Abstract
Children have been neglected in the fight against tuberculosis (TB) for decades. Despite being the number one infectious disease killer, TB does not feature on the child survival agendas partly due to absent and inaccurate data. Quality is a missing ingredient in TB care in children, yet high rates of unfavorable TB outcomes highlight its importance in this age group. Quality care is particularly important for TB affected children in the absence of a point of care sensitive and specific diagnostic test. Using the current models of child TB care, it will take another 200 years to end TB. Without focusing on the quality of child TB care, the ambitious country specific United Nations High Level Meeting for TB targets will carry minimal impact. High TB burden countries must also adopt Universal Health Care (UHC) and ensure that quality TB care is made free and equitable for all children, adolescents and their affected families. We advocate for the importance of evaluating the quality of child TB care, and provide a basic framework for quality in child TB with special attention given to creating differentiated service delivery models for children and families affected by TB.
Keywords: Quality care, Tuberculosis, Children, TB/HIV
1. Introduction
1.1. Global, clinical, and operational difficulties in current child TB care
An estimated 205,000 children died of tuberculosis (TB) in 2018, including 32,000 (16%) children living with HIV, accounting for a mortality rate of 18.3% [1]. Most of these deaths (estimated 80%) were in children who had not yet reached their fifth birthday and 96% were among children who were untreated [2]. Although notification data suggest that TB accounted for just 3.3% of all child deaths in 2018, this is likely a gross underestimate for several reasons. Young children with TB often present with symptoms that overlap and mimic non-TB pneumonia (particularly those living with HIV), and difficult to diagnose extra-pulmonary forms of TB, resulting in under-diagnosis and under-reporting of TB-related child deaths. Further, as microbiological confirmation remains challenging with current diagnostics [3], a significant portion of mortality due to HIV-associated TB is attributed to HIV alone. As a result of these challenges, child TB has been unforgivably missing from the child survival agendas [4].
Of the 10 million people combatting TB in 2018, an estimated 1.12 million were children 0–14 years of age [1]. The TB diagnosis and reporting gap remains unacceptably high across all age groups approaching 33% globally, and remains disproportionately high at 63.3% in children under 5 years of age [1]. Despite some improvement, TB preventive interventions continue to struggle to reach eligible children with recent estimates suggesting that only 23% of eligible children receive TB preventive treatment (TPT) [1]. Eighty-seven percent of the global TB burden is carried by 8 countries alone including India, China, Indonesia, the Philippines, Pakistan, Nigeria, Bangladesh and South Africa. Notified data from these eight countries suggests that children shoulder between 6% (Indonesia) and14% (Nigeria) of the TB burden [1]. Of the 6 of 8 countries [5] with available data, life-saving TPT reaches less than 20% of eligible children, with the exception of South Africa reporting 59–65% [6] TPT coverage in children <5 years and PLHIV. Latent drug resistant DRTB is a growing concern as well, and estimated to affect 3 in every 1000 people globally - with a ten times higher prevalence in children – yet often overlooked or under addressed by TB programs [7]. The global goal of TB elimination will remain well beyond our grasp if resources and efforts are not urgently shifted to ensure that high quality TB care and treatment reaches vulnerable children in TB high burden countries [8].
Children have historically shouldered a disproportionate amount of the global TB burden, largely invisible to TB control programs that focused exclusively on adults with sputum smear positive disease [9]. The traditional model of TB care and treatment relies on referral-based TB disease case finding and treatment at centralized clinical settings, lacking child specific services, and characterized by paternalistic approaches such as national TB program (NTP) controlled access to diagnostics and medications, directly observed therapy (DOT) programs, and passive case finding. Traditional models also place an overreliance on microbiological confirmation to initiate treatment, which disproportionately hinders child TB care as current TB diagnostics lack sensitivity in children. As a result many children are either diagnosed late with severe disease resulting in poor outcomes or never diagnosed. In addition to disempowering patients and their caregivers, these models prevent the much needed integration of child TB into community Integrated Management of Childhood Illnesses (IMCI) and settings such as Maternal, Newborn and Child Health (MNCH) and HIV clinics [1]. Access to TPT for children who are contacts of an adult TB case or living with HIV has been traditionally poor and often difficult for patients to access as it is located only at TB clinics or HIV clinics, often out of stock, and not widely and easily accessible at commonly used health care entry points [9].
Children represent a significant but underappreciated proportion of the DR-TB burden with an estimated 30,000 children becoming sick each year. A meta-analysis of DR-TB treatment outcomes in children showed that 80% had positive outcomes; however, fewer than 5% of children with DR-TB ever start appropriate treatment [10,11].
It is widely recognized that the traditional vertical TB model of passive detection is not sufficient to achieve good outcomes and to reach the WHO's End TB Strategy targets [12]. It is encouraging that countries have committed to the Sustainable Development Goals (SDGs) by 2030 which includes Universal Health Coverage (UHC), financial risk protection, access to quality essential health care services, and access to essential medicines and vaccines for all [13]. UHC provides a critical opportunity in low- and middle-income countries to invigorate child TB services as part of essential health services and integrate them across primary health care and other health platforms such as nutrition, IMNCI, HIV, immunization programs. However, current models of child TB care are poorly aligned with UHC and these goals.
