Short abstract
Microtubules are cytoskeletal elements known as drivers of directed cell migration, vesicle and organelle trafficking, and mitosis. In this review, we discuss new research in the lens that has shed light into further roles for stable microtubules in the process of development and morphogenesis. In the lens, as well as other systems, distinct roles for characteristically dynamic microtubules and stabilized populations are coming to light. Understanding the mechanisms of microtubule stabilization and the associated microtubule post-translational modifications is an evolving field of study. Appropriate cellular homeostasis relies on not only one cytoskeletal element, but also rather an interaction between cytoskeletal proteins as well as other cellular regulators. Microtubules are key integrators with actin and intermediate filaments, as well as cell–cell junctional proteins and other cellular regulators including myosin and RhoGTPases to maintain this balance.
Impact statement
The role of microtubules in cellular functioning is constantly expanding. In this review, we examine new and exciting fields of discovery for microtubule’s involvement in morphogenesis, highlight our evolving understanding of differential roles for stabilized versus dynamic subpopulations, and further understanding of microtubules as a cellular integrator.
Keywords: Microtubules, cytoskeleton, development, lens, myosin, cadherin
Introduction
The cytoskeleton is a structure that helps maintain cell shape,1–6 organizes and transports organelles,1,7–12 provides mechanical support,1,5,13–16 and allows for cell division and movement.2,17–20 Amongst these cytoskeletal elements, microtubules specifically are known regulators of directional cell migration,21–26 vesicle and organelle trafficking,8,27,28 and mitosis.29–32 These polarized hollow cylinders are composed of α-tubulin and β-tubulin heterodimers and nucleated at the microtubule organizing center at their minus ends, with the plus ends growing outward.33–35 Movement along microtubules to allow for trafficking and placement of cellular contents is directed by molecular motor proteins kinesins and dyneins, with kinesins moving toward the microtubule plus end and dyneins moving toward the microtubule minus end.27,36–40
Microtubules are characteristically defined by a constant cycling between growing and shrinking referred to as dynamic instability.41–44 Subpopulations of microtubules can be stabilized by post-translational modifications including acetylation45–53 or detyrosination48,49,54 as well as their interactions with microtubule-associated proteins (MAPs).55–58 This stabilization of microtubules is important for several cell processes, with acetylated microtubules found at the leading edge of actively migrating cells assisting in directional migration.54 Acetylated microtubules are also the foundation of primary cilia, which are generally referred to as the antennae of the cell, involved in sensing the cellular environment, cell signaling, liquid flow, cell polarity and multiple sensory organ functions including smell, sound, and sight.45,47,59–63
Microtubules’ role in cell functioning greatly depends on its ability to coordinate the functioning of other cellular elements, including other cytoskeletal components.64–68 This has proven especially true in the field of cellular migration, with microtubules shown to have both direct and indirect effects on the actomyosin machinery in motile cells, partly through regulation of their stability.64–68 Newer research has also demonstrated that microtubule interaction with cellular elements includes not only cytoskeletal elements and cytoplasmic organelles, but also cell–cell junctional proteins.68–72 Here, we look at the expanding role of microtubules in cellular functions and how microtubules may serve as a keystone of cytoskeletal coordination.
Microtubules in the lens: A developmental role
The lens is a tissue whose structure defines its function. A transparent tissue devoid of vasculature and nerves, it serves to focus light rays onto the retina to allow for vision. While generally looked upon as a simple clear gelatinous structure, the lens is actually defined by a precise cellular arrangement within the lens epithelium and among the differentiated fiber cells as well as between these two primary cell types of the lens.73–76 It is this interaction between and among these two different cell types that allows for the generation and maintenance of a three-dimensional structure that can adapt and accommodate to allow for proper vision.77,78 This precise cytoarchitecture’s establishment and maintenance relies heavily on cytoskeletal signaling networks in conjunction with cell–cell junctions.79–83 This reliance on cell–cell interaction makes the lens an ideal system for studying the processes of development, morphogenesis, and regeneration.
Additionally, the lens is a structure that continues to develop and grow throughout an organism’s life.84–86 Given that the lens lacks blood and nervous supply,87 it is the structural architecture and cell–cell communications that allow for this tissue to retain integrity across the course of an animal’s life. The disruption of this system leads to opacities such as cataracts, often accompanied by changes in protein density, cellular depolarization, osmotic disruption, and oxidative stress damage leading to cellular degeneration, membrane disruption, and loss of tissue architecture.88–92 This makes the lens a system also well-suited to examining pathogenesis, at how cellular and structural changes affect tissue development and maintenance and how this tissue and those around it respond to injury.
Despite early research into the presence and potential role of microtubules in lens culture systems,93–96 very little was understood about the role of microtubules in the in vivo lens. In this early work, microtubules were demonstrated to orient themselves along the axis along which they would elongate and were found to be present in both the epithelium and fiber cells,97 and were abundant in elongated fiber cells.97 Early work from the Beebe lab looked at lens epithelial cell elongation and found that only certain microtubule inhibitors, namely colchicine, prevented epithelial cells in culture from elongating.98 However, these in vivo studies looked at epithelial cells in isolation under the influence of fetal calf serum to promote differentiation rather than looking at how the microtubules in actively elongating fiber cells may play a role in overall lens development. Moreover, microtubule inhibitors caused significant inhibition of lens cell elongation and cataract56,99 and cataractous lenses were often found to demonstrate loss of microtubules97 demonstrating that microtubules must play some role in lens development and maintenance.
More recently, a form of autosomal-recessive congenital cataract was linked to a mutation in a mediator of microtubule plus-end-directed vesicle transport, FYCO1, suggesting that there is a role for microtubule trafficking in proper lens development.56 FYCO1 is also associated with autophagosomes, and thus microtubules’ role may also involve trafficking of autophagosomes for organelle-free zone formation,100 similar to its role in other systems.63,101–103 Additional studies have also suggested that microtubules may play a role in lens fiber cell differentiation related to their known function in transporting proteins, membrane, and organelles.9
New findings using the embryonic lens as a model demonstrate that microtubule isoforms are expressed abundantly in both epithelial and fiber cells68,104 unlike other cytoskeletal elements, in particular lens-specific intermediate filaments, that are greatly enriched in either epithelial or fiber cell compartments.104–108 Instead, it is the post-translational modifications that are highly localized in the lens, with stable, acetylated microtubules concentrated in newly differentiated fiber cells and the apical aspects of lens equatorial epithelial cells.68 Disruption of dynamic microtubules does not impede normal fiber cell elongation and morphogenesis, but depolymerization of the stable acetylated microtubules results in opacities.68 These opacities coincide with separation along the transition zone of the apical surfaces of lens equatorial epithelial cells and newly differentiating fiber cells at the epithelial-fiber interface (EFI),68 revealing a requisite role for the stable microtubule population in lens morphogenesis. In addition, these studies demonstrated that loss of acetylated microtubules resulted in aberrant directionality of movement of the anterior tips of lens fiber cells as they elongated, as well as dysregulation of actin organization along lateral cell–cell borders of both lens epithelial and fiber cells and increased activation of myosin.68 These studies using the lens as a model system both reinforce previously known functions of microtubules, while also providing greater insight into the developmental process as a whole and how cytoskeletal elements’ interactions and interrelation underlie crucial cellular processes.
Microtubule stability and dynamics: Expanding understanding of roles
Microtubules are traditionally characterized by their property of dynamic instability41–44 (Figure 1) as described in a variety of cell populations including brain, kidney, and cell lines. However, tubulin itself is a highly modifiable protein, subject to a variety of post-translational modifications, including detyrosination,109,110 glutamylation,111,112 polygyclation,113 acetylation,114,115 and phosphorylation of serine112,116 and tyrosine117 residues. Many of these have been examined in brain tissue, which greatly relies on axonal plasticity, with dynamic growing and shrinking and directionality, as well as incorporation of a neurofilament network as an essential cytoskeletal component. These modified forms of tubulin are generally found to accumulate in the subpopulation of stabilized microtubules within a cell48,118–121 (Figure 1), as demonstrated in brain, kidney, ovary, intestine, and fibroblast cells. These post-translational modifications and changes in microtubule stability also provide several alterations in terms of microtubule functioning including microtubule interaction with other cytoskeletal elements122,123 as well as microtubule roles in cellular processes such as morphogenesis48,124,125 including brain development and regeneration,126 barrier function127,128 of the epidermis and vasculature, cytokinesis,129 and cell migration130 as described in multiple systems including neurons and fibroblasts.
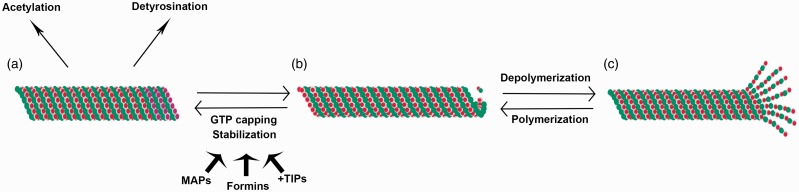
Figure 1.
Microtubule stabilization and dynamic instability. Microtubules are typically characterized by cycles of polymerization and depolymerization, termed dynamic instability (b, c). However, some subpopulations of microtubules can be stabilized, a process that involves GTP capping of terminal α-tubulin monomers and is promoted by microtubule associated proteins (MAPs), formins and microtubule plus-end binding proteins (+TIPs) (a, b). This stabilization can then give rise to post-translational modifications of microtubules including acetylation and detyrosination (a).
Early work initially demonstrated the presence of a distinct stable microtubule population131,132 that proves resistant to depolymerization.133,134 From the beginning, stability was linked to differences in MAPs135 as well as post-translational modification.49,110 In multiple systems, both acetylation as well as detyrosination have been shown to correlate with microtubule stability.49,118,136,137 It has been difficult to identify whether the posttranslational modifications are the cause of increased stability or a consequence of stable microtubules being a longer lived population due to prior modification such as end capping.49,121,138 Tyrosination or detyrosination involves the removal or re-addition of a tyrosine residue on the C-terminus of α-tubulin.48 Detyrosination is increased artificially when microtubules are exposed to stabilizing treatments54,139 and detyrosinated microtubules show resistance to depolymerization by microtubule antagonistic drugs,137 preventing microtubule disassembly by blocking interaction with microtubule depolymerizing motor proteins.140,141 These detyrosinated microtubules have specialized organization and distribution in both morphogenesis and cellular migration. In migrating cells, those cells responsible for leading wound healing have been shown to be rich in detyrosinated microtubules and are responsible for appropriate orientation of migration.54 Additionally, the presence of detyrosinated microtubules has been linked to morphogenetic change and differentiation.142–147 The distribution of detyrosination may act as a cellular cue to help direct cellular polarity.148 More recent work has shown that tyrosination/detyrosination of tubulin impacts other cellular functions, including interaction of microtubules with microtubule plus end tracking proteins50,140 as well as molecular motors, such as kinesin-1.149 As will be discussed further later, these detyrosinated microtubules have also been shown to preferentially interact with intermediate filaments, specifically vimentin, and help to localize vimentin.150
Although detyrosination has been the most studied tubulin modification, acetylation has also been greatly linked to microtubule stabilization. Acetylated microtubules are highly enriched in certain cellular structures including the mitotic spindle, centrioles, and cilia51 as well as being highly accumulated in stable microtubule subpopulations.118,151–153 Changes in acetylation have been linked to several disease states including Huntington’s,154 polycystic kidney disease,155 Alzheimer’s,156 and Parkinson’s.157 Acetylated microtubules have been shown to play a role in cellular polarity52,158,159 as well as contact inhibition of proliferation and cellular adhesion.160 Recent work also shows a functional role for acetylated microtubules in lens fiber cell morphogenesis, elongation, and directionality.68 Acetylated microtubules also show differential interactions with microtubule motors51 and organelles.161–163 Interestingly, formin proteins, traditionally known to function as actin nucleators, have been shown to act as microtubule stabilizers and help control microtubule acetylation.164,165 Formins promote microtubule stabilization by promoting end capping166 while also promoting expression of the tubulin acetylation protein α-TAT1 gene.167,168 The overlapping functions of formin proteins on both actin and microtubule proteins demonstrate a potential coordinate activity between cytoskeletal elements in cellular functioning.167
Beyond these two better studied post-translational modifications associated with microtubule stability, newer work has also investigated the roles for polyamination and transglutamination in microtubule stability.169 This was examined in neuronal axons, where stable microtubules serve as the structural framework and tracks for axonal transport, while dynamic microtubules are more linked to reorganization and repair during neurite growth and remodeling.170–172 Transglutaminase activity and polyamine levels were known to increase with brain maturation and neuronal differentiation,173 and were found to be modulators of microtubules by promoting stability.53,169 Further research will likely further develop our understanding of how post-translational modifications and their link to microtubule stability impact microtubule function. New approaches may help to differentiate between microtubule posttranslational modification and microtubule stability to better understand the differential roles these play in microtubule functions.
