Abstract
The AAA proteins are a family of enzymes that play key roles in diverse dynamic cellular processes, ranging from proteostasis to directional intracellular transport. Dysregulation of AAA proteins has been linked to several diseases, including cancer, suggesting a possible therapeutic role for inhibitors of these enzymes. In the past decade, new chemical probes have been developed for AAA proteins including p97, dynein, midasin and ClpC1. In this review, we discuss how these compounds have been used to study the cellular functions and conformational dynamics of AAA proteins. We discuss future directions for inhibitor development and early efforts to utilize AAA protein inhibitors in the clinical setting.
The ATPases Associated with diverse cellular Activities (AAA proteins) are a family of enzymes that convert the chemical energy of ATP into mechanical work, such as protein unfolding or directional transport1,2. True to their name, the AAA proteins play critical roles across a wide array of cellular processes ranging from ribosome assembly to proteolysis and microtubule-based intracellular transport. Studying the AAA proteins in physiologic contexts has been challenging, as many AAA protein-driven processes are essential for survival and are often carried out on the timescale of seconds to minutes. An additional impediment to their study is that AAA proteins generally function as oligomeric ensembles and often associate with even larger complexes to function as macromolecular machines that can be megadaltons in size (Box 1 summarizes the structural features of AAA proteins). Knock-down of AAA proteins (e.g. by shRNA) can often take longer than many of the cellular processes they are involved in and thereby lead to the accumulation of phenotypes not directly linked to their functions. Destabilization of multi-protein complexes also has the potential to cause dominant negative effects. Similarly, the slowly- or non-hydrolyzable ATP analogs often used to study AAA proteins in vitro are poorly suited for studying these enzymes in cellular contexts, as they are generally unable to cross cell membranes and tend to inhibit multiple different enzymes.
Box 1. Overview of AAA structure and function.
(A) Schematic of AAA domain secondary and tertiary structure showing N-terminal domain (blue), large (dark green), and small (light green) subunits. (B) Hexameric arrangement of AAA domains characteristic of AAA proteins, as viewed from above or below the plane of the ‘ring’, with N-terminal domains omitted. (C) Nucleotide binding site is at junction of adjacent large subunits. The small subunit of one AAA domain (“n”) and the large subunit of the next AAA domain (“n+1”) often associate tightly and move together in a nucleotide binding site occupancy-dependent fashion as ‘rigid bodies.’ N-terminal domains are omitted. (D) Classes of AAA proteins and the corresponding domain arrangement. Class I and II AAA proteins usually form hetero- or homo-hexameric ensembles while dynein and midasin contain six AAA domains in a single polypeptide, with additional unique domains extending from the ring to perform specialized functions (e.g. microtubule and cargo binding in the case of dynein, shown in black). In class I and II AAA proteins, N-domains are diverse in terms of structure and size and often mediate interactions with AAA substrate/client proteins. (E) Unfoldases such as p97 and ClpC1 disassemble protein complexes and unfold protein substrates; unfolded products are threaded toward proteases or recycled for other purposes. (F) Cytoplasmic dynein operates as a dimer and moves cargo toward minus-ends of microtubules.

Cell-permeable chemical inhibitors of AAA proteins have the potential to overcome many of these challenges. They can act on timescales that match the processes driven by these enzymes, limiting the degree to which cells can activate compensatory pathways or accumulate indirect effects. By acting on native enzymes, they can circumvent the need for genetic manipulation of essential genes, which is often cumbersome in cell culture or model organism studies.
The first cell-permeable chemical inhibitors of eukaryotic AAA proteins were reported for p97 (or VCP)3 and dynein4 (Figure 1). There are now over 10 chemically diverse inhibitors for the eukaryotic unfoldase p97, and some of these have been the subject of follow-up studies that shed light on the cellular and biochemical mechanisms of p97 activity (summarized in Figure 1A and reviewed elsewhere3,5). Inhibitors with distinct chemotypes are also available for the microtubule-based motor protein dynein and these have likewise been utilized to elucidate this enzyme’s role in cellular processes. In contrast to p97 and dynein, relatively few probes are available for other AAA enzymes: three peptide-based ligands are known for the prokaryotic unfoldase ClpC1 and heterocycle-based small molecule probes have been identified for the large AAA midasin, as well as katanin, Drg1, LuxO, and, more recently, RUVBL1/2 and Spastin (Figure 1B). In this review, we highlight ways in which AAA inhibitors have been used to dissect cellular mechanisms, focusing on inhibitors of dynein and midasin. We also discuss how chemical inhibitors have enabled mechanistic analysis of the AAA proteins themselves by trapping midasin, p97, and ClpC1 in unique conformational states.
Figure 1. Small molecule ligands of AAA proteins.
