Short abstract
Spectrins are proteins that are responsible for many aspects of cell function and adaptation to changing environments. Primarily the spectrin-based membrane skeleton maintains cell membrane integrity and its mechanical properties, together with the cytoskeletal network a support cell shape. The occurrence of a variety of spectrin isoforms in diverse cellular environments indicates that it is a multifunctional protein involved in numerous physiological pathways. Participation of spectrin in cell–cell and cell–extracellular matrix adhesion and formation of dynamic plasma membrane protrusions and associated signaling events is a subject of interest for researchers in the fields of cell biology and molecular medicine. In this mini-review, we focus on data concerning the role of spectrins in cell surface activities such as adhesion, cell–cell contact, and invadosome formation. We discuss data on different adhesion proteins that directly or indirectly interact with spectrin repeats. New findings support the involvement of spectrin in cell adhesion and spreading, formation of lamellipodia, and also the participation in morphogenetic processes, such as eye development, oogenesis, and angiogenesis. Here, we review the role of spectrin in cell adhesion and cell–cell contact.
Impact statement
This article reviews properties of spectrins as a group of proteins involved in cell surface activities such as, adhesion and cell–cell contact, and their contribution to morphogenesis. We show a new area of research and discuss the involvement of spectrin in regulation of cell–cell contact leading to immunological synapse formation and in shaping synapse architecture during myoblast fusion. Data indicate involvement of spectrins in adhesion and cell–cell or cell–extracellular matrix interactions and therefore in signaling pathways. There is evidence of spectrin’s contribution to the processes of morphogenesis which are connected to its interactions with adhesion molecules, membrane proteins (and perhaps lipids), and actin. Our aim was to highlight the essential role of spectrin in cell–cell contact and cell adhesion.
Keywords: Spectrins, spectrin-based skeleton, cell adhesion, cell–cell contact, adhesion molecules, membrane
Spectrin—A multifunctional protein
Originally identified in erythrocytes, spectrins associate with actin filaments to form a 2D meshwork on the inner surface of the plasma membrane. In mammalian erythrocytes, spectrins exist as large flexible rod-like heterotetramers made of a side-to-side assembly of αI and βI subunits. The tetramers constitute the filaments of the network; they are cross-linked by short actin filaments via the actin binding site present in β-spectrins. In nucleated cells, several spectrin isoforms are expressed, emerging from seven genes, two encoding α-spectrins (αI and αII subunits), five encoding β-spectrins (βI–βV subunits), and by different combinations contributing consequently to numerous spectrin species presenting their specific cellular expression patterns in all metazoan cells.1,2 αI- and βI-spectrin are exclusively expressed in the red blood cells, whereas in nonerythroid cells, arrangements of αII-spectrin and subunits of βII–βV spectrin are the most common.3 Moreover, in nucleated cells, the distribution of spectrins is not limited to the plasma membrane; they have also been identified in endomembranes of the Golgi complex, cytoplasmic vesicles, as well as of the nucleus.4
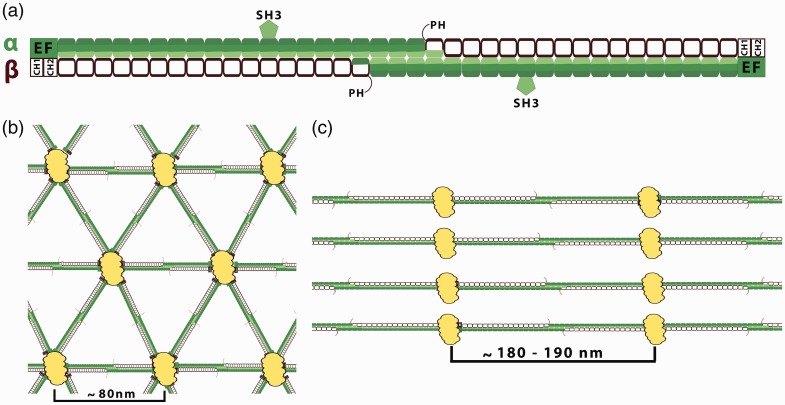
The core structural element of spectrin is a triple-helical spectrin repeat (A–C helixes). Typically, 20 complete repeats can be found in α-spectrin and 16 in the β subunits of spectrin, excluding the longer βV-spectrin subunit which consists of 29 full repeats. The homolog of this heavy βV-spectrin subunit is named βH-spectrin in Drosophila melanogaster and Sma-1 in Caenorhabditis elegans. The α- and β-spectrin subunits differ from each other by several unique domains. The motifs based on the spectrin repeats are the ankyrin-binding domain of β-spectrin and the oligomerization site of α- and β-spectrins. Others, such as actin-binding domain, EF-hand domain (calcium binding) in β-spectrins, pleckstrin homology (PH), Src homology 3 (SH3), and CCC region are non-spectrin-repeat structural motifs.5,6 The PH domain is present only in “long” carboxyl end isoforms of β-spectrin. The SH3 domain is located in the ninth repeat of αII-spectrin. The CCC region is 36-residue insert within the α10 repeat unit of αII-spectrin and is the binding site for calmodulin and the cleavage sites for both caspases and for calpains7–9 (Figure 1(a)).
Figure 1.
Spectrin-based membrane skeleton. (a) Organization of the spectrin tetramers. Spectrins, as flexible long tetramers (∼200 nm length when fully extended) composed of α (filled segments) and β (empty segments) subunits associate side by side then form head-to-head dimer interactions. Each segment represents a 106-amino acid residue repeat unit (folded in a triple α-helical coiled-coil structure). The interconnections of spectrin repeats are thought to be closely associated with spectrin flexibility. α-Spectrin contains 21 repeats plus a single C-helix at the N terminus. A 60 amino acid residues fragment of the ninth repeat of the α-subunit represents an SH3 domain. β-Spectrin consists of 16 repeats plus a partial repeat at the C-terminus which contains just two A and B helices. Marked spectrin domains: PH: pleckstrin homology domain which is present only in “long” carboxyl end isoforms of the β-spectrin domain—except βI isoform; EF: EF-hand domain (calcium binding). Actin and protein 4.1R binding domain (2 CH domain) is located at the N-terminal end of the β-spectrin. (b) The quasi-triangle network of the erythrocytes spectrin-based skeleton. Spectrin tetramers are connected by junctional complexes (containing actin filaments, adducing, tropomodulin, and protein 4.1). The edge length of this network is ∼80 nm.10 The spectrin-based skeleton of resting erythrocytes is in a relaxed state what may be functionally helpful for the dynamics fully reversible deformations of the spectrin skeleton during circulation. (c) Periodicity of membrane skeleton in neuronal axons, where spectrin heterotetramers are connected to actin-based junctional complexes. The spectrin tetramers are spaced along the axon with periodicity of approximately 180–190 nm. This value agrees with the extended length of spectrin tetramers. The synergistic arrangement of bundling spectrin tetramers by actin rings in the same direction may increase the rigidity of spectrin tetramers. The lengths of spectrin tetramers in neuronal cells suggest that spectrin is under constant tensile stress. This force may be provided by the microtubule and neurofilament cytoskeletal systems that jam-pack inside neuronal processes, which are absent in erythrocytes.10(A color version of this figure is available in the online journal.)