1.2. Quality as a missing ingredient in child TB care
Many TB programs do not adequately address and prioritize children due to the misconception that children do not significantly contribute to TB transmission as a result of their paucibacillary disease and limited ability to aerosolize and spread bacilli [14], [15], [16]. These erroneous beliefs have long undermined the right to health in this population, driven underestimation of the TB burden in these age groups [2], and repeatedly demonstrated to be false as older children and adolescents can present with adult forms of cavitated pulmonary disease, especially in high burden HIV settings [17,18]. Today, quality in pediatric TB care not only requires scale-up of TB services to reach all children in need but also restructuring of the traditional model to incorporate child-friendly comprehensive TB care delivery in an accessible, timely, safe, effective, efficient and equitable manner [19].
1.2.1. Challenges to quality child TB care
1.2.1.1. Diagnosis challenges
A commonly perpetuated misconception is that child TB is difficult to diagnose and requires clinical expertise. Bacteriological confirmation of child TB is indeed challenging due to its’ paucibacillary nature coupled with limitations of currently available diagnostic tests that lack sensitivity in children [20]. Nevertheless, the vast majority of child TB can be diagnosed through clinical algorithms based on a combination of findings, including, history of exposure to TB, clinical presentation, and chest radiography [21]. Although this approach has well-recognized limitations [22], no reliable, simple, non-sputum based, point of care test exists to confirm TB diagnosis making accurate clinical diagnosis a clinical important component of the fight against child TB. Of note, rates of bacteriologic confirmation improve in adolescents compared to younger children, with molecular testing, and additive use of non-respiratory samples such as stool and naso-pharyngeal aspirates [23]. To date, despite ongoing research, there are no biomarker diagnostics available or endorsed for TB diagnosis. The real time PCR platforms available [24] and whole genome sequencing [25] offer promising options for detection of drug resistance, but again rely on respiratory sample collection and mycobacterial isolation. Integrated testing and diagnosis of HIV for those with presumptive TB or TB disease also remains poorly realized and large HIV testing gaps remain in this population [26,27].
1.2.1.2. Treatment challenges
Phase 3 trials rarely include children younger than 17 years of age. As a result, children have delayed access to new medications as it only occurs after efficacy and safety is established in adults. This is a major setback for the most vulnerable cohort of patients where shorter simpler regimens are urgently needed. Pediatric formulations for new, shorter preventive regimens containing rifapentine (3HP or 1HP) and key DRTB drugs (bedaquiline and delamanid) are still not accessible. Further, drug-drug interactions of TB and ARV drugs along with high pill burden remain substantial obstacles. The pediatric dispersible fixed dose combinations for first line treatment have helped reduce issues linked with dosage inaccuracies and palatability [28]. Although nearly 100 countries have procured these drugs from the Global Drug Facility [29], children treated outside of national programs are unable to access the drugs, and TB programs report challenges with stock outs and drug availability at the facility level [30,31]. The recent WHO recommendation for an all oral regimen for MDR TB [32] is a major improvement in drug resistant TB management especially for children, yet these regimens are often not accessible to children in high burden settings. Nevertheless, many drugs are still not in pediatric formulations and have to be crushed using adult tablets. Finally, in the era of growing HIV drug resistance, finding optimal, effective treatment regimens for HIV-associated TB is becoming increasingly difficult.
1.2.1.3. Prevention challenges
An adequately efficacious TB vaccine is yet to become reality. Although the M72/AS01E vaccine has demonstrated promise in adults, no evidence has been produced in children [33]. TPT is a cornerstone of TB control in child household contacts and children living with HIV. Despite WHO currently endorsing multiple options for TPT (e.g. 6H, 9H, 3HR, 4R, and 3HP and 1HP), TPT reaches less than a quarter of eligible children [34]. Drug-drug interactions with newer TPT regimens (e.g. 3HP, 3HR, 4R) and ARVs have yet to be adequately addressed [35], [36], [37].
1.2.1.4. Psychosocial support challenges
Social protection in the form of nutrition support, transport incentives, and cash transfers improve TB outcomes [38,39]. Psychosocial support including education for family empowerment is particularly relevant, but is not a routine component of TB programs in high burden settings, and likely the most commonly overlooked component of care in any setting. Limited evidence suggests that the effect of TB-related stigma and discrimination on children is pervasive and poorly addressed globally, resulting in untold social calamities, poorer health outcomes and interrupted education [40,41].
1.2.1.5. Research and development challenges
Children affected by TB have specific age dependent needs that require a focused research agenda as opposed to the current R & D trend of applying adult technologies to children. There is an urgent need to ensure earlier inclusion of children in research, expedite translation of research findings to policy and close policy-practice gaps [42], [43], [44].
1.2.1.6. Political will and funding challenges
Evidence shows overwhelmingly how concrete investments in child TB can decrease morbidity and mortality, fulfill the rights of the child to have medical care and a healthy life, and meaningfully contribute to the End TB strategy globally [44], [45], [46]. Improvements can be driven through collective efforts that gear country domestic funding and attract donor funding. In high TB burden countries, Ministries of Health and implementers can partner to implement comprehensive strategies that combine efforts towards UHC with active case finding through contact investigation seamlessly linked to referral pathways for prevention, diagnosis and management of child TB that leaves no community behind.
