Highlights
-
•
Stability of recorded neural signals is crucial for the clinical viability of implantable brain-computer interfaces (BCIs).
-
•
A fully implantable BCI offers a long-term robust and durable control signal in a person with amyotrophic lateral sclerosis.
-
•
Increasing home use of the electrocorticography (ECoG)-BCI demonstrates user adoption of the BCI for communication.
Keywords: Brain-computer interface, Electrocorticography, Stability, Amyotrophic lateral sclerosis, Implant, Communication
Abstract
Objective
We investigated the long-term functional stability and home use of a fully implanted electrocorticography (ECoG)-based brain-computer interface (BCI) for communication by an individual with late-stage Amyotrophic Lateral Sclerosis (ALS).
Methods
Data recorded from the cortical surface of the motor and prefrontal cortex with an implanted brain-computer interface device was evaluated for 36 months after implantation of the system in an individual with late-stage ALS. In addition, electrode impedance and BCI control accuracy were assessed. Key measures included frequency of use of the system for communication, user and system performance, and electrical signal characteristics.
Results
User performance was high consistently over the three years. Power in the high frequency band, used for the control signal, declined slowly in the motor cortex, but control over the signal remained unaffected by time. Impedance increased until month 5, and then remained constant. Frequency of home use increased steadily, indicating adoption of the system by the user.
Conclusions
The implanted brain-computer interface proves to be robust in an individual with late-stage ALS, given stable performance and control signal for over 36 months.
Significance
These findings are relevant for the future of implantable brain-computer interfaces along with other brain-sensing technologies, such as responsive neurostimulation.
1. Introduction
As a result of brainstem stroke or neuromuscular disease, people may become completely paralyzed while their cognition remains intact (locked-in syndrome, LIS, prevalence ∼0.73–0.8 per 100,000; Pels et al., 2017, Blandin, 2009). For people with LIS, quality of life is correlated with the possibility to communicate adequately (Rousseau et al., 2015). Augmentative and alternative communication (AAC) technology may be a solution for many people with LIS, but existing systems depend on (some level of) muscle control. A relatively new muscle-independent technology to reestablish communication in people with LIS is that of brain-computer interfaces (BCIs). BCIs typically use electrophysiologic signals that can be modulated by mental acts, such as attempted movement, or elicited by external stimuli, to generate a control signal. Recently, we showed that an implanted BCI employing subdural electrocorticographic (ECoG) electrodes can be used for reliable communication in an individual with LIS due to Amyotrophic Lateral Sclerosis (ALS) (Vansteensel et al., 2016). It remained to be determined, however, whether the neuro-electrical signals used for BCI control are stable in the long run and if it is possible to distinguish between brain-states chronically. Such long-term stability is vital for the eventual clinical viability of implantable BCIs since it eliminates the need for repeated surgery to reestablish control.
Prior work on long-term ECoG-recordings indirectly suggests that these signals could provide a stable BCI control signal. Animal studies have shown that placement of ECoG-electrodes does not damage the brain, besides non-harmful indentations underneath contact points and encapsulation of the electrode-strip (Bullara et al., 1979, Yuen et al., 1987, Degenhart et al., 2016). In humans, work has been conducted on deep brain stimulation (DBS) and epilepsy monitoring. It was demonstrated that it is feasible to use ECoG-recordings for at least 6 months for the discrimination between three types of tics in Tourette’s syndrome (Shute et al., 2016), for closed-loop adaptable DBS in patients with essential tremor (Herron et al., 2016), and for seizure detection and reduction in epilepsy patients (Sillay et al., 2013, Morrell et al., 2011, Nurse et al., 2018). However, recently two publications showed that the ECoG-signal recorded for seizure detection is not stable in the first 3–5 months after implantation in epilepsy patients (Sun et al., 2017, Ung et al., 2017). This finding bears relevance to all ECoG-implants, including BCIs.
Here, we report on the long-term stability of ECoG-signals for home-use of BCIs. We extended our implantable BCI study (Vansteensel et al., 2016), and studied home-use, impedance, raw signal dynamics and signal modulation features over 36 months. We conclude that ECoG-based BCIs can be a long-term solution for people with LIS, since the control signal is robust and durable and BCI performance remains high, resulting in a sustainable home-use communication BCI for 36 months.
