Abstract
Childbirth injures muscles and nerves responsible for urinary continence. Mesenchymal stem cells (MSCs) or their secretome given systemically could provide therapeutic benefit for this complex multisite injury. We investigated whether MSCs or their secretome, as collected from cell culture, facilitate recovery from simulated childbirth injury. Age-matched female Sprague-Dawley rats received pudendal nerve crush and vaginal distension (PNC+VD) and a single intravenous (iv) injection of 2 million MSCs or saline. Controls received sham injury and iv saline. Additional rats received PNC+VD and a single intraperitoneal (ip) injection of concentrated media conditioned by MSCs (CCM) or concentrated control media (CM). Controls received a sham injury and ip CM. Urethral and nerve function were assessed with leak point pressure (LPP) and pudendal nerve sensory branch potential (PNSBP) recordings 3 wk after injury. Urethral and pudendal nerve anatomy were assessed qualitatively by blinded investigators. Quantitative data were analyzed using one-way ANOVA and Holm-Sidak post hoc tests with P < 0.05 indicating significant differences. Both LPP and PNSBP were significantly decreased 3 wk after PNC+VD with saline or CM compared with sham-injured rats, but not with MSC or CCM. Elastic fiber density in the urethra increased and changed in orientation after PNC+VD, with a greater increase in elastic fibers with MSC or CCM. Pudendal nerve fascicles were less dense and irregularly shaped after PNC+VD and had reduced pathology with MSC or CCM. MSC and CCM provide similar protective effects after PNC+VD, suggesting that MSCs act via their secretions in this dual muscle and nerve injury.
Keywords: paracrine action, elastin, external urethral sphincter, pudendal nerve, urinary incontinence
vaginal delivery can injure the muscles, nerves, and connective tissues responsible for continence (25). These injuries are correlated with both postpartum stress urinary incontinence (SUI) as well as development of SUI later in life (8). Current treatment options for SUI either have poor long-term efficacy or have a high revision rate (11, 18, 29).
Mesenchymal stem cells (MSCs) can differentiate into mesenchymal cell types and also secrete trophic factors which facilitate regeneration (13). MSCs and their secretions can act systemically as well as locally via mechanisms including immune modulation and metabolic regulation (2), suggesting their potential to facilitate regeneration of complex injuries with multiple mechanisms and sites of injury, such as childbirth.
Intraurethral injection of muscle-derived progenitor cells are currently in clinical trials for SUI (5). However, since the maternal injuries of childbirth are not limited to the urethra, systemic delivery of stem cells has the potential to improve recovery of multiple injury sites and may lead to better long-term outcomes. Systemic delivery of cells has been tested with positive results in a vaginal distension (VD) model of simulated childbirth injury in rats (9, 23); however, this model does not simulate the maternal nerve injuries that occur in childbirth.
We have developed a dual-injury rat model, consisting of VD and pudendal nerve crush (PNC), which better mimics the maternal injuries of childbirth than VD alone since it produces multiple mechanisms and sites of injury, similar to human childbirth (16). This model consistently decreases leak point pressure (LPP) and pudendal nerve function for 4–5 wk, indicative of SUI (16). The aim of this study was to determine whether intraperitoneal (ip) injection of concentrated conditioned media (CCM) cultured from rat bone marrow-derived MSCs or intravenous (iv) injection of rat bone marrow-derived MSCs could prevent SUI and preserve pudendal nerve function in this dual-injury rat model.
MATERIALS AND METHODS
Study Design
This study was approved by the Institutional Animal Care and Use Committee of the Cleveland Veterans Affairs Medical Center. In the first of 2 experiments, 44 adult female Sprague-Dawley rats (250–300 g) underwent sham injury with a 1-ml saline treatment intravenously (iv; n = 15), PNC+VD with 1 ml saline iv (n = 13), or PNC+VD with 2 million MSCs suspended in 1 ml saline iv (n = 16). All treatments were given 1 h after the end of PNC+VD. Five rats in each group were euthanized 3 wk after injury for anatomic assessment of the urethra and pudendal nerve. The remaining rats underwent LPP testing with simultaneous external urethral sphincter (EUS) electromyography (EMG), and pudendal nerve sensory branch potential (PNSBP) recordings 3 wk after injury to assess restoration of pudendal nerve function.
