Short abstract
Cardiomyocytes are large (∼40,000 µm3), rod-shaped muscle cells that provide the working force behind each heartbeat. These highly structured cells are packed with dense cytoskeletal networks that can be divided into two groups—the contractile (i.e. sarcomeric) cytoskeleton that consists of filamentous actin-myosin arrays organized into myofibrils, and the non-sarcomeric cytoskeleton, which is composed of β- and γ-actin, microtubules, and intermediate filaments. Together, microtubules and intermediate filaments form a cross-linked scaffold, and these networks are responsible for the delivery of intracellular cargo, the transmission of mechanical signals, the shaping of membrane systems, and the organization of myofibrils and organelles. Microtubules are extensively altered as part of both adaptive and pathological cardiac remodeling, which has diverse ramifications for the structure and function of the cardiomyocyte. In heart failure, the proliferation and post-translational modification of the microtubule network is linked to a number of maladaptive processes, including the mechanical impediment of cardiomyocyte contraction and relaxation. This raises the possibility that reversing microtubule alterations could improve cardiac performance, yet therapeutic efforts will strongly benefit from a deeper understanding of basic microtubule biology in the heart. The aim of this review is to summarize the known physiological roles of the cardiomyocyte microtubule network, the consequences of its pathological remodeling, and to highlight the open and intriguing questions regarding cardiac microtubules.
Impact statement
Advancements in cell biological and biophysical approaches and super-resolution imaging have greatly broadened our view of tubulin biology over the last decade. In the heart, microtubules and microtubule-based transport help to organize and maintain key structures within the cardiomyocyte, including the sarcomere, intercalated disc, protein clearance machinery and transverse-tubule and sarcoplasmic reticulum membranes. It has become increasingly clear that post translational regulation of microtubules is a key determinant of their sub-cellular functionality. Alterations in microtubule network density, stability, and post-translational modifications are hallmarks of pathological cardiac remodeling, and modified microtubules can directly impede cardiomyocyte contractile function in various forms of heart disease. This review summarizes the functional roles and multi-leveled regulation of the cardiac microtubule cytoskeleton and highlights how refined experimental techniques are shedding mechanistic clarity on the regionally specified roles of microtubules in cardiac physiology and pathophysiology.
Keywords: Microtubule, cytoskeleton, myocytes, heart, heart failure, post-translational modification
Overview—The cardiomyocyte cytoskeleton
Cardiomyocytes are the cellular working units of the heart and utilize coordinated force generation to contract the ventricles and power blood-flow through the circulatory system. Within the myocyte, densely packed myofibrils consisting of actin and myosin (and regulatory and structural proteins like troponin and titin) comprise the sarcomeric cytoskeleton, which converts chemical energy into mechanical work by adenosine triphosphate (ATP) hydrolysis. Since sarcomeres drive contraction, the structural “health” of the sarcomere is integral for cardiac function; and as cardiac myocytes are extremely long-lived (decades1,2), maintaining sarcomere homeostasis presents a tall order to the cell. This order is carried out by a number of specialized support systems that are largely coordinated by the non-sarcomeric cytoskeleton.
Sarcomeric proteins must be readily recycled and replaced, which requires the effective delivery of mRNA and protein, as well as the clearance and degradation of aged constituents. Mitochondria, which provide the ATP to fuel contraction, must be properly positioned and maintained. The transverse tubule (T-tubule) and sarcoplasmic reticulum (SR) membranes must be organized in close apposition to one another to support excitation–contraction (E-C) coupling. Inter-myocyte connections that mediate synchronous contraction must be closely coordinated via cell–cell junctions at the intercalated disc. The proper positioning, regulation, and turnover of each of these components is facilitated by the meshwork of microtubules and intermediate filaments making up the non-sarcomeric cytoskeleton. Microtubules deliver ion channels, translational machinery and facilitate the clearance of misfolded proteins by orchestrating their sorting and delivery to distinct subcellular locales. Scaffolding for the myofilaments and positioning of organelles within the myocyte is also mediated by the non-sarcomeric cytoskeleton. As it forms an interconnected lattice throughout the cell, the non-sarcomeric cytoskeleton is well positioned to sense and transmit mechanical signals3–5 that enable the heart to regulate gene transcription and adapt to changing demands. Furthermore, microtubules are deformed during contraction and can directly regulate contractility by mechanically impeding myocyte shortening. Despite the many known functions of the microtubule cytoskeleton, there are even more unanswered questions regarding its role in myocyte biology. In this review we will focus on the microtubules as a major component of the non-sarcomeric cytoskeleton; for a recent review of other elements, please see Grimes et al.6
Microtubules—What they are, what they do
Microtubules are hollow, 25 nm diameter tubes formed by the polymerization of α/β-tubulin dimers. They can run tens of microns in length within the cell, and are the stiffest of the cytoskeletal filaments—microtubules have a persistence length ∼2–3 orders of magnitude greater than actin filaments,7 which are in turn an order of magnitude stiffer than intermediate filaments.8 Microtubules can be “dynamic,” undergoing rounds of growth (polymerization) and shrinkage (catastrophe), or “stable,” meaning they present as fixed structures over a time scale of minutes to hours.9 The canonical functions of microtubules are to provide cellular structural support, enable chromosome movement during cell division, and serve as the tracks by which cargo is transported throughout the cell.10 Cargo transport is achieved via the microtubule motor proteins kinesin and dynein, with kinesin trafficking cargo towards the growing, plus end of a microtubule, and dynein moving in the opposite, retrograde direction.
Microtubule populations in the heart
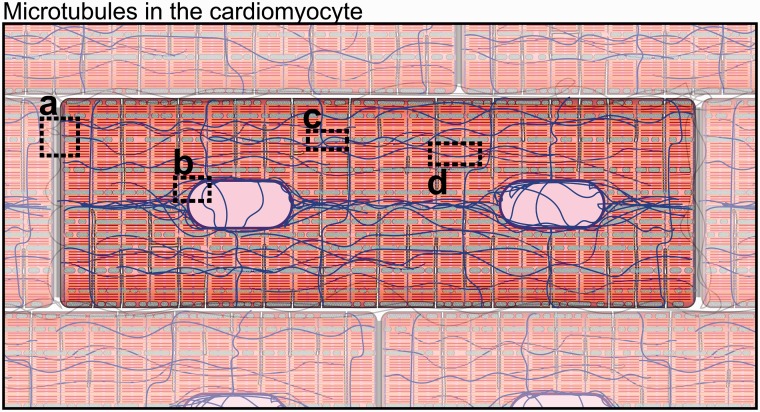
The cardiomyocyte microtubule network can be spatially separated into three distinct (yet overlapping) populations. Early characterization of microtubules in cardiac muscle by electron microscopy identified interfibrillar microtubules running parallel to and in close association with myofibrils and mitochondria, as well as an additional population encircling the nucleus (Figure 1).11 Later, cortical microtubules, which wrap around the cardiomyocyte perpendicular to myofibrils, were implicated in the mechanotransduction of external cues and in the regulation of transmembrane proteins and ion channels.12,13 Interfibrillar microtubules are involved in organelle positioning, T-tubule and SR membrane regulation,5,14,15 maintenance of the intercalated disc,16 and regulation of myofilament mechanics.17–19 Perinuclear microtubules encircle the nucleus (or nuclei) of the cardiomyocyte, organize perinuclear organelles, and directly interact with the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex to mechanically couple the microtubule cytoskeleton with the nucleoskeleton.20–22 A dense highway of microtubules is typically observed spanning between nuclei in cardiomyocytes (Figure 1) but little is known about any unique functionality of this structure. Cortical microtubules, interfibrillar microtubules, and perinuclear microtubules are spatially distinct, vary in stability, post-translational profile and function, but utilize much of the same microtubule-associated machinery to conduct their tasks.
Figure 1.
Microtubules in the cardiomyocyte. (a) Intercalated disc region. (b) Nuclear region. (c) Mitochondrial associated microtubules. (d) Microtubules at the dyad/Z-disc. Microtubules (blue), sarcomeric cytoskeleton (red/orange), mitochondria (green), nuclei (light purple). (A color version of this figure is available in the online journal.)
Microtubules in the failing heart
Consistent with the diverse roles played by the non-sarcomeric cytoskeleton in the myocyte, numerous and impactful modifications to the non-sarcomeric cytoskeleton are observed in heart failure. The density of the microtubule and intermediate filament network increases significantly in end-stage heart failure of diverse etiology23,24 with an accompanying decrease in sarcomeric protein density.23,25,26 There is an accumulation of microtubule post-translational modifications (PTMs) in heart failure that alter the microtubule interactome to likely favor microtubule stabilization by facilitating interactions with microtubule associated proteins (MAPs) and intermediate filaments.17,23,27,28 These cumulative alterations in network density, dynamics, and binding partners interfere with the physiological roles of the microtubule (through incompletely understood mechanisms), affecting E-C coupling, intercalated disc maintenance, myofilament contractility, and proteostasis. As such, treatments to restore the density or dynamics of cardiac microtubules could provide benefit to a number of the maladaptive processes observed in cardiac pathology, but the role of microtubules must be better defined in order to inform specific interventions. Below we will break down the role of microtubules within the cardiomyocyte, and highlight how they are altered in and may contribute to heart disease.
Microtubules and tubulin turnover
In the healthy myocyte, roughly 50% of tubulin is in its free, soluble form, while the remaining half is incorporated into polymerized microtubules.29–31 The half-life of a microtubule can vary greatly based on its interactions with stabilizing factors, but is on the order of minutes to hours,32 while the lifetime of free tubulin is on the order of 1–2 days.33,34 Compared to other cell types, microtubules in mature cardiomyocytes appear particularly stable,35 which can be assessed using nocodazole, a drug that binds free tubulin and prevents it from incorporating into the microtubule lattice.36 Thus, the rate at which the microtubule lattice disintegrates upon nocodazole treatment is an indirect assessment of microtubule stability.37,38 Nanomolar concentrations of nocodazole reduce microtubule dynamicity in most cell types,39 including immature cardiac cells, while higher concentrations are required to depolymerize microtubules in adult cardiomyocytes.37,38 In adult ventricular myocytes the most stable microtubules are largely concentrated around the myocyte nuclei, and remain after nocodazole or colchicine treatment sufficient to eliminate most other microtubules (Belmadani et al.38 and our unpublished observations).
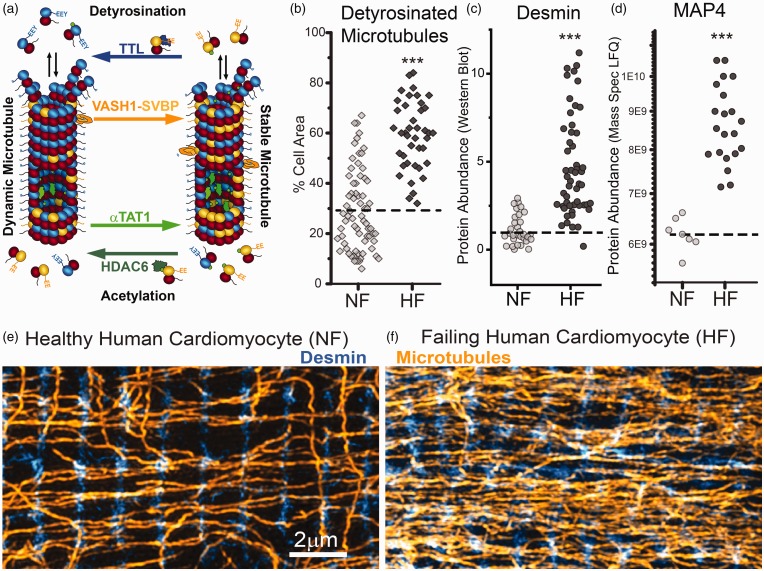
Further stabilization of the microtubule network has been demonstrated in patient tissue and various animal models of cardiac hypertrophy and heart failure (cat pulmonary artery banding19,37; dog left ventricular pressure overload40,41; mouse transverse aortic constriction30,42,43; human patients23,24; see review31). Pressure-overload induced hypertrophy stabilizes cardiac microtubules, as inferred from increased resistance to nocodazole treatment37 and increased tubulin content in insoluble fractions.30,42,43 In patients with end-stage heart failure of ischemic, dilated, or hypertrophic origin, markers of stable microtubules are increased, including PTMs, stabilizing MAPs, and a greater increase in polymerized microtubule density than total tubulin content (Figure 2(b) to (f)).23,24 Microtubule stabilization likely occurs early on in disease progression, as indicated by a rapid rise of tubulin levels and stabilizing MAPs in pressure overload.27,28,37,44,45 Elevated tubulin levels also persists into disease progression, remaining six months following pulmonary artery banding.44 Significant work is still needed to clarify the temporal progression of microtubule stabilization during the onset, progression, and reversion of discrete myopathic etiologies—particularly of those independent of significant pressure-overload—as well as to establish causality between microtubule stabilization and hypertrophy or decompensation.
Figure 2.
Microtubule network stabilization in heart failure. (a) Schematic of C-terminal detyrosination and luminal acetylation. (b to d) Microtubule detyrosination, desmin, and MAP4 are consistently increased in human heart failure (modified from Chen et al.23) (e and f) Immunofluorescence. Microtubules intersect with desmin intermediate filaments at the Z-disc of healthy and failing human cardiomyocytes. (A color version of this figure is available in the online journal.)
