Abstract
Introduction
Transmitted, or any pretreatment drug resistance (TDR, PDR) can compromise efficacy of first‐line antiretroviral therapy (ART). In Peru, genotypic resistance testing is not routinely performed before ART initiation, and estimated PDR prevalence prior to 2012 ranged from 1.0% to 4.7%. We aimed to update estimates of PDR prevalence in men who have sex with men (cis‐MSM) and transgender women (TW).
Methods
We obtained HIV sequences from three studies of ART‐naïve cisgender‐MSM and TW (n = 470) in Lima, Peru from 2013 to 2017, almost two‐thirds of whom had acute or recent infections. Sanger sequences of HIV pol were interrogated for surveillance drug resistance mutations (SDRM) using the Stanford Calibrated Population Resistance (CPR) tool and scored for resistance to nucleoside reverse transcriptase inhibitors (NRTIs) and non‐nucleoside reverse transcriptase inhibitors (NNRTIs) with the HIVdb programme. We calculated binomial proportions and 95% confidence intervals. χ2 and exact or trend tests were used to examine predictors of PDR.
Results
Seventy‐seven (16.4%) individuals had PDR (95% CI: 13.2 to 20.0); most resistance was likely TDR since 63% were incident infections. SDRM were present in 9.8% (7.3 to 12.9). Resistance to any NRTI was present in <1% of individuals, while efavirenz resistance was present in 10% (6.9% to 12.4%). TW were not statistically more likely than cis‐MSM to have PDR (11.4% vs. 9.1%, p = 0.54). Age, incident versus prevalent infection, or residence district did not predict PDR. Prevalence of SDRM increased from 3% in 2013 to 21% 2017 within incident infections (p = 0.04), but not when including prevalent infections.
Conclusions
Prevalence of NNRTI resistance in three studies of ART‐naïve MSM and TW in Lima, Peru reaches 10%. Because our study reports PDR in a population in which most acquired HIV recently, the overall prevalence of PDR, including previously treated persons, is likely underestimated. These results underscore the need for a nationally representative survey of PDR in Peru and consideration of non‐NNRTI anchored first‐line ART options. This study also represents the first evaluation of PDR in cis‐MSM versus TW in South America, and demonstrates that, although TW are at higher risk of acquiring HIV, they are at similar risk of acquiring a virus with resistance mutations.
Keywords: drug resistance, Latin America and the Caribbean, transgender people, men who have sex with men, Peru
1. Introduction
The ability to successfully treat HIV‐1 infection is contingent upon the continued efficacy of antiretroviral drugs (ARVs). Of 36.9 million persons living with HIV (PLWH) worldwide, 21.7 million (or about 60%) were accessing combination antiretroviral therapy (ART) as of 2017 1. Acquisition of much HIV drug resistance (HIVDR) is attributed to poor ART adherence, leading to periods of sub‐therapeutic ARV levels and functional mono‐ or dual‐therapy, which can ultimately result in virologic failure (acquired drug resistance (ADR)) 2, 3. Other causes of ADR include use of the non‐fully suppressive regimens available prior to the mid‐1990s, intermittent provision of ARVs, including prior peripartum prevention approaches 4, 5, 6, 7, or problems with consistent ART supply chain 8, 9. Transmitted drug resistance (TDR) refers to HIVDR present when a new HIV infection is acquired, whether through horizontal or vertical transmission; pretreatment drug resistance (PDR) is the broader term encompassing TDR and any HIVDR present at the initiation or re‐initiation of ART, and is more commonly used to describe infections of unknown duration and when TDR cannot be conclusively ascertained. PDR can result in failure of first‐line regimens, especially when not recognized at time of ART initiation 10. The WHO currently recommends initiation of ART regimens not anchored on non‐nucleoside reverse transcriptase inhibitors (NNRTI) when PDR exceeds 10%, or else genotypic testing for HIVDR prior to ART initiation if not feasible to empirically use non‐NNRTI regimen 11. Testing for PDR is currently the standard of care in high‐income countries including the US 12, 13.
Peru instituted a National ART Programme in 2004 after the introduction of ART in the country in 1999 14. This programme has provided free access to ART for PLWH since 2006. At present, first‐line ART regimens in Peru include a combination of two nucleoside reverse transcriptase inhibitors (NRTI) with one non‐nucleoside reverse transcriptase inhibitor (NNRTI), generally efavirenz (EFV); the estimated ART coverage in Peru in 2018 was 73%, compared with 31% in 2010 15. These regimens are initiated empirically without routine genotypic testing for PDR. Previously reported prevalence in Peru ranges between 1% TDR from a study of a mixed population in Lima with data acquired before 2009 (n = 96) 16 to a high of 4.7% PDR from a study of men who have sex with men (MSM) from several Andean countries, including Peru, in 2009 (n = 149) 17. The Peruvian Sentinel Surveillance Survey from 2002 to 2003 reported a prevalence of 3.3% PDR in MSM from six cities (n = 359) 18. In its most recent report of HIV drug resistance, the WHO reported PDR in nationally‐representative ART naïve populations in Argentina, Brazil, and Colombia to be between 9.8% and 12.8%, with estimates increasing over time 19, although data from Peru have never been included in these reports due to the limited availability of surveillance data.
