Version Changes
Revised. Amendments from Version 2
In response to reviewer suggestions, we added another network-related package to Table 2 and swapped in "TRUE" for "T" in a code snippet.
Abstract
RCy3 is an R package in Bioconductor that communicates with Cytoscape via its REST API, providing access to the full feature set of Cytoscape from within the R programming environment. RCy3 has been redesigned to streamline its usage and future development as part of a broader Cytoscape Automation effort. Over 100 new functions have been added, including dozens of helper functions specifically for intuitive data overlay operations. Over 40 Cytoscape apps have implemented automation support so far, making hundreds of additional operations accessible via RCy3. Two-way conversion with networks from \textit{igraph} and \textit{graph} ensures interoperability with existing network biology workflows and dozens of other Bioconductor packages. These capabilities are demonstrated in a series of use cases involving public databases, enrichment analysis pipelines, shortest path algorithms and more. With RCy3, bioinformaticians will be able to quickly deliver reproducible network biology workflows as integrations of Cytoscape functions, complex custom analyses and other R packages.
Keywords: Bioconductor, Cytoscape, Automation, Scripting, Network biology
Introduction
In the domain of biology, network models serve as useful representations of interactions, whether social, neural or molecular. Since 2003, Cytoscape has provided a free, open-source software platform for network analysis and visualization that has been widely adopted in biological and biomedical research fields 1. The Cytoscape platform supports community-developed extensions, called apps, that can access third-party databases, offer new layouts, add analytical algorithms, support additional data types, and much more 2, 3.
In 2011, the CytoscapeRPC app was created to enable R-based workflows to exercise Cytoscape v2 functionality via functions in the corresponding RCytoscape R package over XML-RPC communications protocols 4. In 2015, the CyREST app was created to enable R-based workflows to exercise Cytoscape v3 functionality. This was achieved by the first version of the RCy3 R package, which re-implemented much of RCytoscape’s organization, data structures and syntax over REST communications protocols 3, 5.
Here, we describe version 2.0 of the RCy3 package, which is better aligned with Cytoscape’s CyREST API. We have rewritten every function, deprecated 43 functions and added over 100 new functions. This work provides a more intuitive and productive experience for Cytoscape users learning about the RCy3 package, and it positions RCy3 to take advantage of future Cytoscape Automation 6 development and evolution. The goal of this paper is to describe the implementation and operation of the updated RCy3 package and to provide detailed use cases relevant to network biology applications in common and advanced bioinformatics workflows.
Methods
Design and Implementation
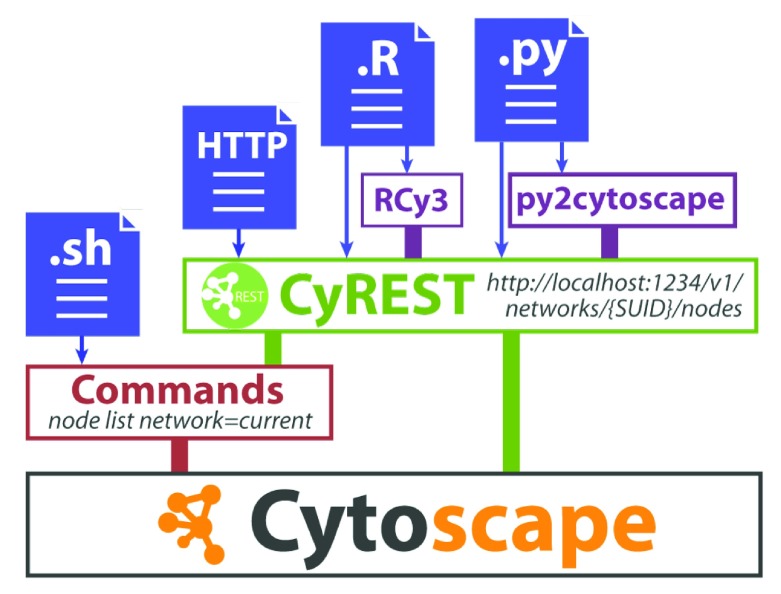
RCy3 is a component of Cytoscape Automation. At the core of Cytoscape Automation is CyREST, which implements an interface to the Cytoscape Java application via the REST protocol 6. A collection of GET, POST, PUT and DELETE operations practically cover the complete feature set of the Cytoscape desktop software. Additional features, including those provided by user-installed Cytoscape apps, are covered by a separate command-line interface called Commands ( Figure 1). For version 2.0, we redesigned the RCy3 package to parallel CyREST and Commands APIs to standardize the syntax and organization of its functions and streamline its future development. RCy3 functions are grouped into categories to aid the parallel development with Cytoscape Automation APIs and to facilitate navigation and comprehension of the overall package ( Table 1).