2. Methods
We present longitudinal data from a 58-year old woman with late-stage ALS who was implanted with an ECoG-based communication BCI (Fig. 1) in 2015 (Vansteensel et al., 2016). Besides focusing on the primary motor cortex electrodes used for BCI control, we also report on the electrodes implanted over the left dorsolateral prefrontal cortex (dlPFC; Fig. 1), which were frequently tested but not used for everyday BCI-control. Regular recordings were obtained during a 2-year research period (November 2015–November 2017). Infrequent system-check visits allowed for additional data acquisition in the subsequent 12 months (last visit 29th of November 2018). The latter are indicated by triangles in the figures.
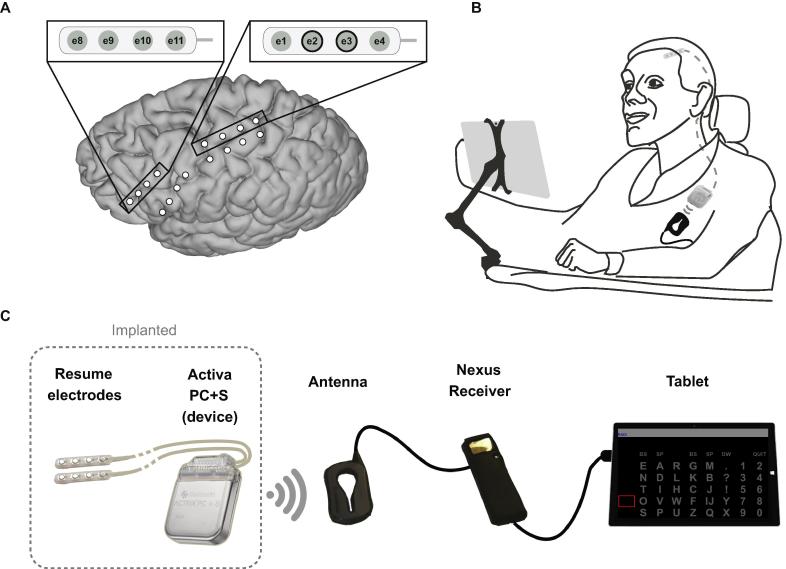
Fig. 1.
Overview of the implanted brain-computer interface system. (A) Electrode-strip locations. During a first surgery, two strips were placed on the primary motor cortex and two on the dorsolateral prefrontal cortex. For both regions, one strip was placed over the primary target area, as determined using presurgical fMRI, and one was placed on a secondary target area as a backup. One strip over each area was connected to the device (see inserts) during a second surgery. These strips were selected based on correlation of the signal with the task condition of Localizer tasks performed during two days of testing between the two surgeries (see Vansteensel et al., 2016 for details). The bipolar electrode pair e2-e3 (indicated with black circles in top right insert) is used for BCI-control. (B and C) Overview of the BCI components and the location of the implanted parts. Adapted with permission from Vansteensel et al. (2016).
The system logs frequency and duration of home use, allowing for assessment of technology adoption between May 2016 (when she received the system at home) and April 2019. User satisfaction with the BCI system was evaluated in May 2019, using a modified Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST 2.0) and Psychosocial Impact of Assistive Devices Questionnaire (PIADS) (see Vansteensel et al., 2016 for details). The study was approved by the local Medical Ethical Committee of the UMC Utrecht. The participant gave informed consent to participate in the study using a dedicated procedure, described earlier (Vansteensel et al., 2016). This trial is registered in clinicaltrials.gov (NCT02224469).
2.1. Tasks and analysis
Three tasks were regularly performed from the start of the study by the participant, while ECoG-data was recorded with the implanted device. Each of these tasks involves a different level of mental activity and engagement of the user and can therefore be used to evaluate the stability of the BCI from a different perspective. The tasks were (1) a ‘Localizer-task’ with alternating 15 s blocks of rest and attempted hand movement, which was used to determine and track the signal features corresponding to attempted hand movement, (2) a one-dimensional continuous cursor-control (‘Target’) task, with attempted hand movement and rest being used to move a cursor up and down respectively. This task was used for BCI training and to study BCI performance accuracy as well as signal features in the presence of visual feedback (Vansteensel et al., 2016) and (3) a ‘Baseline-task’ with 3 min recording of both motor and dlPFC bipolar-pairs during rest with eyes open, which was used to study fluctuations of the baseline signal features over time.
