Abstract
Oligoprogressive disease is a relatively new clinical concept describing progression at only a few sites of metastasis in patients with otherwise controlled widespread disease. In the era of well-tolerated targeted treatments, resistance inevitably occurs and overcoming this is a challenge. Local ablative therapy for oligoprogressive disease may allow the continuation of systemic treatments by overcoming the few sub-clones that have developed resistance. Stereotactic body radiotherapy is now frequently used in treating oligometastatic disease using ablative doses with minimally invasive techniques and acceptable toxicity. We discuss the current retrospective clinical evidence base supporting the use of local ablative therapy for oligoprogression in metastatic patients on targeted treatments within multiple tumour sites. As there is currently a lack of published prospective data available, the best management for these patients remains unclear. We discuss current trials in recruitment and the potential advancements in treating this group of patients with stereotactic radiotherapy.
Key words: metastatic disease, Oligometastases, oligoprogression, stereotactic body radiotherapy, targeted therapy
Statement of Search Strategies Used and Sources of Information
An electronic literature search was carried out using PubMed, Medline and clinicaltrials.gov for current studies in progress. Search terms included ‘oligoprogression’, ‘oligoprogressive disease’, ‘oligometastatic disease’, ‘prostate cancer’, ‘breast cancer’, ‘non-small cell lung cancer’, ‘resistance mechanisms’ and ‘stereotactic body radiotherapy’. Only English language publications were considered. The reference lists of selected publications were manually reviewed to identify additional publications not identified in the initial search.
Oligoprogressive Disease versus Oligometastatic Disease: what is the Difference?
The description of oligometastatic disease (OMD) has evolved from its inception. It was initially described as an intermediate disease state between localised and widespread disease [1]. It is considered as metastatic disease confined to a limited number of sites (often described as up to three or five sites) and can be synchronous or metachronous with the primary tumour presentation. Recognising OMD is of clinical importance, as patients can be considered for local ablative treatments such as stereotactic body radiotherapy (SBRT) or surgery, ideally within a clinical trial. The evidence base suggests a better prognosis with potentially curative outcomes using ablative strategies across a variety of tumour sites. There are many systematic and retrospective reviews presenting survival outcomes for patients treated by surgical resection, SBRT or radiofrequency ablation of oligometastases that suggest long-term survival benefits [2], [3], [4], [5], [6], [7], [8], [9], [10].
In addition, there are two phase II randomised prospective studies of synchronous oligometastatic non-small cell lung cancer (NSCLC). Gomez et al. [11] randomised patients with synchronous oligometastatic NSCLC after a response to first-line systemic treatment to local consolidative treatment with radiotherapy or surgical resection of all lesions or a combination of both ± maintenance treatment versus standard of care (maintenance treatment or observation alone). The trial was terminated early due to significant median progression-free survival (PFS) differences between arms; the median PFS for local consolidative treatment was 11.9 months (90% confidence interval 5.7–20.9) versus 3.9 months in the standard of care arm (90% confidence interval 2.3–6.6). Adverse events were similar between the groups. A similar phase II study [12] randomised patients with up to five sites of OMD to maintenance chemotherapy and SBRT or maintenance chemotherapy alone. This study also stopped early due to finding a significant improvement in PFS (9.7 versus 3.5 months, P = 0.01) within the SBRT arm, with similar toxicity in both groups. Current trials in progress include CORE (NCT02759783) and SABR-COMET (NCT01446744), addressing the clinical question of SBRT use on PFS and overall survival in OMD for multiple tumour sites. SABR-COMET [13] has completed recruitment. Patients with between one and five metastatic lesions were treated with palliative standard of care, which included palliative radiotherapy or palliative chemotherapy as indicated, or arm 2, SBRT to all metastatic lesions ± palliative chemotherapy [58]. Of 99 patients randomised, the median overall survival in the standard arm was 28 months (95% confidence interval 19–33 months) versus 41 months (95% confidence interval 26–not reached months) in the SBRT arm. There were three treatment-related National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 5 events in the SBRT arm. These included death due to radiation pneumonitis, pulmonary abscess and subdural haemorrhage after surgery to repair a SBRT-related perforated gastric ulcer.
