Abstract
The aim of the present study was to use a model of simulated human childbirth in rats to determine the damage to genitourinary structures and behavioral signs of urinary dysfunction induced by vaginal distension (VD) in female rats. In experiment 1, the length of the genitourinary tract and the nerves associated with it were measured immediately after simulated human delivery induced by VD or sham (SH) procedures. Electroneurograms of the dorsal nerve of the clitoris (DNC) were also recorded. In experiment 2, histological characteristics of the bladder and major pelvic ganglion of VD and SH rats were evaluated. In experiment 3, urinary parameters were determined in conscious animals during 6 h of dark and 6 h of light before and 3 days after VD or SH procedures. VD significantly increased distal vagina width (P < 0.001) and the length of the motor branch of the sacral plexus (P < 0.05), DNC (P < 0.05), and vesical nerves (P < 0.01) and decreased DNC frequency and amplitude of firing. VD occluded the pelvic urethra, inducing urinary retention, hematomas in the bladder, and thinness of the epithelial (P < 0.05) and detrusor (P < 0.01) layers of the bladder. Major pelvic ganglion parameters were not modified after VD. Rats dripped urine in unusual places to void, without the stereotyped behavior of micturition after VD. The neuroanatomic injuries after VD occur alongside behavioral signs of urinary incontinence as determined by a new behavioral tool for assessing micturition in conscious animals.
Keywords: external urethral sphincter, dorsal nerve of the clitoris, major pelvic ganglion, micturition
micturition consists of two phases: storage and urine expulsion. During storage, the detrusor is relaxed while the bladder neck and urethra are activated, preventing involuntary bladder emptying (11). Extrinsic elements such as the levator ani muscle also contribute to the maintenance of continence (3). When the bladder reaches its threshold volume, spinal and supraspinal reflexes are triggered to induce bladder contraction and urethral relaxation, and urine flows through the urethra (11). Damage to the lower urinary tract and/or its innervation can induce urinary dysfunction (3, 29, 41).
Urinary dysfunction affects the health of many women (60). Stress urinary incontinence has been described as involuntary loss of urine during effort and is the most prevalent urinary disorder related to vaginal childbirth, which is known to injure the pudendal nerve and denervate the external urethral sphincter (EUS) (3, 15, 57).
Maternal pelvic viscera and nerve damage results from the difficulty of human childbirth due the large fetal head and brain relative to the maternal pelvis size. Neonates at birth have heads that are close to the size of the maternal birth canal through which they must pass during the second stage of parturition (48). Births of fetuses over 4 kg or fetal malposition often prolong parturition (30), retaining the fetus in the pelvic cavity, the main anatomic resistance to fetal expulsion. Prolonged second stage of parturition is not uncommon in primigravid women, with an incidence of 37% (30, 55).
Simulated delivery injury models, including vaginal distension (VD) with a balloon in rats, have been created to better understand the injury process during parturition of women (20, 33). The diameter of the balloon and the duration of the distention can be adjusted to mimic the difficulty of parturition in women. A prolonged parturition can be modeled with VD of greater duration than the duration of parturition in intact rats (19, 34). Using the VD model, investigators have demonstrated bladder, urethral, and vaginal hypoxia (9), anatomic and functional damage to the EUS and its innervation (4, 21), and decreases in urethral resistance (26). Whether these VD-induced structural and functional changes are sufficient to cause signs of voiding dysfunction in awake animals is unknown.
Most of the studies using simulated delivery injury models have focused on urethral neuromuscular injury (4, 21, 58). However, in addition to the pudendal nerve, there are other somatic and autonomic nerves running over or adjacent to the vaginal wall that could also be stretched during childbirth (40). Nonetheless, injury to pelvic, perineal, or bladder nerves has not been investigated in the rat VD model of simulated delivery. Moreover, no behavioral correlates of urinary dysfunction have been investigated after VD in conscious rats.
The aim of the present study was to determine, in anesthetized rats, the VD-induced damage to genitourinary structures and nerves and, in conscious animals, the behavioral signs of urinary dysfunction.
MATERIALS AND METHODS
Experimental design.
Thirty-four adult nulliparous Wistar female rats (250–300 g body wt) were housed with water and food ad libitum and maintained on a 12:12-h light-dark cycle. The experimental protocol was approved by Tlaxcala University Committee on Laboratory Animals, according to the guidelines of the Mexican Council on Laboratory Animals Care (NOM-062-Z00-1999).
Animals were randomized to undergo 4 h of VD (n = 19) or sham VD (SH; n = 13). To simulate the difficulty of the fetus passing though the pelvic cavity and the injury induced by a prolonged second stage of human childbirth, the balloon was filled with 4 ml water, resulting in a balloon diameter of 19 mm, ∼15% greater than the diameter of the cranium of newborn pups (16.5 mm from occipital to nose). To simulate prolonged human second stage of parturition, the duration of VD was 4 h, 45% greater than the average duration of parturition of intact rats (2.5–3.0 h, from bleeding to whole pup expulsion) (19, 34). Considering that during a second stage dystocic parturition, the fetus stays in the pelvic cavity trying to be expelled, the VD was provided tonically for 4 h.
In experiment 1, immediately after VD (n = 6) or SH (n = 6) procedures, the length of the genitourinary tract and the nerves associated with it were determined, and electroneurograms (ENGs) of the dorsal nerve of the clitoris (DNC) were recorded in 4 animals/group. In experiment 2, histological characteristics of the bladder and major pelvic ganglion (MPG) of VD (n = 4) and SH (n = 4) rats were evaluated. In experiment 3, urinary behavior was characterized in conscious animals during 6 h of dark and 6 h of light before and 3 days after VD (n = 9) or SH (n = 3) procedures. All analysis was performed in a blinded manner.
VD.
Rats were anesthetized with a mixture of intraperitoneal ketamine (60 mg/kg) and xylazine (7.5 mg/kg), and additional doses were used as needed. In VD animals, for catheter accommodation, a cotton swab with mineral oil was introduced into the vagina. A modified 10-F Foley balloon catheter was then inserted into the vagina and inflated with 4 ml water for 4 h. In SH animals, the catheter was placed into the vagina but was not inflated. The catheter was secured with a double silk suture at the skin of the perivaginal orifice.
Gross anatomy.
