Abstract
There is no widely accepted standard of care for canine anal sac apocrine gland adenocarcinoma (ASAGAC). Surgery alone is inadequate in many cases, but the benefit of adjuvant chemotherapy is not well-established. The primary objective of this retrospective study was to evaluate the role of carboplatin chemotherapy in the post-operative management of ASAGAC. Seventy-four dogs with naturally-occurring ASAGAC underwent surgery. Forty-four dogs received adjuvant carboplatin and 30 did not. Median overall survival was 703 days. Median time to progression was 384 days. Only primary tumor size and lymph node metastasis at diagnosis significantly impacted outcome. Differences in OS and TTP, between dogs that received adjuvant carboplatin and those that did not, failed to reach statistical significance. Treatment of progressive disease, whilst not limited to chemotherapy, significantly prolonged survival. This study demonstrates that adjuvant carboplatin chemotherapy is well-tolerated and may have a role in the management of dogs with ASAGAC.
Keywords: Carboplatin, Surgery, Outcome, Canine
Introduction
Anal sac apocrine gland adenocarcinoma (ASAGAC) is a malignant tumor arising from the secretory epithelium of the walls of the bilateral anal sacs. ASAGAC accounts for only 2% of all canine skin tumors and 17% of all perianal tumors in this species.1,2
ASAGAC displays aggressive biologic behavior. It is both locally invasive and highly metastatic, with a reported overall metastatic rate of between 40 and 96%.1,3–10 Metastasis to the regional lymph nodes is frequently reported early in the course of the disease,5,6,7,9,10 whilst metastasis to distant sites, such as the lungs, liver, spleen and bone, is typically observed months to years later.1,3–7,9
In addition to the morbidity associated with the tumor’s locally aggressive behavior, systemic clinical signs may occur as a result of paraneoplastic hypercalcemia.4–8,11,12,13 The hypercalcemia associated with ASAGAC occurs because of parathyroid hormone-related peptide (PTH-rp) synthesis and secretion by the tumor, and the protein’s downstream endocrine sequelae.11,12 Paraneoplastic hypercalcemia is reported in approximately 25% of cases of ASAGAC, although documented incidences are as high as 53%, and whilst there exists some discordance in the literature,5,6,8 hypercalcemia has been associated with decreased survival time.4,7,13
Multiple treatment modalities have been utilized in the management of ASAGAC, including surgery, radiation therapy, chemotherapy, electrochemotherapy, and various combinations thereof.5,6,8,9,13–16 Currently, there is no widely accepted standard of care for canine ASAGAC, other than to recommend surgical resection of the primary tumor, and to consider adjuvant therapy. Surgery alone is often inadequate in controlling either local or metastatic disease.4,5,7 With surgery alone, local recurrence occurs in 20 to 50% of dogs with ASAGAC,4,5 and metastasis occurs in 40 to 96%.1,3–10 The longest reported overall survival times were achieved in dogs that received multimodal therapy, consisting of a combination of surgery, radiation therapy and chemotherapy.6
Numerous chemotherapeutic agents have been investigated for both the adjuvant and palliative management of ASAGAC, including the platinum compounds carboplatin and cisplatin, actinomycin-D, the synthetic anthracycline / acenedione mitoxantrone, melphalan, and more recently the tyrosine kinase inhibitor toceranib phosphate (Palladia™).5,6,8,9,14,16 Despite this, the role of adjuvant chemotherapy in the treatment of ASAGAC remains poorly defined. Undoubtedly, this is a consequence of inconsistent treatment, diagnostic and monitoring regimens, study population heterogeneity, and resultant under-powering of statistical analyses and conclusions.
The primary objective of the present study was to retrospectively evaluate the role of adjuvant carboplatin in the management of surgically excised ASAGAC in a relatively homogenous population of dogs with consistent diagnostic, treatment and monitoring regimens. The authors’ hypothesis was that adjuvant carboplatin chemotherapy would improve time to progression and/or overall survival time in dogs with AGASAC relative to dogs treated with surgery alone. The secondary objective of this study was to evaluate prognostic factors that may be used to guide treatment recommendations.
Materials and methods
Case selection and data collection
Cases with a definitive ante-mortem diagnosis of ASAGAC were identified from a search of the medical records of the University of Wisconsin-Madison Veterinary Medical Teaching Hospital (UW-M VMTH), spanning a ten-year period, between September 2001 (9/10/2001) and December 2011 (12/6/2011).
Inclusion criteria applied to the cases required a histopathologically confirmed diagnosis of ASAGAC for which surgery was the primary treatment option employed, as well as complete staging, consisting of history, physical examination including per rectum digital examination, hematologic, biochemical and urine analyses, thoracic radiography and abdominal ultrasound. For those cases receiving adjuvant carboplatin chemotherapy, dogs were included on an intent-to-treat basis. This institution’s standard protocol consisted of an intended four doses of carboplatin to be administered at 300mg/m2 intravenously every three weeks. A 10–20% dose reduction is used for dogs weighing less than 10kg and a 20% dose reduction is used for dogs developing any grade IV toxicity.
Cases were excluded if surgery was not the initial treatment option employed, if radiation therapy was incorporated in the initial treatment plan, or if the adjuvant chemotherapy protocol administered was not the aforementioned standard protocol. Cases suspected of having extra-nodal metastatic disease identified during staging were excluded from the study. Cases were also excluded if inadequate information was available, specifically a lack of detail regarding the diagnostic, staging and monitoring tests, and a duration of follow-up of less than 30 days.
Information retrieved from the medical records included; signalment, presenting clinical signs, relevant physical examination findings, results of clinicopathologic testing, including hematologic, biochemical and urine analyses, radiographic, ultrasonographic and advanced imaging (computed tomography) findings, a surgical description, the details of adjunctive chemotherapy, and any rescue and / or palliative treatments instituted, as well as outcomes. The referring veterinarian(s) and / or owner(s) were contacted for follow-up where additional detail was required.
Toxicities were graded according to the Veterinary cooperative oncology group’s common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1.16
Statistical analysis
For the purposes of fulfilling the study’s primary objective, cases were divided into two sub-groups; those dogs that underwent surgery alone, and those that underwent surgery followed by adjuvant chemotherapy.
Descriptive and comparative statistics were performed for the overall study population, as well as the two main sub-groups. Fisher’s Exact Test was used to compare categorical non-numerical data, such as gender, between groups. The Wilcoxon Rank-Sum Test was used to compare continuous numerical data, such as age and weight, between groups. Results were considered significant at P <0.05.