In addition to the correlation between certain post-translational modifications and microtubule stability, certain MAPs play a role in microtubule stability as well (Figure 1). Among these are several structural MAPs that in binding to microtubules affect their stability and polymerization.174 In particular, all MAP1 proteins have been associated with a stabilization function,58,175–177 with MAP1B playing a larger role in modulating microtubule stability and catastrophe depending on its phosphorylation state.178–180 Other MAPs, including MAP4, also enhance tubulin polymerization and stability, potentially by sterically blocking disassembly,180–182 again with phosphorylation of the MAP regulating its activity.183–185 In addition to these, tau, a MAP highly studied in neurodegeneration, also promotes axonal microtubule stabilization,186–188 and work has demonstrated that tau stabilizes a protofilament conformation that favors the formation of microtubules, and increases microtubule stability.189,190 Recent work has shown that this tau binds at the interface between tubulin heterodimers to promote stability.57,191
Microtubule plus end binding proteins (+TIPs) also have a role to play in microtubule stability. Regulators of prototypical +TIPs including CLIP-170, specifically a family of proteins called CLASPs, have been identified as microtubule stabilizers and also help to orient stable microtubules toward the leading edge of migration.192–194 Other +TIPs involved in microtubule stabilization include APC, which has been shown to be necessary for axonal outgrowth.195,196 Additionally, end binding proteins, particularly EB1, are involved in the formation of the EB-stabilizing cap at microtubule plus ends.197–201 Formins, previously recognized as promoters of microtubule stability, can also interact with +TIP proteins, including EB1, APC, and CLIP-170, and these interactions may also serve to promote microtubule stabilization downstream of Rho activity.152,202,203 Interestingly, new research also demonstrates a role for certain microtubule motor proteins in promoting microtubule stability as well204 and that in particular kinesin Kif4 can induce microtubule stability by interactions with the formin-EB1 microtubule stabilization pathway.205
Importantly, this subpopulation of stable microtubules, as opposed to those more characteristic dynamically unstable microtubules, has differing functions. In many cell types, there is a characteristic distribution of stable versus dynamic microtubules.45,68,129,206 Partly, this works to help establish cellular polarity, with stable microtubule populations generally found at the cell front versus dynamic microtubules more likely to be present at the rear of the cell.48,54 Further work to better understand microtubule stability and its various roles in the cell is essential to global understanding of microtubule function.
Microtubules as a keystone of cytoskeletal coordination
The cytoskeleton is comprised of three major subtypes—microtubules, actin, and intermediate filaments—that have distinct functional and morphological properties that are important for their individual functions. However, despite these distinctions, all three interact to form a complex network necessary for proper cell functioning. Dynamic interactions between these groups are critical for multiple cellular processes as well as maintaining cytoplasmic homeostasis. These interactions are just the beginning of our understanding of how microtubules, through interaction with other cellular elements, play critical roles in a variety of cellular functions.
Previous work has shed light on various coordinate roles for actin and microtubules, with their interaction being critical for such processes as cell division, migration, vesicle and organelle transport, axonal growth, and wound healing67,207,208 (Figure 2). These roles often rely on actin’s ability to guide as well as stabilize microtubules.209 Previous work has shown that microtubules can grow along actin bundles210,211 and when actin becomes disorganized, microtubular growth can be inhibited, with redistribution and misdirectionality.21,195,212,213 More recent studies show that in turn microtubule depolymerization causes upregulation and reorganization of the actin cytoskeleton,68 implying a mutual regulatory process.
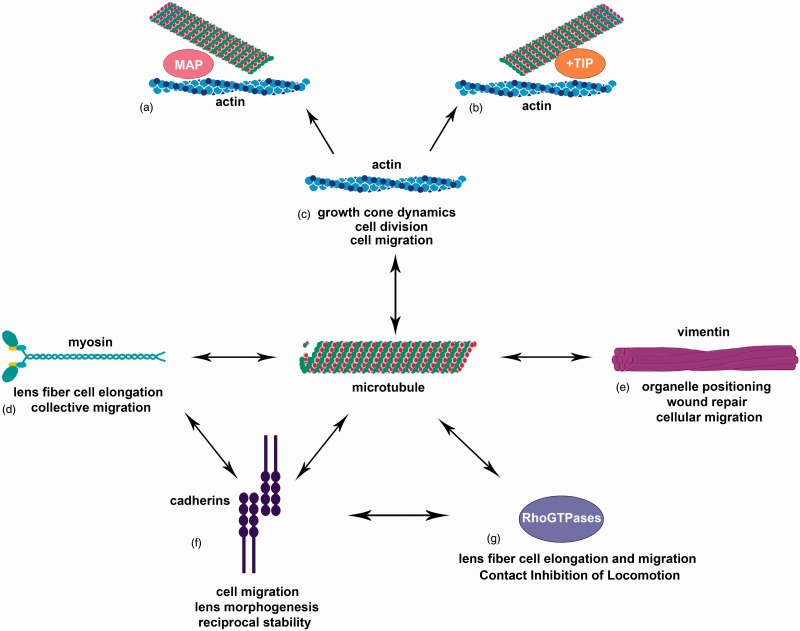
Figure 2.
Microtubules as a cornerstone of cellular interaction. Microtubules interact with a variety of cellular components (a–g). Interaction with actin is important for growth cone dynamics, cell division, and cell migration (c) and is facilitated by microtubule-associated proteins (MAPs) (a) and microtubule plus-end binding proteins (b). Interactions with myosin have been seen in collective migration and lens fiber cell elongation (d). Intermediate filaments, including vimentin, interact with microtubules for organelle positioning, wound repair, and cellular migration (e). Cadherins, especially N-cadherin, have been shown to interact with microtubules as well as myosin and RhoGTPases (d, f, g). Cadherins and microtubules reciprocally regulate one another’s stability (f) and the interaction of cadherins with microtubules as well as myosin and RhoGTPases is important for cell migration and lens morphogenesis (d, f, g). RhoGTPases are also direct interactors with microtubules for processes such as lens fiber cell elongation and migration as well as Contact Inhibition of Locomotion.
One of the more studied aspects of this interaction has been in the field of growth cone steering and dynamics. In growth cones, actin filaments exhibit dynamicity, with a constant cycle of polymerization and severing, that is necessary for rapid axonal outgrowth.214–216 It is this instability of the actin cytoskeleton that is able to facilitate microtubule extension.217 Microtubules in growth cones have been primarily linked as guidance sensors in growth cones controlling directionality as well as forward progression.218–221 For this to occur, F-actin networks provide tracks for microtubule growth, while actin dynamics allow for targeted distribution of microtubules to help oversee directionality.222–226
Actin filaments and microtubules can be crosslinked by direct linkage mediated by crosslinking proteins227,228 or indirectly via protein complexes or signaling molecules. Direct linkage by crosslinking proteins is usually considered to be static interactions, whereas indirect interactions mediated by motor protein complexes or signaling molecules are thought to be dynamic in nature.229 Some of the key mediators of these interactions are MAPs, which come in multiple types. Among these are structural MAPs, including MAP 1, MAP 2, MAP4 and tau, that bind along the surface of microtubules and enhance stability and assembly, motor proteins such as dyneins and kinesins and microtubule plus end tracking proteins (+TIPs) that interact with the growing (plus) end of microtubules as well as other protein complexes.229
For many structural MAPs, their expression is developmentally regulated, including MAP1, MAP2, and tau230–235 particularly in neurons. Previously, it was shown that these proteins indirectly regulated actin by modulating microtubule dynamics and can act as structural links to hold these filaments together.236–238 However, studies have also shown that these proteins can act to bundle as well as crosslink actin filaments independent of their actions on microtubules, with differential abilities among different classes of structural MAPs.229,239,240 The properties of these MAPs, including their regulation and interaction with cytoskeletal proteins, are also impacted by post-translational modifications, similar to microtubules themselves.241,242 Additionally, tau-mediated changes in actin organization have been linked to the development of neurodegenerative disorders such as Alzheimer’s and taupathies.243 Future research to better understand the in vivo role that these proteins play in dynamic coordinated interactions is needed to better understand the mechanisms of their function.
+TIPS are the other major subgroup of microtubule interacting proteins that can regulate crosslinking with other cytoskeletal proteins. These proteins can interact not just with microtubules but also actin filaments and regulatory kinases, which can induce microtubule-actin crosslinking.195,196,223,244–248 They have been linked as mediators of actin-microtubule interaction for growth cone guidance and regulated distribution of +TIPS can promote interaction of F-actin and microtubules that allows for effective growth cone steering.244,246,247,249–251 Some of the more studied of these +TIPS include CLASP, which binds actin and microtubules through a shared domain247 and whose loss results in both disrupted actin morphology as well as impaired axon outgrowth.250 Additionally, +TIPS such as APC can act as microtubule stabilizers as discussed above, which can promote their outgrowth.195,196 These +TIPS do not only act on microtubules, but also may have a role in microtubule-F-actin coupling.252,253 Other studies have looked at end-binding (EB) proteins as regulators of microtubule dynamics254,255 and studies within the lens demonstrate a coordinate localization between EB1 and acetylated stable microtubules.68 These proteins are also important scaffolding proteins that can recruit actin modulators as well as MAPs that alter actin dynamics.175,245,256–258 Of note, one of the major classes of proteins known to interact with both microtubules and actin cytoskeletal elements is spectraplakins.259–264 Some of these essential interactions are facilitated by spectraplakin binding to cytoskeleton through interaction with end binding proteins such as EB1.244–265 Just as importantly, +TIPS can also act as microtubule-actin uncouplers, including LIS1.266
Newer evidence also expands the potential role of +TIPS in terms of its role in actin functioning, including serving as sites that facilitate F-actin nucleation. This is likely related to interactions between APC and mDia, a formin linked to actin remodeling267–269 and also an influencer of microtubule stability.153,270,271 Future research will likely reveal expanding ways by which different cytoskeletal elements impact one another and evolving roles that microtubule-actin crosslinking plays in a variety of cellular processes.