1.2.1.7. Monitoring and evaluation challenges
Robust monitoring and evaluating methodologies are needed to continuously identify gaps in service delivery, identify quality improvement strategies, and advocate for better health services for children with TB. To date, child TB has been neglected in these areas. For example, in the case of reporting, disaggregation of ages among those under 19 years old is not yet the norm in standard indicator in TB and/or TB/HIV programs. The architecture of TB cascades in children is complex, and information analyzing crucial steps such as diagnostic approaches, microbiological diagnosis, and TB outcomes is not routinely available. Moreover, as diagnosis and treatment of TB can happen in different service areas, the information does not flow uniformly in data collection tools, contributing to an unclear picture of quality of care in different age categories.
1.2.1.8. Client-centered care challenges
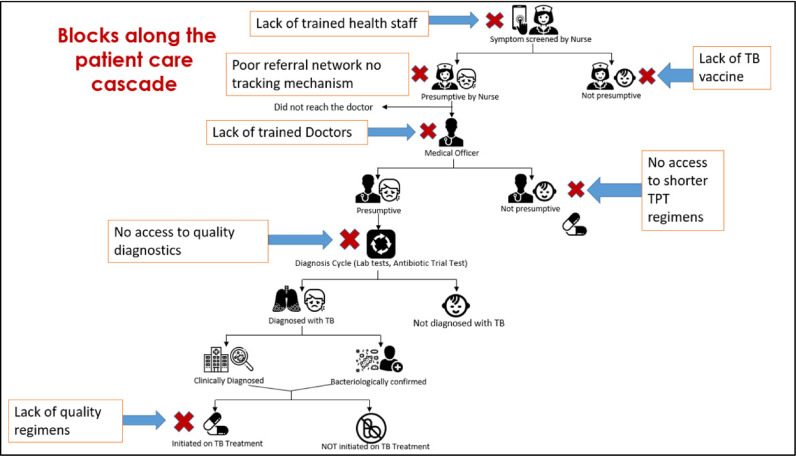
TB care is frequently delivered in vertical programs with little regard to patient and family needs and preferences. Further, TB care often lacks coordination with other services, thus propagating poor access to essential quality-assured services [46,47] (Fig. 1). In Pakistan that ranks 5th in the high TB burden country list, a TB clinic is typically a room in a district/tertiary level public health facility with a medical officer, available for half a day, who dispenses TB medicines, and enters patients in a program register. Case finding relies entirely on passive detection and referrals. The country has a network of over 5000 basic health units each serving 10,000 people, but they lack capacity to effectively suspect TB let alone test, diagnose, and treat it. Even though TB diagnostics of variable quality are available at the secondary level, TB diagnosis in children is limited to tertiary level public hospitals with busy, understaffed, and low quality pediatric services. In Pakistan, similar to high TB burden countries like India and Indonesia, the private sector provides non-standardized, often unaffordable TB care to many patients who remain unreported, and have unknown treatment outcomes [48].
Fig. 1.
Barriers to Quality Care along the TB Patient Care Cascade in high burden settings (Adapted from “Zero TB Cities: Childhood TB Program presentation 2018″ with permission).
When inappropriately addressed, the accumulative negative effect of these challenges promulgates low quality of TB care. Recent data show such challenges have resulted in poor performance in many clinically-important TB indicators such as long diagnostic delays (2 months), high lost to follow up rates (4–38%), high patient costs (half of annual income spent on care), and high TB mortality (1.6 million deaths) and case fatality (16%) worldwide [46].
1.3. Robust data including quality metrics-essential to provide quality care
To achieve the goal of creating integrated, people-centered, equitable, effective and accessible TB services, it is no longer sufficient to simply focus on traditional TB targets and numbers. While the UNHLM created extensive country-level TB diagnostic and treatment targets [49], a thorough assessment of the quality of services - through creation of “TB quality” metrics and indicators - is urgently needed if we are serious about achieving the UNHLM goals. Now more than ever we need commitment to the quality aspect of TB care if we are to advance the development of innovative approaches, improve access to care, and offer needs-driven, evidence-based, highly effective TB services to children and adolescents.
In parallel, children must be considered in TB research and development efforts. Despite the difficulties in including children in research, it is imperative that they are considered when developing diagnostic platforms, new drug formulations, TPT strategies, shortened regimes, TB/HIV treatments, and novel vaccines. These efforts would ideally be augmented by social science research that identifies the most effective and age-appropriate models of care. Operational research is crucial to attain more robust data and to identify gaps in quality of the current systems.