Figure 1. The Cytoscape Automation stack.
The Java desktop version of Cytoscape (bottom) supports Commands (red) and CyREST (green) interfaces for scripting and automation either directly (blue) or via R and Python harmonization libraries (purple).
Table 1. Organization of RCy3 functions.
The categories correspond to separate R files in the package. Brief descriptions and example functions are listed for each category.
| Category | Description | Examples |
|---|---|---|
| Apps | Inspecting and managing apps for Cytoscape | installApp
disableApp getInstalledApps |
| Collections | Getting information about network collections | getCollectionList
getCollectionNetworks |
| Commands | Constructing any arbitrary CyREST API or Commands API method via standard GET, PUT,
POST and DELETE protocols |
cyrestGET
commandsPOST cyrestAPI |
| CyNDEx | Communicating with NDEx from within Cytoscape | importNetworkFromNDEx
exportNetworkToNDEx |
| Cytoscape System | Checking Cytoscape System information, including versions and memory usage | cytoscapePing
cytoscapeVersionInfo |
| Filters | Selecting nodes and edges based on filter criteria | createDegreeFilter
createColumnFilter |
| Groups | Working with groups in Cytoscape | createGroup
collapseGroup |
| Layouts | Performing layouts in addition to getting and setting layout properties | layoutNetwork
getLayoutNames |
| Networks | Creating and managing networks and retrieving information on networks, nodes and edges | createNetworkFrom*
create*FromNetwork getNetworkSuid exportNetwork getAllNodes getEdgeCount getFirstNeighbors |
| Network Selection | Manipulating selection of nodes and edges in networks | selectNodes
invertNodeSelection selectFirstNeighbors |
| Network Views | Performing view operations in addition to getting and setting view properties | getCurrentView
fitContent exportImage toggleGraphicsDetails |
| Session | Managing Cytoscape sessions, including save, open and close | openSession
saveSession closeSession |
| Style Bypasses | Setting and clearing bypass values for visual properties | setNodeColorBypass
setEdgeLineStyleBypass hideNodes |
| Style Defaults | Getting and setting default values for visual properties | setNodeShapeDefault
setEdgeLineWidthDefault |
| Style Dependencies | Getting and setting style dependencies | lockNodeDimensions |
| Style Mappings | Defining mappings between table column values and visual properties | mapVisualProperty
updateStyleMapping setNodeSizeMapping setEdgeColorMapping |
| Style Values | Retrieving current values for visual properties | getNodeWidth
getEdgeColor getNetworkZoom |
| Styles | Managing styles and retrieving general lists of properties relevant to multiple style modes | createVisualStyle
exportVisualStyles getArrowShapes |
| Tables | Managing table columns and table column functions, like map and rename, as well as
loading and extracting table data in Cytoscape |
getTableColumns
renameTableColumn loadTableData mapTableColumn |
| Tools | Performing actions found in the Tools Menu in Cytoscape | cybrowserDialog
diffusionBasic |
| User Interface | Controling the panels in the Cytoscape user interface | hidePanel
floatPanel |
The basics
All RCy3 functions are ultimately implemented as calls to a small set of utility functions that execute the CyREST or Commands REST protocol (e.g., cyrestGET and commandsPOST). The internals of these functions handle the composition of operations and parameters to be sent via httr functions to CyREST, as well as the processing of results from JSON to appropriate R objects using RJSONIO.
In most RCy3 functions there is an optional argument for base.url. This is the URL used by RCy3 to connect to the Cytoscape desktop application via CyREST, and it defaults to "http://localhost:1234/v1". The default CyREST port is 1234, and it can be changed in Cytoscape through Edit/Preferences/Properties or by command-line (see CyREST setup guide). If you change the CyREST port, you should reflect the change in the base.url argument per function call or change each function’s default value using the default package.