(A) Selected p97 inhibitors that were discussed in this review (a more comprehensive overview of p97 inhibitors is available elsewhere3). (B) Small molecule ligands of other AAA proteins. Ligands are generally protein antagonists except for those shown for katanin56, which shows activity consistent with enzymatic activation, and ClpC1, which modulates protein activity. Structures not shown for peptide-based ClpC1 antagonists ecumicin57 and lassomycin58. Other probes not discussed in this review include diazaborine and CV-133, which inhibit Drg1 and LuxO, respectively59,60. Two AAA protein inhibitors were described while this manuscript was under revision, spastazoline and CB-6644, which inhibit spastin and RUVBL1/2 respectively61. Compounds in blue were identified through screening and/or optimization against a purified protein target. Compounds shown in black were identified through cell-based or phenotypic screening with in vitro follow-up. Stereochemical information regarding MSC1094308 is not available and it is likely a racemic mixture.
Using chemical inhibitors to probe intracellular dynamics
Dyneins are AAA proteins that drive directional motion in cells along microtubules. Microtubules are polarized filaments with biochemically distinct ‘plus’ and ‘minus’ ends. Whereas most eukaryotic cells have several motor proteins that move toward microtubule plus ends (e.g. >40 kinesins in humans), minus-end directed transport depends mainly on one dynein isoform, cytoplasmic dynein 1 in eukaryotic cells6. As a result, cytoplasmic dynein 1 transports cargoes ranging from whole organelles to mRNA particles and also plays a role in the assembly and function of microtubule-based subcellular structures such as the mitotic spindle7. Other dynein isoforms have more specialized functions. For instance, cytoplasmic dynein 2 drives motion within microtubule-containing cilia and flagella, a process known as intraflagellar transport (IFT; cytoplasmic dynein 2 is also known as IFT dynein)8. Axonemal dyneins drive the beating of cilia and flagella9.
Given dynein’s role in dynamic cellular processes, fast acting inhibitors that could selectively disrupt dynein-driven motion had long been sought. Yet until this decade, dynein antagonists were limited by the combination of poor selectivity for dynein and poor cell permeability10. An important advance came in 2012 with the discovery that a class of inhibitors of the Hedgehog pathway—which plays roles in metazoan morphogenesis and tumor biology—inhibit cytoplasmic dyneins4,11. These compounds, named the ciliobrevins, were shown to block dynein-driven transport in vitro and in cells, although with limited potency (concentrations of 20–100 μM are generally required for dynein inhibition). Synthesis of close analogs of the ciliobrevins led to derivatives with improved dynein isoform selectivity12 and chemical-structure guided modification of the ciliobrevin scaffold led to the dynapyrazoles, a class of dynein inhibitors with improved potency13. Like the ciliobrevins, dynarrestin, a chemically-unrelated dynein antagonist, was more recently discovered as a Hedgehog pathway inhibitor and subsequent studies demonstrated dynein inhibition in vitro14.
With these chemical inhibitors it has become possible to selectively impair dynein-dependent processes in cells. For instance, two different groups used ciliobrevins to probe the intracellular dynamics that occur when a T-cell interacts with an antigen presenting cell to form an immunological synapse15,16. Inhibition of dynein-driven motion slowed the progress of centrosomes toward the immunological synapse and impaired the T cells’ ability to polarize their cytoplasm in response to immunological synapse formation. In addition, inhibition of dynein by cilibobrevin blocked the ability of natural killer (NK) cells to polarize the majority of their lytic granules away from the immunological synapse, leading to excess killing of bystander cells17. Ciliobrevins have also been used to slow the dynein-dependent flagellar beating of eukaryotic sperm cells and single-celled parasites18,19. Other examples of dynein inhibition using the ciliobrevins have been summarized elsewhere20.
Dynein inhibition by dynapyrazoles caused distinct effects depending on the setting and cargo being observed. Dynapyrazole-A blocked dynein-1-dependent lysosome motion within neuron-like cells, reducing the number of high-velocity lysosome trajectories in both anterograde and retrograde directions (corresponding to microtubule plus and minus ends, respectively, Figure 2A, top)13. In contrast, when cytoplasmic dynein 2-driven intraflagellar transport was studied, dynapyrazole-A selectively impaired retrograde intraflagellar transport, causing 60–70% reduction in retrograde velocity and only 20% reduction in anterograde velocity (Figure 2A, bottom). These distinct effects of dynein inhibition may reflect the differences between dynein 1-driven motion of membrane-bound organelles in the cytosol and dynein 2-driven IFT. Motion in the cytoplasm is thought to require tight ‘tug-of-war’ coupling between plus end- and minus end-directed forces21. In contrast, it is becoming clear that during IFT in a given direction mechanisms exist for inactivation of enzymes that move in the opposite direction8,22. Further studies are needed clarify the different relationships between each direction of microtubule-based transport within the cytoplasm and in cilia.
Figure 2. Small molecule AAA inhibitors block dynamic cellular processes.