In Peru, cisgender MSM (cis‐MSM) and transgender women (TW) comprise the majority of PLWH. The HIV prevalence within TW has been estimated to be as high as 30% 20, 21, while the overall HIV prevalence in the Peruvian population aged 15 to 49 is 0.3% 22. Additionally, the HIV epidemic is concentrated in the capital, where not only the highest concentration of cis‐MSM live, but 22% of cis‐MSM are living with HIV; national HIV prevalence in cis‐MSM is approximately 12% 22, 23. Although cis‐MSM and TW represent the majority of PLWH in Peru, these groups have frequently been grouped together in studies of high‐risk populations, even though they largely have separate sexual networks. Because it is uncommon for TW to partner with cis‐MSM or other TW, and their partners may be less likely to be engaged in HIV care 24, it is possible that there is a differential risk of TDR between cis‐MSM and TW. Demonstration of increasing PDR within Latin American countries emphasizes the need for broader surveillance to inform policy and guidelines, inclusion of data from Peru, and separate reporting of data for unique risk populations 16.
2. Methods
We obtained HIV‐1 sequences and corresponding participant data from three completed parent studies in Lima, Peru: ¿Sabes?: HIV Testing and Treatment to Prevent Onward HIV Transmission among MSM and Transgender Women in Lima, Peru (“Sabes”) 25, Spatial and Phylogenetic Clusters of HIV Microepidemics Among MSM in Lima (“Microepidemics”), and Gender‐Affirmative Transgender Care to Improve the HIV Treatment Cascade (“Feminas”) 26. Sabes is a multi‐step longitudinal study using a Seek‐Test‐Treat‐Retain (STTR) strategy to reduce community viral load to decrease HIV transmission (2013 to 2016, n = 3337). Participants in this study who screened HIV‐negative at Step 1 and consented to Step 2 (n = 2109) underwent monthly antibody/antigen testing and HIV RNA testing if seronegative, such that the timing of most infections was precisely known. Participants with acute or recent (within three months) infection detected in Steps 1 and 2 were eligible to join the Step 3 ART interventional study. We obtained sequence data from all Step 3 eligible participants (n = 256) as well as participants with incident infection of longer or unknown duration (n = 111). Microepidemics employed mobile testing vans dispatched to nightclubs and plazas in 2016 to 2017 to increase testing of high‐risk MSM and characterize HIV transmission “hot‐spots” by combining viral phylogenetics and geospatial mapping of neighborhoods and social venues in Lima. New HIV cases were defined as those for whom no prior positive data relating to HIV care, including ART use, were documented in the Peruvian national laboratory database. In this analysis, only participants with new HIV diagnosis and no prior record in the national ART programme were included to exclude possibility of prior ARV exposure. Lastly, Feminas was a study of gender affirming medical services coupled with HIV testing and ART for TW in Lima (2016 to 2017); transgender women who were naïve to ART and desired feminine hormone therapy were enrolled to assess whether co‐provision of these services could improve engagement in care. As part of each parent study, HIV‐1 sequencing was performed on cryopreserved plasma from the first phlebotomy at HIV diagnosis. We included in this analysis all amplifiable sequences from persons in these three studies who had either been newly diagnosed during the study or who had a recent diagnosis of HIV, determined to be ART‐naïve and viremic.
This study was considered not human subjects research by the University of Washington Human Subjects Division, as all data was previously collected under the parent studies and deidentified prior to transfer to our institution. All parent studies received appropriate ethical approvals by the local and/or national Peruvian and US‐partner institutions.
2.1. Laboratory methods
The cDNA HIV pol sequences were reverse transcribed from viral RNA extracted from previously‐unthawed cryopreserved plasma, with Sanger sequencing as described previously 27. Amplification primers IBR2_M and MozFO_M were used to extend the 2510 to 3209 region of the HIV pol gene. Sequencing included the region of reverse transcriptase (RT) relevant for most clinically relevant drug‐resistance mutations (DRM) to NRTIs and NNTRIs; K238T/N, Y318F, and N348I mutations therefore were unlikely to be captured. Because sequence data were originally obtained for phylogenetic analysis, regions coding protease and integrase were not sequenced.