The second most common argument in RCy3 functions is network. If left as NULL (default), the currently selected network in the Cytoscape application is referenced. Network name or SUID (session unique identifier) can thus be explicitly specified or inferred from the current state of the application. The current network can also be controlled and retrieved by setCurrentNetwork and getCurrentNetwork. Given a base.url and network (when needed), the majority of RCy3 functions simply validate parameters and construct arguments in order to call one of the cyrest* or commands* functions.
The commandsRun function is a special RCy3 function that allows users to directly issue commands via Cytoscape’s command-line syntax (e.g., "node list network=current"), including commands implemented by Cytoscape app developers (see Use cases). This single function can perform hundreds of operations made available by both Cytoscape and automation-enabled apps 6. Over 40 of these RCy3-supported apps are currently registered in the Cytoscape App Store 2. The cyrestAPI and commandsAPI open interactive Swagger documentation for the CyREST and Commands programmatic interfaces. Cytoscape Automation can be performed via these Swagger web pages. The same operations and parameters are supported by the cyrest* and commands* functions in RCy3. Command-line syntax can also be run from the Automation panel in Cytoscape, at manual.cytoscape.org/en/stable/Command_Tool.html.
Generic and specific
The primary goal of RCy3 is to provide wrappers for every feature made available by CyREST and Commands. However, we also have a secondary goal of providing useful and intuitive functions for common workflows in R. So, in addition to the generic functions implemented to parallel the CyREST and Commands APIs, we have also implemented sets of specific helper functions.
As an example, consider the common Cytoscape operation of mapping network data values to visual style properties. CyREST has a POST endpoint for /styles/{style name}/mappings that takes a JSON data structure defining the mapping. We implemented updateStyleMapping which takes a style.name and mapping arguments and sends them out via cyrestPOST. We also implemented mapVisualProperty to help construct the mapping argument. With these generic functions one can perform any of the hundreds of visual style mappings supported by Cytoscape, including new ones added in the future. However, these functions are not simple to use, requiring knowledge of specific property names, like "NODE_FILL_COLOR", and mapping data structures. To simplify usage for common situations, we therefore also implemented specific functions for over a dozen of the most commonly used mappings (e.g., setNodeColorMapping). With autocomplete in tools like RStudio, after just typing setNode... a script author is presented with a series of intuitively named functions with obvious arguments.
Networks in R
Networks are a popular visualization option in R often implemented as graph models by igraph and Biocondutor’s graph (i.e., graphNEL). RCy3 can create networks in Cytoscape from either igraph, graphNEL or dataframe objects ( createNetworkFrom*). Likewise, igraph and graphNEL objects can be created from networks ( create*FromNetwork), and dataframes from node and edge tables in Cytoscape ( getTableColumns).
In the case of createNetworkFromDataFrames, two dataframes are accepted as arguments, one for nodes and one for edges. The nodes dataframe must include a column named "id", and the edges dataframe must include "source" and "target" columns. Additional columns are imported as node and edge attributes into Cytoscape. The function can also work with just one dataframe. If a dataframe of only edges is passed to createNetworkFromDataFrames, then a connected network will be created with all of the nodes. If a dataframe of only nodes is passed, then a network with no connections, only nodes, will be created.
RCy3 can also import network file formats supported by Cytoscape natively (e.g., SIF, xGMML and CX 7) and via user-installed apps (e.g., GPML 8 and adjacency matrices). With these functions RCy3 can interoperate with any other Bioconductor packages that deal with networks in a standardized manner, providing advanced network visualization options and advanced network analytics from the Cytoscape ecosystem (see Table 2).
Table 2. Top 31 network-related Bioconductor packages ordered by rank (as of Sept 2019) and their network models relevant to RCy3.
