Short abstract
Mutations in genes encoding nuclear lamins and associated nuclear envelope proteins have been linked to a broad range of inherited diseases affecting different tissues and organs. These diseases are often referred to as laminopathies. Scientists have yet to elucidate exactly how pathogenic mutations leading to alteration of a nuclear envelope protein cause disease. Our relatively recent research has shown that pathogenic mutations in genes encoding nuclear envelope proteins lead to defective nucleocytoplasmic connections that disrupt proper functioning of the linker of nucleoskeleton and cytoskeleton complex in the establishment of cell polarity. These defects may explain, at least in part, pathogenic mechanisms underlying laminopathies.
Impact statement
Mutations in genes encoding nuclear lamins and associated nuclear envelope proteins have been linked to several diseases affecting different tissues and organs. The pathogenic mechanisms underlying these diseases, often called laminopathies, remain poorly understood. Increased knowledge of the functions of different nuclear envelope proteins and the interactions between them is crucial to elucidate these disease mechanisms. Our research has shown that pathogenic mutations in genes encoding nuclear envelope proteins lead to defective nucleocytoplasmic connections that disrupt proper functioning of the linker of nucleoskeleton and cytoskeleton (LINC) complex in the establishment of cell polarity. These defects may contribute to the pathogenesis of laminopathies and provide novel targets for therapeutics.
Keywords: Lamin, linker of nucleoskeleton and cytoskeleton complex complex, nesprin, nuclear envelope, SUN protein
Introduction
Genetics research has since the middle of the 1990s linked mutations in genes encoding proteins of the nuclear envelope to rare diseases. Most of these diseases involve specific tissues and organs even though the affected proteins are fairly ubiquitously expressed. One notable example is mutations in LMNA encoding lamin A and lamin C, components of the nuclear lamina that are expressed in most terminally differentiated cells. Mutations in LMNA can cause myopathy, cardiomyopathy, partial lipodystrophy, peripheral neuropathy and multi-system disorders, such as progeria, which have features of accelerated aging. Diseases caused by mutations in LMNA and genes encoding B-type lamins and other nuclear envelope proteins, most of which are directly or indirectly associated with lamins, are often referred to as “laminopathies.”1
The nuclear envelope separates the nucleoplasm from the cytoplasm in nucleated eukaryotic cells. In addition to its barrier function, recent data have emerged showing that the nuclear envelope connects the nuclear lamina inside the nucleus to the cytoskeleton in the cytoplasm. The linker of nucleoskeleton and cytoskeleton (LINC) complex mediates these connections. Several laminopathy-causing mutations disrupt LINC complex-mediated nucleocytoplasmic connections, leading to various cellular defects. These defects may relate to disease pathogenesis.
To better understand of potential pathogenic mechanisms underlying the laminopathies, one must first understand basic aspects of the nuclear envelope and the diseases themselves. We have therefore structured this review to first discuss the composition and molecular genetics of the nuclear envelope, with an emphasis on the lamins and LINC complex proteins. We then discuss genetic and clinical aspects of different laminopathies. Finally, we address the potential role of defective nucleocytoplasmic connections in these diseases.
Nuclear envelope and nucleocytoplasmic connections
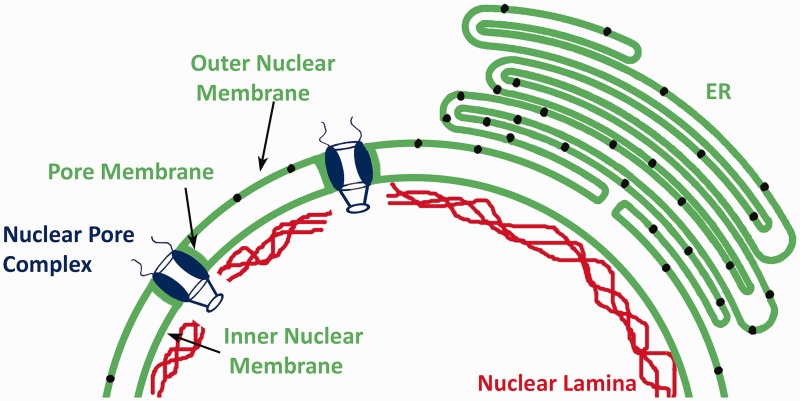
The nuclear envelope is composed of the nuclear pore complexes, nuclear lamina, and nuclear membranes, which are continuous with the rough endoplasmic reticulum (Figure 1). The pore complexes provide routes for passive and active transport between the nucleus and cytoplasm and also function in chromatin organization and regulation of gene expression.2,3 The lamina, which lines the inner aspect of the inner nuclear membrane, is composed of intermediate filament proteins called lamins.4–7 Electron microscopy of Xenopus oocytes originally identified the nuclear lamina as meshwork of filaments 10 nm in diameter, which is similar to cytoplasmic intermediate filaments.8 More recent cryo-electron tomographic analysis of mouse embryonic fibroblasts lacking vimentin revealed that lamin filaments have a globular-decorated fiber appearance of 3.5 nm thickness.9 Hence, in mammalian somatic cells, the lamina may have a distinctly different structure than canonical cytoskeletal intermediate filament networks.
Figure 1.
Schematic diagram of the nuclear envelope showing nuclear pore complexes, the nuclear lamina and the nuclear membranes. The nuclear membranes are morphologically divided into the pore membrane, inner nuclear membrane, and outer nuclear membrane. The outer nuclear membrane is directly continuous with the rough endoplasmic reticulum (ER) membrane and similarly contains ribosomes (represented by small black circles within these membranes). (A color version of this figure is available in the online journal.)
Nuclear membranes are morphologically divided into the pore, inner and outer membrane domains. The pore membranes are small domains of the nuclear envelope that connect the inner and outer membrane and contain transmembrane proteins of the nuclear pore complexes such as gp210 and POM121.10,11 The inner nuclear membrane is associated with the nuclear lamina. Approximately 80 transmembrane proteins have been identified as specifically targeted to the inner nuclear membrane in interphase rat hepatocytes12; however, the transmembrane protein composition of the inner nuclear membrane may vary between cell types.13 Most of these transmembrane proteins are synthesized on rough endoplasmic reticulum-bound ribosomes and reach the inner nuclear membrane by lateral diffusion through the interconnected rough endoplasmic reticulum, outer nuclear, and pore membrane domains. They are then retained in the inner nuclear membrane by binding to the lamina or chromatin. During this diffusion, the nucleocytoplasmic domains of the proteins pass through the 10 nm diameter lateral channels of nuclear pore complexes, which prevents proteins with large nucleocytoplasmic domains from reaching the inner nuclear membrane this way.14–17 However, mechanisms involving active transport have been postulated as alternatives to this diffusion and binding process.18
The outer nuclear membrane has ribosomes on its outer surface and is separated from the inner nuclear membrane by the perinuclear space, a continuation of the endoplasmic reticulum lumen. As there is no barrier for diffusion between the rough endoplasmic reticulum and the directly continuous outer nuclear membrane, both have long been presumed to have identical integral membrane protein content. However, interactions between the luminal domains of transmembrane proteins in the inner nuclear membrane and luminal domains of transmembrane proteins of the rough endoplasmic reticulum could lead to specific retention of proteins in the outer nuclear membrane. This is what happens at the LINC complexes that connect the nucleus and cytoskeleton.19
LINC complexes are composed of outer nuclear membrane KASH (Klarsicht, ANC1, and Syne homology) proteins and inner nuclear membrane SUN (Sad1 and UNC-84) proteins, both of which are type II membrane proteins with a single transmembrane segment. In mammals, somatic cell KASH proteins are generally referred to as nesprins.20 Nesprins contain a KASH domain of approximately 60 amino acids at their carboxyl-termini, which includes a transmembrane segment and up to 30 residues in the perinuclear space. They also contain multiple, clustered spectrin repeats that project into the cytoplasm. SUN proteins contain a conserved SUN domain also located within the perinuclear space. X-ray crystallography analysis shows that three KASH peptides bind to a trimeric arrangement of the SUN domains of SUN2, with a KASH-SUN disulfide bond adding stability to the complex.21,22 The other widely expressed SUN protein, SUN1, may exist in a different oligomeric state.23
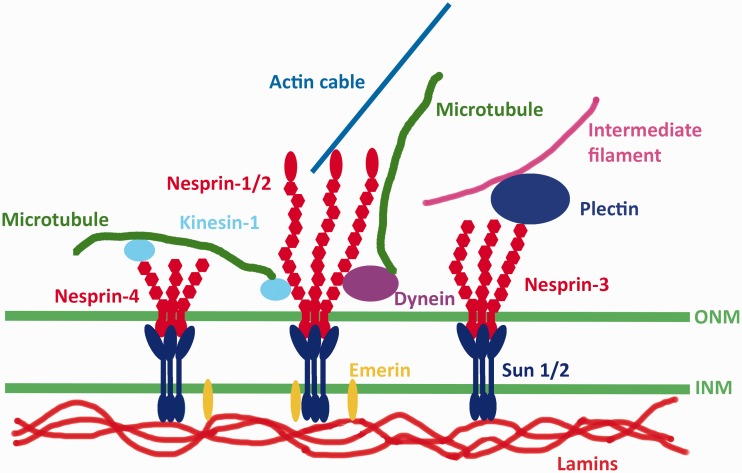
Binding of SUN proteins to nesprins leads to formation of a protein “bridge” between the inner and outer nuclear membranes (Figure 2). Inside the nucleus, SUN proteins interact with the lamina, specifically A-type lamins.19,24 In mammalian cells lacking A-type lamins, SUN proteins still localize to the inner nuclear membrane. This suggests that associations with other nuclear proteins or perhaps chromatin contribute to their localization. However, SUNs and nesprins demonstrate increased diffusional mobility within the nuclear membrane in cells lacking A-type lamins, suggesting that these proteins contribute to stabilizing LINC complexes in the nuclear envelope.25 SUN proteins also interact with emerin and SAMP1, integral proteins of the inner nuclear membrane.26,27 On the cytoplasmic side of the nuclear envelope, LINC complexes, via their different nesprin components, bind to cytoskeletal elements. Different nesprin proteins (see below) bind to different cytoskeletal elements via their variable cytoplasmic domains. Various nesprin-1 and nesprin-2 isoforms interact with the microtubule motors dynein or kinesin-1.27–29 Nesprin-1G and nesprin-2G bind to actin filaments via calponin homology domains near the aminotermini.30,31 Nesprin-2G can additionally bind to actin filaments via the formin FHOD1 and the actin bundling protein fascin.32,33 Nesprin-3 binds to plectin, which in turn interacts with cytoplasmic intermediate filaments.34 Nesprin-4 interacts with kinesin-1.35 These nesprin-mediated connections to different cytoskeletal elements allow LINC complexes to function in diverse cellular processes.