Prevalence of resistance mutations was assigned by the Calibrated Population Resistance Tool (CPR) 28, based on the WHO surveillance drug resistance mutation (SDRM) list 29. Per WHO recommendations, we then used the Genotypic Resistance Interpretation Algorithm – HIVdb Programme (HIVdb, Stanford University, Stanford, CA) to calculate penalty scores for relevant NRTI and NNRTI, and sequences were determined to be either susceptible (<15, including potential low‐level resistance) or resistant (≥15; low‐, medium‐, or high‐resistance) as well as to report all identified DRM (including other polymorphisms) 30. Sequences resulting in a mixed call at a given codon were given the highest score for a corresponding mutation. Consensus sequences from all genotyped individuals were aligned and manually edited, and neighbour‐joining phylogenetic trees were used to seek evidence of laboratory contamination.
2.2. Statistical analysis
We defined incident HIV infections as those in participants who were either seronegative at diagnosis (RNA or p24+ only; acute HIV) or else seropositive with confirmed negative test within the preceding six months. Any infection of known duration >6 months or seropositive without prior testing history was considered a prevalent infection. Kruskal‐Wallis and χ2 tests were used for descriptive statistics. SDRM and total PDR prevalence was determined using binomial proportions and 95% confidence intervals. χ2, trend, and Fisher's exact tests examined relationships between SDRM and gender identity, year of sample acquisition, diagnosis as incident or prevalent infection, age category, and residence district. For analysis of trend by year of sampling, we excluded persons whose first positive test was prior to 2013 (n = 5). We categorized residence district, as reported by Sabes and Feminas participants, into five geographic regions of the metropolitan area (Lima Norte, Lima Centro, Lima Sur, Lima Este, and Callao), containing all 43 administrative districts. We performed a Fisher's exact test to examine if there was heterogeneity between resistance patterns acquired by TW versus cis‐MSM populations. All statistical analysis was completed in the R package 3.5.0 using R‐Studio 1.1.453 31, 32.
3. Results
We obtained 471 successfully‐amplified sequences from plasma. We excluded 1 sample from an individual who reported first diagnosis of HIV infection in 1995. Samples were obtained as part of HIV testing at medical clinics or HIV testing sites (86%) or different LGBTQ social venues or plazas (14%). Two hundred and ninety‐nine (64%) sequences were obtained from participants with acute or incident infections (≤180 days since last negative test), and 171 (36%) were from prevalent infections (>180 days or unknown duration). Characteristics of participants, by parent study, are found in Table 1. Overall, 140 (30%) participants were TW and 330 (70%) were cis‐MSM. HIV‐1 infection was predominately with subtype B (n = 452). Additional subtypes and circulating recombinant forms (CRF) included A (n = 3), BC (n = 1), BF (n = 6), C (n = 1), CRF12_BF (n = 1), CRF44_BF (n = 5) and F (n = 1).
Table 1.
Characteristics of participants from the three parent studies
| Study | p‐value | |||
|---|---|---|---|---|
| Sabes | Microepidemics | Feminas | ||
| Total participants (%) | 367 | 64 | 39 | |
| Gender Identity | ||||
| Cisgender male | 284 (77) | 46 (72) | 0 (0) | <0.0001 |
| Transgender female | 83 (23) | 18 (28) | 39 (100) | |
| Age | ||||
| 18 to 25 | 195 (53) | 34 (53) | 20 (51) | <0.0001 |
| 26 to 35 | 138 (38) | 22 (34) | 15 (38) | |
| 36 to 45 | 25 (7) | 6 (9) | 3 (8) | |
| 46+ | 9 (2) | 2 (3) | 1 (3) | |
| Duration of Infection | ||||
| Incident (≤180 days) | 282 (77) | 6 (9) | 11 (28) | <0.0001 |
| Prevalent (>180 days) | 85 (23) | 58 (91) | 28 (72) | |
| Year of sample | ||||
| 2013 | 61 (17) | 0 (0) | 0 (0) | <0.0001 |
| 2014 | 133 (36) | 0 (0) | 0 (0) | |
| 2015 | 134 (37) | 0 (0) | 0 (0) | |
| 2016 | 39 (11) | 0 (0) | 22 (56) | |
| 2017 | 0 (0) | 64 (100) | 17 (44) | |
| Initial viral load (log10 copies/mL) | ||||
| Median (IQR) | 5.9 (5.1 to 6.7) | 5.2 (5.0 to 5.8) | 4.9 (4.6 to 5.1) | 0.37 |
| Initial CD4 + Count (cells/mL) | ||||
| Median (IQR) | 416 (278 to 567) | 347 (299 to 398) | 323 (260 to 562) | 0.63 |
Percent totals may not equal 100 due to rounding.
3.1. Pre‐treatment drug resistance
Looking first at all DRM (via HIVdb programme), a total of 92 DRM were identified across all sequences; this represented 22 unique base changes, 14 of which confer potential resistance to NNRTIs and 8 to NRTIs. Seventy‐seven individuals (16.4%) had at least one DRM (95% CI: 13.2 to 20.0) (Table 2). SDRM were present in 46 individuals (9.8%; 7.3 to 12.9). The most prevalent SDRM were K103N/S, G190A/E and M184V. Five thymidine analogue mutations (TAMs) were identified within two individuals, including Type II (n = 4), and Type I (n = 1) TAM pathways. Seven (1.5%) individuals had 2 or more unique mutations and Genotype Susceptibility Score (GSS) to first‐line regimens was ≥2 except in three individuals overall. There was no difference in prevalence of TDR between the three parent studies (p = 0.30).
