Abstract
Objective
To examine the association of consumption of dairy foods with risk of total and cause specific mortality in women and men.
Design
Three prospective cohort studies with repeated measures of diet and lifestyle factors.
Setting
Nurses’ Health Study, Nurses’ Health Study II, and the Health Professionals Follow-up Study, in the United States.
Participants
168 153 women and 49 602 men without cardiovascular disease or cancer at baseline.
Main outcome measure
Death confirmed by state vital records, the national death index, or reported by families and the postal system. During up to 32 years of follow-up, 51 438 deaths were documented, including 12 143 cardiovascular deaths and 15 120 cancer deaths. Multivariable analysis further adjusted for family history of cardiovascular disease and cancer, physical activity, overall dietary pattern (alternate healthy eating index 2010), total energy intake, smoking status, alcohol consumption, menopausal status (women only), and postmenopausal hormone use (women only).
Results
Compared to the lowest category of total dairy consumption (average 0.8 servings/day), the multivariate pooled hazard ratio for total mortality was 0.98 (95% confidence interval 0.96 to 1.01) for the second category of dairy consumption (average 1.5 servings/day), 1.00 (0.97 to 1.03) for the third (average 2.0 servings/day), 1.02 (0.99 to 1.05) for the fourth (average 2.8 servings/day), and 1.07 (1.04 to 1.10) for highest category (average 4.2 servings/day; P for trend <0.001). For the highest compared to the lowest category of total dairy consumption, the hazard ratio was 1.02 (0.95 to 1.08) for cardiovascular mortality and 1.05 (0.99 to 1.11) for cancer mortality. For subtypes of dairy products, whole milk intake was significantly associated with higher risks of total mortality (hazard ratio per 0.5 additional serving/day 1.11, 1.09 to 1.14), cardiovascular mortality (1.09, 1.03 to 1.15), and cancer mortality (1.11, 1.06 to 1.17). In food substitution analyses, consumption of nuts, legumes, or whole grains instead of dairy foods was associated with a lower mortality, whereas consumption of red and processed meat instead of dairy foods was associated with higher mortality.
Conclusion
These data from large cohorts do not support an inverse association between high amount of total dairy consumption and risk of mortality. The health effects of dairy could depend on the comparison foods used to replace dairy. Slightly higher cancer mortality was non-significantly associated with dairy consumption, but warrants further investigation.
Introduction
Dairy products are widely consumed worldwide. They are important sources of protein, vitamin D, and calcium, but several constituents of dairy foods such as saturated fat and cholesterol might have adverse effects on health. Previous observational studies have examined associations of dairy foods with multiple health outcomes. In general, the associations between total dairy and risk of hypertension,1 2 type 2 diabetes,3 cardiovascular disease,4 colorectal cancer,5 and breast cancer6 were null or moderately inverse. However, higher intakes of dairy products have also been associated with greater risk of prostate cancer7 and ovarian cancer.8 9
The results have also been inconsistent in prospective observational studies of dairy intake and mortality: positive associations have been seen in some studies,10 11 12 while inverse or null associations have been seen in others.13 14 15 16 17 In two meta-analyses summarizing these findings, overall dairy intake was not significantly associated with risk of mortality but the heterogeneity between studies was high.18 19 Reasons for the heterogeneity across studies included limited sample size in some studies, different types of dairy products, different overall dietary patterns across populations, and inconsistent control for confounding variables.
Therefore, we examined the association of total dairy consumption and dairy subtypes with total and cause specific mortality in three large cohorts, including the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHSII), and Health Professional Follow-up Study (HPFS). The total sample size of our study was 217 755, and we documented 51 438 deaths during 29-32 years of follow-up.
Methods
Study population
The NHS began in 1976, when 121 700 female registered nurses aged 30-55 residing in 11 US states were recruited to complete a baseline questionnaire about their lifestyle and medical history. The NHSII was established in 1989 and consisted of 116 671 younger female registered nurses, aged 25-42 at baseline. The nurses responded to a baseline questionnaire similar to the NHS. The HPFS was initiated in 1986, and was composed of 51 529 male dentists, pharmacists, veterinarians, optometrists, osteopathic physicians, and podiatrists, aged 40-75 at baseline. Participants returned a baseline questionnaire about detailed medical history, lifestyle, and usual diet. Detailed descriptions of the three cohorts are provided elsewhere.20 21 22 In all the three cohorts, questionnaire data were collected at baseline and biennially thereafter, to update information on lifestyle factors and the occurrence of chronic diseases. These cohorts consist of participants with about 95% European ancestry. We excluded participants who reported cardiovascular disease or cancer at baseline for these analyses (1984 for the NHS, 1989 for the NHSII, and 1986 for the HPFS).23 We further excluded participants with extreme and implausible daily energy intakes (<500 or >3500 kcal for women; <800 or >4200 kcal for men; 1 kcal=4.18 kJ=0.00418 MJ). The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T H Chan School of Public Health, and those of participating registries as required.
Assessment of dairy consumption
In 1984, a 116 item food frequency questionnaire was administered to the NHS participants to obtain information on usual intake of food and beverages. Starting in 1986, an expanded 131 item food frequency questionnaire was administered every four years to update diet. Using a similar food frequency questionnaire, dietary data was collected every four years from the HPFS participants starting in 1986 and from the NHSII participants starting in 1989. In all food frequency questionnaires, participants were asked how often (in nine categories, ranging from “never or less than once per month” to “six or more times per day”), on average, they consumed a standard portion size of each food item during the previous year.
The questionnaire items for dairy products included “skim/low fat milk,” “whole milk,” “ice cream,” “sherbert,” “yogurt,” “cottage/ricotta cheese,” “cream cheese,” “other cheese,” and “cream.” The standard serving sizes were a 240 mL glass for skim/low fat milk and whole milk; one tablespoon (6 g) for cream; 1/2 cup (120 mL) for sherbet or frozen yogurt, ice cream, and cottage and ricotta cheese; and 30 mL for cream cheese and other cheese. Butter consumption was not included because it is nutritionally distinct from other dairy foods. Consumption of total dairy foods was calculated as the sum of intakes of all types of dairy products. We further divided the individual dairy foods into four groups: milk, cheese, yogurt, and other types of dairy foods including ice cream, cream, and sherbert. The validity and reproducibility of the food frequency questionnaire have been described in detail elsewhere.24 25 26 27 Briefly, correlation coefficients comparing intakes based on four dietary records, each record lasting one week, collected over a one year period with a single food frequency questionnaire ranged from 0.57 for hard cheese to 0.97 for yogurt.26
Assessment of covariates and effect modifiers
Updated information on age, weight, smoking status, physical activity, family history of cardiovascular disease and cancer, self reported diagnosis of diseases (including hypertension, hypercholesterolemia, cardiovascular disease, and cancer), and cardiovascular drug treatment use (including aspirin, diuretics, calcium blockers, β blockers, other antihypertensive drugs, statins, and other cholesterol lowering drugs) was collected in the biennial follow-up questionnaires in the NHS, NHSII, and HPFS. For female participants, we also ascertained data on menopausal status and postmenopausal hormone use. To measure the overall diet quality using food frequency questionnaire data, we calculated the alternate healthy eating index in the three cohorts.28
Assessment of deaths
Our primary endpoint was death from any cause ascertained by 31 December 2016. In the NHS, NHSII, and HPFS, we performed systematic searches of state vital records and the national death index. This search was supplemented by reports from family members and postal authorities. Using these methods, we were able to ascertain more than 98% of the deaths in each cohort.29 Physicians who were blinded to data on dairy consumption and other risk factors reviewed death certificates and medical records to classify the cause of death according to ICD-8 and ICD-9 (international classification of diseases, 8th and 9th revisions).