Microtubule post-translational modifications
Microtubule turnover is highly affected by the stability, rigidity and dynamics of the network, which is regulated by a combination of the “tubulin code” and MAPs. The “tubulin code” encompasses the diversity of tubulin isoforms and PTMs that can combine in numerous ways to confer unique functionality to subsets of microtubules within the cell.46–49
Typically, the longer lived a microtubule, the more post-translationally modified it becomes. However, the increased PTM of microtubules observed in disease could both arise from, as well as confer increased stability. For example, stable, long-lasting microtubules provide a substrate for luminal acetylation, but acetylation itself also can prolong the lifetime of a microtubule.50 Acetylation occurs within the microtubule lumen by αTAT1, while the tubulin deacetylase HDAC6 predominantly acts on free tubulin (Figure 2(a)).51,52 Highly acetylated microtubules exhibit increased flexural compliance and decreased damage under repetitive bending cycles,53 which can increase their lifetime in the face of mechanical stress. This may be important for maintaining network integrity in the heart, where microtubules are observed to buckle during each contraction.17 If we assume a 60min lifetime for a stable microtubule, this would equate to about 3600 bending cycles per cardiac microtubule in a resting human, and 36,000 bending cycles/microtubule in a mouse. It remains to be shown whether cyclic buckling fatigues or “breaks” microtubules in a myocyte the way repetitive bending does in vitro,54 and whether damaged microtubules repair themselves to maintain network integrity.
The most well studied PTM of the microtubule network in the heart is the C-terminal detyrosination of α-tubulin. The buckling, load-bearing behavior of microtubules is promoted by detyrosination,17 and detyrosination directly increases myocyte viscoelastic stiffness (as expanded upon below) and contributes to contractile dysfunction in cardiomyocytes from patients with heart failure.17,23 Regulation of the “tyrosination cycle” occurs on free and polymerized tubulin, with polymerized tubulin as the primary substrate for detyrosination by vasohibin enzymes or other carboxypeptidases,55,56 and free tubulin as the primary target for tyrosination by tubulin tyrosine ligase (TTL) (Figure 2(a)).57 TUBA4A is the only tubulin isotype translated in its detyrosinated form,58 and interestingly is one of the most highly expressed isotypes in the heart.23 Thus, total levels of detyrosination may reflect a combination of TUBA4A synthesis and PTM of other tubulin isoforms. Microtubule detyrosination is also associated with enhanced microtubule stability,32,38 which may be conferred by promoting interactions with intermediate filaments59,60 such as desmin.17 Increased microtubule detyrosination is observed in end stage human heart failure and is sufficient to increase myocyte viscoelasticity, while reducing detyrosinated tubulin in cardiomyocytes from failing hearts is sufficient to reduce myocyte stiffness and improve contractility.17,30
Other common tubulin PTMs, such as glutamylation and glycylation, are receiving emerging attention for their important regulatory roles in neuronal biology. For example, glutamylation can promote microtubule severing by enzymes such as katanin and spastin to regulate network density,61,62 and aberrant glutamylation perturbs neuronal transport and can cause neurodegeneration.63,64 The role of these PTMs has not been examined in the heart to our knowledge, but as regulators of network stability, organization, and MAP association, they warrant further investigation, which will benefit from the generation of mouse models with cardiac-specific manipulation of tubulin modifying enzymes.
Microtubule associated proteins
There are two primary classes of MAPs: molecular motors and structural MAPs.46,65–67 Two families of microtubule-based motors, kinesin and dynein, drive anterograde and retrograde cargo transport respectively. Structural MAPs (e.g. tau, MAP4, dynactin, end-binding protein 1 (EB1)) can influence the stability of microtubules either by blocking or recruiting severing proteins or by affecting the association of microtubules with other cytoskeletal elements including the sarcomeres.27 MAPs interact with each other, either cooperatively or competitively, and the affinity of MAPs for microtubules is strongly impacted by PTMs.
The balance of intracellular transport can be shifted by PTM of the microtubule and the binding of structural MAPs. While the interactions between different kinesins and detyrosinated microtubules have not been exhaustively explored, detyrosination tends to enhance kinesin recruitment to the microtubule or its processivity68–70 while highly processive dynein complexes are recruited to tyrosinated microtubules.71 Thus, the tyrosination state of the microtubule lattice influences the balance between retrograde (dynein-driven) and anterograde (kinesin-driven) transport, which can have important ramifications for the cell. For example, dynein-mediated retrograde transport is required for autophagy-mediated protein recycling,72 while delivery of cytosolic cargo70 and export of endocytic machinery to the cell surface73 is primarily kinesin driven. Proper patterning of microtubule tyrosination is also necessary for long-range transport, as has been demonstrated in neurons where an intact tyrosination cycle is required for neuronal differentiation and organization.56,74 Furthermore, structural MAPs along the microtubule can also influence the balance of motor-based transport. For example, MAP7 promotes kinesin-based transport while tau inhibits it, but neither impact dynein motility.65 In sum, these examples demonstrate the multiplexed ability to tune microtubule transport via various combinations of PTMs, MAPs, and tubulin isotypes.
While the aforementioned phenomena have largely been characterized in neurons, many of the same MAPs are expressed in the heart. One of the most highly expressed structural MAPs in the heart, MAP4,23,37 is involved in both pathological densification of the microtubule network27,28,43 and inhibition of microtubule-based transport.75 An increase in MAP4 expression is observed in heart disease,37 and cardiac-specific overexpression of MAP4 is sufficient to increase tubulin expression and microtubule stability.76 This increase in network density and stability may also come at the cost of jamming motor-based transport with excessive MAP4 decoration of the microtubules, leading to defective cargo delivery throughout the myocyte.75,77
Phosphorylation of MAPs is a common regulator of MAP-microtubule affinity, which can have a pronounced effect on microtubule dynamics and network architecture.78 MAP phosphorylation is tuned by protein kinases and phosphatases78 that respond to neurohormonal signals elicited by cardiac stress, and that are well-established mediators of cardiac hypertrophy.79–81 For example, dephosphorylation of MAP4, which is demonstrated in pressure-overload hypertrophy, increases its association with and stabilization of cardiac microtubules.28,43 By contrast, phosphorylation of MAP4 reduces its microtubule affinity and can destabilize the network, which may also lead to pathological remodeling, as phosphomimetic-MAP4 knock-in mice develop hypertrophy, fibrosis and systolic dysfunction.82 Consequently, it appears a proper balance of de- and phosphorylated MAP4 (and by extension hyper- and hypo-stabilized microtubules) is optimal for the heart, and shifting of the equilibrium in either direction can lead to pathological remodeling.
Phosphorylation also regulates the microtubule associated protein tau. Famous for its role in neurodegenerative disease, tau is also expressed in the heart23,83,84 (and see proteomic data set PXD008934). Tau can protect microtubules from severing,85 and may provide additional stabilization of cardiac microtubules. A direct interaction between tau and bridging integrator-1 (BIN1) has been characterized in vitro, and phosphorylation of tau destabilizes its interaction with both BIN186 and microtubules,87 but the specific role played by tau in the heart remains to be determined.
A family of structural MAPs and motors known as tip-tracking proteins are responsible for stabilization and steering of the microtubule growing-end (+TIP).88 In the heart +TIP interactions are largely coordinated by EB1, which promotes microtubule stability and recruits a family of CAP-Gly domain-containing tip-tracking proteins including cytoplasmic linker proteins 170, and 115 (CLIP170 and CLIP115) and p150glued.89–91 Tip-tracking proteins are preferentially recruited to tyrosinated microtubules and phosphorylation of EB1 enhances its localization to the +TIP. Also favoring tyrosinated tubulin, tip-tracking kinesins can facilitate microtubule disassembly (kinesin-13 family members, MCAK and KIF2A),92 which may contribute to the decreased stability of tyrosinated microtubules.93 Thus, PTMs to either MAPs or the microtubules on which they bind regulate intrinsic microtubule properties and their interactions within the myocyte, highlighting the complex, multi-layered regulation of the microtubule cytoskeleton.
While not classically considered MAPs, cytoskeletal linker proteins also influence microtubule organization and stability in the cardiomyocyte. Plectins can directly cross-link microtubules and intermediate filaments,94 while microtubule actin cross-linking factor 1 (MACF1) stabilizes and guides microtubule growth along actin filaments. Cardiac specific deletion of MACF1 leads to aberrant microtubule redistribution that is strongly correlated with ventricular hypertrophy and contractile dysfunction upon pressure overload,29 while plectin deletion leads to the disruption of desmin intermediate filaments95 that typically help maintain microtubule network organization.17 These data highlight the interdependence of the cytoskeletal networks in the cardiomyocyte, which must be considered when attempting to perturb specific components.
Microtubules at the membrane and costamere
Beneath the myocyte membrane lies a cortical network of microtubules that wraps around the myocyte and is predominantly aligned with the short axis of the cell. While the functionality of this pool of microtubules is far from fully elucidated, cortical microtubules regulate sarcolemmal ion channel localization, turnover, and activity,96 caveolae formation and signaling via G-protein coupled receptors (GPCRs),97 and mechanosensation and signaling, at least partly via connections to transmembrane complexes such as the dystrophin/dystroglycan complex.3
Vesicular trafficking is dependent on the microtubule network and is essential for the delivery and recycling of proteins between the SR, Golgi, and plasma membrane. Cycling of β-adrenergic receptors98,99 and insulin receptors100 depends on microtubules, while microtubules and actin microfilaments restrict cyclic adenosine monophosphate (cAMP) formation by localizing adenylyl cyclase signaling components in caveolae in adult myocytes.97 Ion channel localization and recycling at the cortex requires intact microtubules. Microtubule depolymerization reduces both kinesin-mediated anterograde101,102 and dynein-mediated retrograde103,104 transport of potassium channels in cardiomyocytes, leading to either decreased or increased surface expression of these channels. Inhibiting dynamic microtubules with nocodazole increases the expression of the chloride channel ClC2,105 while stabilization of the microtubule network with taxol reduces sarcolemmal sodium channel density.106 Microtubules are also implicated in the regulation of channel function independent of surface expression, as is demonstrated for KCNQ1.96 Thus, perturbations to either microtubule density or dynamics can disrupt ion flux in the cardiomyocyte.
Costameres are cortical hubs of mechanotransduction in striated muscle that connect the cell membrane to the sarcomeres at the Z-disc. At the costamere, the dystrophin/dystroglycan complex (DGC) bridges the extracellular matrix and the cytoskeleton.107,108 Dystrophin, the protein compromised in Duchenne muscular dystrophy, facilitates costameric organization,109,110 recruits glycoprotein complexes and associates with intermediate filaments,111 actin112 and microtubules.3,113,114 For a cartoon schematic of the DGC and its interactions with the cytoskeleton please see review.114 Proper localization of dystrophin, dystroglycan, and microtubules at the costamere protects muscle from exercise-induced injury. A functional hierarchy was proposed,115,116 where β2 spectrin localizes ankyrin-B to costameres, and ankyrin-B in turn localizes dynactin-4 and dystrophin, the latter of which likely facilitates dynactin-4 mediated stabilization of the costamere-associated microtubule network. In a commonly used model of Duchenne muscular dystrophy (the mdx mouse), subsarcolemmal microtubule network derangement increases reactive oxygen species (ROS) production and leads to aberrant calcium signaling.4,5,117 Transgenic overexpression of dystrophin or mini-dystrophin, but not utrophin, prevents cortical microtubule disorganization in mdx mice, protects skeletal muscle from eccentric contraction-induced force loss, and improves physical activity of the mice.3 However, further fine-mapping studies of dystrophin domains showed that the microtubule binding domain of dystrophin is dispensable for its microtubule-organizing function in vivo, and that full-length dystrophin is not sufficient to maintain normal microtubule network organization in the absence of other proteins in the dystrophin-glycoprotein complex.3 This suggests dystrophin plays an indirect role in microtubule organization. Loss of dystrophin is coincident with the transition from compensated hypertrophy to heart failure,118 but it remains to be demonstrated if a loss of dystrophin localization at the costamere is related to microtubule disarray and microtubule-dependent dysfunction in the progression of heart disease.
Microtubules at the dyad
Similar to costameric association at the cortex, microtubules also associate with interfibrillar structures at the Z-disc regulating the transport and positioning of a number of key components involved in E-C coupling. The SR and T-tubule membranes are well distributed throughout the myocyte and make regular contact at the Z-line in structures known as dyads (see review119). Dyads are hubs of E-C coupling (see review120), where action potential propagation leads to calcium entry through L-type calcium channels on the T-tubule membrane, which is then amplified by ryanodine receptors (RyRs) via calcium-induced calcium release from SR stores (Figure 3, inset). Efficient E-C coupling requires an extensive and well-ordered network of T-tubules and tight proximal interaction between T-tubules and the SR, as well as normal distribution of L-type calcium channels.
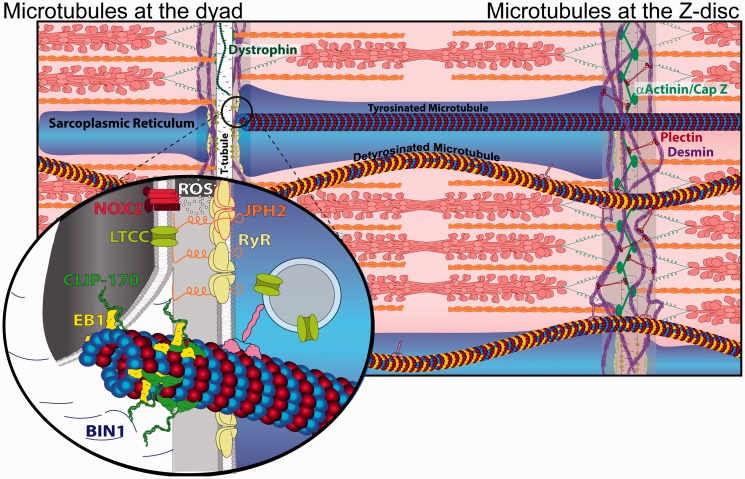
Figure 3.