Spectrins with these domains are elongated organelle-sized proteins forming resilient arrays binding integral membrane proteins (mostly via adaptor peripheral proteins) and phospholipids. Spectrin coupling to ankyrins and actin links this membrane protein with membrane lipid bilayer, microfilaments and microtubule skeletal systems.2,11
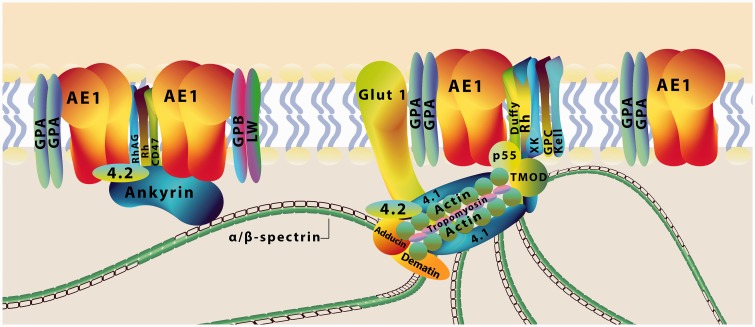
The recent discovery12 of a highly nanostructured and periodic membrane skeleton in neurons via super-resolution microscopy13,14 has changed the traditional view of the spectrin–actin-based membrane skeleton in mammalian cell types including erythrocytes. According to the current model, the erythrocyte membrane skeleton is a 2D triangular network organized by spectrin tetramers which are linked to junctional complexes containing short actin filaments, tropomodulin, tropomyosin, protein 4.1 and adducin and their associated proteins, whereas in major membrane skeleton macrocomplexes ankyrin R with anion exchanger 1 and other integral proteins are anchored near the spectrin self-association site1,5,15,16 (Figure 2). In the spectrin molecule there are particular regions which bind lipids with high affinity. The PH domain of some β isoforms is highly specific toward PIP2, and some spectrin repeats recognize membranes containing phosphatidylserine17 or enriched in phosphatidylethanolamine.18–20 Wolny et al.21 proposed that direct interaction between ankyrin-sensitive spectrin and PE-rich domains stabilizes the structure of spectrin-based membrane skeleton. Recently, Pan et al.10 resolved the membrane skeletal organization in native erythrocytes using super-resolution stochastic optical reconstruction microscopy (STORM), revealing an ∼80 nm junction-to-junction distance that is in agreement with relaxed spectrin tetramers (Figure 1(b)). It also shows that actin filaments and its capping proteins reside at junctional complexes. A different skeletal organization occurs in nonerythroid cells. In neuronal cells it appears as a periodic, 1D lattice of well-defined, ∼180–190 nm periodicity.22–27 In this cytoskeletal network, spectrin tetramers link the adducin-capped actin rings (Figure 1(c)).
Figure 2.
Current model of the red cell membrane. The network of the spectrin skeleton is anchored to the plasma membrane of the erythrocyte via two major membrane skeleton macrocomplexes and through direct interactions with lipids. The spectrin–actin interaction is modulated by accessory proteins such as protein 4.1, together with dematin, adducin, tropomyosin, and tropomodulin. Their functions are to stabilize the actin–spectrin complex, to maintain actin filament length, and to bind the spectrin-based network to the transmembrane proteins (glycophorin C, the anion exchanger AE1) via adapter proteins (protein p55 and protein 4.2). Another major binding site to membrane is mediated via ankyrin, which binds to β-spectrin and the anion exchanger AE1. The Rh/RhAG–ankyrin complex can also be a link between the red cell membrane and the spectrin-based skeleton. Spectrins also interact directly with phospholipids, membrane components actively confined to the inner leaflet of the lipid bilayer. GLUT 1: glucose transporter 1; GPA: glycophorin A; GPB: glycophorin B; Rh: rhesus factor; RhAG: Rh-associated glycoprotein, proteins Duffy; XK: Kell, CD47, LW. (A color version of this figure is available in the online journal.)
Many reports of red blood cells, mainly those in hereditary hemolytic anemia, have evidently determined the importance of spectrin in supporting cell shape, establishing the physical properties of the cell membrane and maintaining cell membrane integrity.28–30
In nonerythroid cells, spectrins may participate in the organization of specialized membrane domains by controlling localization and stability of many surface proteins.2,5 As it has been recently reported, the periodic, ruler-like membrane skeleton based on spectrin and actin serves as a nanoscale scaffold to mediate physical interactions between cell types of the neural stem cell lineage.31
The cell-specific repertoire of spectrin subunits encoding gene defects underlies a new group of disorders, the neuronal spectrinopathies, which includes spectrin-associated autosomal recessive cerebellar ataxia type 1,32,33 spinocerebellar ataxia type 5,34,35 early infantile epileptic encephalopathy type 5,36,37 West syndrome,38 and serious cardiac disorders such as congenital arrhythmias, heart failure, and possibly sudden cardiac death.39 In Drosophila, loss of β-spectrin has been reported to lead to the loss of Na+/K+-ATPase from the basolateral domain of epithelial cells.40 In an extreme case, in mice, it was reported that the loss of a variant of βII-spectrin led to death in utero.41
These diverse cellular environments found in both erythroid and nonerythroid cells and the various protein interactions put spectrin in a multifunctional context with numerous physiological pathways. Pleiotropic effects of spectrin dysfunctions likely reflect different roles depending on the cell type and which particular spectrin molecule is formed from α and β subunits. In this mini-review we focus on data concerning the role of spectrins in cell surface activities such as adhesion, cell–cell contact, and cell–extracellular matrix interactions (Figure 3).
Figure 3.
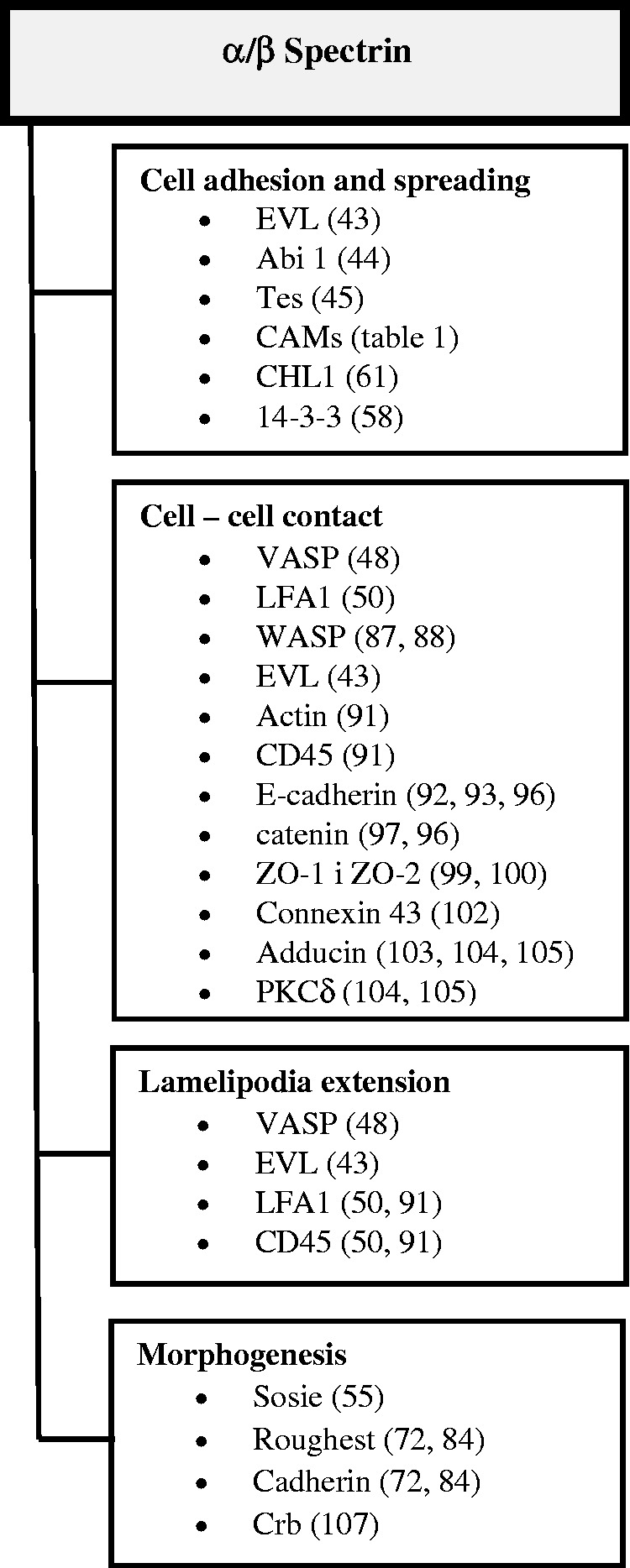
Involving of spectrin and related proteins in cell adhesion processes. CAM: cell adhesion molecule; EVL: Ena-Vasp-like; LFA-1: lymphocytes function-associated antigen 1; VASP: vasodilatator-stimulated phosphoprotein; ZO-1: zonula occludens 1.
