Short abstract
Bone marrow mesenchymal stem cells exist in a multipotential state, where osteogenic and adipogenic genomes are silenced in heterochromatin at the inner nuclear leaflet. Physical force, generated in the marrow space during dynamic exercise exerts control overexpression of differentiation. Mesenchymal stem cells experience mechanical force through their cytoskeletal attachments to substrate, inducing signaling that alters gene expression. The generated force is further transferred from the cytoskeleton to the nucleoskeleton through tethering of actin to Linker of Nucleus and Cytoskeleton (LINC) complexes. Forces exerted on LINC alter the shape and placement of the nucleus within the cell, and are ultimately transferred into the nucleus. LINC complexes transverse the nuclear membrane and connect to the internal nucleoskeleton that is made up of lamin filaments and actin. Force transfer through LINC thus causes structural rearrangements of the nuclear scaffolding upon which chromosomes are arranged. Gene availability is not only modulated through heterochromatin remodeling enzymes and active transcription factors but also by control of nucleoskeletal structure and nuclear enzymes that mediate actin polymerization in the nucleus. Nuclear actin structure may be affected by similar force-activated pathways as those controlling the cytoplasmic actin cytoskeleton and represent a critical determinant of mesenchymal stem cell lineage commitment.
Impact statement
Gene expression is controlled by nuclear structure which is modulated by both internal and external forces exerted on the nucleoskeleton. Extracellular forces experienced through the actin cytoskeleton are transmitted to the internal nucleoskeleton via Linker of Nucleus and Cytoskeleton (LINC) protein connections. LINC complexes directly alter nuclear shape and entry of molecules that regulate transcription. New mechanistic models indicate that nuclear actin is a dynamic component of the filamentous nucleoskeleton and modified by an intranuclear “actin toolbox”, a set of enzymes that regulate linear and branched polymerization of nuclear actin. External stimulation of both biomechanical and biochemical pathways alters nuclear actin structure and has profound effects on gene expression by controlling chromatin architecture and transcription factor access to gene targets. The available data indicate that nucleoskeletal control of gene expression is critical for self-renewal and mesenchymal lineage-allocation in stem cells.
Keywords: Mesenchymal stem cells, Linker of Nucleus and Cytoskeleton, β-catenin, mechanobiology, cytoskeleton, osteoblast
Introduction
Cells in an organism are attuned to perceive, respond, and employ mechanical signals to communicate; this ability to manipulate mechanical stimuli has been recorded as early as when the sperm of a horseshoe crab utilizes forces generated by the actin cytoskeleton to penetrate the egg.1 Sensitivity to mechanical signals is critical to sensing and balancing forces during the gastrulation phases in vivo2 and continues throughout the entire span of an organism. Mesenchymal stem cells (MSC) differentiate to supply cells for musculoskeletal tissue development and continue this process in the adult during tissue regeneration. A key tissue for successful locomotion on land, the skeleton, bears load similar to the rebars and steel beams in modern buildings, but departs from inert building materials by continually remodeling. In adults, MSC harbored in the stem cell niches of bone marrow are recruited to reinforce skeletal tissue, building a skeleton that can withstand physical forces sustained while supporting human activities (e.g. carrying, throwing, running).
At the tissue level, load bearing is anabolic for the skeleton as shown by the greater bone mineral density observed in the playing arm of tennis players when compared to the non-dominant arm, or in the greater tibial BMD of sprinters when compared to non-athletes.3 At the other end of the spectrum, loss of mechanical input is detrimental to the skeleton, for instance the loss of skeletal mass in microgravity.4 Tissue level remodeling of the skeleton requires that MSC are able to sense and respond to physical forces imposed by functional loading in the macro and microenvironment.5 As such, MSCs poised in stem states in different depot locations receive signals for recruitment. The MSC responds to physical and soluble factors with specific gene expression driving expansion of clonal progenitors within an emergent lineage, and then expression of functional proteins reflecting the differentiated cell. For example, to become a bone osteoblast with capacity to secrete bone matrix for mineralization of skeletal tissue, the bone marrow MSC must leave its hematopoietic niche, turning on early genes to allow proliferation, and then, once within the bony matrix, express a set of genes having to do with terminal differentiation and function.6 Alternately, MSCs within the bone marrow also have the capacity to become adipocytes, a necessary cell both for regeneration and energy storage.7
It is recognized that MSCs perceive force via the cytoskeleton, which is a force responsive cell scaffold that undergoes structural remodeling after exogenous mechanical stimulation. A key part of this structural remodeling occurs at the level of the actin cytoskeleton, which in turn profoundly regulates MSC lineage commitment and differentiation.8 Studies focusing on how physical force results in gene expression in the MSC have led to new insights into yet another level of structural remodeling that occurs in the nucleus. Here, we will consider how architectural changes in the nucleus may participate in epigenetic regulation of gene expression and cell function.
Nuclear morphology, and subsequently gene expression, is subject to external mechanical force
The nucleus is the repository of genes, the expression of which determines the identity of the cell and its functionality. For the MSC, this requires genes, which are initially silenced within a “closed” heterochromatin state, to be re-established in an “open” euchromatin state that renders genes available to master transcription factors that induce differentiation. Silenced genes are generally found in the periphery of the nucleus, and move centrally as they become active.9 This hints at an active nuclear landscape where location and structural regulation of chromosomal location are key to gene expression.
The nucleus has discrete mechanical properties. It is the largest and densest organelle in the cell, and its structure—including size, height, area, and stiffness—is determined by its outside self-connections to non-nuclear cellular elements, and inside by nucleoskeletal connections made up of intermediate lamina filaments, lamins A/C and B and the cellular DNA. During differentiation, stem cell nuclei become stiffer even in the absence of outside connections, largely due to increased lamin A/C expression.10 While lamin B1-deficient cells have normal nuclear mechanics, loss of lamin A/C reduces nuclear stiffness, and is thus thought to account for the majority of nuclear stiffness.11 The stiffer nucleus has an increased proportion of genes in the heterochromatin state, and this will modulate the response to incoming signals.12 In this way, both the remodeled nucleoskeleton and nuclear stiffness appear to alter the scaffolding on which chromatin is arrayed. Besides actin contributing to forces placed on the nucleus, our work suggests that actin polymers are key contributors within the nucleus to provide chromatin scaffolding.