Two measures of outcome were evaluated. Time to progression (TTP) was defined as the time (in days) from the date of surgery until the date of confirmed disease progression (either local recurrence or metastatic disease). Overall survival (OS) was defined as the time (in days) from the date of surgery until the date of death as a result of anal sac apocrine gland adenocarcinoma or another cause. Data was considered right-censored at the time of last veterinary contact if the dogs remained alive at the conclusion of the study period, or if they were ultimately lost to follow-up. Kaplan-Meier survival curves were estimated for both outcomes, along with median survival times and confidence intervals. OS and TTP were compared between the study’s two main sub-groups using the Log-Rank Test. Results were considered significant at P <0.05.
The effect of variables, including signalment, primary tumor characteristics, presence or absence of clinical signs at the time of diagnosis, presence or absence of hypercalcemia at the time of diagnosis, presence or absence of metastasis at the time of diagnosis, the location and extent of metastasis, the administration of adjuvant chemotherapy, and the administration of various rescue therapies at the time of progression, on OS and TTP were evaluated using Cox proportional-hazard models. Variables were first fit singly, and those that were significant were then used as a potential predictor set for a backward stepwise procedure. Adjuvant chemotherapy was retained at all steps so that its contribution could be evaluated in the presence of other potentially confounding variables. All other variables that had P <0.05 were considered significant and were retained in the model.
All statistical analyses were performed utilizing two commercially available software packages; Microsoft® Excel® for mac 2011 (version 14.2.5) and R for Mac OS X GUI 2012 (version 2.15.2).
Results
Patient population
One hundred and twenty-four cases of ASAGAC were treated surgically during the study’s time period. Of these cases, 74 dogs fulfilled the study’s inclusion criteria. Thirty dogs (40.5%) received adjuvant carboplatin chemotherapy, whilst 44 dogs (59.5%) did not received adjuvant chemotherapy. Nineteen dogs were excluded due to the primary use of non-carboplatin chemotherapy protocols. Ten dogs were excluded because radiation therapy was incorporated into the initial treatment regimen. In six cases surgery was not the primary treatment modality employed. In only one case the presence of diffuse pulmonary metastatic disease precluded inclusion. Fourteen cases were excluded as a result of inadequate historical, diagnostic, staging or monitoring data, or duration of follow-up not exceeding 30 days.
The median age of all the dogs in the study population was 9.71 years (Range: 6.06 to 16.15 years). Forty-eight dogs (65%) were castrated males and 26 were spayed females (35%). There were no intact dogs in the study population. Twenty-nine different breeds were represented in the study population. Labradors and their crosses predominated (n= 17), followed by golden retrievers (n= 7), German shepherds (n= 7), Siberian huskies (n= 6), springer spaniels (n= 5), dachshunds (n=3), poodles (n= 3), cocker spaniels (n= 2), beagles (n= 2), border collies (n= 2), rottweilers (n= 2), and one each of eighteen other breeds. The median weight for the dogs was 28.8 kg (Range: 2.4 to 51.0 kg). The demographic characteristics were similar between the study’s two main sub-groups (Table 1).
Table 1.
Demographic characteristics for the overall study population and both major sub-groups; dogs with ASAGAC that underwent surgery alone and dogs with ASAGAC that underwent surgery followed by adjuvant carboplatin chemotherapy.
| Parameter evaluated | Overall study population (n = 74) |
Surgery alone (n = 30) |
Surgery with adjuvant chemotherapy (n = 44) |
Significance (P-value) |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 9.71 | 10.17 | 9.45 | P= 0.126 |
| Range | 6.06 – 16.15 | 6.10 – 16.15 | 6.06 – 14.49 | |
| Gender | ||||
| Males | 48 (65%) | 23 (77%) | 25 (57%) | P= 0.090 |
| Females | 26 (35%) | 7 (23%) | 19 (43%) | |
| Weight (kg) | ||||
| Median | 28.8 | 31.2 | 28.6 | P= 0.222 |
| Range | 2.4 – 51.0 | 8.2 – 51.0 | 2.4 – 48.0 | |
| Hypercalcemia | 12 (16%) | 2 (7%) | 10 (23%) | P= 0.107 |
| Mild | 3 | 1 | 2 | P= 0.697 |
| Moderate | 5 | 1 | 4 | |
| Severe | 4 | 0 | 4 |
Diagnostics and staging
All dogs underwent complete staging, consisting of history, physical examination incorporating per rectum digital examination, hematologic, biochemical and urine analyses, thoracic radiography and abdominal ultrasound.
In 43% of the overall study population (n= 32/74), with similar percentages in both major sub-groups (surgery alone n= 14/30; adjuvant chemotherapy n= 18/44), the disease was incidentally identified during a routine clinical examination or as part of the work-up of an unrelated problem. Most common clinical signs reported for the remaining dogs were similar to those previously reported for dogs with ASAGAC, and included scooting or apparent perineal irritation (n= 12), tenesmus (n= 11), polyuria and polydipsia (n= 9) and a noticeable perianal mass or swelling (n= 7). Less frequently reported clinical signs included a dull demeanor (n= 6), inappetence to anorexia (n= 3), hind limb lameness (n= 3), diarrhea or inappropriate defecation (n= 2), and dysuria, stranguria or inappropriate urination (n= 1). The proportion of dogs displaying clinical signs was not statistically different between both major sub-groups (P= 0.809). The presence or absence of specific clinical signs was not statistically analyzed, given small numbers.
Of the overall study population, 12 dogs (16%) were hypercalcemic at the time of diagnosis. Of these, three dogs (25%) were classified as having mild hypercalcemia, five (42%) as having moderate hypercalcemia, and four (33%) with severe hypercalcemia. The degree of hypercalcemia was classified according to previously published ranges of the University of California-Davis School of Veterinary Medicine Endocrinology Laboratory.21 The proportion of hypercalcemic dogs did not statistically differ between the surgery alone and adjuvant chemotherapy sub-groups (n= 2/30, 7%; n= 10/44, 23%) (P = 0.107), nor did the proportion of dogs in each of the extent categories (P = 0.697).
Except for the presence of hypercalcemia, there were no notable or repeatable hematologic or biochemical abnormalities identified at the time of diagnosis for either the overall study population, nor for either of the major sub-groups. Most panels were considered unremarkable. Six dogs had mildly elevated liver enzymes, three dogs were mildly anemic and two dogs had a mild thrombocytosis. One dog diagnosed with International Renal Interest Society (IRIS) stage II chronic kidney disease.
Nineteen dogs (26%) had caudal abdominal lymphadenopathy as determined by abdominal imaging at the time of diagnosis. The proportion of dogs with caudal abdominal lymphadenopathy did not statistically differ for the surgery alone and adjuvant chemotherapy groups (n= 6/31, 20%; n= 13/44, 30%) (P = 0.424). Of the 19 dogs with enlarged lymph nodes identified on imaging, only five had metastasis confirmed by a pre-surgical fine needle aspirate and cytology, but all dogs with suspected lymph node metastasis underwent surgical lymph node extirpation and subsequent histopathologic evaluation for confirmation. Enlarged lymph nodes on imaging were invariably metastatic.