Spectrin is engaged in cell adhesion via interaction with proteins involved in actin dynamics
In the central region of α-spectrin, within repeat 9 between helix B and C the functional SH3 domain is present. The SH3 domain, a compact β-barrel made of five antiparallel β-strands (PBD ID: 1U06)42 is a common structural motif often found in proteins involved in signal transduction and is also related to cell adhesion and migration. A number of published data indicate that the SH3 spectrin domain in nonerythroid cells interacts with proteins involved in actin polymerization and dynamics such as EVL (Ena-Vasp-like), a member of the enabled/vasodilatator-stimulated phosphoprotein (Ena/VASP) family,43 Abi1,44 and proteins of focal adhesion such as Tes.45 Those interactions of αII-spectrin (via its SH3 domain) correspond to a linkage within actin and its polymerization machinery.
αII-Spectrin may also play a role in the mechanism regulating the actin machinery through several ligands. Research conducted by Bialkowska et al.46 indicates a role for the SH3 domain of spectrin in initiating Rac activation in the specialized integrin clusters that lead to cell adhesion and spreading. Furthermore, recent data point to the possibility that spectrin may regulate the invadosome by controlling integrin mobility in the membrane.47 Invadopodia are adhesive mechanosensory structures organized with a central actin-rich core enclosed by an adhesion and scaffold protein ring. Experimental data revealed that, in addition to actin, αII-spectrin is also a highly dynamic component of the invadosomes core. Depletion of αII-spectrin in cells destabilizes invadosome and reduces its ability to degrade the extracellular matrix and to stimulate invasion. These data point to the role played by spectrin in the stability of the invadosome and to the connection between actin regulation and extracellular matrix digestion.47
Benz et al.48 reported an interaction between αII-spectrin and VASP which controls cell–cell contacts. Much recent data have supported this involvement of αII-spectrin in cell adhesion and spreading as well as in the actin skeleton organization in melanoma, neuronal, endothelial, fibroblast, and lymphocyte cell lines.47,49–51 αII-Spectrin turns out to be an important factor for nonerythroid cell shape and cell–matrix adhesion. Depletion of αII-spectrin in different nucleated cells revealed defects in cell adhesion and lamellipodia formation accompanied with marked modifications of the actin-based cellular elements, such as loss of stress fibers and focal contacts. In the WM266 melanoma cell line, partial depletion of αII-spectrin was associated with a loss of cell spreading and defective adhesion, together with a reduced number of focal adhesions, which appeared less well organized and more irregular than wild type. These changes resulted in rounded and spiked cell morphology.51 Down-regulation of αιι-spectrin expression in human neuroblastoma SH-SY5Y cells caused major changes in neurite morphology and cell shape. Neurites were thinner and displayed abnormal adhesiveness during cell migration, and the irregular polygonal cell shape occurred in parallel with a loss of cortical F-actin from neuronal cell bodies.49 In research on an αII-spectrin conditional knock-out mouse model it was demonstrated that αII-spectrin plays a major role in axon initial segment assembly and neuronal migration.52 Also αιι-spectrin depletion and the accompanying decrease in β3-integrin immobilization in a fibroblast MEF cell line was associated with defects of adhesion and migration. This decrease of cell migration indicates that targeting of αιι-spectrin may be essential for control of cell invasion.47 Disruption of the spectrin skeleton organization was associated with a decrease in the number and dynamics of actin-rich lamellipodia and a loss of filopodia extensions upon activation of spectrin-depleted Jurkat T cells. The presence of spectrin in immunological synapses suggests that spectrin contributes to this dynamic of actin filament reorganization, which is essential for immunological synapse formation.50 In Drosophila muscle cells, α/βH-spectrin dynamically accumulates and diffuses in the fusogenic synapse, where an attacking fusion partner invades its receiving partner with actin-propelled protrusions to promote cell fusion.53 In these fusogenic synapses spectrin exhibits mechanosensitive accumulation, functioning as a cellular fence to restrict the diffusion of cell-adhesion molecules and as a cellular filter to constrict invasive protrusions, thereby increasing the mechanical tension to promote cell membrane fusion.53
Severe alterations of cell spreading and adhesion have also been observed early in embryonic fibroblasts from αII-spectrin−/− mice.54 Furthermore, Sptan1−/− mice died before embryonic day 16 with cardiac and neural malformations. These data indicate that the spectrin–ankyrin scaffold is crucial in vertebrates for cell spreading, tissue patterning, and the developing brain and heart, but is not required for cell viability. Likewise, a study on Drosophila indicated similar involvement of βH-spectrin in cell adhesion and migration.55
In summary, the roles of spectrin in adhesion, lamellipodia extension, and cell spreading through several ligands and partners regulating actin dynamics have recently been strongly highlighted thanks to new data obtained from examination of a number of different cell models.
Spectrins directly or indirectly interact with adhesion molecules
Published data indicate that different adhesion proteins directly or indirectly interact with spectrin repeats (Table 1). The cytoplasmic tail of the adhesion glycoprotein Lutheran/basal cell adhesion molecule (Lu/BCAM) interacts with erythroid αI-spectrin.70 Lu/BCAM is a laminin 511/521 unique receptor expressed in red blood cells, endothelial and epithelial tissues, as well as smooth muscle cells. Spectrin regulates adhesive activity of Lu/BCAM. As demonstrated by An et al.,75 disruption of interaction of Lu/BCAM/spectrin in erythrocytes enhances adhesion of red blood cells to laminin. Likewise, in epithelial and endothelial cells (ECs) αII-spectrin interacts with Lu/BCAM and this interaction is required for stress fiber formation during cell spreading on laminin 511/521. Spectrin acts as a signal relay between laminin and actin in which it is involved in actin dynamics.71
Table 1.
Examples of cell adhesion molecules, which bind directly or indirectly via linker proteins to spectrins.
| Cell adhesion molecule | Isoforms of spectrin | Interaction (references) |
|---|---|---|
| NCAM | βI-spectrin | Direct56,57 |
| αII-spectrin | Via 14.3.3β protein58 | |
| L1 | Spectrin | Via ankyrin B59,60 |
| αII-spectrin | Direct61 | |
| Neuroglian | Spectrin | Via ankyrin62,63 |
| CHL1 | βII-spectrin | Direct61 and via ankyrin64 |
| Neurofascin | Spectrin | Via ankyrin G59,65,66 |
| βII-spectrin | 67 | |
| NrCAM | Spectrin | Via ankyrin68 |
| SynCAM 1 | Spectrin | Via band 4.1-like protein 3, also called 4.1B69 |
| Lu/B-CAM | αI-spectrin | Direct70 |
| αII-spectrin | Direct71 | |
| ICAM | αII-spectrin | Not shown, via LFA-150 |
| Ig-CAM Roughest | βH-spectrin (Drosophila) | Direct72 Via annexin B973,74 |
CHL1: close homolog of L1; ICAM: intercellular adhesion molecule; LFA-1: lymphocytes function-associated antigen 1; NCAM: neural cell adhesion molecule.