Figure 2.
Schematic diagram of LINC complexes. Transmembrane SUN proteins of the inner nuclear membrane (INM) interact with nesprins of the outer nuclear membrane (ONM) within the perinuclear space. In the nucleus, SUN proteins bind to A-type lamins and also interact with emerin, another transmembrane protein of the INM. In the cytoplasm, different nesprin proteins bind to different cytoskeletal elements. Different isoforms of nesprin-1 and nepsrin-2 can bind actin filaments directly or associate with microtubules indirectly via dynein or kinesin-1. Nesprin-3 can associate with intermediate filaments by binding to plectin. Nesprin-4 can also associate with microtubules indirectly by binding kinesin-1. (A color version of this figure is available in the online journal.)
Molecular genetics of the lamina and LINC complex
In mammals, three genes encode lamins, the protein building blocks of the nuclear lamina. The proteins are generally divided into two types, A-type and B-type, based on their biochemical properties and primary structures.36 LMNB1 on human chromosome 5 encodes lamin B1, which is expressed in all or most somatic cells.37,38 LMNB2 on human chromosome 19 encodes lamin B2, also expressed in all or most somatic cells.39 In mammals, the gene encoding lamin B2 also gives rise to a male germ cell‐specific isoform, lamin B3, which is generated by alternative RNA splicing.40
LMNA on human chromosome 1 is the human gene encoding the A-type lamins, prelamin A and lamin C, which arise by alternative RNA splicing and are expressed in most terminally differentiated cells.38,41 Prelamin A and lamin C are identical for their first 566 amino acids with prelamin A having 98 unique carboxyl-terminal amino acids and lamin C having 6. In mammals, the same gene that encodes lamin A and C also gives rise to a germ cell‐specific isoform, lamin C2, and a poorly studied minor isoform called lamin AΔ10.42,43
Except for lamin C, mammalian lamins terminate with a CaaX motif, where C is a cysteine, a an aliphatic amino acid, and X any amino acid. This motif at the carboxyl-termini of proteins triggers three sequential enzymatic modifications. First, protein farnesyltransferase catalyzes the addition of a farnesyl moiety to the cysteine.44 Second, an endoprotease that recognizes the farnesylated protein catalyzes cleavage of the peptide bond between the cysteine and -aaX. For B-type lamins and most other CaaX proteins, ras converting CAAX endopeptidase 1 (RCE1) catalyzes this reaction.45 For prelamin A, zinc metallopeptidase, STE24 homolog (ZMPSTE24) can also catalyze the cleavage of the -aaX.46 In the third step, isoprenylcysteine carboxyl methyltransferase catalyzes methylation of the carboxyl-terminal farnesylcysteine.47,48
In the case of prelamin A, but not B-type lamins, the protein undergoes an additional reaction to generate mature lamin A. This is an endoproteolytic cleavage leading to removal of its last 15 amino acids, which includes the farnesylcysteine α-methyl ester.49,50 Indeed, the cysteine must be farnesylated for this cleavage reaction to occur.50 Experiments in both knockout mice and in vitro have shown that ZMPSTE24 catalyzes this endoproteolytic cleavage reaction.46,51,52 The physiological reason for this processing of prelamin A to lamin A remains unknown and the cleaved 15-amino-acid polypeptide has never been detected in cells. However, defects in this processing lead to human disease (see below).
The mammalian genome contains five genes that encode somatic cell KASH-domain proteins, four of which are called nesprins. The human gene symbols for the four nesprins are SYNE1 through SYNE4, for synaptic nuclear envelope, because the encoded proteins were originally identified in the nuclei that lie beneath the postsynaptic membrane at the neuromuscular junction.53 The proteins were subsequently named nesprins for nuclear envelope spectrin-repeat proteins.20 SYNE1 and SYNE2 encode nesprin-1 and nesprin-2, respectively, with multiple isoforms of each arising by alternative RNA splicing and alternative translational start and termination sites.20,54 SYNE1 contains 146 exons and SYNE2 116 exons and the “giant” or “G” isoforms are >800 kDa. Some smaller isoforms reside in the inner nuclear membrane, where they associate with emerin, SUN proteins, and lamins.26,55 SYNE3 encodes nesprin-3, which also has several splice variants and is expressed widely in somatic cells.34 Nesprin-4, encoded by SYNE4, is absent from many tissues and preferentially expressed in secretory epithelia and hair cells of the inner ear.35,56 A fifth human gene, LRMP, encodes Jaw1/LRMP, which contains a KASH domain and interacts with SUN proteins in lymphoid organs, immune cells, taste buds, and melanomas.57 CCDC155 encodes KASH5, which is expressed only in germ cells and associates with telomeres, SUN1, and dynactin during meiosis.58,59
In mammals, five different genes code for SUN proteins. SUN1 and SUN2 encode proteins that are widely expressed in in many cell types.60,61 SUN3, SPAG4, and SUN5 encode testis-specific SUN-domain proteins.62–64
Laminopathies
Laminopathies are rare, inherited diseases caused by mutations in genes encoding nuclear lamins and other nuclear envelope proteins. Here, we focus on those laminopathies affecting A-type nuclear lamins, the SUN and nesprin core proteins of the LINC complex and the LINC complex-associated protein emerin. More details on these diseases and the laminopathies involving other nuclear envelope proteins can be found in our previous review articles.1,65–70
A-type nuclear lamins
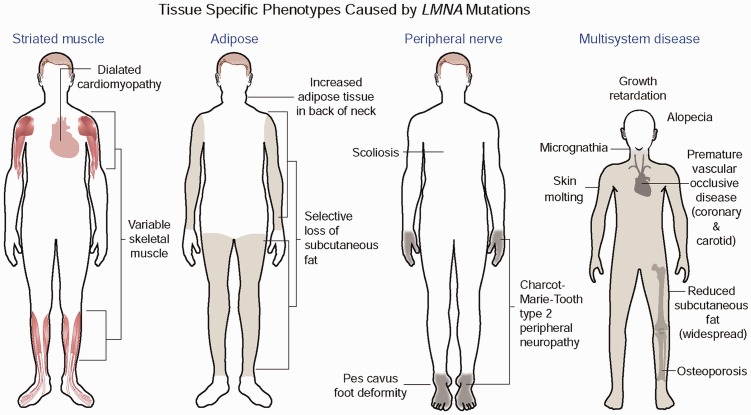
Different mutations in LMNA can generate four major, mostly non-overlapping phenotypes selectively involving striated muscle, adipose tissue or peripheral nerve or involving multiple systems with progeroid features (Figure 3). The first striated muscle disease linked to mutations in LMNA was autosomal dominant Emery-Dreifuss muscular dystrophy, which is characterized by dilated cardiomyopathy and a scapulohumeral-peroneal distribution of skeletal muscle involvement with concurrent tendon contractures.71 Subsequent research showed that the same dominant LMNA mutations that cause amino acid substitutions, small deletions, splicing defects or haploinsufficiency can cause related disorders with dilated cardiomyopathy but variable muscle involvement.72–75 Extremely rare compound heterozygous mutations can cause the same striated muscle phenotypes.76 Dominant mutations that mostly cluster in exon 8 of LMNA cause Dunnigan-type familial partial lipodystrophy, in which there is selective loss of subcutaneous fat from the extremities, excessive fat accumulation in the neck and face, and the subsequent development of insulin resistance, diabetes mellitus, and liver steatosis.77–79 The vast majority of these mutations cause amino acid substitutions that change the surface charge of an immunoglobulin-type fold in lamin A and lamin C.80,81 However, variant lipodystrophy syndromes and metabolic disorders have been described caused by mutations affecting amino acids in different domains of the proteins.82,83 Homozygosity for the R298C LMNA mutation causes Charcot–Marie–Tooth type 2B1, a sensory and motor peripheral neuropathy with chronic distal weakness and often associated with foot and spine deformations.84 This very rare disorder has only been identified in Northwest African families whose affected individuals share a common ancestral haplotype.85 Finally, specific mutations in LMNA cause progeroid syndromes, which involve multiple organ systems and in some respects mimic premature aging.