(A) Dynein inhibition with dynapyrazole blocks intracellular transport. Top row: bidirectional intracellular transport of organelles (e.g. lysosomes) is arrested. Bottom row: intraflagellar transport is blocked in primarily the retrograde direction. Blue ovals: microtubule plus-end directed transport (anterograde); red ovals: microtubule minus-end directed transport (retrograde); blue lines: microtubules with plus-end indicated; green ovals: stationary cargoes. (B) Rbin-1 inhibits midasin-dependent maturation and nuclear export of pre-ribosomal particles, trapping ribosomal precursors in the nucleus. (C) Rbin-1-dependent nuclear accumulation of ribosomal precursors is reversible (top row) and abrogated by Rbin resistance-conferring mutations in midasin (bottom row). Rounded Rectangles represent fission yeast.
Ribosome assembly is an energetically-demanding process requiring the association and remodeling of hundreds of proteins and multiple RNA components under tight spatial and temporal control23. Several AAA proteins are known to contribute to key steps of this process, including midasin, which is a large polypeptide (~5000 amino acids) containing six non-equivalent AAA domains. Midasin was identified as a target of small molecule ribosome biogenesis inhibitors (named ribozinoindoles or Rbins)24. Rbins can inhibit the ATPase activity of recombinant wild-type midasin but a single-point mutation (F1093L) in midasin abrogates Rbin activity in vitro and in cells, indicating that midasin is the direct physiological target of Rbins.
Ribosome assembly begins within the nucleolus, proceeds through the nucleoplasm, and concludes with the maturation of ribosomes in the cytoplasm, requiring the coordinated association and dissociation of several non-ribosomal proteins (eg. Ppp1, Rix7, Ytm1, Rsa4) and processing of rRNAs . Treatment of fission yeast with Rbins causes accumulation of pre-ribosomal particles within the nucleus within 15–30 minutes and impairs processing of rRNAs(Figure 2B)24. The rapid action and reversibility of Rbins made it possible to discern midasin’s contribution to three distinct stages of ribosome biogenesis. The first midasin-dependent step is likely to be the assembly of the Nsa1 particle, an early pre-60s particle. Midasin’s role in this step of ribosome biogenesis had not previously been described. Rbin treatment also disrupted the removal of Ytm1 from the pre-60s particle, leading to its accumulation in the nucleus. Finally, Rbin treatment disrupted processing of the Rix-1 particle and blocked its accumulation in the nucleus.
A major difficulty in the use of small molecules to dissect cellular mechanisms is the possibility that phenotypes arise due to inhibition of unanticipated targets. This limitation was overcome through parallel analyses of phenotypes associated with Rbin treatment carried out in cells that were inhibitor-sensitive (wildtype midasin) or inhibitor-resistant (midasinF1093L). In one example of this approach, Rpl2501, a component of the pre-60s ribosomal particle, accumulated in the nucleus upon Rbin-1 treatment in wildtype cells, but did not accumulate in the nucleus in cells expressing midasinF1093L (Figure 2C, bottom). In another example of this approach, Ppp1 and Rix7 were absent from the Nsa1 particle (as assessed by immunoprecipitation) in the presence of Rbin-1 but present when cells bearing mutant midasinF1039L were treated with Rbin-1. Suppression of Rbin-associated phenotypes in cells with midasinF1093L helps exclude the possibility that these effects are due to off-target inhibition by the compound and helps strongly link the associated processes with midasin’s actions in cells.
Using chemical inhibitors to probe protein dynamics
One key way that small molecules can help examine how dynamic enzymes such as the AAA proteins function is by trapping the enzymes in conformational states that may not otherwise be accessible. The ability to trap intermediates along dynamic reaction pathways has led to insights in the study of other enzyme families such as G-protein coupled receptors (GPCRs), kinases, and polymerases, as well as to the design of “state-specific” inhibitors25–28.
Nucleotides (ATP, ADP) and their analogs (ATPˠS, ADP-vanadate, AMP-PNP) have been used to block proteins’ mechanochemical cycles. Selective chemical probes can help advance the study of AAA proteins beyond what might be accessible with nucleotide analogs in several ways. In contrast to nucleotide analogs, selective probes may distinguish between different active sites. Increasingly, allosteric probes for the AAA proteins are becoming available, and these can trap protein conformations that may not be stabilized by nucleotide analogs. Here we describe three cases in which small molecule inhibitors have been used to dissect AAA protein function in vitro.