The next adhesion molecule directly reacting with βI-spectrin is neural cell adhesion molecule 1. In the mammalian nervous system two transmembrane isoforms, NCAM140 and NCAM180, are present. The NCAM–spectrin–PKCβ2 complex is essential for neurite outgrowth. Overexpression of NCAM leads to a general increase in the level of βI-spectrin in hippocampal neurons of mouse brain, whereas the deficiency of NCAM in these cells results in a decrease in βI-spectrin levels.56 In addition, there are cell adhesion molecules that do not contain intracellular domains but are associated with the plasma membrane via a glycosylphosphatidylinositol (GPI) anchor. In mouse hippocampal neurons βI-spectrin interacts with the GPI-anchored isoform NCAM120.56 Moreover, NCAM–spectrin complex disassembly results in abnormally high numbers of perforated postsynaptic densities and formation of postsynaptic endocytic zones, thus affecting cell–cell contact.76
The spectrin meshwork regulates the removal of L1 family members from the neuronal cell surface by endocytosis. The intracellular domain of CHL1 (close homolog of L1) contains a binding site for ezrin77 and also directly binds to βII-spectrin.61 This ligand-induced clustering of CHL1 prompted palmitoylation of CHL1 and membrane raft-dependent remodeling of the CHL1/βII–spectrin complex, accompanied by CHL1 endocytosis in cultured mouse hippocampal neurons, which is required for CHL1-dependent neurite outgrowth. Knock-down of βII-spectrin encoding gene (SPTBN1) expression using targeted siRNA results in increased endocytosis of CHL1.61 Furthermore, it was found in human neuroblastoma SH-SY5Y cells that αιι-spectrin is implicated in normal morphology and adhesive properties of neuronal cell bodies and neurites, and in cell surface expression and organization of adhesion molecule L1.49 Remarkably, αιι-spectrin depletion in SH-SY5Y cells affected L1- but not NCAM-cell surface expression, and L1 clustering at growth cones. In a recent study, using super-resolution 3D-STORM, a remarkable alignment of the periodic cytoskeletons between abutting cells at axon–axon and axon–oligodendrocyte contacts was reported. Some possible candidates to drive this nanoscale alignment are two adhesion molecules, neurofascin and cell adhesion molecule L1 (L1CAM).31
As previously demonstrated, αII- and βII-spectrin are present in myelinating Schwann cells, where they contribute to myelination.78,79 While testing mice lacking βII-spectrin it was observed that glial spectrins may also contribute to the functions of myelinating glia. Depletion of βII-spectrin in myelinating glial cells disrupted the paranodal cell adhesion complex of glial neurofascin in both peripheral and central nervous systems, resulting in muscle weakness and sciatic nerve conduction slowing in juvenile and middle-aged mice.67 Also, it has been recently documented that L1 coupling to ankyrin and therefore to the spectrin–actin skeleton modulates ethanol inhibition of L1 adhesion and ethanol teratogenesis.80 Furthermore, αII-spectrin interacts with the protein 14-3-3, which is engaged in neuronal migration and synaptic plasticity. This interaction works as a positive/negative switch in NCAM-dependent neurite outgrowth.58
Many studies show that immunoglobulin superfamily CAMs control the cytoskeleton. On the other hand, the cytoskeleton is directly responsible for the regulation of functions and levels of cell adhesion molecules at functionally important domains of the plasma membrane of neurons.81
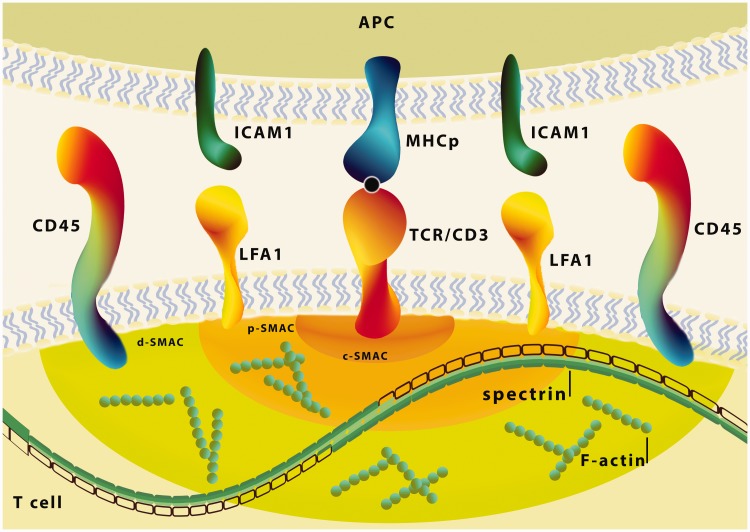
As mentioned above, αII-spectrin accumulates in specialized integrin clusters that initiate cell adhesion.46 Binding of LFA-1 integrin (lymphocytes function-associated antigen 1) on T cells to ICAM (intercellular adhesion molecule 1, also known as CD54) to antigen-presenting cells has been shown to provide a second signal for T cell activation.82 In T cells the polarization of actin toward the cell contact area is accompanied by recruitment of talin, which activates LFA-1.83 Spectrin by direct interactions with VASP indirectly controls activation of talin and in this way may participate in regulation of LFA-1 integrin clustering in the IS region50 (Figure 4).
Figure 4.
The role of spectrin in immunological synapse formation. Schema of the immunological synapse (IS) and representative protein interactions in the synaptic space. In the central SMAC, the T cell receptor (TCR)/CD3 complex interacts with MHC-peptide. The adhesion molecules on the surface of both cells (LFA-1–ICAM-1) are responsible for the formation and stabilization of the IS, as well as for initiating signal transduction pathways activated by TCR. The distal ring of IS is rich in proteins, such as CD45 and F-actin controls lamellipodia and filopodia formation. The spectrin through the presence at sites of immunological synapses in T cells and interaction with actin, CD45 and regulation of LFA-1 integrin clustering, may participate in this dynamic actin-rich process which is essential for immunological synapse formation. c-SMAC: central-SMAC; d-SMAC: distal-SMAC; ICAM-1: intercellular adhesion molecule1; LFA-1: lymphocytes function-associated antigen 1; p-SMAC: peripheral-SMAC; SMAC: supramolecular activation cluster. (A color version of this figure is available in the online journal.)
Also data from Drosophila morphogenesis research suggest that during eye morphogenesis the immunoglobulin superfamily cell adhesion molecule Roughest depends on βH-spectrin (on segment 33 in βH; homolog to mammalian βV-spectrin). Expression of βH33 results in the loss of interommatidial cells, which leads to fragmentation of the zonula adherens (ZA) and disruption of the Roughest molecule. This spectrin genetically and physically interacts with Roughest, maintaining its distribution. Lee et al.72 have suggested that the apical spectrin membrane skeleton serves to coordinate the Cadherin-based (ZA) and Roughest/Ig-CAM adhesion system.84,85 Tjota et al.73 demonstrated that annexin B9 (AnxB9) in Drosophila links to the βH isoform of spectrin and is involved in intermembrane adhesion in multivesicular bodies (MVB). AnxB9 depletion results in increased levels of basolateral βH-spectrin and MVB markers as well as destruction of the apical–lateral boundary. Loss of AnxB9 or βH-spectrin function leads to the redistribution of Drosophila cadherin E to endosomal vesicles. AnxB9 and βH-spectrin participate in endosomal trafficking to the MVB and they are essential for maintaining proper segregation of membrane domains.73,86
As may be concluded from the above, there is substantial evidence provided by the literature that various spectrin isoforms are directly or indirectly involved in interactions with different adhesion molecules.
Spectrins are involved in cell–cell contact and morphogenesis
Spectrin is also found in adhesion complexes that regulate cell–cell contacts. Spectrin interacts with proteins of the WASP family such as the Wiskott–Aldrich syndrome protein. It was proven in the 1990s that T cells from patients with Wiskott–Aldrich syndrome show characteristic cytoskeletal defects87 and some impaired functions.88 Proteins of the VASP and Ena/VASP-like protein (EVL), which belong to the Ena/VASP family, also play a key role in remodeling of actin during activation of T cells. They are important in formation and extension of lamellipodia and join the adapter ADAP, which participates in LFA-1 integrin clustering.89,90 In T lymphocytes, spectrins can regulate the localization and activity of actin, CD45, and LFA1-proteins involved in cell–cell contact and cell signaling.91 Recent studies have shown that spectrins also participate in cell–cell contact and cell adhesion upon immunological synapse formation.50 This study emphasizes the regulatory function of spectrin as a protein engaged in the initial phase of contact between T cells and antigen-presenting cells.