Nuclear position, along with its height and area, is regulated through forces exerted on the nuclear envelope by the cytoplasmic cytoskeleton. In this way, the nucleus of the cell, connected through a network of microtubules and actin struts to integrins on the substrate surface, also gathers information regarding the external environment. Physical substrate strains or fluid flow over the cell as would be induced by mechanical loading of the skeleton induces clustering of integrins, activation of RhoA kinase, and subsequently induction of new focal adhesions.13 Focal adhesions, besides connecting cells to their external substrate, also serve as hubs for the recruitment and clustering of signaling molecules, where the combination of force transmission and signal transduction result in further cytoskeletal remodeling.14 Studying MSCs, our group demonstrated that substrate strain induced rearrangement of focal adhesions and their interconnecting F-actin struts.8 Focal adhesion development requires activation of RhoA, a process that we found devolved from strain-induced FAK/Fyn, with subsequent activation of mTORC2 (mTorc-Rictor) and its Akt target.8,15 An important result of focal adhesion development through dynamic strain is the enhancement of signaling. A repeated bout of mechanical strain augmented force-induced signal pathway as demonstrated by increased phospho-Akt, GSK3β inhibition, β-catenin preservation and, when repeated twice daily over the four days necessary for adipocyte differentiation, significantly enhanced the ability of strain delivery to repress adipogenesis.14 Investigating a potential role of mTORC2 in marrow-derived MSC cell differentiation, we found that deleting mTORC2 function in cells by knocking down Rictor (rapamycin-insensitive companion of mTOR, the component delineating mTORC2 from mTORC1) resulted in adipogenesis.8 Accordingly, Rictor knockout mice have reduced skeletal mass.16
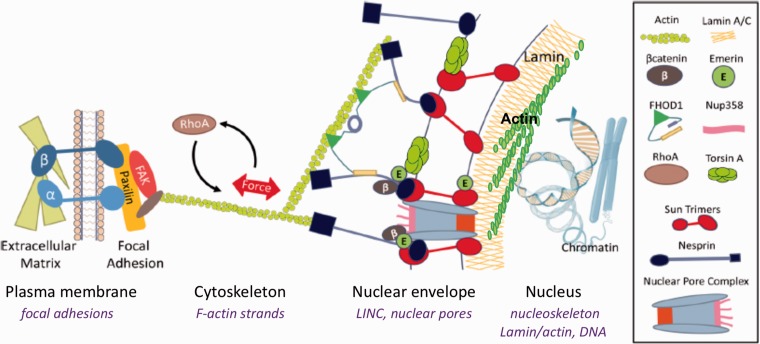
At focal adhesion sites, activation of RhoA causes polymerization of actin, with actin polymers tenting the cell structure both between focal adhesion sites and to the Linker of Nucleus and Cytoskeleton (LINC) connections at the nuclear envelope (Figure 1). LINC complexes provide the physical hardwiring of the cell nucleus to the outside world allowing the nucleus to perceive the extranuclear mechanical environment by tying the nucleus to the cytoplasmic structure through interactions with actin, microtubule, and intermediate filament networks.17 At the cytoplasmic face, LINC complexes are composed of the giant nesprin proteins 1 and 2. N-termini of giant nesprins share a Calponin Homology domain identical to that found in α-actinin that bind actin with high affinity and promote actin polymerization.18 Through interactions with kinesin and dyenin, nesprin on the cytoplasmic face of the nucleus also interacts with microtubules.19 The C-termini of the nesprin proteins protrude through the outer nuclear membrane via a transmembrane domain to end in a highly conserved Klarsicht, ANC-1, Syne Homology (KASH) domain.20 Overexpression of nesprin-mini, a nesprin designed to lack the domains of giant nesprin that differentiate it from smaller nesprins expressed throughout the cell, decreases nuclear size.21 This suggests that giant nesprins might form a filamentous network that wraps around the nucleus and restricts nuclear size. The KASH domain of giant nesprin binds to the Sun proteins that span the nuclear leaflet and emerge within the nucleus where they bind to the inner lamin A/C network. In mammalian cells, two isoforms, Sun-1 and Sun-2 are expressed,22 and may determine the distance between the inner and outer nuclear membranes.23 Structural analysis of Sun proteins reveal that the highly homologous proteins Sun-1 and Sun-2 form trimers which each bind a KASH peptide.24 While not shown to be required for LINC structure, the protein emerin is also included as part of the LINC complex, likely participating in actin polymerization.25 Emerin’s nuclear localization supports its role in nuclear actin assembly, where it appears to regulate the actin dynamics important for the gene regulation by the MKL1-SRF transcription factor.26 Emerin also interacts with β-catenin, playing a role in its nuclear export.27
Figure 1.
LINC complex connects the external actin cytoskeleton to the nucleoskeleton. The nuclear envelope, nucleoskeleton, and their binding partners facilitate mechanical coupling between cytoplasmic and nuclear cytoskeletons. LINC complexes composed of Sun trimers and Giant Nesprin mechanically couple the actin cytoskeleton, and involve FHOD1 and Torsin facilitated LINC assembly. Chromatin interacts with nuclear structural elements to regulate gene expression including lamin B (through lamin B receptor, LBR), lamin A/C (via lamin-associated domains, LADs), and actin filaments. Inside the nucleus, G-actin is assembled into linear rods via mDia and into branched networks via the Arp2/3 complex, and interacts with lamin structure of the nucleoskeleton. (A color version of this figure is available in the online journal.)