No repeatable abnormalities were revealed by thoracic radiography at the time of diagnosis. One dog had sternal lymph node enlargement evident on thoracic radiographs. This dog did have extensive mid to caudal abdominal lymph node enlargement evident on both abdominal ultrasonography and subsequent computed tomography of the abdomen, which was cytologically diagnosed as multifocal metastasis. Cytologic evaluation of a CT-guided fine needle aspirate sample from the sternal lymph node also confirmed metastasis to that site. This dog was one of only two that received advanced imaging (computed tomography). In the other dog, computed tomography of the abdominal cavity was intended to aid surgical planning and merely confirmed the presence of caudal abdominal lymphadenopathy.
Metastasis
The overall metastatic rate at the time of diagnosis was approximately 26% (n= 19/74), and did not statistically differ for the surgery alone and adjuvant chemotherapy groups; approximately 23% (n= 6/30) and 30% (n= 13/44) respectively (P= 0.424). Eighteen dogs (24%) had solely caudal abdominal lymph node metastases, and one dog had metastatic extension to the sternal lymph node.
Surgery
All dogs underwent anal sacculectomy as primary treatment. All 19 (25%) dogs with caudal abdominal lymphadenopathy had concurrent lymph node extirpation performed. The one dog with sternal lymphadenopathy also had a concurrent thoracotomy, reducing the disease burden to microscopic post-operatively. Lymph node extirpation was not performed if the intra-abdominal lymph nodes were clinically assessed as normal, based on ultrasound imaging by a board-certified radiologist. The proportion of dogs that underwent lymph node extirpation was similar for both major sub-groups (surgery alone n= 6/30, 20%; adjuvant chemotherapy n= 13/44, 30%) (P = 0.424).
The study population’s overall reported surgical complication rate was 15% (n= 11/74). The surgical complication rate was once again was not different for each of the major sub-groups (surgery alone n= 4/30, 13%; adjuvant chemotherapy n= 7/44, 16%) (P= 1.0). These comprised peri-operative hemorrhage (n= 4), surgical site seroma formation (n= 3), surgical site dehiscence (n= 3), or herniation (n= 1). One dog, however, underwent a pull-through procedure because of dehiscence, which resulted in unacceptable post-procedural fecal incontinence and ultimately euthanasia. There was an increased complication rate in those dogs that had concurrent caudal abdominal lymph node extirpation (n= 8/19, 42%) compared with those that did not (n= 3/55, 5%) (P= <0.005).
Primary tumor characteristics and histopathology
Of the overall study population, 42 dogs (57%) had left-sided masses, 29 dogs (40%) had right-sided masses, and three dogs (4%) had bilateral disease. Two dogs had bilateral anal sacculectomy, based on per rectum digital examination and suspicion of bilateral disease, although neoplasia was only identified histopathologically in the left anal sac in both dogs. Three dogs had bilateral anal sacculectomy performed because this was the surgeon’s preference, despite disease being clinically suspected and ultimately histopathologically identified on only one side. These proportions were not statistically different between the surgery alone and adjuvant chemotherapy sub-groups (P = 0.765). The median size of the mass(es) at the time of diagnosis was 2.10cm (range: 0.3 to 9.0cm) and there was no significant difference between the two major sub-groups (surgery alone median: 2.20cm, range: 0.8 to 9.0cm; adjuvant chemotherapy median: 2.05cm, range: 0.3 to 6.80cm) (P = 0.611).
Surgical margins were variably described as incomplete or complete. However, for those cases in which the margins were described as “complete”, scrutiny of the histopathology report typically revealed statements such as “1–2mm complete margins” or “completely removed with narrow (unspecified) margins”. Given these histopathologic descriptions, what is known about the surgical technique of anal sacculectomy and the institution’s clinical assumption that anal sacculectomy for removal of ASAGAC is invariably incomplete when the measurable extent of the microscopic margins are critically evaluated, this parameter was not statistically analyzed.
Chemotherapy
For those dogs included in the surgery with adjuvant chemotherapy group, all dogs received at least one dose of carboplatin administered intravenously within thirty-three days of surgery. Median time to commencing chemotherapy was 17 days (Range: 11 to 33 days). Median number of doses administered was four (Range: 2 to 4 doses). The median dose administered was 300mg/m2 (Range: 240 to 300mg/m2).
Forty dogs (91%) completed the planned four doses of carboplatin, with minimal chemotherapy-associated adverse events. The overall rate of chemotherapy-associated adverse events was 30% (n= 13/44), whilst the percentage of dogs that experienced dose-limiting side effects was 18% (n= 8/44). Reported side effects included hematologic abnormalities, both neutropenia and thrombocytopenia, and gastrointestinal upsets, varying from solely inappetence to vomiting and / or diarrhea. Three dogs (7%) developed side-effect(s) resulting in cessation of therapy, including two grade IV neutropenias and associated illness, and one grade III neutropenia in a dog also presenting with neurologic signs. One dog ceased chemotherapy because of progressive disease.
Outcomes
Forty-six dogs (62%) died or were euthanized during the study period, whilst the remaining 28 dogs (38%) were censored either alive (n= 17) or lost to follow-up (n= 11). Thirty dogs (41%) died or were euthanized as a result of progressive disease, 15 dogs (20%) died or were euthanized as a result of unrelated illness or trauma. The cause of death was not recorded for one dog in the surgery with adjuvant chemotherapy group. Of the two major sub-groups, 20 (67%) in the surgery alone and 26 dogs (59%) in the adjuvant chemotherapy groups respectively, died during the study period. This difference was not statistically significant (P= 0.217). The apparent differences in cause of death or censoring between the two major subgroups, where ten (33%) and 20 dogs (45%) in the surgery alone and adjuvant chemotherapy groups respectively died as a result of progressive disease, ten (33%) and five dogs (11%) respectively died of unrelated illness or trauma, and seven (23%) and four dogs (9%) were lost to follow-up, were also not statistically significant (P= 0.086).