Role of microtubules at the dyad and Z-disc. Desmin (purple) wraps myofilaments at the Z-disc where it interacts with α-actinin (green) through plectin (red), and microtubules possibly through kinesin (pink) or plectin. Detyrosinated (yellow) microtubules link to the Z-disc and buckle during contraction while tyrosinated microtubules are not mechanically coupled and slide past myofilaments. Inset: A structural schematic of the dyad: L-type calcium channels and NOX2 on the T-tubule (white, cut open) are held in proximity to RyRs (pale yellow) at the SR (blue) by JPH2 (orange). Reactive oxygen species (ROS) emitted by NOX2 sensitizes RyRs. Microtubules link to the T-tubule via an interaction between CLIP-170 and BIN1. (A color version of this figure is available in the online journal.)
Microtubules maintain the structural and functional integrity of dyads through multiple mechanisms, including targeted delivery of L-type calcium channels and T-tubule scaffolding proteins.121,122 The T-tubule scaffolding protein BIN1 is important for both L-type calcium channel localization121 and T-tubule structural regulation123–125 (see review by Fu and Hong126), while junctophilin-2 (JPH2) directly couples the T-tubule and SR membranes.122 The growing tip of microtubules links to the T-tubule network through an interaction between the tip tracking protein CLIP-170 and BIN1.14 L-type calcium channels and SR membranes (containing RyRs) are moved on microtubules by kinesin motors.127,128 Triadin, named for its role in maintaining the triad (the analogous structures to dyads in skeletal muscle), is involved in shaping and remodeling SR membranes, likely in part through its interaction with microtubules via 63 kDa cytoskeleton-linking membrane protein (CLIMP63).129 In both right and left ventricular pressure overload, microtubule proliferation is associated with reduced expression and localization of JPH2, pathological T-tubule remodeling and RyR disorganization.15,130 T-tubule disruption and calcium mishandling are secondary to microtubule-mediated misregulation of JPH2,131 and reducing microtubule density can restore dyad structure and improve ventricular function, suggesting a causative role of microtubules in the misregulation of E-C coupling in disease.
Microtubules also regulate myocyte sensitivity to mechanical stretch through the E-C coupling machinery. During diastolic stretch, microtubules serve as mechanotransducers to activate the production of ROS by NADPH oxidase 2 (NOX2) in the T-tubule membranes, a process termed X-ROS signaling (Figure 3, inset).5,117 Under normal conditions, ROS can rapidly sensitize nearby RyRs, which primes the E-C coupling machinery at an appropriate phase of the cardiac cycle (late diastole). However, overproduction of ROS can lead to excessive RyR calcium release and subsequent arrhythmia. In disease states such as myopathy arising from Duchenne muscular dystrophy, increased NOX2 activity, microtubule disorganization and detyrosination, and altered mechanical loads may all lead to excessive X-ROS signaling that disrupts calcium homeostasis and causes oxidative stress in both cardiac and skeletal muscle.4,132 Upregulation of the β-tubulin isoform tubb6 has been shown to at least partly underlie microtubule disorganization in dystrophic muscle.133 While the complex interplay between microtubules, NOX2, and dystrophin requires further elucidation, efforts to target this axis—by restoration of microtubule network organization, tyrosination,4,134,135 or NOX2 activity4,136—have each been shown to confer protection in dystrophic muscle.
Microtubules at the intercalated disc
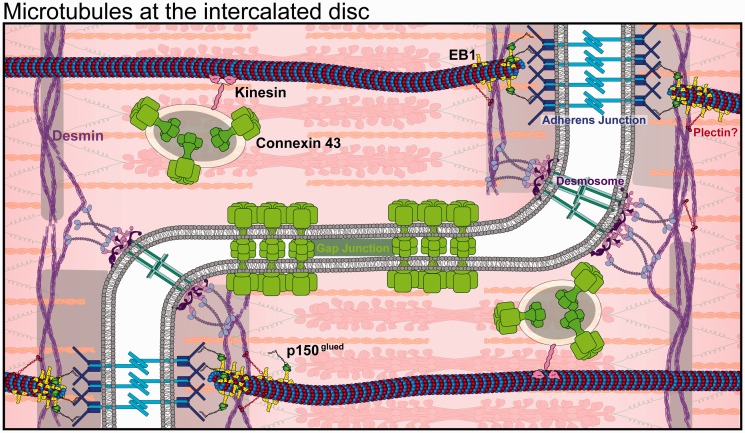
Inter-myocyte coupling is achieved at the intercalated disc, specialized regions at myocyte ends that are enriched in desmosomes, adherens and gap junction complexes137 (Figure 4). Myocytes are electrically coupled through gap junctions, which are large, non-specific transmembrane channels consisting of connexin hexamers that span adjacent myocyte membranes to provide electrical, metabolic, and immunological connectivity. Connexin protein is continuously made, transported, and degraded, having a half-life of only 1–5 h,138,139 and its turnover is regulated by both intracellular and extracellular stresses. In heart disease, for example following myocardial infarction, connexin is readily downregulated and protein localization to the intercalated disc is compromised140. Connexin is also readily post-translationally modified, and its altered phosphorylation is linked to gap junction redistribution and arrhythmias.141,142 Microtubules deliver connexons (oligomers of connexin) to the intercalated disc by motor-based transport, with the help of EB1 and p150glued anchoring of the growing end of microtubules to adherens junctions through N-cadherin.16,139,143 Analogous to the loss of the dyadic structure in heart failure, alterations to the microtubule cytoskeleton are also associated with the disorganization of intercalated discs.144 Perhaps consistently, administration of agents that disrupt microtubule dynamics are associated with ventricular arrhythmias.145–147 Yet the mechanism by which this occurs is far from settled, motivating future studies to evaluate whether reversing microtubule alterations in disease may exert beneficial effects via restoration of cell-cell coupling.
Figure 4.
Microtubule interactions at the intercalated disc. Microtubule +TIPs anchoring to the adherens junctions (blue) via BIN1 and p150glued directs the delivery of connexin 43 (light green) and other intercalated disc machineries. Desmosomes connect to desmin intermediate filaments via desmoplakin. Gap junctions (light green) provide cytoplasmic connectivity between cells. (A color version of this figure is available in the online journal.)
Microtubules and the myofilaments
In contrast to the circumferential cortical network, a longitudinal network of interfibrillar microtubules interacts with the sarcomeres through transverse desmin intermediate filaments at the Z-disc to form a lattice-like scaffolding associated with myofilaments (Figure 3). Coupled to the sarcomeres, microtubules transmit force as they are pulled taut during diastole and compressed during systole.17 Lateral reinforcement of microtubules by cross-links to cytoskeletal proteins enables microtubules to bear compressive loads and mechanically impede sarcomere shortening.148 Microtubules buckling between sarcomeres during contraction of adult cardiomyocytes provides visual evidence for such mechanical coupling between sarcomeres and microtubules, and importantly this sarcomeric mode of buckling is promoted by detyrosination.17 The interaction between microtubules and desmin intermediate filaments and the Z-disc59 could be mediated through Kif5b149 or plectin,94 or may be non-specific and dictated largely by proximity within the cell. Regardless, the lateral reinforcement and thus mechanical behavior of microtubules within the myocyte is dependent on cross-linking between detyrosinated microtubules and other cytoskeletal elements.
Microtubule depolymerization or tyrosination increases myocyte contractility and accelerates relaxation without altering calcium signaling,18,19,23,31 consistent with a mechanical uncoupling of microtubules from myofilaments. Measurements of myocyte and myocardial stiffness support a direct mechanical explanation for this altered contractility,17,18,23,150–153 as myocyte viscoelasticity is reduced by microtubule tyrosination or depolymerization. A viscoelastic contribution of the network implies that the buckling of detyrosinated microtubules dissipates energy (resisting both contraction and relaxation). Due to its viscoelastic nature, this resistance will increase with increasing shortening or lengthening speeds, which has been demonstrated in both human and rat myocytes.18,23
As the microtubule contribution to myocyte mechanics strongly depends on the network density and interactome, it differs significantly in various cellular contexts and pathological states. This manifests as a considerably larger improvement in myocyte mechanical behavior when microtubules are targeted in myocytes from diseased vs. healthy hearts,19,23 and may underlie the inverse correlation between detyrosinated microtubules and ejection fraction observed in human and murine heart disease.17,24,42
It should also be noted that common methodologies used to assess cardiac mechanics—including permeabilizing cells or tissues, isolating myofilaments, or maintaining a preparation in cold relaxing solution—compromises the integrity of the microtubule network, and thus confounds the mechanical assessment. In rat cardiomyocytes, the transverse and shear modulus were found to be decreased by colchicine and increased by taxol, while the tensile elastic modulus was not changed.94 In a separate study, colchicine was found to have variable effects on the tensile stiffness of cardiomyocytes, but on average accounted for roughly 10% of the passive tension of the cell.154 These findings are consistent with more recent work demonstrating that differences in cardiomyocyte tensile properties only arise at physiological strain rates18 and are almost undetectable in healthy cells upon a slow application of stretch. Thus, the effective mechanical response depends on the pathological context, technical conditions of the preparation, and the orientation and speed of the mechanical test. These complexities have likely contributed to past confusion regarding the mechanical role of cardiac microtubules.
In tissue and organ level studies, a similar pathology-dependent mechanical contribution has been observed in some,41,152,155 but not all156,157 investigations. Colchicine treatment significantly improved myocardial performance after severe pressure overload in the right ventricle of cats152,155 and the left ventricle of dogs,41 with little impact on normal myocardium. Colchicine-dependent preservation of myocardial function in vivo has also been observed in a rat model of pulmonary hypertension130 and upon left ventricular pressure overload in rats158 and mice.15
Taken together, a picture emerges where the microtubule contribution to cardiac mechanics is highly context dependent; there are subtle, yet measurable phenotypes in healthy cardiomyocytes, and an increasing relative contribution in disease that appears most prominent upon severe pressure overload or advanced heart failure. While in our opinion the contribution to isolated myocyte mechanics has been reasonably well defined, more work is needed to delineate how this contribution scales to the tissue and organ level.
Microtubules and mitochondria
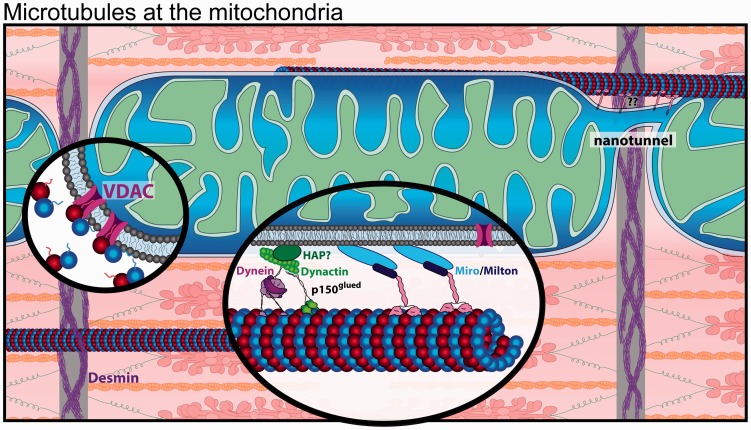
In non-muscle cells, variable energy demand is met by rapid remodeling and redistribution of the mitochondrial network,159–161 in what is largely a microtubule driven process.162,163 Perturbing microtubules is sufficient to remodel the mitochondrial network, likely by changing the balance of mitochondrial fission and fusion,164,165 or by disrupting microtubule motor-dependent forces that shape mitochondrial morphology. Fusion is supported by mitochondrial motility,160 which is driven by the microtubule motors kinesin for anterograde166 and dynein for retrograde distribution of mitochondria and is regulated by the motor adaptor complex Miro/Milton in a calcium depending manner167–169 (Figure 5, inset, middle).
Figure 5.
Microtubules are in close proximity to the mitochondria in the cardiomyocyte. Nanotunnels (top right) span adjacent mitochondria. Inset (middle): Mitochondria interact with both kinesin and dynein motors. Inset (left): VDAC is inhibited by free tubulin through binding of the β-tubulin tail. (A color version of this figure is available in the online journal.)
In comparison to non-muscle cells, mitochondria in cardiomyocytes are less mobile. A constant high energy demand is met by a dense population of mitochondria that are packed between myofibrils, occupying 35–40% of the cell volume.170,171 Dense packing of mitochondria restricts their mobility, fission and fusion,172,173 and leads to spatially distinct populations of subsarcolemmal, inter-myofibrillar, and perinuclear mitochondria.174–176 The subsarcolemmal mitochondria lie beneath membrane crests spanning between costameres, and loss of cortical microtubules may displace subsarcolemmal mitochondria.177 Distinct from fusion, cardiac mitochondria do form direct connections via nanotunnel extensions that permit exchange of matrix content (Figure 5).171,173,178 The association of nanotunnels with microtubules in electron micrographs suggests nanotunnels may be generated or stabilized by an interaction between the mitochondrial membrane and microtubules.178 Further, recent evidence suggests mitochondria also undergo fusion and fission at higher rates than previously thought, and these dynamics are likely regulated by several relevant stimuli.173 Thus, the interplay between mitochondrial and microtubule dynamics in various pathophysiological contexts warrants future investigation.