Figure 3.
LMNA mutations cause four main disease phenotypes. Most dominant LMNA mutations cause striated muscle disease. The diagram shows the classical Emery-Dreifuss muscular dystrophy, which is characterized by dilated cardiomyopathy and a scapulohumeral-peroneal distribution of skeletal muscle involvement with concurrent tendon contractures. However, the same mutations can result in dilated cardiomyopathy with variable skeletal muscle involvement. Other dominant mutations most often leading to a change in the surface charge of the immunoglobulin fold in lamins A and C cause Dunnigan-type partial lipodystrophy, which has selective loss of subcutaneous fat from the extremities, excessive fat accumulation in the neck and face with the subsequent development of insulin resistance, diabetes mellitus, and liver steatosis. The autosomal recessive R298C LMNA mutation causes a Charcot–Marie–Tooth type 2 peripheral neuropathy characterized by a stocking-glove sensory neuropathy, an associated pes cavus foot deformity and additional variable features such as scoliosis. The de novo dominant G608G LMNA mutation causes the multisystem disease HGPS, with progeroid features including growth retardation, micrognathia, reduced subcutaneous fat, alopecia, osteoporosis, skin mottling, and premature vascular occlusive disease. Other rare dominant LMNA mutations also cause progeriod syndromes with similar features; the recessive R527H mutation causes mandibuloacral dysplasia, a disorder with a combination of progeroid features and partial lipodystrophy. Reprinted from Dauer and Worman66 with permission from Elsevier. (A color version of this figure is available in the online journal.)
Hutchinson-Gilford progeria syndrome (HGPS) is the prototypical progeroid syndrome. Characteristic features are growth retardation, micrognathia, reduced subcutaneous fat, alopecia, osteoporosis, skin mottling and premature vascular occlusive disease, with affected children dying in their teenage years usually from complications of cardiovascular disease.86 It is caused by a de novo G608G (ggc > ggt) or G608S (ggc > agc) mutation in LMNA.87,88 These mutations lead to the creation of an aberrant splice donor site in a portion of RNA encoded by exon 11, which leads to an in-frame deletion of 50 amino acids in prelamin A. This internally truncated prelamin A, which is called progerin, retains the farnesylated and methylated carboxyl-terminal cysteine of prelamin A. Considerable evidence points to progerin, specifically farnesylated progerin, as being the major driver of pathology in HGPS. Accumulation of progerin in cells leads to alterations in nuclear architecture.89 Treatment of cultured cells expressing progerin with a protein farnesyltransferase inhibitor (FTI), which blocks protein farnesylation, reverses the abnormal nuclear architecture.90–95 More significantly, FTI treatment improves the phenotype of genetically-modified mice that express progerin.96,97 An FTI has since been studied in clinical trials of children with HGPS.98,99
Deletion of Zmpste24 leads to expression of full-length but unprocessed, permanently farnesylated prelamin A and progeroid phenotypes in mice.51,52 FTI treatment significantly improves the progeroid phenotype in these mice, demonstrating that the farnesyl moiety of the unprocessed prelamin A contributes to pathology.100 In humans, mutations in ZMPSTE24 cause a range of progeroid disorders from the relatively mild mandibuloacral dysplasia type B to a severe progeria syndrome to the neonatal lethal restrictive dermopathy.101–105 ZMPSTE24 has other functions than the proteolytic processing of prelamin A106 but a correlation between residual proteolytic activity in mutant forms of ZMPSTE24 and the severity of the progeroid disorders they cause further emphasizes the role of unprocessed prelamin A in these diseases.105 We have also described one patient with a progeroid disorder and a point mutation that abolishes the ZMPSTE24 recognition site in prelamin A. Cultured dermal fibroblasts from this individual have abnormal nuclear morphology that is reversed when treated with a FTI.107
The evidence is strong that protein farnesylation contributes to pathology in most of the progeroid disorders associated with prelamin A. However, there are several cases of mutations in LMNA causing progeroid disorders with similar features without evidence of accumulation of a farnesylated prelamin A variant. Mandibuloacral dysplasia type A has features of both progeria and lipodystrophy and is caused by a recessive LMNA R527H mutation.108 Cases classified as atypical Werner syndrome have also been associated with LMNA mutations and farnesylated prelamin A variants similarly do not appear to be expressed.109 Hence, rare mutations in LMNA leading to alterations in A-type lamins other than those causing accumulation of farnesylated prelamin A variants can also cause progeroid phenotypes.
SUN and nesprin core proteins of the LINC complex
Various diseases have been linked to mutations in the genes encoding SUN proteins and nesprins, some robustly and some more tentatively.110 Mutations in SYNE1 encoding nesprin-1 cause autosomal recessive cerebellar ataxia.111 Since the original publication, subsequent studies have confirmed that mutations in SYNE1 may be one of the more common recessive ataxias worldwide.112,113
SYNE1 mutations also cause recessive arthrogryposis multiplex congenita, a disorder characterized by congenital joint contractures and reduced fetal movements, and the genetic linkage is robust with pathogenic alleles shown to segregate with affected individuals in different families.114–116 Homozygosity for a mutation in SYNE4, which encodes a truncated nesprin-4 variant, also segregates with affected individuals in two families of Iraqi-Jewish ancestry with progressive high-frequency hearing loss.56 An autosomal dominantly inherited point mutation in SYNE2 leading to an amino acid substitution in nesprin-2β1 segregates among first-degree relatives with an Emery-Dreifuss muscular dystrophy-like phenotype.117
Other mutations in genes encoding SUN proteins and nesprins have been linked to cardiomyopathy and muscular dystrophy. Autosomal dominant sequence variations in SYNE1 have been reported in individuals with cardiomyopathy, sometimes associated with Emery-Dreifuss muscular dystrophy-like skeletal muscle involvement.117–119 Sequence variations in SUN1 and SUN2 have also been reported in individuals with phenotypes similar to Emery-Dreifuss muscular.120 However, these SYNE1, SUN1, and SUN2 sequence variants have not been shown to segregate between affected and unaffected individuals within families, making these associations with cardiomyopathy and muscular dystrophy tentative.