By contrast, oligoprogressive disease (OPD) is a relatively new clinical concept and is distinct from OMD as defined by Hellman and Weichselbaum [1]. OPD develops on a background of polymetastatic disease. OPD occurs following an initial response to systemic treatment where disease progression only occurs in a limited number of sites. OPD is increasingly encountered in clinical practice due to the widespread use of first- and second-generation molecular targeted treatments [14], [15] with the potential for the development of sub-clones of drug resistance. OPD patients are considered to have a more advanced disease status compared with patients with OMD, by virtue of the fact that they initially present with polymetastatic disease [16], [17], [18], [19].
Identifying patients with OPD may be important because there is potential to prevent the development of widespread resistant disease from the propagation of drug-resistant clones. Removing or ablating OPD sites may slow or prevent this process, allowing prolonged therapeutic benefit from the current line of systemic therapy. This benefit may persist until a further stochastic clonal event occurs or already disseminated resistant clones become clinically or radiologically apparent, or until treatment becomes intolerable [20].
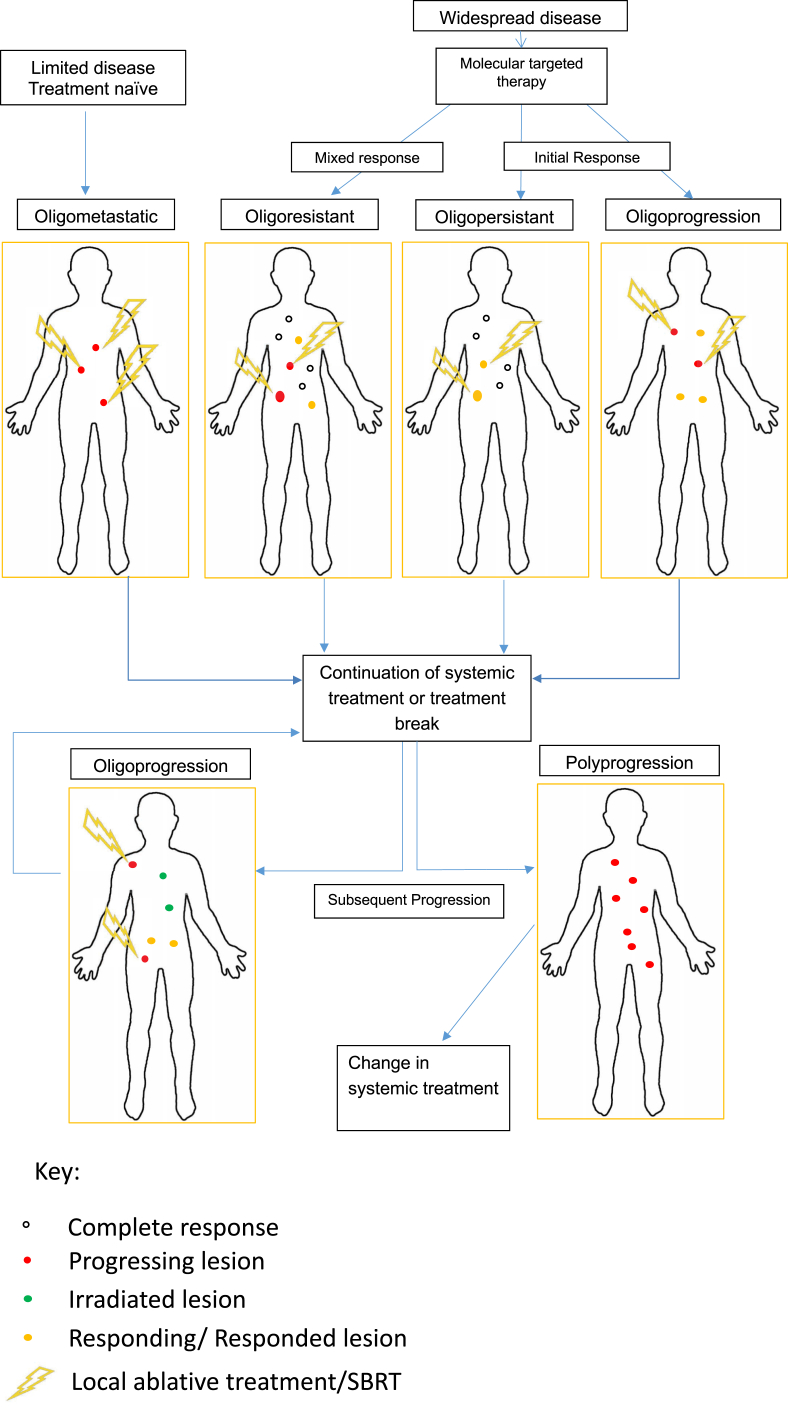
There remain clinical controversies in the optimal management of OPD due to a lack of prospective data. It is important to recognise and distinguish between the different disease patterns as management strategies differ. This review will examine the evidence base regarding the treatment of OPD, with a particular focus on lung and prostate cancer, and will highlight the current issues with OPD management, specifically whether to treat disease with a change of systemic therapies (‘spray the whole lawn’) or offer local ablative treatment (‘selective weeding’) [21]. Figure 1 highlights the different disease patterns and time points when local ablative therapy could be considered in a patient's pathway and can be used as a framework and vocabulary for discussing OPD.