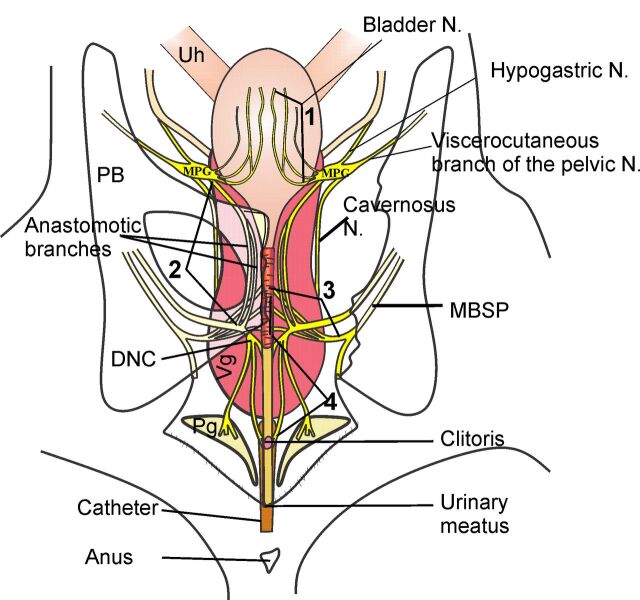
In experiment 1, immediately after VD or SH procedures, with the catheter in place and before balloon deflation, a longitudinal skin incision was made at the midline of the abdominal and pelvic region. Using a stereomicroscope (MZ6 Leica), a laparotomy was performed, and the pelvic bones were uncovered. The width of the distal vagina and length of the bladder were determined. The volume of urine in the bladder was measured with an insulin syringe. A portion of the right ilium, ischium, and pubic bones was then dissected, and, in an anteroposterior direction, the lengths of the vesical nerves, of the DNC, and of nerves running over the ventral wall of the vagina (1–3 in Fig. 1) were measured (4 in Fig. 1). The length of the motor branch of the sacral plexus (MBSP) was measured in a ventrodorsolateral direction. To measure the length of the nerves, a thread was placed over each one (in the anatomic direction described above) and cut with the endings aligned with anatomical reference points showed in Fig. 1. The length of the cut thread was then measured with a caliper. Drawings were made, and digital photographs were taken.
Fig. 1.
Drawing of the lower urogenital tract and its innervation of the female rat during vaginal distension (VD). Note that beside the motor branch of the sacral plexus (MBSP; 3) that innervates the striated urethral muscle, there are other nerves stretched during VD, such as the vesical nerves (1), the anastomotic branches (2), and the dorsal nerve of the clitoris (DNC; 4). The lines associated with the numbers indicate the points of nerve measurement. MPG, major pelvic ganglia; Uh, uterine horn; Ub, bladder; Vg, vagina; Pg, preputial gland.
ENGs.
The DNC was dissected at the level of the ischiatic arch. The right DNC was transected, and bipolar platinum hook electrodes were placed on the distal portion of the sectioned nerve. The electrodes were connected through a Grass 7P511 amplifier (bandpass filtered at 100 Hz to 3 kHz) to an electrophysiological recording system (Digidata 1440A, 10-kHz sampling connected to a computer running AxoScope software) to store and print the action potentials. Warm mineral oil was applied. To stimulate activity of sensory fibers, ENGs were recorded before, during, and after gently squeezing the clitoral hood with forceps for ∼1 s (4.8-in. stainless steel Adson forceps without teeth). This was performed at least three times.
Histology of the bladder and MPG.
In experiment 2, immediately after VD or SH procedures, using a stereoscopic microscope, the bladder and right MPG were harvested, fixed in formalin (4%), embedded in paraffin, and sectioned with a microtome at 7 μm thickness. Sections were stained with hematoxylin and eosin or Masson's trichrome, examined with an optical microscope (Axio Imager A1, Carl Zeiss, Thornwood, NY), and photographed with a digital camera (Cannon PowerShot S50, Canon USA, Lake Success, NY).
The thickness of the bladder layers was determined in three cross sections of the middle region using AxioVision digital image processing software (version 4.6, Carl Zeiss). Two values were taken per section: at the midline of the dorsal and ventral walls. The two values were averaged for each animal, and the mean was used to create a group average.
The area of the MPG was determined measuring three sections of the middle of the ganglion per rat and averaging the data. From these sections, the area of the soma of 20 neurons localized in the middle of the MPG was also determined. Neurons with a visible nucleus were chosen. The area was measured by tracing the outline of the MPG or cell body and calculating the enclosed area using AxioVision. MPG thickness was determined by adding the thickness of the 7-μm sections where neurons were found.
Micturition recording.
Micturition was recorded as previously described (24). The recordings took place in the last 6 h of dark and the first 6 h of light. Rats were habituated in an observational cage during the pretest before VD. The observational cage consisted of a large (43 × 53 × 20 cm) transparent cage, with water and food in the middle of the cage, and the Plexiglas floor was replaced with a wire grid supported 4 cm above a glass-topped stand. A camera was placed in front of the cage. A urine collector plate was placed in the 4-cms space and could slide out from below the cage to facilitate manual measurements of voided volume.
A closed circuit video with infrared cameras was used to record urinary parameters and voiding behavior. Rats were observed via digital video, with the monitor in a room adjacent to the animal room. When urine was observed in the collector plate, the observer entered the animal room and measured the urine volume using an insulin syringe and wiped the urine collector. The urinary behavior parameters recorded were voiding frequency, defined as number of voids during 6 h (dark or light), and voided volume, defined as the mean volume collected per voiding in a 6-h period. The videos were replayed to characterize the female rat's behavior during urine expulsion. In previous studies, it has been described that male rats void at the edges of the cage, mostly in the corners (24, 25). To urinate, the rat moves to the edge of the cage, places the rump toward the wall, raises the tail, and expels the urine (25). In the present study, the criteria to classify a urine expulsion as a void was the stereotyped behavior of micturition: placing the rump facing the wall of the cage to expel urine (in any edge of the cage). Any urine expelled in another posture was considered to be a result of urinary dysfunction or urinary leakage.
Data analysis.
Results are presented as means ± SE. Statistical analysis was performed using Sigma Plot Software (version 12, Systat Software). In experiments 1 and 2, means among VD and SH groups were compared using Student's t-test.
For the ENGs, a 1-s recording sample during clitoral hood stimulation was segmented and archived using AxoScope (Axon instruments). Quantitative assessment of ENG signals was performed by determining the mean rectified amplitude and firing rate or mean frequency of firing. Mean values from three samples in each rat were calculated. Values for each animal were used to calculate means and SEs. Student's t-test was used to determine significant differences between groups.