LINC complex-associated protein emerin
Positional cloning in families with X-linked Emery-Dreifuss muscular dystrophy identified EMD encoding emerin as the causative gene.121 Subsequently, emerin was localized to the inner nuclear membrane and shown to be lacking from the nuclear envelope of affected patients.122,123 Almost all cases are associated with loss of emerin expression.122–125 The phenotype of X-linked Emery-Dreifuss muscular dystrophy is virtually identical to that of the autosomal form caused by LMNA mutations, with dilated cardiomyopathy as a predominant feature. Mutations in EMD causing lack of emerin expression can also cause dilated cardiomyopathy with different patterns of skeletal muscle involvement.126,127
Defective nucleocytoplasmic connections in laminopathies
To gain novel insights into pathogenesis, our groups have been investigating how pathogenic mutations that cause laminopathies affect nucleocytoplasmic connections. We have used a modified wounded fibroblast monolayer system to examine the initial polarization of cells preparing for migration.128,129 Addition of serum or the serum factor lysophosphatidic acid (LPA) to serum-starved, wounded monolayers triggers rapid polarization of the wound-edge cells, which is characterized by reorientation of the centrosome between the nucleus and the wound. Centrosome reorientation in wounded monolayers of fibroblasts does not involve movement of the centrosome but rather movement of the nucleus past a stationary centrosome by myosin-directed retrograde actin flow.130
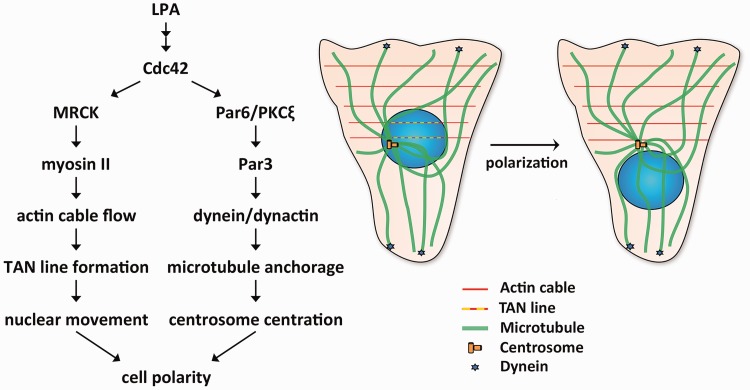
LPA stimulation of wounded fibroblast monolayers activates two pathways, one that induces this nuclear movement and a second that holds the centrosome in place (Figure 4). LPA stimulation leads to activation of Cdc42.131 In the nuclear movement pathway, Cdc42 activates myotonic dystrophy kinase-related Cdc42-binding kinase, which phosphorylates and activates myosin II.130 This leads to retrograde actin cable flow originated from the wound edge. The nucleus is coupled to these moving actin filaments via the LINC complex, specifically nesprin-2G-SUN2 which binds to actin via the calponin homology domains of nesprin. Linear arrays of nesprin-2G and SUN2 assemble to form transmembrane actin-associated nuclear (TAN) lines to move the nucleus rearward.31 Cdc42 simultaneously activates a second pathway involving Par6/PKCζ, Par3, dynein/dynactin, and microtubules that holds the centrosome in the cell centroid.130,132 These pathways acting together lead to proper cell polarity. Defects in either of these pathways lead to different cellular phenotypes. If the nuclear movement pathway is blocked, both the centrosome and nucleus are positioned at the cell centroid. If the centrosome centration pathway is blocked, both are positioned rearward of the cell centroid.
Figure 4.
Mechanisms that generate cell polarity in wounded monolayers of fibroblasts. LPA-treatment of wounded monolayers leads to activation of Cdc42. Cdc42 activates two pathways that lead to cell polarity. In the nuclear movement pathway (left arm of diagram at left), Cdc42 activates myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK), which phosphorylates and activates myosin II, leading to actin cable flow and TAN line formation to move the nucleus. Cdc42 simultaneously activates a second centrosome centration pathway (right arm in the diagram at left), which involves Par6/PKCζ, Par3, dynein/dynactin, and microtubules and holds the centrosome in the centroid of the cell. The schematic diagram at the right shows microtubules, actin cables, TAN lines, and dynein during cell polarization. (A color version of this figure is available in the online journal.)
Striated muscle disease
We initially examined how lamin A variants expressed in either striated muscle disease or Dunnigan-type partial lipodystrophy affect fibroblasts in wounded monolayers polarizing for migration.133 Expression of lamin A variants found in patients with striated muscle diseases blocked actin-dependent nuclear movement, whereas most of the partial lipodystrophy variants inhibited microtubule-dependent centrosome centration. Depletion of A-type lamins similarly blocked nuclear movement, showing that lamin A variants affecting striated muscle generate a null phenotype. This is consistent with in vivo findings that germline deletion of A-type lamins in mice leads to striated muscle disease but not lipodystrophy.134,135 TAN lines assembled within cells expressing striated muscle disease-associated lamin A variants or depleted of A-type lamins; however, they appeared less stable and slipped over the nucleus rather than moving with it. Hence, alterations in A-type lamins that occur in striated muscle disease cause defective anchoring of TAN lines and inhibit cell polarization by blocking actin-dependent nuclear movement.
In subsequent experiments, we examined how loss of the LINC complex-associated integral membrane protein emerin, which occurs in X-linked Emery-Dreifuss muscular dystrophy, affects fibroblasts polarizing for migration.136 In fibroblasts without emerin, TAN lines formed normally but nuclei moved randomly due to nondirectional actin cable flow. Slippage of TAN lines was also observed. Depletion of myosin IIB from cells generated similar nondirectional nuclear movement. Emerin interacts with myosin IIB and is required for myosin IIB to localize near the nucleus. These results are consistent with a model in which emerin is necessary for the proper organization of cytoplasmic actin flow through localization of myosin IIB. Consistent with its role, emerin is not uniquely localized to the inner nuclear membrane but a small quantity is normally in the outer nuclear membrane.137
Although the mechanisms may be different, genetic alterations that occur in striated muscle diseases caused by LMNA and EMD mutations both prevent effective establishment of polarity in wounded monolayers of fibroblasts polarizing for migration. SUN1 and SUN2 variants tentatively linked to striated muscle disease also block nuclear movement and cause polarity defects in the same assay.120 However, defects in fibroblast polarity and subsequent migration toward a wound edge cannot be obviously linked to the striated muscle pathology in these diseases. Yet, establishing polarity in migrating myoblasts is essential for skeletal muscle development and repair. In experiments using wounded monolayers of cultured myoblasts, we established that essentially the same processes are involved in the establishment of polarity as in fibroblasts, including stimulation by LPA and rearward movement of the nucleus while the centrosome is maintained at the cell centroid.138 As in fibroblasts, nuclear movement was dependent on actin, the formation of nesprin-2G and SUN2 TAN lines and the presence of A-type lamins. Furthermore, abolishing polarization by depleting nesprin-2G interfered with directed myoblast migration and fusion into myotubes. Defects in actin-dependent nuclear movement in myoblasts, a process first identified in fibroblasts, may therefore play a role in the pathogenesis of striated muscle diseases caused by mutations in LMNA and EMD. Still, myoblast dysfunction cannot explain all of the pathology, as mice with germline deletions of A-type lamins, emerin or even both proteins are born with skeletal muscle, which in the case of emerin loss alone is only minimally abnormal well into adulthood.134,139–141 Furthermore, affected humans usually do not develop symptoms until later childhood or early adulthood. A plausible mechanism, which remains to be tested experimentally, would be that alterations in A-type lamins or emerin also make differentiated myofibers more susceptible to mechanical damage and that partially defective myoblasts cannot adequately replace the damaged fibers.
HGPS
More recently, we have examined nucleocytoplasmic connections in HGPS.142 LPA-stimulated wounded monolayers of fibroblasts form children with HGPS, as well as NIH3T3 fibroblasts expressing progerin, demonstrated defective nuclear movement and polarity establishment. These defects were reversed by blocking progerin farnesylation. Farnesylated progerin inhibited rearward nuclear movement by both weakening TAN line anchorage, as occurs in cells lacking A-type lamins, and disturbing retrograde actin flow.
SUN1 over accumulates in cells from children with HGPS.26,143 We showed that progerin expression increases SUN1 accumulation in NIH3T3 fibroblasts and SUN1 overexpression prevents rearward nuclear movement and polarization in wounded monolayers.142 Furthermore, SUN1 depletion by siRNA rescues the defects in rearward nuclear movement and polarization in fibroblasts from children with HGPS. SUN1 accumulation inhibits nuclear movement in these cells by inducing excessive association of microtubules with nuclei. This agrees with our previous finding that SUN1 is involved in microtubule-dependent nuclear positioning.29 Consistently, inhibition of dynein rescues actin-dependent nuclear movement in fibroblasts from children with HGPS and NIH3T3 cells overexpressing progerin. Hence, imbalanced nucleocytoplasmic connections resulting from increased SUN1 expression and the resultant preferential engagement of microtubules over actin by LINC complexes underlie the polarity defects in fibroblasts from children with HGPS. Intriguingly, fibroblasts from human subjects older than approximately 60 years exhibit similar nuclear movement and cell polarity defects as fibroblasts from children with HGPS and these defects can be rescued by reducing SUN1 levels or inhibiting dynein.142
Experiments in mouse models further suggest that elevated level of SUN1 in progeroid disorder leads to physiological defects. Progeroid mice with the LmnaΔ9 mutation express a truncated, farnesylated prelamin A, albeit different than progerin, and live approximately 30 days.144 However, genetic deletion of Sun1 in these mice improves growth and prolongs survival.143 Mice with an Lmna point mutation corresponding to that causing HGPS express abundant amounts of progerin and have a progeroid phenotype, including a distinctive aortic pathology with loss of smooth muscle cells in the arterial media and fibrosis of the adventitia. Expression of a dominant-negative KASH-domain construct that disrupts the LINC complex in smooth muscle cells ameliorates the toxic effects of progerin in these cells and limits the accompanying adventitial fibrosis.145 These results suggest that modulating SUN1 expression or disrupting LINC complexes in progeroid disorders in which there are excessive connections to microtubules could be effective therapies in children with HGPS and even the cardiovascular complications of physiological aging.