Statistical analysis
We followed the participants from the date of the return of the baseline questionnaire until their date of death or the end of follow-up (31 December 2016), whichever came first. We used Cox proportional hazards regression models to examine the associations between total dairy consumption and risk of mortality. The regression models included follow-up time as the time scale and were stratified by age in years. In the multivariable analysis, we further adjusted for family history of cardiovascular disease and cancer, physical activity, overall dietary pattern (alternate healthy eating index 2010), total energy intake, smoking status, alcohol consumption, menopausal status (women only), and postmenopausal hormone use (women only), all of which were updated from follow-up questionnaires. We additionally adjusted for baseline body mass index, hypertension, and hypercholesterolemia in both men and women. We used the cumulative average of dietary intakes from baseline to the censoring events to best represent long term diet and minimize within-person variation,23 and we stopped updating dietary information after self reported diagnosis of intermediate outcomes, including type 2 diabetes, cardiovascular disease, and cancer. We modelled dairy intakes as categorical variables, and tested for trend by modelling the median value within each category as a continuous variable.
All analyses were performed separately in each cohort, and then pooled to obtain the overall effect using a fixed effect model. We used random effects models if significant heterogeneity across cohorts was found. Statistical heterogeneity across studies was assessed by Cochrane Q test, with P<0.1 indicating significant heterogeneity between studies.30 We examined dose-response associations of dairy intake with risk of mortality by pooling individual level data in the three cohorts. We used restricted cubic splines with three knots to flexibly model the non-linear association. To test for a potential non-linear association between dairy consumption and risk of mortality, we used a likelihood ratio test to compare the model with cubic spline terms to the model with a linear term of dairy consumption, with P<0.05 denoting significant non-linearity. A significant P value for non-linearity indicated that the cubic spline model fitted the data better; therefore, we further tested for overall significance of curve using a likelihood ratio test by comparing the model with cubic spline terms to the model only with intercept. Otherwise, a non-significant P value for non-linearity indicated that the linear model fitted the data better.
Stratified analyses were conducted according to development of cardiovascular disease or cancer, cardiovascular drug treatment use, follow-up time (≤14 years or >14 years), calendar year (≤2000 or >2000), body mass index (≤25 or >25), age (≤75 or >75), smoking status (current smoker or never/past smoker), alternate healthy eating index (median score or less or more than median score), physical activity (median score or less or more than median score), total fat intake (median score or less or more than median score), calcium intake (median score or less or more than median score), and vitamin D intake (median score or less or more than median score). We treated the effect modifiers as time varying variables, and tested for potential effect modification by including an interaction term between the exposure and potential effect modifier in the Cox model and conducting a likelihood ratio test comparing the models with and without the interaction term.
We estimated the associations of substituting one serving per day of nuts and legumes, whole grain, poultry, or red and processed meat for total dairy by including both variables in continuous form in the same multivariate model. The difference in their regression coefficients were used to estimate the hazard ratios for the substitution and the covariance to estimate the 95% confidence intervals.31 All statistical tests were two sided at type I error rate of 0.05 and performed using SAS version 9.2 for UNIX (SAS Institute, Cary, NC). Because we tested multiple types of dairy foods and several death related outcomes, we conservatively corrected for multiple testing using Bonferroni correction and controlled for type I error rate at 0.05/60=0.001.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study participants or the relevant patient community.
Results
Our original analysis included 217 755 participants, of whom 74 805 were from the NHS, 93 348 from the NHSII, and 49 602 from the HPFS; 168 153 women and 49 602 men without cardiovascular disease or cancer at baseline. Table 1 shows the baseline characteristics of the participants by frequency of total dairy consumption. Those who consumed higher amounts of dairy foods drank less alcohol, were more likely to be physically active, were less likely to be current smokers, and had a slightly lower alternate healthy eating index.
Table 1.
Baseline characteristics of participants by fifths of total dairy consumption in the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study. Data are mean values unless stated otherwise
| Characteristics | Nurses’ Health Study (1984) | Nurses’ Health Study (1986) | Health Professionals Follow-up Study (1991) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (n=20 497) | Q3 (n=13 378) | Q5 (n=15 260) |
Q1 (n=16 316) | Q3 (n=14 967) | Q5 (n=28 883) |
Q1 (n=10 868) | Q3 (n=8970) |
Q5 (n=10 936) |
|||
| Dairy intake (servings/day)* | 0.67 (0.28) | 1.88 (0.16) | 4.09 (1.15) | 0.96 (0.37) | 2.62 (0.24) | 6.43 (2.52) | 0.52 (0.24) | 1.61 (0.15) | 4.00 (1.29) | ||
| Age | 49.9 | 50.4 | 50.5 | 36.8 | 36.3 | 35.7 | 54.1 | 54.2 | 54.6 | ||
| Physical activity (MET-h/week) | 12.9 | 14.7 | 15.4 | 23.4 | 24.1 | 25.5 | 19.5 | 20.9 | 21.3 | ||
| Diet quality score† | 48.1 | 48.1 | 46.6 | 49.7 | 48.8 | 47.6 | 54.0 | 53.1 | 50.7 | ||
| Total energy intake (kcal/day)‡ | 1449 | 1781 | 2101 | 1450 | 1723 | 2049 | 1656 | 1964 | 2384 | ||
| Alcohol (g/day) | 7.8 | 6.7 | 6.1 | 2.7 | 3.0 | 3.5 | 12.2 | 11.3 | 10.4 | ||
| Body mass index | 23.6 | 23.9 | 23.8 | 24.2 | 24.3 | 23.9 | 24.7 | 25.0 | 25.0 | ||
| White ethnicity (No (%)) | 19 677 (96) | 13 110 (98) | 14 955 (98) | 15 174 (93) | 14 518 (97) | 28 017 (97) | 9999 (92) | 8611 (96) | 10 608 (97) | ||
| Current smokers (No (%)) | 6149 (30) | 2809 (21) | 3510 (23) | 2447 (15) | 1796 (12) | 3466 (12) | 1195 (11) | 807 (9) | 1203 (11) | ||
| Hypertension (No (%)) | 1845 (9) | 1070 (8) | 1221 (8) | 1142 (7) | 1048 (7) | 1733 (6) | 2608 (24) | 1973 (22) | 2297 (21) | ||
| Hypercholesterolemia (No (%)) | 820 (4) | 401 (3) | 458 (3) | 2774 (17) | 2245 (15) | 3755 (13) | 1739 (16) | 1076 (12) | 1094 (10) | ||
| Postmenopausal (No (%)) | 9429 (46) | 6421 (48) | 7477 (49) | 653 (4) | 599 (4) | 866 (3) | NA | NA | NA | ||
| Postmenopausal hormone use (No (%))§ | 2665 (13) | 1873 (14) | 2136 (14) | 653 (4) | 449 (3) | 866 (3) | NA | NA | NA | ||
MET-h/week=metabolic equivalent hours per week; NA=not available.
Data are mean values and standard deviations.
Alternate healthy eating index (range 0-100) was used, with a higher score indicating healthier diet.
1 kcal=4.18 kJ=0.00418 MJ.
Percentage of current postmenopausal hormone use among total women.
During 29-32 years of follow-up, 51 438 deaths were documented in the three cohorts, including 12 143 cardiovascular deaths and 15 120 cancer deaths. In an age adjusted model, dairy intake was positively associated with risk of total mortality in the NHSII and HPFS, but not NHS (table 2), which might be due to the different distribution of covariates according to dairy intake across the three cohorts as shown in table 1. However, after adjusting for covariates, dairy intake was positively associated with risk of mortality across the three cohorts (P for heterogeneity >0.1). Compared to the lowest category of total dairy consumption, the multivariable pooled hazard ratio for death was 1.07 (95% confidence interval 1.04 to 1.10) for the highest category of dairy consumption (P for linear trend <0.001; table 2). The findings remained significant after correcting for multiple testing. We further investigated the associations of dairy intake with risk of mortality using a dose-response analysis. We saw a non-linear relation between total dairy intake and risk of total mortality (P for non-linearity <0.001; P for overall significance of the curve <0.001; P for linear association <0.001) and risk of cardiovascular mortality (P=0.001; P=0.004; P=0.60; fig 1), such that moderate intake was associated with a slightly lower risk, whereas high intake was associated with an increased risk. Total dairy intake was associated with higher risk of cancer mortality in a linear fashion (P=0.10; P=0.04; P=0.048).