It is not only actin that has been shown to interact with microtubules, but also certain intermediate filament subtypes, in particular, vimentin (Figure 2). In earlier work, it was demonstrated that distribution of vimentin corresponded to microtubule distribution272 and depolymerization of microtubules causes retraction of the vimentin network to the perinuclear region of the cell.273,274 Additionally, studies suggest that vimentin as well as other intermediate filaments, including neurofilaments, can be rearranged by microtubule-associated motor proteins, kinesin, and dynein, implying that vimentin distribution relies on microtubule transport.275–280 Further research demonstrates that vimentin filament transport is dependent on microtubules and their acetylation state but not impacted by microtubule dynamics or polymerization.281 Additionally, these studies also showed that vimentin motility was also linked to interactions between vimentin and polymerized actin that may in turn be impacted by microtubules.281–283
This interaction between microtubules and vimentin is necessary for several cellular processes. This includes proper positioning of organelles requires the combined efforts of microtubules as well as intermediate filaments such as vimentin and neurofilaments.284–287 Studies in a mock cataract surgery model demonstrated that collapse of intermediate filaments, and in particular, vimentin, following microtubule depolymerization results in impaired wound closure.288 These studies, as well as others, demonstrate a role for vimentin-microtubule interaction in cellular migration. Vimentin-null fibroblasts demonstrate impaired locomotion and it is possible that normal locomotion relies on the associations between vimentin and MTs and their molecular motors to provide for rapid turnover and reorganization of the vimentin network.276,289,290 Recent work on astrocytes has shown that APC, already known to interact with microtubules and control their organization in migrating astrocytes291–293 is necessary for microtubule interaction with intermediate filaments in these cells.294 More importantly, it also controls the microtubule-dependent rearrangement of IFs during astrocyte migration, as well as having a role in vimentin organization in other cell types as well.294
Beyond interaction with cytoskeleton, microtubule functioning through reciprocal regulation with cell–cell adhesion molecules is becoming better understood. Both N-cadherin and microtubules have been demonstrated to be critical in regulating cellular migration processes.38,66,67,70,72,295–299 In many instances, the cell–cell adhesions and cytoskeleton have been shown to work coordinately in regulating these processes, with classical and non-classical cadherins traditionally associated with the actin and intermediate filament elements of the cytoskeleton. However, there are several insights into how microtubules and cadherin junctions may interact to function together in cellular processes.245
Microtubules have been shown to directly interact with cadherin junctions in other systems300,301 and potentially regulate the presence of cadherin junctions at the cell surface for cell–cell contact302,303 (Figure 2). In addition to this, microtubule depolymerization has also been shown to disrupt integrity of cadherin junctions as well.304–306 This effect is not limited solely to microtubule depolymerization but also applies to impedance of microtubule dynamics or overstabilization.303 It is possible that the function microtubules play in maintaining cadherin junction integrity is intrinsic to the role that microtubules play in regulating vesicle transport, with N-cadherin having been shown to traffic along microtubules.307,308 Clearly, there is a dependence of N-cadherin on microtubules for proper function.
Conversely, cadherin junctions have also been shown to regulate microtubules. Microtubule polymerization has been linked to interaction with the actin cytoskeleton for guidance, with cadherin junctions playing roles in anchoring actin bundles at the cell surface.309,310 Additionally, cadherins have been shown to also play direct roles in recruiting microtubules to sites of cell–cell contact.303 Microtubules have been shown to anchor to cadherin junctions304,305 and p120 catenin, an atypical catenin found at cadherin junctions, has also been demonstrated to directly interact with microtubules.311,312 These interactions can play a role in regulating microtubule dynamics by either increasing or decreasing microtubule stabilization.69,313–315 New work has shed insight into how interaction between cadherins and microtubules, along with actin, may be critical in how cells sense guidance cues and underlie mechanoresponsive behavior316,317 and suggest that polarization of cell–cell junctions as well as the actin cytoskeleton that is crucial cellular processes including migration and angiogenesis are dependent on microtubule dynamics as well as RhoGTPases.318
It is therefore not surprising that systems such as the lens would have interactions between the microtubule cytoskeleton and N-cadherin junctions. Recent findings show that microtubules, especially acetylated microtubules, and N-cadherin junctions interact strongly in the cortical fiber cell region, the site of active lens fiber cell elongation, and that stable microtubules are necessary for appropriate localization and likely functioning of N-cadherin junctions.68 However, loss of N-cadherin does not disrupt the presence and assembly of these acetylated microtubules, nor does the presence of these microtubules alone prevent dysmorphogenesis demonstrating the necessity of both proteins.319
Since the loss of either N-cadherin or stable microtubules also causes alteration in actin distribution as well as an aberrant overactivation of myosin II, it is likely that the regulation of the actomyosin network plays a critical role in N-cadherin and microtubule coordinate activity within the lens68,319 and plays a part in the process of lens fiber cell elongation and migration, given active myosin’s role in cell contractility and movement.79,320–323 Microtubule functioning therefore relies not just on interactions with other cytoskeletal proteins but also further expands to include other cellular networks (Figure 2).
Myosin II activity has been shown to serve as a regulator of both microtubule stability and N-cadherin distribution,64,295,324–326 both of which have proven necessary for lens fiber cell elongation. Myosin activation has been shown to antagonize microtubule acetylation, with the converse also proven true.64 Additionally, myosin activity has been shown to weaken the ability of cells to migrate coordinately when intercellular junctions are compromised; a block in myosin activity can rescue collective migration even when cadherins are lost.325 Beyond this general role in the process of collective migration, active myosin II has been shown to regulate the concentration of cadherin junctions at cell–cell interfaces326,327 and this myosin activity may depend on dynamic microtubules.303 This may explain the stable microtubule dependent, myosin activity mediated N-cadherin distribution in the lens epithelium and fiber cells.
The roles myosin may play in relation to microtubules and N-cadherin are likely also dependent on actin regulation. While myosin can be activated by myosin light chain kinase, its activity is also regulated by Rho-dependent kinase (ROCK). Through this downstream regulation, ROCK has been shown to mediate effects on the stability of microtubules65 and the distribution of N-cadherin326 (Figure 2). In addition to this downstream regulation of myosin activity, RhoGTPases also play a large role in modulating N-cadherin and microtubule functions.
Interestingly, the regulation of the stabilization of microtubules has been linked to the RhoGTPase family.152,153,270,328–333 Multiple reports have shown that RhoA can promote the stabilization of microtubules,270,328 while Rac1 can inhibit the destabilization of microtubules.334–336 The active growth and stabilization of microtubules have been shown to promote activation of Rac1 and lamellipodial protrusions,315,337,338 creating a potential feedback loop that allows for cellular migration which may tie microtubules to Rac1’s role in lens fiber cell elongation and migration.
Rac1 involvement in lens fiber cell elongation and migration has been previously documented,82 with lens conditional deletion of Rac1 preventing elongation of lens fiber cells along both the posterior lens capsule and the epithelial fiber cell interface and is now confirmed in our N-cadherin knockout system. RhoGTPases are also linked to regulation of cadherin cell–cell adhesion.339–345 It is likely therefore that there may exist an integral role for the multifold regulation of these RhoGTPases by cell–cell adhesion molecules, the cytoskeleton as well as signaling cascades including FGF346 that allows for proper lens fiber cell elongation and migration, which may explain the high association of N-cadherin junctions and microtubules particularly in the area of fiber cell elongation.68 This interconnectivity may also provide insight into the effects of microtubule depolymerizing agents on the stability and solubility of N-cadherin junctions within the lens.
The RhoGTPases’ role in migration has also been greatly tied to Contact Inhibition of Locomotion (CIL), a process that relies on both cadherins and microtubules as mediators. In CIL, N-cadherin-mediated cell–cell contact formation regulates RhoGTPase activity, causing a switching in Rac1 versus RhoA localization and activation within the cell that can help determine the directionality of further motion.296–298,347–349 This requires an alteration of cell polarity, mediated by RhoA, Rac1, and ROCK regulation of microtubule dynamics, causing a switch in the localization of stable microtubules to lead the new directionality of motion.350–352 Given the importance of this mechanism for collective movement of cells during development, it is possible that this interplay between N-cadherin and microtubules mediated through RhoGTPases may be a critical part of overall lens morphogenesis.
Conclusions
Microtubules are a critical aspect of the cellular cytoskeleton. Newer studies in the lens have given insight into the continuously evolving roles of microtubules, both dynamic and stable subpopulations, in a variety of cellular processes. The dynamic versus stable microtubular interplay is an evolving field that leads to a deeper look into how microtubule modifications and interaction with other cellular elements also are integral in cellular functioning. More and more research points to the cytoskeletal network of the cell as highly interactive. Microtubules are becoming more and more understood as a key integrator with other cytoskeletal proteins including actin and intermediate filaments, as well as cell–cell junctional proteins and other cellular regulators including myosin and RhoGTPases. For appropriate cellular homeostasis, all of these elements must interact in precise balance, with microtubules playing an integral role in this balance.
Authors’ contributions
CML wrote the manuscript and designed the figures with critical oversight input, and review from ASM.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by grants EY014258 and EY 026478 from NEI to ASM.
References
- 1.Fletcher DA, Mullins RD. Cell mechanics and the cytoskeleton. Nature 2010; 463:485–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heng YW, Koh CG. Actin cytoskeleton dynamics and the cell division cycle. Int J Biochem Cell Biol 2010; 42:1622–33 [DOI] [PubMed] [Google Scholar]
- 3.Rafferty NS, Scholz DL. Actin in polygonal arrays of microfilaments and sequestered actin bundles (SABs) in lens epithelial cells of rabbits and mice. Curr Eye Res 1985; 4:713–8 [DOI] [PubMed] [Google Scholar]
- 4.Rangamani P, Xiong GY, Iyengar R. Multiscale modeling of cell shape from the actin cytoskeleton. Prog Mol Biol Translat Sci 2014; 123:143–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao PV, Maddala R. Ankyrin-B in lens architecture and biomechanics: just not tethering but more. Bioarchitecture 2016; 6:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tokuda S, Higashi T, Furuse M. ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: involvement of ZO-1 in the regulation of cytoskeleton and cell shape. PLoS One 2014; 9:e104994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barak P, Rai A, Dubey AK, Rai P, Mallik R. Reconstitution of microtubule-dependent organelle transport. Meth Enzymol 2014; 540:231–48 [DOI] [PubMed] [Google Scholar]