RCy3 can provide and consume network models to and from these packages (checkmarks). Asterisks indicate where a Cytoscape app is required (* aMatReader, ** WikiPathways).
| Package | Description | Network model | RCy3 |
|---|---|---|---|
| graph | Essential graph data structures | graphNEL | ✔ |
| RBGL | Graph algorithms contained in the BOOST library | graphNEL | ✔ |
| Rgraphviz | Plotting with graphviz library | graphNEL | ✔ |
| pathview | Data integration and visualization on pathways | graphNEL | ✔ |
| KEGGgraph | Interface between KEGG pathways and graphs | graphNEL | ✔ |
| Gostats | Tools for interacting with Gene Ontology | graphNEL | ✔ |
| TCGAbiolinks | Integrative analysis with GDC data | igraph | ✔ |
| graphite | Graph objects from pathway databases | graphNEL | ✔ |
| minet | Infers mutual information networks | graphNEL | ✔ |
| SPIA | Signaling pathway impact analysis | graphNEL | ✔ |
| RDAVIDWebService | Retrieves data from DAVID | graphNEL | ✔ |
| STRINGdb | Retrieves interaction networks from STRING | igraph | ✔ |
| GENIE3 | Gene regulatory network inference | adjacency matrix | ✔ * |
| EnrichmentBrowser | Set and network-based enrichment analysis | graphNEL | ✔ |
| BioNet | Integrated network analysis and module detection | igraph, graphNEL | ✔ |
| GDCRNATools | Integrative analysis of lncRNA, mRNA and miRNA | dataframes | ✔ |
| CEMiTool | Analysis of coexpression gene modules | adjacency matrix | ✔ * |
| Linnorm | Linear model and normality based transformation | igraph | ✔ |
| RedeR | Interactive visualization of nested networks | igraph | ✔ |
| qpgraph | Estimate gene and eQTL networks | graphNEL | ✔ |
| RTN | Reconstruction of transcriptional networks | igraph | ✔ |
| rWikiPathways | Access WikiPathways web services | GPML | ✔ ** |
| hypergraph | Representing and manipulating hypergraphs | graphNEL | ✔ |
| apComplex | Bipartite graphs of complexes from AP-MS data | graphNEL | ✔ |
| cellTree | scRNA-seq data as a hierarchical tree structure | igraph | ✔ |
| FGNet | Functional gene networks from enrichment analyses | igraph | ✔ |
| SpidermiR | Integrative network analysis with miRNA data | igraph | ✔ |
| paxtoolsr | Access to BioPAX models in Pathway Commons | igraph, SIF | ✔ |
| netbiov | Visualization of large biological networks | igraph | ✔ |
| rsbml | Link to libsbml for SBML parsing | graphNEL | ✔ |
| ndexr | Interface to NDEx servers | igraph, CX | ✔ |
Operation
In order to work with RCy3 you must have Cytoscape v3.7 or later installed and running. Cytoscape can be installed from cytoscape.org. The RCy3 package can be installed from Bioconductor:
if(!requireNamespace("BiocManager", quietly=TRUE)) install.packages("BiocManager") BiocManager::install("RCy3") library(RCy3)
Launch Cytoscape and keep it running whenever using RCy3. Confirm that you have everything installed and that RCy3 is communicating with Cytoscape via CyREST:
cytoscapePing() #[1] "You are connected to Cytoscape!"
As with any R package, one can access the documentation and browse over a dozen vignettes included in the RCy3 package:
help(package=RCy3) browseVignettes("RCy3")
Use cases
The following sections demonstrate a variety of common and advanced network biology use cases as runnable R code snippets. The code for these use cases is also available as an online Rmd notebook and Rmd file in the Cytoscape Automation repository (see Data availability). The first set focuses on fundamental Cytoscape operations that are common to most use cases:
Loading networks (from R objects, Cytoscape files and public databases)
Visualizing network data
Filtering by node degree or data
Saving and exporting networks
Additionally, there are examples that demonstrate analytical workflows, relying not only on Cytoscape, but also on Cytoscape apps and other R packages:
Building maps of enrichment analysis results using EnrichmentMap and AutoAnnotate
Visualizing integrated network analysis using BioNet
Performing advanced graph analytics using RBGL
Loading Networks. Networks come in all shapes and sizes, in multiple formats from multiple sources. The following code snippets demonstrate just a few of the myriad ways to load networks into Cytoscape using RCy3.
From R objects. . .