In the second experiment, 54 adult female Sprague-Dawley rats underwent sham injury with concentrated control media (CM) given intraperitoneally (ip; n = 18), PNC+VD with CM ip (n = 19), or PNC+VD with CCM ip (n = 17). As above, all treatments were given 1 h after the end of PNC+VD, and five rats in each group were euthanized 3 wk after injury for anatomic assessment. The remaining rats in each group underwent LPP measurement with simultaneous EUS EMG and PNSBP recordings 3 wk after injury.
Stem Cell Harvest and Culture
Bone marrow from donor female Sprague-Dawley rats was used to obtain MSCs (9). Cells were aspirated from the femur and tibia by flushing the bone with saline and were sorted using intracellular adhesion molecule 1 (ICAM-1) to select for MSCs. The MSCs were transfected with lentivirus to express green fluorescent protein (GFP), and GFP-positive (GFP+) MSCs were cultured to passage 16 (P16) before being injected or used to prepare CCM.
MSC Characterization
As described previously (9), P16 MSCs were sorted using flow cytometry for CD29, CD90, CD54, and CD45 to confirm expression of stem cell surface makers; P16 MSCs were cultured in chrondrogenic media, osteogenic media, or adipogenic media for 20–35 days to confirm the ability of these MSCs to differentiate into other mesenchymal cell types.
CCM Preparation
To create CCM, P16 MSCs were incubated in 15 ml serum-free DMEM for 24 h, and conditioned media was extracted and centrifuged at 4,000 rpm for 75 min and filtered with a sterile 0.22-μm filter to create a 50× concentrated solution of 0.3 ml (9). A cell count assay was performed to confirm the absence of cells in CCM.
PNC+VD
Rats were anesthetized with 2% isoflurane and underwent PNC followed by VD as described previously (16). The pudendal nerve was isolated bilaterally and crushed with a Castroviejo needle holder twice for 30 s. The vagina was then accommodated with increasing sizes of bouge a boule urethral dilators (24–32Fr). A modified 10F Foley catheter was inserted into the vagina, and the balloon was inflated with 3 ml water for 4 h. Sham injury was created by making an incision in the dorsal skin, accommodating the vagina with the urethral dilators, and inserting a Foley catheter for 4 h without inflation. One hour after the end of VD or sham VD, rats received either saline or MSCs iv via the tail vein, or CM or CCM ip.
In Vivo Urethral Functional Testing
Functional recordings, including LPP, EUS EMG, and PNSBP, were performed as described previously (16). Under isoflurane inhalant anesthesia, a transvesical catheter was inserted into the bladder dome and fixed with a suture. The abdominal wall was closed, and the catheter was connected to both a pressure transducer (model PT300; Astro-Med, Providence, RI) and syringe pump (5 ml/h, model 200; KD Scientific, New Hope, PA,). Bipolar parallel platinum electrodes (30-gauge needles 2 mm apart) were placed on the surface of the midurethra and were connected to an amplifier (band-pass frequencies: 3 Hz–3 kHz, model P511; AC Amplifier, Astro-Med) and electrophysiological recording system (10-kHz sampling rate, PowerLab 8/35, ADInstruments, Colorado Springs, CO). The pudendal nerve sensory branch was identified on the ventral side of the pudendal canal and was suspended over recording electrodes connected to an amplifier and the electrophysiological recording system.
Before the recordings, the animals were anesthetized with urethane (1.2 g/kg ip), and the isoflurane anesthesia was removed since urethane better preserves reflexes of continence and voiding (3). Intravesical pressure was increased for LPP testing with simultaneous EUS EMG recording with the bladder approximately half full by gradually pressing on the bladder with the rat in a supine position (3). When leakage was visualized, the external pressure was rapidly removed. LPP testing was repeated four to six times for each rat. If an active bladder contraction occurred, the results were not analyzed and the test was repeated. After LPP testing, the clitoris was brushed gently with a cotton swab or gauze bilaterally with moderate speed while PNSBP was recorded. The test was repeated six to nine times in each animal.
Anatomy
The urethra and pudendal nerve were dissected, stored at −80°C, and sectioned transversely (14 μm). Transverse pudendal nerve sections underwent immunofluorescence to identify axons with primary antibodies, anti-neurofilament 200 and anti-neurofilament 68 (1:100 dilution, Sigma-Aldrich, St. Louis, MO), and the secondary antibody, Alexa Fluor 488-conjugated donkey anti-mouse IgG (1:400 dilution, Invitrogen, Carlsbad, CA).