In epithelial cells, knock-down of either βII-spectrin or ankyrin G leads to loss of the lateral membrane, increase of the apical and basal membrane surface, and a change of cell morphology from columnar to squamous.92,93 These proteins are necessary for the concentration and accumulation of E-cadherin in epithelial cell–cell contact and the delivery of phospholipids and proteins to the lateral membrane.92 Loss of minus end capping protein Tmod3 function leads to destabilization and disassembly of tropomyosin-coated actin filaments followed by disorganization of the spectrin-based membrane skeleton on lateral membranes. Tmod3-capped tropomyosin–actin filaments provide crucial links in the spectrin membrane skeleton of polarized epithelial cells, enabling the membrane skeleton to maintain cell shape.94 Also CAMSAP3 is crucial for epithelial architecture. CAMSAP3 (also known as Nezha) is a member of the calmodulin-regulated spectrin-associated protein (CAMSAP)/Nezha/Patronin family proteins, which bind and stabilize the ends of microtubules in epithelial cells. In intestinal epithelial cells, the microtubule minus-end binding protein CAMSAP3 tethers non-centrosomal microtubules to the apical cortex, leading to their longitudinal orientation. These findings demonstrate that apically localized CAMSAP3 determines proper orientation of microtubules, and in turn disposition/localization of organelles, in mature mammalian epithelial cells.95
Increased abundance of spectrins was reported in cellular contacts such as adherens, tight, and gap junctions. In adherens junctions spectrin directly interacts with the E-cadherin/β-catenin complex96 or α-catenin.97 Interactions between TGF-β signaling/ELF(βII) and E-cadherin/β-catenin mediate tumor suppression,98 which revealed among other things a loss of cell–cell adhesion in cells of a βII (ELF)+/− Smad4+/− mouse model. The interaction between ankyrin G that recruits βII-spectrin to E-cadherin–α-catenin complexes, providing a connection between E-cadherin and spectrin/actin skeleton, is involved in morphogenesis of the lateral membrane of kidney epithelial cells.93 In tight junctions spectrin co-localizes with zonula occludens 1 (ZO-1)99 and interacts with ZO-2 via 4.1 protein.100 These interactions are also involved in maintaining the epithelial cells,101 whereas in gap junctions a particular isoform of αII-spectrin (isoform αIIΣ1) interacts with connexin 43 and stabilizes this protein, which may suggest a putative role of spectrin in cell signaling by modulating cell–cell contact.102
Another member of the spectrin–actin junctional complex is adducin. This protein via interaction with βII-spectrin stabilizes preformed lateral membranes of human bronchial epithelial cells. Depletion of βII-spectrin resulted in loss of adducin from the lateral membrane. Abdi and Bennett103 found that adducin functions to stabilize and promote long-range organization of the lateral membrane, in contrast to βII-spectrin and ankyrin G, which are required for formation of the lateral membrane. They concluded that adducin acting through spectrin provided a novel mechanism to regulate global properties of the lateral membrane of bronchial epithelial cells. Wu et al.104 suggested that Ca2+ plays an important role in regulating the expression and function of β-adducin to sustain normal organization of the spectrin-based cytoskeleton and the differentiation properties in keratinocytes through the calmodulin/EGFR/cadherin signaling pathway. They observed that siRNA transfection of β-adducin in differentiating keratinocytes resulted in significant reduction of not only β-adducin protein, but also spectrin and PKCδ proteins. It led to disruption of the spectrin-based skeleton and the abnormal cytoskeletal arrangements of both adducin and PKCδ in keratinocytes.105 The above-mentioned data and new findings reveal a novel function of adducin as a negative regulator of non-small cell lung cancer cell migration and invasion, which could be essential for limiting lung cancer progression and metastasis.106
Recent research has demonstrated that spectrins also participate in biological processes, among them in morphogenesis. Urwyler et al.55 reported that cortical βH-spectrin mediates some of the functions of sosie, which is a novel gene required in various morphogenetic processes in Drosophila oogenesis. sosie contributes to normal cortical localization of βH-spectrin, interacts with βH-spectrin and is required for normal localization of spectrin. It is involved in maintenance of the structure of the spectrin and actin skeletons during oogenesis.
Chen et al.107 found that spectrins (α- and β-spectrins) are required for controlling photoreceptor morphogenesis in Drosophila via modulations of cell membrane domains. The spectrins are dispensable for retinal differentiation in eye imaginal discs during the larval stage. They are specifically required for photoreceptor polarity during pupal eye development. The authors show that overexpression of β-spectrin causes strong shrinkage of apical membrane domains, while loss of β-spectrin causes an expansion of apical domains, implying an antagonistic relationship between β-spectrin and βH-spectrin. βH-spectrin localizes apically, whereas β-spectrin is preferentially distributed in the basolateral region. α/β-spectrins are essential for the apical and basolateral membrane compartment modulations and for the morphogenesis of the developing photoreceptors.
Machnicka et al. have made the similar observation that αII-spectrin appears to be involved in the expression of proteins closely involved in angiogenesis in physiological as well as in pathological conditions in an EC model and an in vitro model of angiogenesis (unpublished data). It was found that αII-spectrin is involved in cell integrity, actin remodeling and cell adhesion and spreading in the primary human umbilical vein endothelial cells as well as in the HMEC-1 EC line. Moreover, a deficiency in αII-spectrin may affect complex mechanisms such as in vitro capillary tube formation, a dynamic process mimicking angiogenesis. These findings support the participation of αII-spectrin in angiogenesis by modulating integrins and adhesion molecules, highlighting a new crucial function of αII-spectrin in regulation of angiogenesis.
The above-mentioned data indicate involvement of spectrin not only in cell–cell and cell–extracellular matrix interactions, but also in morphogenesis, which at least in part is related to its interactions with adhesion molecules and with membrane proteins (and perhaps lipids) and/or with elements of actin or microtubular systems. Also some of those interactions may participate in signal transduction, and some signal transduction pathway proteins may be regulated by interactions with spectrins.
Conclusion
Spectrins are proteins that are responsible for many aspects of the function of cells and their adaptation to changing environments. Primarily the spectrin-dependent cytoskeleton supports cell shape and maintains cell membrane integrity and its mechanical properties. It is involved in cell architecture, morphology, and plasma membrane stability. Additionally, spectrins play multiple roles in cell physiology. They function as an interface for signal transduction mediation and interact with membrane channels, adhesion molecules, receptors, and transporters. They are involved in cell adhesion, lamellipodia extension, and cell spreading through several ligands and partners regulating actin dynamics. In most cells spectrins are known to be engaged in cell–cell contact. New findings support the participation of spectrins in the processes of angiogenesis and morphogenesis.
Authors’ contribution
All authors participated in conceptual development, design, and writing of the review.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
Supported by National Science Centre, Poland, Project No. 2015/19/B/NZ5/03469.