Beyond regulating mitochondrial positioning and exchange of matrix content, microtubule-based transport is important for the functional regulation of mitochondria. Microtubules mediate the delivery of purinosomes to the mitochondria,179,180 multienzyme complexes that regulate metabolic flux and provide precursors for DNA synthesis. Additionally, free tubulin can directly regulate mitochondrial respiration through reversible blockage of the mitochondrial voltage-dependent anion channel (VDAC) by β-tubulin c-terminal tails (Figure 5, inset, left).181–184 Thus, the interplay between microtubule dynamics and mitochondrial dynamics may elicit complex effects on metabolism and ATP production. Myocytes from failing hearts exhibit shifts in mitochondrial fusion and fission balance that affect myocyte bioenergetics,185–187 which we speculate may at least partly arise due to altered microtubule dynamics, but this is not well understood.
Microtubules and the nucleus
Cardiomyocytes are often multi-nucleated, with nuclei aligned in the interior of the myofibrils and connected by a dense highway of microtubules (Figure 1). This differs from skeletal muscle, where nuclei are extruded to the cell periphery. The perinuclear microtubules in a cardiomyocyte are amongst the most stable in the cell resisting treatments with colchicine or nocodazole,38 which may indicate that they are protected by heavy decoration with interacting partners.
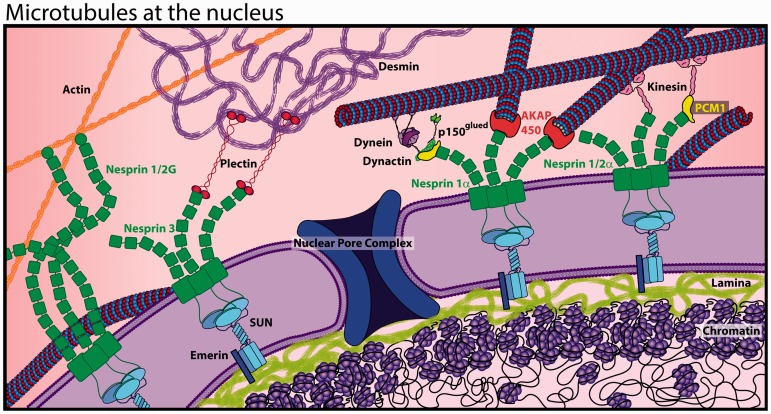
In most eukaryotic cells, microtubules emanate from a microtubule organizing center (MTOC) at juxta-nuclear centrosomes, where the minus (−) ends of microtubules originate near the nucleus and plus (+) ends radiate toward the periphery.188 Yet in cardiomyocytes, centrosomes disintegrate shortly after birth,189 and non-centrosomal MTOCs decorate the nuclear envelope, associated Golgi and Golgi outposts in muscle cells.190 While microtubules are still concentrated around the nucleus, the lack of a centrosomal MTOC creates a more widely distributed and less uniformly polarized microtubule network (Figure 1).
Regardless, centrosomal proteins such as PCM-1, AKAP450, pericentrin, and γ-tubulin localize to the nuclear periphery and can nucleate microtubule growth,191–193 recruit motor proteins194 and facilitate interactions between the “cage” of perinuclear microtubules and the nuclear mechanotransduction machinery known as the LINC complex. The LINC complex consists of the outer nuclear membrane nesprin proteins and inner nuclear membrane SUN proteins that connect the cytoskeleton to the nuclear lamina (Figure 6). These nuclear intermediate filaments (lamins) couple with regions of chromatin to form lamin-associated domains (LADs),195 which are associated with silenced genes.196 The LINC complex is thus capable of transmitting mechanical signals from the cytoskeleton to LADs to regulate gene expression, an attractive mechanism for the myocyte to sense changes in environmental stress and respond accordingly. Microtubules interact with the LINC complex via nesprin 1α, nesprin 2α,197 and nesprin 4198 by binding the microtubule motors kinesin and dynein,194,199 and considerable evidence supports a nesprin 1α interaction in the cardiomyocyte.200,201 The importance of these LINC complex interactions is highlighted by mutations in LINC components causing diverse cardiomyopathies (see review22,196), yet much remains unknown about the mechanistic underpinnings of these connections and their role in nuclear homeostasis and cardiac mechanotransduction.
Figure 6.
The LINC complex links the nucleoskeleton with the cytoskeleton. Cytoskeletal filaments (actin, desmin, and microtubules) are anchored to nesprins at the outer nuclear membrane, which couple to the SUN family of proteins spanning the inner nuclear membrane to connect with the nucleoskeleton (lamins). (A color version of this figure is available in the online journal.)
Microtubules and hypertrophy
Mechanotransduction in the myocyte facilitates a remarkable plasticity of the heart to adapt to chronic changes in load, as occurs with frequent exercise or elevated blood pressure. Thickening of the ventricular walls (hypertrophy) helps minimize wall stress under increased load, while atrophy of the heart occurs in response to unloading. Each requires individual myocytes to quickly and efficiently change their size, as myocyte proliferation is not sufficient to drive hypertrophy.2,202,203
Microtubule proliferation is closely associated with cardiac hypertrophy induced by pressure overload on the left and right ventricle in various small and large animal studies,15,42,44,45,158 and seems to scale with the severity of wall stress.40,204 Microtubules appear required for hypertrophy in response to increased external stress, as treatment with colchicine significantly blunts the hypertrophic response to pressure overload.42,45,130,158 In a more subtle, clinically relevant setting of chronic hypertension, colchicine treatment has been demonstrated to arrest the progression of hypertrophy in spontaneously hypertensive rats.205 Microtubules have also been shown to be required for phenylephrine-induced hypertrophy in vitro, and the anti-hypertrophic effects of adenosine have been attributed to a reduction in stable, detyrosinated microtubules.30 In a clever approach exploiting transgenic mice harboring hyper- or hypo-stable forms of tubulin, Cheng et al.206 showed that stabilization of tubulin is sufficient to induce modest cardiac hypertrophy, which is then further exacerbated upon pressure overload. These findings appear consistent with that of Fassett et al.,30,42 where increased microtubule stability upon genetic manipulation of adenosine metabolism is associated with compensated hypertrophy at baseline and worsening cardiac function upon modest aortic constriction of murine hearts. Thus, there is evidence in the literature that microtubules are both necessary and sufficient for the cardiac hypertrophic response, although we consider the evidence stronger for the former.
Given their promiscuity in the cell, it is likely that microtubules could mediate the hypertrophic response via multiple independent pathways. For example, microtubules help transport sarcomeric mRNAs for local translation and incorporation into myofibrils, which may be important for building new sarcomeres during hypertrophic growth.75,207 They can also serve as mechanotransducers, transmitting signals such as cell stretch to downstream effectors79–81,208 that may regulate hypertrophic gene expression. Dissecting the molecular mechanisms by which microtubules mediate cardiomyocyte hypertrophy will require more nuanced investigation into the numerous components involved in prosecuting a hypertrophic stimulus, and the specific subsets of microtubules that may enable this response. While there is considerable evidence that destabilizing microtubules can blunt the hypertrophic response, it remains an open question as to whether destabilization could reverse established cardiac remodeling, and whether such a treatment may be tolerated.
Microtubules and protein turnover
Continuous elimination of misfolded or damaged proteins and organelles is of particular importance in the cardiomyocyte, given its long lifetime (see review209). If the recycling machinery becomes overwhelmed, the accumulation of misfolded protein is associated with and can cause heart failure.210 The degradation of proteins in cardiac muscle is predominately carried out by two proteolytic systems: the ubiquitin-proteasome system (UPS) and autophagy (see review211), both of which depend on microtubule-based transport for the collection and clearance of waste material.
Proteins are marked for elimination by the UPS system by post-translational ubiquitination, but if ubiquitinated proteins are not degraded by proteasomes they can accumulate and are transported into perinuclear structures known as aggresomes.212 Aggresome formation is a microtubule-dependent process requiring dynein-mediated transport, and typically occurs in close proximity to MTOCs.213–216 Mutations in UPS components are associated with hereditary hypertrophic and dilated cardiomyopathy, and can result in proteotoxicity, endoplasmic reticulum (ER) stress and autophagic activation (see review217).
The other primary method of waste clearance is autophagy, which is driven by the fusion of lysosomes with autophagosomes to form an autolysosome that degrades proteins and organelles. Microtubules regulate multiple stages in both basal and stress-induced autophagy (see review218), including the formation of autophagosomes,219,220 dynein-mediated retrograde transport of the autophagosome,221 and autophagosome-lysosome fusion.222–224 Thus, an intact microtubule network is necessary at multiple levels to maintain autophagic flux.
As a regulator of microtubule-based transport, there is increasing evidence that microtubule PTMs can potently influence autophagic flux. Tubulin hyperacetylation was found to be protective against cardiac proteotoxicity by promoting autophagy in a mouse model of desminopathy.210 Microtubule acetylation may activate autophagy by increasing the polymerized microtubule pool, enhancing the recruitment and movement of kinesin 1 and dynein,225 and triggering phosphorylation of JNK which promotes the association of factors necessary for initiating autophagosome formation.220,226 Microtubule detyrosination has also been implicated in autophagy during starvation.224,227 A small subset of detyrosinated microtubules was found to be optimal to recruit and concentrate lysosomes via kinesin-1, which encouraged fusion with autophagosomes and protein degradation. In this model, an appropriate level of detyrosination allows for optimal autophagic flux, as too much or too little compromises the process by either dispersing and stalling lysosomes across the microtubule network, or by preventing their recruitment to microtubules, respectively.
While considered as largely independent systems targeting proteins for degradation in the proteasome and lysosome, the UPS and autophagy execute coordinated protein degradation with shared molecular machinery.228 Methods to increase the efficiency of proteasomal and/or autophagic activity have demonstrated cardioprotective potential,229–233 which should be considered when designing any microtubule-based approach to combat cardiac disease states, particularly given the regulation of autophagy by tubulin PTMs.
Microtubule-based therapies
Extrapolating from the literature reviewed above, tailored tuning of the microtubule network could provide a means to increase power output, blunt hypertrophy, enhance protein recycling, reduce oxidative stress, and prevent arrhythmia. Yet accomplishing all this in a single therapy seems a tall order (or perhaps pure fantasy), and at minimum will require a highly nuanced understanding of microtubules in the heart.
To date, therapeutic interventions targeting microtubules for the treatment of cardiovascular disease have been restricted to colchicine treatment. While this is a blunt tool, beneficial effects on the heart have been demonstrated in numerous animal studies, such as alleviating hypertrophy44,126,153,199 and improving systolic function.41,42,130 More granular manipulations, such as pharmaceutical inhibition of detyrosination with parthenolide, can acutely lower stiffness and improve contractile function of failing human cardiomyocytes, but still produces off-target alterations to E-C coupling,23 and is associated with complex immunological231 and oxidative responses234 that may or may not be due to its inhibition of detyrosination. Taxanes (microtubule stabilizers) commonly used in chemotherapy are associated with cardiomyopathy, conduction abnormalities, and impaired contractility, particularly when combined with anthracyclines.235,236 Longitudinal, in vivo imaging of non-failing patient hearts before and after this chemotherapeutic regiment indicate compromised diastolic function,237 suggesting that stabilizing cardiac microtubules can be detrimental in humans.
In patients, meta-analysis suggests long-term, low-dose colchicine is associated with reduced risk of overall cardiovascular events, although establishing efficacy for modifying the course of coronary artery disease or heart failure requires further investigation.238 Moreover, these benefits in patients likely arise largely due to colchicine's anti-inflammatory effects mediated through non-muscle cells. To achieve an appreciable depolymerization of cardiac microtubules (measured as an increase in the ratio of free: polymerized tubulin), a dosage of more than 0.4 mg/kg/day was required with either chronic or acute administration in animal models.41,42,45,130,158,205 This is more than ∼20 times the maximal recommended dosage of colchicine (1.2 mg/day) approved for humans for the treatment of gout,239 which must be kept low to minimize gastrointestinal side-effects. With a half-life of less than 20 min, plasma colchicine peaks at ∼2 µM within minutes and rapidly drops to 0.25 µM within an hour of treatment at maximal dosage.240 Thus, plasma levels suggest it unlikely that the dosage of colchicine tolerated in humans will significantly depolymerize cardiac microtubules and explain the beneficial effects observed in patients. We should note, however, that intracellular concentrations may easily differ from plasma levels, and subtle destabilization of myocyte microtubules could still have significant consequences.
Regardless, grossly targeting the microtubule network could lead to numerous undesirable effects, and more specific pharmacologic and genetic therapies should be pursued. Cardiac decompensation is associated with the stabilization of microtubules, a shift in their post-translational profile, and altered associations with MAPs, which has diverse implications for microtubule regulation of the dyad, myofilaments, mitochondria, and intercalated disc (to name a few). A clearer picture of how microtubules, their PTMs and MAPs impact these processes in the myocyte will likely inform more effective and specific therapies to treat patients with heart disease.
Closing thoughts
Not surprisingly, as cytoskeletal directors of cargo transport through the myocyte, microtubules are implicated in diverse cellular processes, and several areas appear particularly ripe for further investigation in cardiac microtubule biology. The role of microtubules in mechanotransduction and proteostasis appears critical for the acute and chronic adaptation of the heart to changing loads, and may be central to delineating between physiological and pathological remodeling. A more detailed understanding of how microtubules shape and maintain the unique membrane systems of the cardiomyocyte—and the effect this has on E-C coupling and arrhythmia—is required. Single cell studies and the cell-free reductionist assays that are the bulwark of the cytoskeletal field are instrumental in revealing molecular mechanisms and should be applied to the problems above. Yet it is also essential to test how targeting microtubules, MAPs and their modifying enzymes impact cardiac function at the organ level and in vivo, and to evaluate the long-term efficacy of more refined microtubule-based therapies.