# From graph objects (graphNEL) g<-makeSimpleGraph() createNetworkFromGraph(g) ## And round-trip back from Cytoscape to graph g2<-createGraphFromNetwork() # From igraph objects library(igraph) ig<-igraph::make_graph("Zachary") createNetworkFromIgraph(ig) ## And round-trip back from Cytoscape to igraph ig2<-createIgraphFromNetwork() ## Note that the Cytoscape model infers directionality # From dataframes nodes<-data.frame(id=c( "node 0","node 1","node 2","node 3") , group=c( "A","A","B","B"),#categorical strings score=as.integer( c( 20,10,15,5)),#integers stringsAsFactors=FALSE) edges <-data.frame( source=c( "node 0","node 0","node 0","node 2"), target=c("node 1","node 2","node 3","node 3"), interaction=c("inhibits","interacts", "activates","interacts"),#optional weight=c(5.1,3.0,5.2,9.9),#numerics stringsAsFactors=FALSE) createNetworkFromDataFrames(nodes, edges)
From Cytoscape-supported file formats. . .
# From Cytoscape session files ## Will erase and replace all data from current session! openSession()# default file = galFiltered.cys # From local network files importNetworkFromFile()# default file = galFiltered.sif ## Supported file formats: SIF, GML, xGMML, graphML, CX, plus # From NDEx (https://ndexbio.org), the network database importNetworkFromNDEx("5be85817-1e5f-11e8-b939-0ac135e8bacf") ## Account information or accessKey are required arguments only ## when accessing private content
From public databases via Cytoscape apps. . .
# From STRING (https://string-db.org), starting with a list of genes/proteins installApp("stringApp")# http://apps.cytoscape.org/apps/stringapp gene.list<-c("T53" ,"AKT1" ,"CDKN1A") gene.str<-paste(gene.list, collapse=",") string.cmd<-paste("string protein query cutoff=0.99 limit=40 query", gene.str, sep="=") commandsRun(string.cmd) # From WikiPathways (https://wikipathways.org), starting with a keyword library(rWikiPathways)# install from Bioconductor installApp("WikiPathways")# http://apps.cytoscape.org/apps/wikipathways keyword<-"glioblastoma" gbm.pathways<-findPathwaysByText(keyword) gbm.wpid<-gbm.pathways[[1]]$id# let’s just take the first one wikipathways.cmd<-paste( "wikipathways import-as-pathway id", gbm.wpid, sep="=") commandsRun(wikipathways.cmd)
Visualizing data on networks. Cytoscape excels at generating publication-quality network visualization with data overlays. This vignette demonstrates just one of the hundreds of visual style mapping options using RCy3.
# Load sample network closeSession(FALSE)# clears all session data wihtout saving importNetworkFromFile()# default file = galFiltered.sif # Load sample data csv<-system.file("extdata","galExpData.csv", package="RCy3") data<-read.csv(csv, stringsAsFactors=FALSE) loadTableData(data,data.key.column="name") # Prepare data-mapping points gal80Rexp.min<-min(data$gal80Rexp, na.rm=TRUE) gal80Rexp.max<-max(data$gal80Rexp, na.rm=TRUE) ## For a balanced color gradient, pick the largest absolute value gal80Rexp.max.abs<-max(abs(gal80Rexp.min),abs(gal80Rexp.max)) # Set node color gradient from blue to white to red setNodeColorMapping('gal80Rexp',c(-gal80Rexp.max.abs,0, gal80Rexp.max.abs), c('#5577FF','#FFFFFF','#FF7755'), default.color='#999999')
Filtering networks by degree and by data. Network topology and associated node or edge data can be used to make selections in Cytoscape that enable filtering and subnetworking. The filters are added to the Select tab in the Control Panel of Cytoscape’s GUI and saved in session files.
# Load demo Cytoscape session file openSession()# default file = galFiltered.cys net.suid<-getNetworkSuid()# get SUID for future reference # Filter for neighbors of high degree nodes createDegreeFilter(filter.name = "degree filter", criterion=c(0,9), predicate="IS_NOT_BETWEEN") selectFirstNeighbors()# expand selection to first neighbors createSubnetwork(subnetwork.name="first neighbors of high degree nodes") # Filter for high edge betweenness createColumnFilter(filter.name="edge betweenness", type="edges", column="EdgeBetweenness", 4000, "GREATER_THAN", network=net.suid) createSubnetwork(subnetwork.name="high edge betweenness")
Saving and exporting networks. There are local and cloud-hosted options for saving and sharing network models and images. The Cytoscape session file (CYS) includes all networks, collections, tables and styles. It retains every aspect of your session, including the size of the application window. Network and image exports include only the currently active network. Export to NDEx requires account information you can obtain from ndexbio.org. Files are saved to the current working directory by default, unless a full path is provided.