In experiment 3, the number of urine expulsions (voids and leaks) per rat were counted per light/dark phase (6-h dark or light). From those values, means and SEs were calculated. ANOVA for repeated measures was used to compare data of urinary behavior parameters before (dark and light phases) and after (dark and light phases) SH or VD. Urinary parameters were voiding frequency, defined as number of voidings during the 6 h; voiding interval, defined as time (in min) between one voiding and the next; voided volume, defined as the volume collected per voiding; percentage of voids; and percentage of leaks. Tukey's post hoc test was used to compare individual groups. P values of <0.05 indicated a statistically significant difference for all statistical comparisons.
RESULTS
Experiment 1: genitourinary tract and nerve stretching.
VD significantly increased distal vagina width (SH: 9.8 ± 0.2 mm vs. VD: 17.0 ± 0.3 mm, P < 0.0001; Fig. 2) and bladder length (SH: 12.0 ± 0.5 mm vs. VD: 25.0 ± 1.1 mm, P < 0.0001; Fig. 2) but not urethral length (SH: 22.5 ± 0.5 mm vs. VD: 27.0 ± 0.6 mm, P = 0.07). In VD animals, the balloon pressed the middle urethra against the pelvic bone, inducing occlusion of that structure, which prevented micturition and, in consequence, produced temporary urinary retention during VD (urine volume: 0.40 ± 0.15 ml for SH animals vs. 1.67 ± 0.25 ml for VD animals, P < 0.001). Hematomas were present only in bladders of VD rats (Fig. 2).
Fig. 2.

Photos of the urogenital tract during sham distension (SH; A) or VD (B). Note that during VD, the balloon distends the distal genitourinary tract and compresses the pelvic urethra against the pubic bone, leading to bladder overdistension as a consequence of urinary retention induced by the outlet obstruction (B). Compared with the bladder of SH rats (C), only bladders of VD rats presented with hematomas (He; D).
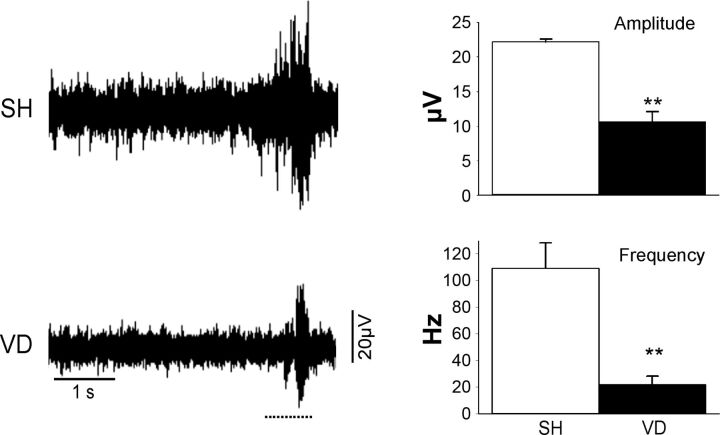
VD significantly increased the length of the genitourinary nerves. The vesical nerves were elongated by ∼56%, the anastomotic branches that originate from MPG by 36%, and the MBSP and DNC by 50% (Fig. 1 and Table 1). ENG amplitude and frequency visibly increased during clitoral hood stimulation, but both were significantly decreased after VD compared with SH (Fig. 3).
Table 1.
Effect of VD on the length of genitourinary nerves
| SH | VD | |
|---|---|---|
| Vesical branches | 16 ± 0.26 | 25 ± 0.5† |
| Anastomotic branches | 11 ± 0.45 | 15 ± 1.7* |
| Motor branch of the sacral plexus | 11.6 ± 1.67 | 17.5 ± 0.05* |
| Dorsal nerve of the clitoris | 10 ± 1.0 | 15 ± 1.0* |
Values are mean ± SEs of nerve length (in mm).
VD, vaginal distension; SH, sham.
P < 0.05 and
P < 0.01 by Student's t-test.
Fig. 3.
Example electrical activity recorded from the DNC of a SH or VD rat before or while the clitoris was being squeezed. The horizontal dashed line below each electroneurogram recording indicates the duration of the stimulus (squeezing). Values are means ± SE of data from 4 SH animals and 4 VD animals. **Significant difference vs. SH animals with P < 0.01.
Experiment 2: bladder and MPG features.
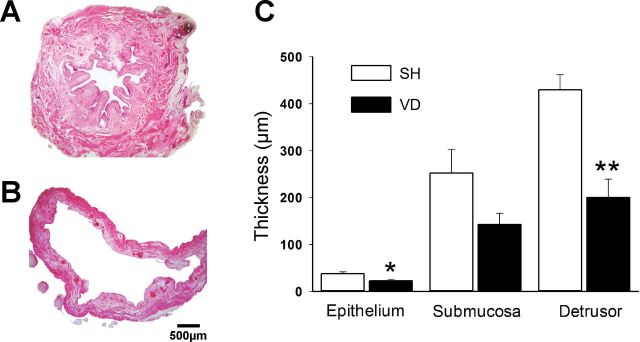
VD overdistended the bladder and significantly decreased the thickness of the epithelial (P < 0.05) and detrusor layers (P < 0.01) but not the submucosa (Fig. 4). Blood and leucocyte extravasation were observed in VD rat bladder tissue. MPG area (SH: 0.397 ± 0.050 mm2 vs. VD: 0.419 ± 0.061 mm2) and thickness (SH: 653.0 ± 51.0 μm vs. VD: 808.0 ± 128.0 μm) and the area and diameter of MPG neurons (P > 0.05; Fig. 5) did not change with VD.
Fig. 4.
Photos of transverse sections of the bladder (hematoxylin and eosin stain) in SH (A) and VD (B) animals. Note that the thicknesses of the epithelium and detrusor layers were significantly decreased in VD animals (C). Values are means ± SE of data from 4 animals. *Significant difference vs. SH animals with P < 0.05; **significant difference vs. SH animals with P < 0.01.
Fig. 5.