Fig 1.
Schematic of nomenclature in the metastatic state.
Systemic Drug Resistance and Oligoprogressive Disease
There is an expanding armamentarium of molecularly targeted systemic treatments available to patients with metastatic cancers across a number of different tumour types. Anti-androgen targeted therapies have proven overall survival and PFS benefit in prostate cancer patients [22], [23], [24], [25]. Anti-HER2 targeted treatments, such as pertuzumab in combination with trastuzumab and docetaxel chemotherapy, for metastatic HER-2-positive breast cancer have shown an improvement in median overall survival of 15.7 months compared with placebo [26]. Tyrosine kinase inhibitor (TKI) treatment in epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) mutation-positive metastatic NSCLC patients has resulted in a PFS of 8–13 months, an improvement compared with standard chemotherapy with higher response rates [27], [28], [29], [30].
Although patients may show an initial response to targeted therapies, secondary failure due to drug resistance remains a problem. Acquired resistance to TKI in NSCLC patients develops after a median of 8–13 months [27], [29]. Three main genomic resistance mechanisms have been identified, including target receptor alteration, activation of bypass signalling pathways and phenotypic transformation [31], [32]. Other causes include increased drug efflux and change in drug absorption [33]. In a similar manner to tumour genomic heterogeneity [34], intra-patient drug resistance can be variable and therefore isolated sites of distinct drug-resistant clones can develop [31], [35]. Similar mechanisms of resistance involving androgen receptors and the activation of androgen deprivation therapy resistance pathways have been described in patients with castrate-resistant prostate cancer (CRPC) treated with abiraterone or enzalutamide [36]. Multiple mechanisms of resistance, including poor internalisation of trastuzumab and HER-2 receptor complex at the cell surface and intracellular transportation of trastuzumab in metastatic breast cancer patients, have also been explored [37].
Ablative treatments are able to effectively target resistant clones regardless of the mutational genotype or load. Therefore this may be an attractive additional treatment option for patients before considering systemic treatment change [38]. In practice, the conventional approach to patients presenting with OPD varies depending on a number of patient factors, the tumour type and the number of subsequent lines of systemic treatments available. In many cases, at the onset of OPD the current line of systemic treatment is discontinued and a switch to a further line of systemic therapy is considered. The alternative strategy is to continue the systemic treatment beyond progression. NSCLC patients with EGFR mutations who continue TKI beyond first progression have been suggested to have a survival benefit compared with patients who stop TKI in retrospective studies [39], [40]. Tumour flare, which can occur with the interruption of TKI, may play a role in this [41]. A prospective phase II study enrolled 208 patients in this setting. There were 171 progressive disease events by Response Evaluation Criteria In Solid Tumours (RECIST) v1.1 while on erlotinib. Of these patients, clinicians chose to continue erlotinib in 93 (54%) patients beyond progression. Post-hoc analysis found that the median time from starting erlotinib to first progression by RECIST v1.1 in these 93 patients was 11 months, with an addition of a median of 3.1 months if continuing erlotinib until further progression [42]. The Prostate Cancer Clinical Trials Working Group 3 (PCWG3) advised to consider continuation of systemic targeted treatments for patients with prostate cancer who are continuing to benefit symptomatically, even in the presence of prostate-specific antigen or imaging progression [43]. PCWG3 also discussed the option of surgery or radiotherapy to ablate oligoprogressing metastases.