Both focal adhesions and the actin polymers which emerge to ultimately connect to other focal adhesions8 or LINC contacts on the nucleus,28 or to course over the nucleus as TAN lines,29 are controlled by external forces. Static and dynamic forces affect RhoA, both through its activation through a specific GEF, LARG.30,31 Induced actin structure thus affects cell shape and eventually nuclear shape by altering force on the nucleus. Force transmission is largely thought to require actin connections to LINC, for instance, interfering with the LINC protein nesprin’s ability to bind actin polymers untethers the nucleus from aspects of cytoplasmic architecture, resulting in loss of force transmission into the nucleus.32
Importantly, the ability to sense the environment and transmit force into the nucleus alters differentiation on MSC. Embedded in this canon is the influential work of Engler et al. showing that cytoskeletal sensing of substrate force directed MSC differentiation: hard substrates promote the osteoblast cell fate and soft surfaces encourage the adipocyte phenotype.33 It has since been accepted that genetic elements within the nucleus respond to mechanical challenges indirectly through their transduction into intermediary biochemical cascades, for instance with activation of signals such as β-catenin34 or yes-associated protein (YAP),35 both which translocate to the nucleus. Mounting evidence suggests that applied forces might also directly alter chromosomal conformations, thus influencing the accessibility of genetic information for binding of transcriptional enhancers or repressors.36,37 The ultimate target of LINC connectivity and transfer of structural information is the nuclear lamin nucleoskeleton packed against the inner nuclear leaflet. In this way, alteration of LINC by changes in intracellular forces is expected to modulate gene expression. For example, depleting LINC element Nesprin-2 disrupts the localization and reduces levels of the heterochromatin protein HP1β38 which regulates levels of trimethylated histone (H3K9Me3).39 Heterochromatin loss mediated by decreased HP140 levels are implicated in aging41,42 and in premature aging syndromes.43 In yeast, deletion of the Sun analog Csm4 unravels chromatin organization increasing its diffusivity and preventing DNA repair.44 Decreased HP1β levels in MSCs with non-functional LINC complex suggest that a disorganized nucleus experiences deregulated transcription.
The ultimate target of LINC connectivity and transfer of structural information is the nuclear lamin nucleoskeleton packed against the inner nuclear leaflet. In this way, alteration of LINC by changes in the intracellular force is expected to modulate gene expression. For example, our group recently reported that simulated microgravity, which disrupts F-actin contractility,45 alters nuclear morphology and decreased lamin A/C as well as LINC elements.46 Conversely, protecting F-actin contractility by applying exogenous low intensity vibrations protects the lamin A/C and LINC expression from the effects of simulated microgravity.47
The structure of the inner nuclear leaflet modulates its association with silenced genes
Genes are highly organized and compacted within the nucleus in nucleosomal units, that further assemble into larger structures (e.g. 30-nm fiber) and chromatin loops. In mammals, specific genes, or regions of genes, can directly interact with the nucleoskeleton at the level of DNA or “closed” heterochromatin.48 Chromatin associated with the inner nuclear leaflet tends to be either gene-poor or transcriptionally silenced.49 Thus, genes activated during late stages of differentiation are expected to be peripherally localized at earlier stages of differentiation. Indeed, nuclear architecture is rearranged during differentiation to facilitate transcription of specific genes.50 For instance, PPAR-γ (PPARG), the master transcription factor for adipogenesis, and other adipogenic genes are located in the nuclear periphery in bone marrow MSCs, but move centrally in response to an adipogenic stimulus.51 The mechanisms controlling such structural rearrangements of genes are currently unknown.
The inner nuclear leaflet that harbors portions of chromatin in the heterochromatin state is composed of lamins, specifically lamin B and lamin A/C. Lamin B (LMNB1) is constitutively expressed in all cells, and is associated with promoters of differentiation,52 while the expression of lamins A and C, different splice variants of the LMNA gene, is variably controlled with respect to differentiation in mammalian cells.53 While pluripotent embryonic stem cells do not express lamins A and C, in somatic MSCs both types of lamins are present and tether heterochromatin to the inside nuclear leaflet,54 thus have effects on chromatin structure and gene expression. Further, lamins control localization of polycomb proteins, maintaining gene repression through compartmentalization.55
The portions of chromatin associated with the lamina or the lamina-associated domains are largely considered to be transcriptionally repressed in a heterochromatin state. Hence, any alterations in the nuclear lamina, e.g. in Emery–Dreifuss muscular dystrophy, can be reflected in aberrations in chromatin state to potentially disrupt gene position and access by transcription factors. One study observed that after mutation of lamin A, chromosomes 13 and 18 relocated from the nuclear periphery to the interior.56 Further, disruption of the inner nuclear leaflet due to loss of lamin A/C not only has effects on chromosomal state but can alter actin dynamics. The latter may have profound effects on nuclear-cytoplasmic movement of transcription factor MKL-1, suggesting that lamin A/C can indirectly control gene expression through effects on dynamic actin structure.26
Nuclear access of transcription factors is governed by dynamic structures in the cell
The nuclear envelope functions as a barrier to transcription factors that shuttle from the cytoplasm to the nucleus and back. As covered above, nuclear shape is defined by cytoplasmic structures that exert tension on the nucleus to control the size of nuclear pores.57 Actin, through binding to LINC, is part of this structural network. Actin structure can also influence the activity of transcription factors through their sequestration or release. Currently, the most data available in this regard is for the mechanically active transcription factor YAP, which is released by polymerized cytoplasmic actin to be transported into the nucleus.58 The nuclear entry of YAP stimulates multicellular growth of organs.35 Exit of transcription factors from the nucleus leading to decreased target expression can also be regulated by changes in cytoplasmic actin, for instance, MKL1 transport from the nucleus to bind actin monomers in the cytoplasm appears to remove constraints on PPARG to promote adipocyte lineage commitment.59 Hence, actin structure external to the nucleus clearly affects many types of control on gene expression by allowing for import and export of transcription factors.