# Saving sessions saveSession("MySession")#.cys ## Leave filename blank to update previously saved session file # Exporting images and networks exportNetwork()#.sif ## Optionally specify filename, default is network name ## Optionally specify type: SIF(default), CX, cyjs, graphML, NNF, SIF, xGMML exportImage()#.png ## Optionally specify filename, default is network name ## Optionally specify type: PNG (default), JPEG, PDF, PostScript, SVG # Exporting to NDEx, a.k.a. “Dropbox” for networks exportNetworkToNDEx(username, password,TRUE) ## Account information (username and password) is required to upload ## Use updateNetworkInNDEx if the network has previously been uploaded
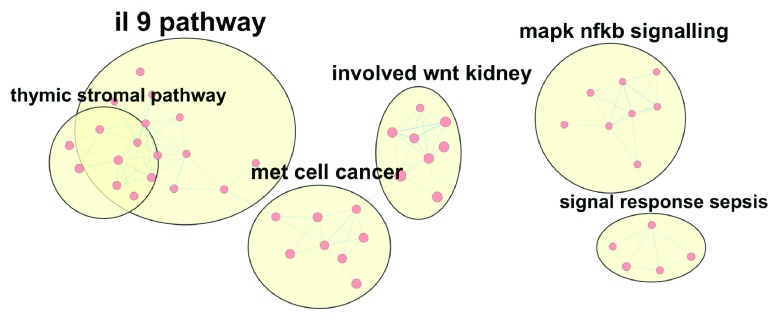
Building maps of enrichment analysis results. This workflow illustrates how to plot an annotated map of enrichment results using the EnrichmentMap Pipeline Collection of apps in Cytoscape 9. An enrichment map is a network visualization of related genesets in which nodes are gene sets (or pathways) and edge weight indicates the overlap in member genes 10. Following the construction of the enrichment map, AutoAnnotate clusters redundant gene sets and uses WordCloud 11 to label the resulting cluster ( Figure 2). The code uses the Commands interface to invoke EnrichmentMap and AutoAnnotate apps. After installing apps, run commandsAPI() to open the live Swagger documentation to browse and execute command-line syntax.
installApp("EnrichmentMap Pipeline Collection")# installs 4 apps # Download sample gmt file of human pathways gmt.file<-"rcy3_enrichmentmap.gmt" download.file(file.path("http://download.baderlab.org/EM_Genesets", "September_01_2019/Human/symbol/Pathways", "Human_WikiPathways_September_01_2019_symbol.gmt"), gmt.file) # Run EnrichmentMap build command em_command<-paste('enrichmentmap build analysisType="generic"', "gmtFile=",paste(getwd(), gmt.file, sep="/"), "pvalue=",1, "qvalue=",1, "similaritycutoff=",0.25, "coefficients=","JACCARD") print(em_command) commandsGET(em_command) # Run the AutoAnnotate command aa_command<-paste("autoannotate annotate-clusterBoosted", "clusterAlgorithm=MCL", "labelColumn=EnrichmentMap::GS_DESCR", "maxWords=3") print(aa_command) commandsGET(aa_command) # Annotate a subnetwork createSubnetwork(c(1:4),"__mclCluster") commandsGET(aa_command)
Figure 2. Annotated enrichment map of pathways.
A few of the largest clusters of pathways from WikiPathways are displayed as a network with WordCloud-based labels annotating groups (yellow areas). The size of the labels correspond to the size of the groups.