Electrophysiological data was collected from bipolar pairs of electrodes using the implanted device. During the Localizer- and Baseline-tasks, time-domain data was collected at 200 Hz (‘raw signal’). High-frequency band (HFB) power was calculated on an external computer with a multi-taper time-frequency transformation based on multiplication in the frequency domain (average power over 65–95 Hz, 1 Hz bins, 0.5 s Hanning window) (Oostenveld et al., 2011). For the Localizer-task, correlation (R2) of mean per trial HFB-power with the task condition (active vs rest) was calculated. Baseline-task HFB-power was averaged over time, resulting in a single value per run. During the Target-task, bandwidth filtered data, calculated on board of the device (center-frequency 80 Hz, bandwidth 2.5 Hz, using on-device analog filters; effective frequency range at these settings is 80 ± ∼10 Hz) was collected at 5 Hz (‘power data’). Note that, because of the different nature of the online and offline power conversion, HFB power may differ somewhat between these methods. Yet, because for each task, HFB power was computed always using the same approach, the longitudinal HFB power profile per task can be accurately described. Target-tasks (5-min duration) were typically preceded by a 3-min calibration period, after which one or more Target-task runs were performed. Performance (percent correct hits) was calculated for each run. Empirical chance level was 48.4%, based on permutation testing. Besides performance scores, mean HFB-power during the feedback-period of active and rest trials was calculated. Clear outlier data samples were removed.
2.2. Impedance measurements
Regular impedance measurements were obtained by applying a short (80 μs, 100 Hz) pulse to each bipolar electrode-pair of the motor strip with the device (Activa PC+S, Medtronic).
2.3. Statistical testing
To evaluate whether or not the BCI was stable over time, the presence or absence of a significant linear increase or decrease in HFB-power, BCI performance accuracy and impedance over time was computed by entering the respective values over time as dependent variables in linear regression models with time as the independent variable. For each model, the R2 value and significance (p < 0.05) was calculated in SPSS (version 25, IBM).
3. Results
The user received the home-system in May 2016. She reported high satisfaction with the system after several weeks of use, with scores on the PIADS of 1.1, 2.2, 1.0 for the domains competence, adaptability, and self-esteem, respectively (Vansteensel et al., 2016; score range is −3 to 3, larger values indicate a more positive effect of the device). Three years later (May 2019), these scores increased significantly to 2.5, 2.5 and 2.3. On the modified QUEST 2.0 questionnaire, scores on 9 of 12 items were ‘very satisfied’. Two items were scored ‘satisfied’ and one item (‘cables’) was scored ‘unsatisfied’. Five items received a higher score than in 2016.
System log-files showed that, initially, home use was higher during summer (April–September) than during winter (October–March), which is related to her using the system mainly outside in the first two years. Frequency and duration of BCI-system home use per month were as follows: May–September, 2016: 12.5 h and 16 times, October 2016–March 2017: 6.5 h and 6 times, April–September 2017: 10.5 h and 7 times, October 2017–March 2018: 1 h, 1 time. Starting in spring 2018, home use increased, leading to an average per month of 37.7 h (spread over 15 sessions) in April-September 2018, 98.7 h (37 sessions) in October 2018-March 2019, and 148 h (65 sessions) in April 2019. Notably, signal processing settings for home use required no changes between September 2016 and April 2019, except for a short period with fever due to reasons unrelated to the device. This increase coincided with progressive loss of eye movement control, leading the user to increasingly replace her eye tracker with the BCI system for daily communication at home. When asked about the added value of the BCI 43 months after implantation, the user expressed (literally translated from Dutch) that she was “very happy with the UNP” (UNP = Utrecht NeuroProsthesis, the implanted BCI) as “my eyes have become too slow for my eye tracking system” and with the BCI system “I can speak preprogrammed sentences and create my own words and sentences”. The value of the system is further illustrated by her statement "without the UNP I would be without words".