Improvements in quality of care in pediatric TB will generate robust operational research approaches and informative data collection, which will then be used to further bolster the quality of pediatric TB care. The 2018 United Nations High-Level Meeting (UNHLM) on TB created important, ambitious targets (Fig. 2) and commitments that can help guide the creation of robust monitoring, evaluation, and learning systems for measuring quality of child TB efforts [50]. Specifically, these targets include “successfully treating 3.5 million children with tuberculosis by 2022,” preventing tuberculosis for those most at risk, “including 4 million children under five years of age” through preventative treatment by 2022, addressing sociocultural barriers to tuberculosis to mitigate stigma and discrimination (including for children), and increasing commitment to “child-friendly diagnostics” and “safer, more effective, and shorter treatment regimens for adolescents and children.”
Fig. 2.
Global Targets of the UN General Assembly High Level Meeting on TB- Political declaration September 2018.
Reaching these ambitious targets will require a coordinated, dedicated, multisectoral approach, and novel robust data collection and analysis which must recognize and evaluate not just numbers, but the quality of care being delivered. Quality of TB care must be measured, incorporated, and addressed along each step of the TB treatment and prevention cascades, within innovative health system strengthening initiatives, and throughout financing and R&D efforts [19]. While to date, published analyses of these TB cascades of care remains limited, early findings show promise in identifying gaps and areas for improvement. A systematic analysis of the adult LTBI cascade successfully identified the cascade steps with major losses and most amenable to targeted improvement efforts [51]. Likewise, data from adult TB (DR and MDR) cascades in India [52] and South Africa [53] revealed major gaps along multiple steps, including diagnosis, treatment initiation, and treatment/post-treatment follow up, all needing additional attention. Data examining the TB diagnostic cascade in HIV-positive adults in Uganda demonstrated a huge drop off between the positive TB screens and obtaining sputum for testing [54]. These early studies highlight major gaps in the adult TB care cascades and allow for targeted areas of improvement. Child specific data is limited to one recent systematic review that demonstrated a greater than 50% loss of children at each step in the child contact management cascade of care in the majority of studies examined [55]. The use of cascades of care to illustrate quality (or lack thereof) in child TB care can be a game changer for services: gaps in TB treatment or TPT initiation rates can be highlighted to increase access, while a focused attention on TB treatment or TPT completion rates can help improving service delivery [55]. Excellent resources exist for constructing and analyzing TB cascades of care across settings and populations [56,57], and the call to action to utilize these to improve the quality of TB care for child TB has never been stronger or more urgent.
1.4. The role of differentiated care in child TB and creating the “Ideal child TB services model”
Differentiated service delivery (DSD) is defined as a client-centered approach that simplifies and adapts services across the cascade of care in ways that both serve the needs of those receiving the service and reduce burdens on the health system [58]. Quality of services are inherent to a successful DSD model, and this approach is being widely used in HIV programs, with early encouraging results showing improved quality of HIV care, improved client outcomes, and improved cost effectiveness for the health system. For example, the use of differentiated care thinking has been instrumental to reach more people for HIV testing in high burden countries [59], [60], [61], incrementally increasing number of new cases identified, or designing programmatic models of care that support HIV treatment follow up, spacing clinic visits every 3 to 6 months for thousands of people on chronic care [62], [63], [64], [65].
Despite a growing consensus among experts calling for adoption of DSD and tailored models of care in child TB services [46], no such models are formalized or described in the literature. Differentiated TB care for children offers a unique opportunity to enhance the quality of care along the child TB care cascade.
Applying differentiated frameworks that have been successful in HIV programs can promote the creation of context-adapted solutions to “what” package of interventions are needed for success, “how” TB care is delivered in different contexts, “who” provides such care, and “when” various services are delivered. Finer differentiation will likely be needed to effectively consider the unique needs of important patient populations such as younger children vs. adolescents, DSTB vs. DRTB cases, HIV/TB co-infection vs. TB only, and/or patients with socioeconomic vulnerabilities.
The following presents a general framework to approaching the design of child TB DSD models:
WHAT: Integrated, comprehensive, and age-appropriate TB services across the entire prevention, diagnostic, and treatment cascade should be available at every visit. Appropriate, targeted TB screening and diagnostic workup for children and adolescents, coupled with prompt treatment initiation, and patient-centered and patient-preferred treatment monitoring practices need to be offered. A combination of facility vs. community, clinician vs. peer, and traditional vs. novel IT delivery of services should be available to offer affected families a variety of options that can best fit their needs. Flexible clinic hours and follow up schedules (including appointment spacing and fast tracking) also have the potential to improve quality of services.
The essential package of child TB services must also include the following:
-
•
Adequate and adapted screening and diagnostic algorithms that maximize the options to reach a microbiological diagnosis.
-
•
Age appropriate, family centric psychosocial support, education, empowerment opportunities and when possible social protection and travel incentives.
-
•
Age appropriate TB and ancillary testing, DS and DR TB treatment and TPT services for children, utilizing age-disaggregated data (0–2, 3–5, 6–10, 11–14 and 15–19 years)
-
•
Inpatient services for children with severe disseminated forms of TB with appropriate infectious disease isolation, access to advanced imaging and microbiologic testing, linkage to subspecialty services, and client-centered counseling and education.
-
•
TB services integrated at prenatal, antenatal/newborn, and well child clinics, offering TB screening, diagnosis, and treatment, HIV testing, BCG and TPT as routine.