Table 2.
Hazard ratios of total, cardiovascular, and cancer mortality according to fifths of total dairy intake in the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study
| Frequency of dairy consumption* | Hazard ratio (95% CI)† | P for trend | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Total mortality | |||||||
| Nurses’ Health Study | |||||||
| No of deaths | 4755 | 5021 | 5024 | 5347 | 5035 | — | — |
| Age adjusted | 1.00 | 0.90 (0.87 to 0.94) | 0.86 (0.83 to 0.90) | 0.88 (0.84 to 0.91) | 0.94 (0.91 to 0.98) | 0.99 (0.97 to 1.00) | 0.07 |
| Multivariate adjusted‡ | 1.00 | 0.99 (0.95 to 1.03) | 0.98 (0.94 to 1.02) | 1.02 (0.97 to 1.06) | 1.08 (1.03 to 1.13) | 1.03 (1.02 to 1.05) | <0.001 |
| Nurses’ Health Study II | |||||||
| No of deaths | 624 | 521 | 549 | 484 | 518 | ||
| Age adjusted | 1.00 | 0.84 (0.75 to 0.95) | 0.93 (0.83 to 1.05) | 0.90 (0.80 to 1.02) | 1.12 (1.00 to 1.26) | 1.03 (1.01 to 1.06) | 0.008 |
| Multivariate adjusted‡ | 1.00 | 0.96 (0.85 to 1.07) | 1.02 (0.91 to 1.15) | 1.02 (0.90 to 1.15) | 1.16 (1.02 to 1.32) | 1.04 (1.01 to 1.06) | 0.01 |
| Health Professionals Follow-up Study | |||||||
| No of deaths | 3969 | 4260 | 4806 | 5264 | 5261 | — | — |
| Age adjusted | 1.00 | 0.96 (0.92 to 1.00) | 0.97 (0.93 to 1.01) | 0.99 (0.95 to 1.03) | 1.03 (0.99 to 1.07) | 1.02 (1.01 to 1.03) | 0.005 |
| Multivariate adjusted‡ | 1.00 | 0.98 (0.94 to 1.03) | 1.02 (0.97 to 1.06) | 1.03 (0.98 to 1.07) | 1.05 (1.00 to 1.10) | 1.02 (1.01 to 1.03) | 0.006 |
| Pooled analysis | |||||||
| Age adjusted | 1.00 | 0.92 (0.90 to 0.95) | 0.91 (0.89 to 0.94) | 0.93 (0.90 to 0.95) | 0.99 (0.96 to 1.02) | 1.01 (1.00 to 1.02) | 0.10 |
| Multivariate adjusted‡ | 1.00 | 0.98 (0.96 to 1.01) | 1.00 (0.97 to 1.03) | 1.02 (0.99 to 1.05) | 1.07 (1.04 to 1.10) | 1.03 (1.02 to 1.04) | <0.001 |
| Cardiovascular mortality | |||||||
| Nurses’ Health Study | |||||||
| No of deaths | 902 | 896 | 842 | 885 | 893 | — | — |
| Age adjusted | 1.00 | 0.86 (0.79 to 0.95) | 0.78 (0.71 to 0.86) | 0.78 (0.71 to 0.86) | 0.88 (0.80 to 0.96) | 0.96 (0.93 to 0.99) | 0.01 |
| Multivariate adjusted‡ | 1.00 | 0.97 (0.88 to 1.06) | 0.91 (0.82 to 1.00) | 0.94 (0.85 to 1.04) | 1.04 (0.94 to 1.16) | 1.02 (0.98 to 1.05) | 0.34 |
| Nurses’ Health Study II | |||||||
| No of deaths | 59 | 46 | 47 | 52 | 54 | — | — |
| Age adjusted | 1.00 | 0.79 (0.54 to 1.17) | 0.85 (0.58 to 1.24) | 1.01 (0.69 to 1.47) | 1.18 (0.81 to 1.71) | 1.06 (0.98 to 1.15) | 0.15 |
| Multivariate adjusted‡ | 1.00 | 0.88 (0.61 to 1.27) | 0.93 (0.63 to 1.37) | 1.01 (0.67 to 1.51) | 1.22 (0.81 to 1.83) | 1.06 (0.97 to 1.15) | 0.22 |
| Health Professionals Follow-up Study | |||||||
| No of deaths | 1293 | 1358 | 1565 | 1616 | 1635 | — | — |
| Age adjusted | 1.00 | 0.95 (0.88 to 1.02) | 0.98 (0.91 to 1.05) | 0.93 (0.87 to 1.01) | 0.96 (0.89 to 1.03) | 0.99 (0.97 to 1.01) | 0.38 |
| Multivariate adjusted‡ | 1.00 | 0.97 (0.90 to 1.05) | 1.01 (0.94 to 1.09) | 0.96 (0.89 to 1.04) | 0.99 (0.91 to 1.08) | 1.00 (0.97 to 1.02) | 0.88 |
| Pooled analysis | |||||||
| Age adjusted | 1.00 | 0.91 (0.86 to 0.96) | 0.89 (0.84 to 0.95) | 0.88 (0.83 to 0.93) | 0.93 (0.88 to 0.99) | 0.98 (0.97 to 1.00) | 0.08 |
| Multivariate adjusted‡ | 1.00 | 0.97 (0.91 to 1.03) | 0.97 (0.92 to 1.03) | 0.96 (0.90 to 1.02) | 1.02 (0.95 to 1.08) | 1.00 (0.99 to 1.03) | 0.49 |
| Cancer mortality | |||||||
| Nurses’ Health Study | |||||||
| No of deaths | 1557 | 1497 | 1514 | 1579 | 1494 | — | — |
| Age adjusted | 1.00 | 0.86 (0.80 to 0.93) | 0.84 (0.78 to 0.90) | 0.85 (0.79 to 0.91) | 0.88 (0.82 to 0.94) | 0.97 (0.94 to 0.99) | 0.004 |
| Multivariate adjusted‡ | 1.00 | 0.95 (0.88 to 1.02) | 0.96 (0.90 to 1.04) | 0.99 (0.92 to 1.07) | 1.02 (0.94 to 1.10) | 1.02 (0.99 to 1.04) | 0.29 |
| Nurses’ Health Study II | |||||||
| No of deaths | 253 | 221 | 248 | 208 | 227 | — | — |
| Age adjusted | 1.00 | 0.88 (0.74 to 1.06) | 1.03 (0.87 to 1.23) | 0.94 (0.79 to 1.14) | 1.20 (1.00 to 1.43) | 1.05 (1.01 to 1.09) | 0.02 |
| Multivariate adjusted‡ | 1.00 | 0.86 (0.71 to 1.03) | 1.06 (0.89 to 1.27) | 1.04 (0.86 to 1.26) | 1.20 (0.98 to 1.47) | 1.06 (1.01 to 1.10) | 0.01 |
| Health Professionals Follow-up Study | |||||||
| No of deaths | 1115 | 1140 | 1262 | 1424 | 1381 | — | — |
| Age adjusted | 1.00 | 0.95 (0.88 to 1.03) | 0.98 (0.90 to 1.06) | 1.05 (0.97 to 1.13) | 1.04 (0.96 to 1.13) | 1.03 (1.00 to 1.05) | 0.04 |
| Multivariate adjusted‡ | 1.00 | 0.97 (0.89 to 1.05) | 1.02 (0.94 to 1.11) | 1.09 (1.00 to 1.18) | 1.06 (0.97 to 1.15) | 1.03 (1.00 to 1.06) | 0.05 |
| Pooled analysis | |||||||
| Age adjusted | 1.00 | 0.90 (0.85 to 0.95) | 0.91 (0.86 to 0.96) | 0.93 (0.89 to 0.98) | 0.96 (0.92 to 1.01) | 1.00 (0.99 to 1.02) | 0.66 |
| Multivariate adjusted‡ | 1.00 | 0.95 (0.90 to 1.00) | 1.00 (0.94 to 1.05) | 1.04 (0.98 to 1.09) | 1.05 (0.99 to 1.11) | 1.03 (1.01 to 1.05) | 0.003 |
Mean dairy intake for each fifth is: 0.8 servings/day for Q1, 1.5 servings/day for Q2, 2.0 servings/day for Q3, 2.8 servings/day for Q4, and 4.2 servings/day for Q5.