References
- 1.Lux SE. Anatomy of the red cell membrane skeleton: unanswered questions. Blood 2016; 127:187–99 [DOI] [PubMed] [Google Scholar]
- 2.Bennett V, Lorenzo DN. An adaptable spectrin/ankyrin-based mechanism for long-range organization of plasma membranes in vertebrate tissues. Curr Top Membr 2016;77:143–84 [DOI] [PubMed]
- 3.Goodman SR, Zagon IS, Kulikowski RR. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci USA 1981; 78:7570–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med 2008; 14:28–36 [DOI] [PubMed] [Google Scholar]
- 5.Machnicka B, Czogalla A, Hryniewicz-Jankowska A, Bogusławska DM, Grochowalska R, Heger E, Sikorski AF. Spectrins: a structural platform for stabilization and activation of membrane channels, receptors and transporters. Biochim Biophys Acta 2014; 1838:620–34 [DOI] [PubMed] [Google Scholar]
- 6.Delalande O, Czogalla A, Hubert J-F, Sikorski A, Le Rumeur E. Dystrophin and spectrin, two highly dissimilar sisters of the same family. Subcell Biochem 2017; 82:373–403 [DOI] [PubMed] [Google Scholar]
- 7.Nedrelow JH, Cianci CD, Morrow JS. c-Src binds alpha II spectrin’s Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176. J Biol Chem 2003; 278:7735–41 [DOI] [PubMed] [Google Scholar]
- 8.Nicolas G, Fournier CM, Galand C, Malbert-Colas L, Bournier O, Kroviarski Y, Bourgeois M, Camonis JH, Dhermy D, Grandchamp B, Lecomte MC. Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol Cell Biol 2002; 22:3527–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotter B, Kroviarski Y, Nicolas G, Dhermy D, Lecomte M-C. AlphaII-spectrin is an in vitro target for caspase-2, and its cleavage is regulated by calmodulin binding. Biochem J 2004; 378:1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan L, Yan R, Li W, Xu K. Super-resolution microscopy reveals the native ultrastructure of the erythrocyte cytoskeleton. Cell Reports 2018; 22:1151–8 [DOI] [PubMed] [Google Scholar]
- 11.De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J Cell Sci 2000; 113:2331–43 [DOI] [PubMed] [Google Scholar]
- 12.Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 2013; 339:4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang B, Babcock H, Zhuang X. Breaking the diffraction barrier: super-resolution imaging of cells. Cell 2010; 143:1047–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sahl SJ, Hell SW, Jakobs S. Fluorescence nanoscopy in cell biology. Nat Rev Mol Cell Biol 2017; 18:685–701 [DOI] [PubMed] [Google Scholar]
- 15.Baines AJ. The spectrin-ankyrin-4.1-adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life. Protoplasma 2010; 244:99–131 [DOI] [PubMed] [Google Scholar]
- 16.Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol 1993; 9:27–66 [DOI] [PubMed] [Google Scholar]
- 17.An X, Guo X, Sum H, Morrow J, Gratzer W, Mohandas N. Phosphatidylserine binding sites in erythroid spectrin: location and implications for membrane stability. Biochemistry 2004; 43:310–5 [DOI] [PubMed] [Google Scholar]
- 18.Hryniewicz-Jankowska A, Bok E, Dubielecka P, Chorzalska A, Diakowski W, Jezierski A, Lisowski M, Sikorski AF. Mapping of an ankyrin-sensitive, phosphatidylethanolamine/phosphatidylcholine mono- and bi-layer binding site in erythroid beta-spectrin. Biochem J 2004; 382:677–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bok E, Plazuk E, Hryniewicz-Jankowska A, Chorzalska A, Szmaj A, Dubielecka PM, Stebelska K, Diakowski W, Lisowski M, Langner M, Sikorski AF. Lipid-binding role of betaII-spectrin ankyrin-binding domain. Cell Biol Int 2007; 31:1482–94 [DOI] [PubMed] [Google Scholar]
- 20.Ray S, Chakrabarti A. Membrane interaction of erythroid spectrin: surface-density-dependent high-affinity binding to phosphatidylethanolamine. Mol Membr Biol 2004; 21:93–100 [DOI] [PubMed] [Google Scholar]
- 21.Wolny M, Grzybek M, Bok E, Chorzalska A, Lenoir M, Czogalla A, Adamczyk K, Kolondra A, Diakowski W, Overduin M, Sikorski AF. Key amino acid residues of ankyrin-sensitive phosphatidylethanolamine/phosphatidylcholine-lipid binding site of βI-spectrin. PLoS One 2011; 6:e21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Este E, Kamin D, Göttfert F, El-Hady A, Hell SW. STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Reports 2015; 10:1246–51 [DOI] [PubMed] [Google Scholar]
- 23.Han B, Zhou R, Xia C, Zhuang X. Structural organization of the actin-spectrin–based membrane skeleton in dendrites and soma of neurons. Proc Natl Acad Sci 2017; 114:6678–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C-M, Zhang C, Zollinger DR, Leterrier C, Rasband MN. An αII spectrin-based cytoskeleton protects large-diameter myelinated axons from degeneration. J Neurosci 2017; 37:11323–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J, Zhou R, Wu Z, Carrasco MA, Kurshan PT, Farley JE, Simon DJ, Wang G, Han B, Hao J, Heller E, Freeman MR, Shen K, Maniatis T, Tessier-Lavigne M, Zhuang X. Prevalent presence of periodic actin–spectrin-based membrane skeleton in a broad range of neuronal cell types and animal species. Proc Natl Acad Sci 2016; 113:6029–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Este E, Kamin D, Balzarotti F, Hell SW. Ultrastructural anatomy of nodes of Ranvier in the peripheral nervous system as revealed by STED microscopy. Proc Natl Acad Sci USA 2017; 114:191–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Susuki K, Otani Y, Rasband MN. Submembranous cytoskeletons stabilize nodes of Ranvier. Exp Neurol 2016; 283:446–51 [DOI] [PubMed] [Google Scholar]
- 28.Delaunay J. The molecular basis of hereditary red cell membrane disorders. Blood Rev 2007; 21:1–20 [DOI] [PubMed] [Google Scholar]
- 29.Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet 2008; 372:1411–26 [DOI] [PubMed] [Google Scholar]
- 30.Machnicka B, Grochowalska R, Bogusławska DM, Sikorski AF, Lecomte MC. Spectrin-based skeleton as an actor in cell signaling. Cell Mol Life Sci 2012; 69:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauser M, Yan R, Li W, Repina NA, Schaffer DV, Xu K. The spectrin-actin-based periodic cytoskeleton as a conserved nanoscale scaffold and ruler of the neural stem cell lineage. Cell Rep 2018; 24:1512–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins E, Suminaite D, Jackson M. Cerebellar ataxias: β-III spectrin’s interactions suggest common pathogenic pathways. J Physiol 2016; 594:4661–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lise S, Clarkson Y, Perkins E, Kwasniewska A, Sadighi Akha E, Parolin Schnekenberg R, Suminaite D, Hope J, Baker I, Gregory L, Green A, Allan C, Lamble S, Jayawant S, Quaghebeur G, Cader MZ, Hughes S, Armstrong RJ, Kanapin A, Rimmer A, Lunter G, Mathieson I, Cazier JB, Buck D, Taylor JC, Bentley D, McVean G, Donnelly P, Knight SJ, Jackson M, Ragoussis J, Németh AH. Recessive mutations in SPTBN2 implicate β-III spectrin in both cognitive and motor development. PLoS Genetics 2012; 8:1003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, Stevanin G, Dürr A, Zühlke C, Bürk K, Clark HB, Brice A, Rothstein JD, Schut LJ, Day JW, Ranum LP. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet 2006; 38:184–90 [DOI] [PubMed] [Google Scholar]
- 35.Perkins EM Clarkson YL Sabatier N Longhurst DM Millward CP Jack J, Toraiwa J, Watanabe M, Rothstein JD, Lyndon AR, Wyllie DJ, Dutia MB, Jackson M.. Loss of -III spectrin leads to Purkinje cell dysfunction recapitulating the behavior and neuropathology of spinocerebellar ataxia type 5 in humans. J Neurosci 2010; 30:4857–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Ji T, Nelson AD, Glanowska K, Murphy GG, Jenkins PM, Stevanin G, Dürr A, Zühlke C, Bürk K, Clark HB, Brice A, Rothstein JD, Schut LJ, Day JW, Ranum LP. Critical roles of αII spectrin in brain development and epileptic encephalopathy. J Clin Investig 2018; 128:760–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tohyama J, Nakashima M, Nabatame S, Gaik-Siew C, Miyata R, Rener-Primec Z, Kato M, Matsumoto N, Saitsu H. SPTAN1 encephalopathy: distinct phenotypes and genotypes. J Hum Genet 2015; 60:167–73 [DOI] [PubMed] [Google Scholar]