HOW: Health teams should strive to simplify access to TB services across all levels of the health system. Priority must be placed on creating “patient/child-friendly” TB services, such as comprehensive (‘one-stop-shop’) clinics that include TB diagnostics and TB treatment under one roof. TB services must be accessible to the patient/parent/guardian every working day of the week, with patient-friendly hours and locations. When possible, TB work up, treatment initiation and monitoring should be available longitudinally at a single “comprehensive care clinic” and all clinicians at the site should be able to diagnose and treat TB. Linkages to locally-available social support systems also need to be offered to all children with TB, and active case finding efforts with their homes must be done consistently. Due to wide variations in available resources at health care settings in high TB burden countries, a differentiated, adaptable approach to implementing these efforts creatively using available resources is needed.
WHO: As staffing cadres vary site to site, and country to country, it is important that all clinicians and health care cadres interacting with pediatric patients be able to assess children for TB appropriately and consistently, and ensure correct and appropriate follow up and/or linkages occur for children found to have presumptive TB, TB infection/exposure or TB disease so that appropriate workup and/or treatment can be promptly accessed.
WHEN: Health care workers at all sites and locations must consider the possibility of TB infection or TB disease during every encounter and every visit with a pediatric patient. This includes routine visits, unscheduled sick visits, and hospitalizations. By having TB disease in the differential for sick children, and considering eligibility of TPT during well visits, TB will be better prioritized, recognized, and addressed by health care workers, and make TB assessment a more routine part of all pediatric encounters. In high TB burden settings, a diagnosis of TB must be considered, screened and evaluated for by clinicians in all health care settings where children commonly receive care. These include prenatal clinics, MNCH clinics, immunization clinics, outpatient and inpatient departments (e.g. pediatric clinics and pediatric wards), malnutrition programs, and HIV clinics.
Reframing child TB care from a ‘specialized’ service to a ‘routine, simplified standard-of-care’ service will allow clinicians across the spectrum to be empowered to accurately identify, diagnose and confidently treat child TB, including referrals within the health system when needed. Further, combined with robust active case finding in the community, a successful shift of child TB care from central to peripheral settings promises to dramatically narrow the abysmally large child TB detection gap of nearly 63% and enhance the quality of services [66], [67], [68], [69].
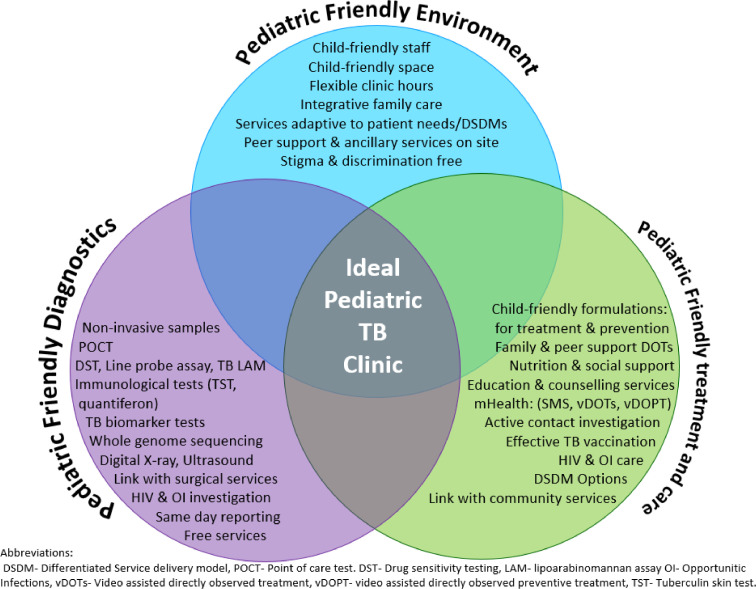
The ideal child TB clinic model (Fig. 3) is inspired by a wish list of integrated services that would effectively capitalize upon diagnostic and preventive services currently out of children's grasp. This model can thrive in both peripheral and central care settings and afford comprehensive prevention, diagnosis and treatment integrated within a child friendly primary care setting. As certain technologies such as genome sequencing, biomarker testing, and DST may never be available at peripheral sites, strengthening health systems to support specimen or patient transport and timely mHealth result reporting between peripheral labs and sentinel sites will be required.
Fig. 3.
The Ideal Pediatric TB Clinic model.
2. Conclusion and a quality way forward
Although TB continues to be a formidable public health challenge whose full extent remains unknown in children, the goal of ending TB in children is attainable. Access to quality TB diagnosis, prevention and treatment services and advances is a basic right for every child. Nevertheless, there are many challenges and gaps that plague health systems of high burden countries and expose the poorest and most vulnerable children to the possibility of death or disability from this preventable disease. Early investment, leveraging local innovation to implement the ideal child TB service model, presented here, can assist countries in reaching the UNHLM targets with lasting impact and pave the road to ending TB in children. As the ideal child TB service model reaches scale and matures as an integral part of the health system through the UHC platform, the quality of services must also advance.
Declaration of Competing Interest
The authors have no competing interests to declare.
Contributor Information
Farhana Amanullah, Email: farhana.maqbool@ird.global.
Jason Michael Bacha, Email: bacha@bcm.edu.