Per increment of 1 serving/day of dairy.
Multivariable adjusted model was further adjusted for family history of cancer (yes, no), family history of cardiovascular disease (yes, no), baseline disease status (hypertension, hypercholesterolemia), baseline body mass index (<20.9, 21.0-22.9, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≥35.0), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalent hours per week), overall dietary pattern (alternate healthy eating index score, in fifths), total energy intake (fifths), smoking status (Nurses’ Health Study and Health Professionals Follow-up Study: never, former (categorized into 1-4, 5-14, 15-24, 25-34, 35-44, ≥45 cigarettes/day, or unknown), current (categorized in to 1-4, 5-14, 15-24, 25-34, 35-44, ≥45 cigarettes/day, or unknown), alcohol consumption (0, ≥0-5, ≥5-10, ≥10-15, ≥15 g/day), postmenopausal status (yes, no; women only), and current postmenopausal hormone use (yes, no; women only).
Fig 1.
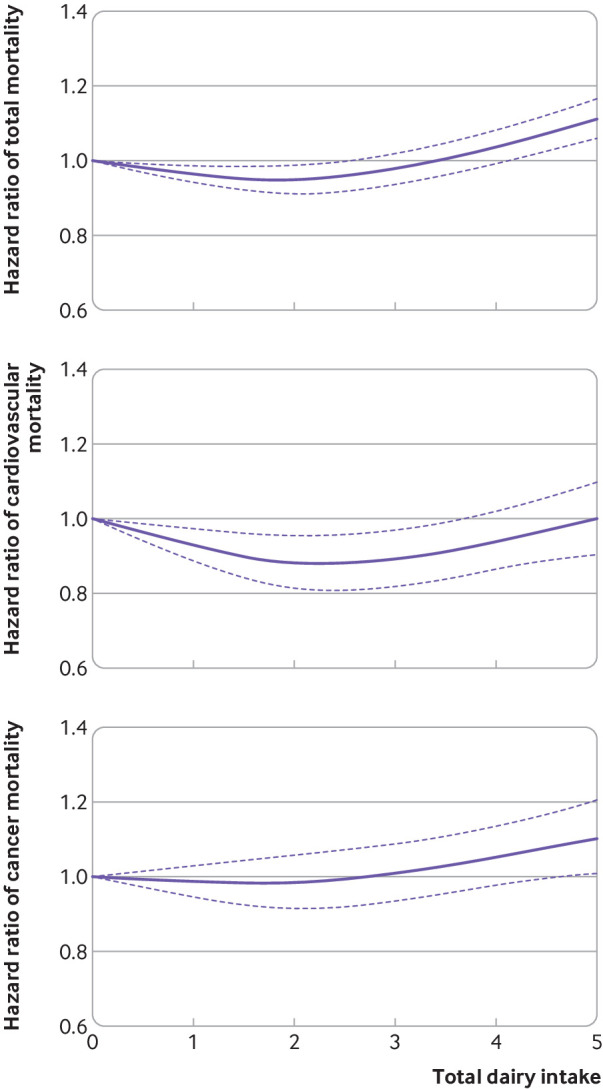
Dose-response associations of total dairy intake with risks of total mortality and mortality due to cardiovascular disease and cancer, based on pooled analyses of cohorts from the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study. Multivariable adjusted model was further adjusted for family history of cancer (yes, no), family history of cardiovascular disease (yes, no), baseline disease status (hypertension, hypercholesterolemia), baseline body mass index (<20.9, 21.0-22.9, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≥35.0), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalent hours per week), overall dietary pattern (alternate healthy eating index score, in fifths), total energy intake (fifths), smoking status (Nurses’ Health Study and Health Professionals Follow-up Study: never, former (categorized into 1-4, 5-14, 15-24, 25-34, 35-44, ≥45 cigarettes/day, or unknown), current (categorized in to 1-4, 5-14, 15-24, 25-34, 35-44, ≥45 cigarettes/day, or unknown), alcohol consumption (0, ≥0-5, ≥5-10, ≥10-15, ≥15 g/day), postmenopausal status (yes, no; women only), and current postmenopausal hormone use (yes, no; women only)
We further examined associations of subtypes of dairy foods with risk of total mortality and mortality due to cardiovascular disease and cancer (table 3, table S1). We categorized individual dairy products into four groups, and used different cutoff values for different types of dairy foods owing to low amounts of consumption for some specific dairy products. Intake of skimmed or low fat milk was associated with slightly higher risk of total mortality and cardiovascular mortality, and with a lower risk of colorectal cancer. Whole milk intake was significantly associated with higher risk of total mortality, cardiovascular mortality, and cancer mortality, including lung cancer, ovarian cancer, and prostate cancer. The associations of whole milk intake and risk of total mortality and cancer mortality remained significant after correcting for multiple testing (P for trend <0.001). Cheese and yogurt intake was not associated with risk of total or cause specific mortality. Further adjustment for smoking pack years and other dietary factors such as red meat did not appreciably change the results.
Table 3.
Hazard ratios of total mortality and mortality due to cardiovascular disease and cancer according to consumptions of dairy food subtypes, based on pooled analyses of cohorts from the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study*†
| Dairy intake (by serving category) | Hazard ratio (95% CI)‡ | P for trend | ||||
|---|---|---|---|---|---|---|
| Category 1 | Category 2 | Category 3 | Category 4 | |||
| Skimmed or low fat milk (serving categories: <1/week, 1-4/week, 4/week-1.5/day, ≥1.5/day) | ||||||
| Total mortality | ||||||
| NHS | 1.00 | 0.95 (0.92 to 0.99) | 0.98 (0.94 to 1.02) | 1.05 (1.01 to 1.10) | 1.02 (1.01 to 1.03) | <0.001 |
| NHSII | 1.00 | 0.89 (0.80 to 1.00) | 0.88 (0.78 to 0.98) | 0.93 (0.82 to 1.06) | 0.99 (0.97 to 1.02) | 0.64 |
| HPFS | 1.00 | 0.98 (0.94 to 1.01) | 1.01 (0.97 to 1.05) | 1.04 (1.00 to 1.09) | 1.01 (1.00 to 1.02) | 0.01 |
| Pooled analysis | 1.00 | 0.96 (0.93 to 0.99) | 0.99 (0.96 to 1.02) | 1.04 (1.01 to 1.07) | 1.01 (1.00 to 1.02) | <0.001 |
| Cardiovascular mortality | ||||||
| NHS | 1.00 | 1.02 (0.93 to 1.11) | 0.97 (0.89 to 1.06) | 1.07 (0.97 to 1.19) | 1.01 (0.99 to 1.04) | 0.22 |
| NHSII | 1.00 | 0.81 (0.57 to 1.13) | 0.79 (0.56 to 1.12) | 0.79 (0.53 to 1.19) | 0.96 (0.88 to 1.05) | 0.39 |
| HPFS | 1.00 | 0.97 (0.90 to 1.04) | 1.05 (0.98 to 1.13) | 1.05 (0.97 to 1.13) | 1.01 (1.00 to 1.02) | 0.10 |
| Pooled analysis | 1.00 | 0.98 (0.93 to 1.04) | 1.02 (0.96 to 1.07) | 1.05 (0.99 to 1.12) | 1.01 (1.00 to 1.02) | 0.06 |