A careful reader may note that much of our knowledge on cardiac microtubules has been inferred from studies using blunt pharmacological reagents to depolymerize or artificially stabilize the network. While these tools are practical and useful in identifying a contribution of the microtubule network to the process at hand, it is often difficult to glean mechanistic clarity, due largely to the fact that microtubules contribute to multiple, intermingling processes in the cell. Greater mechanistic insight requires that these approaches be combined (or replaced) with more refined techniques, such as live-cell monitoring of specific populations of dynamic and stable microtubules, genetically modulating the enzymes, MAPs and tubulin isotypes that confer unique functionality to subsets of microtubules, or perturbing specific microtubule motors and their ability to traffic particular cargo throughout the cell. An excellent toolkit for interrogating microtubule biology has been developed over the past 20 years, but its application to cardiomyocyte biology has somewhat lagged. We hope that beyond serving as a useful reference point, this review will help stimulate investigation into such a rich area of myocyte biology, with broad implications for cardiac health and disease.
ACKNOWLEDGEMENTS
The authors thank Dr. Patrick Robison for useful discussion and editing of the manuscript.
Authors’ contributions
MAC, CYC and BLP contributed to the design and writing of this manuscript; MAC illustrated the manuscript with direction from CYC and BLP.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by NHLBI grant R01-HL133080 to BLP, NIAMS grant 5T32AR053461 to MAC, and American Heart Association Fellowship 19POST34400012 to CYC.
References
- 1.Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc National Acad Sci 2014; 111:8850–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisén J. Dynamics of cell generation and turnover in the human heart. Cell 2015; 161:1566–75 [DOI] [PubMed] [Google Scholar]
- 3.Belanto JJ, Mader TL, Eckhoff MD, Strandjord DM, Banks GB, Gardner MK, Lowe DA, Ervasti JM. Microtubule binding distinguishes dystrophin from utrophin. Proc National Acad Sci 2014; 111:5723–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr JP, Robison P, Shi G, Bogush AI, Kempema AM, Hexum JK, Becerra N, Harki DA, Martin SS, Raiteri R, Prosser BL, Ward CW. Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat Commun 2015; 6:8526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prosser BL, Ward CW, Lederer W. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 2011; 333:1440–5 [DOI] [PubMed] [Google Scholar]
- 6.Grimes KM, Prasad V, McNamara JW. Supporting the heart: functions of the cardiomyocyte’s non-sarcomeric cytoskeleton. J Mol Cell Cardiol. 2019; 131:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gittes F, Mickey B, Nettleton J, Howard J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J Cell Biology 1993; 120:923–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block J, Schroeder V, Pawelzyk P, Willenbacher N, Köster S. Physical properties of cytoplasmic intermediate filaments. Biochim Biophys Acta Mol Cell Res 2015; 1853:3053–64 [DOI] [PubMed] [Google Scholar]
- 9.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 1997; 13:83–117 [DOI] [PubMed] [Google Scholar]
- 10.Franker MA, Hoogenraad CC. Microtubule-based transport – basic mechanisms, traffic rules and role in neurological pathogenesis. J Cell Sci 2013; 126:2319–29 [DOI] [PubMed] [Google Scholar]
- 11.Goldstein M, Entman M. Microtubules in mammalian heart muscle. J Cell Biol 1979; 80:183–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steele DF, Fedida D. Cytoskeletal roles in cardiac ion channel expression. Biochim Biophys Acta Biomembr 2014; 1838:665–73 [DOI] [PubMed] [Google Scholar]
- 13.Steele DF, Eldstrom J, Fedida D. Mechanisms of cardiac potassium channel trafficking. J Physiology 2007; 582:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meunier B, Quaranta M, Daviet L, Hatzoglou A, Leprince C. The membrane-tubulating potential of amphiphysin 2/BIN1 is dependent on the microtubule-binding cytoplasmic linker protein 170 (CLIP-170). Eur J Cell Biol 2009; 88:91–102 [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Chen B, Guo A, Zhu Y, Miller JD, Gao S, Yuan C, Kutschke W, Zimmerman K, Weiss RM, Wehrens XH, Hong J, Johnson FL, Santana LF, Anderson ME, Song L-S. Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure. Circulation 2014; 129:1742–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S-S, Shaw RM. Trafficking highways to the intercalated disc: new insights unlocking the specificity of connexin 43 localization. Cell Commun Adhes 2014; 21:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robison P, Caporizzo MA, Ahmadzadeh H, Bogush AI, Chen C, Margulies KB, Shenoy VB, Prosser BL. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 2016; 352:aaf0659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporizzo M, Chen C, Salomon A, Margulies KB, Prosser BL. Microtubules provide a viscoelastic resistance to myocyte motion. Biophys J 2018; 115:1796–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsutsui H, Ishihara K, Cooper G. Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science 1993; 260:682–7 [DOI] [PubMed] [Google Scholar]
- 20.Padmakumar V, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou I. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci 2005; 118:3419–30 [DOI] [PubMed] [Google Scholar]
- 21.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm. J Cell Biol 2006; 172:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroud MJ. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiomyopathy. Biophysical Rev 2018; 10:1033–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C, Caporizzo MA, Bedi K, Vite A, Bogush AI, Robison P, Heffler JG, Salomon AK, Kelly NA, Babu A, Morley MP, Margulies KB, Prosser BL. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat Med 2018; 260:682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zile MR, Green GR, Schuyler GT, Aurigemma GP, Miller DC, Cooper G. Cardiocyte cytoskeleton in patients with left ventricular pressure overload hypertrophy. J Am Coll Cardiol 2001; 37:1080–4 [DOI] [PubMed] [Google Scholar]
- 25.Witjas-Paalberends RE, Güçlü A, Germans T, Knaapen P, Harms HJ, Vermeer A, Christiaans I, Wilde A, Remedios C, Lammertsma AA, van Rossum AC, Stienen G, van Slegtenhorst M, Schinkel AF, Michels M, Ho CY, Poggesi C, van der Velden J. Gene-specific increase in the energetic cost of contraction in hypertrophic cardiomyopathy caused by thick filament mutations. Cardiovasc Res 2014; 103:248–57 [DOI] [PubMed] [Google Scholar]
- 26.Bollen IA, van der Meulen M, de Goede K, Kuster DW, Dalinghaus M, van der Velden J. Cardiomyocyte hypocontractility and reduced myofibril density in end-stage pediatric cardiomyopathy. Front Physiol 2017; 8:1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinnakkannu P, Samanna V, Cheng G, Ablonczy Z, Baicu CF, Bethard JR, Menick DR, Kuppuswamy D, Cooper G. Site-specific microtubule-associated protein 4 dephosphorylation causes microtubule network densification in pressure overload cardiac hypertrophy. J Biol Chem 2010; 285:21837–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng G, Takahashi M, Shunmugavel A, Wallenborn GJ, DePaoli-Roach AA, Gergs U, Neumann J, Kuppuswamy D, Menick DR, Cooper G. Basis for MAP4 dephosphorylation-related microtubule network densification in pressure overload cardiac hypertrophy. J Biol Chem 2010; 285:38125–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fassett JT, Xu X, Kwak D, Wang H, Liu X, Hu X, Bache RJ, Chen Y. Microtubule actin cross-linking factor 1 regulates cardiomyocyte microtubule distribution and adaptation to hemodynamic overload. PLoS One 2013; 8:e73887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fassett J, Xu X, Kwak D, Zhu G, Fassett EK, Zhang P, Wang H, Mayer B, Bache RJ, Chen Y. Adenosine kinase attenuates cardiomyocyte microtubule stabilization and protects against pressure overload-induced hypertrophy and LV dysfunction. J Mol Cell Cardiol 2019; 130:49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper G. Cytoskeletal networks and the regulation of cardiac contractility: microtubules, hypertrophy, and cardiac dysfunction. Am J Physiol Heart Circ Physiol 2006; 291:H1003–14 [DOI] [PubMed] [Google Scholar]
- 32.Webster D, Gundersen G, Bulinski J, Borisy G. Differential turnover of tyrosinated and detyrosinated microtubules. Proc National Acad Sci 1987; 84:9040–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Tian G, Cowan NJ, Cabral F. Mutations affecting β-tubulin folding and degradation. J Biol Chem 2006; 281:13628–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voelzmann A, Hahn I, Pearce SP, Sánchez-Soriano N, Prokop A. A conceptual view at microtubule plus end dynamics in neuronal axons. Brain Res Bull 2016; 126:226–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuel J, Schwartz K, Lompre A, Delcayre C, Marotte F, Swynghedauw B, Rappaport L. Immunological quantitation and localization of tubulin in adult rat heart isolated myocytes. Eur J Cell Biol 1983; 31:99–106 [PubMed] [Google Scholar]
- 36.Hoebeke J, Nijen VG, Brabander DM. Interaction of oncodazole (R 17934), a new anti-tumoral drug, with rat brain tubulin. Biochem Biophys Res Commun 1976; 69:319–24 [DOI] [PubMed] [Google Scholar]
- 37.Sato H, Nagai T, Kuppuswamy D, Narishige T, Koide M, Menick D, Cooper G. Microtubule stabilization in pressure overload cardiac hypertrophy. J Cell Biol 1997; 139:963–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belmadani S, Poüs C, Fischmeister R, Méry P-F. Post-translational modifications of tubulin and microtubule stability in adult rat ventricular myocytes and immortalized HL-1 cardiomyocytes. Mol Cell Biochem 2004; 258:35–48 [DOI] [PubMed] [Google Scholar]
- 39.Vasquez R, Howell B, Yvon A, Wadsworth P, Cassimeris L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. MBoC 1997; 8:973–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tagawa H, Koide M, Sato H, Zile MR, Carabello BA, George CI. Cytoskeletal role in the transition from compensated to decompensated hypertrophy during adult canine left ventricular pressure overloading. Circ Res 1998; 82:751–61 [DOI] [PubMed] [Google Scholar]
- 41.Koide M, Hamawaki M, Narishige T, Sato H, Nemoto S, DeFreyte G, Zile MR, George CI, Carabello BA. Microtubule depolymerization normalizes in vivo myocardial contractile function in dogs with pressure-overload left ventricular hypertrophy. Circulation 2000; 102:1045–52 [DOI] [PubMed] [Google Scholar]
- 42.Fassett JT, Xu X, Hu X, Zhu G, French J, Chen Y, Bache RJ. Adenosine regulation of microtubule dynamics in cardiac hypertrophy. Am J Physiol Heart Circ Physiol 2009; 297:H523–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fassett JT, Hu X, Xu X, Lu Z, Zhang P, Chen Y, Bache RJ. AMPK attenuates microtubule proliferation in cardiac hypertrophy. Am J Physiol Heart Circ Physiol 2013; 304:H749–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tagawa H, Rozich JD, Tsutsui H, Narishige T, Kuppuswamy D, Sato H, Rmott PJ, Koide M, Cooper G. Basis for increased microtubules in pressure-hypertrophied cardiocytes. Circulation 1996; 93:1230–43 [DOI] [PubMed] [Google Scholar]
- 45.Takahashi M, Tsutsui H, Tagawa H, Igarashi-Saito K, Imanaka-Yoshida K, Takeshita A. Microtubules are involved in early hypertrophic responses of myocardium during pressure overload. Am J Physiol Heart Circ Physiol 1998; 275:H341–8 [DOI] [PubMed] [Google Scholar]
- 46.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle 2007; 6:2152–60 [DOI] [PubMed] [Google Scholar]
- 47.Gadadhar S, Bodakuntla S, Natarajan K, Janke C. The tubulin code at a glance. J Cell Sci 2017; 130:199471jcs. [DOI] [PubMed] [Google Scholar]
- 48.Yu I, Garnham CP, Roll-Mecak A. Writing and reading the tubulin code. J Biol Chem 2015; 290:17163–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park JH, Roll-Mecak A. The tubulin code in neuronal polarity. Curr Opin Neurobiol 2018; 51:95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Portran D, Schaedel L, Xu Z, Théry M, Nachury MV. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol 2017; 19:391–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perdiz D, Mackeh R, Poüs C, Baillet A. The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell Signal 2011; 23:763–71 [DOI] [PubMed] [Google Scholar]
- 52.Howes SC, Alushin GM, Shida T, Nachury MV, Nogales E. Effects of tubulin acetylation and tubulin acetyltransferase binding on microtubule structure. MBoC 2014; 25:257–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Z, Schaedel L, Portran D, Aguilar A, Gaillard J, Marinkovich PM, Théry M, Nachury MV. Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 2017; 356:328–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schaedel L, John K, Gaillard J, Nachury MV, Blanchoin L, Théry M. Microtubules self-repair in response to mechanical stress. Nat Mater 2015; 14:1156–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nieuwenhuis J, Adamopoulos A, Bleijerveld OB, Mazouzi A, Stickel E, Celie P, Altelaar M, Knipscheer P, Perrakis A, Blomen VA, Brummelkamp TR. Vasohibins encode tubulin detyrosinating activity. Science 2017; 358:1453–6 [DOI] [PubMed] [Google Scholar]