Lucia Gonzalez Fernandez, Email: lucia.gonzalez@iasociety.org.
Anna Maria Mandalakas, Email: anna.mandalakas@bcm.edu.
References
- 1.WHO . 2019. Global TB Report. 2019 October 17. Report No. [Google Scholar]
- 2.Dodd P.J., Yuen C.M., Sismanidis C., Seddon J.A., Jenkins H.E. The global burden of tuberculosis mortality in children: a mathematical modelling study. The Lancet Global Health. 2017;5(9):e898–e906. doi: 10.1016/S2214-109X(17)30289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicol M.P., Zar H.J. New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects. Paediatr Respir Rev. 2011;12(1):16–21. doi: 10.1016/j.prrv.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham S.M., Sismanidis C., Menzies H.J., Marais B.J., Detjen A.K., Black R.E. Importance of tuberculosis control to address child survival. The Lancet. 2014;383(9928):1605–1607. doi: 10.1016/S0140-6736(14)60420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. TB country data profiles. 2018.
- 6.WHO. TB country profile-South Africa. 2018.
- 7.Knight G.M., McQuaid C.F., Dodd P.J., Houben R.M. Global burden of latent multidrug-resistant tuberculosis: trends and estimates based on mathematical modelling. Lancet Infect Dis. 2019 doi: 10.1016/S1473-3099(19)30307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organization WH . World Health Organization; 2018. Global tuberculosis report 2018. [Google Scholar]
- 9.Marais B.J. Improving access to tuberculosis preventive therapy and treatment for children. Int J Infect Dis. 2017;56:122–125. doi: 10.1016/j.ijid.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Harausz E.P., Garcia-Prats A.J., Law S., Schaaf H.S., Kredo T., Seddon J.A. Treatment and outcomes in children with multidrug-resistant tuberculosis: a systematic review and individual patient data meta-analysis. PLoS Med. 2018;15(7) doi: 10.1371/journal.pmed.1002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodd P.J., Sismanidis C., Seddon J.A. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16(10):1193–1201. doi: 10.1016/S1473-3099(16)30132-3. [DOI] [PubMed] [Google Scholar]
- 12.End TB strategy[cited 2019]. Available from:https://www.who.int/tb/post2015_strategy/en/.
- 13.Ghebreyesus T.A. All roads lead to universal health coverage. The Lancet Global Health. 2017;5(9):e839–ee40. doi: 10.1016/S2214-109X(17)30295-4. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Childhood TB: Training Toolkit 2014[cited 2019]. Available from:www.who.int/tb/challenges/childtbtraining_manual/en/.
- 15.Newton S.M., Brent A.J., Anderson S., Whittaker E., Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008;8(8):498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.TB in Children - Getting, diagnosing, preventing TBAvailable from: https://www.tbfacts.org/tb-children/.
- 17.Roy R.B., Brandt N., Moodie N., Motlagh M., Rasanathan K., Seddon J.A. Why the convention on the rights of the child must become a guiding framework for the realization of the rights of children affected by tuberculosis. BMC Int Health Hum Rights. 2016;16(1):32. doi: 10.1186/s12914-016-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marais B.J., Schaaf H.S. Tuberculosis in children. Cold Spring Harb Perspect Med. 2014;4(9) doi: 10.1101/cshperspect.a017855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cazabon D., Alsdurf H., Satyanarayana S., Nathavitharana R., Subbaraman R., Daftary A. Quality of tuberculosis care in high burden countries: the urgent need to address gaps in the care cascade. Int J Infecti Dis. 2017;56:111–116. doi: 10.1016/j.ijid.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiNardo A.R., Detjen A., Ustero P., Ngo K., Bacha J., Mandalakas A.M. Culture is an imperfect and heterogeneous reference standard in pediatric tuberculosis. Tuberculosis. 2016;101:S105–S1S8. doi: 10.1016/j.tube.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Bacha J.M., Ngo K., Clowes P., Draper H.R., Ntinginya E.N., DiNardo A. Why being an expert–despite xpert–remains crucial for children in high TB burden settings. BMC Infect Dis. 2017;17(1):123. doi: 10.1186/s12879-017-2236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Organization WH . World Health Organization; 2014. Guidance for national tuberculosis programmes on the management of tuberculosis in children. [PubMed] [Google Scholar]
- 23.Organization WH . WHO; Geneva: 2013. Policy update: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. [PubMed] [Google Scholar]
- 24.Nikam C., Kazi M., Nair C., Jaggannath M., Manoj M., Vinaya R. Evaluation of the Indian TrueNAT micro RT-PCR device with GeneXpert for case detection of pulmonary tuberculosis. Int J Mycobacteriol. 2014;3(3):205–210. doi: 10.1016/j.ijmyco.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Satta G., Lipman M., Smith G., Arnold C., Kon O., McHugh T. Mycobacterium tuberculosis and whole-genome sequencing: how close are we to unleashing its full potential? Clin Microbiol Infect. 2018;24(6):604–609. doi: 10.1016/j.cmi.2017.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A.M., Gupta D., Kumar A., Gupta R., Kanchar A., Rao R. HIV testing among patients with presumptive tuberculosis: how do we implement in a routine programmatic setting? Results of a large operational research from India. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0156487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velen K., Lewis J.J., Charalambous S., Page-Shipp L., Popane F., Churchyard G.J. Household HIV testing uptake among contacts of TB patients in South Africa. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0155688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.2018. Available from:https://www.who.int/tb/FDC_Factsheet.pdf?ua=1.