| Cancer mortality | ||||||
| NHS | 1.00 | 0.97 (0.91 to 1.04) | 0.99 (0.92 to 1.05) | 1.04 (0.96 to 1.12) | 1.01 (0.99 to 1.03) | 0.19 |
| NHSII | 1.00 | 1.04 (0.87 to 1.24) | 1.00 (0.84 to 1.20) | 1.06 (0.87 to 1.29) | 1.01 (0.97 to 1.05) | 0.70 |
| HPFS | 1.00 | 0.97 (0.90 to 1.05) | 0.99 (0.92 to 1.06) | 1.00 (0.92 to 1.08) | 1.00 (0.98 to 1.02) | 0.83 |
| Pooled analysis | 1.00 | 0.98 (0.93 to 1.03) | 0.99 (0.94 to 1.04) | 1.02 (0.97 to 1.08) | 1.00 (0.99 to 1.02) | 0.28 |
| Whole milk (serving categories: <1/month, 1-3/month, 3/month-2/week, ≥2/week) | ||||||
| Total mortality | ||||||
| NHS | 1.00 | 1.01 (0.97 to 1.05) | 1.03 (0.99 to 1.07) | 1.15 (1.10 to 1.19) | 1.13 (1.10 to 1.17) | <0.001 |
| NHSII | 1.00 | 0.99 (0.84 to 1.16) | 1.10 (0.93 to 1.29) | 1.26 (1.08 to 1.48) | 1.28 (1.10 to 1.49) | 0.002 |
| HPFS | 1.00 | 1.00 (0.95 to 1.05) | 1.01 (0.96 to 1.06) | 1.11 (1.07 to 1.16) | 1.09 (1.05 to 1.13) | <0.001 |
| Pooled analysis | 1.00 | 1.00 (0.97 to 1.04) | 1.03 (0.99 to 1.06) | 1.13 (1.10 to 1.17) | 1.11 (1.09 to 1.14) | <0.001 |
| Cardiovascular mortality | ||||||
| NHS | 1.00 | 1.05 (0.95 to 1.16) | 0.98 (0.89 to 1.09) | 1.17 (1.07 to 1.28) | 1.14 (1.05 to 1.24) | 0.001 |
| NHSII | 1.00 | 0.88 (0.52 to 1.49) | 1.04 (0.61 to 1.76) | 1.18 (0.71 to 1.94) | 1.18 (0.73 to 1.91) | 0.49 |
| HPFS | 1.00 | 0.98 (0.90 to 1.07) | 1.07 (0.98 to 1.17) | 1.06 (0.98 to 1.14) | 1.05 (0.98 to 1.12) | 0.15 |
| Pooled analysis | 1.00 | 1.01 (0.95 to 1.08) | 1.03 (0.96 to 1.10) | 1.10 (1.04 to 1.17) | 1.09 (1.03 to 1.15) | 0.001 |
| Cancer mortality | ||||||
| NHS | 1.00 | 1.01 (0.94 to 1.09) | 1.04 (0.96 to 1.12) | 1.12 (1.05 to 1.21) | 1.11 (1.04 to 1.19) | <0.001 |
| NHSII | 1.00 | 1.02 (0.80 to 1.32) | 1.06 (0.82 to 1.37) | 1.32 (1.03 to 1.70) | 1.30 (1.03 to 1.65) | 0.03 |
| HPFS | 1.00 | 0.98 (0.89 to 1.07) | 0.98 (0.89 to 1.08) | 1.12 (1.03 to 1.22) | 1.10 (1.02 to 1.17) | 0.006 |
| Pooled analysis | 1.00 | 1.00 (0.94 to 1.06) | 1.02 (0.96 to 1.08) | 1.13 (1.07 to 1.19) | 1.11 (1.06 to 1.17) | <0.001 |
| Cheese (serving categories: <1/week, 1-4/week, 4/week-1.5/day, ≥1.5/day) | ||||||
| Total mortality | ||||||
| NHS | 1.00 | 1.03 (0.98 to 1.08) | 1.04 (0.98 to 1.09) | 1.09 (1.00 to 1.18) | 1.02 (1.00 to 1.04) | 0.07 |
| NHSII | 1.00 | 1.01 (0.88 to 1.14) | 1.13 (0.98 to 1.31) | 1.20 (0.96 to 1.51) | 1.08 (1.02 to 1.14) | 0.009 |
| HPFS | 1.00 | 0.90 (0.87 to 0.94) | 0.92 (0.88 to 0.96) | 0.92 (0.85 to 0.99) | 0.98 (0.96 to 1.00) | 0.04 |
| Pooled analysis | 1.00 | 0.95 (0.92 to 0.98) | 0.97 (0.94 to 1.01) | 1.00 (0.95 to 1.06) | 1.00 (0.99 to 1.02) | 0.69 |
| Cardiovascular mortality | ||||||
| NHS | 1.00 | 1.05 (0.93 to 1.17) | 1.05 (0.94 to 1.19) | 1.15 (0.95 to 1.39) | 1.03 (0.98 to 1.08) | 0.23 |
| NHSII | 1.00 | 0.86 (0.58 to 1.27) | 1.41 (0.93 to 2.16) | 1.40 (0.70 to 2.79) | 1.23 (1.04 to 1.45) | 0.01 |
| HPFS | 1.00 | 0.90 (0.84 to 0.96) | 0.91 (0.84 to 0.98) | 0.86 (0.75 to 0.99) | 0.96 (0.93 to 1.00) | 0.05 |
| Pooled analysis | 1.00 | 0.93 (0.88 to 0.99) | 0.96 (0.90 to 1.02) | 0.96 (0.86 to 1.08) | 0.99 (0.96 to 1.03) | 0.61 |
| Cancer mortality | ||||||
| NHS | 1.00 | 0.99 (0.91 to 1.08) | 1.05 (0.96 to 1.15) | 1.13 (0.98 to 1.30) | 1.05 (1.01 to 1.09) | 0.01 |
| NHSII | 1.00 | 0.96 (0.79 to 1.16) | 1.02 (0.82 to 1.26) | 1.09 (0.76 to 1.55) | 1.04 (0.95 to 1.13) | 0.41 |
| HPFS | 1.00 | 0.96 (0.89 to 1.03) | 0.98 (0.90 to 1.06) | 0.91 (0.78 to 1.07) | 0.98 (0.95 to 1.02) | 0.39 |
| Pooled analysis | 1.00 | 0.97 (0.92 to 1.02) | 1.01 (0.95 to 1.07) | 1.03 (0.93 to 1.14) | 1.02 (0.99 to 1.05) | 0.16 |
NHS=Nurses’ Health Study; NHSII=Nurses’ Health Study II; HPFS=Health Professionals Follow-up Study.
Associations for yogurt not shown to avoid duplication with another study.
Multivariable adjusted model was further adjusted for family history of cancer (yes, no), family history of cardiovascular disease (yes, no), baseline disease status (hypertension, hypercholesterolemia), baseline body mass index (<20.9, 21.0-22.9, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≥35.0), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalent hours per week), overall dietary pattern (alternate healthy eating index score, in fifths), total energy intake (fifths), smoking status (Nurses’ Health Study and Health Professionals Follow-up Study: never, former (categorized into 1-4, 5-14, 15-24, 25-34, 35-44, ≥45 cigarettes/day, or unknown), current (categorized in to 1-4, 5-14, 15-24, 25-34, 35-44, ≥45 cigarettes/day, or unknown), alcohol consumption (0, ≥0-5, ≥5-10, ≥10-15, ≥15 g/day), postmenopausal status (yes, no; women only), and current postmenopausal hormone use (yes, no; women only). Subtypes of dairy foods were adjusted for each other.
Per increment of 0.5 servings/day.