- 56.Aillaud C, Bosc C, Peris L, Bosson A, Heemeryck P, Dijk JV, Friec JL, Boulan B, Vossier F, Sanman LE, Syed S, Amara N, Couté Y, Lafanechère L, Denarier E, Delphin C, Pelletier L, Humbert S, Bogyo M, Andrieux A, Rogowski K, Moutin M-J. Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 2017; 358:1448–53 [DOI] [PubMed] [Google Scholar]
- 57.Prota AE, Magiera MM, Kuijpers M, Bargsten K, Frey D, Wieser M, Jaussi R, Hoogenraad CC, Kammerer RA, Janke C, Steinmetz MO. Structural basis of tubulin tyrosination by tubulin tyrosine ligase. J Cell Biol 2013; 200:259–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Redeker V. Mass spectrometry analysis of C-terminal posttranslational modifications of tubulins. Methods Cell Biol 2010; 95:77–103 [DOI] [PubMed] [Google Scholar]
- 59.Kreitzer G, Liao G, Gundersen GG. Detyrosination of tubulin regulates the interaction of intermediate filaments with microtubules in vivo via a kinesin-dependent mechanism. MBoC 1999; 10:1105–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments: selective binding of kinesin to detyrosinated tubulin and vimentin. J Biol Chem 1998; 273:9797–803 [DOI] [PubMed] [Google Scholar]
- 61.Valenstein ML, Roll-Mecak A. Graded control of microtubule severing by tubulin glutamylation. Cell 2016; 164:911–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vemu A, Szczesna E, Zehr EA, Spector JO, Grigorieff N, Deaconescu AM, Roll-Mecak A. Severing enzymes amplify microtubule arrays through lattice GTP-tubulin incorporation. Science 2018; 361:eaau1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Magiera MM, Bodakuntla S, Žiak J, Lacomme S, Sousa P, Leboucher S, Hausrat TJ, Bosc C, Andrieux A, Kneussel M, Landry M, Calas A, Balastik M, Janke C. Excessive tubulin polyglutamylation causes neurodegeneration and perturbs neuronal transport. Embo J 2018; 37:e100440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shashi V, Magiera MM, Klein D, Zaki M, Schoch K, Rudnik‐Schöneborn S, Norman A, Neto OLA, Dusl M, Yuan X, Bartesaghi L, Marco PD, Alfares AA, Marom R, Arold ST, Guzmán‐Vega FJ, Pena LDM, Smith EC, Steinlin M, Babiker MOE, Mohassel P, Foley AR, Donkervoort S, Kaur R, Ghosh PS, Stanley V, Musaev D, Nava C, Mignot C, Keren B, Scala M, Tassano E, Picco P, Doneda P, Fiorillo C, Issa MY, Alassiri A, Alahmad A, Gerard A, Liu P, Yang Y, Ertl‐Wagner B, Kranz PG, Wentzensen IM, Stucka R, Stong N, Allen AS, Goldstein DB, Schoser B, Rösler KM, Alfadhel M, Capra V, Chrast R, Strom TM, Kamsteeg E-J, Bönnemann CG, Gleeson JG, Martini R, Janke C, Senderek J. Loss of tubulin deglutamylase CCP1 causes infantile‐onset neurodegeneration. Embo J 2018; 37:e100540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Monroy BY, Sawyer DL, Ackermann BE, Borden MM, Tan TC, Ori-McKenney KM. Competition between microtubule-associated proteins directs motor transport. Nat Commun 2018; 9:1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodson HV, Jonasson EM. Microtubules and microtubule-associated proteins. Cold Spring Harb Perspect Biol 2018; 10:a022608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magiera MM, Singh P, Gadadhar S, Janke C. Tubulin posttranslational modifications and emerging links to human disease. Cell 2018; 173:1323–7 [DOI] [PubMed] [Google Scholar]
- 68.Barisic M, e, Sousa R, Tripathy SK, Magiera MM, Zaytsev AV, Pereira AL, Janke C, Grishchuk EL, Maiato H. Microtubule detyrosination guides chromosomes during mitosis. Science 2015; 348:799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaul N, Soppina V, Verhey KJ. Effects of α-tubulin K40 acetylation and detyrosination on kinesin-1 motility in a purified system. Biophys J 2014; 106:2636–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dunn S, Morrison EE, Liverpool TB, Molina-París C, Cross RA, Alonso MC, Peckham M. Differential trafficking of Kif5c on tyrosinated and detyrosinated microtubules in live cells. J Cell Sci 2008; 121:1085–95 [DOI] [PubMed] [Google Scholar]
- 71.Nirschl JJ, Magiera MM, Lazarus JE, Janke C, Holzbaur E. α-Tubulin tyrosination and CLIP-170 phosphorylation regulate the initiation of dynein-driven transport in neurons. Cell Reports 2016; 14:2637–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Webb JL, Ravikumar B, Rubinsztein DC. Microtubule disruption inhibits autophagosome-lysosome fusion: implications for studying the roles of aggresomes in polyglutamine diseases. Int J Biochem Cell Biology 2004; 36:2541–50 [DOI] [PubMed] [Google Scholar]
- 73.Lin SX, Gundersen GG, Maxfield FR. Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (Glu) microtubules and kinesin. MBoC 2002; 13:96–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erck C, Peris L, Andrieux A, Meissirel C, Gruber AD, Vernet M, Schweitzer A, Saoudi Y, Pointu H, Bosc C, Salin PA, Job D, Wehland J. A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc National Acad Sci 2005; 102:7853–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scholz D, Baicu CF, Tuxworth WJ, Xu L, Kasiganesan H, Menick DR, George CI. Microtubule-dependent distribution of mRNA in adult cardiocytes. Am J Physiol Heart Circ Physiol 2008; 294:H1135–44 [DOI] [PubMed] [Google Scholar]
- 76.Takahashi M, Shiraishi H, Ishibashi Y, Blade KL, Rmott PJ, Menick DR, Kuppuswamy D, Cooper G. Phenotypic consequences of β1-tubulin expression and MAP4 decoration of microtubules in adult cardiocytes. Am J Physiol Heart Circ Physiol 2003; 285:H2072–83 [DOI] [PubMed] [Google Scholar]
- 77.Scholz D, Rmott P, Garnovskaya M, Gallien TN, Huettelmaier S, DeRienzo C, Cooper G. Microtubule-associated protein-4 (MAP-4) inhibits microtubule-dependent distribution of mRNA in isolated neonatal cardiocytes. Cardiovasc Res 2006; 71:506–16 [DOI] [PubMed] [Google Scholar]
- 78.Ramkumar A, Jong BY, Ori‐McKenney KM. ReMAPping the microtubule landscape: how phosphorylation dictates the activities of microtubule‐associated proteins. Dev Dyn 2018; 247:138–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molkentin JD. Calcineurin–NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res 2004; 63:467–75 [DOI] [PubMed] [Google Scholar]
- 80.Zhang T, Brown J. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res 2004; 63:476–86 [DOI] [PubMed] [Google Scholar]
- 81.Dorn GW, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest 2005; 115:527–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li L, Zhang Q, Zhang X, Zhang J, Wang X, Ren J, Jia J, Zhang D, Jiang X, Zhang J, Mei H, Chen B, Hu J, Huang Y. Microtubule associated protein 4 phosphorylation leads to pathological cardiac remodeling in mice. Ebiomedicine 2018; 37:221–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Betrie AH, Ayton S, Bush AI, Angus JA, Lei P, Wright CE. Evidence of a cardiovascular function for microtubule-associated protein tau. J Alzheimer’s Dis 2017; 56:849–60 [DOI] [PubMed] [Google Scholar]
- 84.Gu Y, Oyama F, Ihara Y. Tau is widely expressed in rat tissues. J Neurochem 1996; 67:1235–44 [DOI] [PubMed] [Google Scholar]
- 85.Sudo H, Baas PW. Acetylation of microtubules influences their sensitivity to severing by katanin in neurons and fibroblasts. J Neurosci 2010; 30:7215–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lasorsa A, Malki I, Cantrelle F-X, Merzougui H, Boll E, Lambert J-C, Landrieu I. Structural basis of tau interaction with BIN1 and regulation by tau phosphorylation. Front Mol Neurosci 2018; 11:421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang J, Grundke‐Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in alzheimer neurofibrillary degeneration. Eur J Neurosci 2007; 25:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bearce EA, Erdogan B, Lowery L. TIPsy tour guides: how microtubule plus-end tracking proteins (+TIPs) facilitate axon guidance. Front Cell Neurosci 2015; 9:241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ran J, Luo Y, Zhang Y, Yang Y, Chen M, Liu M, Li D, Zhou J. Phosphorylation of EB1 regulates the recruitment of CLIP-170 and p150 glued to the plus ends of astral microtubules. Oncotarget 2017; 8:9858–9867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dixit R, Barnett B, Lazarus JE, Tokito M, Goldman YE, Holzbaur EL. Microtubule plus-end tracking by CLIP-170 requires EB1. Proc Natl Acad Sci USA 2009; 106:492–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peris L, Thery M, Fauré J, Saoudi Y, Lafanechère L, Chilton JK, Gordon-Weeks P, Galjart N, Bornens M, Wordeman L, Wehland J, Andrieux A, Job D. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J Cell Biol 2006; 174:839–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peris L, Wagenbach M, Lafanechère L, Brocard J, Moore AT, Kozielski F, Job D, Wordeman L, Andrieux A. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol 2009; 185:1159–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wloga D, Joachimiak E, Fabczak H. Tubulin post-translational modifications and microtubule dynamics. Int J Mol Sci 2017; 18:2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Svitkina T, Verkhovsky A, Borisy G. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biology 1996; 135:991–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Konieczny P, Fuchs P, Reipert S, Kunz WS, Zeöld A, Fischer I, Paulin D, Schröder R, Wiche G. Myofiber integrity depends on desmin network targeting to Z-disks and costameres via distinct plectin isoforms. J Cell Biol 2008; 181:667–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nicolas CS, Park K-H, Harchi A, Camonis J, Kass RS, Escande D, Mérot J, Loussouarn G, Bouffant F, Baró I. IKs response to protein kinase A-dependent KCNQ1 phosphorylation requires direct interaction with microtubules. Cardiovasc Res 2008; 79:427–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 2006; 281:26391–9 [DOI] [PubMed] [Google Scholar]
- 98.Limas C, Limas C. Rapid recovery of cardiac beta-adrenergic receptors after isoproterenol-induced ‘down’-regulation. Circ Res 1984; 55:524–31 [DOI] [PubMed] [Google Scholar]
- 99.Limas CJ, Limas C. Involvement of microtubules in the isoproterenol-induced ‘down’-regulation of myocardial β-adrenergic receptors. Biochim Biophys Acta Biomembr 1983; 735:181–4 [DOI] [PubMed] [Google Scholar]
- 100.Eckel J, Reinauer H. Effect of vinblastine on the insulin-receptor interaction in mammalian heart muscle. Biochem Bioph Res Co 1980; 92:1403–8 [DOI] [PubMed] [Google Scholar]
- 101.Akhavan A. Motorized traffic of a cardiac ion channel: implication of conventional kinesin in transport of Kv1.5 channels to the plasma membrane. J Physiol (Lond) 2010; 588:903–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Drum B, Yuan C, Li L, Liu Q, Wordeman L, Santana FL. Oxidative stress decreases microtubule growth and stability in ventricular myocytes. J Mol Cell Cardiol 2016; 93:32–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi W, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res 2005; 97:363–71 [DOI] [PubMed] [Google Scholar]
- 104.Loewen ME, Wang Z, Eldstrom J, Zadeh A, Khurana A, Steele DF, Fedida D. Shared requirement for dynein function and intact microtubule cytoskeleton for normal surface expression of cardiac potassium channels. Am J Physiol Heart Circ Physiol 2009; 296:H71–83 [DOI] [PubMed] [Google Scholar]
- 105.Dhani SU, Mohammad-Panah R, Ahmed N, Ackerley C, Ramjeesingh M, Bear CE. Evidence for a functional interaction between the ClC-2 chloride channel and the retrograde motor dynein complex. J Biol Chem 2003; 278:16262–70 [DOI] [PubMed] [Google Scholar]
- 106.Casini S, Tan HL, Demirayak I, Remme C, Amin AS, Scicluna BP, Chatyan H, Ruijter JM, Bezzina CR, van Ginneken A, Veldkamp MW. Tubulin polymerization modifies cardiac sodium channel expression and gating. Cardiovasc Res 2010; 85:691–700 [DOI] [PubMed] [Google Scholar]
- 107.Peter AK, Cheng H, Ross RS, Knowlton KU, Chen J. The costamere bridges sarcomeres to the sarcolemma in striated muscle. Prog Pediatr Cardiol 2011; 31:83–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Craig SW, Pardo JV. Gamma actin, spectrin, and intermediate filament proteins colocalize with vinculin at costameres, myofibril‐to‐sarcolemma attachment sites. Cell Motil 1983; 3:449–62 [DOI] [PubMed] [Google Scholar]
- 109.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature 1990; 345:315–9 [DOI] [PubMed] [Google Scholar]
- 110.Ervasti JM. Costameres: the achilles’ heel of herculean muscle. J Biol Chem 2003; 278:13591–4 [DOI] [PubMed] [Google Scholar]
- 111.Rezniczek GA, Konieczny P, Nikolic B, Reipert S, Schneller D, Abrahamsberg C, Davies KE, Winder SJ, Wiche G. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with β-dystroglycan. J Cell Biol 2007; 176:965–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rybakova I, Amann K, Ervasti J. A new model for the interaction of dystrophin with F-actin. J Cell Biol 1996; 135:661–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prins KW, Humston JL, Mehta A, Tate V, Ralston E, Ervasti JM. Dystrophin is a microtubule-associated protein. J Cell Biol 2009; 186:363–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rahimov F, Kunkel LM. Cellular and molecular mechanisms underlying muscular dystrophy. J Cell Biol 2013; 201:499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 2008; 135:1189–200 [DOI] [PubMed] [Google Scholar]
- 116.Ayalon G, Hostettler JD, Hoffman J, Kizhatil K, Davis JQ, Bennett V. Ankyrin-B interactions with spectrin and dynactin-4 are required for dystrophin-based protection of skeletal muscle from exercise injury. J Biol Chem 2011; 286:7370–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen Y-W, Raiteri R, Lederer JW, Dorsey SG, Ward CW. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal. 2012;5:ra56 [DOI] [PMC free article] [PubMed]