Immunofluorescence was also used to assess innervation of neuromuscular junctions (NMJ) of the EUS using primary and secondary antibodies as above to identify axons and 4 μg/ml of tetramethylrhodamine-conjugated α-bungarotoxin (Rh-α-BTX; Invitrogen) to identify NMJs. Alexa Fluor 350-conjugated phalloidin (5 μl in 100 μl PBS, Invitrogen) was used to identify striated muscle of the EUS. Additional near sections of the urethra were stained with elastin von Giesson (EVG) stain. Additional urethral sections underwent immunofluorescence to localize GFP+MSC with primary antibody, rabbit anti-GFP (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), and secondary antibody, Alexa Fluor 488-conjugated goat anti-rabbit (1:500, Invitrogen).
Data Analysis
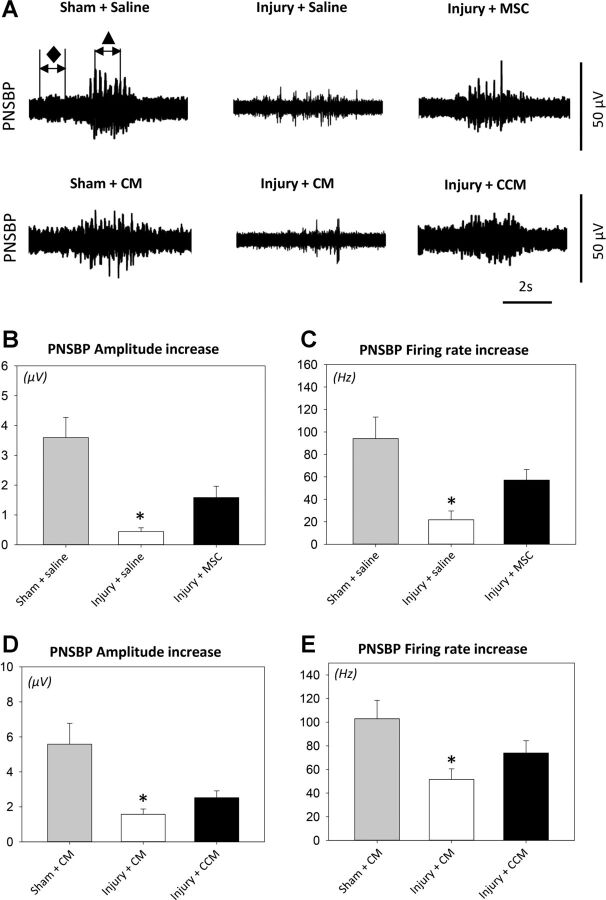
LPP was defined as baseline pressure just before testing subtracted from peak pressure at leakage. Quantitative assessment of EUS EMG and PNSBP signals was performed by selecting four to six 1-s segments for each rat at baseline and peak activity (Figs. 1A and 2A) and determining the mean amplitude and frequency of muscle and nerve activity, as done previously (9). An increase in amplitude and frequency at peak activity was calculated by subtracting baseline from peak activity values. Mean values were calculated for each animal and were used to calculate a mean and SE for each experimental group. One-way ANOVA followed by the Holm-Sidak post hoc test was used to compare LPP, EUS EMG, and PNSBP results when data passed normality testing. If normality failed, ANOVA on ranks followed by Dunn's test was used. P < 0.05 was used to indicate a statistically significant difference between groups in all cases.
Fig. 1.
Leak point pressure (LPP) with simultaneous external urethral sphincter electromyography (EUS EMG) results. Examples demonstrate the two 1-s segments selected from baseline (⧫) and peak activity (▲) that were used to calculate amplitude increase (μV) and firing rate increase (Hz) from baseline to peak pressure in EUS EMG recordings (A). LPP was recorded after pudendal nerve crush and vaginal distension (injury) or sham injury with mesenchymal stem cell (MSC) or saline treatment (B) as well as with concentrated conditioned media (CCM) or concentrated control media (CM) treatment (E). Also shown are EUS EMG amplitude increase after injury or sham injury with MSC or saline treatment (C) as well as with CCM or CM treatment (F) and firing rate increase after injury or sham injury with MSC or saline treatment (D) as well as with CCM or CM treatment (G). Values are means± SE of data from 8–14 animals. ⧫, Baseline EMG; ▲, LPP EMG.*Significant difference compared with corresponding sham-injured animals, P < 0.05.