Transcription factor β-catenin (CTNNB1) must enter the nucleus to affect genes involved in lineage. β-catenin lacks a classical nuclear localization signal, and is thought to directly interact with Nuclear Pore Complexes (NPCs) during nuclear entry.60 This nuclear import of β-catenin is likely to be preceded by its ability to form complexes with the LINC component nesprin at the nuclear envelope,61 suggesting that the cell cytoskeleton interacts with the LINC complex to provide a scaffold to localize β-catenin in close proximity to NPCs. Consistent with a potential regulatory role of LINC complexes for β-catenin nuclear transfer, progeroid mutations involving LINC and nucleoskeleton elements62 are marked by increased adipogenic infiltration in musculoskeletal tissues indicating reductions in Wnt activity and cellular β-catenin.63
Our laboratories have directly addressed how structural LINC complexes regulate β-catenin trafficking into the nucleus. Initial studies revealed that depletion of the LINC components Sun-1 and Sun-2 causes Nesprin-2 dislocation from the nuclear envelope and disrupts focal adhesion kinase (FAK/PTK2) signaling due to loss of actin tethering to nesprin.64 We next found that both mechanical and soluble factor activation of β-catenin, each requiring the intermediary step of GSK3β inactivation, led to β--catenin association with the nucleoskeleton. This association preceded localization of β--catenin in the soluble nuclear compartment.65 Interestingly, KASH-less isoforms of nesprin also form complexes with β-catenin at cell–cell junctions and can regulate β-catenin availability in the cytoplasm.66 To address the possibility that LINC connections were involved in β-catenin trafficking, we co-depleted LINC elements Sun-1 and Sun-2 to find that loss of Sun proteins prevented β-catenin’s association with the nucleoskeleton. In the absence of nucleoskeletal association, β-catenin nuclear entry was reduced, resulting in decreases in nuclear β-catenin levels and expression of the known β--catenin target Axin-2.65 As such, not only does cytoskeletal structure, which is subject to regulation by dynamic and static external mechanical factors, impose a cytomechanical checkpoint at the level of LINC complexes to regulate β-catenin access to the inner nucleus but the insoluble structural nucleoskeleton also actively participates in β-catenin dynamics.
Convergence of signaling molecules and cell actin structures to control gene expression
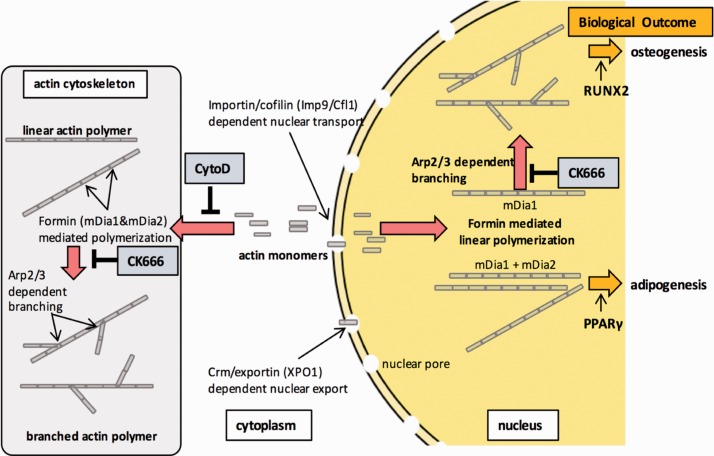
The same proximal strain-activated pathway that leads to inhibition of GSK3β and preservation of β-catenin34 is also involved in regulating actin polymerization through RhoA.15 Similarly, both β--catenin and increased F-actin are associated with decreased adipogenesis from MSCs.31,67 As such we became interested in parsing control of differentiation as resulting from either the cytoskeleton or signaling cascades. In a study aimed at separating effects of β-catenin from that of cytoskeletal regulation of differentiation, we disrupted the MSC cytoskeleton using continuous cytochalasin D over the several days necessary to induce differentiation from the multipotent state.14,58 Expecting that reduced cytoskeletal structure induces MSCs to enter the adipogenic lineage,68 we instead found that marrow-derived MSC rapidly and robustly entered the osteogenic lineage, eventually mineralizing with formation of hydroxyapatite.69 This osteogenic response to continuous cytochalasin D was replayed in MSCs derived from adipose depots70 and even occurred in the absence of osteogenic medium (which promotes the osteogenic gene program through ascorbate-directed formation of an extracellular matrix). Importantly, injection of the tibial space with cytochalasin D led to abundant formation of and cortical bone by one week in live mice.69
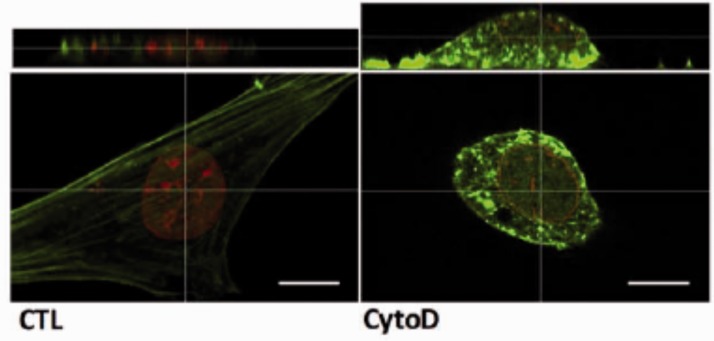
To understand the mechanism leading to rapid MSC differentiation, we noted that cytochalasin D actin disruption resulted in mass transport of cellular actin into the nucleus. After CytoD treatment, stress fibers disassembled and accumulated in the nucleus within 30 min.69 Visually, there is a profound alteration in nuclear structure after actin polymerization, with increased height, partially due to loss of LINC connectivity, and in part due to increased nuclear actin (Figure 2). Actin transport into the nucleus is dependent on the level of monomeric actin substrate and the co-transporters importin-9 and the actin binding protein cofilin-1.71 Knock out of either importin-9 or cofilin-1 prevented actin translocation into the nucleus and most tellingly, prevented osteogenesis altogether. In sum, the depolymerized actin state induced by cytochalasin D profoundly affects gene expression,72 promoting differentiation, with osteogenesis of MSC outpacing adipogenesis (Figure 3).
Figure 2.
Nuclear changes after depolymerizing actin. Confocal images of MSC before and after treatment with cytochalasin D. Nuclear height is increased due to CytoD which untethers LINC from cytoplasmic actin and causes actin transfer into the nucleus. As CytoD does not enter the nucleus, there is an increase in intranuclear F-actin. F-actin is green, stained with phalloidin, lamin B is stained with red, Scale bars = 25 µm. (A color version of this figure is available in the online journal.)