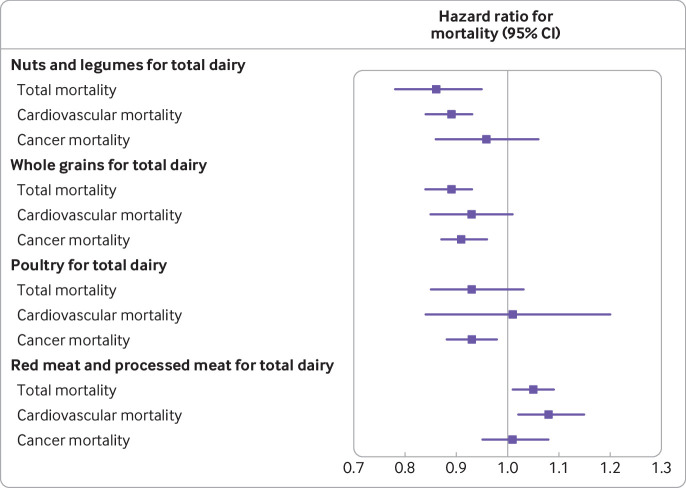
We estimated that substituting one serving per day of total dairy for nuts and legumes was associated with a 14% lower risk of total mortality (hazard ratio 0.86, 95% confidence interval 0.78 to 0.95; fig 2). Substituting one dairy serving per day for whole grains was associated with an 11% lower risk of total mortality (0.89, 0.84 to 0.93). However, a substitution for red and processed meat was also associated with a 5% higher risk of total mortality (1.05, 1.01 to 1.09).
Fig 2.
Hazard ratios for total mortality and mortality due to cardiovascular disease and cancer associated with replacement of total dairy with other foods in the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study. Error bars=95% confidence intervals. Multivariable adjusted model was further adjusted for family history of cancer (yes, no), family history of cardiovascular disease (yes, no), baseline disease status (hypertension, hypercholesterolemia), baseline body mass index (<20.9, 21.0-22.9, 23.0-24.9, 25.0-29.9, 30.0-34.9, ≥35.0), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalent hours per week), overall dietary pattern (alternate healthy eating index score, in fifths), total energy intake (fifths), smoking status (Nurses’ Health Study and Health Professionals Follow-up Study: never, former (categorized into 1-4, 5-14, 15-24, 25-34, 35-44, ≥45 cigarettes/day, or unknown), current (categorized in to 1-4, 5-14, 15-24, 25-34, 35-44, ≥45 cigarettes/day, or unknown), alcohol consumption (0, ≥0-5, ≥5-10, ≥10-15, ≥15 g/day), postmenopausal status (yes, no; women only), and current postmenopausal hormone use (yes, no; women only)
In sensitivity analyses, we used time varying dairy intake updated every four years as exposure to provide more weight to the most recent diet. We found that dairy intake was not significantly associated with total mortality and mortality due to cancer and cardiovascular disease (table S2). To minimize reverse causation, we excluded participants who died during the first four years of follow-up, and results did not change (table S3). Significant interactions were found between dairy consumption and risk of mortality by body mass index (P value for interaction=0.03; table S4). A positive association was found among non-obese participants and no association was found among obese participants. We saw no significant differences in the associations between dairy consumption and risk of total mortality when stratified by disease status; cardiovascular drug treatment use; follow-up time; calendar year; age; smoking; diet quality; physical activity; and intakes of fat, calcium, and vitamin D (table S4).
Discussion
Principal findings
The present analysis of three large cohort studies involving 217 755 participants and 51 438 deaths did not support an inverse association between dairy intake and mortality. The robustness of our findings was confirmed by using time varying intake of dairy that was updated every four years as the exposure, excluding deaths in the first four years of follow-up, and the results were unchanged. A dose-response analysis suggested that two servings per day of dairy consumption was associated with lowest total and cardiovascular disease mortality. Substitution analyses suggested that replacing dairy with nuts or legumes and whole grains could lower mortality risk, but replacing total dairy with red and processed meat could increase mortality risk.
Comparison with other studies
The association between dairy intake and risk of mortality has been extensively investigated. Some early studies showed positive associations between dairy intake and risk of mortality,10 11 12 while others showed inverse or null associations.13 14 15 16 17 By summarizing these studies, one meta-analysis showed that overall dairy intake was not associated with risk of mortality.18 19 More prospective cohort studies with large sample sizes have since been conducted worldwide but with conflicting conclusions. For example, in two Swedish cohorts including 106 772 participants, dairy intake was associated with higher risk of mortality,11 but in a Danish cohort of 74 241 participants and a Dutch cohort of 34 409 participants, dairy intake was not associated with higher risk of mortality.32 33 In addition, in a Japanese cohort of 94 980 participants34 and in the Prospective Urban Rural Epidemiology study including 136 384 participants from 21 countries and five continents, dairy intake was associated with lower risk of mortality.35 36
In our study, we found that total dairy intake was associated with slightly higher mortality, and the positive association remained even after correcting for multiple testing. The divergent findings across studies could be due to multiple reasons. For example, specific types of dairy foods varied across studies, the degree of residual confounding could have differed across studies because the covariates adjusted for were varied, and the effects of dairy foods could have depended on the nutritional quality of the background diet. However, the most important reason for the inconsistent findings could be the varied amount of dairy intake across populations—northern European countries consume the largest amount of dairy products worldwide whereas Asian countries such as Japan and China consume the least amount owing to high prevalence of lactose intolerance.37 38
In our study, whole milk intake was associated with higher risk of cancer mortality, including ovarian cancer and prostate cancer mortality. The positive associations of whole milk intake and cancer remained significant after correcting for multiple testing. Previous studies also showed that whole milk intake was associated with higher risks of prostate and ovarian cancer.8 9 39 40 The effects of milk consumption on plasma insulin-like growth factor 1,41 42 which predicts higher risks of prostate and breast cancer,43 provides a plausible mechanism. Galactose, a lactose metabolite contained in milk, has been thought to increase risk of ovarian cancer, but no relation was seen in a pooled analysis.44
We also observed that whole milk intake was associated with higher risk of cardiovascular mortality. This finding could be explained by saturated fatty acids from whole milk affecting the blood lipid profile and promoting atherosclerosis. Results from short term intervention studies indicated that a diet higher in saturated fatty acids from whole milk and butter increased low density lipoprotein cholesterol when substituted for carbohydrates or unsaturated fatty acids.34 Furthermore, the Dietary Approaches to Stop Hypertension dietary pattern lowered systolic blood pressure, and the high content of low fat dairy products in that diet might have contributed to the effect.45 Observational studies also showed that saturated fat intake was associated with higher risk of coronary heart disease,46 although the effects depended on the substitution of unsaturated fats.47 One cohort study found that the replacement of dairy fat with polyunsaturated fat was significantly associated with lower risk of cardiovascular disease.48 Moreover, whole milk could be a marker of unhealthy diet and lifestyle.
Although we adjusted for a wide range of diet and lifestyle factors, residual confounding remains a possibility. However, further adjustment for smoking pack years and other dietary factors such as red meat did not appreciably change the results. As for skimmed or reduced fat milk, participants who developed hypertension, hypercholesterolemia, or cardiovascular disease could change to adopt a healthier lifestyle by consuming more skimmed or reduced milk instead of whole milk. Although we stopped updating dietary intakes after the development of cardiovascular disease and cancer, reverse causation could remain and potentially explain a slightly positive association observed between skim milk intake and risk of cardiovascular mortality. Reverse causation might also be the reason for non-significant association between time-varying dairy intake and risk of total mortality in the sensitivity analysis.
Our study found that intake of skimmed or low fat milk was associated with lower risk of colorectal cancer mortality, and calcium contained in dairy foods might have an anti-carcinogenic effect. In previous meta-analyses and pooled analyses, milk consumption was inversely associated with risk of colorectal cancer, possibly because of its high calcium content.5 49 50 Many prospective cohort studies showed that calcium intake was associated with lower risk of colorectal cancer,40 51 52 and clinical trials also showed that calcium supplementation reduced the recurrence of colorectal adenoma.53 Animal studies showed that calcium can suppress colonic epithelial cell proliferation, induce apoptosis in colonic epithelium cells, and inhibit mutations of the colonic k ras gene.54 55
Our finding that dairy intake was not associated with lower risk of mortality was also consistent with mendelian randomisation studies using lactase persistence genotype rs4988235 as the instrumental variable. Two studies conducted in a Danish population of 97 811 participants found that the T allele of rs4988235 was not associated with lower risks of type 2 diabetes, total mortality, and mortality due to cardiovascular disease and cancer.56 57 Two studies conducted within the CHARGE consortium involving more than 171 000 participants showed that the T allele of rs4988235 was associated with slightly higher body mass index and systolic blood pressure, two important risk factors of mortality.58 59
We conducted substitution analyses to predict the effects of replacing dairy products with other foods on mortality. We found that replacing dairy with nuts or legumes, poultry, and whole grains was associated with lower mortality. However, replacing total dairy with red and processed meat was associated with increased mortality. These data suggest that dietary recommendations on dairy intake should consider healthier alternatives. Moreover, the health effects of dairy intake could depend on the background diet. For example, in populations with high carbohydrate intake and poor quality diets, dairy foods and other protein sources might add important nutritional value.