Forty-one dogs (55%) developed progressive disease during the study’s follow-up period. Of the two major sub-groups, 15 dogs (50%) in the surgery alone and 26 dogs (59%) in the adjuvant chemotherapy groups respectively, developed progressive disease. This difference was not statistically significant (P= 0.638). Manifestations of progressive disease included local recurrence (n= 18, 44%), locoregional lymph node metastasis (n= 27, 66%) and pulmonary progression (n= 7, 17%). In regards to locoregional recurrence or metastasis, nine dogs (22%) developed local recurrence, sixteen dogs (39%) developed locoregional lymph node metastasis, and five dogs (12%) a combination of both. For dogs developing distant metastasis, one dog (2.5%) had pulmonary metastasis only, one dog (2.5%) both pulmonary metastasis and local recurrence, two dogs (5%) both pulmonary and locoregional lymph node metastasis, and two dogs (5%) all three. One dog (2.5%) developed locoregional lymph node metastasis and pelvic osteolytic lesions consistent with bone metastasis. One dog (2.5%) developed renal metastasis in additional to locoregional lymph node and pulmonary metastasis. In three dogs (7%) the site of metastasis was not recorded. The proportion of dogs with locoregional lymph node metastasis or pulmonary metastasis did not differ between the two sub-groups (P= 0.457, P= 0.972 respectively). Interestingly, there was a statistically significant difference in the proportion of dogs that developed local recurrence between the two sub-groups, with dogs that received carboplatin being less likely to develop local recurrence (surgery alone n= 10/15, 67%; adjuvant chemo n= 8/26, 31%) (P= 0.016).
Not all dogs that developed progressive disease died (n= 6), and despite having progressive disease three dogs died of unrelated causes (n= 3). Treatment for progressive disease was pursued in twenty-eight cases (n= 28/41, 68%). Importantly, treatment for progression was far more likely to be undertaken in the group of dogs that initially received adjunctive chemotherapy (P= 0.001). Treatment for progressive disease included various combinations of repeated surgery, radiation therapy and chemotherapy, in addition to palliative measures, such as analgesia. Of the dogs with progressive disease, those that received treatment had a significantly longer overall survival (P: <0.005) compared to those that did not, with a median survival time from progression to death of 374 days for those dogs that received treatment compared to 47 days for those that did not.
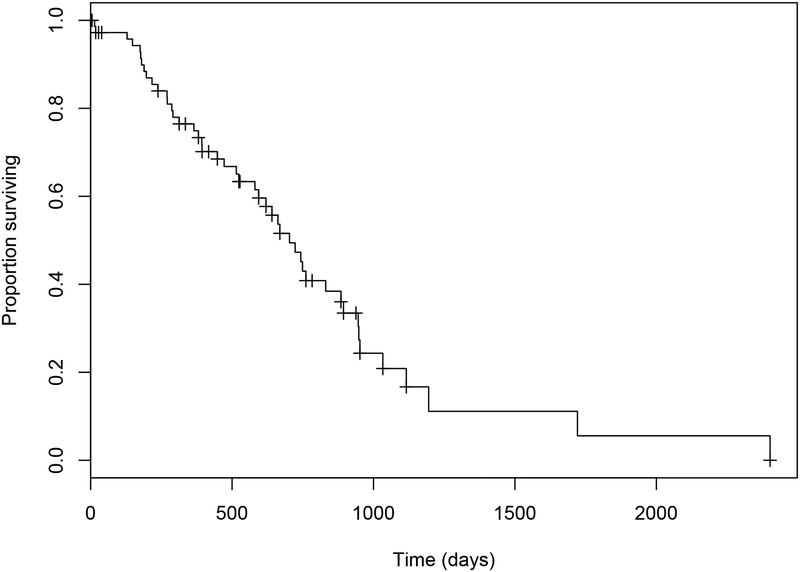
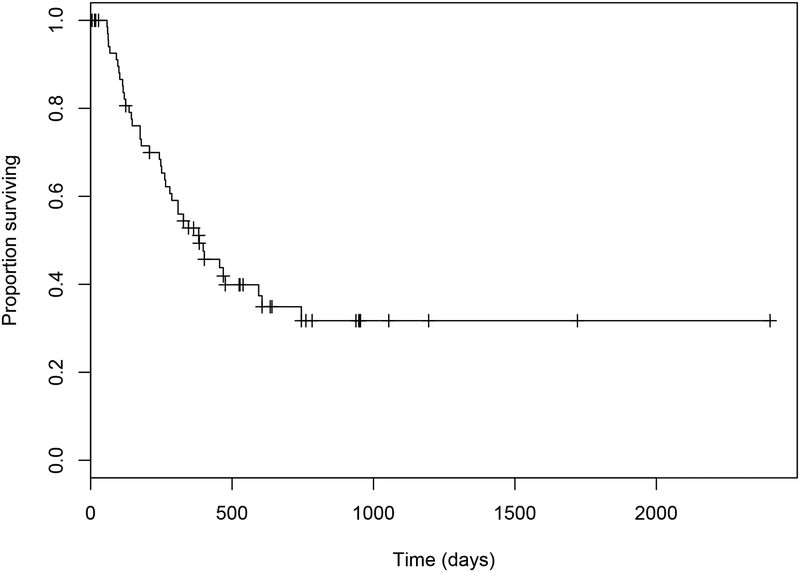
Median OS was 703 days (95% confidence interval (CI): 594 to 894) with a range of five to 2402 days (Figure 1.). Median overall TTP was 384 days (95% CI: 280 to 745) with a range of 58 to 745 days in those dogs with confirmed disease progression (Figure 2.). The median OS and TTP in those dogs that received adjuvant chemotherapy were 723 days (95% CI: 620 to 1116) (Range: 28 to 1721 days) and 382 days (95% CI: 287 to NA) (Range: 58 to 745 days) respectively. The median OS and TTP in those dogs that underwent only excisional surgery were 581 days (95% CI: 394 to 948) (Range: 5 to 2402 days) and 402 days (95% CI: 243 to NA) (Range: 91 to 469 days) respectively. These differences were not statistically significant (OS P= 0.174; TTP P= 0.370).
Figure 1.
Estimated Kaplan-Meier survival curve for overall survival times in 74 dogs with ASAGAC treated with surgery +/−adjuvant carboplatin chemotherapy.
Figure 2.
Estimated Kaplan-Meier survival curve for time to progression in 74 dogs with ASAGAC treated with surgery +/− adjuvant carboplatin chemotherapy.