- 118.Prado FP, dos Santos DO, Blefari V, Silva CA, Machado J, do Kettelhut I, Ramos SG, Baruffi M, Salgado HC, Prado CM. Early dystrophin loss is coincident with the transition of compensated cardiac hypertrophy to heart failure. PLoS One 2017; 12:e0189469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Scriven D, Asghari P, Moore E. Microarchitecture of the dyad. Cardiovasc Res 2013; 98:169–76 [DOI] [PubMed] [Google Scholar]
- 120.Bers DM. Cardiac excitation-contraction coupling. Nature 2002; 415:415198a [DOI] [PubMed] [Google Scholar]
- 121.Hong T-T, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM. BIN1 localizes the L-type calcium channel to cardiac t-tubules. Plos Biol 2010; 8:e1000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beavers DL, Landstrom AP, Chiang DY, Wehrens X. Emerging roles of junctophilin-2 in the heart and implications for cardiac diseases. Cardiovasc Res 2014; 103:198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa G-C, Farsad K, Wenk MR, Camilli P. Amphiphysin 2 (Bin1) and t-tubule biogenesis in muscle. Science 2002; 297:1193–6 [DOI] [PubMed] [Google Scholar]
- 124.Hong T, Yang H, Zhang S-S, Cho H, Kalashnikova M, Sun B, Zhang H, Bhargava A, Grabe M, Olgin J, Gorelik J, Marbán E, Jan LY, Aw R. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med 2014; 20:624–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mata A, Tajada S, O’Dwyer S, Matsumoto C, Dixon RE, Hariharan N, Moreno CM, Santana L. BIN1 Induces the formation of t‐tubules and adult‐like Ca2+ release units in developing cardiomyocytes. Stem Cells 2019; 37:54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fu Y, Hong T. BIN1 regulates dynamic t-tubule membrane. Biochim Biophys Acta Mol Cell Res 2016; 1863:1839–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Basheer WA, Shaw RM. Connexin 43 and CaV1.2 ion channel trafficking in healthy and diseased myocardium. Circ Arrhythm Electrophysiol 2018; 9:e001357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vega AL, Yuan C, Votaw SV, Santana LF. Dynamic changes in sarcoplasmic reticulum structure in ventricular myocytes. J Biomed Biotechnol 2011; 2011:382586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Osseni A, Sébastien M, Sarrault O, Baudet M, Couté Y, Fauré J, Fourest-Lieuvin A, Marty I. Triadin and CLIMP-63 form a link between triads and microtubules in muscle cells. J Cell Sci 2016; 129:3744–55 [DOI] [PubMed] [Google Scholar]
- 130.Prins KW, Tian L, Wu D, Thenappan T, Metzger JM, Archer SL. Colchicine depolymerizes microtubules, increases junctophilin‐2, and improves right ventricular function in experimental pulmonary arterial hypertension. J Am Heart Assoc 2017;6:6 [DOI] [PMC free article] [PubMed]
- 131.Prins KW, Asp ML, Zhang H, Wang W, Metzger JM. Microtubule-mediated misregulation of junctophilin-2 underlies t-tubule disruptions and calcium mishandling in mdx mice. JACC Basic Transl Sci 2016; 1:122–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Prosser BL, Ward CW. Mechano-chemo transduction tunes the heartstrings. Sci Signal 2014; 7:pe7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Randazzo D, Khalique U, Belanto JJ, Kenea A, Talsness DM, Olthoff JT, Tran MD, Zaal KJ, Pak K, Pinal-Fernandez I, Mammen AL, Sackett D, Ervasti JM, Ralston E. Persistent upregulation of the β-tubulin tubb6, linked to muscle regeneration, is a source of microtubule disorganization in dystrophic muscle. Hum Mol Genet 2019; 28:1117–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Belanto JJ, Olthoff JT, Mader TL, Chamberlain CM, Nelson DM, McCourt PM, Talsness DM, Gundersen GG, Lowe DA, Ervasti JM. Independent variability of microtubule perturbations associated with dystrophinopathy. Hum Mol Genet 2016; 25:ddw318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nelson DM, Lindsay A, Judge LM, Duan D, Chamberlain JS, Lowe DA, Ervasti JM. Variable rescue of microtubule and physiological phenotypes in mdx muscle expressing different miniaturized dystrophins. Hum Mol Genet 2018; 27:2090–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Loehr J, Wang S, Cully TR, Pal R, Larina IV, Larin KV, Rodney GG. NADPH oxidase mediates microtubule alterations and diaphragm dysfunction in dystrophic mice. Elife 2018; 7:e31732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hu C, Tian Y, Xu H, Pan B, Terpstra EM, Wu P, Wang H, Li F, Liu J, Wang X. Inadequate ubiquitination-proteasome coupling contributes to myocardial ischemia-reperfusion injury. J Clin Invest 2018; 128:5294–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res 1998; 83:629–35 [DOI] [PubMed] [Google Scholar]
- 139.Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan Y-N, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 2007; 128:547–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res 2009; 104:1103–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc National Acad Sci 2007; 104:20512–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Matsushita S, Tran V, Pelleg A, Wechsler AS, Kresh YJ. Pacing-induced cardiac gap junction remodeling: modulation of connexin43 phosphorylation state. Am J Ther 2009; 16:224–30 [DOI] [PubMed] [Google Scholar]
- 143.Smyth JW, Shaw RM. The gap junction life cycle. Heart Rhythm 2012; 9:151–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Macquart C, Jüttner R, Dour C, Chatzifrangkeskou M, Schmitt A, Gotthardt M, Bonne G, Muchir A. Microtubule cytoskeleton regulates Connexin 43 localization and cardiac conduction in cardiomyopathy caused by mutation in A-type lamins gene. Hum Mol Genet. 2018;ddy227 [DOI] [PubMed]
- 145.Parker K, Taylor LK, Atkinson B, Hansen DE, Wikswo JP. The effects of tubulin-binding agents on stretch-induced ventricular arrhythmias. Eur J Pharmacol 2001; 417:131–40 [DOI] [PubMed] [Google Scholar]
- 146.Rowinsky EK, Donehower RC. Paclitaxel (Taxol). N Engl J Med 1995; 332:1004–14 [DOI] [PubMed] [Google Scholar]
- 147.Madias C, Maron RJ, Supron S, Estes MN, Link MS. Cell membrane stretch and chest blow‐induced ventricular fibrillation: commotio cordis. J Cardiovasc Electr 2008; 19:1304–9 [DOI] [PubMed] [Google Scholar]
- 148.Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, Parker KK, Ingber DE, Weitz DA. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol 2006; 173:733–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Z, Cui J, Wong W, Li X, Xue W, Lin R, Wang J, Wang P, Tanner JA, Cheah KS, Wu W, Huang J-D. Kif5b controls the localization of myofibril components for their assembly and linkage to the myotendinous junctions. Development 2013; 140:617–26 [DOI] [PubMed] [Google Scholar]
- 150.Nishimura S, Nagai S, Katoh M, Yamashita H, Saeki Y, Okada J, Hisada T, Nagai R, Sugiura S. Microtubules modulate the stiffness of cardiomyocytes against shear stress. Circ Res 2006; 98:81–7 [DOI] [PubMed] [Google Scholar]
- 151.Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper G. Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ Res 1997; 80:281–9 [DOI] [PubMed] [Google Scholar]
- 152.Yamamoto S, Tsutsui H, Takahashi M, Ishibashi Y, Tagawa H, Imanaka-Yoshida K, Saeki Y, Takeshita A. Role of microtubules in the viscoelastic properties of isolated cardiac muscle. J Mol Cell Cardiol 1998; 30:1841–53 [DOI] [PubMed] [Google Scholar]
- 153.Zile MR, Richardson K, Cowles M, Buckley MJ, Koide M, Cowles BA, Gharpuray V, George CI. Constitutive properties of adult mammalian cardiac muscle cells. Circulation 1998; 98:567–79 [DOI] [PubMed] [Google Scholar]
- 154.Granzier HL, Wang K. Passive tension and stiffness of vertebrate skeletal and insect flight muscles: the contribution of weak cross-bridges and elastic filaments. Biophys J 1993; 65:2141–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zile MR, Koide M, Sato H, Ishiguro Y, Conrad CH, Buckley JM, Morgan JP, Cooper G. Role of microtubules in the contractile dysfunction of hypertrophied myocardium. J Am Coll Cardiol 1999; 33:250–60 [DOI] [PubMed] [Google Scholar]
- 156.Cicogna AC, Robinson KG, Conrad CH, Singh K, Squire R, Okoshi MP, Bing OH. Direct effects of colchicine on myocardial function. Hypertension 1999; 33:60–5 [DOI] [PubMed] [Google Scholar]
- 157.Collins JF, Pawloski DC, Davis MG, Ball N, Gerald DW, Walsh RA. The role of the cytoskeleton in left ventricular pressure overload hypertrophy and failure. J Mol Cell Cardiol 1996; 28:1435–43 [DOI] [PubMed] [Google Scholar]
- 158.Scopacasa BS, Teixeira VP, Franchini KG. Colchicine attenuates left ventricular hypertrophy but preserves cardiac function of aortic-constricted rats. J Appl Physiol 2003; 94:1627–33 [DOI] [PubMed] [Google Scholar]
- 159.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci 2007; 120:838–48 [DOI] [PubMed] [Google Scholar]
- 160.Liu X, Weaver D, Shirihai O, Hajnóczky G. Mitochondrial ‘kiss‐and‐run’: interplay between mitochondrial motility and fusion–fission dynamics. Embo J 2009; 28:3074–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang C, Du W, Su Q, Zhu M, Feng P, Li Y, Zhou Y, Mi N, Zhu Y, Jiang D, Zhang S, Zhang Z, Sun Y, Yu L. Dynamic tubulation of mitochondria drives mitochondrial network formation. Cell Res 2015; 25:1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yaffe MP, Stuurman N, Vale RD. Mitochondrial positioning in fission yeast is driven by association with dynamic microtubules and mitotic spindle poles. Proc National Acad Sci 2003; 100:11424–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fu C, Jain D, Costa J, Velve-Casquillas G, Tran PT. Mmb1p binds mitochondria to dynamic microtubules. Curr Biol 2011; 21:1431–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mehta K, Chacko L, Chug M, Jhunjhunwala S, Ananthanarayanan V. Association of mitochondria with microtubules inhibits mitochondrial fission by precluding assembly of the fission protein Dnm1. J Biol Chem 2019; 294:3385–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Woods LC, Berbusse GW, Naylor K. Microtubules are essential for mitochondrial dynamics–fission, fusion, and motility–in dictyostelium discoideum. Frontiers Cell Dev Biology 2016; 4:19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Al-Mehdi A-B, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC, Pastukh VV, Alexeyev MF, Gillespie MN. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal 2012; 5:ra47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saotome M, Safiulina D, Szabadkai G, Das S, Fransson Å, Aspenstrom P, Rizzuto R, Hajnóczky G. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci USA 2008; 105:20728–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nemani N, Carvalho E, Tomar D, Dong Z, Ketschek A, Breves SL, Jaña F, Worth AM, Heffler J, Palaniappan P, Tripathi A, Subbiah R, Riitano MF, Seelam A, Manfred T, Itoh K, Meng S, Sesaki H, Craigen WJ, Rajan S, Shanmughapriya S, Caplan J, Prosser BL, Gill DL, Stathopulos PB, Gallo G, Chan DC, Mishra P, Madesh M. MIRO-1 determines mitochondrial shape transition upon GPCR activation and Ca2+ Stress. Cell Reports 2018; 23:1005–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sheng Z-H. Mitochondrial trafficking and anchoring in neurons: new insight and implications. J Cell Biol 2014; 204:1087–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Page E, McCallister LP. Quantitative electron microscopic description of heart muscle cells application to normal, hypertrophied and thyroxin-stimulated hearts. Am J Cardiol 1973; 31:172–81 [DOI] [PubMed] [Google Scholar]
- 171.Huang X, Sun L, Ji S, Zhao T, Zhang W, Xu J, Zhang J, Wang Y, Wang X, Franzini-Armstrong C, Zheng M, Cheng H. Kissing and nanotunneling mediate intermitochondrial communication in the heart. Proc National Acad Sci 2013; 110:2846–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chen Y, Liu Y, Dorn GW. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 2011; 109:1327–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eisner V, Cupo RR, Gao E, Csordás G, Slovinsky WS, Paillard M, Cheng L, Ibetti J, Chen WS, Chuprun KJ, Hoek JB, Koch WJ, Hajnóczky G. Mitochondrial fusion dynamics is robust in the heart and depends on calcium oscillations and contractile activity. Proc Natl Acad Sci USA 2017; 114:E859–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kuznetsov AV, Troppmair J, Sucher R, Hermann M, Saks V, Margreiter R. Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: possible physiological role? Biochim Biophys Acta Bioenerg 2006; 1757:686–91 [DOI] [PubMed] [Google Scholar]
- 175.Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol 2005; 288:C1074–C1082 [DOI] [PubMed] [Google Scholar]
- 176.Hoppel CL, Tandler B, Fujioka H, Riva A. Dynamic organization of mitochondria in human heart and in myocardial disease. Int J Biochem Cell Biol 2009; 41:1949–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Miragoli M, Sanchez-Alonso JL, Bhargava A, Wright PT, Sikkel M, Schobesberger S, Diakonov I, Novak P, Castaldi A, Cattaneo P, Lyon AR, Lab MJ, Gorelik J. Microtubule-dependent mitochondria alignment regulates calcium release in response to nanomechanical stimulus in heart myocytes. Cell Reports 2016; 14:140–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lavorato M, Iyer RV, Dewight W, Cupo RR, Debattisti V, Gomez L, la Fuente S, Zhao Y-T, Valdivia HH, Hajnóczky G, Franzini-Armstrong C. Increased mitochondrial nanotunneling activity, induced by calcium imbalance, affects intermitochondrial matrix exchanges. Proc Natl Acad Sci USA 2017; 114:E849–E858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.An S, Kumar R, Sheets ED, Benkovic SJ. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 2008; 320:103–6 [DOI] [PubMed] [Google Scholar]