Strengths and limitations of study
Our study has several strengths. Firstly, dairy intake was measured repeatedly in all three cohorts, and we calculated cumulative averages for dairy intake to minimize the random measurement error caused by within-person variation. Furthermore, we stopped updating dairy intake on the diagnosis of potential intermediate conditions, which minimized the potential for reverse causation due to change of lifestyle factors because of chronic diseases. Secondly, our study included 217 755 participants with 51 438 deaths. The large sample size and number of deaths provided sufficient power for the analyses.
Several limitations also need to be considered. Firstly, the NHS, NHSII, and HPFS are predominantly white healthcare professionals, which could limit the generalizability of the findings to other nationalities and races. However, their occupations are a distinct advantage that allows us to collect high quality data using self reported questionnaires and enhance the internal validity of the study by reducing confounding. Secondly, given the observational study design, we could not directly establish a causal relation between dairy intake and mortality.
Conclusions and policy implications
In conclusion, our data did not support an inverse association between high amount of dairy consumption and risk of mortality. A dose-response analysis suggested that two servings per day of dairy consumption was associated with lowest cardiovascular mortality, but higher intake was associated with a slightly higher mortality, especially cancer mortality. Substitution analyses suggested that the health effects of dairy products could depend on the comparison foods used to replace dairy.
What is already known on this topic
The relation between dairy intake and various health outcomes including type 2 diabetes, cardiovascular disease, and cancer have been extensively examined, and most studies have shown no appreciable beneficial or adverse associations
However, evidence on the association between dairy intake and mortality from prospective cohort studies is more limited
What this paper adds
Total dairy intake was not associated with lower risk of total mortality
The health effects of dairy could depend on the comparison foods used to replace dairy
Slightly higher cancer mortality was non-significantly associated with dairy consumption, but warrants further investigation
Acknowledgments
We thank the participants and staff of the NHS, NHSII, and HPFS cohorts for their valuable contributions as well as the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nevada, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary tables
Contributors: MD, JL, LQ, CE, XZ, JEM, MS, JEC, KMR, PK, DC, WCW, and FBH designed the study and collected data. MD mainly conducted analyses and wrote the manuscript. FBH supervised the data analysis and reviewed and edited the manuscript. All authors contributed substantially to the interpretation of data and the drafting or critical revision of the manuscript for important intellectual content. The authors assume full responsibility for analyses and interpretation of these data. MD and FBH are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The NHS, NHSII, and HPFS are supported by grants (UM1 CA186107, P01 CA87969, R01 CA49449, R01 HL034594, R01 HL088521, UM1 CA176726, R01 CA67262, UM1 CA167552, R01 HL35464, R01 HL60712) from the National Institutes of Health. The funding sources did not participate in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review, or approval of the manuscript.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Institutes of Health for the submitted work; FBH reports support from grants HL60712, HL118264, and DK112940 from the National Institutes of Health, research support from the California Walnut Commission, honorariums for lectures from Metagenics and Standard Process, and honorariums from Diet Quality Photo Navigation, outside the submitted work; the remaining authors report no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and the Harvard T H Chan School of Public Health. The completion of the self administered questionnaire was considered to imply informed consent.
Data sharing: No additional data available.
The lead authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. Ralston RA, Lee JH, Truby H, Palermo CE, Walker KZ. A systematic review and meta-analysis of elevated blood pressure and consumption of dairy foods. J Hum Hypertens 2012;26:3-13. 10.1038/jhh.2011.3 [DOI] [PubMed] [Google Scholar]
- 2. Soedamah-Muthu SS, Verberne LD, Ding EL, Engberink MF, Geleijnse JM. Dairy consumption and incidence of hypertension: a dose-response meta-analysis of prospective cohort studies. Hypertension 2012;60:1131-7. 10.1161/HYPERTENSIONAHA.112.195206 [DOI] [PubMed] [Google Scholar]
- 3. Gao D, Ning N, Wang C, et al. Dairy products consumption and risk of type 2 diabetes: systematic review and dose-response meta-analysis. PLoS One 2013;8:e73965. 10.1371/journal.pone.0073965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qin LQ, Xu JY, Han SF, Zhang ZL, Zhao YY, Szeto IM. Dairy consumption and risk of cardiovascular disease: an updated meta-analysis of prospective cohort studies. Asia Pac J Clin Nutr 2015;24:90-100. [DOI] [PubMed] [Google Scholar]
- 5. Aune D, Lau R, Chan DS, et al. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol 2012;23:37-45. 10.1093/annonc/mdr269 [DOI] [PubMed] [Google Scholar]
- 6. Dong JY, Zhang L, He K, Qin LQ. Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast Cancer Res Treat 2011;127:23-31. 10.1007/s10549-011-1467-5 [DOI] [PubMed] [Google Scholar]
- 7. Aune D, Navarro Rosenblatt DA, Chan DS, et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 2015;101:87-117. 10.3945/ajcn.113.067157 [DOI] [PubMed] [Google Scholar]
- 8. Liu J, Tang W, Sang L, et al. Milk, yogurt, and lactose intake and ovarian cancer risk: a meta-analysis. Nutr Cancer 2015;67:68-72. 10.1080/01635581.2014.956247 [DOI] [PubMed] [Google Scholar]
- 9. Larsson SC, Orsini N, Wolk A. Milk, milk products and lactose intake and ovarian cancer risk: a meta-analysis of epidemiological studies. Int J Cancer 2006;118:431-41. 10.1002/ijc.21305 [DOI] [PubMed] [Google Scholar]
- 10. Tognon G, Nilsson LM, Shungin D, et al. Nonfermented milk and other dairy products: associations with all-cause mortality. Am J Clin Nutr 2017;105:1502-11. 10.3945/ajcn.116.140798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michaëlsson K, Wolk A, Langenskiöld S, et al. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ 2014;349:g6015. 10.1136/bmj.g6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldbohm RA, Chorus AM, Galindo Garre F, Schouten LJ, van den Brandt PA. Dairy consumption and 10-y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am J Clin Nutr 2011;93:615-27. 10.3945/ajcn.110.000430 [DOI] [PubMed] [Google Scholar]
- 13. Farvid MS, Malekshah AF, Pourshams A, et al. Dairy food intake and all-cause, cardiovascular disease, and cancer mortality: the Golestan cohort study. Am J Epidemiol 2017;185:697-711. 10.1093/aje/kww139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonthuis M, Hughes MC, Ibiebele TI, Green AC, van der Pols JC. Dairy consumption and patterns of mortality of Australian adults. Eur J Clin Nutr 2010;64:569-77. 10.1038/ejcn.2010.45 [DOI] [PubMed] [Google Scholar]
- 15. Ness AR, Smith GD, Hart C. Milk, coronary heart disease and mortality. J Epidemiol Community Health 2001;55:379-82. 10.1136/jech.55.6.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soedamah-Muthu SS, Masset G, Verberne L, Geleijnse JM, Brunner EJ. Consumption of dairy products and associations with incident diabetes, CHD and mortality in the Whitehall II study. Br J Nutr 2013;109:718-26. 10.1017/S0007114512001845 [DOI] [PubMed] [Google Scholar]