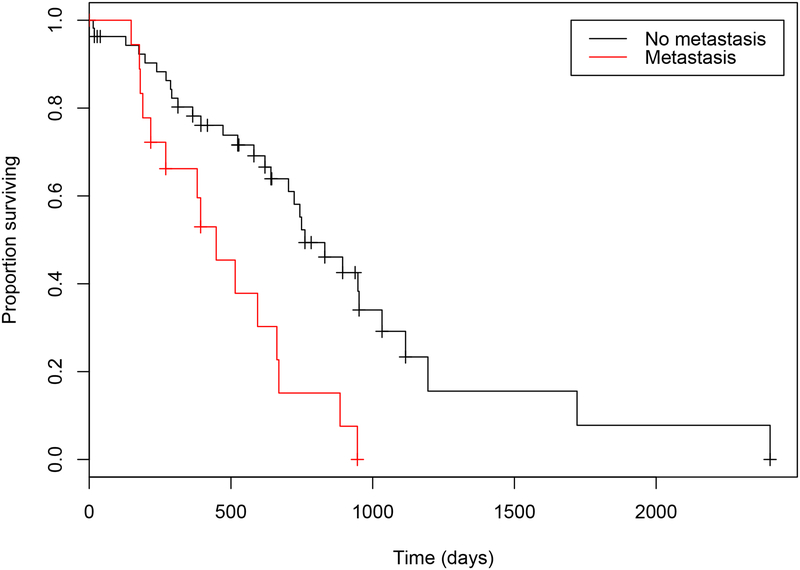
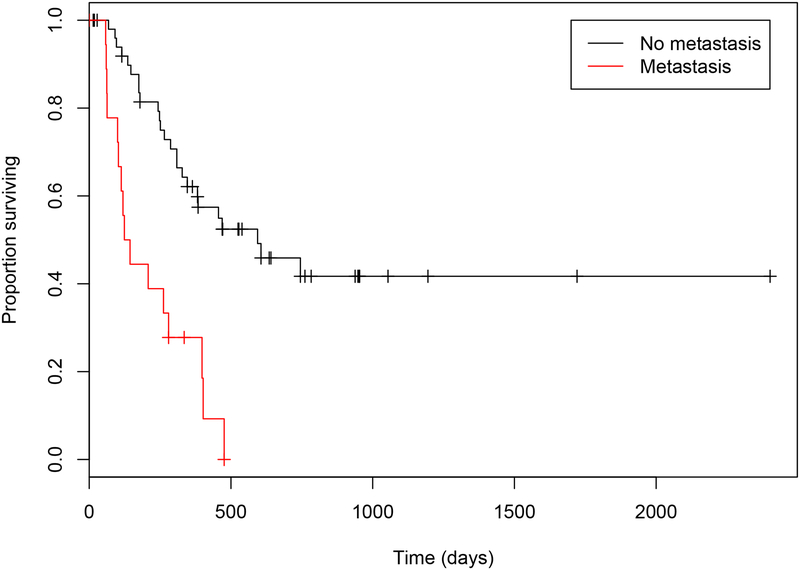
Several clinical variables were identified as related to outcome on univariate analysis, including the presence of clinical signs at diagnosis (Table 2.). However, only size of the primary tumor (OS P= <0.021; TTP P= 0.050) and presence of metastasis at the time of diagnosis (OS P= 0.042; TTP P= 0.018) were significant in the multivariate model (Figures 3 & 4.). The median OS and TTP in those dogs that did not have metastasis were 761 days (95% CI: 703 to 1116) (Range: 14 to 2402 days) and 594 days (95% CI: 346 to NA) (Range: 68 to 745 days) respectively. The median OS and TTP in those dogs that did have metastasis were 448 days (95% CI: 270 to 885) (Range: 5 to 946 days) and 134 days (95% CI: 103 to NA) (Range: 58 to 476 days) respectively.
Table 2.
Results of the univariate analysis with respect to overall survival (OS) and time to progression (TTP).
| OS | TTP | |||
|---|---|---|---|---|
| VARIABLE | exp(coef)ŧ | P value | exp(coef)ŧ | P value |
| Age | 1.1113 | 0.142 | 1.0159 | 0.833 |
| Gender | 0.8126 | 0.516 | 1.3302 | 0.420 |
| Weight | 1.0098 | 0.413 | 1.0007 | 0.956 |
| Clinical signs | 1.9885 | 0.036* | 1.7104 | 0.106 |
| Hypercalcemia | 1.8522 | 0.149 | 1.4900 | 0.342 |
| Metastasis (Lymph node) | 3.0245 | 0.001* | 3.8686 | <0.001* |
| Side | 1.2767 | 0.594 | 1.5037 | 0.453 |
| Size | 1.2984 | <0.001* | 1.2378 | 0.007* |
| Lymph node extirpation | 3.0245 | 0.001* | 3.8686 | <0.001* |
| Complications | 3.4844 | <0.001* | 11.5647 | <0.001* |
| Chemotherapy | 0.7415 | 0.324 | 1.0163 | 0.960 |
| Start days | 1.0027 | 0.933 | 1.0368 | 0.251 |
| No of treatments | 0.6078 | 0.193 | 0.9033 | 0.783 |
| Toxicity | 0.7175 | 0.485 | 0.7421 | 0.501 |
| Dose Reductions | 0.6356 | 0.416 | 0.6615 | 0.448 |
| Progression | 2.8555 | 0.004* | NA | NA |
| Local progression | 0.4651 | 0.047* | 0.4782 | 0.034* |
| Lymph node progression | 1.1806 | 0.690 | 1.2625 | 0.520 |
| Pulmonary progression | 1.3102 | 0.539 | 1.7632 | 0.187 |
| Progression elsewhere | 2.1453 | 0.306 | 1.5109 | 0.579 |
| Treatment for progression | 0.4043 | 0.020* | 1.1461 | 0.697 |
Exponential (coefficient) is defined as the multiplied relative risk of an event (death or progression) occurring for a unit change in the variable.
Statistically significant P= <0.05
Figure 3.
Estimated Kaplan-Meier survival curves for overall survival times in dogs with ASAGAC diagnosed with (red) or without (black) metastasis at the time of initial diagnosis. The median overall survival time of 448 days in dogs with metastasis at the time of initial diagnosis was significantly shorter (P= 0.042) than the 761-day median overall survival time in dogs that did not have metastasis.
Figure 4.
Estimated Kaplan-Meier survival curves for time to progression in dogs with ASAGAC, with (red) or without (black) metastasis at the time of initial diagnosis. The median time to progression of 134 days in dogs with metastasis at the time of initial diagnosis was significantly shorter (P= 0.018) than the 448-day median time to progression in dogs that did not have metastasis at the time of initial diagnosis.
Lymph node extirpation, and the related issue of post-operative complications, were both significantly associated with poorer outcomes. Dogs that underwent lymph node extirpation had a median OS and TTP of 448 and 134 days respectively (OS P= 0.001; TTP P= <0.001), whilst dogs that did not undergo lymph node extirpation had a median OS and TTP of 764 and 594 days respectively (P= <0.001; P= <0.001).
The administration of chemotherapy had no statistically detectable impact on OS or TTP in dogs with ASAGAC, although the group that did not have metastasis at the time of diagnosis and did receive adjuvant chemotherapy had the longest median TTP and OS (Figures 5 and 6.).