- 8.Fu MM, Holzbaur EL. Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol 2014; 24:564–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo WK, Wen XJ, Zhou CJ. Microtubule configuration and membranous vesicle transport in elongating fiber cells of the rat lens. Exp Eye Res 2003; 77:615–26 [DOI] [PubMed] [Google Scholar]
- 10.Serpinskaya AS, Tuphile K, Rabinow L, Gelfand VI. Protein kinase darkener of apricot and its substrate EF1gamma regulate organelle transport along microtubules. J Cell Sci 2014; 127:33–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Bergeijk P, Hoogenraad CC, Kapitein LC. Right time, right place: probing the functions of organelle positioning. Trends Cell Biol 2016; 26:121–34 [DOI] [PubMed] [Google Scholar]
- 12.Xiang X, Qiu R, Yao X, Arst HN, Jr., Penalva MA, Zhang J. Cytoplasmic dynein and early endosome transport. Cell Mol Life Sci 2015; 72:3267–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorland YL, Huveneers S. Cell-cell junctional mechanotransduction in endothelial remodeling. Cell Mol Life Sci 2017; 74:279–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pritchard RH, Huang YY, Terentjev EM. Mechanics of biological networks: from the cell cytoskeleton to connective tissue. Soft Matter 2014; 10:1864–84 [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki M, Furuike S, Ito T. Mechanical response of single filamin A (ABP-280) molecules and its role in the actin cytoskeleton. J Muscle Res Cell Motil 2002; 23:525–34 [DOI] [PubMed] [Google Scholar]
- 16.Yu D, Pessino V, Kuei S, Valentine MT. Mechanical and functional properties of epothilone-stabilized microtubules. Cytoskeleton 2013; 70:74–84 [DOI] [PubMed] [Google Scholar]
- 17.Le Bras S, Le Borgne R. Epithelial cell division – multiplying without losing touch. J Cell Sci 2014; 127:5127–37 [DOI] [PubMed] [Google Scholar]
- 18.Ong K, Wloka C, Okada S, Svitkina T, Bi E. Architecture and dynamic remodelling of the septin cytoskeleton during the cell cycle. Nat Commun 2014; 5:5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen CG, Wright AJ, Muller S. The role of the cytoskeleton and associated proteins in determination of the plant cell division plane. Plant J 2013; 75:258–69 [DOI] [PubMed] [Google Scholar]
- 20.Roubinet C, Tran PT, Piel M. Common mechanisms regulating cell cortex properties during cell division and cell migration. Cytoskeleton 2012; 69:957–72 [DOI] [PubMed] [Google Scholar]
- 21.Adams G, Jr., Zhou J, Wang W, Wu H, Quan J, Liu Y, Xia P, Wang Z, Zhou S, Jiang J, Mo F, Zhuang X, Thomas K, Hill DL, Aikhionbare FO, He P, Liu X, Ding X, Yao X. The microtubule plus end tracking protein TIP150 interacts with cortactin to steer directional cell migration. J Biol Chem 2016; 291:20692–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barth AI, Caro-Gonzalez HY, Nelson WJ. Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization. Sem Cell Development Biol 2008; 19:245–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao D, Su Z, Wang W, Wu H, Liu X, Akram S, Qin B, Zhou J, Zhuang X, Adams G, Jin C, Wang X, Liu L, Hill DL, Wang D, Ding X, Yao X. Signaling scaffold protein IQGAP1 interacts with microtubule plus-end tracking protein SKAP and links dynamic microtubule plus-end to steer cell migration. J Biol Chem 2015; 290:23766–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francis R, Xu X, Park H, Wei CJ, Chang S, Chatterjee B, Lo C. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS One 2011; 6:e26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George SP, Chen H, Conrad JC, Khurana S. Regulation of directional cell migration by membrane-induced actin bundling. J Cell Sci 2013; 126:312–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakida NM, Botvinick EL, Lin J, Berns MW. An intact centrosome is required for the maintenance of polarization during directional cell migration. PLoS One 2010; 5:e15462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bryantseva SA, Zhapparova ON. Bidirectional transport of organelles: unity and struggle of opposing motors. Cell Biol Int 2012; 36:1–6 [DOI] [PubMed] [Google Scholar]
- 28.Hume AN, Seabra MC. Melanosomes on the move: a model to understand organelle dynamics. Biochem Soc Trans 2011; 39:1191–6 [DOI] [PubMed] [Google Scholar]
- 29.Bahmanyar S, Nelson WJ, Barth AI. Role of APC and its binding partners in regulating microtubules in mitosis. Adv Exp Med Biol 2009; 656:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarangapani KK, Asbury CL. Catch and release: how do kinetochores hook the right microtubules during mitosis? Trends Genet 2014; 30:150–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka K. Dynamic regulation of kinetochore-microtubule interaction during mitosis. J Biochem 2012; 152:415–24 [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K. Regulatory mechanisms of kinetochore-microtubule interaction in mitosis. Cell Mol Life Sci 2013; 70:559–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fishel EA, Dixit R. Role of nucleation in cortical microtubule array organization: variations on a theme. Plant J 2013; 75:270–7 [DOI] [PubMed] [Google Scholar]
- 34.Luders J, Stearns T. Microtubule-organizing centres: a re-evaluation. Nat Rev Mol Cell Biol 2007; 8:161–7 [DOI] [PubMed] [Google Scholar]
- 35.Rieder CL, Faruki S, Khodjakov A. The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol 2001; 11:413–9 [DOI] [PubMed] [Google Scholar]
- 36.Capitanio M, Vanzi F, Broggio C, Cicchi R, Normanno D, Romano G, Sacconi L, Pavone FS. Exploring molecular motors and switches at the single-molecule level. Microsc Res Tech 2004; 65:194–204 [DOI] [PubMed] [Google Scholar]
- 37.Fu MM, Nirschl JJ, Holzbaur E. LC3 binding to the scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Development Cell 2014; 29:577–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Current Opin Cell Biol 2000; 12:63–71 [DOI] [PubMed] [Google Scholar]
- 39.Hancock WO. Mitotic kinesins: a reason to delve into kinesin-12. Curr Biol 2014; 24:R968–70 [DOI] [PubMed] [Google Scholar]
- 40.Hancock WO. Bidirectional cargo transport: moving beyond tug of war. Nat Rev Mol Cell Biol 2014; 15:615–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bre MH, Kreis TE, Karsenti E. Control of microtubule nucleation and stability in Madin-Darby canine kidney cells: the occurrence of noncentrosomal, stable detyrosinated microtubules. J Cell Biol 1987; 105:1283–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotani H, Horio T. Dynamics of microtubules visualized by darkfield microscopy: treadmilling and dynamic instability. Cell Motil Cytoskeleton 1988; 10:229–36 [DOI] [PubMed] [Google Scholar]
- 43.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA 1993; 90:9552–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature 1984; 312:237–42 [DOI] [PubMed] [Google Scholar]
- 45.Akisaka T, Yoshida H, Takigawa T. Differential distribution of posttranslationally modified microtubules in osteoclasts. J Histochem Cytochem 2011; 59:630–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belmadani S, Pous C, Fischmeister R, Mery PF. Post-translational modifications of tubulin and microtubule stability in adult rat ventricular myocytes and immortalized HL-1 cardiomyocytes. Mol Cell Biochem 2004; 258:35–48 [DOI] [PubMed] [Google Scholar]
- 47.Bouquet C, Soares S, von BY, Ravaille VM, Propst F, Nothias F. Microtubule-associated protein 1B controls directionality of growth cone migration and axonal branching in regeneration of adult dorsal root ganglia neurons. J Neurosci 2004; 24:7204–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulinski JC, Gundersen GG. Stabilization of post-translational modification of microtubules during cellular morphogenesis. Bioessays 1991; 13:285–93 [DOI] [PubMed] [Google Scholar]
- 49.Bulinski JC, Richards JE, Piperno G. Posttranslational modifications of alpha tubulin: detyrosination and acetylation differentiate populations of interphase microtubules in cultured cells. J Cell Biol 1988; 106:1213–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikegami K, Setou M. Unique post-translational modifications in specialized microtubule architecture. Cell Struct Funct 2010; 35:15–22 [DOI] [PubMed] [Google Scholar]
- 51.Perdiz D, Mackeh R, Pous C, Baillet A. The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell Signall 2011; 23:763–71 [DOI] [PubMed] [Google Scholar]
- 52.Quinones GB, Danowski BA, Devaraj A, Singh V, Ligon LA. The posttranslational modification of tubulin undergoes a switch from detyrosination to acetylation as epithelial cells become polarized. Mol Biol Cell 2011; 22:1045–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song Y, Kirkpatrick LL, Schilling AB, Helseth DL, Chabot N, Keillor JW, Johnson GV, Brady ST. Transglutaminase and polyamination of tubulin: posttranslational modification for stabilizing axonal microtubules. Neuron 2013; 78:109–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci USA 1988; 85:5946–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartolini F, Ramalingam N, Gundersen GG. Actin-capping protein promotes microtubule stability by antagonizing the actin activity of mDia1. Mol Biol Cell 2012; 23:4032–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, Kantorow M, Pras E, Frydman M, Pras E, Riazuddin S, Riazuddin SA, Hejtmancik JF. Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am J Hum Genet 2011; 88:827–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, Zweckstetter M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc Natl Acad Sci USA 2015; 112:7501–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tegha-Dunghu J, Bausch E, Neumann B, Wuensche A, Walter T, Ellenberg J, Gruss OJ. MAP1S controls microtubule stability throughout the cell cycle in human cells. J Cell Sci 2014; 127:5007–13 [DOI] [PubMed] [Google Scholar]
- 59.Badding MA, Dean DA. Highly acetylated tubulin permits enhanced interactions with and trafficking of plasmids along microtubules. Gene Ther 2013; 20:616–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prescott AR, Webb SF, Rawlins D, Shaw PJ, Warn RM. Microtubules rich in post-translationally modified alpha-tubulin form distinct arrays in frog lens epithelial cells. Exp Eye Res 1991; 52:743–53 [DOI] [PubMed] [Google Scholar]
- 61.Vo NT, Bols NC. Demonstration of primary cilia and acetylated alpha-tubulin in fish endothelial, epithelial and fibroblast cell lines. Fish Physiol Biochem 2016; 42:29–38 [DOI] [PubMed] [Google Scholar]
- 62.Wang W, Brautigan DL. Phosphatase inhibitor 2 promotes acetylation of tubulin in the primary cilium of human retinal epithelial cells. BMC Cell Biol 2008; 9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie R, Nguyen S, McKeehan WL, Liu L. Acetylated microtubules are required for fusion of autophagosomes with lysosomes. BMC Cell Biol 2010; 11:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Joo EE, Yamada KM. MYPT1 regulates contractility and microtubule acetylation to modulate integrin adhesions and matrix assembly. Nat Commun 2014; 5:3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ. Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS One 2010; 5:e8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, Olshevskaja LV. Effect of colcemid on the locomotory behaviour of fibroblasts. J Embryol Exp Morphol 1970; 24:625–40 [PubMed] [Google Scholar]
- 67.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol 2003; 5:599–609 [DOI] [PubMed] [Google Scholar]
- 68.Logan CM, Bowen CJ, Menko AS. Functional role for stable microtubules in lens fiber cell elongation. Exp Cell Res 2018; 362:477–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Plestant C, Strale PO, Seddiki R, Nguyen E, Ladoux B, Mege RM. Adhesive interactions of N-cadherin limit the recruitment of microtubules to cell-cell contacts through organization of actomyosin. J Cell Sci 2014; 127:1660–71 [DOI] [PubMed] [Google Scholar]
- 70.Revenu C, Streichan S, Dona E, Lecaudey V, Hufnagel L, Gilmour D. Quantitative cell polarity imaging defines leader-to-follower transitions during collective migration and the key role of microtubule-dependent adherens junction formation. Development 2014; 141:1282–91 [DOI] [PubMed] [Google Scholar]
- 71.Peglion F, Etienne-Manneville S. p120catenin alteration in cancer and its role in tumour invasion. Philos Trans R Soc Lond 2013; 368:20130015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peglion F, Llense F, Etienne-Manneville S. Adherens junction treadmilling during collective migration. Nat Cell Biol 2014; 16:639–51 [DOI] [PubMed] [Google Scholar]
- 73.Kuszak JR. The ultrastructure of epithelial and fiber cells in the crystalline lens. Int Rev Cytol 1995; 163:305–50 [DOI] [PubMed] [Google Scholar]
- 74.Kuszak JR, Novak LA, Brown HG. An ultrastructural analysis of the epithelial-fiber interface (EFI) in primate lenses. Exp Eye Res 1995; 61:579–97 [DOI] [PubMed] [Google Scholar]