Conclusions
Genetic mutations causing laminopathies can generate defective nucleocytoplasmic connections. These in turn lead to nuclear positioning and cell polarity defects. Proper nuclear positioning and establishing and maintaining polarity are necessary for productive cell migration and the proper development and function of diverse organs.146–148 The relatively recent data generated from studies of defective nucleocytoplasmic connections in laminopathies can therefore lead to compelling hypotheses about pathogenesis for further experimental testing. These may explain, at least in part, pathogenic mechanisms underlying diseases caused by mutations in genes encoding nuclear envelope proteins.
ACKNOWLEDGMENTS
The authors thank the Guest Editor of this Thematic Issue for the invitation to contribute this review.
Authors’ contributions
All of the authors contributed to this review.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HJW declares the following potential conflicts of interest: scientific advisory board and equity owner AlloMek Therapeutics, scientific advisory board and consulting income MNG Laboratories, consulting income Eiger BioPharmaceuticals, sponsored research funding (to Columbia University) Navitor Pharmaceuticals. The other authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
FUNDING
The author(s) disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health [grant numbers R01AR048997, R01AR068636].
References
- 1.Worman HJ, Bonne G. Laminopathies”: a wide spectrum of human diseases. Exp Cell Res 2007; 313:2121–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wente SR, Rout MP. The nuclear pore complex and nuclear transport. Cold Spring Harb Perspect Biol 2010; 2:a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capelson M, Doucet C, Hetzer MW. Nuclear pore complexes: guardians of the nuclear genome. Cold Spring Harb Symp Quant Biol 2010; 75:585–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerace L, Blum A, Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol 1978; 79:546–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher DZ, Chaudhary N, Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci USA 1986; 83:6450–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman AE, Maul G, Steinert PM, Yang HY, Goldman RD. Keratin-like proteins that coisolate with intermediate filaments of BHK-21 cells are nuclear lamins. Proc Natl Acad Sci USA 1986; 83:3839–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature 1986; 319:463–8 [DOI] [PubMed] [Google Scholar]
- 8.Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature 1986; 323:560–4 [DOI] [PubMed] [Google Scholar]
- 9.Turgay Y, Eibauer M, Goldman AE, Shimi T, Khayat M, Ben-Harush K, Dubrovsky-Gaupp A, Sapra KT, Goldman RD, Medalia O. The molecular architecture of lamins in somatic cells. Nature 2017; 543:261–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greber UF, Senior A, Gerace L. A major glycoprotein of the nuclear pore complex is a membrane-spanning polypeptide with a large lumenal domain and a small cytoplasmic tail. EMBO J 1990; 9:1495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallberg E, Wozniak RW, Blobel G. An integral membrane protein of the pore membrane domain of the nuclear envelope contains a nucleoporin-like region. J Cell Biol 1993; 122:513–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science 2003; 301:1380–2 [DOI] [PubMed] [Google Scholar]
- 13.Worman HJ, Schirmer EC. Nuclear membrane diversity: underlying tissue-specific pathologies in disease? Curr Opin Cell Biol 2015; 34:101–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soullam B, Worman HJ. The amino-terminal domain of the lamin B receptor is a nuclear envelope targeting signal. J Cell Biol 1993; 120:1093–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soullam B, Worman HJ. Signals and structural features involved in integral membrane protein targeting to the inner nuclear membrane. J Cell Biol 1995; 130:15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol 1997; 138:1193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Östlund C, Ellenberg J, Hallberg E, Lippincott-Schwartz J, Worman HJ. Intracellular trafficking of emerin, the Emery-Dreifuss muscular dystrophy protein. J Cell Sci 1999; 112:1709–19 [DOI] [PubMed] [Google Scholar]
- 18.Zuleger N, Kerr AR, Schirmer EC. Many mechanisms, one entrance: membrane protein translocation into the nucleus. Cell Mol Life Sci 2012; 69:2205–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 2006; 172:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci 2001; 114:4485–98 [DOI] [PubMed] [Google Scholar]
- 21.Sosa BA, Rothballer A, Kutay U, Schwartz TU. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell 2012; 149:1035–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Z, Du X, Cai Z, Song X, Zhang H, Mizuno T, Suzuki E, Yee MR, Berezov A, Murali R, Wu SL, Karger BL, Greene MI, Wang Q. Structure of Sad1-UNC84 homology (SUN) domain defines features of molecular bridge in nuclear envelope. J Biol Chem 2012; 287:5317–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hennen J, Saunders CA, Mueller JD, Luxton G. Fluorescence fluctuation spectroscopy reveals differential SUN protein oligomerization in living cells. Mol Biol Cell 2018; 29:1003–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol 2006; 26:3738–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Östlund C, Folker ES, Choi JC, Gomes ER, Gundersen GG, Worman HJ. Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J Cell Sci 2009; 122:4099–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, Shackleton S. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem 2010; 285:3487–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borrego-Pinto J, Jegou T, Osorio DS, Auradé F, Gorjánácz M, Koch B, Mattaj IW, Gomes ER. Samp1 is a component of TAN lines and is required for nuclear movement. J Cell Sci 2012; 125:1099–105 [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 2009; 64:173–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu R, Antoku S, Gundersen GG. Centrifugal displacement of nuclei reveals multiple LINC complex mechanisms for homeostatic nuclear positioning. Curr Biol 2017; 27:3097–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, Korenbaum E. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res 2004; 295:330–9 [DOI] [PubMed] [Google Scholar]
- 31.Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010; 329:956–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutscheidt S, Zhu R, Antoku S, Luxton GW, Stagljar I, Fackler OT, Gundersen GG. FHOD1 interaction with nesprin-2G mediates TAN line formation and nuclear movement. Nat Cell Biol 2014; 16:708–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jayo A, Malboubi M, Antoku S, Chang W, Ortiz-Zapater E, Groen C, Pfisterer K, Tootle T, Charras G, Gundersen GG, Parsons M. Fascin regulates nuclear movement and deformation in migrating cells. Dev Cell 2016; 38:371–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol 2005; 171:799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci USA 2009; 106:2194–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adam SA, Goldman RD. Insights into the differences between the A- and B-type nuclear lamins. Adv Biol Regul 2012; 52:108–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin F, Worman HJ. Structural organization of the human gene (LMNB1) encoding nuclear lamin B1. Genomics 1995; 27:230–6 [DOI] [PubMed] [Google Scholar]
- 38.Wydner KL, McNeil JA, Lin F, Worman HJ, Lawrence JB. Chromosomal assignment of human nuclear envelope protein genes LMNA, LMNB1, and LBR by fluorescence in situ hybridization. Genomics 1996; 32:474–8 [DOI] [PubMed] [Google Scholar]
- 39.Biamonti G, Giacca M, Perini G, Contreas G, Zentilin L, Weighardt F, Guerra M, Della Valle G, Saccone S, Riva S, Falaschi A. The gene for a novel human lamin maps at a highly transcribed locus of chromosome 19 which replicates at the onset of S-phase. Mol Cell Biol 1992; 12:3499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furukawa K, Hotta Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. EMBO J 1993; 12:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem 1993; 268:16321–6 [PubMed] [Google Scholar]
- 42.Furukawa K, Inagaki H, Hotta Y. Identification and cloning of an mRNA coding for a germ cell‐specific A‐type lamin in mice. Exp Cell Res 1994; 212:426–30 [DOI] [PubMed] [Google Scholar]