3.1. Task-Related signal modulation and BCI performance
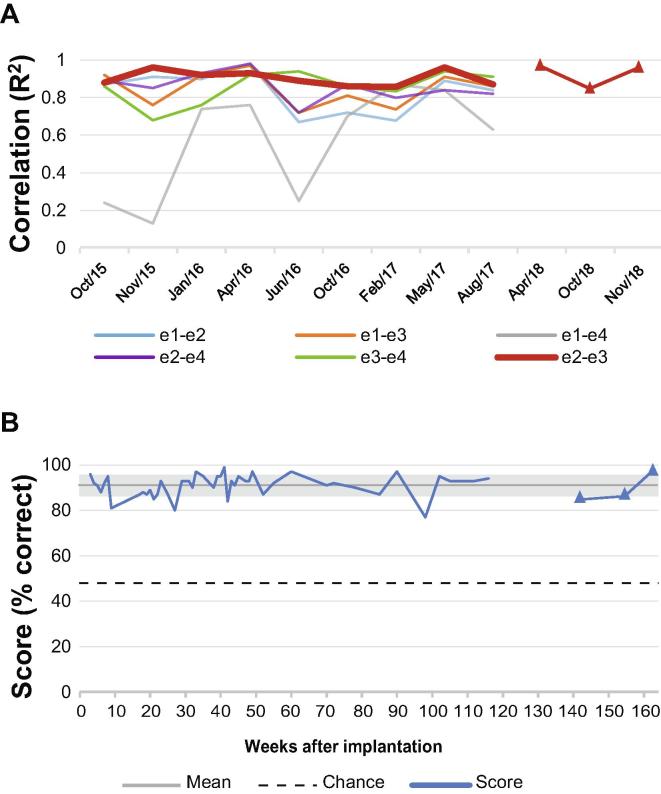
The Localizer-task resulted in a consistently high R2 between HFB-power and active versus rest conditions for all but one pair on the motor strip (Fig. 2A), indicating sustained task-related modulation of HFB-power. Linear regression for all pairs did not show a change in R2 values over 36 months. Changes induced in HFB power by attempted hand movement could be used reliably for BCI control: performance on the Target-task (where the participant controlled vertical cursor movement on a computer screen using HFB-power from electrode pair e2-e3) was consistently high (91%, chance 48.4%, no significant trend, Fig. 2B).
Fig. 2.
Task-related signal modulation over 36 months. (A) Correlation (R2) of HFB-power (65–95 Hz) with the Localizer task condition (rest vs. attempted hand movement), for all pairs on the motor electrode strip. The correlation was calculated between the mean HFB-power (over time) per trial and the task condition (active vs. rest). In red, the pair used for BCI control and home use. (B) Mean score per week on the Target-task using HFB-power of pair e2-e3 on the motor electrode strip. The Target-task was mainly used to familiarize the user with brain control. Therefore, the number of instances was higher in the first months after implantation than in the last months of this graph. Chance level is 48.4%, overall mean score was 91% (grey line), grey shading indicates the standard deviation of the mean. For both panels, the triangles represent the data collected after the research period (from November 2017).