Fig. 2.
Pudendal nerve sensory branch potential (PNSBP) results. Examples demonstrate the two 1-s segments selected from baseline (⧫) and brush activity (▲) that were used to calculate PNSBP amplitude difference (μV) and firing rate difference (Hz) during brush stimuli to the rat clitoris (A). Shown are PNSBP amplitude increase after pudendal nerve crush and vaginal distension (injury) or sham injury with MSC or saline treatment (B) as well as with CCM or CM treatment (D) and firing rate increase after injury or sham injury with MSC or saline treatment (C) as well as with CCM or CM treatment (E). Values are means± SE of data from 8–14 animals. ⧫, Baseline PNSBP; ▲, brush PNSBP. *Significant difference compared with corresponding sham-injured animals, P < 0.05.
Histology and immunofluorescence were evaluated qualitatively by a blinded observer according to the following criteria. EUS striated muscle morphology and elastin fibers as well as their location, orientation, and quantity of elastin fibers were assessed on urethral EVG-stained slides. Thickness of innervating axons, concentration of motor endplates, and their innervation status were used to qualitatively assess innervation of the EUS. Density of nerve fascicles and axon morphology were used to qualitatively assess pudendal nerves. GFP+ cells were counted at a ×20 field and scanned for 10 fields.
RESULTS
Characterization and Differentiation of MSC
MSCs expressed CD29, CD54, and CD90, but not CD45. Differentiation assays confirmed that MSCs can differentiate into adipogenic, chrondrogenic, and osteogenic cells as we have shown previously (9).
MSC or CCM Treatment Improves Urethral Function After Simulated Childbirth Injury
LPP was significantly decreased 3 wk after PNC+VD with saline or CM treatment compared with sham-injured rats (Fig. 1), indicative of urethral injury and decreased function, as previously demonstrated (16). LPP in rats that underwent PNC+VD and were treated with MSCs or CCM was not significantly different from sham-injured rats, demonstrating that both MSCs and CCM facilitate functional recovery of the urethra. The increase in amplitude of EUS EMG with LPP was significantly decreased 3 wk after PNC+VD in rats treated with saline or MSCs (Fig. 1).
In contrast, there were no significant differences between the experimental groups in the increase in EUS EMG firing rate with LPP testing, suggesting that MSC treatment did not affect EUS EMG recovery after PNC+VD. Similarly, the increase in both EUS EMG amplitude and firing rate were not significantly different among the experimental groups treated with CCM or CM (Fig. 1). The increase in amplitude and firing rate of PNSBP during clitoral brushing were both significantly decreased 3 wk after PNC+VD animals treated with either saline or CM compared with sham-injured animals; however, they were not significantly different when injured animals were treated with either MSCs or CCM (Fig. 2), indicating that both MSCs and CCM preserved nerve function after PNC+VD.
Anatomy
MSC or CCM treatment promotes urethral elastogenesis.
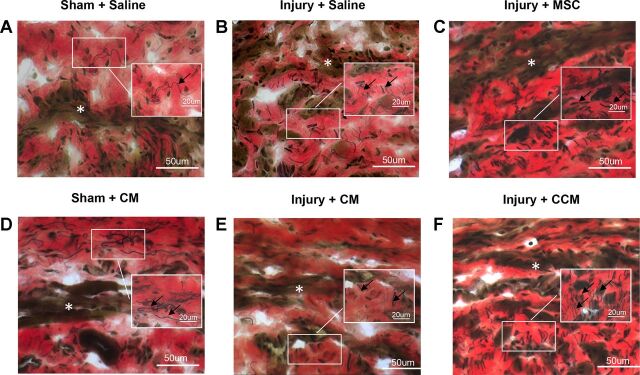
Under microscopic examination of the urethra, striated muscle fibers of the EUS had fewer striations and were atrophied 3 wk after PNC+VD compared with sham-injured animals. After treatment with MSCs or CCM, the EUS had increased striated muscle layers than those treated with saline or CM. Elastin fibers were observed as consecutive long fibers in sham-injured animals, mainly outside the EUS, and most were parallel to the striated muscle fibers of the EUS (Fig. 3). Elastin fibers external to the EUS were disrupted and twisted after PNC+VD. The density of elastin fibers interior to the EUS increased, and their orientation became radial in all injured animals, regardless of treatment. Density of elastin fibers increased further with either MSC or CCM treatment, indicating that both MSCs and CCM stimulate elastogenesis (Fig. 3).