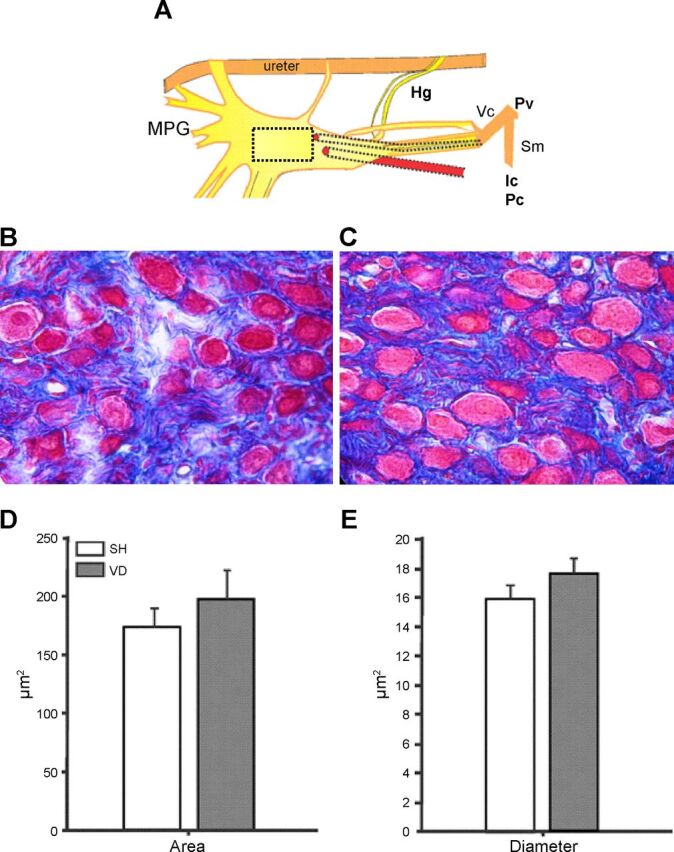
MPG neurons. The drawing in A represents the MPG; the square shows the site where tissue for the microphotographs was taken. Representative postganglionic neurons of SH (B) and VD (C) groups are shown. The graphs indicate the area (D) and diameter (E) of 20 neurons/rat. Values are means ± SE of data from 4 animals. Hg, hypogastric nerve; Pv, pelvic nerve; Vc, viscerocutaneous branch of the Pv; Sm, somatomotor branch of the Pv.
Experiment 3: micturition behavior.
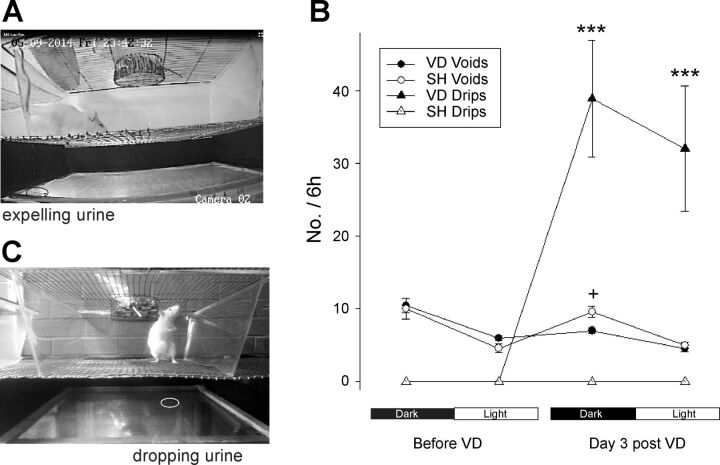
SH rats voided more frequently in the dark phase than in the light phase (1.65 ± 0.15 vs. 0.8 ± 0.06 voids/h, P < 0.01; Tables 2 and 3). Voiding interval (P < 0.01) and voided volume (P < 0.05) were higher in the light phase than in the dark phase (Tables 2 and 3). To void, 100% of the SH rats walked to the edges of the cage (urine was found in the four edges but mainly on the corners), placed the rump toward the wall, and expelled urine, flowing in a stream. In 50% of the voids, rats had a pronounced rise of the tail (Fig. 6A). SH rats did not drip urine in any body posture. The pattern of the urinary parameters in the dark-light cycle (Tables 2 and 3) or the voiding behavior did not change 3 days after SH procedures.
Table 2.
Urinary parameters of female rats with VD during 6 h of the dark phase
| Voiding Frequency, number/6
h |
Voiding Interval, min |
Voiding Volume, ml |
|||||
|---|---|---|---|---|---|---|---|
| Number of Rats/Group | Before | After | Before | After | Before | After | |
| SH | 5 | 10 ± 1.8 | 10.5 ± 1.2 | 41 ± 3.3 | 39 ± 7 | 0.65 ± 0.09 | 0.59 ± 0.1 |
| VD | 9 | 11 ± 1.0 | 7 ± 0.5* | 39.5 ± 3.8 | 54 ± 6* | 0.6 ± 0.06 | 0.68 ± 0.1 |
Values are means ± SE.
P < 0.05 vs. before VD.
Table 3.
Urinary parameters of female rats with VD during 6 h of the light phase
| Voiding Frequency, number/6
h |
Voiding Interval, min |
Voiding Volume, ml |
|||||
|---|---|---|---|---|---|---|---|
| Number of Rats/Group | Before | After | Before | After | Before | After | |
| SH | 5 | 4.5 ± 0.6 | 5 ± 0.8 | 69 ± 5.4 | 68 ± 2.5 | 0.85 ± 0.06 | 0.89 ± 0.1 |
| VD | 9 | 6 ± 0.3 | 5 ± 0.4 | 73 ± 6.6 | 84 ± 5.8 | 0.86 ± 0.1 | 0.74 ± 0.06 |
Values are means ± SE.
Fig. 6.
Photos of a female rat in the observational cage of micturition. Rats void at the edge of the cage (A). VD rats dripped urine during some behaviors implicating stress, such as standing up on the hind limbs during vertical exploration (C). The graph indicates the percentage of urine expelled as voids or drips before and 3 days after VD (n = 9) or SH (n = 3) procedures during the last 6 h of the dark phase and the first 6 h of the light phase. Values are means ± SE. ***P < 0.0001 vs. voids of the same phase.
The pattern of urinary behavior parameters and micturition behavior was similar to those of SH rats before VD. However, 3 days after VD, the animals dripped urine in the absence of the stereotyped voiding behavior during the dark phase (38 ± 7 drips/6 h) and light phase (12 ± 3 drips/6 h). In the dark phase, 72 ± 7% of the urine expulsions were dripped in unusual places for voiding (Fig. 6); from this number, ∼69% were 1–3 drops (20–30 μl) expelled during behaviors implicating stress such as standing to reach food, sneezing, scratching, running, leaning the body to lick the vaginal orifice, or standing on their hind legs for vertical exploration (Fig. 6C), and the other 3% were >7 drops expelled while the rats were rushing to the edge of the cage. Only 28 ± 7% of the urine expulsed were voids. Compared with the number of voids before VD, the number of voids during the dark phase decreased significantly (P < 0.05) and the voiding interval increased after VD (Table 2).