Visualizing integrated network analysis using BioNet. The BioNet package implements analytical methods to perform integrated network analysis, for example, of gene expression data and clinical survival data in the context of protein-protein interaction networks. Partnered with RCy3, the analytical results from BioNet can be visualized in Cytoscape with vastly more options for customization. Starting with the "Quick Start" tutorial from BioNet, we pass the results directly to Cytoscape for visualization:
library(BioNet)# install from Bioconductor library(DLBCL)# install from Bioconductor data(dataLym) data(interactome) ## The following steps are from BioNet's Quick Start tutorial: pvals<-cbind(t=dataLym$t.pval, s=dataLym$s.pval) rownames(pvals)<-dataLym$label pval<-aggrPvals(pvals, order=2, plot=FALSE) subnet<-subNetwork(dataLym$label, interactome) subnet<-rmSelfLoops(subnet) fb<-fitBumModel(pval, plot=FALSE) scores<-scoreNodes(subnet, fb, fdr=0.001) module<-runFastHeinz(subnet, scores) logFC<-dataLym$diff names(logFC)<-dataLym$label plotModule(module, scores=scores, diff.expr=logFC) # Using RCy3 we can generate a custom visualization of the same output ## Create network from graphNEL object and load data calculated above createNetworkFromGraph(module,"module","BioNet") loadTableData(as.data.frame(scores)) loadTableData(as.data.frame(logFC)) ## Set styles setNodeLabelMapping("geneSymbol") setNodeFontSizeDefault(18) setNodeBorderWidthDefault(3.0) logFC.max.abs<-max(abs(min(logFC)),abs(max(logFC))) setNodeColorMapping('logFC',c(-logFC.max.abs,0, logFC.max.abs), c('#5577FF','#FFFFFF','#FF7755'), default.color='#999999') createColumnFilter("Positive scores","scores",c(0,max(scores)),"BETWEEN") setNodeShapeBypass(getSelectedNodes(),"ELLIPSE")
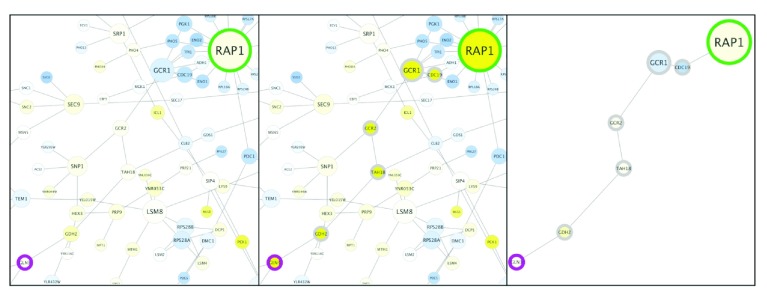
Performing advanced graph analytics using RBGL. As an interface to the BOOST library, the RBGL Bioconductor package offers an impressive array of analytical functions for graphs. Here we will start with a network in Cytoscape, load it into R as a graph object, perform shortest path calculation using RBGL and then visualize the results back in Cytoscape ( Figure 3).
library(RBGL)# install from Bioconductor # Convert a sample Cytoscape network to a graph object openSession() g<-createGraphFromNetwork() # Identify start and finish nodes (styling is optional) start<-"YNL216W" finish<-"YER040W" setNodeBorderWidthBypass(c(start,finish),20) setNodeBorderColorBypass(start,"#00CC33") setNodeBorderColorBypass(finish,"#CC00CC") # Use RBGL to perform shortest path calculation shortest<-sp.between(g, start, finish) shortest$`YNL216W:YER040W`$length #[1] 6 shortest.path<-shortest$`YNL216W:YER040W`$path_detail # Visualize results in Cytoscape selectNodes(shortest.path,"name") setNodeBorderWidthBypass(shortest.path,20) createSubnetwork()
Figure 3. Visualizing shortest path results.
In Cytoscape, the start and finish nodes were colored green and magenta, respectively (left panel). The shortest path calculated by RBGL was then selected and nodes along the path were highlighted with thick borders (middle panel). Finally, a subnetwork was created from the selected path (right panel). Note that while common gene names are displayed as node labels, official yeast identifiers are the actual node names that are referenced in the script.