Figure 3.
Nuclear actin controls MSC differentiation. Cytochalasin D treatment of MSC causes rapid cofilin/importin-9 dependent transfer of G-actin into the nucleus. Actin elongation inside the nucleus supports both osteogenesis and adipogenesis, while secondary branching of actin polymers is specifically necessary for osteogenic differentiation. (A color version of this figure is available in the online journal.)
Interestingly, β-catenin might eventually be found to be indirectly involved in controlling actin state through its association with α-catenin in cadherin junctions.73 α-catenin does not bind both β-catenin and actin at the same time,74 thus presumably when β-catenin is “activated” and moves toward the nucleus, α-catenin is freed from sequestration and dimerizes. In this state, it can suppress Arp2/3-mediated actin branching. As such, activating β--catenin may indirectly contribute to cytoplasmic actin structure by promoting α-catenin’s bundling of linear actin.75
Intranuclear actin affects gene expression
Actin is known to play a role in gene transcription, at the very least through altering chromatin architecture76 and transcriptional processes.77,78 This certainly provides a solid teleological rationale for why actin nuclear transport is tightly regulated.71 Further, as discussed above, nuclear shape is controlled through the tethering of the nuclear membrane within the cell through cytoplasmic cytoskeletal connections to the LINC complex.17 Importantly, LINC complex connects to internal nuclear chromatin, such that changes in nuclear shape are thought to be able to modulate gene silencing and activation through regulating the internal nucleoskeleton, largely made up of lamin.76 The idea that intranuclear actin itself may participate in structural rearrangements of chromatin and heterochromatin is an exciting new concept that deserves consideration in mechanistic models for lineage commitment of stem cells.
In addition to its monomeric form, intranuclear actin also exists in filamentous forms79,80 and as actin-cofilin rods.81 Polymerized actin is likely the minority of intranuclear actin, but turns over rapidly82 suggesting a susceptibility to dynamic control. The role of intranuclear actin and actin-based structures in controlling gene transcription is poorly understood.83 In our studies of MSC treated with cytochalasin D, we were able to see phalloidin staining within the nucleus, a sign of actin bundling.84 Intranuclear actin is protected from the depolymerizing effects of cytochalasin D, since this fungal metabolite is not transferred into the nucleus.85 Moreover, a rise in intranuclear actin concentration, such as that seen with cytochalasin D treatment, should promote substrate-regulated polymerization.25 Interestingly, one of the few genes that is consistently upregulated by cytochalasin D is Vestigial-Like 4,72 a regulator of the Hippo pathway that controls the interactions of YAP and TAZ with TEAD transcription factors.
Importantly, within the nucleus are to be found all the generally accepted members of the actin tool box that allow polymerization and depolymerization of actin monomers.86 These components include the formins which catalyze end-on-end actin polymerization as well as key members of the Arp2/3 complex necessary for initiating secondary branching.87 Several reports indicate that the polymerized state of intranuclear actin guides targeting of some transcription factors. For instance, MLK1 (i.e. MAL) binds monomeric actin in the nucleus thereby preventing its binding to and co-activation of serum response factor.78 Upon exposure to serum, MLK1 shuttles into the nucleus. Once inside, formin activated actin polymerization ensures MLK1 retention where it promotes serum-induced transcriptional responses.
Polymeric actin also influences differentiation of mesenchymal stem cells. Progression into the osteogenic lineage requires the master osteogenic transcription factor, RUNX2, which although present within the nucleus, does not actively interact with its target cistrome until the MSC is induced to leave the multipotent state and enters osteogenesis.6 The PY motif of RUNX2 has been previously shown to recruit YAP to RUNX2 binding sites at heterochromatin, where its presence represses RUNX2 activity.88 Our data suggest that RUNX2 activation may be regulated through nuclear availability of YAP, consistent with previous studies.69 Another possibility is that internal nuclear structure itself controls heterochromatization, a mechanism supported by the binding of lamin A/C to DNA causing specific silencing, perhaps through recruiting polycomb complexes.89,90
Actin in the nucleus has also been directly implicated in gene transcription because of its association with RNA polymerase II. Nuclear actin co-localizes with and can be immune-precipitated along with RNA polymerase II.91 Further, it was observed that removing the pool of monomeric nuclear actin by polymerizing monomers into filamentous forms disrupted gene expression and global transcription.92 Monomeric actin may also exert some of its activity by interacting with proteins in the histone deacetylase 1 (HDAC1) complex. In one study, increased concentrations of monomeric actin limits HDAC function, while loss of the monomeric pool to polymeric actin filaments allowed for a greater HDAC activity which could potentially reduce transcription.93 In yeast, monomeric actin has also been observed in the chromatin remodeling complex INO80 along with Arp4, Arp5, and Arp 6, and as a complex may be involved in gene transcription.77,94 As such, the intranuclear state of actin controls not only availability of genes to their transcription factors but transcription itself.
Inhibition of actin polymerization also reduces subtelomeric dynamics, suggesting actin structure protects telomere integrity.44 Furthermore, Sun-1 and the sheltering subunit RAP-1 mediate physical tethering of telomeres to the nuclear envelope during postmitotic genome reorganization,95 suggesting that nucleoskeletal composition imposes a powerful influence on telomere function and maintenance. As telomeres protect chromosomal termini from being processing as “damaged” DNA fragments,96 it is possible that force induced changes in nucleoskeletal composition and architecture contribute to stem cell tissue regeneration.