Table 2.
Prevalence of Drug Resistance Mutations among 470 ART‐naive participants
| Type | # With mutations | Prevalence (95% CI) (n = 470) | |
|---|---|---|---|
| Total with any HIVDR | 77 | 16.4% (13.2 to 20.0) | |
| Total with SDRM | 46 | 9.8% (7.3 to 12.9) | |
| NNRTI SDRMs | 9.3% (6.9 to 12.4) | ||
| K103N/S | 34 | 7.4% (5.2 to 10.2) | |
| G190A/E | 6 | 1.3% (0.5 to 2.8) | |
| K101E | 3 | 0.6% (0.1 to 1.9) | |
| Y181C | 1 | 0.2% (0.0 to 1.2) | |
| Y188C | 1 | 0.2% (0.0 to 1.2) | |
| NRTI SDRMs | 1.3% (0.5 to 2.8) | ||
| M184V | 4 | 0.9% (0.2 to 2.2) | |
| T215F/Y | 2 | 0.4% (0.1 to 1.5) | |
| D67N | 1 | 0.2% (0.0 to 1.2) | |
| K70R | 1 | 0.2% (0.0 to 1.2) | |
| L74I | 1 | 0.2% (0.0 to 1.2) | |
| V75M | 1 | 0.2% (0.0 to 1.2) | |
| K219Q | 1 | 0.2% (0.0 to 1.2) | |
| Any TAM | 5 | 1.1% (0.3 to 2.5) | |
HIVDR, HIV Drug Resistance; SDRM, Surveillance Drug Resistance Mutations; TAMs, Thymidine analogue mutations, including both Type I and Type II.
Incorporating the cumulative effects of all DRM in a sequence, each participant was scored by HIVdb as either susceptible or resistant against 5 NRTIs currently used or considered for future use in Peru (abacavir (ABC), azidothymidine (AZT), emtricitabine (FTC), lamivudine (3TC), tenofovir disoproxil fumarate (TDF)) and 4 NNRTIs (efavirenz (EFV), nevirapine (NVP), rilpivirine (RPV) and doravirine (DOR); Table 3). Resistance to any NNRTI ranged from 5.0% (RPV) to 12% (NVP), while NRTI resistance was substantially lower, between 0.2% (TDF) and 0.9% (ABC, 3TC/FTC).
Table 3.
Frequency of resistance to commonly used nucleoside and non‐nucleoside reverse transcriptase inhibitors (NRTI and NNRTI)
| Antiretroviral drugs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| NRTI | NNRTI | ||||||||
| ARV | ABC | AZT | FTC | 3TC | TDF | EFV | NVP | RPV | DOR |
| Susceptible | 466 | 468 | 466 | 466 | 469 | 426 | 418 | 448 | 441 |
| Resistant | 4 | 2 | 4 | 4 | 1 | 44 | 52 | 22 | 29 |
| Overall % Resistance (95% CI) | 0.9 (0.2 to 2.2) | 0.5 (0.1 to 1.5) | 0.9 (0.2 to 2.2) | 0.9 (0.2 to 2.1) | 0.2 (0.0 to 1.2) | 10.0 (6.9 to 12.4) | 12.0 (8.4 to 14.3) | 5.0 (2.0 to 7.01) | 6.2 (4.2 to 8.7) |
ABC, abacavir; AZT, azidothymidine; FTC, emtricitabine; 3TC, lamivudine; TDF, tenofovir disoproxil fumarate; EFV, efavirenz; NVP, nevirapine; RPV, rilpivirine; DOR, doravirine.
3.2. Predictors of SDRM
There was no difference in the likelihood of SDRM between incident and prevalent infections (9.4% vs. 10.5%, p = 0.75). There was 91.3% concordance between year of HIV diagnosis and year of sample acquisition. After excluding five samples from participants who reported HIV diagnosis prior to 2013, there was no significant trend in overall SDRM prevalence between 2013 and 2017 (p = 0.33 for trend, Figure 1). Restricting this analysis to incident HIV diagnoses (n = 299), there was a significant increase across this period, (3% in 2013, 7% in 2014, 11% in 2015, 11% in 2016 and 21% in 2017; p = 0.04). The prevalence of SDRM in TW was 11.4% (95% CI: 6.7 to 17.9), which was not significantly higher than cis‐MSM at 9.1% (95% CI: 6.2 to 12.7), p = 0.54. However, we detected heterogeneity in the pattern of DRM between TW and cis‐MSM, meaning that observed mutations were not evenly distributed between gender identity groups (p < 0.0001). The following mutations were more common in the cis‐MSM population V108I, E138A and V179D; V179E and G190A were more common in TW.