3.2. Raw signal dynamics
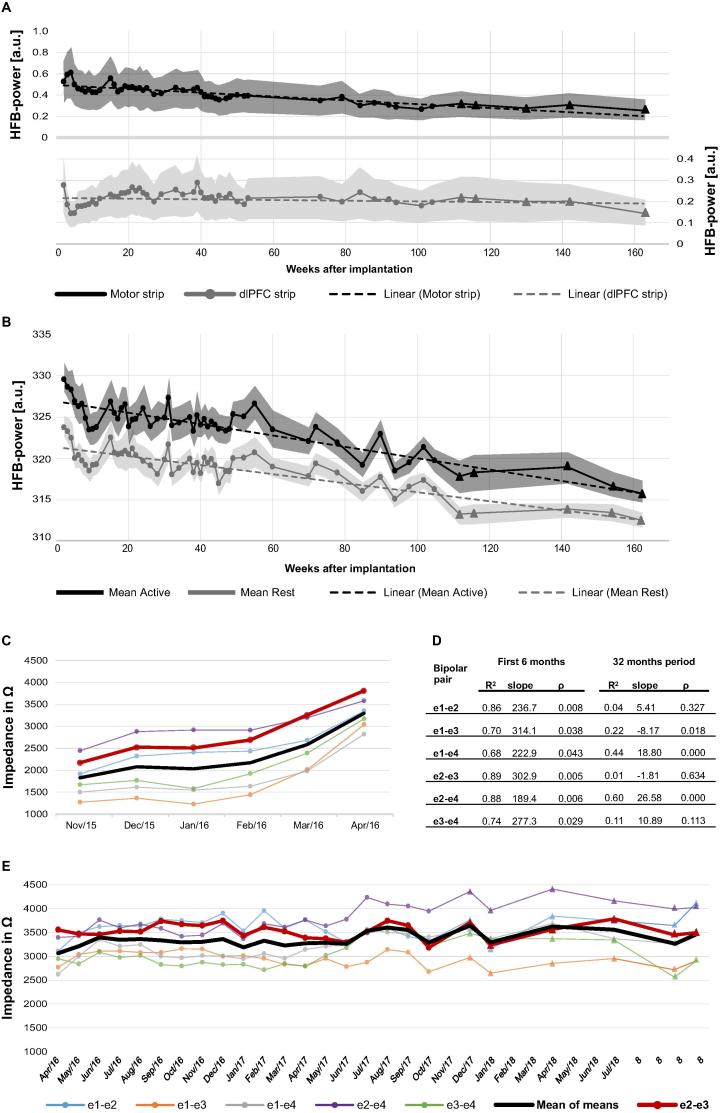
To track the baseline signal features over time, 3 min baseline recordings were frequently obtained. Baseline-task HFB-power of the bipolar pair over the sensorimotor cortex used for BCI-control (e2-e3) showed a slow but significant decrease over 36 months (Fig. 3A; linear regression, slope −2 × 10−3, R2 = 0.74, p < 0.001). In contrast, HFB-power of the best performing bipolar pair on the dlPFC-strip (selected based on highest HFB correlation with a Localizer-task) was stable (slope 0.00, R2 = 0.032, p = 0.21). In correspondence with the findings from the Baseline task, mean HFB-power during both the active and rest trials of the Target-task (electrode pair e2-e3 over the sensorimotor cortex; Fig. 3B), averaged per week, showed a declining trend (active: slope −0.065, R2 = 0.79, p < 0.001; rest: −0.052, R2 = 0.747, p < 0.001).
Fig. 3.
Chronic evaluation of ECoG-electrode parameters and HFB-power. (A) Mean HFB-power per run (65–95 Hz) HFB-power (mean per run) during the Baseline-task in the motor strip (home use pair e2-e3) in black and dlPFC-strip (best performing pair, e9-e11) in grey. The dotted lines are the linear trend lines. Shaded area surrounding the line represents the standard deviation per run. Triangles represent data acquired after the research period. Note: motor and dlPFC-strips have independent analog amplifiers on the device, therefore the absolute HFB-power values of motor and dlPFC cannot be compared. (B) HFB-power (pair e2-e3 of the motor strip) during the active (black) and rest (grey) phase of the Target-task. Note that due to the analogue filtering on board of the device (center frequency 80 Hz, bandwidth 2.5 Hz; effective frequency range at these settings is 80 ± ∼10 Hz) the power values differ from the calculated power values in panel A. The dotted lines are the linear trend lines, the shaded area surrounding the line represents the standard deviation, and the triangles represent data acquired after the research period. Note that HFB power appears to stabilize after week 112. (C and E) Electrode pair impedances over time. Colored lines indicate values per electrode pair, averaged per month. In black the mean over all pairs of the motor strip, in red the pair used for control. Panel A shows the impedance values obtained using stimulation at 0.25 V. Panel C shows the values obtained after stimulation settings were increased to 0.7 V in April 2016 when some recordings using 0.25 V reached the maximum displayable value of 4000 Ω. Triangles represent the data collected after the research period (from November 2017). (D) Linear regression results of the impedances in panels A and C. During the first 5 months, a significant increase in impedance was found in all electrode pairs. From April 2016 onwards the impedances stabilized for all but 4 pairs (e1-e3, e1-e4, e2-e4 and e3-e4).