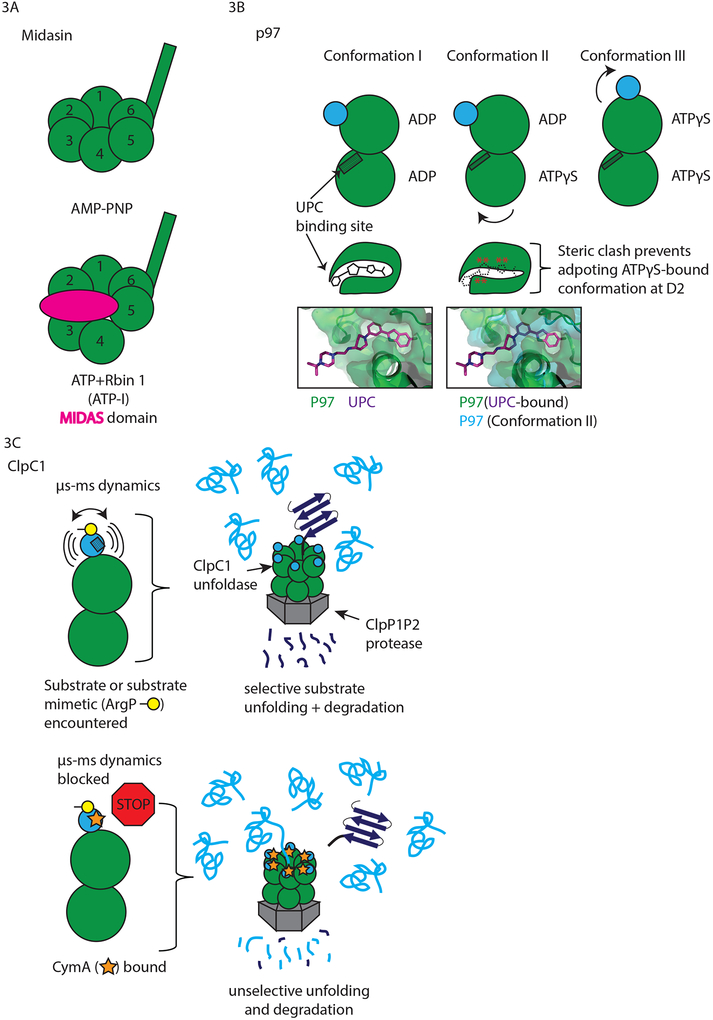
In a first step toward teasing apart the mechanistic details of how midasin contributes to ribosome biogenesis and how Rbins block this process, the cryo-EM structures of midasin in the presence of a non-hydrolyzable ATP analog (AMP-PNP) or in the presence of ATP and Rbin-1 (ATP-I) were solved (Figure 3A)29. These structures provided snapshots of an enzyme undergoing dynamic nucleotide-driven rearrangements: In the AMP-PNP structure, the six AAA domains of midasin were nearly coplanar. By contrast, in the ATP-I structure, two rigid bodies (see Box 1) corresponding to tightly associated portions of adjacent AAA domains moved ~15Å below the plane established by the other domains (Figure 3A, compare position of domains labeled 3 and 4 in top and bottom panels). In contrast to the rearrangements observed within the AAA ring, a long segment of the C-terminal tail was positioned similarly in the two structures, extending ~20nm away from the AAA ring of midasin in each case. The MIDAS domain, which was located at the extreme C-terminus of midasin separated from the AAA domains by ~2000 amino acids, is known to mediate contact with several putative substrates of this enzyme30. Densities corresponding to this domain were not observed in the AMP-PNP structure but were observed in the ATP-I structure. In the ATP-I structure, the MIDAS domain docks onto the AAA ring (shown in pink, Figure 3A, bottom). This intramolecular docking is possible most likely due to the existence of a flexible linker that connects the MIDAS domain to rest of the molecule, allowing the MIDAS domain to explore a large volume and find its binding site on the AAA ring.
Figure 3. Small molecule AAA protein ligands trap biochemically distinct states.
(A) Cryoelectron microscopy analysis of Midasin: AMP-PNP bound Midasin has six coplanar AAA domains (top row); in Rbin-1- and ATP-bound midasin (ATP-I), two rigid bodies (3 and 4) corresponding to the large subunit of a given AAA domain and the small subunit of the previous AAA domain have moved below the plane and a new density corresponding with the C-terminal MIDAS domain (pink oval) is found in the plane of the other AAA domains. The MIDAS domain is not observed in the AMP-PNP structure of midasin. (B) Cryo-EM at 2.3Å resolution reveals that that allosteric p97 inhibitor UPCDC30245 (UPC) blocks the nucleotide-dependent molecular rearrangements of p97 (conformations I, II, and III, top row). Lower row, left: UPC (carbons in purple, heteroatoms in blue) in its binding cavity (green surface). Lower row, right: contour of the p97 binding cavity in conformation II (blue surface) overlaid on structure of UPC-bound p97. (C) Cyclomarin A (CymA) blocks μs-ms dynamics of ClpC1. Top row: N-terminal domain dynamics are stimulated by substrate mimetic ArgP (yellow); these dynamics are thought to contribute to selective substrate degradation by ClpC1P1P2 protease. Bottom row: CymA binding to the N-terminal domain abolishes these dynamics and leads to unselective protein unfolding and degradation.