- 43.Machiels BM, Zorenc AH, Endert JM, Kuijpers HJ, van Eys GJ, Ramaekers FC, Broers JL. An alternative splicing product of the lamin A/C gene lacks exon 10. J Biol Chem 1996; 271:9249–53 [DOI] [PubMed] [Google Scholar]
- 44.Reiss Y, Goldstein JL, Seabra MC, Casey PJ, Brown MS. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell 1990; 62:81–8 [DOI] [PubMed] [Google Scholar]
- 45.Maske CP, Hollinshead MS, Higbee NC, Bergo MO, Young SG, Vaux DJ. A carboxyl-terminal interaction of lamin B1 is dependent on the CAAX endoprotease Rce1 and carboxymethylation. J Cell Biol 2003; 162:1223–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corrigan DP, Kuszczak D, Rusinol AE, Thewke DP, Hrycyna CA, Michaelis S, Sinensky MS. Prelamin A endoproteolytic processing in vitro by recombinant Zmpste24. Biochem J 2005; 387:129–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke S, Vogel JP, Deschenes RJ, Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci USA 1988; 85:4643–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai Q, Choy E, Chiu V, Romano J, Slivka SR, Steitz SA, Michaelis S, Philips MR. Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J Biol Chem 1998; 273:15030–4 [DOI] [PubMed] [Google Scholar]
- 49.Weber K, Plessmann U, Traub P. Maturation of nuclear lamin A involves a specific carboxy-terminal trimming, which removes the polyisoprenylation site from the precursor; implications for the structure of the nuclear lamina. FEBS Lett 1989; 257:411–4 [DOI] [PubMed] [Google Scholar]
- 50.Beck LA, Hosick TJ, Sinensky M. Isoprenylation is required for the processing of the lamin A precursor. J Cell Biol 1990; 110:1489–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, Mohr A, Meta M, Genant H, Jiang Y, Wisner ER, Van Bruggen N, Carano RA, Michaelis S, Griffey SM, Young SG. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci USA 2002; 99:13049–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pendás AM, Zhou Z, Cadiñanos J, Freije JM, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodríguez F, Tryggvason K, López-Otín C. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet 2002; 31:94–9 [DOI] [PubMed] [Google Scholar]
- 53.Apel ED, Lewis RM, Grady RM, Sanes JR. Syne-1, a dystrophin- and Klarsicht-related protein associated with synaptic nuclei at the neuromuscular junction. J Biol Chem 2000; 275:31986–95 [DOI] [PubMed] [Google Scholar]
- 54.Rajgor D, Mellad JA, Autore F, Zhang Q, Shanahan CM. Multiple novel nesprin-1 and nesprin-2 variants act as versatile tissue-specific intracellular scaffolds. PLoS One 2012; 7:e40098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci 2005; 118:673–87 [DOI] [PubMed] [Google Scholar]
- 56.Horn HF, Brownstein Z, Lenz DR, Shivatzki S, Dror AA, Dagan-Rosenfeld O, Friedman LM, Roux KJ, Kozlov S, Jeang KT, Frydman M, Burke B, Stewart CL, Avraham KB. The LINC complex is essential for hearing. J Clin Invest 2013; 123:740–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kozono T, Tadahira K, Okumura W, Itai N, Tamura-Nakano M, Dohi T, Tonozuka T, Nishikawa A. Jaw1/LRMP has a role in maintaining nuclear shape via interaction with SUN proteins. J Biochem 2018; 164:303–11 [DOI] [PubMed] [Google Scholar]
- 58.Morimoto A, Shibuya H, Zhu X, Kim J, Ishiguro K, Han M, Watanabe Y. A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J Cell Biol 2012; 198:165–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horn HF, Kim DI, Wright GD, Wong ES, Stewart CL, Burke B, Roux KJ. A mammalian KASH domain protein coupling meiotic chromosomes to the cytoskeleton. J Cell Biol 2013; 202:1023–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hodzic DM, Yeater DB, Bengtsson L, Otto H, Stahl PD. Sun2 is a novel mammalian inner nuclear membrane protein. J Biol Chem 2004; 279:25805–12 [DOI] [PubMed] [Google Scholar]
- 61.Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou I. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci 2005; 118:3419–30 [DOI] [PubMed] [Google Scholar]
- 62.Shao X, Tarnasky HA, Lee JP, Oko R, van der Hoorn FA. Spag4, a novel sperm protein, binds outer dense-fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev Biol 1999; 211:109–23 [DOI] [PubMed] [Google Scholar]
- 63.Göb E, Schmitt J, Benavente R, Alsheimer M. Mammalian sperm head formation involves different polarization of two novel LINC complexes. PLoS One 2010; 5:e12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frohnert C, Schweizer S, Hoyer-Fender S. SPAG4L/SPAG4L-2 are testis-specific SUN domain proteins restricted to the apical nuclear envelope of round spermatids facing the acrosome. Mol Hum Reprod 2011; 17:207–18 [DOI] [PubMed] [Google Scholar]
- 65.Worman HJ, Courvalin JC. Nuclear envelope, nuclear lamina, and inherited disease. Int Rev Cytol 2005; 246:231–79 [DOI] [PubMed] [Google Scholar]
- 66.Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev Cell 2009; 17:626–38 [DOI] [PubMed] [Google Scholar]
- 67.Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest 2009; 119:1825–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Worman HJ, Östlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol 2010; 2:a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Worman HJ. Nuclear lamins and laminopathies. J Pathol 2012; 226:316–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Méndez-López I, Worman HJ. Inner nuclear membrane proteins: impact on human disease. Chromosoma 2012; 121:153–67 [DOI] [PubMed] [Google Scholar]
- 71.Bonne G, Di Barletta MR, Varnous S, Bécane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet 1999; 21:285–8 [DOI] [PubMed] [Google Scholar]
- 72.Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ, Jr, Spudich S, De Girolami U, Seidman JG, Seidman C, Muntoni F, Müehle G, Johnson W, McDonough B. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med 1999; 341:1715–24 [DOI] [PubMed] [Google Scholar]
- 73.Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, de Visser M, Schwartz K. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum Mol Genet 2000; 9:1453–9 [DOI] [PubMed] [Google Scholar]
- 74.Bonne G, Mercuri E, Muchir A, Urtizberea A, Bécane HM, Recan D, Merlini L, Wehnert M, Boor R, Reuner U, Vorgerd M, Wicklein EM, Eymard B, Duboc D, Penisson-Besnier I, Cuisset JM, Ferrer X, Desguerre I, Lacombe D, Bushby K, Pollitt C, Toniolo D, Fardeau M, Schwartz K, Muntoni F. Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann Neurol 2000; 48:170–80 [PubMed] [Google Scholar]
- 75.Brodsky GL, Muntoni F, Miocic S, Sinagra G, Sewry C, Mestroni L. Lamin A/C gene mutation associated with dilated cardiomyopathy with variable skeletal muscle involvement. Circulation 2000; 101:473–6 [DOI] [PubMed] [Google Scholar]
- 76.Raffaele Di Barletta M, Ricci E, Galluzzi G, Tonali P, Mora M, Morandi L, Romorini A, Voit T, Orstavik KH, Merlini L, Trevisan C, Biancalana V, Housmanowa-Petrusewicz I, Bione S, Ricotti R, Schwartz K, Bonne G, Toniolo D. Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery-Dreifuss muscular dystrophy. Am J Hum Genet 2000; 66:1407–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 2000; 9:109–12 [DOI] [PubMed] [Google Scholar]
- 78.Shackleton S, Lloyd DJ, Jackson SN, Evans R, Niermeijer MF, Singh BM, Schmidt H, Brabant G, Kumar S, Durrington PN, Gregory S, O'Rahilly S, Trembath RC. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 2000; 24:153–6 [DOI] [PubMed] [Google Scholar]
- 79.Speckman RA, Garg A, Du F, Bennett L, Veile R, Arioglu E, Taylor SI, Lovett M, Bowcock AM. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet 2000; 66:1192–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhe-Paganon S, Werner ED, Chi YI, Shoelson SE. Structure of the globular tail of nuclear lamin. J Biol Chem 2002; 277:17381–4 [DOI] [PubMed] [Google Scholar]
- 81.Krimm I, Östlund C, Gilquin B, Couprie J, Hossenlopp P, Mornon JP, Bonne G, Courvalin JC, Worman HJ, Zinn-Justin S. The Ig-like structure of the C-terminal domain of lamin A/C, mutated in muscular dystrophies, cardiomyopathy, and partial lipodystrophy. Structure 2002; 10:811–23 [DOI] [PubMed] [Google Scholar]
- 82.Caux F, Dubosclard E, Lascols O, Buendia B, Chazouillères O, Cohen A, Courvalin JC, Laroche L, Capeau J, Vigouroux C, Christin-Maitre S. A new clinical condition linked to a novel mutation in lamins A and C with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy. J Clin Endocrinol Metab 2003; 88:1006–13 [DOI] [PubMed] [Google Scholar]