- 17. van Aerde MA, Soedamah-Muthu SS, Geleijnse JM, et al. Dairy intake in relation to cardiovascular disease mortality and all-cause mortality: the Hoorn Study. Eur J Nutr 2013;52:609-16. 10.1007/s00394-012-0363-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo J, Astrup A, Lovegrove JA, Gijsbers L, Givens DI, Soedamah-Muthu SS. Milk and dairy consumption and risk of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol 2017;32:269-87. 10.1007/s10654-017-0243-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Soedamah-Muthu SS, Ding EL, Al-Delaimy WK, et al. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: dose-response meta-analysis of prospective cohort studies. Am J Clin Nutr 2011;93:158-71. 10.3945/ajcn.2010.29866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med 2005;165:997-1003. 10.1001/archinte.165.9.997 [DOI] [PubMed] [Google Scholar]
- 21. Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006;29:650-6. 10.2337/diacare.29.03.06.dc05-1961 [DOI] [PubMed] [Google Scholar]
- 22. Malik VS, Sun Q, van Dam RM, et al. Adolescent dairy product consumption and risk of type 2 diabetes in middle-aged women. Am J Clin Nutr 2011;94:854-61. 10.3945/ajcn.110.009621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531-40. 10.1093/oxfordjournals.aje.a009849 [DOI] [PubMed] [Google Scholar]
- 24. Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790-6. 10.1016/0002-8223(93)91754-E [DOI] [PubMed] [Google Scholar]
- 25. Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114-26, discussion 1127-36. 10.1093/oxfordjournals.aje.a116211 [DOI] [PubMed] [Google Scholar]
- 26. Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858-67. 10.1093/ije/18.4.858 [DOI] [PubMed] [Google Scholar]
- 27. Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51-65. 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 28. McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr 2006;9(1A):152-7. 10.1079/PHN2005938 [DOI] [PubMed] [Google Scholar]
- 29. Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the national death index and Equifax nationwide death search. Am J Epidemiol 1994;140:1016-9. 10.1093/oxfordjournals.aje.a117191 [DOI] [PubMed] [Google Scholar]
- 30. Petitti DB. Approaches to heterogeneity in meta-analysis. Stat Med 2001;20:3625-33. 10.1002/sim.1091 [DOI] [PubMed] [Google Scholar]
- 31. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876-83. 10.1161/CIRCULATIONAHA.109.915165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bergholdt HKM, Nordestgaard BG, Varbo A, Ellervik C. Lactase persistence, milk intake, and mortality in the Danish general population: a Mendelian randomization study. Eur J Epidemiol 2018;33:171-81. 10.1007/s10654-017-0328-x [DOI] [PubMed] [Google Scholar]
- 33. Praagman J, Dalmeijer GW, van der Schouw YT, et al. The relationship between fermented food intake and mortality risk in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Br J Nutr 2015;113:498-506. 10.1017/S0007114514003766 [DOI] [PubMed] [Google Scholar]
- 34. Huth PJ, Park KM. Influence of dairy product and milk fat consumption on cardiovascular disease risk: a review of the evidence. Adv Nutr 2012;3:266-85. 10.3945/an.112.002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang C, Yatsuya H, Tamakoshi K, Iso H, Tamakoshi A. Milk drinking and mortality: findings from the Japan collaborative cohort study. J Epidemiol 2015;25:66-73. 10.2188/jea.JE20140081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dehghan M, Mente A, Rangarajan S, et al. Prospective Urban Rural Epidemiology (PURE) study investigators Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet 2018;392:2288-97. 10.1016/S0140-6736(18)31812-9 [DOI] [PubMed] [Google Scholar]
- 37. Wang Y, Li S. Worldwide trends in dairy production and consumption and calcium intake: is promoting consumption of dairy products a sustainable solution for inadequate calcium intake? Food Nutr Bull 2008;29:172-85. 10.1177/156482650802900303 [DOI] [PubMed] [Google Scholar]
- 38. Hjartåker A, Lagiou A, Slimani N, et al. Consumption of dairy products in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort: data from 35 955 24-hour dietary recalls in 10 European countries. Public Health Nutr 2002;5(6B):1259-71. 10.1079/PHN2002403 [DOI] [PubMed] [Google Scholar]
- 39. Ma J, Giovannucci E, Pollak M, et al. Milk intake, circulating levels of insulin-like growth factor-I, and risk of colorectal cancer in men. J Natl Cancer Inst 2001;93:1330-6. 10.1093/jnci/93.17.1330 [DOI] [PubMed] [Google Scholar]
- 40. Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst 2004;96:1015-22. 10.1093/jnci/djh185 [DOI] [PubMed] [Google Scholar]
- 41. Qin LQ, He K, Xu JY. Milk consumption and circulating insulin-like growth factor-I level: a systematic literature review. Int J Food Sci Nutr 2009;60(Suppl 7):330-40. 10.1080/09637480903150114 [DOI] [PubMed] [Google Scholar]
- 42. Harrison S, Lennon R, Holly J, et al. Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis. Cancer Causes Control 2017;28:497-528. 10.1007/s10552-017-0883-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shi R, Yu H, McLarty J, Glass J. IGF-I and breast cancer: a meta-analysis. Int J Cancer 2004;111:418-23. 10.1002/ijc.20233 [DOI] [PubMed] [Google Scholar]
- 44. Genkinger JM, Hunter DJ, Spiegelman D, et al. Dairy products and ovarian cancer: a pooled analysis of 12 cohort studies. Cancer Epidemiol Biomarkers Prev 2006;15:364-72. 10.1158/1055-9965.EPI-05-0484 [DOI] [PubMed] [Google Scholar]
- 45. Sacks FM, Svetkey LP, Vollmer WM, et al. DASH-Sodium Collaborative Research Group Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344:3-10. 10.1056/NEJM200101043440101 [DOI] [PubMed] [Google Scholar]
- 46. Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491-9. 10.1056/NEJM199711203372102 [DOI] [PubMed] [Google Scholar]
- 47. Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ 2018;361:k2139. 10.1136/bmj.k2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen M, Li Y, Sun Q, et al. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am J Clin Nutr 2016;104:1209-17. 10.3945/ajcn.116.134460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cho E, Smith-Warner SA, Spiegelman D, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst 2004;96:1015-22. 10.1093/jnci/djh185 [DOI] [PubMed] [Google Scholar]
- 50.World Cancer Research Fund/American Institute for Cancer Research. Second expert report: food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC, 2007. https://www.wcrf.org/dietandcancer
- 51. Murphy N, Norat T, Ferrari P, et al. Consumption of dairy products and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS One 2013;8:e72715. 10.1371/journal.pone.0072715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med 2009;169:391-401. 10.1001/archinternmed.2008.578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther 2010;32:789-803. 10.1016/j.clinthera.2010.04.024 [DOI] [PubMed] [Google Scholar]
- 54. Holt PR, Atillasoy EO, Gilman J, et al. Modulation of abnormal colonic epithelial cell proliferation and differentiation by low-fat dairy foods: a randomized controlled trial. JAMA 1998;280:1074-9. 10.1001/jama.280.12.1074 [DOI] [PubMed] [Google Scholar]
- 55. Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci 2001;952:73-87. 10.1111/j.1749-6632.2001.tb02729.x [DOI] [PubMed] [Google Scholar]
- 56. Bergholdt HKM, Nordestgaard BG, Varbo A, Ellervik C. Lactase persistence, milk intake, and mortality in the Danish general population: a Mendelian randomization study. Eur J Epidemiol 2018;33:171-81. 10.1007/s10654-017-0328-x [DOI] [PubMed] [Google Scholar]
- 57. Bergholdt HK, Nordestgaard BG, Ellervik C. Milk intake is not associated with low risk of diabetes or overweight-obesity: a Mendelian randomization study in 97,811 Danish individuals. Am J Clin Nutr 2015;102:487-96. 10.3945/ajcn.114.105049 [DOI] [PubMed] [Google Scholar]
- 58. Mendelian Randomization of Dairy Consumption Working Group Dairy consumption and body mass index among adults: mendelian randomization analysis of 184802 individuals from 25 studies. Clin Chem 2018;64:183-91. 10.1373/clinchem.2017.280701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L, CHARGE Consortium Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study [correction in: BMJ 2017;358:j3550]. BMJ 2017;356:j1000. 10.1136/bmj.j1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary tables