Figure 1.
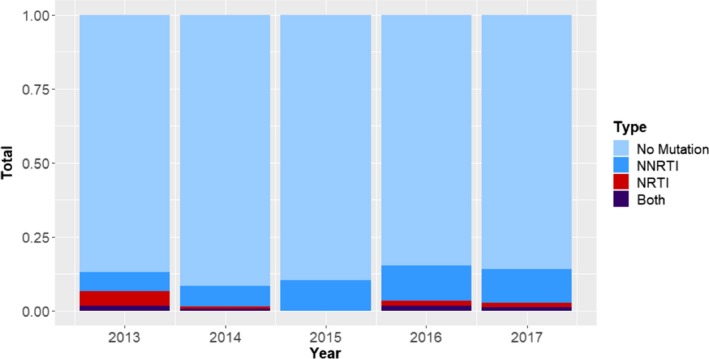
Surveillance drug resistance mutation prevalence by year. Percent and type of mutation for each sample year excluding five individuals with known positive date preceding study years.
Analysis by age likewise suggested no difference in PDR by age group (p = 0.82 for trend across age groups). Four‐hundred and five participants (86%) had residence district information available. Whether evaluated by individual residence district or the larger five regions of Lima, we also found no heterogeneity in risk of PDR (p = 0.57), including no single district with a statistically elevated risk of PDR.
4. Discussion
This study leveraged HIV sequence data from three studies of cis‐MSM and TW to complete the largest and most recent evaluation of PDR to our knowledge in Peru. Compared to prior studies in Peru estimating TDR or PDR at 4% or below, we observed 9.8% prevalence of SDRM and 16.4% prevalence of all DRM – nearly threefold higher than the previously documented results in 2009 17. The prevalence of resistance to the two currently available NNRTIs, EFV and NVP, were 10% and 12% respectively. Additionally, while prior estimates from Peru were lower than those from neighbouring countries, our observed PDR approximates the most recent nationally representative WHO PDR estimates for counties such as Argentina 12.8% (9.2 to 17.4), Brazil 9.8% (8.1 to 12.0) and Colombia 9.9% (7.5 to 12.9), despite the fact that our study only includes ART‐naïve persons, does not include PI mutations, and excludes cisgender women, who have the highest rate of PDR globally 19. In addition to increased PDR prevalence in our study compared to older data, the prevalence also increased across study years within the present report.
As in many other low‐ and middle‐income countries that adopted broad use of ART only after highly active combinations were available in the mid‐2000s, the most common DRMs detected confer resistance to NNRTIs, rather than NRTI or other classes. Higher prevalence of NNRTI compared to NRTI resistance is expected due to factors such as the pharmacokinetics of these drug classes, differential impact of DRM on viral fitness and therefore persistence of these strains, and less significantly in Peru, the prior use of single‐dose nevirapine in prevention of mother‐to‐child transmission of HIV (PMTCT). Because the epidemic in Peru is largely in persons assigned male sex at birth, and HIV prevalence in cisgender women is 0.2% overall 21, the high rate of NNRTI resistance is likely more attributable to adherence and supply‐chain consistency 8, 9. Because at the time of this study, HIV pre‐exposure prophylaxis (PrEP) with oral TDF/FTC was only available through studies and demonstration projects in Lima, our data is unlikely to show influence of PrEP on TDR, but is reassuring that TDF/FTC should retain high efficacy given the <1% observed prevalence of NRTI resistance.
We acknowledge certain limitations of this study. Our data examine HIVDR related to NRTI and NNRTI, but we were unable to investigate mutations that confer resistance to protease inhibitors (PI) or INSTI as available sequences lacked the gag/pol region coding for protease or integrase genes. At present however, neither PIs nor INSTIs are included in first‐line ART for adults in Peru, and so PDR to these classes is unlikely. Additionally, these data are based on high‐risk populations in Lima most of whom had incident HIV and were confirmed to be ART‐naive, and do not comprise a systematic national‐level PDR survey, which should include cisgender women and persons re‐initiating ART. However, with 90% of the HIV epidemic believed to be encompassed within MSM and TW communities, and largely within Lima, this sample is likely representative of TDR in PLWH at present in Peru 22, 23, 33. Additionally, two of three prior studies on PDR in Peru were focused in these same populations; thus, our estimates are comparable to these published data. Because we limited our analysis to newly diagnosed individuals most of whom had recently acquired HIV, we likely underestimate PDR, which can include previously treated individuals. Inclusion of such individuals would likely increase estimates to levels at or above other Latin American countries. Although not unique to our study, social desirability bias can cause participants to omit report of previous ART receipt. We mitigated this bias by cross‐referencing prevalent infections with the national ART programme database.