Diagnosis and Frequency of Oligoprogressive Disease
Identifying OPD poses a challenge as the conventional approach of using RECIST (a commonly used international standard to determine a patient's outcome from treatment) has not been adapted to report on OPD [44]. For example, a patient with progression of over 50% in a solitary lesion with all other metastatic lesions showing continued response by a reduction of more than 75% could be reported as a partial response or stable disease after summing all the longest diameters of the individual lesions. In practice, however, knowing if there is progression of a solitary lesion in the context of response or stable disease elsewhere, compared with widespread progression, would highlight different treatment options. Similarly, many patients who have progressed by RECIST v1.1 may continue treatment with targeted therapies beyond progression, indicating the need for further radiological criteria to identify OPD and aid treatment decisions [45]. A recent review [46] discussed the limitations of RECIST v1.1 in the era of targeted treatments with regards to both extra-cranial OPD (EC-OPD) and intra-cranial metastases.
There is additional uncertainty regarding which imaging modality best distinguishes OPD from widespread progression and hence which patients may benefit from local therapy. The most commonly used imaging modalities in routine clinical practice for patients with widespread disease for most tumour sites include computed tomography and bone scans. This poses a considerable challenge in certain tumour sites such as prostate cancer, where bone progression is difficult to assess and is unmeasurable by RECIST v1.1 [47].
Assessing how often the OPD pattern arises clinically is challenging due to its relatively recent description as a clinical disease state and its variable definitions in the literature. In addition, due to the lack of clear imaging criteria for the disease pattern, the large systemic therapy studies of molecular targeted agents have not reported the proportion of patients who develop OPD or the suitability of any oligoprogressive lesions for ablative approaches.
A retrospective review of computed tomography scans for EGFR mutation-positive NSCLC patients enrolled in prospective TKI trials documented patterns of progression [14]. The study included 49 patients with serial computed tomography scans and measurable disease; 11/49 (22.5%) patients had progression isolated initially to the primary site alone and not in distant metastases. A further retrospective review included 104 patients who had progressed on TKI by RECIST v1.1, 16 (15%) of these patients had an asymptomatic solitary site of EC-OPD [48]. A similar retrospective review investigated the pattern of progression in 108 oestrogen/progesterone-positive patients with metastatic breast cancer who had progressed on endocrine therapy [15]. OPD was defined as three or fewer sites of progression on a background of having more than six sites of disease before therapy. The study reported that 23/108 (21%) patients developed OPD; 14 of these patients were deemed suitable for local therapy. The most common site of OPD was in bone (nine), liver (five), locoregional (three) and lung (two) metastases. OPD patterns in 35 men with chemotherapy naïve metastatic CRPC on abiraterone and 20 men on abiraterone post-docetaxel chemotherapy were reported in a retrospective analysis [49]. Progression was assessed using RECIST v1.1 and/or PCWG2 criteria, and OPD was considered if five or fewer sites of disease were progressing. PCWG2 criteria emphasise the need for studies to use end points such as failure to progress or delay of disease progression for non-cytotoxic drugs, as demonstrating the shrinkage of lesions, especially bone lesions, is not feasible or necessary. In total, 12/35 (34%) chemotherapy naïve metastatic CRPC patients met the criteria for OPD. By contrast, within the cohort of 20 patients who had previously received chemotherapy, only one patient presented with OPD. It is probable that OPD will become less common after subsequent lines of therapy, due to the increased heterogeneity of the tumour with time and exposure to different drug treatments.