- 83.Decaudain A, Vantyghem MC, Guerci B, Hécart AC, Auclair M, Reznik Y, Narbonne H, Ducluzeau PH, Donadille B, Lebbé C, Béréziat V, Capeau J, Lascols O, Vigouroux C. New metabolic phenotypes in laminopathies: LMNA mutations in patients with severe metabolic syndrome. J Clin Endocrinol Metab 2007; 92:4835–44 [DOI] [PubMed] [Google Scholar]
- 84.De Sandre-Giovannoli A, Chaouch M, Kozlov S, Vallat JM, Tazir M, Kassouri N, Szepetowski P, Hammadouche T, Vandenberghe A, Stewart CL, Grid D, Lévy N. Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am J Hum Genet 2002; 70:726–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hamadouche T, Poitelon Y, Genin E, Chaouch M, Tazir M, Kassouri N, Nouioua S, Chaouch A, Boccaccio I, Benhassine T, De Sandre-Giovannoli A, Grid D, Lévy N, Delague V. Founder effect and estimation of the age of the c.892C>T (p.Arg298Cys) mutation in LMNA associated to Charcot-Marie-Tooth subtype CMT2B1 in families from North Western Africa. Ann Hum Genet 2008; 72:590–7 [DOI] [PubMed] [Google Scholar]
- 86.Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B, Brooks BP, Gerber LH, Turner ML, Domingo DL, Hart TC, Graf J, Reynolds JC, Gropman A, Yanovski JA, Gerhard-Herman M, Collins FS, Nabel EG, Cannon RO, 3rd Gahl WA, Introne WJ. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med 2008; 358:592–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, Lévy N. Lamin A truncation in Hutchinson-Gilford progeria. Science 2003; 300:2055. [DOI] [PubMed] [Google Scholar]
- 88.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 2003; 423:293–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, Gruenbaum Y, Khuon S, Mendez M, Varga R, Collins FS. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 2004; 101:8963–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang SH, Bergo MO, Toth JI, Qiao X, Hu Y, Sandoval S, Meta M, Bendale P, Gelb MH, Young SG, Fong LG. Blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson-Gilford progeria syndrome mutation. Proc Natl Acad Sci USA 2005; 102:10291–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toth JI, Yang SH, Qiao X, Beigneux AP, Gelb MH, Moulson CL, Miner JH, Young SG, Fong LG. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc Natl Acad Sci USA 2005; 102:12873–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Capell BC, Erdos MR, Madigan JP, Fiordalisi JJ, Varga R, Conneely KN, Gordon LB, Der CJ, Cox AD, Collins FS. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 2005; 102:12879–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mallampalli MP, Huyer G, Bendale P, Gelb MH, Michaelis S. Inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 2005; 102:14416–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Glynn MW, Glover TW. Incomplete processing of mutant lamin A in Hutchinson-Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum Mol Genet 2005; 14:2959–69 [DOI] [PubMed] [Google Scholar]
- 95.Wang Y, Östlund C, Worman HJ. Blocking protein farnesylation improves nuclear shape abnormalities in keratinocytes of mice expressing the prelamin A variant in Hutchinson-Gilford progeria syndrome. Nucleus 2010; 1:432–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang SH, Meta M, Qiao X, Frost D, Bauch J, Coffinier C, Majumdar S, Bergo MO, Young SG, Fong LG. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J Clin Invest 2006; 116:2115–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Capell BC, Olive M, Erdos MR, Cao K, Faddah DA, Tavarez UL, Conneely KN, Qu X, San H, Ganesh SK, Chen X, Avallone H, Kolodgie FD, Virmani R, Nabel EG, Collins FS. A farnesyltransferase inhibitor prevents both the onset and late progression of cardiovascular disease in a progeria mouse model. Proc Natl Acad Sci USA 2008; 105:15902–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gordon LB, Kleinman ME, Miller DT, Neuberg DS, Giobbie-Hurder A, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland R, Snyder BD, Fligor B, Bishop WR, Statkevich P, Regen A, Sonis A, Riley S, Ploski C, Correia A, Quinn N, Ullrich NJ, Nazarian A, Liang MG, Huh SY, Schwartzman A, Kieran MW. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 2012; 109:16666–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gordon LB, Kleinman ME, Massaro J, D'Agostino RB, Sr, Shappell H, Gerhard-Herman M, Smoot LB, Gordon CM, Cleveland RH, Nazarian A, Snyder BD, Ullrich NJ, Silvera VM, Liang MG, Quinn N, Miller DT, Huh SY, Dowton AA, Littlefield K, Greer MM, Kieran MW. Clinical trial of the protein farnesylation inhibitors lonafarnib, pravastatin, and zoledronic acid in children with Hutchinson-Gilford progeria syndrome. Circulation 2016; 134:114–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fong LG, Frost D, Meta M, Qiao X, Yang SH, Coffinier C, Young SG. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science 2006; 311:1621–3 [DOI] [PubMed] [Google Scholar]
- 101.Agarwal AK, Fryns JP, Auchus RJ, Garg A. Zinc metalloproteinase, ZMPSTE24, is mutated in mandibuloacral dysplasia. Hum Mol Genet 2003; 12:1995–2001 [DOI] [PubMed] [Google Scholar]
- 102.Moulson CL, Go G, Gardner JM, van der Wal AC, Smitt JH, van Hagen JM, Miner JH. Homozygous and compound heterozygous mutations in ZMPSTE24 cause the laminopathy restrictive dermopathy. J Invest Dermatol 2005; 125:913–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Navarro CL, Cadiñanos J, De Sandre-Giovannoli A, Bernard R, Courrier S, Boccaccio I, Boyer A, Kleijer WJ, Wagner A, Giuliano F, Beemer FA, Freije JM, Cau P, Hennekam RC, López-Otín C, Badens C, Lévy N. Loss of ZMPSTE24 (FACE-1) causes autosomal recessive restrictive dermopathy and accumulation of Lamin A precursors. Hum Mol Genet 2005; 14:1503–13 [DOI] [PubMed] [Google Scholar]
- 104.Shackleton S, Smallwood DT, Clayton P, Wilson LC, Agarwal AK, Garg A, Trembath RC. Compound heterozygous ZMPSTE24 mutations reduce prelamin A processing and result in a severe progeroid phenotype. J Med Genet 2005; 42:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barrowman J, Wiley PA, Hudon-Miller SE, Hrycyna CA, Michaelis S. Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity. Hum Mol Genet 2012; 21:4084–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ast T, Michaelis S, Schuldiner M. The protease Ste24 clears clogged translocons. Cell 2016; 164:103–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Lichter-Konecki U, Anyane-Yeboa K, Shaw JE, Lu JT, Östlund C, Shin JY, Clark LN, Gundersen GG, Nagy PL, Worman HJ. A mutation abolishing the ZMPSTE24 cleavage site in prelamin A causes a progeroid disorder. J Cell Sci 2016; 129:1975–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Novelli G, Muchir A, Sangiuolo F, Helbling-Leclerc A, D'Apice MR, Massart C, Capon F, Sbraccia P, Federici M, Lauro R, Tudisco C, Pallotta R, Scarano G, Dallapiccola B, Merlini L, Bonne G. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet 2002; 71:426–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen L, Lee L, Kudlow BA, Dos Santos HG, Sletvold O, Shafeghati Y, Botha EG, Garg A, Hanson NB, Martin GM, Mian IS, Kennedy BK, Oshima J. LMNA mutations in atypical Werner's syndrome. Lancet 2003; 362:440–5 [DOI] [PubMed] [Google Scholar]
- 110.Nagy PL, Worman HJ. Next-generation sequencing and mutational analysis: implications for genes encoding LINC complex proteins. Methods Mol Biol 2018; 1840:321–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gros-Louis F, Dupré N, Dion P, Fox MA, Laurent S, Verreault S, Sanes JR, Bouchard JP, Rouleau GA. Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat Genet 2007; 39:80–5 [DOI] [PubMed] [Google Scholar]
- 112.Noreau A, Bourassa CV, Szuto A, Levert A, Dobrzeniecka S, Gauthier J, Forlani S, Durr A, Anheim M, Stevanin G, Brice A, Bouchard JP, Dion PA, Dupré N, Rouleau GA. SYNE1 mutations in autosomal recessive cerebellar ataxia. JAMA Neurol 2013; 70:1296–301 [DOI] [PubMed] [Google Scholar]
- 113.Synofzik M, Smets K, Mallaret M, Di Bella D, Gallenmüller C, Baets J, Schulze M, Magri S, Sarto E, Mustafa M, Deconinck T, Haack T, Züchner S, Gonzalez M, Timmann D, Stendel C, Klopstock T, Durr A, Tranchant C, Sturm M, Hamza W, Nanetti L, Mariotti C, Koenig M, Schöls L, Schüle R, de Jonghe P, Anheim M, Taroni F, Bauer P. SYNE1 ataxia is a common recessive ataxia with major non-cerebellar features: a large multi-centre study. Brain 2016; 139:1378–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Attali R, Warwar N, Israel A, Gurt I, McNally E, Puckelwartz M, Glick B, Nevo Y, Ben-Neriah Z, Melki J. Mutation of SYNE-1, encoding an essential component of the nuclear lamina, is responsible for autosomal recessive arthrogryposis. Hum Mol Genet 2009; 18:3462–9 [DOI] [PubMed] [Google Scholar]