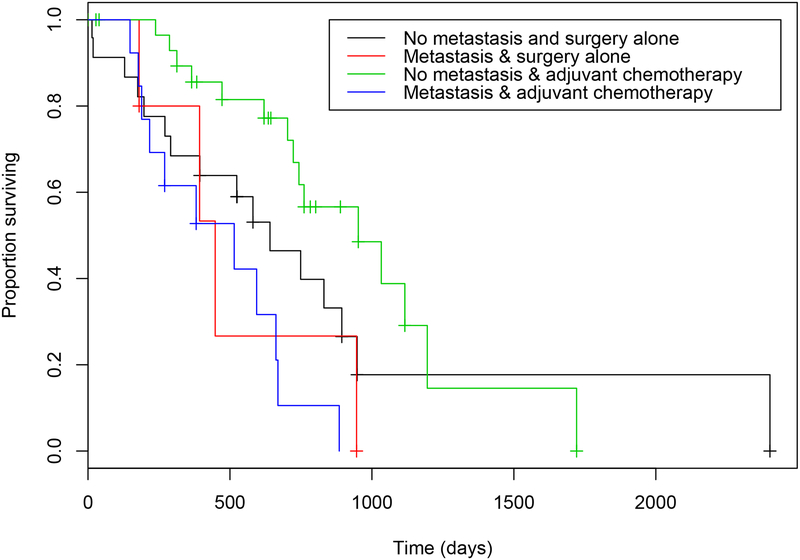
Figure 5.
Estimated Kaplan-Meier survival curves for overall survival times in dogs with ASAGAC, with or without metastasis at the time of initial diagnosis, and that did or did not receive adjuvant carboplatin chemotherapy. Dogs that did not have metastasis at the time of diagnosis and received adjuvant carboplatin chemotherapy (green) had the longest median survival time of 952 days, whilst dogs that did have metastasis at the time of initial diagnosis and did not receive adjuvant carboplatin chemotherapy (red) had the shortest median survival time of 448 days.
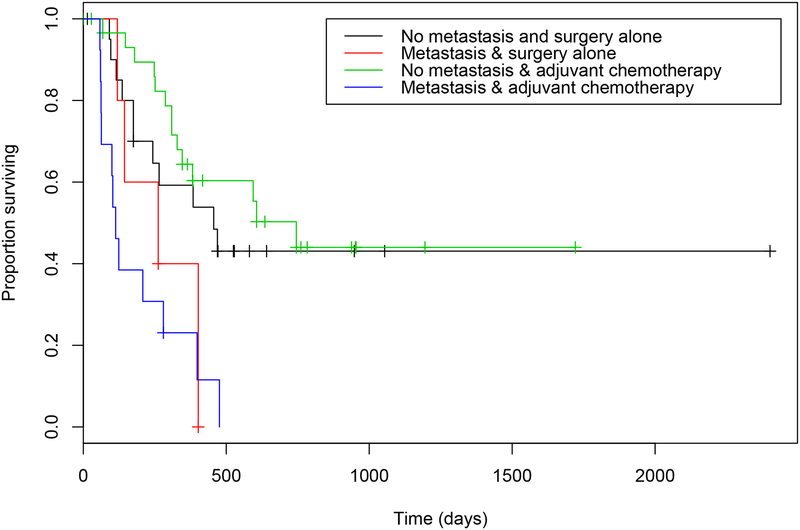
Figure 6.
Estimated Kaplan-Meier survival curves for time to progression in dogs with ASAGAC, with or without metastasis at the time of initial diagnosis, and that did or did not receive adjuvant carboplatin chemotherapy. Dogs that did not have metastasis at the time of diagnosis and received adjuvant carboplatin chemotherapy (green) had the longest median time to progression of 745 days, whilst dogs that did have metastasis at the time of initial diagnosis and did receive adjuvant carboplatin chemotherapy (blue) had the shortest median time to progression of 113 days.
Discussion
The primary objective of the present study was to investigate the role of carboplatin chemotherapy in the microscopic disease setting following anal sacculectomy +/− metastatic lymph node extirpation. Survival analyses, however, failed to unequivocally prove the authors’ primary hypothesis; that adjuvant carboplatin chemotherapy would improve measures of outcome in dogs with AGASAC. Median OS in the study population was, however, longer than earlier reports of between six and eleven months,4,5 and more comparable to the recent studies of Williams et al.7 and Turek et al.6 Median TTP was also longer in the present study compared to previous reports.5,7 Although comparison of outcomes between dissimilar studies is suboptimal, the prolonged values reported in this study might reflect improvements in the diagnosis, monitoring and treatment of canine ASAGAC over time, as well as a change in society’s attitude towards dog ownership and veterinary care. The significant impact of treatment on survival time, even in the face of progressive disease, supports this theory, and is important information for veterinarian and owners.
The currently recommended adjunctive chemotherapy protocol employed at UW-M VMTH and utilized in this study consists of four doses of carboplatin administrated intravenously every three weeks. The use of carboplatin is based on the previously documented efficacy (33%) of the platinum agents in macroscopic ASAGAC.5 Generally, chemotherapy is considered more effective against microscopic disease. It is interesting, therefore, that the administration of platinum agents for metastatic AGASAC in the microscopic disease setting has failed to improve survival compared to surgery alone.7,9 In both the aforementioned studies the population numbers were small, and the chemotherapy protocols were not fully described and / or standardized. In Williams et al. retrospective evaluation of 113 dogs with ASAGAC either carboplatin or cisplatin was administered to 28 dogs, but the details of the protocols were not described.7 In Polton and Brearley’s retrospective evaluation of 80 dogs with ASAGAC approximately half the dogs received carboplatin administered in the adjuvant microscopic setting, the neoadjuvant perioperative setting, and the palliative macroscopic setting, but once again the details of the protocols were not elaborated further.9 To the authors’ knowledge, this is the first study investigating the efficacy of a standardized carboplatin protocol as adjunctive treatment in the management of surgically excised AGASAC, and comparing measures of outcomes between dogs receiving adjunctive chemotherapy and a contemporaneous cohort of dogs, with similar clinical characteristics, that did not receive adjunctive chemotherapy.
Carboplatin was well tolerated by the dogs in the present study. Despite an overall adverse event rate of 18%, most were mild, with only two dogs developing grade IV toxicities (neutropenia), and 91% of dogs completing the standard protocol. The incidence of dose-limiting side effects was 18%, which is higher than the incidence of dose-limiting side-effect(s) for carboplatin when used as adjunctive therapy for osteosarcoma as previously reported by Saam et al., but seemingly lower than that reported by Phillips et al.20,21 During the study period, hemograms were routinely assessed seven to ten days after treatment. More recent studies suggest that hemograms obtained on days 14 and 21 may provide more information about treatment-induced myelosuppression relative to hemograms assessed at seven to ten days.21 Consequently, the incidence of neutropenia and thrombocytopenia may have been underestimated in the present study.