Contrasting the AMP-PNP and ATP-I structures, a model emerges in which different nucleotide states within four distinct ATP binding sites in the AAA ring drive rearrangements of the AAA domains themselves that can facilitate the capture and release of the MIDAS domain, which directly interacts with other pre-ribosomal factors that are midasin ‘substrates’. These successive steps of MIDAS domain capture and release coupled with rearrangements of the AAA ring could underpin midasin’s dynamic role in releasing components from different pre-ribosomal particles. This model is supported by docking of the ATP-I midasin structure into a previously solved cyro-EM map of a ribosomal precursor particle31, which readily accommodates the midasin density and positions the MIDAS domain between the AAA ring and a known midasin substrate, Rsa4. This arrangement could allow direct conformational coupling between the AAA ring and midasin’s substrate. Although Rbin could not be directly observed in the midasin structure at the resolution obtained (~8Å), it is likely to be present in the ATP-I structure as the concentration used (1μM) was well above both the GI50 observed in cells and the concentration required for half-maximal inhibition of recombinant midasin ATPase activity. Therefore, it is likely that theses findings depended on Rbin’s ability to trap a state of midasin that may not have been accessible using ATP analogs alone.
p97 is a multi-functional AAA protein needed for degradation of unfolded and ubiquitylated proteins and also contributes to several cellular processes through its ability to extract proteins from large multi-protein complexes5. p97 has two AAA domains per polypeptide protomer, leading to a stacked double ring configuration upon hexamerization2. The first well-validated p97 inhibitors to be discovered, DBeQ and its derivatives, act in an ATP-competitive manner and several analogs in the series have been shown to be selective for the D2 AAA domain32–34. Other inhibitors that bind p97 at an allosteric site wedged between the two AAA domains of a single protomer have been identified (Figure 1A)35,36. Proteomic analyses of two such compounds have confirmed the binding site, located at the periphery of the D2 domain in a groove between an alpha helix and the core beta sheet motif.
Using cyro-electron microscopy (cryo-EM), Banerjee et al confirmed that a third allosteric inhibitor (UPCDC30245, hereafter UPC) binds within the same groove and provided high resolution (~2.3Å) information on binding site contacts37. They then compared the geometry of UPC-bound p97 to that of p97 in the presence and absence of ATPˠS. Analysis of UPC’s binding mode revealed that the transitions involved with ATPˠS binding would cause several steric clashes with the inhibitor in its binding site (Figure 3B, Conformations I, II). Although ATPˠS is an imperfect model for ATP, the authors observed p97 in two of its well-known confirmations (N-domain ‘up’ and ‘down’38), suggesting the ATPˠS-p97 interaction faithfully recapitulates at least some aspects of the enzyme’s interaction with natural nucleotide. However, further work is needed to understand why the authors observed three distinct conformational states of p97 within one experiment (Conformations I, II, and III, schematized in Figure 3B, top row), in which p97 was incubated with ATPˠS. These may reflect stepwise association of ATPˠS molecules with p97 or, conversely, the effect of hydrolysis of the ˠ-thiophosphate moiety. Nevertheless, binding of UPC likely blocks the molecular rearrangements necessary for p97’s function by blocking nucleotide-state dependent motion of several helices. These results imply that binding of UPC to p97 should trap this enzyme in the compact conformation it adopts in the presence of ADP only (i.e. Conformation I, Figure 3B, top left) and block its nucleotide-dependent dynamics.
Like its homolog p97, ClpC1 is an AAA protein that initiates the degradation of proteins by unfolding them and shuttling them toward a protease (ClpP1/P2 in prokaryotes, forming the active protease complex ClpC1P1P2). Both ClpC1 and ClpP1P2 are essential in mycobacterium tuberculosis but are not required for viability in bacteria in general39–41. Thus ClpC1P1P2 has been recognized as an attractive anti-mycobacterial target. Through pulldown experiments in mycobacterial lysates, ClpC1 was identified as the target of the natural product Cyclomarin A (hereafter CymA)42. CymA and close derivatives bind ClpC1 (Kd ~20nM) and stimulate ClpC1P1P2-driven degradation of model substrates in mycobacteria. Subsequent biochemical studies and x-ray crystallography demonstrated that CymA binds to the N-terminal domain of ClpC1, which is structurally analogous to the N-terminal domain of p9743.
The ClpC1P1P2 protease is thought to degrade substrates via two separate pathways. One degradation pathway relies on recognition of proteins bearing phosphorylated arginines, which are proposed to mark proteins for degradation in prokaryotes analogously to ubiquitylation in eukaryotes. The other pathway degrades unfolded proteins without the requirement for a specific tag (this pathway relies on the adaptor protein MecA in other bacteria, but not in mycobacteria; as reviewed elsewhere41,44). Recent structural studies with a homolog of ClpC1 from another bacterium showed that the N-terminus of ClpC has a specific binding site for peptides bearing phosphorylated arginines, and that degradation reliant on this binding site can be inhibited by the addition of phosphorylated arginine as a free amino acid (ArgP)45.