3.3. Impedance
Impedance displayed a significant (p < 0.05, linear regression) increase for all motor bipolar pairs in the first months after implantation (Fig. 3C/D). After 5 months, slow trends were still observed but impedances generally stabilized around 3500 Ohm (Fig. 3E).
4. Discussion
BCI-control based on ECoG signals from the motor cortex is consistently high, and supports sustainable BCI home use for 36 months. Despite a slow decline in HFB-power in active and resting states, discrimination between states remains excellent, as demonstrated by stable high R2 values, high performance (91%), and system adoption by the user, as evidenced by increasing home use. A decline in baseline HFB-power (54–86 Hz) has been shown for non-human primates (Ryapolova-Webb et al., 2014), and was attributed to regrowth of dura between electrode and leptomeningeal surface. This is unlikely to explain the decline in HFB-power in our study, since we did not observe this phenomenon in the dlPFC-strip. One explanation could be that the patient improved downregulation of HFB-power during baseline and needed less increase for reliable control. Indeed, the user reported decreased effort over time (Vansteensel et al., 2016). Alternatively, the HFB-power decline could reflect neurodegenerative characteristics of ALS, where the motor cortex degenerates and Betz cells, which are likely to contribute to HFB-power (Rouse et al., 2013), are depleted (Nihei et al., 1993). Impedance stabilized 5 months after implantation. The initial rise and subsequent stabilization of impedance values corresponds to earlier reports (Sillay et al., 2013, Swann et al., 2018).
5. Conclusion
In conclusion, our data show that, despite small changes in impedance and HFB-power, accurate and stable long-term ECoG-based BCI control was feasible in a person with LIS due to ALS. These findings bear relevance for the growing field of sensing neurotechnology, including BCIs and responsive DBS, but should be confirmed in other individuals and brain areas, and in different etiologies.
Acknowledgments
Acknowledgments
We thank the participant for her contribution and feedback.
Funding
This study was supported by grants from the Netherlands (UGT7685, Economic Affairs SSM06011 and STW 12803, cosponsored by Medtronic), the European Union (ERC-Adv 320708) and the National Institute On Deafness And Other Communication Disorders of the National Institutes of Health under Award Number U01DC016686. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding sources had no role in the design of the study, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the article for publication.
Declaration of Competing Interest
T. Denison was an employee of Medtronic Inc, which co-sponsored the STW-grant. He is a shareholder of Medtronic and holds patents related to patient directed therapy control (8,380,314), a chopper-stabilized instrumentation amplifier (8,354,881), and a therapy control based on a patient movement state (8,121,694), all licensed to Medtronic. TD did not influence the clinical conclusions of this paper. E.G.M. Pels, E.J. Aarnoutse, S. Leinders, Z.V. Freudenburg, M.P. Branco, B.H. van der Vijgh, T.J. Snijders, M.J. Vansteensel and N.F. Ramsey report no disclosures.
References
- Blandin V. ALIS; Association du Locked-In Syndrome. 12 ans d’expérience en France. http://www.uzleuven.be/sites/default/files/revalidatiecentrum/wittebols/ALIS Leuven 1 - veronique blandin.pdf (published 2009).