In the light phase, most of the time the rat was sleeping but periodically awoke to stretch its body, void, eat, and/or drink. After VD, the rats dripped urine while stretching the body, scratching the head, or leaning the body to lick the vaginal orifice. Although ∼50% of the urine expulsions were drips (Fig. 6B), the number of voids, voiding interval, or voiding volume during the light phase did not significantly change after VD (Table 2). VD rats did not dripped urine with every effort behavior but only occasionally, for example, 1 drip of six vertical explorations.
DISCUSSION
Parturition is considered as an important risk factor for pelvic floor dysfunction in women (3, 47). To test the effect of vaginal delivery, an animal model with vaginal balloon dilatation has been proposed (33). VD induces hypoxia to the urogenital organs (9) and anatomic and functional damage of the urethra (4, 21, 26, 58).
The present study adds information about the regions of the genitourinary tract that are distended by the rodent VD model, the grade of elongation of the genitourinary nerves during VD, the effect of VD on urinary retention and bladder layers, and the consequence of this procedure on micturition in awake animals. In fact, this is the first study that describes correlation of VD-induced genitourinary neuroanatomy with behavioral signs of urinary dysfunction.
During VD, the inflated balloon did not stay completely in the pelvic cavity but protruded to the distal vagina, which made it necessary to suture the vaginal orifice to avoid balloon expulsion. In that position, the balloon compressed the organs of the pelvic area (pelvic vagina, pelvic urethra, rectum, pelvic floor muscles, and its vessels and nerves) against the pelvic bone and distended the perineal genitourinary tract (distal region of the vagina and urethra and the vessels and nerves related to them). Thus, the VD model in rats produces direct mechanical damage to the pelvic urethra and distal genitourinary tract, which may explain the resultant disorganization and thinness of the EUS (4, 39) and the physiological impairment observed after VD, such as hypoxia of the genitourinary organs, indicating a reduction in blood flow (9), abolished EUS activity (21), decreased leak point pressure, and/or urine leakage with effort, suggesting urethral closure incompetence (26, 33, 56). VD-induced physiological urethral impairment may result from damage of the EUS muscle fibers, urethral smooth muscle, and their innervation, since these factors contribute to urinary continence (23).
VD distends the distal vagina and the somatic nerves running along it, such as the DNC and MBSP. These nerves were elongated by 50%, greater than that reported in a simulation model of pudendal nerve stretching during second stage of parturition in women (32). This nerve elongation may denervate the clitoris and EUS. Stretching nerve injury may also decrease microtubules and tau protein, important elements in axonal function (51). DNC and MBSP stretching due to VD may induce sexual and urinary dysfunction, since denervation of the clitoris induces signs of coital urinary incontinence (5) and damage of the EUS innervation induced signs of urinary incontinence in rats (6, 29, 42).
Manual stretching of the motor branch of the pudendal nerve, ∼74% of its length, temporarily abolished the activity of the EUS, which returned 30 min after the stretching (52). However, this procedure was performed at the level of the Alcock's canal and stretched the complete nerve before branching. In contrast, VD may also stretch terminal nerves, those thin fascicles arriving at their targets that may get easily broken and disrupt motor endplates.
We observed urinary retention and bladder overdistension after VD, presumably as an indirect effect of the pelvic urethra occlusion while the balloon compressed the urethra against the pubic bone, preventing micturition. The hematomas in the bladder, thinness of the epithelium and detrusor layers, and stretching of vesical nerves may result in sensory and motor bladder dysfunction. Sensory receptors localized in the epithelium and muscular layers (27) may decrease, affecting the transference of bladder information to the central nervous system. Motor nerves also may diminish and lead to an underactive bladder. Decrease in the adrenergic and cholinergic innervation of the bladder has been observed after induced urinary retention and bladder overdistension in rats (31, 59). Whether women suffer neuroanatomic changes in the bladder with labor is unknown, although it is possible, since urinary retention is a well-known condition after childbirth, with epidural analgesia, prolonged labor, episiotomy, and high birth weight as risk factors (28, 37, 38). The epidural analgesia commonly used to avoid pain during labor causes bladder hypotonia and eliminates the normal sensation to void, leading to bladder overdistension (62), which may damage the detrusor and consequently induce long-term voiding difficulties (64). Thus, for physiological or pharmacological reasons, bladder overdistension is not uncommon during labor (5) and because it is dangerous for women's urinary health, physicians try to avoid bladder overdistension by catheterizing the urethra at some stage in labor (14). The fact that the VD model also induces bladder overdistension is positive for the model because it mimics what happen clinically.
There are also some reports of bladder rupture during labor (63), which can lead to underactive bladder. Thus, impaired detrusor contraction is greater in women with a history of urinary retention (1). Symptoms of underactive bladder are common in both older men and women (7, 61); however, its prevalence in people of <65 yr old is higher in women than men (20.6% vs. 9.3%) (61).
The MPG is sexually dimorphic in rats: bigger in male rats than in female rats (44). The MPG in female rats is within adipose tissue attached on the rostral vaginal tract, almost lateral to the cervix (36). It has been estimated to contain ∼5,000 neurons (13, 17).
Considering that the vagina is a large hollow organ, during VD, the balloon could pull the rostral region of the vaginal tract down, stretching and damaging the MPG. However, our results did not show any significant differences in the MPG or area of neurons between SH and VD animals. These data suggest that VD does not significantly affect the morphometry of MPG neurons.
In contrast, a previous study (33) reported a decreased number of MPG neurons in VD rats, from three to eight neurons in SH animals to zero to one neurons in VD rats. The controversy can be related to the method used to obtain the tissue to be analyzed. As described in materials and methods, we cut the complete ganglion, analyzed 3 sections of the middle of it, and found ∼100 neurons/section but analyzed only those with a visible nucleus. The previous study reported that the genitourinary tract was collected and analyzed. Considering that the female MPG is very thin and hard to visualize, it is possible that they did not collect the complete MPG, since they reported very few neurons (3–8 neurons) in sham animals.