To understand how CymA binding to the N-terminal domain of ClpC1 modulates this protein’s function, Weinhäupl et al performed a series of structural analyses of the enzyme in solution46 (Figure 3C). First, they verified that ArgP binds the N-terminus of ClpC1 and that it blocks the interaction between ClpC1 and a model substrate bearing phosphorylated arginines (lysozyme-P). CymA does not disrupt ClpC1’s interaction with lysozyme-P. Binding of ArgP induced an increase in the dynamics of the protein in solution, as assessed by NMR, and partially blocked the degradation of FITC-casein, another model ClpC1P1P2 substrate whose degradation does not require arginine phosphorylation. Pre-treatment with CymA completely abolished ArgP-induced dynamics, returning the N-terminal domain to a relatively static apo-like state. Surprisingly, in the presence of both CymA and ArgP, ClpC1P1P2 degraded FITC-casein at rates similar to those observed in the absence of ArgP. This is not due to simple displacement of ArgP by CymA, as they have distinct binding sites on the ClpC1 N-terminus43. Rather, these data are consistent with a model in which ArgP-induced conformational dynamics at the N-terminal domain of ClpC1 are required for both selecting substrates with phosphorylated arginines and for limiting access of non-specific unfolded protein substrates to the proteolytic core of the complex, possibly acting as an ‘entropic brush’. By blocking ClpC1 dynamics, CymA may lead to cell death by disrupting bacterial protein quality control machinery both through dysregulation of phospho-arginine dependent proteolysis and possibly through unregulated degradation of misfolded or unfolded proteins.
Future directions
The development of AAA inhibitors has been delayed compared to other large enzyme families, possibly owing to the difficulty of purifying intact and active oligomeric complexes. It is now becoming clear, however, that AAA proteins, as a family, are no less “druggable” than other enzyme classes. It is likely that the progress will only accelerate in the coming years. Beyond making probes for studying AAA proteins in cells and in vitro, progress toward developing inhibitors of these essential enzymes has potential to lead to clinically useful compounds. CB-5083, a potent p97 antagonist that showed improved efficacy compared to FDA-approved proteasome inhibitors in both solid and liquid tumor models is the first AAA protein inhibitor to reach clinical trials in humans34. It was generally well-tolerated, but had to be withdrawn due to unforeseen retinal toxicity likely related to off-target inhibition of a phosphodiesterase47.
Other AAA proteins may make promising cancer targets as well. Overexpression of RUVBL1 and RUVBL248, several subunits of the eukaryotic helicase Mcm2–749, the transcription regulator ATAD250, and the spindle assembly checkpoint regulator Trip1351–54, have all been associated with poor prognosis in several cancer types, suggesting a possible therapeutic rationale for their inhibition. Mechanistically, these trends suggest that cancers are not ‘addicted’ to the function of AAA proteins, but rather that the aberrant physiologic demands involved in tumor proliferation make cancers relatively more reliant on the actions of essential enzymes such as AAA proteins than normal tissues. One example of this is the ‘proteotoxic crisis’ present in some types of hematologic cancers, which opens a therapeutic window for inhibition of otherwise essential enzymes such as the proteasome and p9755. Assessing the potential of AAA proteins as therapeutic targets will require the development of drug-like antagonists whose therapeutic index can be carefully assessed. Even before such compounds are available, the experience with AAA inhibitors thus far has demonstrated how they can be powerful tools for studying the roles of these enzymes in cellular biology and understanding the key mechanistic details of their activity. As more inhibitors with new modes of action are discovered, more unique cellular states and biochemical conformations can be trapped and analyzed.
Acknowledgements
JBS was supported by NIH grant T32GM007739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program. TMK was supported by the NIH/NIGMS (R01GM98579 and R35GM130234).
References
•• of outstanding interest
• of special interest
- 1.Hanson PI & Whiteheart SW AAA+ proteins: have engine, will work. Nat. Rev. Mol. Cell Biol 6, 519–529 (2005). [DOI] [PubMed] [Google Scholar]
- 2.White SR & Lauring B AAA+ ATPases: achieving diversity of function with conserved machinery. Traffic 8, 1657–1667 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Chapman E, Maksim N, de la Cruz F & La Clair JJ Inhibitors of the AAA+ chaperone p97. Molecules 20, 3027–3049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firestone AJ et al. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature 484, 125–129 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Mechanistic analysis of Hedgehog pathway inhibitors reveals that they are cell-permeable inhibitors of cytoplasmic dyneins.
- 5.Stach L & Freemont PS The AAA+ ATPase p97, a cellular multitool. Biochem. J 474, 2953–2976 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vale RD The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Allan VJ Cytoplasmic dynein. Biochem. Soc. Trans 39, 1169–1178 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Prevo B, Scholey JM & Peterman EJG Intraflagellar transport: mechanisms of motor action, cooperation, and cargo delivery. FEBS J. 284, 2905–2931 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King SM Axonemal Dynein Arms. Cold Spring Harb. Perspect. Biol 8, a028100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinman JB & Kapoor TM Chemical probes for dynein. in Dyneins 172–191 (2018). [Google Scholar]
- 11.Hyman JM et al. Small-molecule inhibitors reveal multiple strategies for Hedgehog pathway blockade. Proc. Natl. Acad. Sci. U. S. A 106, 14132–14137 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.See SK et al. Cytoplasmic Dynein Antagonists with Improved Potency and Isoform Selectivity. ACS Chem. Biol 11, 53–60 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman JB et al. Chemical structure-guided design of dynapyrazoles, cell-permeable dynein inhibitors with a unique mode of action. Elife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Rational chemical approach to development of dynein inhibitors with improved potency compared to ciliobrevins; demonstration of inhibition of cytoplasmic dyneins 1 and 2 in cells.