- 75.Kuszak JR, Peterson KL, Brown HG. Electron microscopic observations of the crystalline lens. Microsc Res Tech 1996; 33:441–79 [DOI] [PubMed] [Google Scholar]
- 76.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Development Biol 2005; 280:1–14 [DOI] [PubMed] [Google Scholar]
- 77.Dawes LJ, Sugiyama Y, Lovicu FJ, Harris CG, Shelley EJ, McAvoy JW. Interactions between lens epithelial and fiber cells reveal an intrinsic self-assembly mechanism. Dev Biol 2014; 385:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugiyama Y, Lovicu FJ, McAvoy JW. Planar cell polarity in the mammalian eye lens. Organogenesis 2011; 7:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao PV, Maddala R. The role of the lens actin cytoskeleton in fiber cell elongation and differentiation. Sem Cell Development Biol 2006; 17:698–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens. Dev Biol 2011; 349:363–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sue Menko A. Lens epithelial cell differentiation. Exp Eye Res 2002; 75:485–90 [DOI] [PubMed] [Google Scholar]
- 82.Maddala R, Chauhan BK, Walker C, Zheng Y, Robinson ML, Lang RA, Rao PV. Rac1 GTPase-deficient mouse lens exhibits defects in shape, suture formation, fiber cell migration and survival. Dev Biol 2011; 360:30–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maddala R, Nagendran T, Lang RA, Morozov A, Rao PV. Rap1 GTPase is required for mouse lens epithelial maintenance and morphogenesis. Dev Biol 2015; 406:74–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res 2010; 90:643–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Augusteyn RC. Growth of the lens: in vitro observations. Clin Exp Optometry 2008; 91:226–39 [DOI] [PubMed] [Google Scholar]
- 86.Augusteyn RC. Growth of the human eye lens. Mol Vis 2007; 13:252–7 [PMC free article] [PubMed] [Google Scholar]
- 87.Lang RA. Apoptosis in mammalian eye development: lens morphogenesis, vascular regression and immune privilege. Cell Death Differ 1997; 4:12–20 [DOI] [PubMed] [Google Scholar]
- 88.Berthoud VM, Beyer EC. Oxidative stress, lens gap junctions, and cataracts. Antioxidants Redox Signal 2009; 11:339–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Graw J. Cataract mutations and lens development. Prog Retin Eye Res 1999; 18:235–67 [DOI] [PubMed] [Google Scholar]
- 90.Michael R, Bron AJ. The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc Lond 2011; 366:1278–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shichi H. Cataract formation and prevention. Exp Opin Investig Drugs 2004; 13:691–701 [DOI] [PubMed] [Google Scholar]
- 92.Yan Q, Liu JP, Li DW. Apoptosis in lens development and pathology. Differentiation 2006; 74:195–211 [DOI] [PubMed] [Google Scholar]
- 93.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation 1981; 19:134–53 [DOI] [PubMed] [Google Scholar]
- 94.Piatigorsky J. Lens cell elongation in vitro and microtubules. Ann N Y Acad Sci 1975; 253:333–47 [DOI] [PubMed] [Google Scholar]
- 95.Piatigorsky J, Rothschild SS, Milstone LM. Differentiation of lens fibers in explanted embryonic chick lens epithelia. Dev Biol 1973; 34:334–45 [DOI] [PubMed] [Google Scholar]
- 96.Piatigorsky J, Webster Hde F, Wollberg M. Cell elongation in the cultured embryonic chick lens epithelium with and without protein synthesis. Involvement of microtubules. J Cell Biol 1972; 55:82–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuwabara T. Microtubules in the lens. Arch Ophthalmol 1968; 79:189–95 [DOI] [PubMed] [Google Scholar]
- 98.Beebe DC, Feagans DE, Blanchette-Mackie EJ, Nau ME. Lens epithelial cell elongation in the absence of microtubules: evidence for a new effect of colchicine. Science 1979; 206:836–8 [DOI] [PubMed] [Google Scholar]
- 99.Mikuni I, Fujiwara T, Obazawa H. Microtubules in experimental cataracts: disappearance of microtubules of epithelial cells and lens fibers in colchicine-induced cataracts. Tokai J Exp Clin Med 1981; 6:297–303 [PubMed] [Google Scholar]
- 100.Basu S, Rajakaruna S, Reyes B, Van Bockstaele E, Menko AS. Suppression of MAPK/JNK-MTORC1 signaling leads to premature loss of organelles and nuclei by autophagy during terminal differentiation of lens fiber cells. Autophagy 2014; 10:1193–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kenific CM, Wittmann T, Debnath J. Autophagy in adhesion and migration. J Cell Sci 2016; 129:3685–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mackeh R, Perdiz D, Lorin S, Codogno P, Pous C. Autophagy and microtubules – new story, old players. J Cell Sci 2013; 126:1071–80 [DOI] [PubMed] [Google Scholar]
- 103.Monastyrska I, Rieter E, Klionsky DJ, Reggiori F. Multiple roles of the cytoskeleton in autophagy. Biol Rev Cambridge Philos Soc 2009; 84:431–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhao Y, Wilmarth PA, Cheng C, Limi S, Fowler VM, Zheng D, David LL, Cvekl A. Proteome-transcriptome analysis and proteome remodeling in mouse lens epithelium and fibers. Exp Eye Res 2019; 179:32–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ireland ME, Mrock LK. Differentiation of chick lens epithelial cells: involvement of the epidermal growth factor receptor and endogenous ligand. Investi Ophthalmol Vis Sci 2000; 41:183–90 [PubMed] [Google Scholar]
- 106.Simirskii VN, Lee RS, Wawrousek EF, Duncan MK. Inbred FVB/N mice are mutant at the cp49/Bfsp2 locus and lack beaded filament proteins in the lens. Invest Ophthalmol Vis Sci 2006; 47:4931–4 [DOI] [PubMed] [Google Scholar]
- 107.Sun J, Rockowitz S, Xie Q, Ashery-Padan R, Zheng D, Cvekl A. Identification of in vivo DNA-binding mechanisms of Pax6 and reconstruction of Pax6-dependent gene regulatory networks during forebrain and lens development. Nucl Acids Res 2015; 43:6827–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song C, Nguyen NT, Tan SH, Asundi AK. Modelling and optimization of micro optofluidic lenses. Lab Chip 2009; 9:1178–84 [DOI] [PubMed] [Google Scholar]
- 109.Barra HS, Arce CA, Argarana CE. Posttranslational tyrosination/detyrosination of tubulin. Mol Neurobiol 1988; 2:133–53 [DOI] [PubMed] [Google Scholar]
- 110.Barra HS, Arce CA, Rodriguez JA, Caputto R. Some common properties of the protein that incorporates tyrosine as a single unit and the microtubule proteins. Biochem Biophys Res Commun 1974; 60:1384–90 [DOI] [PubMed] [Google Scholar]
- 111.Edde B, Rossier J, Le Caer JP, Desbruyeres E, Gros F, Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science 1990; 247:83–5 [DOI] [PubMed] [Google Scholar]
- 112.Alexander SP, Rieder CL. Chromosome motion during attachment to the vertebrate spindle: initial saltatory-like behavior of chromosomes and quantitative analysis of force production by nascent kinetochore fibers. J Cell Biol 1991; 113:805–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bre MH. Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science 1994; 266:1688–91 [DOI] [PubMed] [Google Scholar]
- 114.L'Hernault SW, Rosenbaum JL. Reversal of the posttranslational modification on Chlamydomonas flagellar alpha-tubulin occurs during flagellar resorption. J Cell Biol 1985; 100:457–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.L'Hernault SW, Rosenbaum JL. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 1985; 24:473–8 [DOI] [PubMed] [Google Scholar]
- 116.Eipper BA. Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci USA 1972; 69:2283–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matten WT, Aubry M, West J, Maness PF. Tubulin is phosphorylated at tyrosine by pp60c-src in nerve growth cone membranes. J Cell Biol 1990; 111:1959–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Webster DR, Borisy GG. Microtubules are acetylated in domains that turn over slowly. J Cell Sci 1989; 92:57–65 [DOI] [PubMed] [Google Scholar]
- 119.Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci USA 1987; 84:9040–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Assembly and turnover of detyrosinated tubulin in vivo. J Cell Biol 1987; 105:265–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol 1988; 106:141–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kreitzer G, Marmorstein A, Okamoto P, Vallee R, Rodriguez-Boulan E. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nat Cell Biol 2000; 2:125–7 [DOI] [PubMed] [Google Scholar]
- 123.Kreitzer G, Myat MM. Microtubule motors in establishment of epithelial cell polarity. Cold Spring Harb Perspect Biol 2018; 10:a027896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kirkpatrick LL, Brady ST. Modulation of the axonal microtubule cytoskeleton by myelinating Schwann cells. J Neurosci 1994; 14:7440–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kirkpatrick LL, Witt AS, Payne HR, Shine HD, Brady ST. Changes in microtubule stability and density in myelin-deficient shiverer mouse CNS axons. J Neurosci 2001; 21:2288–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brady ST. A kinesin medley: biochemical and functional heterogeneity. Trends Cell Biol 1995; 5:159–64 [DOI] [PubMed] [Google Scholar]
- 127.Hsu CY, Lecland N, Pendaries V, Viode C, Redoules D, Paul C, Merdes A, Simon M, Bierkamp C. Stabilization of microtubules restores barrier function after cytokine-induced defects in reconstructed human epidermis. J Dermatol Sci 2018; 91:87–96 [DOI] [PubMed] [Google Scholar]
- 128.Mu S, Liu Y, Jiang J, Ding R, Li X, Li X, Ma X. Unfractionated heparin ameliorates pulmonary microvascular endothelial barrier dysfunction via microtubule stabilization in acute lung injury. Respir Res 2018; 19:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Foe VE, von Dassow G. Stable and dynamic microtubules coordinately shape the myosin activation zone during cytokinetic furrow formation. J Cell Biol 2008; 183:457–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaverina I, Straube A. Regulation of cell migration by dynamic microtubules. Semin Cell Dev Biol 2011; 22:968–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brady ST, Tytell M, Lasek RJ. Axonal tubulin and axonal microtubules: biochemical evidence for cold stability. J Cell Biol 1984; 99:1716–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sahenk Z, Brady ST. Axonal tubulin and microtubules: morphologic evidence for stable regions on axonal microtubules. Cell Motil Cytoskeleton 1987; 8:155–64 [DOI] [PubMed] [Google Scholar]
- 133.Black MM, Keyser P. Acetylation of alpha-tubulin in cultured neurons and the induction of alpha-tubulin acetylation in PC12 cells by treatment with nerve growth factor. J Neurosci 1987; 7:1833–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sale WS, Besharse JC, Piperno G. Distribution of acetylated alpha-tubulin in retina and in vitro-assembled microtubules. Cell Motil Cytoskeleton 1988; 9:243–53 [DOI] [PubMed] [Google Scholar]
- 135.Chapin SJ, Bulinski JC. Microtubule stabilization by assembly-promoting microtubule-associated proteins: a repeat performance. Cell Motil Cytoskeleton 1992; 23:236–43 [DOI] [PubMed] [Google Scholar]
- 136.Wade RH. On and around microtubules: an overview. Mol Biotechnol 2009; 43:177–91 [DOI] [PubMed] [Google Scholar]
- 137.Kreis TE. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J 1987; 6:2597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chapin SJ, Bulinski JC. Cellular microtubules heterogeneous in their content of microtubule-associated protein 4 (MAP4). Cell Motil Cytoskeleton 1994; 27:133–49 [DOI] [PubMed] [Google Scholar]
- 139.Bulinski JC, Gundersen GG, Webster DR. A function for tubulin tyrosination? Nature 1987; 328:676. [DOI] [PubMed] [Google Scholar]
- 140.Peris L, Thery M, Faure J, Saoudi Y, Lafanechere L, Chilton JK, Gordon-Weeks P, Galjart N, Bornens M, Wordeman L, Wehland J, Andrieux A, Job D. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J Cell Biol 2006; 174:839–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Peris L, Wagenbach M, Lafanechere L, Brocard J, Moore AT, Kozielski F, Job D, Wordeman L, Andrieux A. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol 2009; 185:1159–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wehland J, K. Turnover Of W. the carboxy-terminal tyrosine of alpha-tubulin and means of reaching elevated levels of detyrosination in living cells. J Cell Sci 1987; 88:185–203 [DOI] [PubMed] [Google Scholar]
- 143.Wehland J, Weber K. Tubulin-tyrosine ligase has a binding site on beta-tubulin: a two-domain structure of the enzyme. J Cell Biol 1987; 104:1059–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baas PW. Microtubule stability in the axon: new answers to an old mystery. Neuron 2013; 78:3–5 [DOI] [PubMed] [Google Scholar]