- 115.Laquérriere A, Maluenda J, Camus A, Fontenas L, Dieterich K, Nolent F, Zhou J, Monnier N, Latour P, Gentil D, Héron D, Desguerres I, Landrieu P, Beneteau C, Delaporte B, Bellesme C, Baumann C, Capri Y, Goldenberg A, Lyonnet S, Bonneau D, Estournet B, Quijano-Roy S, Francannet C, Odent S, Saint-Frison MH, Sigaudy S, Figarella-Branger D, Gelot A, Mussini JM, Lacroix C, Drouin-Garraud V, Malinge MC, Attié-Bitach T, Bessieres B, Bonniere M, Encha-Razavi F, Beaufrère AM, Khung-Savatovsky S, Perez MJ, Vasiljevic A, Mercier S, Roume J, Trestard L, Saugier-Veber P, Cordier MP, Layet V, Legendre M, Vigouroux-Castera A, Lunardi J, Bayes M, Jouk PS, Rigonnot L, Granier M, Sternberg D, Warszawski J, Gut I, Gonzales M, Tawk M, Melki J. Mutations in CNTNAP1 and ADCY6 are responsible for severe arthrogryposis multiplex congenita with axoglial defects. Hum Mol Genet 2014; 23:2279–89 [DOI] [PubMed] [Google Scholar]
- 116.Baumann M, Steichen-Gersdorf E, Krabichler B, Petersen BS, Weber U, Schmidt WM, Zschocke J, Müller T, Bittner RE, Janecke AR. Homozygous SYNE1 mutation causes congenital onset of muscular weakness with distal arthrogryposis: a genotype-phenotype correlation. Eur J Hum Genet 2017; 25:262–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM. Nesprin-1 and -2 are involved in the pathogenesis of Emery-Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet 2007; 16:2816–33 [DOI] [PubMed] [Google Scholar]
- 118.Puckelwartz MJ, Kessler EJ, Kim G, Dewitt MM, Zhang Y, Earley JU, Depreux FF, Holaska J, Mewborn SK, Pytel P, McNally EM. Nesprin-1 mutations in human and murine cardiomyopathy. J Mol Cell Cardiol 2010; 48:600–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou C, Li C, Zhou B, Sun H, Koullourou V, Holt I, Puckelwartz MJ, Warren DT, Hayward R, Lin Z, Zhang L, Morris GE, McNally EM, Shackleton S, Rao L, Shanahan CM, Zhang Q. Novel nesprin-1 mutations associated with dilated cardiomyopathy cause nuclear envelope disruption and defects in myogenesis. Hum Mol Genet 2017; 26:2258–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meinke P, Mattioli E, Haque F, Antoku S, Columbaro M, Straatman KR, Worman HJ, Gundersen GG, Lattanzi G, Wehnert M, Shackleton S. Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet 2014; 10:e1004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet 1994; 8:323–7 [DOI] [PubMed] [Google Scholar]
- 122.Nagano A, Koga R, Ogawa M, Kurano Y, Kawada J, Okada R, Hayashi YK, Tsukahara T, Arahata K. Emerin deficiency at the nuclear membrane in patients with Emery-Dreifuss muscular dystrophy. Nat Genet 1996; 12:254–9 [DOI] [PubMed] [Google Scholar]
- 123.Manilal S, Nguyen TM, Sewry CA, Morris GE. The Emery-Dreifuss muscular dystrophy protein, emerin, is a nuclear membrane protein. Hum Mol Genet 1996; 5:801–8 [DOI] [PubMed] [Google Scholar]
- 124.Manilal S, Recan D, Sewry CA, Hoeltzenbein M, Llense S, Leturcq F, Deburgrave N, Barbot J, Man N, Muntoni F, Wehnert M, Kaplan J, Morris GE. Mutations in Emery-Dreifuss muscular dystrophy and their effects on emerin protein expression. Hum Mol Genet 1998; 7:855–64 [DOI] [PubMed] [Google Scholar]
- 125.Yates JR, Bagshaw J, Aksmanovic VM, Coomber E, McMahon R, Whittaker JL, Morrison PJ, Kendrick-Jones J, Ellis JA. Genotype-phenotype analysis in X-linked Emery-Dreifuss muscular dystrophy and identification of a missense mutation associated with a milder phenotype. Neuromuscul Disord 1999; 9:159–65 [PubMed] [Google Scholar]
- 126.Muntoni F, Lichtarowicz-Krynska EJ, Sewry CA, Manilal S, Recan D, Llense S, Taylor J, Morris GE, Dubowitz V. Early presentation of X-linked Emery-Dreifuss muscular dystrophy resembling limb-girdle muscular dystrophy. Neuromuscul Disord 1998; 8:72–6 [DOI] [PubMed] [Google Scholar]
- 127.Astejada MN, Goto K, Nagano A, Ura S, Noguchi S, Nonaka I, Nishino I., Hayashi YK. Emerinopathy and laminopathy clinical, pathological and molecular features of muscular dystrophy with nuclear envelopathy in Japan. Acta Myol 2007; 26:159–64 [PMC free article] [PubMed] [Google Scholar]
- 128.Gundersen GG, Kim I, Chapin CJ. Induction of stable microtubules in 3T3 fibroblasts by TGFbeta and serum. J Cell Sci 1994; 107:645–59 [DOI] [PubMed] [Google Scholar]
- 129.Cook TA, Nagasaki T, Gundersen GG. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J Cell Biol 1998; 141:175–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin and actin flow establishes MTOC polarization in migrating cells. Cell 2005; 121:451–63 [DOI] [PubMed] [Google Scholar]
- 131.Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr Biol 2001; 11:1536–41 [DOI] [PubMed] [Google Scholar]
- 132.Schmoranzer J, Fawcett JP, Segura M, Tan S, Vallee RB, Pawson T, Gundersen GG. Par3 and dynein associate to regulate local microtubule dynamics and centrosome orientation during migration. Curr Biol 2009; 19:1065–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Folker ES, Östlund C, Luxton GW, Worman HJ, Gundersen GG. Proc Natl Acad Sci USA 2011; 108:131–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol 1999; 147:913–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cutler DA, Sullivan T, Marcus-Samuels B, Stewart CL, Reitman ML. Characterization of adiposity and metabolism in Lmna-deficient mice. Biochem Biophys Res Commun 2002; 291:522–7 [DOI] [PubMed] [Google Scholar]
- 136.Chang W, Folker ES, Worman HJ, Gundersen GG. Emerin organizes actin flow for nuclear movement and centrosome orientation in migrating fibroblasts. Mol Biol Cell 2013; 24:3869–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, Hussey PJ, Hutchison CJ. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol 2007; 178:897–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chang W, Antoku S, Östlund C, Worman HJ, Gundersen GG. Linker of nucleoskeleton and cytoskeleton (LINC) complex-mediated actin-dependent nuclear positioning orients centrosomes in migrating myoblasts. Nucleus 2015; 6:77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Melcon G, Kozlov S, Cutler DA, Sullivan T, Hernandez L, Zhao P, Mitchell S, Nader G, Bakay M, Rottman JN, Hoffman EP, Stewart CL. Loss of emerin at the nuclear envelope disrupts the Rb1/E2F and MyoD pathways during muscle regeneration. Hum Mol Genet 2006; 15:637–51 [DOI] [PubMed] [Google Scholar]
- 140.Ozawa R, Hayashi YK, Ogawa M, Kurokawa R, Matsumoto H, Noguchi S, Nonaka I, Nishino I. Emerin-lacking mice show minimal motor and cardiac dysfunctions with nuclear-associated vacuoles. Am J Pathol 2006; 168:907–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Y, Shin JY, Nakanishi K, Homma S, Kim GJ, Tanji K, Joseph LC, Morrow JP, Stewart CL, Dauer WT, Worman HJ. Postnatal development of mice with combined genetic depletions of lamin A/C, emerin and lamina-associated polypeptide 1. Hum Mol Genet 2019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang W, Wang Y, Luxton GWG, Östlund C, Worman HJ, Gundersen GG. Imbalanced nucleocytoskeletal connections create common polarity defects in progeria and physiological aging. Proc Natl Acad Sci U S A 2019; 116:3578–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen CY, Chi YH, Mutalif RA, Starost MF, Myers TG, Anderson SA, Stewart CL, Jeang KT. Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies. Cell 2012; 149:565–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hernandez L, Roux KJ, Wong ES, Mounkes LC, Mutalif R, Navasankari R, Rai B, Cool S, Jeong JW, Wang H, Lee HS, Kozlov S, Grunert M, Keeble T, Jones CM, Meta MD, Young SG, Daar IO, Burke B, Perantoni AO, Stewart CL. Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Dev Cell 2010; 19:413–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim PH, Luu J, Heizer P, Tu Y, Weston TA, Chen N, Lim C, Li RL, Lin PY, Dunn JCY, Hodzic D, Young SG, Fong LG. Disrupting the LINC complex in smooth muscle cells reduces aortic disease in a mouse model of Hutchinson-Gilford progeria syndrome. Sci Transl Med 2018; 10:eaat7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gundersen GG, Worman HJ. Nuclear positioning. Cell 2013; 152:1376–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Butler MT, Wallingford JB. Planar cell polarity in development and disease. Nat Rev Mol Cell Biol 2017; 18:375–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Campanale JP, Sun TY, Montell DJ. Development and dynamics of cell polarity at a glance. J Cell Sci 2017; 130:1201–7 [DOI] [PMC free article] [PubMed] [Google Scholar]