- 14.Höing S et al. Dynarrestin, a Novel Inhibitor of Cytoplasmic Dynein. Cell Chem Biol 25, 357–369.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Kapoor TM, Chen JK & Huse M Diacylglycerol promotes centrosome polarization in T cells via reciprocal localization of dynein and myosin II. Proc. Natl. Acad. Sci. U. S. A 110, 11976–11981 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi J et al. Centrosome repositioning in T cells is biphasic and driven by microtubule end-on capture-shrinkage. J. Cell Biol 202, 779–792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu H-T et al. NK cells converge lytic granules to promote cytotoxicity and prevent bystander killing. J. Cell Biol 215, 875–889 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada Y, Baba SA & Kamimura S Effects of the dynein inhibitor ciliobrevin on the flagellar motility of sea urchin spermatozoa. Cytoskeleton 72, 182–192 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Reddy GS, Mukhopadhyay AG & Dey CS Characterization of ciliobrevin A mediated dynein ATPase inhibition on flagellar motility of Leishmania donovani. Mol. Biochem. Parasitol 214, 75–81 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Roossien DH, Miller KE & Gallo G Ciliobrevins as tools for studying dynein motor function. Front. Cell. Neurosci 9, 252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barlan K, Rossow MJ & Gelfand VI The journey of the organelle: teamwork and regulation in intracellular transport. Curr. Opin. Cell Biol 25, 483–488 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts AJ Emerging mechanisms of dynein transport in the cytoplasm versus the cilium. Biochem. Soc. Trans 46, 967–982 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konikkat S & Woolford JL Jr. Principles of 60S ribosomal subunit assembly emerging from recent studies in yeast. Biochem. J 474, 195–214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawashima SA et al. Potent, Reversible, and Specific Chemical Inhibitors of Eukaryotic Ribosome Biogenesis. Cell 167, 512–524.e14 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Identification of inhibitors of ribosome biogenesis in fission yeast; molecular target identification by analysis of resistance-conferring mutants. Analysis of compound action demonstrates role for midasin in multiple steps of ribosome biogenesis.
- 25.Latorraca NR, Venkatakrishnan AJ & Dror RO GPCR Dynamics: Structures in Motion. Chem. Rev 117, 139–155 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Müller S, Chaikuad A, Gray NS & Knapp S The ins and outs of selective kinase inhibitor development. Nat. Chem. Biol 11, 818–821 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Bae B et al. CBR antimicrobials inhibit RNA polymerase via at least two bridge-helix cap-mediated effects on nucleotide addition. Proc. Natl. Acad. Sci. U. S. A 112, E4178–87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma C, Yang X & Lewis PJ Bacterial Transcription as a Target for Antibacterial Drug Development. Microbiol. Mol. Biol. Rev 80, 139–160 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z et al. Structural Insights into Mdn1, an Essential AAA Protein Required for Ribosome Biogenesis. Cell 175, 822–834.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Cryo-EM analysis of midasin in the presence of non-hydrolyzable nucleotide and inhibitor Rbin-1; demonstration that MIDAS domain interacts with the AAA ring in some conformational states.
- 30.Ulbrich C et al. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell 138, 911–922 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Barrio-Garcia C et al. Architecture of the Rix1-Rea1 checkpoint machinery during pre-60S-ribosome remodeling. Nat. Struct. Mol. Biol 23, 37–44 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Chou T-F et al. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc. Natl. Acad. Sci. U. S. A 108, 4834–4839 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• High throughput screen identifies DBeQ as an ATP competitive inhibitor of p97 and demonstrates inhibition of p97-dependent intracellular processes.
- 33.Chou T-F et al. Specific inhibition of p97/VCP ATPase and kinetic analysis demonstrate interaction between D1 and D2 ATPase domains. J. Mol. Biol 426, 2886–2899 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson DJ et al. Targeting the AAA ATPase p97 as an Approach to Treat Cancer through Disruption of Protein Homeostasis. Cancer Cell 28, 653–665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Biochemical, cell-based, and mouse-model analysis of optimized DBeQ derivative demonstrating resistance conferring mutations arising in p97 ATPase site as well as improved anticancer activity compared to standard-of-care compounds in solid and liquid tumors.
- 35.Magnaghi P et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat. Chem. Biol 9, 548–556 (2013). [DOI] [PubMed] [Google Scholar]; • Efforts to identify improved p97 inhibitors including optimization of previously disclosed inhibitors; ATP-competitive, covalent, and allosteric ligands all identified.
- 36.Pöhler R et al. A Non-Competitive Inhibitor of VCP/p97 and VPS4 Reveals Conserved Allosteric Circuits in Type I and II AAA ATPases. Angew. Chem. Int. Ed Engl. 57, 1576–1580 (2018). [DOI] [PubMed] [Google Scholar]; • Identification of allosteric inhibitor of p97 and observation that it inhibits another AAA protein (VPS4B); mass-spectrometry based identification of allosteric binding site.