Conclusions
The nucleus itself is not a compartment that merely contains genetic information. Rather, it is a full participant in cell behavior and function, with an internal nucleoskeleton subject to regulation by external forces. Gene expression in MSCs is dependent on the nucleoskeleton allowing access of transcription factors to cistromic targets required for acquisition of cell phenotype. Mechanical forces, transmitted through the nuclear membrane via LINC complexes, not only modulate the inward flow of active transcription factors but also control heterochromatization. In the cytoplasm, actin is critical to the cytoskeletal scaffolding that transmits force into the nucleus and regulates nuclear shape. Mounting evidence suggests that structural elements of the nucleoskeleton are themselves dynamic. Along with changes in lamin shown during stem cell differentiation, the presence of actin-modifying enzymes within the nucleus indicates that intranuclear actin polymerization is subject to regulation. Dynamic actin polymerization is likely to change gene availability by directly altering how nucleoskeletal lamins are arranged and through direct effects on chromosomal structure. We conclude that dynamic actin modifications alter the nuclear landscape and that nuclear actin structure is a key architectural parameter that supports and regulates gene expression and stem cell differentiation.
Authors’ contributions
All authors have contributed to the authorship of this review article.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The authors are grateful to the NIH for awards AR066616 (JR), P20GM109095 (GU), R01 AR049069 (AvW).
References
- 1.Sanders MC, Way M, Sakai J, Matsudaira P. Characterization of the actin cross-linking properties of the scruin-calmodulin complex from the acrosomal process of Limulus sperm. J Biol Chem 1996; 271:2651–7 [DOI] [PubMed] [Google Scholar]
- 2.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 2005; 6:622. [DOI] [PubMed] [Google Scholar]
- 3.Ozcivici E, Luu YK, Adler B, Zin YX, Rubin J, Judex S, Rubin CT. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol 2010; 6:50–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm D, Grosse J, Wehland M, et al. The impact of microgravity on bone in humans. Bone 2016; 87:44–56 [DOI] [PubMed] [Google Scholar]
- 5. Pagnotti GM, Styner M, Uzer G, Patel VS, Wright LE, Ness KK, Guise TA, Rubin J, Rubin CT. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat Rev Endocrinol 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer MB, Benkusky NA, Sen B, Rubin J, Pike JW. Epigenetic plasticity drives adipogenic and osteogenic differentiation of marrow-derived mesenchymal stem cells. J Biol Chem 2016; 291:17829–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, Xie Z, Zong X, Styner MA, Rubin C, Rubin J. Exercise decreases marrow adipose tissue through beta-oxidation in obese running mice. J Bone Miner Res 2017; 32:1692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen B, Xie Z, Case N, Thompson WR, Uzer G, Styner M, Rubin J. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J Bone Miner Res 2014; 29:78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalli G, Misteli T. Functional implications of genome topology. Nat Struct Mol Biol 2013; 20:290–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hampoelz B, Lecuit T. Nuclear mechanics in differentiation and development. Curr Opin Cell Biol 2011; 23:668–75 [DOI] [PubMed] [Google Scholar]
- 11.Lammerding J, Fong LG, Ji JY, Reue K, Steward CL, Young SG, Lee RT. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem 2006; 281:25768–80 [DOI] [PubMed] [Google Scholar]
- 12.Heo SJ, Driscoll TP, Thorpe SD, Nerukar NL, Baker BM, Yang MT, Chen CS, Lee DA, Mauck RL. Differentiation alters stem cell nuclear architecture, mechanics, and mechanosensitivity. Elife 2016; 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 1996; 133:1403–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen B, Guilluy C, Xie Z, Case N, Styner M, Thomas J, Oguz I, Rubin CT, Burridge K, Rubin J. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells 2011; 29:1829–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson WR, Guilluy C, Xie Z, Sen B, Brobst KE, Yen SS, Uzer G, Styner M, Case N, Burridge K, Rubin J. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells 2013; 31:2528–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Holguin N, Shi Y, Silva MJ, Long F. mTORC2 signaling promotes skeletal growth and bone formation in mice. J Bone Miner Res 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 2006; 172:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J Cell Sci 2002; 115:3207–22 [DOI] [PubMed] [Google Scholar]
- 19.Wilson MH, Holzbaur EL. Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 2015; 142:218–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 2002; 298:406–9 [DOI] [PubMed] [Google Scholar]
- 21. Lu W, Schneider M, Neumann S, Jaeger VM, Taranum S, Munck M, Cartwright S, Richarson C, Carthew J, Noh K, Goldberg M, Noegel AA, Karakesisoglou I. Nesprin interchain associations control nuclear size. Cell Mol Life Sci 2012; 69:3493–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodzic DM, Yeater DB, Bengtsson L, Otto H, Stahl PD. Sun2 is a novel mammalian inner nuclear membrane protein. J Biol Chem 2004; 279:25805–12 [DOI] [PubMed] [Google Scholar]
- 23.Cain NE, Starr DA. SUN proteins and nuclear envelope spacing. Nucleus 2015; 6:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sosa BA, Rothballer A, Kutay U, Schwartz TU. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell 2012; 149:1035–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holaska JM, Kowalski AK, Wilson KL. Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLoS Biol 2004; 2:E231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013; 497:507–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilgner K, Wojciechowicz K, Jahoda C, Hutchison C, Markiewicz E. Dynamic complexes of A-type lamins and emerin influence adipogenic capacity of the cell via nucleocytoplasmic distribution of beta-catenin. J Cell Sci 2009; 122:401–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uzer G, Rubin CT, Rubin J. Cell mechanosensitivity is enabled by the LINC nuclear complex. Curr Mol Bio Rep 2016; 2:36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luxton GW, Gomes ER, Folker ES, Worman HJ, Gundersen GG. TAN lines: a novel nuclear envelope structure involved in nuclear positioning. Nucleus 2011; 2:173–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol 2011; 13:722–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson WR, Yen SS, Uzer G, Xie Z, Sen B, Styner M, Burridge K, Rubin J. LARG GEF and ARHGAP18 orchestrate RhoA activity to control mesenchymal stem cell lineage. Bone 2018; 107:172–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem 2011; 286:26743–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126:677–89 [DOI] [PubMed] [Google Scholar]