The female rats in this study demonstrated stereotyped behavior of micturition, similar to that described previously in male rats (24, 25). The design of the top of the cage with the food and water container in the center of the jumbo cage enabled us to determine that normal rats do not void in the center of the cage. To void, rats walked to an edge of the cage and placed the rump facing the wall of the cage, sometimes just walking backward until reaching the wall, and expelling urine. Expression of this behavior suggests rats feel bladder fullness and volition to expel urine then move to the edges of the cage. Urine marking behavior has been described in rodents (35, 43). To mark, the rat placed the urinary meatus close to the object to be marked (35). Considering that the cage of our rats did not contain objects for marking, any urine expelled without the stereotyped behavior of micturition was considered as a sign of micturition dysfunction or leakage.
Other methods used to analyze micturition in normal and experimental rats have analyzed voiding place preference by placing paper below the grid of the cage to collect the urine (16, 29). Marks in the front of the cage, after pudendal nerve injury, are considered to indicate urinary incontinence (29). However, in the present study, we noticed that rats voided in the four edges of the cage. So, although a detailed study is necessary to determine whether the rats void more frequently in a special edge of the cage, urine in the front of the cage seems to not be an accurate method to determine urinary incontinence.
The metabolic cage has also been widely used to study micturition in awake animals (8, 53). In this cage, the urine slips on the wall of the cage to the urine container. If the container is placed on a force transducer connected to a computer, voiding frequency and voided volume may be automatically recorded (10, 29). Certainly this system seems to be good to study micturition in control animals but not leakage. Indeed, the finding that frequency of voiding in intact rats varied in relation to day-night corroborates prior studies using metabolic cages (∼60% of the voids are in the dark phase, the active period of the rats) (29), indicating that our findings are not an artifact of our methods. However, 1–3 drops alone do not slip along the wall to reach the urine funnel of the metabolic cage, so they are not recorded. Thus, although it requires more work, the analysis of the behavior of micturition seems to be reliable to determine signs of urinary dysfunction in conscious rats.
Considering that the stereotyped behavior of micturition suggests that sensory information of bladder fullness reaches the somatosensory cortex of the brain and the rat maintains urinary continence until reaching the stereotyped posture for voiding, similar to people, expulsion of urine without that posture can indicate urinary dysfunction. Dripping small volumes of urine in unusual places to void correlated with stress behaviors (sneezing, stretching the body, standing in hind limbs, etc.) and may be an indicator of induced stress urinary incontinence. Expulsion of a large amount of urine before reaching the edge to perform the stereotyped behavior may suggest urgency and overactive bladder. Increased number of voids during the rest period (light) may indicate signs of nocturia. According to this behavioral correlation, most (69%) of the urine drips in VD rats are considered signs of stress urinary incontinence (they expelled 1–3 drops of 20–30 μl released during effort behavior) and 3% of urgency (200–300 μl of urine released during daily activities or while walking to the edge). Since we observed bladder overdistension, it would be important in future studies to discern the effect of direct damage from VD versus the effect of VD-induced bladder overdistension on the physiology of micturition of these awake animals.
Bladder overdistension, and acute urinary retention, has been modeled with different techniques: acute complete bladder outlet obstruction with a silk ligature of the urethra, middle bladder neck obstruction with ligature and catheter, bladder overdistension with an inflated balloon into the bladder, and bladder infusion after clamping the distal urethra (12, 49, 50). In these models, structural changes and bladder dysfunction have been reported. Thus, partial outlet obstruction increased voiding frequency and reduced voiding volume, lasting 2 wk. On the other hand, direct urethral VD damage on the physiology of micturition has been modeled by denervating the EUS in rats (22). Lesion of the pudendal nerve in anesthetized rats decreases the urethral pressure and leak point pressure, suggesting stress urinary incontinence. Considering that in our study the rats had the two VD effects: urethral damage and bladder acute urinary retention-overdistension together, we would expect increased voiding frequency as well as urine leakage during effort. However, our rats dripped urine but the voiding frequency did not increase, a sign of urethral dysfunction by weakness of urethral closure. To determine whether urethral dysfunction was more prominent than bladder dysfunction, masking the bladder dysfunction or bladder overdistension was not enough to affect the function, requiring further studies out of the scope of the present study.
Some limitations to be considered are as follows. First, micturition behavior does not indicate whether the bladder concurrently contracted with each urine expulsion. Cystometry in conscious animals could add important information. Second, the hormonal condition of the rats was unknown as the phase of the estrous cycle was not determined, although the estrous cycle did not previously affect VD-induced stress urinary incontinence in mice (18). Finally, the rats were not pregnant, which means that the anatomical and physiological parameters could be different than postpartum since relaxation of pelvic ligaments, cervical ripening, estrogen peak preceding parturition (54), or the hypoestrogenic state of nursing could diminish or increase trauma and recovery from vaginal delivery. It has been proposed that estrogen treatment at the time of pudendal nerve injury facilitates nerve regeneration (2). Even though there are differences between multiparous and nulliparous rats, VD is not innocuous in multiparous animals. It increases the percent of rats with urine leakage and the damage to urethral striated muscle and nerve fibers (45, 46), from which we conclude that the VD model in nulliparous animals is suitable to mimic prolonged vaginal parturition, with the consideration that in some variables, the magnitude of the VD-induced damage may differ between nulliparous vs delivery groups.
In conclusion, the present study demonstrated that VD produces direct and indirect injuries on the genitourinary organs and their innervation in female rats. Direct mechanical damage occurs as the balloon compresses the pelvic region of the genitourinary tract and distends the distal region, including the DNC and MBSP nerves. Indirectly, the inflated balloon damages the bladder, likely as a consequence of urinary retention. The neuroanatomic injuries occur alongside behavioral signs of stress urinary incontinence. The behavior of micturition may be an important tool in the studies of the physiology of micturition as well as for long-term studies of nerve plasticity and to test treatments that could facilitate urinary function recovery.