This study has several strengths, including the large sample size drawn from three different source studies, including participants recruited from studies offering HIV testing in both the clinic setting and at social venues, including dance clubs, saunas, plazas, and a programme featuring outreach specifically to TW. Therefore, we were able to access hidden populations that may not be included in testing of routine samples from ART‐initiation visits, which biases towards groups more likely to link to care. Additionally, the large proportion of incident infections and careful attention to verifying prior HIV testing history allowed us to largely exclude persons with long‐standing infections or prior ART exposure. The high concordance between year of sample with probable timing of infection allowed us to evaluate trends in new infections over time. We also performed all sequencing in an expert reference lab, which is currently otherwise unavailable in Peru. Lastly, we present the first differentiated estimates for TW and cis‐MSM populations in South America, which is important given the low overlap between these two populations. Since TW mainly partner with heterosexual‐identifying cisgender men 24, TDR in this population may better reflect infections that bridge to non‐MSM populations.
5. Conclusions
These data highlight the importance of ongoing monitoring of HIVDR as suggested by the WHO 34. The high prevalence of NNRTI mutations suggests that adoption of INSTI‐based regimens for first‐line therapy could be prudent, especially for cis‐MSM and TW. In the case of dolutegravir, the concern about possible neural tube defects would not be relevant in these populations 35. The risk of NRTI mutations remains low, even in comparison to Brazil 36, which recently reported a similar estimate for overall TDR but a threefold higher proportional risk for NRTI DRM. Our data therefore show little threat to the efficacy of NRTIs in current use in ART or as PrEP. Overall, the prevalence of TDR in cis‐MSM and TW in Lima is much higher that previously recognized, and despite different socioeconomic status, HIV incidence, and access of health and HIV services between these two populations, we found little difference in the risk of transmitted drug resistance in recent infections. Further geographically representative data from cisgender women, children and heterosexual men should be collected to inform national and regional ART programmes.
Competing interests
No authors report competing interests.
Authors' contributions
WLT performed all statistical analysis and wrote the initial manuscript. JRL, AD, HS, RC, TG, SLR and KHM designed and executed the parent studies, provided data and gave input to results and implications. PS provided input from the national ART programme. JM performed all laboratory work for the parent studies and assisted in interpretation of sequence data. RBI designed and assisted in the analysis and wrote the manuscript. All authors have read and contributed to the final manuscript.
Acknowledgements
We acknowledge the participants and staff of the Sabes, Microepidemics, and Feminas Studies in Lima, Peru. We appreciate the statistical support of Edward White PhD and Sayan Dasgupta PhD, as well as data management by IMPACTA PERU Clinical Trials Unit and Siavash Pasalar PhD.
Funding
Funding for this project was supported by the National Institute on Drug Abuse (NIDA) R01DA032106 (AD, JRL, JM) and National Institute of Allergy and Infectious Diseases K23AI129659 (RBI); UW/Fred Hutchinson Centers for AIDS Research Supplement 2P30‐AI027757 (AD, RC, HS, JRL); amfAR, The Foundation for AIDS Research 109071‐57‐HGMM (JRL, SR, KHM); HIV Medical Association – Medical Scholars Programme (WLT). TG received International AIDS Society (IAS), (NIDA) and French National Agency for Research on AIDS and Viral Hepatitis (ANRS) as a recipient of the HIV and Drug Use Research Fellowship; The Sabes study gratefully acknowledges donated ART from Gilead Sciences, Inc, and Merck & Co.
Trebelcock, W. L. , Lama, J. R. , Duerr, A. , Sanchez, H. , Cabello, R. , Gilada, T. , Segura, P. , Reisner, S. L. , Mayer, K. H. , Mullins, J. , Bender Ignacio, R. A. HIV pretreatment drug resistance among cisgender MSM and transgender women from Lima, Peru. J Int AIDS Soc. 2019; 22:e25411
Contributor Information
William L Trebelcock, Email: btrebelc@uw.edu.
Javier R Lama, Email: jrlama@impactaperu.org.
Ann Duerr, Email: aduerr@fredhutch.org.
Hugo Sanchez, Email: hsanchez@epicentro.org.pe.
Robinson Cabello, Email: rcabello@vialibre.org.pe.
Trupti Gilada, Email: trupti_gilada@yahoo.com.
Patricia Segura, Email: patita_segura@yahoo.com.
Sari L Reisner, Email: sreisner@fenwayhealth.org.
Kenneth H Mayer, Email: kmayer@fenwayhealth.org.
James Mullins, Email: jmullins@uw.edu.
Rachel A Bender Ignacio, Email: rbi13@uw.edu.