Breed, body weight and age of the dogs in the present study were similar to previous reports of dogs with ASAGAC.1,3,4,5,7,10 However, 65% of the dogs in the present study were castrated males. This seems in contrast to older studies in which females were overrepresented,1,3,4 and even to more recent studies in which the proportions of males and females were approximately equal.5,7,10,23 However, our study population was not compared to all hospital admissions during the time period, and males may have exceeded females, thereby explaining this anomaly.
ASAGAC was incidentally identified in 43% of the cases in this study. This is a much higher percentage of cases compared to previous publications, in which although 50% of dogs presented with no clinical signs obviously suggestive of anal sac disease, only 7% were diagnosed in dogs on routine examination with no owner complaints of any clinical signs attributable to either primary or metastatic ASAGAC.5 This may be a result of an increasing awareness of the disease by veterinarians, as well as individuals peripherally involved in canine care, such as dog groomers.
Only 16% of the dogs in this study were hypercalcemic at the time of diagnosis. Again, this is in contrast to previous studies in which the reported incidences of paraneoplastic hypercalcemia were higher, ranging between 25% and 53%.4–8 The lower incidence of hypercalcemia in this study may be due to the higher proportion of dogs diagnosed incidentally, implying minimal tumor burdens and / or less advanced disease. Although there has been no previously reported association between tumor size and hypercalcemia, this might not apply with incidentally identified disease, which perhaps infers an even smaller tumor burden. Selection bias may also have influenced this parameter, as it is possible that the owners of a proportion of dogs diagnosed with AGASAC during the study’s time period may have elected not to pursue surgery at the outset. All hypercalcemic dogs became eucalcemic following surgery, irrespective of adjuvant chemotherapy, which supports the previous recommendation to perform excision of the mass and affected lymph nodes, even in a palliative setting, in order to control the pathophysiologic sequelae associated with hypercalcemia.11,12,13 Hypercalcemia was ultimately not associated with measures of outcome in this study. This issue remains unresolved in the literature.5–8,13
The metastatic rate reported in the present study is similar to that reported previously.1,3–10 Moreover, the pattern of progression observed in this study, characterized by early local lymph node metastasis and later extension to distant sites, is also similar to that described previously.1,3–7,9,10 It was interesting that dogs that received adjuvant carboplatin were less likely to develop local recurrence, but a prospective randomized clinically-controlled trial would be required to confirm the role of chemotherapy in inhibiting local disease recurrence.
In the present study, all dogs with suspected lymph node metastases had lymph node extirpation performed concurrently with anal sacculectomy. This study did not address the potential for microscopic metastases in normal-appearing lymph nodes and the clinical implications thereof, as lymph nodes appearing ultrasonographically normal were not investigated further. The impact of microscopic metastases is yet to be investigated in dogs with ASAGAC. Previous studies have demonstrated that, despite grossly apparent metastases to the iliac lymph nodes, dogs with ASAGAC can have long-term survival when surgery incorporates excision of the metastatic lesion(s).9,13,18,19 Lymphadenectomy also assists in controlling the paraneoplastic hypercalcemia associated with ASAGAC.13,18,19 These prior surgical reports had a low complication rate, even in dogs in which pubic osteotomy was performed.13,18,19 Pubic osteotomy was not necessary in any of the dogs in the present study. The overall surgical complication rate was low in the present study, but there was an increased complication rate in those dogs that underwent lymph node extirpation compared with those that did not. However, most complications were minor and readily managed. In agreement with previous reports,13,18,19 therefore, the authors recommend excision of metastatic lymph nodes.
This study did not analyze the impact of incomplete or apparently complete surgical margins on measures of outcome. The lack of assessment is due to our institution operating under the clinical assumption that margins for surgical excision of ASAGAC are invariably incomplete, when the measurable extent of the microscopic margins are critically evaluated.7 The anal sacs are cutaneous diverticula of the stratified squamous epithelium originating at the anocutaneous junction, in between the internal and external anal sphincters, into which adjacent apocrine glands empty their secretions.6 Given the complex anatomy of the region, anal sacculectomy is a necessarily marginal procedure, in order to avoid undesirable post-operative adverse events, such as fecal incontinence.
The present study possesses several limitations. A limitation inherent to all retrospective studies is the lack of case standardization and deficits in medical record keeping. In the present study, the investigators endeavored to standardize the study population, diagnostics, treatment and monitoring, such that there were no statistically appreciable differences between the study’s two main sub-groups. Cox regression models theoretically account for the statistical impact of intertwining clinical variables, but they cannot completely eliminate bias. Given that the study’s two main sub-groups were not randomized nor blinded, there did exist the potential for selection and client biases. Individual clients may be more inclined to treat aggressively, more likely to pursue treatment in the setting of progressive disease, and less likely to euthanatize early. Such client bias can confound the analysis of benefit conferred by therapeutic interventions, and in this study this may be particularly applicable to the apparent benefit of treating progressive disease. A randomized, blinded and clinically-controlled study would be necessary to investigate this clinical question further. Selection bias can also confound statistical analyses, and may be one reason why adjuvant chemotherapy did not ultimately confer a statistically significant survival advantage.
It is argued that OS is not a valid end-point for veterinary studies given the aforementioned client bias, and that measures of outcome, such as TTP and disease free survival (DFI), are more objective measures of therapeutic response. Even TTP and DFI, however, are not without problems, because non-uniform and infrequent reevaluation and follow-up of patients can result in the false impression of increases in outcome.
Conclusion
Dogs with metastatic ASAGAC at the time of initial diagnosis had a poorer prognosis than those dogs without metastases. Whilst surgical extirpation of metastatic regional lymph nodes is associated with an increased risk of post-operative complications, these are typically only minor, and aggressive surgical management is warranted in such cases to improve survival time. In the present study, adjuvant carboplatin chemotherapy was deemed safe and well tolerated, however, the administration of chemotherapy was not associated with any statistically significant improvement in OS or TTP. The decrease in local recurrence in dogs receiving adjuvant chemotherapy is an interesting finding, however, as noted the TTP was not different between the two groups, nor was the development of locoregional metastatic disease. The positive impact of treating progressive disease, albeit not limited to adjuvant chemotherapy, on survival time is an important finding. The limitations of the present study serve to emphasize the need for prospective randomized clinical trials to firmly establish the efficacy and benefit of the various chemotherapeutic options for canine ASAGAC, and to facilitate comparison of chemotherapeutic protocols and multimodality regimens in the management of the disease.
Acknowledgements
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427 (TJS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The authors would also like to acknowledge the invaluable contribution of the University of Wisconsin-Madison Veterinary Medical Teaching Hospital’s clients and referring veterinarians in collating the data for this study.
This work was completed at the University of Wisconsin-Madison.