- 29.2019. Available from:https://www.tballiance.org/news/one-million-child-friendly-tuberculosis-medicines.
- 30.Hwang B., Shroufi A., Gils T., Steele S.J., Grimsrud A., Boulle A. Stock-outs of antiretroviral and tuberculosis medicines in South Africa: a national cross-sectional survey. PLoS ONE. 2019;14(3) doi: 10.1371/journal.pone.0212405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swaminathan S., Rekha B. Pediatric tuberculosis: global overview and challenges. Clin Infect Dis. 2010;50(Supplement_3):S184–SS94. doi: 10.1086/651490. [DOI] [PubMed] [Google Scholar]
- 32.WHO. MDR TB treatment guidelines2019. Available from:https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/.
- 33.Van Der Meeren O., Hatherill M., Nduba V., Wilkinson R.J., Muyoyeta M., Van Brakel E. Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. N Eng J Med. 2018;379(17):1621–1634. doi: 10.1056/NEJMoa1803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Organization WH. … . World Health Organization; Geneva, Switzerland: 2018. Global tuberculosis report 2018.http://apps.who.int/iris/bitstream 2018. WHO/CDS/TB/2018.20. Available from: [Google Scholar]
- 35.Charan J., Goyal J.P., Reljic T., Emmanuel P., Patel A., Kumar A. Isoniazid for the prevention of tuberculosis in HIV-infected children: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(8):773–780. doi: 10.1097/INF.0000000000001879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zunza M., Gray D.M., Young T., Cotton M., Zar H.J. Isoniazid for preventing tuberculosis in HIV-infected children. Cochrane Database Syst Rev. 2017;(8) doi: 10.1002/14651858.CD006418.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Organization WH . World Health Organization; 2018. Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. [PubMed] [Google Scholar]
- 38.Hargreaves J.R., Boccia D., Evans C.A., Adato M., Petticrew M., Porter J.D. The social determinants of tuberculosis: from evidence to action. Am J Public Health. 2011;101(4):654–662. doi: 10.2105/AJPH.2010.199505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter D.J., Glaziou P., Lönnroth K., Siroka A., Floyd K., Weil D. The impact of social protection and poverty elimination on global tuberculosis incidence: a statistical modelling analysis of Sustainable Development Goal 1. The Lancet Global Health. 2018;6(5):e514–ee22. doi: 10.1016/S2214-109X(18)30195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daftary A., Frick M., Venkatesan N., Pai M. Fighting TB stigma: we need to apply lessons learnt from HIV activism. BMJ Specialist J. 2017 doi: 10.1136/bmjgh-2017-000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Craig G., Daftary A., Engel N., O'Driscoll S., Ioannaki A. Tuberculosis stigma as a social determinant of health: a systematic mapping review of research in low incidence countries. Int J Infect Dis. 2017;56:90–100. doi: 10.1016/j.ijid.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Organization WH. Roadmap towards ending TB in children and adolescents. 2018.
- 43.WHO. Pediatric TB research priorities2018. Available from:http://www.treatmentactiongroup.org/sites/default/files/pediatric_tb_research_priorities_9_24.pdf.
- 44.Seddon J.A., Whittaker E., Kampmann B., Lewinsohn D.A., Osman M., Hesseling A.C. The evolving research agenda for paediatric tuberculosis infection. The Lancet Infect. Dis. 2019 doi: 10.1016/S1473-3099(18)30787-4. [DOI] [PubMed] [Google Scholar]
- 45.Marais B.J., Graham S.M., Maeurer M., Zumla A. Progress and challenges in childhood tuberculosis. The Lancet Infect Dis. 2013;13(4):287–289. doi: 10.1016/S1473-3099(13)70031-8. [DOI] [PubMed] [Google Scholar]
- 46.Reid M.J., Arinaminpathy N., Bloom A., Bloom B.R., Boehme C., Chaisson R. Building a tuberculosis-free world: the lancet commission on tuberculosis. The Lancet. 2019;393(10178):1331–1384. doi: 10.1016/S0140-6736(19)30024-8. [DOI] [PubMed] [Google Scholar]
- 47.Hanson C., Osberg M., Brown J., Durham G., Chin D.P. Finding the missing patients with tuberculosis: lessons learned from patient-pathway analyses in 5 countries. J Infect Dis. 2017;216(suppl_7):S686–SS95. doi: 10.1093/infdis/jix388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fatima R., Haq M.U., Yaqoob A., Mahmood N., Ahmad K.L., Osberg M. Delivering patient-centered care in a fragile state: using patient-pathway analysis to understand tuberculosis-related care seeking in Pakistan. J Infect Dis. 2017;216(suppl_7):S733–S7S9. doi: 10.1093/infdis/jix380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.UNHLM TB country commitments2018. Available from:http://www.stoptb.org/assets/documents/global/advocacy/unhlm/1.%20UNHLM%20on%20TB%20-%20TB%20Country%20Targets.pdf.