- 34.Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J Biol Chem 2009; 284:34607–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013; 154:1047–59 [DOI] [PubMed] [Google Scholar]
- 36.Le HQ, Ghatak S, Yeung CC, Tellkamp F, Gunschmann C, Dieterich C, Yeroslaviz A, Habermann B, Pombo A, Niessen CM, Wickstrom SA. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol 2016 [DOI] [PubMed] [Google Scholar]
- 37.Makhija E, Jokhun DS, Shivashankar GV. Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc Natl Acad Sci USA 2016; 113:E32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashmi RN, Eckes B, Glockner G, Groth M, Neumann S, Gloy J, Sellin L, Walz G, Schneider M, Karakesisoglou I, Eichinger L Noegel AA. The nuclear envelope protein Nesprin-2 has roles in cell proliferation and differentiation during wound healing. Nucleus 2012; 3:172–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischle W, Wang Y, Jacobs SA, Kim Y, Allis CD, Khorasanizadeh S. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev 2003; 17:1870–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maison C, Almouzni G. HP1 and the dynamics of heterochromatin maintenance. Nat Rev Mol Cell Biol 2004; 5:296–304 [DOI] [PubMed] [Google Scholar]
- 41.Pal S, Tyler JK. Epigenetics and aging. Sci Adv 2016; 2:e1600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wood JG, Hillenmeyer S, Lawrence C, Chang C, Hosier S, Lightfoot W, Mukherjee E, Jiang N, Schorl C, Brodsky AS, Neretti N, Helfand SL. Chromatin remodeling in the aging genome of Drosophila. Aging Cell 2010; 9:971–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, Jenuwein T, Goldman RD. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci USA 2006; 103:8703–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spichal M, Brion A, Herbert S, Cournac A, Marbouty M, Zimmer C, Koszul R, Fabre E. Evidence for a dual role of actin in regulating chromosome organization and dynamics in yeast. J Cell Sci 2016; 129:681–92 [DOI] [PubMed] [Google Scholar]
- 45.Chen Z, Luo Q, Lin C, Kuang D, Song G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Sci Rep 2016; 6:30322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Touchstone H, Bryd R, Loisate S, Thompson M, Kim S, Puranam K, Senthilnathan AN, Pu S, Beard R, Rubin J, Alwood J, Oxford JT, Uzer G. Recovery of stem cell proliferation by low intensity vibration under simulated microgravity requires intact LINC complex npj. Microgravity 2019, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uzer G, Pongkitwitoon S, Ete Chan M, Judex S. Vibration induced osteogenic commitment of mesenchymal stem cells is enhanced by cytoskeletal remodeling but not fluid shear. J Biomech 2013; 46:2296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kind J, van Steensel B. Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol 2010; 22:320–5 [DOI] [PubMed] [Google Scholar]
- 49.Underwood JM, Becker KA, Stein GS, Nickerson JA. The ultrastructural signature of human embryonic stem cells. J Cell Biochem 2017; 118:764–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joffe B, Leonhardt H, Solovei I. Differentiation and large scale spatial organization of the genome. Curr Opin Genet Dev 2010; 20:562–9 [DOI] [PubMed] [Google Scholar]
- 51.Szczerbal I, Foster HA, Bridger JM. The spatial repositioning of adipogenesis genes is correlated with their expression status in a porcine mesenchymal stem cell adipogenesis model system. Chromosoma 2009; 118:647–63 [DOI] [PubMed] [Google Scholar]
- 52.Kim Y, Sharov AA, McDole K, Cheng M, Hao H, Fan CM, Gaiano N, Ko MS, Zheng Y. Mouse B-type lamins are required for proper organogenesis but not by embryonic stem cells. Science 2011; 334:1706–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 2006; 24:177–85 [DOI] [PubMed] [Google Scholar]
- 54.Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, Herrmann H, Blum H, Engelkamp D, Stewart CL, Leonhardt H, Joffe B. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 2013; 152:584–98 [DOI] [PubMed] [Google Scholar]
- 55.Cesarini E, Mozzetta C, Marullo F, Gregoretti F, Garguilo A, Columbaro M, Cortesi A, Antonelli L, Di Pelino S, Squarzoni S, Palacios D, Zippo A, Bodega B, Oliva G, Lanzuolo C. Lamin A/C sustains PcG protein architecture, maintaining transcriptional repression at target genes. J Cell Biol 2015; 211:533–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meaburn KJ, Cabuy E, Bonne G, Levy N, Morris GE, Novelli G, Kill IR, Bridger JM. Primary laminopathy fibroblasts display altered genome organization and apoptosis. Aging Cell 2007; 6:139–53 [DOI] [PubMed] [Google Scholar]
- 57.Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz, M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, Roca-Cusachs P. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 2017; 171:1397–410.e14 [DOI] [PubMed] [Google Scholar]
- 58.Dupont S, Morsut L, Aragona M, Enzo E, Guilitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474:179–83 [DOI] [PubMed] [Google Scholar]
- 59.Nobusue H, Onishi N, Shimizu T, Sugihara E, Oki Y, Sumikawa Y, Chiyoda T, Akashi K, Saya H, Kano K. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat Commun 2014; 5:3368. [DOI] [PubMed] [Google Scholar]
- 60.Sharma M, Jamieson C, Johnson M, Molloy MP, Henderson BR. Specific armadillo repeat sequences facilitate beta-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J Biol Chem 2012; 287:819–31 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Neumann S, Schneider M, Daugherty RL, Gottardi CJ, Eming SA, Beijer A, Noegel AA, Karakesisoglou I. Nesprin-2 interacts with {alpha}-catenin and regulates Wnt signaling at the nuclear envelope. J Biol Chem 2010; 285:34932–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol 2005; 6:21–31 [DOI] [PubMed] [Google Scholar]