- 180.Chan C, Pedley AM, Kim D, Xia C, Zhuang X, Benkovic SJ. Microtubule-directed transport of purine metabolons drives their cytosolic transit to mitochondria. Proc National Acad Sci 2018; 115:201814042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, Saks V, Bezrukov SM, Sackett DL. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc National Acad Sci 2008; 105:18746–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rostovtseva TK, Gurnev PA, Hoogerheide DP, Rovini A, Sirajuddin M, Bezrukov SM. Sequence diversity of tubulin isotypes in regulation of the mitochondrial voltage-dependent anion channel. J Biol Chem 2018; 293:10949–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sheldon KL, Gurnev PA, Bezrukov SM, Sackett DL. Tubulin tail sequences and post-translational modifications regulate closure of mitochondrial voltage-dependent anion channel (VDAC). J Biol Chem 2015; 290:26784–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Maldonado EN, Sheldon KL, DeHart DN, Patnaik J, Manevich Y, Townsend DM, Bezrukov SM, Rostovtseva TK, Lemasters JJ. Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells – regulation by free tubulin and erastin. J Biol Chem 2013; 288:11920–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arbustini E, Diegoli M, Fasani R, Grasso M, Morbini P, Banchieri N, Bellini O, Bello B, Pilotto A, Magrini G, Campana C, Fortina P, Gavazzi A, Narula J, Viganò M. Mitochondrial DNA mutations and mitochondrial abnormalities in dilated cardiomyopathy. Am J Pathology 1998; 153:1501–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sabbah HN, Sharov V, Riddle JM, Kono T, Lesch M, Goldstein S. Mitochondrial abnormalities in myocardium of dogs with chronic heart failure. J Mol Cell Cardiol 1992; 24:1333–47 [DOI] [PubMed] [Google Scholar]
- 187.Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh S, Cleland JG, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ, Gheorghiade M. Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol 2017; 14:238–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Folker ES, Baylies MK. Nuclear positioning in muscle development and disease. Front Physiol 2013; 4:363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zebrowski DC, Vergarajauregui S, Wu C-C, Piatkowski T, Becker R, Leone M, Hirth S, Ricciardi F, Falk N, Giessl A, Just S, Braun T, Weidinger G, Engel FB. Developmental alterations in centrosome integrity contribute to the post-mitotic state of mammalian cardiomyocytes. Elife 2015; 4:e05563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Oddoux S, Zaal KJ, Tate V, Kenea A, Nandkeolyar SA, Reid E, Liu W, Ralston E. Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. J Cell Biol 2013; 203:205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bugnard E, Zaal K, Ralston E. Reorganization of microtubule nucleation during muscle differentiation. Cell Motil Cytoskeleton 2005; 60:1–13 [DOI] [PubMed] [Google Scholar]
- 192.Gimpel P, Lee Y, Sobota RM, Calvi A, Koullourou V, Patel R, Mamchaoui K, Nédélec F, Shackleton S, Schmoranzer J, Burke B, Cadot B, Gomes ER. Nesprin-1α-dependent microtubule nucleation from the nuclear envelope via akap450 is necessary for nuclear positioning in muscle cells. Curr Biol 2017; 27:2999–3009.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tassin A, Maro B, Bornens M. Fate of microtubule-organizing centers during myogenesis in vitro. J Cell Biology 1985; 100:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Espigat-Georger A, Dyachuk V, Chemin C, Emorine L, Merdes A. Nuclear alignment in myotubes requires centrosome proteins recruited by nesprin-1. J Cell Sci 2016; 129:4227–37 [DOI] [PubMed] [Google Scholar]
- 195.van Steensel B, Belmont AS. Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell 2017; 169:780–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cheedipudi SM, Matkovich SJ, Coarfa C, Hu X, Robertson MJ, Sweet M, Taylor M, Mestroni L, Cleveland J, Willerson JT, Gurha P, Marian AJ. Genomic reorganization of lamin-associated domains in cardiac myocytes is associated with differential gene expression and dna methylation in human dilated cardiomyopathy. Circ Res 2019; 124:1198–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wilson MH, Holzbaur EL. Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 2015; 142:218–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc National Acad Sci 2009; 106:2194–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 2009; 64:173–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Puckelwartz MJ, Kessler EJ, Kim G, DeWitt MM, Zhang Y, Earley JU, Depreux F, Holaska J, Mewborn SK, Pytel P, McNally EM. Nesprin-1 mutations in human and murine cardiomyopathy. J Mol Cell Cardiol 2010; 48:600–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhou C, Rao L, Warren DT, Anahan C, Zhang Q. Mouse models of nesprin-related diseases. Biochem Soc Trans 2018; 46:669–81 [DOI] [PubMed] [Google Scholar]
- 202.Alkass K, Panula J, Westman M, Wu T-D, Guerquin-Kern J-L, Bergmann O. No evidence for cardiomyocyte number expansion in preadolescent mice. Cell 2015; 163:1026–36 [DOI] [PubMed] [Google Scholar]
- 203.Anversa P, Olivetti G, Melissari M, Loud AV. Stereological measurement of cellular and subcellular hypertrophy and hyperplasia in the papillary muscle of adult rat. J Mol Cell Cardiol 1980; 12:781–95 [DOI] [PubMed] [Google Scholar]
- 204.Koide M, Nagatsu M, Zile M, Hamawaki M, Indle M, Keech G, DeFreyte G, Tagawa H, Cooper G. Carabello Premorbid determinants of left ventricular dysfunction in a novel model of gradually induced pressure overload in the adult canine. Circulation 1997; 95:1601–10 [DOI] [PubMed] [Google Scholar]
- 205.Tsutsui H, Ishibashi Y, Takahashi M, Namba T, Tagawa H, Imanaka-Yoshida K, Takeshita A. Chronic colchicine administration attenuates cardiac hypertrophy in spontaneously hypertensive rats. J Mol Cell Cardiol 1999; 31:1203–13 [DOI] [PubMed] [Google Scholar]
- 206.Cheng G, Zile MR, Takahashi M, Baicu CF, Bonnema DD, Cabral F, Menick DR, Cooper G. A direct test of the hypothesis that increased microtubule network density contributes to contractile dysfunction of the hypertrophied heart. Am J Physiol Heart Circ Physiol 2008; 294:H2231–41 [DOI] [PubMed] [Google Scholar]
- 207.Lewis YE, Moskovitz A, Mutlak M, Heineke J, Caspi LH, Kehat I. Localization of transcripts, translation, and degradation for spatiotemporal sarcomere maintenance. J Mol Cell Cardiol 2018; 116:16–28 [DOI] [PubMed] [Google Scholar]
- 208.Davis J, Davis CL, Correll RN, Makarewich CA, Schwanekamp JA, Moussavi-Harami F, Wang D, York AJ, Wu H, Houser SR, Seidman CE, Seidman JG, Regnier M, Metzger JM, Wu JC, Molkentin JD. A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell 2016; 165:1147–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McLendon PM, Robbins J. Proteotoxicity and cardiac dysfunction. Circ Res 2015; 116:1863–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McLendon PM, Ferguson BS, Osinska H, Bhuiyan M, James J, McKinsey TA, Robbins J. Tubulin hyperacetylation is adaptive in cardiac proteotoxicity by promoting autophagy. Proc National Acad Sci 2014; 111:E5178–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lyon RC, Lange S, Sheikh F. Breaking down protein degradation mechanisms in cardiac muscle. Trends Mol Med 2013; 19:239–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol 1998; 143:1883–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Johnston JA, Illing ME, Kopito RR. Cytoplasmic dynein/dynactin mediates the assembly of aggresomes. Cell Motil Cytoskeleton 2002; 53:26–38 [DOI] [PubMed] [Google Scholar]
- 214.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao T-P. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 2003; 115:727–38 [DOI] [PubMed] [Google Scholar]
- 215.García-Mata R, Bebök Z, Sorscher EJ, Sztul ES. Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J Cell Biol 1999; 146:1239–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wójcik C, Schroeter D, Wilk S, Lamprecht J, Paweletz N. Ubiquitin-mediated proteolysis centers in HeLa cells: indication from studies of an inhibitor of the chymotrypsin-like activity of the proteasome. Eur J Cell Biol 1996; 71:311–8 [PubMed] [Google Scholar]
- 217.Schlossarek S, Frey N, Carrier L. Ubiquitin-proteasome system and hereditary cardiomyopathies. J Mol Cell Cardiol 2014; 71:25–31 [DOI] [PubMed] [Google Scholar]
- 218.Mackeh R, Perdiz D, Lorin S, Codogno P, Poüs C. Autophagy and microtubules – new story, old players. J Cell Sci 2013; 126:1071–80 [DOI] [PubMed] [Google Scholar]
- 219.Fass E, Shvets E, Degani I, Hirschberg K, Elazar Z. Microtubules support production of starvation-induced autophagosomes but not their targeting and fusion with lysosomes. J Biol Chem 2006; 281:36303–16 [DOI] [PubMed] [Google Scholar]
- 220.Geeraert C, Ratier A, Pfisterer SG, Perdiz D, Cantaloube I, Rouault A, Pattingre S, Proikas-Cezanne T, Codogno P, Poüs C. Starvation-induced hyperacetylation of tubulin is required for the stimulation of autophagy by nutrient deprivation. J Biol Chem 2010; 285:24184–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jahreiss L, Menzies FM, Rubinsztein DC. The itinerary of autophagosomes: from peripheral formation to kiss‐and‐run fusion with lysosomes. Traffic 2008; 9:574–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xie R, Nguyen S, McKeehan WL, Liu L. Acetylated microtubules are required for fusion of autophagosomes with lysosomes. Bmc Cell Biol 2010; 11:89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kimura S, Noda T, Yoshimori T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct Funct 2008; 33:109–22 [DOI] [PubMed] [Google Scholar]
- 224.Mohan N, Sorokina EM, Verdeny I, Alvarez A, Lakadamyali M. Detyrosinated microtubules spatially constrain lysosomes facilitating lysosome–autophagosome fusion. J Cell Biol 2018; 218:jcb.201807124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bánréti Á, Sass M, Graba Y. The emerging role of acetylation in the regulation of autophagy. Autophagy 2013; 9:819–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, Rapoport TA, Margolis B. Cargo of kinesin identified as jip scaffolding proteins and associated signaling molecules. J Cell Biol 2001; 152:959–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lytvyn D, Yemets A, Blume Y. α-Tubulin acetylation and detyrosination correlate with starvation-induced autophagy in tobacco cells. Biorxiv 2017;111484 [Google Scholar]
- 228.Nam T, Han J, Devkota S, Lee H-W. Emerging paradigm of crosstalk between autophagy and the ubiquitin-proteasome system. Mol Cells 2017; 40:897–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pan B, Zhang H, Cui T, Wang X. TFEB activation protects against cardiac proteotoxicity via increasing autophagic flux. J Mol Cell Cardiol 2017; 113:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wu P, Yuan X, Li F, Zhang J, Zhu W, Wei M, Li J, Wang X. Myocardial upregulation of cathepsin d by ischemic heart disease promotes autophagic flux and protects against cardiac remodeling and heart failure. Circulation Hear Fail 2018; 10:e004044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Su H, Li J, Zhang H, Ma W, Wei N, Liu J, Wang X. COP9 signalosome controls the degradation of cytosolic misfolded proteins and protects against cardiac proteotoxicity. Circ Res 2015; 117:956–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lee Y, Kwon I, Jang Y, Song W, Cosio-Lima LM, Roltsch MH. Potential signaling pathways of acute endurance exercise-induced cardiac autophagy and mitophagy and its possible role in cardioprotection. J Physiol Sci 2017; 67:639–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 2007; 13:619–24 [DOI] [PubMed] [Google Scholar]
- 234.Mao W, Zhu Z. Parthenolide inhibits hydrogen peroxide-induced osteoblast apoptosis. Mol Med Rep 2018; 17:8369–76 [DOI] [PubMed] [Google Scholar]
- 235.Gehl J, Boesgaard M, Paaske T, Jensen VB, Dombernowsky P. Combined doxorubicin and paclitaxel in advanced breast cancer: effective and cardiotoxic. Ann Oncol 1996; 7:687–93 [DOI] [PubMed] [Google Scholar]
- 236.Gianni L, Kearns C, Giani A, Capri G, Viganó L, Lacatelli A, Bonadonna G, Egorin M. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 1995; 13:180–90 [DOI] [PubMed] [Google Scholar]
- 237.Altin C, Sade L, Demirtas S, Karacaglar E, Kanyilmaz S, Simsek V, Ayhan A, Muderrisoglu H. Effects of paclitaxel and carboplatin combination on mechanical myocardial and microvascular functions: a transthoracic doppler echocardiography and two‐dimensional strain imaging study. Echocardiogr 2015; 32:238–47 [DOI] [PubMed] [Google Scholar]
- 238.Mohee K, Zhang J, Cleland JG, Clark AL. Use of colchicine in cardiovascular diseases – a systematic review. Heart 2015; 101:A91 [Google Scholar]
- 239.Slobodnick A, Shah B, Krasnokutsky S, Pillinger MH. Update on colchicine, 2017. Rheumatology (Oxford) 2018; 57:i4–i11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wallace S, Ertel N. Occupancy approach to colchicine dosage. Lancet 1970; 296:1250–1 [DOI] [PubMed] [Google Scholar]