Abbreviations:
- ASAGAC
anal sac apocrine gland adenocarcinoma
- OS
overall survival
- TTP
time to progression
- UW-VMTH
University of Wisconsin-Madison, Veterinary Medical Teaching Hospital
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Berrocal A, Vos JH, van den Ingh TS, Molenbeek RF and van Sluijs FJ. Canine perineal tumors, Journal of Veterinary Medicine Series A 1989; 36: 739–749. [DOI] [PubMed] [Google Scholar]
- 2.Goldschmidt MH and Hendrick MJ. Anal sac gland carcinoma In: Meuten DJ, ed. Tumors in Domestic Animals, 4th ed. Ames, Iowa: Iowa State Press; 2002: 74–76. [Google Scholar]
- 3.Goldschmidt MH and Zoltowski C. Anal sac adenocarcinoma in the dog: 14 cases. The Journal of Small Animal Practice 1981; 22: 119–128. [DOI] [PubMed] [Google Scholar]
- 4.Ross JT, Scavelli TD, Matthieson DT and Patnaik AK. Adenocarcinoma of the apocrine glands of the anal sac in dogs: a review of 32 cases. Journal of the American Animal Hospital Association 1991; 27: 349–355. [Google Scholar]
- 5.Bennett PF, DeNicola DB, Bonney P, Glickman NW and Knapp DW. Canine anal sac adenocarcinomas: clinical presentation and response to therapy. Journal of Veterinary Internal Medicine 2002; 16: 100–104. [DOI] [PubMed] [Google Scholar]
- 6.Turek MM, Forrest LJ, Adams WM, Helfand SC, and Vail DM. Postoperative radiotherapy and mitoxantrone for anal sac adenocarcinoma in the dog: 15 cases. Veterinary Comparative Oncology 2003; 1: 94–104. [DOI] [PubMed] [Google Scholar]
- 7.Williams LE, Gliatto JM, Dodge RK, Johnson JL, Gamblin RM, Thamm DH, Lana SE, Szymkowski M and Moore AS. Carcinoma of the apocrine glands of the anal sac in dogs: 113 cases (1985 – 1995). Journal of the American Veterinary Medical Association 2003; 223: 825–831. [DOI] [PubMed] [Google Scholar]
- 8.Emms SG. Anal sac tumors of the dog and their response to cytoreductive surgery and chemotherapy. Australian Veterinary Journal 2005; 83: 340–343. [DOI] [PubMed] [Google Scholar]
- 9.Polton GA and Brearley MJ. Clinical stage, therapy, and prognosis in canine anal sac carcinoma. Journal of Veterinary Internal Medicine 2007; 21: 274–280. [DOI] [PubMed] [Google Scholar]
- 10.Polton GA, Brearley MJ, Green LM and Scase TJ. Expression of E-cadherin in canine anal sac gland carcinoma and its association with survival. Veterinary Comparative Oncology 2007; 5: 232–238. [DOI] [PubMed] [Google Scholar]
- 11.Meuten DJ, Cooper BJ, Capen CC, Chew DJ and Kociba GJ. Hypercalcemia Associated with an Adenocarcinoma Derived from the Apocrine Glands of the Anal Sac. Veterinary Pathology 1981; 18: 454–471. logy 1981; 18: 454–471. [DOI] [PubMed] [Google Scholar]
- 12.Rosol TJ, Capen CC, Danks JA, Suva LJ, Steinmeyer CL, Hayman J, Ebeling PR and Martin TJ. Identification of parathyroid hormone-related protein in canine apocrine gland adenocarcinoma of the anal sac. Veterinary Pathology 1990; 27: 89–95. [DOI] [PubMed] [Google Scholar]
- 13.Hobson HP, Brown MR and Rogers KS. Surgery of metastatic anal sac adenocarcinoma in five dogs. Veterinary Surgery 2006; 35: 267–270. [DOI] [PubMed] [Google Scholar]
- 14.Hammer AS, Couto CG, Ayl RD and Shank KA. Treatment of tumor-bearing dogs with actinomycin-D. Journal of Veterinary Internal Medicine 1994; 8: 236–239. [DOI] [PubMed] [Google Scholar]
- 15.Spugnini EP, Dotsinsky I, Mudrov N, Bufalini M, Giannini G, Citro G, Feroce F and Baldi A. Adjuvant electrochemotherapy for incompletely excised anal sac carcinoma in a dog. In Vivo 2008; 22: 47–50. [PubMed] [Google Scholar]
- 16.London C, Mathie T, Stingle N, Clifford C, Haney S, Klein MK, Beaver L, Vickery K, Vail DM, Hershey B, Ettinger S, Vaughan A, Alvarez F, Hillman L, Kiselow M, Thamm D, Higginbotham ML, Gauthier M, Krick E, Phillips B, Ladue T, Jones P, Bryan J, Gill V, Novasad A, Fulton L, Carreras J, McNeill C, Henry C, and Gillings S. Preliminary evidence for biologic activity of toceranib phosphate (Palladia™) in solid tumors. Veterinary Comparative Oncology 2012; 10: 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veterinary cooperative oncology group – common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Veterinary Comparative Oncology 2011; doi: 10.1111/j.1476-5829.2011.00283.x. [DOI] [PubMed] [Google Scholar]
- 18.Jeffery N, Phillips SM and Brearley MJ. Surgical management of metastases from anal sac apocrine gland adenocarcinoma of dogs. The Journal of Small Animal Practice 2000; 41: 390. [Google Scholar]
- 19.White RA and Gorman NT. The clinical diagnosis and management of rectal and pararectal tumors in the dog. The Journal of Small Animal Practice 1987; 28: 87–107. [Google Scholar]
- 20.Phillips B, Powers BE, Dernell WS, Straw RC, Khanna C, Hogge GS and Vail DM. Use of Single-Agent Carboplatin as Adjuvant or Neoadjuvant Therapy in Conjunction With Amputation for Appendicular Osteosarcoma in Dogs. Journal of the American Animal Hospital Association 2009; 45: 33–38. [DOI] [PubMed] [Google Scholar]
- 21.Saam DE, Liptak JM, Stalker MJ and Chun R. Predictors of outcome in dogs treated with adjuvant carboplatin appendicular osteosarcoma: 65 cases (1996–2006). Journal of the American Veterinary Medical Association 2011; 238: 195–206. [DOI] [PubMed] [Google Scholar]
- 22.Hypercalcemia and Primary Hyperparathyroidism. In: Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction, 3rd ed. St Louis, Missouri; Saunders, Elsevier; 2004: 660–715. [Google Scholar]
- 23.Polton GA, Mowat V, Lee HC, Mckee KA and Scase TJ. Breed, gender and neutering status of British dogs with anal sac gland carcinoma. Veterinary Comparative Oncology 2006; 4: 125–131. [DOI] [PubMed] [Google Scholar]