- Bullara L.A., Agnew W.F., Yuen T.G.H., Jacques S., Pudenz R.H. Evaluation of electrode array material for neural prostheses. Neurosurgery. 1979;5(6):681–686. doi: 10.1227/00006123-197912000-00006. [DOI] [PubMed] [Google Scholar]
- Degenhart A.D., Eles J., Dum R., Mischel J.L., Smalianchuk I., Endler B. Histological evaluation of a chronically-implanted electrocorticographic electrode grid in a non-human primate. J Neural Eng. 2016;13(4):046019. doi: 10.1088/1741-2560/13/4/046019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron J.A., Thompson M.C., Brown T., Chizeck H.J., Ojemann J.G., Ko A.L. Chronic electrocorticography for sensing movement intention and closed-loop deep brain stimulation with wearable sensors in an essential tremor patient. J Neurosurg. 2016:1–8. doi: 10.3171/2016.8.JNS16536. [DOI] [PubMed] [Google Scholar]
- Morrell M.J., Group RNSS in ES Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- Nihei K., McKee A.C., Kowall N.W. Patterns of neuronal degeneration in the motor cortex of amyotrophic lateral sclerosis patients. Acta Neuropathol. 1993;86(1):55–64. doi: 10.1007/BF00454899. [DOI] [PubMed] [Google Scholar]
- Nurse E.S., John S.E., Freestone D.R., Oxley T.J., Ung H., Berkovic S.F., O'Brien T.J., Cook M.J., Grayden D.B. Consistency of long-term subdural electrocorticography in humans. IEEE Trans Biomed Eng. 2018;65:344–352. doi: 10.1109/TBME.2017.2768442. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pels E.G.M., Aarnoutse E.J., Ramsey N.F., Vansteensel M.J. Estimated prevalence of the target population for brain-computer interface neurotechnology in the Netherlands. Neurorehabil Neural Repair. 2017;31(7):677–685. doi: 10.1177/1545968317714577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse A.G., Williams J.J., Wheeler J.J., Moran D.W. Cortical adaptation to a chronic micro-electrocorticographic brain computer interface. J Neurosci. 2013;33(4):1326–1330. doi: 10.1523/JNEUROSCI.0271-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau M.-C., Baumstarck K., Alessandrini M., Blandin V., Billette de Villemeur T., Auquier P. Quality of life in patients with locked-in syndrome: evolution over a 6-year period. Orphanet J Rare Dis. 2015;10:88. doi: 10.1186/s13023-015-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryapolova-Webb E., Afshar P., Stanslaski S., Denison T., de Hemptinne C., Bankiewicz K. Chronic cortical and electromyographic recordings from a fully implantable device: preclinical experience in a nonhuman primate. J Neural Eng. 2014;11(1):016009. doi: 10.1088/1741-2560/11/1/016009. [DOI] [PubMed] [Google Scholar]
- Shute J.B., Okun M.S., Opri E., Molina R., Rossi P.J., Martinez-Ramirez D. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. NeuroImage Clin. 2016;12:165–172. doi: 10.1016/j.nicl.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillay K.A., Rutecki P., Cicora K., Worrell G., Drazkowski J., Shih J.J. Long-term measurement of impedance in chronically implanted depth and subdural electrodes during responsive neurostimulation in humans. Brain Stimul. 2013;6(5):718–726. doi: 10.1016/j.brs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Sun F.T., Arcot Desai S., Tcheng T.K., Morrell M.J. Changes in the electrocorticogram after implantation of intracranial electrodes in humans: the implant effect. Clin Neurophysiol. 2017;129(3):676–686. doi: 10.1016/j.clinph.2017.10.036. [DOI] [PubMed] [Google Scholar]
- Swann N.C., de Hemptinne C., Miocinovic S., Qasim S., Ostrem J.L., Galifianakis N.B. Chronic multisite brain recordings from a totally implantable bidirectional neural interface: experience in 5 patients with Parkinson's disease. J Neurosurg. 2018;128(2):605–616. doi: 10.3171/2016.11.JNS161162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteensel M.J., Pels E.G., Bleichner M.G., Branco M.P., Denison T., Freudenburg Z.V. Fully implanted brain-computer interface in a locked-in patient with ALS. N Engl J Med. 2016;375(21) doi: 10.1056/NEJMoa1608085. NEJMoa1608085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung H., Baldassano S.N., Bink H., Krieger A.M., Williams S., Vitale F. Intracranial EEG fluctuates over months after implanting electrodes in human brain. J Neural Eng. 2017;14(5):056011. doi: 10.1088/1741-2552/aa7f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen T.G.H.H., Agnew W.F., Bullara L.A. Tissue response to potential neuroprosthetic materials implanted subdurally. Biomaterials. 1987;8(2):138–141. doi: 10.1016/0142-9612(87)90103-7. [DOI] [PubMed] [Google Scholar]