References
- 1. Fact sheet ‐ Latest global and regional statistics on the status of the AIDS epidemic. [Internet]. [cited 2018 Aug 16]. Available from: http://www.unaids.org/en/resources/documents/2018/UNAIDS_FactSheet
- 2. Kuritzkes DR, Lalama CM, Ribaudo HJ, Marcial M, Meyer WA, Shikuma C, et al. Preexisting resistance to nonnucleoside reverse‐transcriptase inhibitors predicts virologic failure of an efavirenz‐based regimen in treatment‐naive HIV‐1–infected subjects. J Infect Dis. 2008;197(6):867–70. [DOI] [PubMed] [Google Scholar]
- 3. Walsh JC, Pozniak AL, Nelson MR, Mandalia S, Gazzard BG. Virologic rebound on HAART in the context of low treatment adherence is associated with a low prevalence of antiretroviral drug resistance. J Acquir Immune Defic Syndr. 2002;30(3):278. [DOI] [PubMed] [Google Scholar]
- 4. Larder B, Kemp S. Multiple mutations in HIV‐1 reverse‐transcriptase confer high‐level resistance to zidovudine (AZT). Science. 1989;246(4934):1155–8. [DOI] [PubMed] [Google Scholar]
- 5. Richman DD, Havlir D, Corbeil J, Looney D, Ignacio C, Spector SA, et al. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. J Virol. 1994;68(3):1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schuurman R, Nijhuis M, van Leeuwen R, Schipper P, de Jong D, Collis P, et al. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug‐resistant virus populations in persons treated with lamivudine (3TC). J Infect Dis. 1995;171(6):1411–9. [DOI] [PubMed] [Google Scholar]
- 7. Eshleman SH, Mracna M, Guay LA, Deseyve M, Cunningham S, Mirochnick M, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV‐1 vertical transmission (HIVNET 012). AIDS. 2001;15(15):1951. [DOI] [PubMed] [Google Scholar]
- 8. Pasquet A, Messou E, Gabillard D, Minga A, Depoulosky A, Deuffic‐Burban S, et al. Impact of drug stock‐outs on death and retention to care among HIV‐infected patients on combination antiretroviral therapy in Abidjan, Côte d'ivoire. PLoS ONE. 2010;5:e13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Global AIDS Monitoring 2019 ‐ Indicators for monitoring the 2016 United Nations Political Declaration on Ending AIDS [Internet]. [cited 2019 Apr 18]. Available from: http://www.unaids.org/en/resources/documents/2018/Global-AIDS-Monitoring
- 10. Milne RS, Silverman RA, Beck IA, Mckernan‐Mullin J, Deng W, Sibley TR, et al. Minority and majority pretreatment HIV‐1 drug resistance associated with failure of first‐line nonnucleoside reverse‐transcriptase inhibitor antiretroviral therapy in Kenyan women. AIDS. 2019;33(6):941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO . Global action plan on HIV drug resistance 2017–2021 [Internet]. WHO; [cited 2019 Apr 8]. Available from: http://www.who.int/hiv/pub/drugresistance/hivdr-action-plan-2017-2021/en/ [Google Scholar]
- 12. Hirsch MS, Günthard HF, Schapiro JM, Brun‐Vézinet F, Clotet B, Hammer SM, et al. Antiretroviral drug resistance testing in adult HIV‐1 infection: 2008 recommendations of an International AIDS Society‐USA panel. Clin Infect Dis Off Publ Infect Dis Soc Am. 2008;47(2):266–85. [DOI] [PubMed] [Google Scholar]
- 13. What's New in the Guidelines? Adult and Adolescent ARV [Internet]. AIDSinfo. [cited 2018 Aug 16]. Available from: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0
- 14. Cáceres CF, Mendoza W. The national response to the HIV/AIDS epidemic in Peru: accomplishments and gaps–a review. J Acquir Immune Defic Syndr. 1999;2009 51 Suppl 1:S60–66. [DOI] [PubMed] [Google Scholar]
- 15. UNAIDS . Global AIDS Monitoring 2019. Peru: | UNAIDS. [Google Scholar]
- 16. Soria J, Bull M, Mitchell C, La Rosa A, Dross S, Kraft K, et al. Transmitted HIV resistance to first‐line antiretroviral therapy in lima, Peru. AIDS Res Hum Retroviruses. 2012;28(4):333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guanira J, Lama JR, Montoya O, Segura P, Ramos E, Ganoza C, et al. HIV drug resistance in the Andean Region. A look after the universal access to HAART in the Andean Region. Poster Presentation presented at: XVIII International HIV Drug Resistance Workshop: Basic Principles and Clinical Implications. 2009.