- 145.Baas PW, Black MM. Individual microtubules in the axon consist of domains that differ in both composition and stability. J Cell Biol 1990; 111:495–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baas PW, Slaughter T, Brown A, Black MM. Microtubule dynamics in axons and dendrites. J Neurosci Res 1991; 30:134–53 [DOI] [PubMed] [Google Scholar]
- 147.Gundersen GG, Khawaja S, Bulinski JC. Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J Cell Biol 1989; 109:2275–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci 2009; 12:559–67 [DOI] [PubMed] [Google Scholar]
- 149.Dunn S, Morrison EE, Liverpool TB, Molina-Paris C, Cross RA, Alonso MC, Peckham M. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J Cell Sci 2008; 121:1085–95 [DOI] [PubMed] [Google Scholar]
- 150.Gurland G, Gundersen GG. Stable, detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J Cell Biol 1995; 131:1275–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Palazzo A, Ackerman B, Gundersen GG. Cell biology: tubulin acetylation and cell motility. Nature 2003; 421:230. [DOI] [PubMed] [Google Scholar]
- 152.Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 2004; 303:836–9 [DOI] [PubMed] [Google Scholar]
- 153.Palazzo AF, Joseph HL, Chen YJ, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr Biol 2001; 11:1536–41 [DOI] [PubMed] [Google Scholar]
- 154.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J Neurosci 2007; 27:3571–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Berbari NF, Sharma N, Malarkey EB, Pieczynski JN, Boddu R, Gaertig J, Guay-Woodford L, Yoder BK. Microtubule modifications and stability are altered by cilia perturbation and in cystic kidney disease. Cytoskeleton 2013; 70:24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Govindarajan N, Rao P, Burkhardt S, Sananbenesi F, Schluter OM, Bradke F, Lu J, Fischer A. Reducing HDAC6 ameliorates cognitive deficits in a mouse model for Alzheimer's disease. EMBO Mol Med 2013; 5:52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pellegrini L, Wetzel A, Granno S, Heaton G, Harvey K. Back to the tubule: microtubule dynamics in Parkinson's disease. Cell Mol Life Sci 2017; 74:409–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hammond JW, Blasius TL, Soppina V, Cai D, Verhey KJ. Autoinhibition of the kinesin-2 motor KIF17 via dual intramolecular mechanisms. J Cell Biol 2010; 189:1013–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell 2010; 21:572–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aguilar A, Becker L, Tedeschi T, Heller S, Iomini C, Nachury MV. Alpha-tubulin K40 acetylation is required for contact inhibition of proliferation and cell-substrate adhesion. Mol Biol Cell 2014; 25:1854–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol 2010; 190:363–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Skoufias DA, Burgess TL, Wilson L. Spatial and temporal colocalization of the Golgi apparatus and microtubules rich in detyrosinated tubulin. J Cell Biol 1990; 111:1929–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burgess TL, Skoufias DA, Wilson L. Disruption of the Golgi apparatus with brefeldin A does not destabilize the associated detyrosinated microtubule network. Cell Motil Cytoskeleton 1991; 20:289–300 [DOI] [PubMed] [Google Scholar]
- 164.Chesarone MA, DuPage AG, Goode BL. Unleashing formins to remodel the actin and microtubule cytoskeletons. Nat Rev Mol Cell Biol 2010; 11:62–74 [DOI] [PubMed] [Google Scholar]
- 165.Thurston SF, Kulacz WA, Shaikh S, Lee JM, Copeland JW. The ability to induce microtubule acetylation is a general feature of formin proteins. PLoS One 2012; 7:e48041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bartolini F, Andres-Delgado L, Qu X, Nik S, Ramalingam N, Kremer L, Alonso MA, Gundersen GG. An mDia1-INF2 formin activation cascade facilitated by IQGAP1 regulates stable microtubules in migrating cells. Mol Biol Cell 2016; 27:1797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fernandez-Barrera J, Alonso MA. Coordination of microtubule acetylation and the actin cytoskeleton by formins. Cell Mol Life Sci 2018; 75:3181–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fernandez-Barrera J, Bernabe-Rubio M, Casares-Arias J, Rangel L, Fernandez-Martin L, Correas I, Alonso MA. The actin-MRTF-SRF transcriptional circuit controls tubulin acetylation via alpha-TAT1 gene expression. J Cell Biol 2018; 217:929–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Song B, Ao Q, Wang Z, Liu W, Niu Y, Shen Q, Zuo H, Zhang X, Gong Y. Phosphorylation of tau protein over time in rats subjected to transient brain ischemia. Neural Regeneration Res 2013; 8:3173–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bloom GS, Richards BW, Leopold PL, Ritchey DM, Brady ST. GTP gamma S inhibits organelle transport along axonal microtubules. J Cell Biol 1993; 120:467–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brady ST. Motor neurons and neurofilaments in sickness and in health. Cell 1993; 73:1–3 [DOI] [PubMed] [Google Scholar]
- 172.Brady ST, Richards BW, Leopold PL. Assay of vesicle motility in squid axoplasm. Methods Cell Biol 1993; 39:191–202 [DOI] [PubMed] [Google Scholar]
- 173.Bailey CD, Johnson GV. Developmental regulation of tissue transglutaminase in the mouse forebrain. J Neurochem 2004; 91:1369–79 [DOI] [PubMed] [Google Scholar]
- 174.Vallee RB, Borisy GG. The non-tubulin component of microtubule protein oligomers. Effect on self-association and hydrodynamic properties. J Biol Chem 1978; 253:2834–45 [PubMed] [Google Scholar]
- 175.Sharma VM, Litersky JM, Bhaskar K, Lee G. Tau impacts on growth-factor-stimulated actin remodeling. J Cell Sci 2007; 120:748–57 [DOI] [PubMed] [Google Scholar]
- 176.Vandecandelaere A, Pedrotti B, Utton MA, Calvert RA, Bayley PM. Differences in the regulation of microtubule dynamics by microtubule-associated proteins MAP1B and MAP2. Cell Motil Cytoskeleton 1996; 35:134–46 [DOI] [PubMed] [Google Scholar]
- 177.Bondallaz P, Barbier A, Soehrman S, Grenningloh G, Riederer BM. The control of microtubule stability in vitro and in transfected cells by MAP1B and SCG10. Cell Motil Cytoskeleton 2006; 63:681–95 [DOI] [PubMed] [Google Scholar]
- 178.Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Development Cell 2003; 4:521–33 [DOI] [PubMed] [Google Scholar]
- 179.Kawauchi T, Chihama K, Nishimura YV, Nabeshima Y, Hoshino M. MAP1B phosphorylation is differentially regulated by Cdk5/p35, Cdk5/p25, and JNK. Biochem Biophys Res Commun 2005; 331:50–5 [DOI] [PubMed] [Google Scholar]
- 180.Ramkumar A, Jong BY, Ori-McKenney KM. ReMAPping the microtubule landscape: how phosphorylation dictates the activities of microtubule-associated proteins. Dev Dyn 2018; 247:138–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nguyen HL, Chari S, Gruber D, Lue CM, Chapin SJ, Bulinski JC. Overexpression of full- or partial-length MAP4 stabilizes microtubules and alters cell growth. J Cell Sci 1997; 110:281–94 [DOI] [PubMed] [Google Scholar]
- 182.Xiao H, Wang H, Zhang X, Tu Z, Bulinski C, Khrapunovich-Baine M, Hogue Angeletti R, Horwitz SB. Structural evidence for cooperative microtubule stabilization by Taxol and the endogenous dynamics regulator MAP4. ACS Chem Biol 2012; 7:744–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mori A, Aizawa H, Saido TC, Kawasaki H, Mizuno K, Murofushi H, Suzuki K, Sakai H. Site-specific phosphorylation by protein kinase C inhibits assembly-promoting activity of microtubule-associated protein 4. Biochemistry 1991; 30:9341–6 [DOI] [PubMed] [Google Scholar]
- 184.Illenberger S, Drewes G, Trinczek B, Biernat J, Meyer HE, Olmsted JB, Mandelkow EM, Mandelkow E. Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110mark. Phosphorylation sites and regulation of microtubule dynamics. J Biol Chem 1996; 271:10834–43 [DOI] [PubMed] [Google Scholar]
- 185.Ebneth A, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of MAP2c and MAP4 by MARK kinases leads to the destabilization of microtubules in cells. Cell Motil Cytoskeleton 1999; 44:209–24 [DOI] [PubMed] [Google Scholar]
- 186.Drubin D, Kirschner M. Purification of tau protein from brain. Meth Enzymol 1986; 134:156–60 [DOI] [PubMed] [Google Scholar]
- 187.Drubin D, Kobayashi S, Kirschner M. Association of tau protein with microtubules in living cells. Ann N Y Acad Sci 1986; 466:257–68 [DOI] [PubMed] [Google Scholar]
- 188.Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol 1986; 103:2739–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature 1998; 391:199–203 [DOI] [PubMed] [Google Scholar]
- 190.Dye RB, Fink SP, Williams RC., Jr. Taxol-induced flexibility of microtubules and its reversal by MAP-2 and Tau. J Biol Chem 1993; 268:6847–50 [PubMed] [Google Scholar]
- 191.Kadavath H, Jaremko M, Jaremko L, Biernat J, Mandelkow E, Zweckstetter M. Folding of the Tau Protein on Microtubules. Angew Chem Int 2015; 54:10347–51 [DOI] [PubMed] [Google Scholar]
- 192.Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld F, Galjart N. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 2001; 104:923–35 [DOI] [PubMed] [Google Scholar]
- 193.Schuyler SC, Pellman D. Microtubule “plus-end-tracking proteins”: the end is just the beginning. Cell 2001; 105:421–4 [DOI] [PubMed] [Google Scholar]
- 194.Schuyler SC, Pellman D. Search, capture and signal: games microtubules and centrosomes play. J Cell Sci 2001; 114:247–55 [DOI] [PubMed] [Google Scholar]
- 195.Zhou FQ, Cohan CS. How actin filaments and microtubules steer growth cones to their targets. J Neurobiol 2004; 58:84–91 [DOI] [PubMed] [Google Scholar]
- 196.Zhou J, Liu M, Aneja R, Chandra R, Joshi HC. Enhancement of paclitaxel-induced microtubule stabilization, mitotic arrest, and apoptosis by the microtubule-targeting agent EM012. Biochem Pharmacol 2004; 68:2435–41 [DOI] [PubMed] [Google Scholar]
- 197.Tirnauer JS. Coupled zones of f-actin and microtubule movement in polarized cells. Development Cell 2002; 3:152–3 [DOI] [PubMed] [Google Scholar]
- 198.Tirnauer JS, Canman JC, Salmon ED, Mitchison TJ. EB1 targets to kinetochores with attached, polymerizing microtubules. Mol Biol Cell 2002; 13:4308–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tirnauer JS, Grego S, Salmon ED, Mitchison TJ. EB1-microtubule interactions in Xenopus egg extracts: role of EB1 in microtubule stabilization and mechanisms of targeting to microtubules. Mol Biol Cell 2002; 13:3614–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Maurer SP, Cade NI, Bohner G, Gustafsson N, Boutant E, Surrey T. EB1 accelerates two conformational transitions important for microtubule maturation and dynamics. Curr Biol 2014; 24:372–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nehlig A, Molina A, Rodrigues-Ferreira S, Honore S, Nahmias C. Regulation of end-binding protein EB1 in the control of microtubule dynamics. Cell Mol Life Sci 2017; 74:2381–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Watanabe S, Okawa K, Miki T, Sakamoto S, Morinaga T, Segawa K, Arakawa T, Kinoshita M, Ishizaki T, Narumiya S. Rho and anillin-dependent control of mDia2 localization and function in cytokinesis. Mol Biol Cell 2010; 21:3193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol 2004; 6:820–30 [DOI] [PubMed] [Google Scholar]
- 204.Gundersen GG, Gomes ER, Wen Y. Cortical control of microtubule stability and polarization. Curr Opin Cell Biol 2004; 16:106–12 [DOI] [PubMed] [Google Scholar]
- 205.Morris EJ, Nader GP, Ramalingam N, Bartolini F, Gundersen GG. Kif4 interacts with EB1 and stabilizes microtubules downstream of Rho-mDia in migrating fibroblasts. PLoS One 2014; 9:e91568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Saxton WM, Stemple DL, Leslie RJ, Salmon ED, Zavortink M, McIntosh JR. Tubulin dynamics in cultured mammalian cells. J Cell Biol 1984; 99:2175–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Griffith LM, Pollard TD. Evidence for actin filament-microtubule interaction mediated by microtubule-associated proteins. J Cell Biol 1978; 78:958–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yarm F, Sagot I, Pellman D. The social life of actin and microtubules: interaction versus cooperation. Curr Opin Microbiol 2001; 4:696–702 [DOI] [PubMed] [Google Scholar]
- 209.Dogterom M, Koenderink GH. Actin-microtubule crosstalk in cell biology. Nat Rev Mol Cell Biol 2019; 20:38–54 [DOI] [PubMed] [Google Scholar]
- 210.Waterman-Storer CM, Salmon ED. Microtubule dynamics: treadmilling comes around again. Curr Biol 1997; 7:R369–72 [DOI] [PubMed] [Google Scholar]
- 211.Letourneau PC. Differences in the organization of actin in the growth cones compared with the neurites of cultured neurons from chick embryos. J Cell Biol 1983; 97:963–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhou FQ, Cohan CS. Growth cone collapse through coincident loss of actin bundles and leading edge actin without actin depolymerization. J Cell Biol 2001; 153:1071–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou FQ, Waterman-Storer CM, Cohan CS. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J Cell Biol 2002; 157:839–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lin CH, Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron 1995; 14:763–71 [DOI] [PubMed] [Google Scholar]