Fig. 3.
Examples of transverse sections of the urethra stained with elastin von Giesson stain. Three weeks after pudendal nerve crush and vaginal distension (Injury) striated muscle fibers of the EUS (white *) had fewer striations and were atrophied (B, C, E, and F) compared with sham-injured animals (A and D). Elastin fibers (black arrows) were long and consecutive and mainly seen outside the EUS in sham-injured animals. Three weeks after injury, the elastin fibers closest to the urethral serosa were disrupted and twisted. Density of elastin fibers was increased in the EUS near urethral smooth muscle, and their orientation changed to be radial rather than circumferential after injury (B, C, E, and F). With MSC or CCM treatment (C and F), there was an additional increase in density of elastin fibers (black arrows) compared with saline or CM treatment (B and E).
MSC or CCM treatment promotes NMJ and pudendal nerve regeneration.
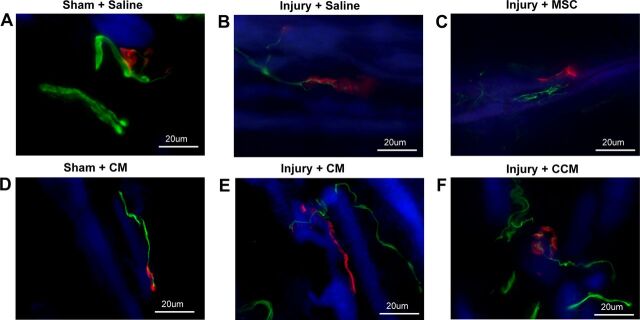
Axons located in the EUS in sham-injured animals were consistently straight, thick, and innervated discrete organized NMJs (Fig. 4). Three weeks after PNC+VD treated with saline or CM, innervating axons were thinner than in sham-injured animals, and NMJs were less organized and diffusely stained along the edge of striated muscle fibers, indicative of denervation. Some NMJs were visibly not well innervated (Fig. 4). In contrast, after treatment with MSC or CCM, axons innervating the EUS took a more torturous course and had multiple collaterals, indicating ongoing neuroregeneration. The degree of NMJ diffusion along striated muscle fibers visibly decreased (Fig. 4), indicating that MSC and CCM accelerated neuromuscular recovery after simulated childbirth injury.
Fig. 4.
Examples of immunofluorescence of the EUS showing neuromuscular junctions (red), innervating nerves (green), and striated muscle (blue). Sham-injured animals demonstrated discrete organized motor endplates innervated by straight thick innervating axons (A and D). Three weeks after pudendal nerve crush and vaginal distension (Injury) with saline or CM treatment, innervating axons were thinner and motor endplates were less organized and diffuse (B and E). With MSC or CCM treatment, innervating axons took a more torturous course and had multiple collaterals (C and F).
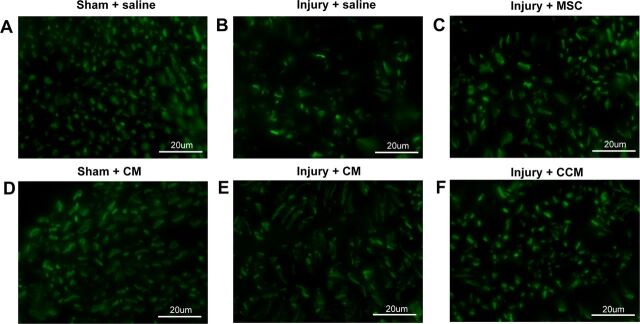
Pudendal nerve sensory branch fascicles in sham-injured animals were circular and had small compact axons (Fig. 5). Three weeks after PNC+VD and treatment with saline or CM, nerve fascicles were less dense and axons had irregular shapes, indicating injury and the beginning of neuroregeneration. In contrast, after treatment with MSC or CCM, the pudendal nerve demonstrated both normal and abnormal nerve fascicles with more normal fascicles than when treated with saline or CM (Fig. 5), indicating facilitated neuroregeneration or neuropreservation. No GFP+ stem cells were observed in the urethra or vagina 3 wk after injury, suggesting the cells did not permanently engraft and differentiate in this location.