- 18. Lama JR, Sanchez J, Suarez L, Caballero P, Laguna A, Sanchez JL, et al. Linking HIV and antiretroviral drug resistance surveillance in Peru: a model for a third‐generation HIV sentinel surveillance. J Acquir Immune Defic Syndr. 2006;42(4):501–5. [DOI] [PubMed] [Google Scholar]
- 19. WHO . HIV drug resistance report 2017 [Internet]. WHO; [cited 2018 Aug 27]. Available from: http://www.who.int/hiv/pub/drugresistance/hivdr-report-2017/en/ [Google Scholar]
- 20. Bautista CT, Sanchez JL, Montano SM, Laguna‐Torres VA, Lama JR, Sanchez JL, et al. Seroprevalence of and risk factors for HIV‐1 infection among South American men who have sex with men. Sex Transm Infect. 2004;80(6):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silva‐Santisteban A, Raymond HF, Salazar X, Villayzan J, Leon S, McFarland W, et al. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero‐epidemiologic study using respondent driven sampling. AIDS Behav. 2012;16(4):872–81. [DOI] [PubMed] [Google Scholar]
- 22. Country Factsheets: Peru [Internet]. 2017. [cited 2018 Dec 31]. Available from: http://www.unaids.org/en/regionscountries/countries/peru
- 23. Sanchez J, Lama JR, Kusunoki L, Manrique H, Goicochea P, Lucchetti A, et al. HIV‐1, sexually transmitted infections, and sexual behavior trends among men who have sex with men in Lima, Peru. J Acquir Immune Defic Syndr. 2007;44(5):578. [DOI] [PubMed] [Google Scholar]
- 24. Long J, Sanchez H, Garcia DC, Castillo LH, Lama JR, Duerr A, et al. Little or no overlap of sexual networks of transgender women and MSM in Lima, Peru [Internet]. CROI Conference; 2019 [cited 2019 Apr 8]. Available from: http://www.croiconference.org/sessions/little-or-no-overlap-sexual-networks-transgender-women-and-msm-lima-peru
- 25. Lama JR, Brezak A, Dobbins JG, Sanchez H, Cabello R, Rios J, et al. Design strategy of the Sabes study: diagnosis and treatment of early HIV infection among men who have sex with men and transgender women in Lima, Peru, 2013–2017. Am J Epidemiol. 2018;187(8):1577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lama JR, Mayer KH, Perez‐Brumer AG, Huerta L, Sanchez H, Clark JL, et al. Integration of gender‐affirming primary care and peer navigation with hiv prevention and treatment services to improve the health of transgender women: protocol for a prospective longitudinal Cohort study. JMIR Res Protoc. 2019;8:e14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campbell MS, Mullins JI, Hughes JP, Celum C, Wong KG, Raugi DN, et al. Viral linkage in HIV‐1 seroconverters and their partners in an HIV‐1 prevention clinical trial. PLoS ONE. 2011;6:e16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gifford RJ, Liu TF, Rhee S‐Y, Kiuchi M, Hue S, Pillay D, et al. The calibrated population resistance tool: standardized genotypic estimation of transmitted HIV‐1 drug resistance. Bioinforma Oxf Engl. 2009;25(9):1197–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bennett DE, Camacho RJ, Otelea D, Kuritzkes DR, Fleury H, Kiuchi M, et al. Drug resistance mutations for surveillance of transmitted HIV‐1 drug‐resistance: 2009 update. PLoS ONE. 2009;4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu TF, Shafer RW. Web resources for HIV type 1 genotypic‐resistance test interpretation. Clin Infect Dis. 2006;42(11):1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. RStudio Team . RStudio: Integrated Development for R. [Internet]. Boston, MA: RStudio, Inc.; 2015. [cited 2019 Feb 20]. Available from: http://www.rstudio.com/ [Google Scholar]
- 32. R Core Team . R: A language and environment for statistical computing. [Internet]. Vienna, Austria: R Foundation for Statistical Computing; 2018. [cited 2019 Feb 20]. Available from: https://www.R-project.org/ [Google Scholar]
- 33. Lama JR, Lucchetti A, Suarez L, Laguna‐Torres VA, Guanira JV, Pun M, et al. Association of herpes simplex virus type 2 infection and syphilis with human immunodeficiency virus infection among men who have sex with men in Peru. J Infect Dis. 2006;194(10):1459–66. [DOI] [PubMed] [Google Scholar]
- 34. WHO . Surveillance of transmitted HIV drug resistance [Internet]. WHO; [cited 2018 Aug 21]. Available from: http://www.who.int/hiv/topics/drugresistance/surveillance/en/ [Google Scholar]
- 35. Zash R, Makhema J, Shapiro RL. Neural‐tube defects with dolutegravir treatment from the time of conception. N Engl J Med. 2018;379(10):979–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arruda MB, Boullosa LT, Cardoso CC, da Costa CM, Brites C, de Lima STS, et al. Brazilian network for HIV Drug Resistance Surveillance (HIV‐BresNet): a survey of treatment‐naive individuals. J Int AIDS Soc [Internet]. 2018. Mar 5 [cited 2019 Apr 10];21(3). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5835841/ [DOI] [PMC free article] [PubMed] [Google Scholar]