Discussion
Every operation exposed by Cytoscape’s REST API has now been implemented as a function in RCy3 2.0. Furthermore, RCy3 provides dozens of higher-level helper functions in support of common usage, such as setNodeColorMapping, to make script writing more intuitive and efficient. The issue trackers for CyREST and RCy3 are linked for functions pending implementation, such as mergeNetworks, as well as for bug fixes. Thus, RCy3 is expected to keep pace with future development of Cytoscape Automation. More broadly, RCy3 is an integral part of the Cytoscape ecosystem, which includes the network repository NDEx 7, Cytoscape apps and services, and web components like cytoscape.js 12. The ecosystem has also defined interfaces and standard formats to interoperate with interaction databases and annotation services. Adopting RCy3 for network analysis will establish a connection to the Cytoscape ecosystem and enable Cytoscape Automation workflows 6. Furthermore, RCy3 enables reproducible scripting of integrated network biology workflows that otherwise rely on transient interactions with Cytoscape’s GUI. As the sharing and publishing of analysis scripts and workflows become more routine (if not mandated), software tools designed to work in an open and accessible ecosystem are becoming essential.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
RCy3 vignettes: https://bioconductor.org/packages/release/bioc/html/RCy3.html
RCy3 Rmd notebooks: https://cytoscape.org/cytoscape-automation/for-scripters/R/notebooks/
RCy3 workshop presentations: http://tutorials.cytoscape.org/#automation
Video demonstrations of RCy3: https://www.youtube.com/playlist
Cytoscape Automation training: http://automation.cytoscape.org/
Cytoscape Automation code repository: https://github.com/cytoscape/cytoscape-automation
Software availability
RCy3 is available from Bioconductor: https://bioconductor.org/packages/release/bioc/html/RCy3.html
Source code available from: https://github.com/cytoscape/RCy3
Archived source code at time of publication: https://doi.org/10.5281/zenodo.3473421
Issue tracker: https://github.com/cytoscape/RCy3/issues
License: MIT License.
Acknowledgments
We would like to acknowledge contributions by other developers on the original implementation of RCy3 by Paul Shannon, Tanja Muetze and Georgi Kolishkovski. We also greatly appreciate the input from Mark Grimes (testing), Martin Morgan (Bioconductor), and the excellent work on CyREST and Cytoscape Commands by David Otasek and John “Scooter” Morris.
Funding Statement
AP, BD, RI and SP were supported by NIGMS P41GM103504 for the National Resource for Network Biology.JAG was supported by Google Summer of Code.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; peer review: 3 approved]
References
- 1. Shannon P, Markiel A, Ozier O, et al. : Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lotia S, Montojo J, Dong Y, et al. : Cytoscape app store. Bioinformatics. 2013;29(10):1350–1351. 10.1093/bioinformatics/btt138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Demchak B, Otasek D, Pico AR, et al. : The Cytoscape Automation app article collection [version 1; peer review: not peer reviewed]. F1000Res. 2018;7:800. 10.12688/f1000research.15355.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shannon PT, Grimes M, Kutlu B, et al. : RCytoscape: tools for exploratory network analysis. BMC Bioinformatics. 2013;14:217. 10.1186/1471-2105-14-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ono K, Muetze T, Kolishovski G, et al. : CyREST: Turbocharging Cytoscape Access for External Tools via a RESTful API [version 1; peer review: 2 approved]. F1000Res. 2015;4:478. 10.12688/f1000research.6767.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Otasek D, Morris JH, Bouças J, et al. : Cytoscape Automation: empowering workflow-based network analysis. Genome Biol. 2019;20(1):185. 10.1186/s13059-019-1758-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pratt D, Chen J, Pillich R, et al. : NDEx 2.0: A Clearinghouse for Research on Cancer Pathways. Cancer Res. 2017;77(21):e58–e61. 10.1158/0008-5472.CAN-17-0606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Iersel MP, Kelder T, Pico AR, et al. : Presenting and exploring biological pathways with PathVisio. BMC Bioinformatics. 2008;9:399. 10.1186/1471-2105-9-399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reimand J, Isserlin R, Voisin V, et al. : Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat Protoc. 2019;14(2):482–517. 10.1038/s41596-018-0103-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Merico D, Isserlin R, Stueker O, et al. : Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5(11):e13984. 10.1371/journal.pone.0013984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oesper L, Merico D, Isserlin R, et al. : WordCloud: a Cytoscape plugin to create a visual semantic summary of networks. Source Code Biol Med. 2011;6:7. 10.1186/1751-0473-6-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franz M, Lopes CT, Huck G, et al. : Cytoscape.js: a graph theory library for visualisation and analysis. Bioinformatics. 2016;32(2):309–311. 10.1093/bioinformatics/btv557 [DOI] [PMC free article] [PubMed] [Google Scholar]