- 215.Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harbor Perspect Biol 2011; 3:1–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol 2006; 8:215–26 [DOI] [PubMed] [Google Scholar]
- 217.Bradke F, Dotti CG. The role of local actin instability in axon formation. Science 1999; 283:1931–4 [DOI] [PubMed] [Google Scholar]
- 218.Marsh L, Letourneau PC. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol 1984; 99:2041–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tanaka E, Ho T, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol 1995; 128:139–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tanaka E, Sabry J. Making the connection: cytoskeletal rearrangements during growth cone guidance. Cell 1995; 83:171–6 [DOI] [PubMed] [Google Scholar]
- 221.Gordon-Weeks PR. Microtubules and growth cone function. J Neurobiol 2004; 58:70–83 [DOI] [PubMed] [Google Scholar]
- 222.Burnette DT, Schaefer AW, Ji L, Danuser G, Forscher P. Filopodial actin bundles are not necessary for microtubule advance into the peripheral domain of Aplysia neuronal growth cones. Nat Cell Biol 2007; 9:1360–9 [DOI] [PubMed] [Google Scholar]
- 223.Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J Cell Sci 2009; 122:3595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J Cell Biol 2002; 158:139–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schaefer AW, Schoonderwoert VT, Ji L, Mederios N, Danuser G, Forscher P. Coordination of actin filament and microtubule dynamics during neurite outgrowth. Development Cell 2008; 15:146–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lee AC, Decourt B, Suter D. Neuronal cell cultures from aplysia for high-resolution imaging of growth cones. J Vis Exp 2008; 12:pii:662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sider JR, Mandato CA, Weber KL, Zandy AJ, Beach D, Finst RJ, Skoble J, Bement WM. Direct observation of microtubule-f-actin interaction in cell free lysates. J Cell Sci 1999; 112:1947–56 [DOI] [PubMed] [Google Scholar]
- 228.Lee E, Pang K, Knecht D. The regulation of actin polymerization and cross-linking in Dictyostelium. Biochim Biophys Acta 2001; 1525:217–27 [DOI] [PubMed] [Google Scholar]
- 229.Mohan R, John A. Microtubule-associated proteins as direct crosslinkers of actin filaments and microtubules. IUBMB Life 2015; 67:395–403 [DOI] [PubMed] [Google Scholar]
- 230.Halpain S, Dehmelt L. The MAP1 family of microtubule-associated proteins. Genome Biol 2006; 7:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tortosa E, Montenegro-Venegas C, Benoist M, Hartel S, Gonzalez-Billault C, Esteban JA, Avila J. Microtubule-associated protein 1B (MAP1B) is required for dendritic spine development and synaptic maturation. J Biol Chem 2011; 286:40638–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kwei SL, Clement A, Faissner A, Brandt R. Differential interactions of MAP2, tau and MAP5 during axogenesis in culture. Neuroreport 1998; 9:1035–40 [DOI] [PubMed] [Google Scholar]
- 233.Morales M, Fifkova E. Distribution of MAP2 in dendritic spines and its colocalization with actin. An immunogold electron-microscope study. Cell Tissue Res 1989; 256:447–56 [DOI] [PubMed] [Google Scholar]
- 234.Shirao T, Gonzalez-Billault C. Actin filaments and microtubules in dendritic spines. J Neurochem 2013; 126:155–64 [DOI] [PubMed] [Google Scholar]
- 235.Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J Neurosci 1996; 16:5583–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Maccioni RB, Cambiazo V. Role of microtubule-associated proteins in the control of microtubule assembly. Physiol Rev 1995; 75:835–64 [DOI] [PubMed] [Google Scholar]
- 237.Slep KC, Rogers SL, Elliott SL, Ohkura H, Kolodziej PA, Vale RD. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J Cell Biol 2005; 168:587–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Johne C, Matenia D, Li XY, Timm T, Balusamy K, Mandelkow EM. Spred1 and TESK1–two new interaction partners of the kinase MARKK/TAO1 that link the microtubule and actin cytoskeleton. Mol Biol Cell 2008; 19:1391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kotani S, Nishida E, Kumagai H, Sakai H. Calmodulin inhibits interaction of actin with MAP2 and Tau, two major microtubule-associated proteins. J Biol Chem 1985; 260:10779–83 [PubMed] [Google Scholar]
- 240.Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Los MJ. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 2014; 112:24–49 [DOI] [PubMed] [Google Scholar]
- 241.Sattilaro RF. Interaction of microtubule-associated protein 2 with actin filaments. Biochemistry 1986; 25:2003–9 [DOI] [PubMed] [Google Scholar]
- 242.Selden SC, Pollard TD. Interaction of actin filaments with microtubules is mediated by microtubule-associated proteins and regulated by phosphorylation. Ann N Y Acad Sci 1986; 466:803–12 [DOI] [PubMed] [Google Scholar]
- 243.Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, Feany MB. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol 2007; 9:139–48 [DOI] [PubMed] [Google Scholar]
- 244.Alves-Silva J, Sanchez-Soriano N, Beaven R, Klein M, Parkin J, Millard TH, Bellen HJ, Venken KJ, Ballestrem C, Kammerer RA, Prokop A. Spectraplakins promote microtubule-mediated axonal growth by functioning as structural microtubule-associated proteins and EB1-dependent +TIPs (tip interacting proteins). J Neurosci 2012; 32:9143–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 2008; 9:309–22 [DOI] [PubMed] [Google Scholar]
- 246.Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR. Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol 2008; 10:1181–9 [DOI] [PubMed] [Google Scholar]
- 247.Tsvetkov AS, Samsonov A, Akhmanova A, Galjart N, Popov SV. Microtubule-binding proteins CLASP1 and CLASP2 interact with actin filaments. Cell Motil Cytoskeleton 2007; 64:519–30 [DOI] [PubMed] [Google Scholar]
- 248.Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Development Cell 2004; 7:871–83 [DOI] [PubMed] [Google Scholar]
- 249.Engel U, Zhan Y, Long JB, Boyle SN, Ballif BA, Dorey K, Gygi SP, Koleske AJ, Vanvactor D. Abelson phosphorylation of CLASP2 modulates its association with microtubules and actin. Cytoskeleton 2014; 71:195–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Marx A, Godinez WJ, Tsimashchuk V, Bankhead P, Rohr K, Engel U. Xenopus cytoplasmic linker-associated protein 1 (XCLASP1) promotes axon elongation and advance of pioneer microtubules. Mol Biol Cell 2013; 24:1544–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hur EM, Saijilafu, Zhou FQ. Growing the growth cone: remodeling the cytoskeleton to promote axon regeneration. Trends Neurosci 2012; 35:164–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Moseley GW, Roth DM, DeJesus MA, Leyton DL, Filmer RP, Pouton CW, Jans DA. Dynein light chain association sequences can facilitate nuclear protein import. Mol Biol Cell 2007; 18:3204–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Marshall TW, Lloyd IE, Delalande JM, Nathke I, Rosenblatt J. The tumor suppressor adenomatous polyposis coli controls the direction in which a cell extrudes from an epithelium. Mol Biol Cell 2011; 22:3962–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Komarova Y, De Groot CO, Grigoriev I, Gouveia SM, Munteanu EL, Schober JM, Honnappa S, Buey RM, Hoogenraad CC, Dogterom M, Borisy GG, Steinmetz MO, Akhmanova A. Mammalian end binding proteins control persistent microtubule growth. J Cell Biol 2009; 184:691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Vitre B, Coquelle FM, Heichette C, Garnier C, Chretien D, Arnal I. EB1 regulates microtubule dynamics and tubulin sheet closure in vitro. Nat Cell Biol 2008; 10:415–21 [DOI] [PubMed] [Google Scholar]
- 256.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, Jelesarov I, Winkler FK, Wuthrich K, Akhmanova A, Steinmetz MO. An EB1-binding motif acts as a microtubule tip localization signal. Cell 2009; 138:366–76 [DOI] [PubMed] [Google Scholar]
- 257.Pedrotti B, Francolini M, Cotelli F, Islam K. Modulation of microtubule shape in vitro by high molecular weight microtubule associated proteins MAP1A, MAP1B, and MAP2. FEBS Lett 1996; 384:147–50 [DOI] [PubMed] [Google Scholar]
- 258.Sharma S, Ganesh T, Kingston DG, Bane S. Promotion of tubulin assembly by poorly soluble taxol analogs. Anal Biochem 2007; 360:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Leung CL, Sun D, Zheng M, Knowles DR, Liem RK. Microtubule actin cross-linking factor (MACF): a hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J Cell Biol 1999; 147:1275–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fuchs E, Karakesisoglou I. Bridging cytoskeletal intersections. Genes Develop 2001; 15:1–14 [DOI] [PubMed] [Google Scholar]
- 261.Kodama A, Karakesisoglou I, Wong E, Vaezi A, Fuchs E. ACF7: an essential integrator of microtubule dynamics. Cell 2003; 115:343–54 [DOI] [PubMed] [Google Scholar]
- 262.Applewhite DA, Grode KD, Keller D, Zadeh AD, Slep KC, Rogers SL. The spectraplakin Short stop is an actin-microtubule cross-linker that contributes to organization of the microtubule network. Mol Biol Cell 2010; 21:1714–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Stroud MJ, Kammerer RA, Ballestrem C. Characterization of G2L3 (GAS2-like 3), a new microtubule- and actin-binding protein related to spectraplakins. J Biol Chem 2011; 286:24987–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Suozzi KC, Wu X, Fuchs E. Spectraplakins: master orchestrators of cytoskeletal dynamics. J Cell Biol 2012; 197:465–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Applewhite DA, Grode KD, Duncan MC, Rogers SL. The actin-microtubule cross-linking activity of Drosophila Short stop is regulated by intramolecular inhibition. Mol Biol Cell 2013; 24:2885–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Grabham PW, Seale GE, Bennecib M, Goldberg DJ, Vallee RB. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. J Neurosci 2007; 27:5823–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gupton SL, Eisenmann K, Alberts AS, Waterman-Storer CM. mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J Cell Sci 2007; 120:3475–87 [DOI] [PubMed] [Google Scholar]
- 268.Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep 2007; 8:1019–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Okada K, Bartolini F, Deaconescu AM, Moseley JB, Dogic Z, Grigorieff N, Gundersen GG, Goode BL. Adenomatous polyposis coli protein nucleates actin assembly and synergizes with the formin mDia1. J Cell Biol 2010; 189:1087–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Palazzo AF, Cook TA, Alberts AS, Gundersen GG. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol 2001; 3:723–9 [DOI] [PubMed] [Google Scholar]
- 271.Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol 2008; 181:523–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ball EH, Singer SJ. Association of microtubules and intermediate filaments in normal fibroblasts and its disruption upon transformation by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci USA 1981; 78:6986–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Goldman RD. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol 1971; 51:752–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Goldman RD, Clement S, Khuon S, Moir R, Trejo-Skalli A, Spann T, Yoon M. Intermediate filament cytoskeletal system: dynamic and mechanical properties. Biolog Bull 1998; 194:361–2; discussion: 2–3 [DOI] [PubMed] [Google Scholar]
- 275.Gyoeva FK, Gelfand VI. Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature 1991; 353:445–8 [DOI] [PubMed] [Google Scholar]
- 276.Helfand BT, Chang L, Goldman RD. Intermediate filaments are dynamic and motile elements of cellular architecture. J Cell Sci 2004; 117:133–41 [DOI] [PubMed] [Google Scholar]
- 277.Helfand BT, Mikami A, Vallee RB, Goldman RD. A requirement for cytoplasmic dynein and dynactin in intermediate filament network assembly and organization. J Cell Biol 2002; 157:795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Yoon M, Moir RD, Prahlad V, Goldman RD. Motile properties of vimentin intermediate filament networks in living cells. J Cell Biol 1998; 143:147–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Theiss C, Napirei M, Meller K. Impairment of anterograde and retrograde neurofilament transport after anti-kinesin and anti-dynein antibody microinjection in chicken dorsal root ganglia. Eur J Cell Biol 2005; 84:29–43 [DOI] [PubMed] [Google Scholar]