- 63.Hernandez L, Roux KJ, Wong ES, Mounkes LD, Mutalif R, Navasankari R, Rai B, Cool S, Jeong JW, Wang H, Lee HS, Kozlov S, Grunert M, Keeble T, Jones CM, Meta MD, Young SG, Daar IO, Burke B, Perantoni AO, Stewart CL. Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Dev Cell 2010; 19:413–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uzer G, Thompson WR, Sen B, Xie Z, Yen SS, Miller S, Bas G, Styner M, Rubin CT, Judex S, Burridge K, Rubin J. Cell mechanosensitivity to extremely low-magnitude signals is enabled by a LINCed nucleus. Stem Cells 2015; 33:2063–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uzer G, Bas G, Sen B, Xie Z, Birks S, Olcum M, McGrath C, Styner M, Rubin J. Sun-mediated mechanical LINC between nucleus and cytoskeleton regulates betacatenin nuclear access. J Biomech 2018; 74:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Q, Minaisah RM, Ferraro E, Li C, Porter LJ, Zhou C, Gao F, Zhang J, Rajgor D, Autore F, Shanahan CM, Warren DT. N-terminal nesprin-2 variants regulate beta-catenin signalling. Exp Cell Res 2016; 345:168–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology 2008; 149:6065–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004; 6:483–95 [DOI] [PubMed] [Google Scholar]
- 69.Sen B, Xie Z, Uzer G, Thompson WR, Styner M, Wu X, Rubin J. Intranuclear actin regulates osteogenesis. Stem Cells 2015; 33:3065–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samsonraj RM, Dudakovic A, Manzar B, Sen B, Dietz AB, Cool SM, Rubin J, van Wijnen AJ. Osteogenic stimulation of human adipose-derived mesenchymal stem cells using a fungal metabolite that suppresses the polycomb group protein EZH2. Stem Cells Transl Med 2018; 7:197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dopie J, Skarp KP, Rajakyla EK, Tanhuanpaa K, Vartiainen MK. Active maintenance of nuclear actin by importin 9 supports transcription. Proc Natl Acad Sci U S A 2012; 109:E544–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samsonraj RM, Paradise CR, Dudakovic A, Sen B, Nair AA, Dietz AB, Deyle DR, Cool SM, Rubin J, van Wijnen AJ. Validation of osteogenic properties of cytochalasin D by high-resolution RNA-sequencing in mesenchymal stem cells derived from bone marrow and adipose tissues. Stem Cells Dev 2018; 27:1136–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCrea PD, Gu D. The catenin family at a glance. J Cell Sci 2010; 123:637–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell 2005; 123:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 2005; 123:903–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martins RP, Finan JD, Guilak F, Lee DA. Mechanical regulation of nuclear structure and function. Annu Rev Biomed Eng 2012; 14:431–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kapoor P, Chen M, Winkler DD, Luger K, Shen X. Evidence for monomeric actin function in INO80 chromatin remodeling. Nat Struct Mol Biol 2013; 20:426–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vartiainen MK, Guettler S, Larijani B, Treisman R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 2007; 316:1749–52 [DOI] [PubMed] [Google Scholar]
- 79.Baarlink C, Wang H, Grosse R. Nuclear actin network assembly by formins regulates the SRF coactivator MAL. Science 2013; 340:864–7 [DOI] [PubMed] [Google Scholar]
- 80.Belin BJ, Cimini BA, Blackburn EH, Mullins RD. Visualization of actin filaments and monomers in somatic cell nuclei. Mol Biol Cell 2013; 24:982–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Munsie LN, Desmond CR, Truant R. Cofilin nuclear-cytoplasmic shuttling affects cofilin-actin rod formation during stress. J Cell Sci 2012; 125:3977–88 [DOI] [PubMed] [Google Scholar]
- 82.McDonald D, Carrero G, Andrin C, de Vries G, Hendzel MJ. Nucleoplasmic beta-actin exists in a dynamic equilibrium between low-mobility polymeric species and rapidly diffusing populations. J Cell Biol 2006; 172:541–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baarlink C, Grosse R. Formin’ actin in the nucleus. Nucleus 2014; 5:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sen B, Uzer G, Samsonraj RM, Zie Z, McGrat C, Styner M, Dudakovic A, van Wijnen AJ, Rubin J. Intranuclear actin structure modulates mesenchymal stem cell differentiation. Stem Cells 2017; 35:1624–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Munter S, Enninga J, Vazquez-Martinez R, Delbarre E, David-Watine B, Nehrbas U, Shorte SL. Actin polymerisation at the cytoplasmic face of eukaryotic nuclei. BMC Cell Biol 2006; 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grosse R, Vartiainen MK. To be or not to be assembled: progressing into nuclear actin filaments. Nat Rev Mol Cell Biol 2013; 14:693–7 [DOI] [PubMed] [Google Scholar]
- 87.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol 2006; 173:383–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. Embo J 2004; 23:790–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramdas NM, Shivashankar GV. Cytoskeletal control of nuclear morphology and chromatin organization. J Mol Biol 2015; 427:695–706 [DOI] [PubMed] [Google Scholar]
- 90.Verboon JM, Rincon-Arano H, Werwie TR, Delrow JJ, Scalzo D, Nandakumar V, Groudine M, Parkhurst SM. Wash interacts with lamin and affects global nuclear organization. Curr Biol 2015; 25:804–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu X, Zeng X, Huang B, Hao S. Actin is closely associated with RNA polymerase II and involved in activation of gene transcription. Biochem Biophys Res Commun 2004; 321:623–30 [DOI] [PubMed] [Google Scholar]
- 92.Serebryannyy LA, Parilla M, Annibale P, Cruz CM, Laster K, Gratton E, Kudryashov D, Kosak ST, Gottardi CJ, de Lanerolle P. Persistent nuclear actin filaments inhibit transcription by RNA polymerase II. J Cell Sci 2016; 129:3412–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Serebryannyy LA, Cruz CM, De Lanerolle P. A role for nuclear actin in HDAC 1 and 2 regulation. Sci Rep 2016; 6:28460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen X, Mizuguchi G, Hamiche A, Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature 2000; 406:541. [DOI] [PubMed] [Google Scholar]
- 95.Crabbe L, Cesare AJ, Kasuboski JM, Fitzpatrick JA, Karlseder J. Human telomeres are tethered to the nuclear envelope during postmitotic nuclear assembly. Cell Rep 2012; 2:1521–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Czapiewski R, Robson MI, Schirmer EC. Anchoring a Leviathan: how the nuclear membrane tethers the genome. Front Genet 2016; 7:82. [DOI] [PMC free article] [PubMed] [Google Scholar]