- 50.TB S. UNHLM TB targets & commitments2018[July 26 2019,]. Available from:http://www.stoptb.org/assets/documents/global/advocacy/unhlm/UNHLM_Targets&Commitments.
- 51.Alsdurf H., Hill P.C., Matteelli A., Getahun H., Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. The Lancet Infect Dis. 2016;16(11):1269–1278. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- 52.Subbaraman R., Nathavitharana R.R., Satyanarayana S., Pai M., Thomas B.E., Chadha V.K. The tuberculosis cascade of care in India's public sector: a systematic review and meta-analysis. PLoS Med. 2016;13(10) doi: 10.1371/journal.pmed.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naidoo P., Theron G., Rangaka M.X., Chihota V.N., Vaughan L., Brey Z.O. The South African tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis. 2017;216(suppl_7):S702–SS13. doi: 10.1093/infdis/jix335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy M., Muyindike W., Vijayan T., Kanyesigye M., Bwana M., Wenger M. Use of symptom screening and sputum microscopy testing for active tuberculosis case detection among HIV-infected patients in real-world clinical practice in Uganda. J Acquir Immune Defic Syndr. 2016;72(5):e86. doi: 10.1097/QAI.0000000000001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szkwarko D., Hirsch-Moverman Y., Du Plessis L., Du Preez K., Carr C., Mandalakas A.M. Child contact management in high tuberculosis burden countries: a mixed-methods systematic review. PLoS ONE. 2017;12(8) doi: 10.1371/journal.pone.0182185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.2018GWHO. Cascade data use manual: to identify gaps in HIV and health services for programme improvement.
- 57.2019IS. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. ≤https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6392267/≥. [DOI] [PMC free article] [PubMed]
- 58.Differentiated service delivery2019. Available from:http://www.differentiatedcare.org.
- 59.WHO. HIV testing workshop.
- 60.Macdonald V., Verster A., Baggaley R. A call for differentiated approaches to delivering HIV services to key populations. J Int AIDS Soc. 2017;20:21658. doi: 10.7448/IAS.20.5.21658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harichund C., Karim Q.A., Kunene P., Simelane S., Moshabela M. HIV self-testing as part of a differentiated HIV testing approach: exploring urban and rural adult experiences from KwaZulu-Natal, South Africa using a cross-over study design. BMC Pub Health. 2019;19(1):53. doi: 10.1186/s12889-018-6366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selke H.M., Kimaiyo S., Sidle J.E., Vedanthan R., Tierney W.M., Shen C. Task-shifting of antiretroviral delivery from health care workers to persons living with HIV/AIDS: clinical outcomes of a community-based program in Kenya. JAIDS J Acquir Immune Defic Syndr. 2010;55(4):483–490. doi: 10.1097/QAI.0b013e3181eb5edb. [DOI] [PubMed] [Google Scholar]
- 63.Jaffar S., Amuron B., Foster S., Birungi J., Levin J., Namara G. Rates of virological failure in patients treated in a home-based versus a facility-based HIV-care model in jinja, Southeast Uganda: a cluster-randomised equivalence trial. The Lancet. 2009;374(9707):2080–2089. doi: 10.1016/S0140-6736(09)61674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vu L., Waliggo S., Zieman B., Jani N., Buzaalirwa L., Okoboi S. Annual cost of antiretroviral therapy among three service delivery models in Uganda. J Int AIDS Soc. 2016;19:20840. doi: 10.7448/IAS.19.5.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mutasa‐Apollo T., Ford N., Wiens M., Socias M.E., Negussie E., Wu P. Effect of frequency of clinic visits and medication pick-up on antiretroviral treatment outcomes: a systematic literature review and meta-analysis. J Int AIDS Soc. 2017;20:21647. doi: 10.7448/IAS.20.5.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brunetti M., Rajasekharan S., Ustero P., Ngo K., Sikhondze W., Mzileni B. Leveraging tuberculosis case relative locations to enhance case detection and linkage to care in Swaziland. Global Health Res Policy. 2018;3(1):3. doi: 10.1186/s41256-018-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ustero P.A., Kay A.W., Ngo K., Golin R., Tsabedze B., Mzileni B. School and household tuberculosis contact investigations in Swaziland: active TB case finding in a high HIV/TB burden setting. PLoS ONE. 2017;12(6) doi: 10.1371/journal.pone.0178873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mandalakas A.M., Ngo K., Ustero P.A., Golin R., Anabwani F., Mzileni B. BUTIMBA: intensifying the hunt for child TB in Swaziland through household contact tracing. PLoS ONE. 2017;12(1) doi: 10.1371/journal.pone.0169769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khan A.J., Khowaja S., Khan F.S., Qazi F., Lotia I., Habib A. Engaging the private sector to increase tuberculosis case detection: an impact evaluation study. Lancet Infect Dis. 2012;12(8):608–616. doi: 10.1016/S1473-3099(12)70116-0. [DOI] [PubMed] [Google Scholar]