Fig. 5.
Examples of immunofluorescence of transections of the sensory branch of the pudendal nerve distal to the injury. Sham-injured animals demonstrated mostly circular nerve fascicles with small dense compact axons (A and D). Three weeks after pudendal nerve crush and vaginal distension (Injury) and treatment with saline or CM, nerve fascicles were less dense and axons had irregular shapes (B and E). With MSC or CCM treatment, the pudendal nerve demonstrated both normal and abnormal nerve fascicles, with a greater number of normal fascicles than when treated with saline or CM (C and F).
DISCUSSION
Cell-based therapies have shown promising results in both preclinical and clinical studies for bone marrow transplantation (31), wound healing (4), inflammation control (1), and neovascularization (10), as well as for SUI (21), through their direct and indirect effects on tissue regeneration. The mechanism by which stem cells repair and regenerate tissues is not entirely known but includes secretion of paracrine factors in addition to differentiation and proliferation of cells (24). Consistent with our study, the number of cells that engraft and differentiate locally is often too few to explain significantly improved functional and anatomic results (22, 23), suggesting that the effects of their secretions on innate tissues dominates the mechanism (4). Bi et al. (2) reported that MSCs and their conditioned media have similar protective effects on acute kidney injury when given ip and concluded that secretome from stem cells protects against acute tubular injury even at a distance from the target organ, inspiring our administration of CCM ip.
Both preclinical studies and clinical trials of cell therapy for SUI primarily investigate local injection of stem cells into the urethra, with the expectation that these cells will engraft and differentiate at this site of injury to restore urethral function (5, 23). However, systemic delivery has the potential to better promote healing at multiple sites. Therefore, in this study we investigated the therapeutic effects of iv injection of rat MSCs or ip injection of CCM. We demonstrated that both MSCs and CCM lead to similar levels of recovery and regeneration after PNC+VD.
We have previously demonstrated that MSCs or their secretions improve urethral function after VD (9). In that study, MSCs were given iv and CCM was delivered periurethrally, leaving open the question of whether CCM could have a beneficial effect if given systemically. In the current study, we expanded the investigation of MSCs and MSC secretome treatment to a more complex dual-injury model which better represents the multiple sites and type of maternal injuries during childbirth than either injury alone, since muscles, organs, and connective tissues of the pelvic floor and their innervating nerves are damaged in delivery, leading to both postpartum SUI and redevelopment of SUI later in life (8, 15). Consistent with a multiple injury mechanism, PNC+VD produces a more severe injury that takes longer to recover from than either single injury alone (16). In the current study, we delivered CCM ip to determine whether it could have a beneficial effect even if not delivered locally to the site of injury.
LPP and PNSBP demonstrated accelerated recovery with both iv MSC and ip CCM treatment, indicating an effect of the cells and their secretions on the urethra and its innervation by the pudendal nerve. In contrast, neither MSC nor CCM treatment significantly improved EUS EMG despite improved preservation of the innervating axons and motor endplates with either MSC or CCM treatment. Since the EUS demonstrated greater atrophy after PNC+VD compared with sham-injured rats, we conclude that muscle and NMJ regeneration were still ongoing, as we have shown previously (7, 30).
Abnormal elastin fibers have been observed in periurethral tissues of parous women with SUI (14). In addition, lysyl oxidase like-1 knockout mice, which are unable to assemble elastin fibers properly, develop SUI (20), suggesting a role for disruption of elastin in the pathogenesis of SUI. In this study, we observed disruption of urethral elastin fibers 3 wk after PNC+VD. The elastin fibers were reoriented and increased in density near the EUS with either MSC or CCM treatment, as also observed by Lin et al. (23), suggesting that both MSC and CCM facilitate elastin repair via a paracrine or systemic effect. The elastin fibers changed in orientation after PNC+VD and were not as highly organized as in sham-injured animals, suggesting that the formation of elastin fibers is a complex process and elastin fibers only partially account for improvement in LPP.