- 37.Banerjee S et al. 2.3 Å resolution cryo-EM structure of human p97 and mechanism of allosteric inhibition. Science 351, 871–875 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Structural analysis of binding of allosteric p97 inhibitor demonstrating the mechanism by which compound binding in the allosteric site inhibits the mechanical cycle of p97.
- 38.Xia D, Tang WK & Ye Y Structure and function of the AAA+ ATPase p97/Cdc48p. Gene 583, 64–77 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik IT & Brötz-Oesterhelt H Conformational control of the bacterial Clp protease by natural product antibiotics. Nat. Prod. Rep 34, 815–831 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Sassetti CM, Boyd DH & Rubin EJ Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol 48, 77–84 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Alhuwaider AAH & Dougan DA AAA+ Machines of Protein Destruction in Mycobacteria. Front Mol Biosci 4, 49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitt EK et al. The natural product cyclomarin kills Mycobacterium tuberculosis by targeting the ClpC1 subunit of the caseinolytic protease. Angew. Chem. Int. Ed Engl 50, 5889–5891 (2011). [DOI] [PubMed] [Google Scholar]; • Pull-down experiments demonstrating that ClpC1 is the molecular target of the antimicrobial Cyclomarin A with cellular analysis showing that this compound increases bacterial proteolysis of model substrates.
- 43.Vasudevan D, Rao SPS & Noble CG Structural basis of mycobacterial inhibition by cyclomarin A. J. Biol. Chem 288, 30883–30891 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; • X-ray crystallographic study demonstrating the binding site and mode of inhibition of ClpC1 by cyclomarin A; demonstration that ClpC1 is the relevant in vivo target for cyclomarin A-dependent killing of mycobacteria.
- 44.Kuhlmann NJ & Chien P Selective adaptor dependent protein degradation in bacteria. Curr. Opin. Microbiol 36, 118–127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trentini DB et al. Arginine phosphorylation marks proteins for degradation by a Clp protease. Nature 539, 48–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinhäupl K et al. The antibiotic cyclomarin blocks arginine-phosphate–induced millisecond dynamics in the N-terminal domain of ClpC1 fromMycobacterium tuberculosis. J. Biol. Chem 293, 8379–8393 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• NMR and small-angle X-ray scattering experiments demonstrating that cyclomarin A acts by blocking the dynamics of the N-terminal domain of ClpC1.
- 47.Doroshow JH, Parchment R, Moscow J NCI Experimental Therapeutics (NExT) Program. Division of Extramural Activities, National Cancer Institute (2018). Available at: https://deainfo.nci.nih.gov/advisory/fac/0518/Doroshow.pdf. (Accessed: 8th October 2018) [Google Scholar]
- 48.Mao Y-Q & Houry WA The Role of Pontin and Reptin in Cellular Physiology and Cancer Etiology. Front Mol Biosci 4, 58 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon NE & Schwacha A The Mcm2–7 replicative helicase: a promising chemotherapeutic target. Biomed Res. Int 2014, 549719 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussain M et al. ATAD2 in cancer: a pharmacologically challenging but tractable target. Expert Opin. Ther. Targets 22, 85–96 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Banerjee R et al. TRIP13 promotes error-prone nonhomologous end joining and induces chemoresistance in head and neck cancer. Nat. Commun 5, 4527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao Y et al. TRIP13 impairs mitotic checkpoint surveillance and is associated with poor prognosis in multiple myeloma. Oncotarget 8, 26718–26731 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou K et al. Loss of thyroid hormone receptor interactor 13 inhibits cell proliferation and survival in human chronic lymphocytic leukemia. Oncotarget 8, 25469–25481 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheng N et al. TRIP13 promotes tumor growth and is associated with poor prognosis in colorectal cancer. Cell Death Dis 9, 402 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deshaies RJ Proteotoxic crisis, the ubiquitin-proteasome system, and cancer therapy. BMC Biol 12, 94 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuo T-C et al. Purine-Type Compounds Induce Microtubule Fragmentation and Lung Cancer Cell Death through Interaction with Katanin. J. Med. Chem 59, 8521–8534 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Gao W et al. Discovery and characterization of the tuberculosis drug lead ecumicin. Org. Lett 16, 6044–6047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavrish E et al. Lassomycin, a Ribosomally Synthesized Cyclic Peptide, Kills Mycobacterium tuberculosis by Targeting the ATP-Dependent Protease ClpC1P1P2. Chem. Biol 21, 509–518 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loibl M et al. The Drug Diazaborine Blocks Ribosome Biogenesis by Inhibiting the AAA-ATPase Drg1. J. Biol. Chem 289, 3913–3922 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyaci H et al. Structure, Regulation, and Inhibition of the Quorum-Sensing Signal Integrator LuxO. PLoS Biol 14, e1002464 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Assimon VA et al. CB-6644 Is a Selective Inhibitor of the RUVBL1/2 Complex with Anticancer Activity. ACS Chem. Biol (2019). doi: 10.1021/acschembio.8b00904 [DOI] [PubMed] [Google Scholar]