- 38.Saitsu H, Tohyama J, Kumada T, Egawa K, Hamada K, Okada I, Mizuguchi T, Osaka H, Miyata R, Furukawa T, Haginoya K, Hoshino H, Goto T, Hachiya Y, Yamagata T, Saitoh S, Nagai T, Nishiyama K, Nishimura A, Miyake N, Komada M, Hayashi K, Hirai S, Ogata K, Kato M, Fukuda A, Matsumoto N. Dominant-negative mutations in alpha-II spectrin cause West syndrome with severe cerebral hypomyelination, spastic quadriplegia, and developmental delay. Am J Hum Genet 2010; 86:881–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derbala MH, Guo AS, Mohler PJ, Smith SA. The role of βII spectrin in cardiac health and disease. Life Sci 2018; 192:278–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubreuil RR, Wang P, Dahl S, Lee J, Goldstein LS. Drosophila beta spectrin functions independently of alpha spectrin to polarize the Na, K ATPase in epithelial cells. J Cell Biol 2000; 149:647–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang Y, Katuri V, Dillner A, Mishra B, Deng C-X, Mishra L. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science 2003; 299:574–7 [DOI] [PubMed] [Google Scholar]
- 42.Musacchio A, Noble M, Pauptit R, Wierenga R, Saraste M. Crystal structure of a Src-homology 3 (SH3) domain. Nature 1992; 359:851–5 [DOI] [PubMed] [Google Scholar]
- 43.Bournier O, Kroviarski Y, Rotter B, Nicolas G, Lecomte MC, Dhermy D. Spectrin interacts with EVL (Enabled/vasodilator-stimulated phosphoprotein-like protein), a protein involved in actin polymerization. Biol Cell 2006; 98:279–93 [DOI] [PubMed] [Google Scholar]
- 44.Ziemnicka-Kotula D, Xu J, Gu H, Potempska A, Kim KS, Jenkins EC, Trenkner E, Kotula L. Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton. J Biol Chem 1998; 273:13681–92 [DOI] [PubMed] [Google Scholar]
- 45.Rotter B, Bournier O, Nicolas G, Dhermy D, Lecomte M-C. AlphaII-spectrin interacts with Tes and EVL, two actin-binding proteins located at cell contacts. Biochem J 2005; 388:631–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bialkowska K, Saido TC, Fox J. SH3 domain of spectrin participates in the activation of Rac in specialized calpain-induced integrin signaling complexes. J Cell Sci 2005; 118:381–95 [DOI] [PubMed] [Google Scholar]
- 47.Ponceau A, Albigès-Rizo C, Colin-Aronovicz Y, Destaing O, Lecomte MC. αII-spectrin regulates invadosome stability and extracellular matrix degradation. PLoS One 2015; 10:e0120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benz PM, Blume C, Moebius J, Oschatz C, Schuh K, Sickmann A, Walter U, Feller SM, Renné T. Cytoskeleton assembly at endothelial cell–cell contacts is regulated by αII-spectrin–VASP complexes. J Cell Biol 2008; 180:205–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trinh-Trang-Tan M-M, Bigot S, Picot J, Lecomte M-C, Kordeli E. AlphaII-spectrin participates in the surface expression of cell adhesion molecule L1 and neurite outgrowth. Exp Cell Res 2014; 322:365–80 [DOI] [PubMed] [Google Scholar]
- 50.Meissner JM, Sikorski AF, Nawara T, Grzesiak J, Marycz K, Bogusławska DM, Michalczyk I, Lecomte MC, Machnicka B. αII-spectrin in T cells is involved in the regulation of cell-cell contact leading to immunological synapse formation? PLoS One 2017; 12:e0189545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, Lecomte M-C. αII-spectrin is critical for cell adhesion and cell cycle. J Biol Chem 2009; 284:2409–18 [DOI] [PubMed] [Google Scholar]
- 52.Huang C-M, Zhang C, Ho T-Y, Oses-Prieto J, Burlingame AL, Lalonde J, Noebels JL, Leterrier C, Rasband MN. αII spectrin forms a periodic cytoskeleton at the axon initial segment and is required for nervous system function. J Neurosci 2017; 37:11311–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duan R, Kim JH, Shilagardi K, Schiffhauer ES, Lee DM, Son S, Li S, Thomas C, Luo T, Fletcher DA, Robinson DN, Chen EH. Spectrin is a mechanoresponsive protein shaping fusogenic synapse architecture during myoblast fusion. Nat Cell Biol 2018; 20:688–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stankewich MC, Cianci CD, Stabach PR, Ji L, Nath A, Morrow JS. Cell organization, growth, and neural and cardiac development require αII-spectrin. J Cell Sci 2011; 124:3956–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urwyler O, Cortinas-Elizondo F, Suter B. Drosophila sosie functions with β(H)-Spectrin and actin organizers in cell migration, epithelial morphogenesis and cortical stability. Biol Open 2012; 1:994–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leshchyns’ka I, Sytnyk V, Morrow JS, Schachner M. Neural cell adhesion molecule (NCAM) association with PKCbeta2 via betaI spectrin is implicated in NCAM-mediated neurite outgrowth. J Cell Biol 2003; 161:625–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pollerberg GE, Schachner M, Davoust J. Differentiation state-dependent surface mobilities of two forms of the neural cell adhesion molecule. Nature 1986; 324:462–5 [DOI] [PubMed] [Google Scholar]
- 58.Ramser EM, Buck F, Schachner M, Tilling T. Binding of αII spectrin to 14-3-3β is involved in NCAM-dependent neurite outgrowth. Mol Cell Neurosci 2010; 45:66–74 [DOI] [PubMed] [Google Scholar]
- 59.Garver TD, Ren Q, Tuvia S, Bennett V. Tyrosine phosphorylation at a site highly conserved in the L1 family of cell adhesion molecules abolishes ankyrin binding and increases lateral mobility of neurofascin. J Cell Biol 1997; 137:703–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buhusi M, Schlatter MC, Demyanenko GP, Thresher R, Maness PF. L1 interaction with ankyrin regulates mediolateral topography in the retinocollicular projection. J Neurosci 2008; 28:177–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian N, Leshchyns’ka I, Welch JH, Diakowski W, Yang H, Schachner M, Sytnyk V. Lipid raft-dependent endocytosis of close homolog of adhesion molecule L1 (CHL1) promotes neuritogenesis. J Biol Chem 2012; 287:44447–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouley M, Tian MZ, Paisley K, Shen YC, Malhotra JD, Hortsch M. The L1-type cell adhesion molecule neuroglian influences the stability of neural ankyrin in the Drosophila embryo but not its axonal localization. J Neurosci 2000; 20:4515–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Enneking E-M, Kudumala SR, Moreno E, Stephan R, Boerner J, Godenschwege TA, Pielage J. Transsynaptic coordination of synaptic growth, function, and stability by the L1-type CAM Neuroglian. PLoS Biol 2013; 11:e1001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buhusi M, Midkiff BR, Gates AM, Richter M, Schachner M, Maness PF. Close homolog of L1 is an enhancer of integrin-mediated cell migration. J Biol Chem 2003; 278:25024–31 [DOI] [PubMed] [Google Scholar]
- 65.Tuvia S, Garver TD, Bennett V. The phosphorylation state of the FIGQY tyrosine of neurofascin determines ankyrin-binding activity and patterns of cell segregation. Proc Natl Acad Sci USA 1997; 94:12957–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Davis JQ, Carpenter S, Bennett V. Structural requirements for association of neurofascin with ankyrin. J Biol Chem 1998; 273:30785–94 [DOI] [PubMed] [Google Scholar]
- 67.Susuki K Zollinger DR Chang K-J Zhang C Huang C-M Tsai C-R, Galiano MR, Liu Y, Benusa SD, Yermakov LM, Griggs RB, Dupree JL, Rasband MN. Glial βII spectrin contributes to paranode formation and maintenance. J Neurosci 2018; 38:6063–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis JQ, Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem 1994; 269:27163–6 [PubMed] [Google Scholar]
- 69.Yageta M, Kuramochi M, Masuda M, Fukami T, Fukuhara H, Maruyama T, Shibuya M, Murakami Y. Direct association of TSLC1 and DAL-1, two distinct tumor suppressor proteins in lung cancer. Cancer Res 2002; 62:5129–33 [PubMed] [Google Scholar]
- 70.Kroviarski Y, El Nemer W, Gane P, Rahuel C, Gauthier E, Lecomte MC, Cartron JP, Colin Y, Le Van Kim C. Direct interaction between the Lu/B-CAM adhesion glycoproteins and erythroid spectrin. Br J Haematol 2004; 126:255–64 [DOI] [PubMed] [Google Scholar]
- 71.Collec E, Lecomte M-C, El Nemer W, Colin Y, Le Van Kim C. Novel role for the Lu/BCAM–spectrin interaction in actin cytoskeleton reorganization. Biochem J 2011; 436:699–708 [DOI] [PubMed] [Google Scholar]