- 280.Uchida A, Alami NH, Brown A. Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol Biol Cell 2009; 20:4997–5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hookway C, Ding L, Davidson MW, Rappoport JZ, Danuser G, Gelfand VI. Microtubule-dependent transport and dynamics of vimentin intermediate filaments. Mol Biol Cell 2015; 26:1675–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Murray ME, Mendez MG, Janmey PA. Substrate stiffness regulates solubility of cellular vimentin. Mol Biol Cell 2014; 25:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gupton SL, Salmon WC, Waterman-Storer CM. Converging populations of f-actin promote breakage of associated microtubules to spatially regulate microtubule turnover in migrating cells. Curr Biol 2002; 12:1891–9 [DOI] [PubMed] [Google Scholar]
- 284.Styers ML, Kowalczyk AP, Faundez V. Intermediate filaments and vesicular membrane traffic: the odd couple's first dance? Traffic 2005; 6:359–65 [DOI] [PubMed] [Google Scholar]
- 285.Mose-Larsen P, Bravo R, Fey SJ, Small JV, Celis JE. Putative association of mitochondria with a subpopulation of intermediate-sized filaments in cultured human skin fibroblasts. Cell 1982; 31:681–92 [DOI] [PubMed] [Google Scholar]
- 286.Collier NC, Sheetz MP, Schlesinger MJ. Concomitant changes in mitochondria and intermediate filaments during heat shock and recovery of chicken embryo fibroblasts. J Cell Biochem 1993; 52:297–307 [DOI] [PubMed] [Google Scholar]
- 287.Chang L, Barlan K, Chou YH, Grin B, Lakonishok M, Serpinskaya AS, Shumaker DK, Herrmann H, Gelfand VI, Goldman RD. The dynamic properties of intermediate filaments during organelle transport. J Cell Sci 2009; 122:2914–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Menko AS, Bleaken BM, Libowitz AA, Zhang L, Stepp MA, Walker JL. A central role for vimentin in regulating repair function during healing of the lens epithelium. Mol Biol Cell 2014; 25:776–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci 2000; 113:2455–62 [DOI] [PubMed] [Google Scholar]
- 290.Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvee A, Koteliansky V, Babinet C, Krieg T. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci 1998; 111:1897–907 [DOI] [PubMed] [Google Scholar]
- 291.Etienne-Manneville S. In vitro assay of primary astrocyte migration as a tool to study Rho GTPase function in cell polarization. Meth Enzymol 2006; 406:565–78 [DOI] [PubMed] [Google Scholar]
- 292.Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature 2003; 421:753–6 [DOI] [PubMed] [Google Scholar]
- 293.Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol 2005; 170:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sakamoto Y, Boeda B, Etienne-Manneville S. APC binds intermediate filaments and is required for their reorganization during cell migration. J Cell Biol 2013; 200:249–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Menko AS, Bleaken BM, Walker JL. Regional-specific alterations in cell-cell junctions, cytoskeletal networks and myosin-mediated mechanical cues coordinate collectivity of movement of epithelial cells in response to injury. Exp Cell Res 2014; 322:133–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Priya R, Yap AS. Making a choice: how cadherin switching controls cell migration. Development Cell 2015; 34:383–4 [DOI] [PubMed] [Google Scholar]
- 297.Theveneau E, Marchant L, Kuriyama S, Gull M, Moepps B, Parsons M, Mayor R. Collective chemotaxis requires contact-dependent cell polarity. Development Cell 2010; 19:39–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Theveneau E, Mayor R. Cadherins in collective cell migration of mesenchymal cells. Curr Opin Cell Biol 2012; 24:677–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Theveneau E, Mayor R. Can mesenchymal cells undergo collective cell migration? The case of the neural crest. Cell Adhes Migrat 2011; 5:490–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic 2007; 8:808–19 [DOI] [PubMed] [Google Scholar]
- 301.Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol 2001; 3:913–7 [DOI] [PubMed] [Google Scholar]
- 302.Stehbens SJ, Akhmanova A, Yap AS. Microtubules and cadherins: a neglected partnership. Front Biosci 2009; 14:3159–67 [DOI] [PubMed] [Google Scholar]
- 303.Stehbens SJ, Paterson AD, Crampton MS, Shewan AM, Ferguson C, Akhmanova A, Parton RG, Yap AS. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J Cell Sci 2006; 119:1801–11 [DOI] [PubMed] [Google Scholar]
- 304.Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell 2008; 135:948–59 [DOI] [PubMed] [Google Scholar]
- 305.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harbor Perspect Biol 2009; 1:a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Yap AS, Stevenson BR, Abel KC, Cragoe EJ, Jr, Manley SW. Microtubule integrity is necessary for the epithelial barrier function of cultured thyroid cell monolayers. Exp Cell Res 1995; 218:540–50 [DOI] [PubMed] [Google Scholar]
- 307.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol 2003; 163:547–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mary S, Charrasse S, Meriane M, Comunale F, Travo P, Blangy A, Gauthier-Rouviere C. Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol Biol Cell 2002; 13:285–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Krylyshkina O, Anderson KI, Kaverina I, Upmann I, Manstein DJ, Small JV, Toomre DK. Nanometer targeting of microtubules to focal adhesions. J Cell Biol 2003; 161:853–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Scott JA, Yap AS. Cinderella no longer: alpha-catenin steps out of cadherin's shadow. J Cell Sci 2006; 119:4599–605 [DOI] [PubMed] [Google Scholar]
- 311.Franz CM, Ridley AJ. p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem 2004; 279:6588–94 [DOI] [PubMed] [Google Scholar]
- 312.Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. J Biol Chem 2004; 279:9512–21 [DOI] [PubMed] [Google Scholar]
- 313.Chausovsky A, Bershadsky AD, Borisy GG. Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol 2000; 2:797–804 [DOI] [PubMed] [Google Scholar]
- 314.Shtutman M, Chausovsky A, Prager-Khoutorsky M, Schiefermeier N, Boguslavsky S, Kam Z, Fuchs E, Geiger B, Borisy GG, Bershadsky AD. Signaling function of alpha-catenin in microtubule regulation. Cell Cycle 2008; 7:2377–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Waterman-Storer CM, Salmon E. Positive feedback interactions between microtubule and actin dynamics during cell motility. Curr Opin Cell Biol 1999; 11:61–7 [DOI] [PubMed] [Google Scholar]
- 316.Tabdanov ED, Puram VV, Win Z, Alamgir A, Alford PW, Provenzano PP. Bimodal sensing of guidance cues in mechanically distinct microenvironments. Nat Commun 2018; 9:4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ito S, Okuda S, Abe M, Fujimoto M, Onuki T, Nishimura T, Takeichi M. Induced cortical tension restores functional junctions in adhesion-defective carcinoma cells. Nat Commun 2017; 8:1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cao J, Ehling M, Marz S, Seebach J, Tarbashevich K, Sixta T, Pitulescu ME, Werner AC, Flach B, Montanez E, Raz E, Adams RH, Schnittler H. Polarized actin and VE-cadherin dynamics regulate junctional remodelling and cell migration during sprouting angiogenesis. Nat Commun 2017; 8:2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Logan CM, Rajakaruna S, Bowen C, Radice GL, Robinson ML, Menko AS. N-cadherin regulates signaling mechanisms required for lens fiber cell elongation and lens morphogenesis. Dev Biol 2017; 428:118–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Aranjuez G, Burtscher A, Sawant K, Majumder P, McDonald JA. Dynamic myosin activation promotes collective morphology and migration by locally balancing oppositional forces from surrounding tissue. Mol Biol Cell 2016; 27:1898–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat Commun 2015; 6:8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Mui KL, Chen CS, Assoian RK. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J Cell Sci 2016; 129:1093–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Maddala R, Skiba N, Vasantha Rao P. Lens fiber cell elongation and differentiation is associated with a robust increase in myosin light chain phosphorylation in the developing mouse. Differentiation 2007; 75:713–25 [DOI] [PubMed] [Google Scholar]
- 324.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol 2007; 9:299–309 [DOI] [PubMed] [Google Scholar]
- 325.Ng MR, Besser A, Danuser G, Brugge JS. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J Cell Biol 2012; 199:545–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Yashiro H, Loza AJ, Skeath JB, Longmore GD. Rho1 regulates adherens junction remodeling by promoting recycling endosome formation through activation of myosin II. Mol Biol Cell 2014; 25:2956–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell 2005; 16:4531–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Cook TA, Nagasaki T, Gundersen GG. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J Cell Biol 1998; 141:175–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol 2004; 265:23–32 [DOI] [PubMed] [Google Scholar]
- 330.Ridley A, Heald R. Cell structure and dynamics. Curr Opin Cell Biol 2011; 23:1–3 [DOI] [PubMed] [Google Scholar]
- 331.Ridley AJ. Rho GTPases and cell migration. J Cell Sci 2001; 114:2713–22 [DOI] [PubMed] [Google Scholar]
- 332.Ridley AJ. Rho proteins, PI 3-kinases, and monocyte/macrophage motility. FEBS Lett 2001; 498:168–71 [DOI] [PubMed] [Google Scholar]
- 333.Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol 2005; 15:76–83 [DOI] [PubMed] [Google Scholar]
- 334.Daub H, Gevaert K, Vandekerckhove J, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate the microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem 2001; 276:1677–80 [DOI] [PubMed] [Google Scholar]
- 335.Kuntziger T, Gavet O, Manceau V, Sobel A, Bornens M. Stathmin/Op18 phosphorylation is regulated by microtubule assembly. Mol Biol Cell 2001; 12:437–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci 2001; 114:3795–803 [DOI] [PubMed] [Google Scholar]
- 337.Small JV, Geiger B, Kaverina I, Bershadsky A. How do microtubules guide migrating cells? Nat Rev Mol Cell Biol 2002; 3:957–64 [DOI] [PubMed] [Google Scholar]
- 338.Wittmann T, Bokoch GM, Waterman-Storer CM. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J Cell Biol 2003; 161:845–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Betson M, Lozano E, Zhang J, Braga VM. Rac activation upon cell-cell contact formation is dependent on signaling from the epidermal growth factor receptor. J Biol Chem 2002; 277:36962–9 [DOI] [PubMed] [Google Scholar]
- 340.Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Chem 1999; 10:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol 1997; 137:1421–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat Rev Mol Cell Biol 2001; 2:887–97 [DOI] [PubMed] [Google Scholar]
- 343.Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, Kaibuchi K. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J Biol Chem 1999; 274:26044–50 [DOI] [PubMed] [Google Scholar]
- 344.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 2001; 22:32–9 [DOI] [PubMed] [Google Scholar]
- 345.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem 1999; 68:459–86 [DOI] [PubMed] [Google Scholar]
- 346.Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol Vision 2003; 9:329–36 [PubMed] [Google Scholar]
- 347.Roycroft A, Mayor R. Molecular basis of contact inhibition of locomotion. Cell Mol Life Sci 2016; 73:1119–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Scarpa E, Szabo A, Bibonne A, Theveneau E, Parsons M, Mayor R. Cadherin switch during EMT in neural crest cells leads to contact inhibition of locomotion via repolarization of forces. Development Cell 2015; 34:421–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Theveneau E, Mayor R. Integrating chemotaxis and contact-inhibition during collective cell migration: small GTPases at work. Small GTPases 2010; 1:113–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Kadir S, Astin JW, Tahtamouni L, Martin P, Nobes CD. Microtubule remodelling is required for the front-rear polarity switch during contact inhibition of locomotion. J Cell Sci 2011; 124:2642–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Moore R, Theveneau E, Pozzi S, Alexandre P, Richardson J, Merks A, Parsons M, Kashef J, Linker C, Mayor R. Par3 controls neural crest migration by promoting microtubule catastrophe during contact inhibition of locomotion. Development 2013; 140:4763–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Nagasaki T, Chapin CJ, Gundersen GG. Distribution of detyrosinated microtubules in motile NRK fibroblasts is rapidly altered upon cell-cell contact: implications for contact inhibition of locomotion. Cell Motil Cytoskeleton 1992; 23:45–60 [DOI] [PubMed] [Google Scholar]