Stem cells home to injury sites following gradients of chemokines (28), and we have previously demonstrated homing of MSCs to the pelvic organs of rats after VD (6). In contrast, in the current study we were unable to locate MSCs in the urethra and vagina 3 wk after PNC+VD. It is possible that few MSCs injected intravenously migrate to the injury site (26), and most of them might have reached the end of their lifespan 3 wk later (22). Nonetheless, they facilitated regeneration and functional recovery from the injury via either local or systemic effects.
We have previously demonstrated that local neurotrophin therapy facilitates continence recovery after PNC+VD in rats (12). The results of the current study suggest that local delivery of trophic factors is not necessary, as also observed by Voulgari-Kokota et al. (32) in a mouse model of glutamate excitotoxicity. Similarly, Lee et al. (19) demonstrated that secretions released by MSCs distant from the injury site suffice to modulate inflammation, improve tissue repair, and protect against neural dysfunction. In addition, it was recently demonstrated that cell-free derivatives from stem cells have the potential to reduce pulmonary embolism and infarcts from iv injection of stem cells, suggesting utility in treatment with both cells and their derivatives (17). However, since most factors present in CCM have short half-lives (2), multiple injections instead of a single treatment could further improve tissue repair and continence recovery.
Our findings suggest that MSCs or CCM could be beneficial for treating postpartum SUI, which occurs after vaginal childbirth in premenopausal women. This treatment also has the potential to be of benefit for premenopausal women with a history of vaginal childbirth. However, to demonstrate the idea that MSCs or CCM could be used to treat SUI months or years after delivery, one would need to conduct a new experiment using delayed injection of MSCs or CCM to determine whether delayed treatment could also facilitate recovery, as has previously been observed after cavernosal nerve injury (27).
Despite our findings of equal effectiveness of CCM and MSCs on recovery after PNC+VD, we did not identify the active cytokines in CCM. Further investigations are needed to delineate the content of CCM, the roles of secreted factors, and their interactions. Our study is also limited by the use of a quadruped animal model of simulated childbirth trauma to investigate SUI. However, no animal model simulates human vaginal delivery and animal models of chronic SUI are not physiologically representative (15), suggesting the need for confirmation in multiple animal models.
Conclusions
Systemically delivered MSCs and CCM prevented SUI after a multifactorial simulated childbirth injury, likely mediated by cell secretions acting via preservation and regeneration of the pudendal nerve, elastogenesis, and enhanced neuromuscular recovery. Both MSC and their secretions independently have the potential to prevent or reduce SUI systemically and may have the potential to treat and/or prevent SUI.
GRANTS
This work was supported by Rehabilitation Research and Development Service of the Department of Veterans Affairs Merit Review Award B7225R and the Cleveland Clinic. K. Deng was a visiting scholar from Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, and was supported by the China Scholarship Council to study at the Cleveland Clinic.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.D., D.L.L., B.H., B.M.B., and M.J.K. performed experiments; K.D., B.H., M.S.P., M.J.K., H.Z., and M.S.D. analyzed data; K.D., B.H., M.S.P., M.J.K., H.Z., and M.S.D. interpreted results of experiments; K.D. prepared figures; K.D., H.Z., and M.S.D. drafted manuscript; K.D., D.L.L., B.H., B.M.B., M.S.P., M.J.K., Z.H., Z.Y., H.Z., and M.S.D. edited and revised manuscript; K.D., D.L.L., B.H., B.M.B., M.S.P., M.J.K., Z.H., Z.Y., H.Z., and M.S.D. approved final version of manuscript; M.S.P., H.Z., and M.S.D. provided conception and design of research.
ACKNOWLEDGMENTS
We thank Giulio Cossu's laboratory at San Raffaele Hospital (Milan, Italy) for the pCCLsin.ppt.hPGK.GFP.pre vector used in this experiment.