GRANTS
This work was funded by Consejo Nacional de Ciencia y Tecnología Grant YCG 183446, José Luis Palacios Galicia scholarship 488223, and Secretaría de Educación Pública-Subsecretaría de Educación Superior, Dirección General de Estudios Superiores Grant PADES 2016 Folio 243. This work was also supported in part by the Cleveland Clinic and Rehabilitation Research and Development Service of the United States Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.P., M.J., and N.X. performed experiments; J.L.P., M.S.D., and Y.C. interpreted results of experiments; J.L.P. and N.X. prepared figures; J.L.P. drafted manuscript; J.L.P., M.J., C.M., N.X., M.S.D., and Y.C. approved final version of manuscript; M.J. and Y.C. analyzed data; C.M., N.X., M.S.D., and Y.C. edited and revised manuscript; Y.C. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Julio Cuatecontzi and José Luis Tlachi for technical assistance and artwork.
REFERENCES
- 1.Abarbanel J, Marcus EL. Impaired detrusor contractility in community-dwelling elderly presenting with lower urinary tract symptoms. Urology 69: 436–440, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed Y, Lin DL, Ferguson C, Esparza N, Damaser MS. Effect of estrogen on urethral function and nerve regeneration following pudendal nerve crush in the female rat. J Urol 175: 1948–1952, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Ashton-Miller JA, Howard D, DeLancey JO. The functional anatomy of the female pelvic floor and stress continence control system. Scand J Urol Nephrol Suppl 2001: 1–7, discussion 106–125, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int 90: 403–407, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Cruz Y, Juarez R, Medel A, Corona-Quintanilla DL, Pacheco P, Juarez M. Coital urinary incontinence induced by impairment of the dorsal nerve of the clitoris in rats. J Urol 195: 507–514, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Cruz Y, Pastelin CF, Balog BM, Zaszczurynski PJ, Damaser MS. Somatomotor and sensory urethral control of micturition in female rats. Am J Physiol Renal Physiol 307: F1207–F1214, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapple CR, Osman NI, Birder L, van Koeveringe GA, Oelke M, Nitti VW, Drake MJ, Yamaguchi O, Abrams P, Smith PP. The underactive bladder: a new clinical concept? Eur Urol 68: 351–353, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Chun AL, Wein AJ, Harkaway R, Levin RM. Comparison of urinary bladder function in sexually mature and immature male and female rats. J Urol 143: 1267–1271, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Damaser MS, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol 98: 1884–1890, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Elkelini MS, Aitken K, Bagli DJ, Hassouna MM. Effects of doxycycline on voiding behaviour of rats with bladder outlet obstruction. BJU Int 103: 537–540, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabella G, Uvelius B. Structural changes in the rat bladder after acute outlet obstruction. Scand J Urol Nephrol Suppl 201: 32–37, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood D, Coggeshall RE, Hulsebosch CE. Sexual dimorphism in the numbers of neurons in the pelvic ganglia of adult rats. Brain Res 340: 160–162, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Gyampoh B, Crouch N, O'Brien P, O'Sullivan C, Cutner A. Intrapartum ultrasound estimation of total bladder volume. BJOG 111: 103–108, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Hale DS, Benson JT, Brubaker L, Heidkamp MC, Russell B. Histologic analysis of needle biopsy of urethral sphincter from women with normal and stress incontinence with comparison of electromyographic findings. Am J Obstet Gynecol 180: 342–348, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Heidkamp MC, Leong FC, Brubaker L, Russell B. Pudendal denervation affects the structure and function of the striated, urethral sphincter in female rats. Int Urogynecol J Pelvic Floor Dysfunct 9: 88–93, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Houdeau E, Prud'homme MJ, Rousseau A, Rousseau JP. Distribution of noradrenergic neurons in the female rat pelvic plexus and involvement in the genital tract innervation. J Auton Nerv Syst 54: 113–125, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Daneshgari F, Liu G. Successful induction of stress urinary incontinence in mice by vaginal distension does not depend on the estrous cycle. Urology 83: 958 e951–9562014. [DOI] [PubMed] [Google Scholar]
- 19.Hudson R, Cruz Y, Lucio A, Ninomiya J, Martinez-Gomez M. Temporal and behavioral patterning of parturition in rabbits and rats. Physiol Behav 66: 599–604, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Jiang HH, Damaser MS. Animal models of stress urinary incontinence. Handb Exp Pharmacol 45–67, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang HH, Gustilo-Ashby AM, Salcedo LB, Pan HQ, Sypert DF, Butler RS, Damaser MS. Electrophysiological function during voiding after simulated childbirth injuries. Exp Neurol 215: 342–348, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang HH, Pan HQ, Gustilo-Ashby MA, Gill B, Glaab J, Zaszczurynski P, Damaser M. Dual simulated childbirth injuries result in slowed recovery of pudendal nerve and urethral function. Neurourol Urodyn 28: 229–235, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang HH, Salcedo LB, Damaser MS. Quantification of neurological and other contributors to continence in female rats. Brain Res 1382: 198–205, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juarez M, Hernandez I, Cruz Y. Genitourinary dysfunction in male rats after bilateral neurectomy of the motor branch of the sacral plexus. Neurourol Urodyn 31: 1288–1293, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Juarez R, Cruz Y. Urinary and ejaculatory dysfunction induced by denervation of specific striated muscles anatomically related to the urethra in male rats. Neurourol Urodyn 33: 437–442, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Kamo I, Kaiho Y, Canon TW, Chancellor MB, de Groat WC, Prantil RL, Vorp DA, Yoshimura N. Functional analysis of active urethral closure mechanisms under sneeze induced stress condition in a rat model of birth trauma. J Urol 176: 2711–2715, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Kanai AJ. Afferent mechanism in the urinary tract. Hand Exp Pharmacol 2001: 171–205, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Kekre AN, Vijayanand S, Dasgupta R, Kekre N. Postpartum urinary retention after vaginal delivery. Int J Gynaecol Obstet 112: 112–115, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, Brubaker L. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn 19: 53–69, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Kjaergaard H, Olsen J, Ottesen B, Dykes AK. Incidence and outcomes of dystocia in the active phase of labor in term nulliparous women with spontaneous labor onset. Acta Obstet Gynecol Scand 88: 402–407, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Lasanen LT, Tammela TL, Kallioinen M, Waris T. Effect of acute distension on cholinergic innervation of the rat urinary bladder. Urol Res 20: 59–62, 1992. [DOI] [PubMed] [Google Scholar]
