Unfortunately, an error was introduced during the redrawing of the Fig. 3. The error concerned the labelling and orientation of Fig. 3B, which was not correctly reflected in the respective figure legend. Please find the corrected legend and Fig. 3 below. During further scrutiny, the authors noticed a few minor typographical errors in the published article. The corrections are as follows:
Fig. 3.
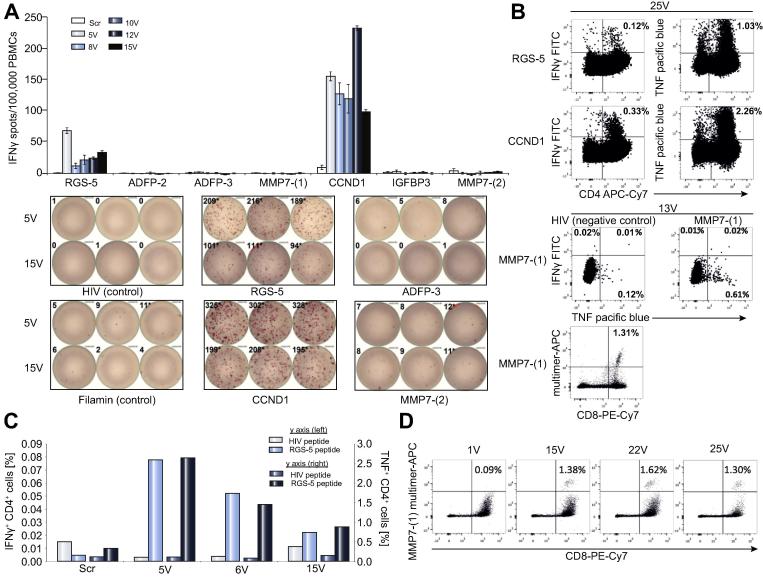
Immunomonitoring of patient PBMCs for vaccine induced T cell responses. PBMCs were pre-stimulated using either peptide pool I (RGS-5, ADFP-2, ADFP-3, MMP7-(1) and HIV-A03) or peptide pool II (CCND1, IGFBP3, MMP7-(2) and Filamin-A), and expanded for 12 days using IL-2. (A) 300,000 cells (Class I binding peptides) or 200,000 (for class II binding peptides) were re-stimulated in triplicates (duplicates for pre-vaccination ‘scr’ time point) in an IFN-γ ELISPOT assay using individual peptides. The top panel shows the normalized spot counts for individual peptide stimulations and the bottom panel shows examples of scanned ELISPOT wells. At least 700,000 cells were re-stimulated with the respective peptides, in the presence of Brefeldin A and GolgiStop and 12 h later, intracellular IFN-γ and TNF were stained and analysed for B and C. (B) Exemplary dot plots of IFN-γ and TNF production by CD4(+) cells in response to RGS-5 and CCND1 peptides, collected at the 25th vaccination (25V) are shown in the top panel. TNF production by CD8(+) cells in response to MMP7-(1) peptide and the respective negative control (HIV) are shown in the bottom panel. MMP7-(1) multimer staining of the corresponding vaccination time point (13V) is shown below in the bottom panel. (C) Frequencies of the RGS-5 peptide induced cytokine(+) live CD4(+) lymphocytes are shown (left y axis: IFN-γ; right y axis: TNF). (D) At least 600,000 cells were analyzed by HLA-peptide multimers (class I). MMP7-(1) multimer(+) cells within live CD4(−) lymphocytes are shown and the % of multimer(+) CD8(+) lymphocytes are indicated. (This figure appears in colour on the web.)
Page 850 – Materials and methods
Next-generation-sequencing
As part of a research project (IndividuaLIVER, approved by the Institutional Review Board at the University Hospital of Tübingen), WES of tumor and normal tissue was performed for L06/10, L04/12 and P03/13 (Fig. 1). In addition, the transcriptome of tumor tissue of L06/12 and P03/13 was sequenced.
Should have read:
As part of a research project (IndividuaLIVER, approved by the Institutional Review Board at the University Hospital of Tübingen), WES of tumor and normal tissue was performed for L06/10, L04/12 and P03/13. In addition, the transcriptome of tumor tissue of L06/10 and P03/13 was sequenced.
Mass spectrometry
HLA ligands were immunoprecipitated from cryopreserved tumor tissues and liver tissue (L03/10) as previously described [12] using the pan-HLA class I antibody, W6/32 (manufactured in-house).
Should have read:
HLA ligands were immunoprecipitated from cryopreserved tumor tissues and liver tissue (L06/10) as previously described [12] using the pan-HLA class I antibody, W6/32 (manufactured in-house).
T cell in vitro immunomonitoring
Responses to HLA class I peptides were monitored by IFN-γ ELISPOT and HLA-peptide multimer staining, while responses to HLA class II peptides and RGS-5 peptide were determined by IFN-γ ELISPOT and ICS (for IFN-γ, TNF-β, IL-2 and IL-10) (all techniques are elaborated in the Supplementary materials and methods).
Should have read:
Responses to HLA class I peptides were monitored by IFN-γ ELISPOT and HLA-peptide multimer staining, while responses to HLA class II peptides and RGS-5 peptide were determined by IFN-γ ELISPOT and ICS (for IFN-γ, TNF-α, IL-2 and IL-10) (all techniques are elaborated in the Supplementary materials and methods).
Page 851 (Table 1)
The column heading P03/12 in the published version, should read P03/13.
Page 852 (Results)
Vaccination, further clinical course and immunomonitoring; 4th paragraph
T cell cytokine response was detected against CCND1 by IFN-γ ELISPOT (Fig. 3A) and was confirmed to be a CD4+ response by intracellular cytokine staining (ICS) (Fig. 3B left lower panel).
Should have read:
T cell cytokine response was detected against CCND1 by IFN-γ ELISPOT (Fig 3A) and was confirmed to be a CD4+ response by intracellular cytokine staining (ICS) (Fig. 3B top panel).
Both the journal and the authors apologise for any inconvenience that these errors may have caused.