- 72.Lee H-G, Zarnescu DC, MacIver B, Thomas GH. The cell adhesion molecule roughest depends on heavy-spectrin during eye morphogenesis in Drosophila. J Cell Sci 2010; 123:277–85 [DOI] [PubMed] [Google Scholar]
- 73.Tjota M, Lee S-K, Wu J, Williams JA, Khanna MR, Thomas GH. Annexin B9 binds to β(H)-spectrin and is required for multivesicular body function in Drosophila. J Cell Sci 2011; 124:2914–26 [DOI] [PubMed] [Google Scholar]
- 74.Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, Vitols E, Vijayadamodar G, Pochart P, Machineni H, Welsh M, Kong Y, Zerhusen B, Malcolm R, Varrone Z, Collis A, Minto M, Burgess S, McDaniel L, Stimpson E, Spriggs F, Williams J, Neurath K, Ioime N, Agee M, Voss E, Furtak K, Renzulli R, Aanensen N, Carrolla S, Bickelhaupt E, Lazovatsky Y, DaSilva A, Zhong J, Stanyon CA, Finley RL, Jr, White KP, Braverman M, Jarvie T, Gold S, Leach M, Knight J, Shimkets RA, McKenna MP, Chant J, Rothberg JM. A protein interaction map of Drosophila melanogaster. Science 2003; 302:1727–36 [DOI] [PubMed] [Google Scholar]
- 75.An X, Gauthier E, Zhang X, Guo X, Anstee DJ, Mohandas N, Chasis JA. Adhesive activity of Lu glycoproteins is regulated by interaction with spectrin. Blood 2008; 112:5212–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puchkov D, Leshchyns’ka I, Nikonenko AG, Schachner M, Sytnyk V. NCAM/spectrin complex disassembly results in PSD perforation and postsynaptic endocytic zone formation. Cerebral Cortex 2011; 21:2217–32 [DOI] [PubMed] [Google Scholar]
- 77.Schlatter MC, Buhusi M, Wright AG, Maness PF. CHL1 promotes Sema3A-induced growth cone collapse and neurite elaboration through a motif required for recruitment of ERM proteins to the plasma membrane. J Neurochem 2008; 104:731–44 [DOI] [PubMed] [Google Scholar]
- 78.Voas MG, Lyons DA, Naylor SG, Arana N, Rasband MN, Talbot WS. alphaII-spectrin is essential for assembly of the nodes of Ranvier in myelinated axons. Curr Biol 2007; 17:562–8 [DOI] [PubMed] [Google Scholar]
- 79.Susuki K, Raphael AR, Ogawa Y, Stankewich MC, Peles E, Talbot WS, Rasband MN. Schwann cell spectrins modulate peripheral nerve myelination. Proc Natl Acad Sci USA 2011; 108:8009–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dou X, Menkari C, Mitsuyama R, Foroud T, Wetherill L, Hammond P, Suttie M, Chen X, Chen SY, Charness ME. L1 coupling to ankyrin and the spectrin-actin cytoskeleton modulates ethanol inhibition of L1 adhesion and ethanol teratogenesis. FASEB J 2018; 32:1364–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leshchyns’ka I, Sytnyk V. Reciprocal interactions between cell adhesion molecules of the immunoglobulin superfamily and the cytoskeleton in neurons. Front Cell Dev Biol 2016; 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell 2004; 15:2932–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tabdanov E, Gondarenko S, Kumari S, Liapis A, Dustin ML, Sheetz MP, Kam LC, Iskratsch T. Micropatterning of TCR and LFA-1 ligands reveals complementary effects on cytoskeleton mechanics in T cells. Integr Biol 2015; 7:1272–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas GH, Zarnescu DC, Juedes AE, Bales MA, Londergan A, Korte CC, Kiehart DP. Drosophila betaHeavy-spectrin is essential for development and contributes to specific cell fates in the eye. Development 1998; 125:2125–34 [DOI] [PubMed] [Google Scholar]
- 85.Williams JA, MacIver B, Klipfell EA, Thomas GH. The C-terminal domain of Drosophila (beta) heavy-spectrin exhibits autonomous membrane association and modulates membrane area. J Cell Sci 2004; 117:771–82 [DOI] [PubMed] [Google Scholar]
- 86.Wu J, Bakerink KJ, Evangelista ME, Thomas GH. Cytoplasmic capes are nuclear envelope intrusions that are enriched in endosomal proteins and depend upon βH-spectrin and Annexin B9. PLoS One 2014; 9:e93680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell 1994; 79:922. [PubMed] [Google Scholar]
- 88.Nonoyama S, Ochs HD. Characterization of the Wiskott-Aldrich syndrome protein and its role in the disease. Curr Opin Immunol 1998; 10:407–12 [DOI] [PubMed] [Google Scholar]
- 89.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol 2000; 1:23–9 [DOI] [PubMed] [Google Scholar]
- 90.Dustin ML. The immunological synapse. Cancer Immunol Res 2014; 2:1023–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cairo CW, Das R, Albohy A, Baca QJ, Pradhan D, Morrow JS, Coombs D, Golan DE. Dynamic regulation of CD45 lateral mobility by the spectrin-ankyrin cytoskeleton of T cells. J Biol Chem 2010; 285:11392–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BLM, Bennett V. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem 2007; 282:26552–61 [DOI] [PubMed] [Google Scholar]
- 93.Kizhatil K, Yoon W, Mohler PJ, Davis LH, Hoffman JA, Bennett V. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J Biol Chem 2007; 282:2029–37 [DOI] [PubMed] [Google Scholar]
- 94.Weber KL, Fischer RS, Fowler VM. Tmod3 regulates polarized epithelial cell morphology. J Cell Sci 2007; 120:3625–32 [DOI] [PubMed] [Google Scholar]
- 95.Toya M, Kobayashi S, Kawasaki M, Shioi G, Kaneko M, Ishiuchi T, Misaki K, Meng W, Takeichi M. CAMSAP3 orients the apical-to-basal polarity of microtubule arrays in epithelial cells. Proc Natl Acad Sci USA 2016; 113:332–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sormunen RT, Leong AS, Vääräniemi JP, Fernando SS, Eskelinen SM. Immunolocalization of the fodrin, E-cadherin, and beta-catenin adhesion complex in infiltrating ductal carcinoma of the breast-comparison with an in vitro model. J Pathol 1999; 187:416–23 [DOI] [PubMed] [Google Scholar]
- 97.Pradhan D, Lombardo CR, Roe S, Rimm DL, Morrow JS. alpha-Catenin binds directly to spectrin and facilitates spectrin-membrane assembly in vivo. J Biol Chem 2001; 276:4175–81 [DOI] [PubMed] [Google Scholar]
- 98.Katuri V, Tang Y, Li C, Jogunoori W, Deng C-X, Rashid A, Sidawy AN, Evans S, Reddy EP, Mishra B, Mishra L. Critical interactions between TGF-beta signaling/ELF, and E-cadherin/beta-catenin mediated tumor suppression. Oncogene 2006; 25:1871–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsukamoto T, Nigam SK. Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J Biol Chem 1997; 272:16133–9 [DOI] [PubMed] [Google Scholar]
- 100.Mattagajasingh SN, Huang SC, Hartenstein JS, Benz EJ. Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton. J Biol Chem 2000; 275:30573–85 [DOI] [PubMed] [Google Scholar]
- 101.Beck KA, Nelson WJ. The spectrin-based membrane skeleton as a membrane protein-sorting machine. Am J Physiol 1996; 270:C1263–1270 [DOI] [PubMed] [Google Scholar]
- 102.Ursitti JA, Petrich BG, Lee PC, Resneck WG, Ye X, Yang J, Randall WR, Bloch RJ, Wang Y. Role of an alternatively spliced form of alphaII-spectrin in localization of connexin 43 in cardiomyocytes and regulation by stress-activated protein kinase. J Mol Cell Cardiol 2007; 42:572–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abdi KM, Bennett V. Adducin promotes micrometer-scale organization of beta2-spectrin in lateral membranes of bronchial epithelial cells. Mol Biol Cell 2008; 19:536–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu J, Masci PP, Chen C, Chen J, Lavin MF, Zhao K-N. β-Adducin siRNA disruption of the spectrin-based cytoskeleton in differentiating keratinocytes prevented by calcium acting through calmodulin/epidermal growth factor receptor/cadherin pathway. Cell Signal 2015; 27:15–25 [DOI] [PubMed] [Google Scholar]
- 105.Zhao K-N, Masci PP, Lavin MF. Disruption of spectrin-like cytoskeleton in differentiating keratinocytes by PKCδ activation is associated with phosphorylated adducin. PLoS One 2011; 6:e28267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lechuga S, Amin PH, Wolen AR, Ivanov AI. Adducins inhibit lung cancer cell migration through mechanisms involving regulation of cell-matrix adhesion and cadherin-11 expression. Biochim Biophys Acta Mol Cell Res 2019; 1866:395–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen TW, Chen G, Funkhouser LJ, Nam S-C. Membrane domain modulation by spectrins in Drosophila photoreceptor morphogenesis. Genesis 2009; 47:744–50 [DOI] [PubMed] [Google Scholar]