- 32.Lien KC, Morgan DM, Delancey JO, Ashton-Miller JA. Pudendal nerve stretch during vaginal birth: a 3D computer simulation. Am J Obstet Gynecol 192: 1669–1676, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology 52: 143–151, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Gomez M, Cruz Y, Pacheco P, Aguilar-Roblero R, Hudson R. The sensory but not muscular pelvic nerve branch is necessary for parturition in the rat. Physiol Behav 63: 929–932, 1998. [DOI] [PubMed] [Google Scholar]
- 35.McIntosh TK, Davis PG, Barfield RJ. Urine marking and sexual behavior in the rat (Rattus norvegicus). Behav Neural Biol 26: 161–168, 1979. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell BS. Morphology and neurochemistry of the pelvic, and paracervical ganglia. Histol Histopathol 8: 761–773, 1993. [PubMed] [Google Scholar]
- 37.Mulder FE, Hakvoort RA, Schoffelmeer MA, Limpens J, Van der Post JA, Roovers JP. Postpartum urinary retention: a systematic review of adverse effects and management. Int Urogynecol J 25: 1605–1612, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Mulder FE, Oude Rengerink K, van der Post JA, Hakvoort RA, Roovers JP. Delivery-related risk factors for covert postpartum urinary retention after vaginal delivery. Int Urogynecol J 27: 55–60, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan HQ, Kerns JM, Lin DL, Liu S, Esparza N, Damaser MS. Increased duration of simulated childbirth injuries results in increased time to recovery. Am J Physiol Regul Integr Comp Physiol 292: R1738–R1744, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastelin CF, Juarez R, Damaser MS, Cruz Y. Neural pathways of somatic and visceral reflexes of the external urethral sphincter in female rats. J Comp Neurol 520: 3120–3134, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Pauwels E, De Wachter S, Wyndaele JJ. Evaluation of different techniques to create chronic urinary incontinence in the rat. BJU Int 103: 782–785, discussion 785–786, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn 25: 388–396, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Price EO. Hormonal control of urine-marking in wild and domestic Norway rats. Horm Behav 6: 393–397, 1975. [DOI] [PubMed] [Google Scholar]
- 44.Purinton PT, Fletcher TF, Bradley WE. Gross and light microscopic features of the pelvic plexus in the rat. Anat Rec 175: 697–705, 1973. [DOI] [PubMed] [Google Scholar]
- 45.Rocha MA, Sartori MG, De Jesus Simoes M, Herrmann V, Baracat EC, Rodrigues de Lima G, Girao MJ. The impact of pregnancy and childbirth in the urethra of female rats. Int Urogynecol J Pelvic Floor Dysfunct 18: 645–651, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Rocha MA, Sartori MG, De Jesus Simoes M, Herrmann V, Baracat EC, Rodrigues de Lima G, Girao MJ. Impact of pregnancy and childbirth on female rats' urethral nerve fibers. Int Urogynecol J Pelvic Floor Dysfunct 18: 1453–1458, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Vaginal delivery parameters and urinary incontinence: the Norwegian EPINCONT study. Am J Obstet Gynecol 189: 1268–1274, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberg K, Trevathan W. Birth, obstetrics and human evolution. BJOG 109: 1199–1206, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Saito M, Shimizu S, Kinoshita Y, Satoh I, Shomori K, Dimitriadis F, Satoh K. Bladder dysfunction after acute urinary retention in the rats: a novel over active bladder model. Mol Cell Biochem 333: 109–114, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Saito M, Suzuki H, Yamada M, Hikita K, Kobayashi N, Kinoshita Y, Houri D, Miyagawa I, Satoh K. Effect of cyclohexenonic long-chain fatty alcohol on rat overactive bladder induced by bladder neck obstruction. Eur J Pharmacol 501: 143–149, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Sajadi KP, Gill BC, Damaser MS. Neurogenic aspects of stress urinary incontinence. Curr Opin Obstet Gynecol 22: 425–429, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sajadi KP, Lin DL, Steward JE, Balog B, Dissaranan C, Zaszczurynski P, Gill BC, Jiang HH, Kerns JM, Damaser MS. Pudendal nerve stretch reduces external urethral sphincter activity in rats. J Urol 188: 1389–1395, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt F, Yoshimura Y, Ni RX, Kneesel S, Constantinou CE. Influence of gender on the diurnal variation of urine production and micturition characteristics of the rat. Neurourol Urodyn 20: 287–295, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Shaikh AA. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol Reprod 5: 297–307, 1971. [DOI] [PubMed] [Google Scholar]
- 55.Shields SG, Ratcliffe SD, Fontaine P, Leeman L. Dystocia in nulliparous women. Am Fam Physician 75: 1671–1678, 2007. [PubMed] [Google Scholar]
- 56.Sievert KD, Emre Bakircioglu M, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol 166: 311–317, 2001. [PubMed] [Google Scholar]
- 57.Smith AR, Hosker GL, Warrell DW. The role of pudendal nerve damage in the aetiology of genuine stress incontinence in women. Br J Obstet Gynaecol 96: 29–32, 1989. [DOI] [PubMed] [Google Scholar]
- 58.Song QX, Balog BM, Kerns J, Lin DL, Sun Y, Damaser MS, Jiang HH. Long-term effects of simulated childbirth injury on function and innervation of the urethra. Neurourol Urodyn 34: 381–386, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tammela T, Lasanen L, Waris T. Effect of distension on adrenergic innervation of the rat urinary bladder. Urol Res 18: 345–348, 1990. [DOI] [PubMed] [Google Scholar]
- 60.Thom DH, Nygaard IE, Calhoun EA. Urologic diseases in America project: urinary incontinence in women-national trends in hospitalizations, office visits, treatment and economic impact. J Urol 173: 1295–1301, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Valente S, DuBeau C, Chancellor D, Okonski J, Vereecke A, Doo F, Lajiness M, Diokno A, Chancellor M. Epidemiology and demographics of the underactive bladder: a cross-sectional survey. Int Urol Nephrol 46, Suppl 1: S7–S10, 2014. [DOI] [PubMed] [Google Scholar]
- 62.Weil A, Reyes H, Rottenberg RD, Beguin F, Herrmann WL. Effect of lumbar epidural analgesia on lower urinary tract function in the immediate postpartum period. Br J Obstet Gynaecol 90: 428–432, 1983. [DOI] [PubMed] [Google Scholar]
- 63.Yang B. Bladder rupture associated with uterine rupture at delivery. Int Urogynecol J 22: 625–627, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Yip SK, Hin LY, Chung TK. Effect of the duration of labor on postpartum postvoid residual bladder volume. Gynecol Obstet Invest 45: 177–180, 1998. [DOI] [PubMed] [Google Scholar]