materials
Not applicable.
Authors’ contributions
R.H. and Y.L. designed the study, Y.L., B.L., X.G. performed the experiments and analyzed data, Y.C., Q.C., W.Y. and Y.S. performed the experiments, W.L., M.T. and Y.S. provided the clinical samples, R.H. and Y.L. wrote the manuscript, and all authors have read the manuscript critically.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Major Special Projects of the Ministry of Science and Technology (No.2018ZX10302207), National Key Research and Program of Shanghai Academic/Technology Research Leader No.19XD1400200 (to R.H.), National Natural Science Foundation of China Grant No.91642112 (to R.H.), No.31600715 (to Y.L.), No.81602665 (to X.Y.), No.81472674 (to Y.S.) and Development Project of Shanghai Peak Disciplines-Integrative Medicine (No.20180101).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2019.09.004.
Contributor Information
Yinghong Shi, Email: shi.yinghong@zs-hospital.sh.cn.
Rui He, Email: ruihe@fudan.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bridgewater J., Galle P.R., Khan S.A., Llovet J.M., Park J.W., Patel T., Pawlik T.M., Gores G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Fujita T. Analyzing risk factors for intrahepatic cholangiocarcinoma. Hepatology. 2013;58(5):1862–1863. doi: 10.1002/hep.26448. [DOI] [PubMed] [Google Scholar]
- 3.Razumilava N., Gores G.J. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulouarn C., Clement B. Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J Hepatol. 2014;60(6):1306–1309. doi: 10.1016/j.jhep.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Sirica A.E. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;9(1):44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]
- 6.Sirica A.E., Gores G.J. Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology. 2014;59(6):2397–2402. doi: 10.1002/hep.26762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuaysri C., Thuwajit P., Paupairoj A., Chau-In S., Suthiphongchai T., Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21(4):957–969. doi: 10.3892/or_00000309. [DOI] [PubMed] [Google Scholar]
- 8.Fingas C.D., Bronk S.F., Werneburg N.W., Mott J.L., Guicciardi M.E., Cazanave S.C., Mertens J.C., Sirica A.E., Gores G.J. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54(6):2076–2088. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohira S., Sasaki M., Harada K., Sato Y., Zen Y., Isse K., Kozaka K., Ishikawa A., Oda K., Nimura Y. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168(4):1155–1168. doi: 10.2353/ajpath.2006.050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claperon A., Mergey M., Aoudjehane L., Ho-Bouldoires T.H., Wendum D., Prignon A., Merabtene F., Firrincieli D., Desbois-Mouthon C., Scatton O. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology. 2013;58(6):2001–2011. doi: 10.1002/hep.26585. [DOI] [PubMed] [Google Scholar]
- 11.Claperon A., Mergey M., Nguyen Ho-Bouldoires T.H., Vignjevic D., Wendum D., Chretien Y., Merabtene F., Frazao A., Paradis V., Housset C. EGF/EGFR axis contributes to the progression of cholangiocarcinoma through the induction of an epithelial-mesenchymal transition. J Hepatol. 2014;61(2):325–332. doi: 10.1016/j.jhep.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Aishima S., Nishihara Y., Iguchi T., Taguchi K., Taketomi A., Maehara Y., Tsuneyoshi M. Lymphatic spread is related to VEGF-C expression and D2–40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol. 2008;21(3):256–264. doi: 10.1038/modpathol.3800985. [DOI] [PubMed] [Google Scholar]
- 13.Fiori M.E., Di Franco S., Villanova L., Bianca P., Stassi G., De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18(1):70. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scanlan M.J., Raj B.K., Calvo B., Garin-Chesa P., Sanz-Moncasi M.P., Healey J.H., Old L.J., Rettig W.J. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci U S A. 1994;91(12):5657–5661. doi: 10.1073/pnas.91.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J.E., Lenter M.C., Zimmermann R.N., Garin-Chesa P., Old L.J., Rettig W.J. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274(51):36505–36512. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 16.Lo A., Wang L.C.S., Scholler J., Monslow J., Avery D., Newick K., O'Brien S., Evans R.A., Bajor D.J., Clendenin C. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 2015;75(14):2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos A.M., Jung J., Aziz N., Kissil J.L., Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119(12):3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J., Fassnacht M., Nair S., Boczkowski D., Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65(23):11156–11163. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- 19.Kraman M., Bambrough P.J., Arnold J.N., Roberts E.W., Magiera L., Jones J.O., Gopinathan A., Tuveson D.A., Fearon D.T. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 20.Feig C., Jones J.O., Kraman M., Wells R.J., Deonarine A., Chan D.S., Connell C.M., Roberts E.W., Zhao Q., Caballero O.L. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(50):20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X., Lin Y., Shi Y., Li B., Liu W., Yin W., Dang Y., Chu Y., Fan J., He R. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res. 2016;76(14):4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 22.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasita H., Komohara Y., Okabe H., Masuda T., Ohnishi K., Lei X.F., Beppu T., Baba H., Takeya M. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci. 2010;101(8):1913–1919. doi: 10.1111/j.1349-7006.2010.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan S., Zhao E., Kryczek I., Vatan L., Sadovskaya A., Ludema G., Simeone D.M., Zou W., Welling T.H. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. doi: 10.1053/j.gastro.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murdoch C., Muthana M., Coffelt S.B., Lewis C.E. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8(8):618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 27.Yeung O.W., Lo C.M., Ling C.C., Qi X., Geng W., Li C.X., Ng K.T., Forbes S.J., Guan X.Y., Poon R.T. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62(3):607–616. doi: 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C., Bai D.S., Huang X.Y., Shi G.M., Ke A.W., Yang L.X., Yang X.R., Zhou J., Fan J. Prognostic significance of Capn4 overexpression in intrahepatic cholangiocarcinoma. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0054619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J.P., Yan J., Xu J., Pang X.H., Chen M.S., Li L., Wu C., Li S.P., Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50(5):980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Karakasheva T.A., Lin E.W., Tang Q., Qiao E., Waldron T.J., Soni M., Klein-Szanto A.J., Sahu V., Basu D., Ohashi S. IL-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res. 2018;78(17):4957–4970. doi: 10.1158/0008-5472.CAN-17-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coussens L.M., Tinkle C.L., Hanahan D., Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103(3):481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croft A.P., Campos J., Jansen K., Turner J.D., Marshall J., Attar M., Savary L., Wehmeyer C., Naylor A.J., Kemble S. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570(7760):246–251. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huelsken J., Hanahan D. A subset of cancer-associated fibroblasts determines therapy resistance. Cell. 2018;172(4):643–644. doi: 10.1016/j.cell.2018.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen F et al. CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 2018, 172(4):841–856 e816. [DOI] [PubMed]
- 35.Ostrand-Rosenberg S., Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ene-Obong A., Clear A.J., Watt J., Wang J., Fatah R., Riches J.C., Marshall J.F., Chin-Aleong J., Chelala C., Gribben J.G. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145(5):1121–1132. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watt J., Kocher H.M. The desmoplastic stroma of pancreatic cancer is a barrier to immune cell infiltration. Oncoimmunology. 2013;2(12) doi: 10.4161/onci.26788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tchou J., Zhang P.J., Bi Y., Satija C., Marjumdar R., Stephen T.L., Lo A., Chen H., Mies C., June C.H. Fibroblast activation protein expression by stromal cells and tumor-associated macrophages in human breast cancer. Hum Pathol. 2013;44(11):2549–2557. doi: 10.1016/j.humpath.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H.E., Hamson E.J., Koczorowska M.M., Tholen S., Chowdhury S., Bailey C.G., Lay A.J., Twigg S.M., Lee Q., Roediger B. Identification of novel natural substrates of fibroblast activation protein-alpha by differential degradomics and proteomics. Mol Cell Proteomics. 2019;18(1):65–85. doi: 10.1074/mcp.RA118.001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koczorowska M.M., Tholen S., Bucher F., Lutz L., Kizhakkedathu J.N., De Wever O., Wellner U.F., Biniossek M.L., Stahl A., Lassmann S. Fibroblast activation protein-alpha, a stromal cell surface protease, shapes key features of cancer associated fibroblasts through proteome and degradome alterations. Mol Oncol. 2016;10(1):40–58. doi: 10.1016/j.molonc.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawahara N., Ono M., Taguchi K., Okamoto M., Shimada M., Takenaka K., Hayashi K., Mosher D.F., Sugimachi K., Tsuneyoshi M. Enhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinoma. Hepatology. 1998;28(6):1512–1517. doi: 10.1002/hep.510280610. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: a foe or ally? Cell Mol Immunol. 2018;15(4):335–345. doi: 10.1038/cmi.2017.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuyada A., Chow A., Wu J., Somlo G., Chu P., Loera S., Luu T., Li A.X., Wu X., Ye W. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res. 2012;72(11):2768–2779. doi: 10.1158/0008-5472.CAN-11-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto H., Sangai T., Ishii G., Ikehara A., Nagashima T., Miyazaki M., Ochiai A. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125(6):1276–1284. doi: 10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- 45.Wang X.M., Yu D.M., McCaughan G.W., Gorrell M.D. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology. 2005;42(4):935–945. doi: 10.1002/hep.20853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.