REFERENCES
- 1.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 18: 2486–2496, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci 69: 1193–1202, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell 9: 11–15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr LK, Robert M, Kultgen PL, Herschorn S, Birch C, Murphy M, Chancellor MB. Autologous muscle derived cell therapy for stress urinary incontinence: a prospective, dose ranging study. J Urol 189: 595–601, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Cruz M, Dissaranan C, Cotleur A, Kiedrowski M, Penn M, Damaser M. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet Gynecol Int 2012: 612946, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damaser MS, Samplaski MK, Parikh M, Lin DL, Rao S, Kerns JM. Time course of neuroanatomical and functional recovery after bilateral pudendal nerve injury in female rats. Am J Physiol Renal Physiol 293: F1614–F1621, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delancey JO. Why do women have stress urinary incontinence? Neurourol Urodyn 29, Suppl 1: S13–S17, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dissaranan C, Cruz MA, Kiedrowski MJ, Balog BM, Gill BC, Penn MS, Goldman HB, Damaser MS. Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury. Cell Transplant 23: 1395–1406, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamaziere JM, Couffinhal T, Duplaa C. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells 26: 2991–3001, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Gilchrist AS, Rovner ES. Managing complications of slings. Curr Opin Urol 21: 291–296, 2011. [DOI] [PubMed] [Google Scholar]
- 12.Gill BC, Balog BM, Dissaranan C, Jiang HH, Steward JB, Lin DL, Damaser MS. Neurotrophin therapy improves recovery of the neuromuscular continence mechanism following simulated birth injury in rats. Neurourol Urodyn 32: 82–87, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 103: 1204–1219, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goepel C, Thomssen C. Changes in the extracellular matrix in periurethral tissue of women with stress urinary incontinence. Acta Histochem 108: 441–445, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Jiang HH, Damaser MS. Animal models of stress urinary incontinence. Handb Exp Pharmacol 202: 45–67, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang HH, Pan HQ, Gustilo-Ashby MA, Gill B, Glaab J, Zaszczurynski P, Damaser M. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol Urodyn 28: 229–235, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung JW, Kwon M, Choi JC, Shin JW, Park IW, Choi BW, Kim JY. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med J 54: 1293–1296, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr LA. Bulking agents in the treatment of stress urinary incontinence: history, outcomes, patient populations, and reimbursement profile. Rev Urol 7, Suppl 1: S3–S11, 2005. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5: 54–63, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee UJ, Gustilo-Ashby AM, Daneshgari F, Kuang M, Vurbic D, Lin DL, Flask CA, Li T, Damaser MS. Lower urogenital tract anatomical and functional phenotype in lysyl oxidase like-1 knockout mice resembles female pelvic floor dysfunction in humans. Am J Physiol Renal Physiol 295: F545–F555, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Gu C, Gao Y, Amano S, Koizumi S, Tokuyama T, Namba H. Bystander effect in glioma suicide gene therapy using bone marrow stromal cells. Stem Cell Res 9: 270–276, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Lin CS, Lue TF. Stem cell therapy for stress urinary incontinence: a critical review. Stem Cells Dev 21: 834–843, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, Lue TF, Lin CS. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy 12: 88–95, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makridakis M, Roubelakis MG, Vlahou A. Stem cells: insights into the secretome. Biochim Biophys Acta 1834: 2380–2384, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Memon HU, Handa VL. Vaginal childbirth and pelvic floor disorders. Womens Health (Lond Engl) 9: 265–277, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol 287: H2670–H2676, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Qiu X, Fandel TM, Ferretti L, Albersen M, Orabi H, Zhang H, Lin G, Lin CS, Schroeder T, Lue TF. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol 62: 720–727, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, McCarthy PM, Penn MS. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells 25: 245–251, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med 148: 459–473, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Song QX, Balog B, Kerns JM, Lin DL, Sun Y, Damaser MS, Jiang HH. Long-term effects of simulated childbirth injury on function and innervation of the urethra. Neurourol Urodyn, doi: 10.1002/nau.22561, 2014. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sudres M, Norol F, Trenado A, Gregoire S, Charlotte F, Levacher B, Lataillade JJ, Bourin P, Holy X, Vernant JP, Klatzmann D, Cohen JL. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol 176: 7761–7767, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Voulgari-Kokota A, Fairless R, Karamita M, Kyrargyri V, Tseveleki V, Evangelidou M, Delorme B, Charbord P, Diem R, Probert L. Mesenchymal stem cells protect CNS neurons against glutamate excitotoxicity by inhibiting glutamate receptor expression and function. Exp Neurol 236: 161–170, 2012. [DOI] [PubMed] [Google Scholar]