Prospective studies documenting the frequency and characteristics of OPD in different tumour types are needed to identify patients who may benefit from local ablative approaches. Advanced imaging techniques, such as positron emission tomography/computed tomography (PET/CT) or diffusion-weighted magnetic resonance imaging (MRI) are not currently widely used in the metastatic setting. Although they may help to distinguish patients who are truly oligoprogressive from those who are likely to display widespread progression relatively soon after local ablative therapy, these imaging modalities are costly and unproven in this setting. The use of functional imaging modalities to assist with assessing bone disease progression is expanding, including exploring combined PET/MRI scans [50]. There is also the need for more cost-effective methods of assessing patients who may benefit from local therapy. Tumour DNA from re-biopsy or circulating tumour DNA are potential predictive markers that require further study.
Clinical Experience of Local Ablative Therapy for Oligoprogressive Disease
Our limited understanding of outcomes after local ablative treatment for OPD comes mainly from small retrospective studies in the NSCLC population, with more limited data in prostate and renal cell cancers [20], [51], [52], [53], [54], [55], [56]. These studies are summarised in Table 1. The studies all include patients with OPD treated with local ablative treatment or palliative radiotherapy for local control. The studies found a median PFS of 3.3–14 months from the time of local treatment for OPD to further progression, with prostate and renal cell cancer patients having the longest PFS after local ablative treatments. Lung and bone metastases were the most common EC-OPD sites found across all patients in most of the studies, with no clear indication of any particular sites of disease providing a better prognosis. Univariate and multivariate analyses in some of the studies show trends suggesting that a shorter PFS from starting systemic treatment to first progression/local ablative treatment is an important poor prognostic indicator [20], [51], [52]. Overall, ablative treatments for OPD seem to be relatively safe. Six of the seven studies reported on toxicity and noted minimal toxicities, mostly reported as grade 1/2 CTCAE toxicities. Grade 3 toxicities were reported in five studies, ranging from 4 to 27% [20], [51], [52], [54], [56], and only one grade 4 toxicity due to postoperative complications was reported [56].
Table 1.
Retrospective studies of local ablative therapy for oligoprogression
| Reference | Patient tumour type | No. OPD sites included | No. patients with PD | No. patients with EC-OPD | Targeted therapy | Local treatment received | Median PFS 2 (months) | Systemic treatment-free survival (months) | No. patients (%) with grade 3/4 toxicity |
|---|---|---|---|---|---|---|---|---|---|
|
[20] Single centre 2005–2011 |
ALK +, EGFR + NSCLC | ≤4 EC or any number of CNS | n = 25 | n = 15 (3 patients CNS + EC-OPD) | Crizotinib or erlotinib | SBRT: 12 XRT: 2 Surgery: 1 |
4 (CI 2.7–7.4) (3 patients CNS + EC-OPD) | Grade 3 fatigue: 2 (8) | |
|
[32] Single centre |
EGFR + NSCLC |
≤4 EC | n = 184 | n = 18 | Erlotinib or gefitinib | RFA: 2 SBRT: 1 XRT: 2 Surgery: 13 |
10 (95% CI 2–27) | 22 (95% CI 6–30) | Grade 3 pneumonia: 1 (5.5) Grade 4 pneumonia: 1 (5.5) |
|
[52] Cohort analysis two centres 2013–2015 |
EGFR + NSCLC |
SBRT cohort: ≤ 3 EC or CNS No SBRT cohort: any number of sites |
n = 25 TKI + SBRT versus n = 25 SACT switched |
n = 25 (includes CNS disease) | TKI not specified | SRS: 4 SBRT: 15 XRT: 6 |
7 versus 4.1 (CI NR) (TKI + SBRT versus chemotherapy for EC-OPD and CNS) | Grade 3 oesophagitis: 1 (4) | |
|
[51] Single centre 2009–2014 |
EGFR + NSCLC |
≤5 EC or CNS | n = 46 | n = 22 | TKI not specified | SBRT: 8 XRT: 12 RFA: 2 |
7 (CI NR) (CNS and EC-OPD) | Grade 3 pneumonitis: 2 (4.3) Grade 3 neutropenia: 10 (21.7) Grade 4 skin rash: 2 (4.3) |
|
|
[54] Multicentre 2009–2015 |
NSCLC | ≤4 EC (oligometastases or OPD or local control of dominant tumour) | n = 108 | n = 20 | Erlotinib, afatinib, gefitinib, pemetrexed No SACT |
SBRT: 20 | 3.3 (CI NR) | Grade 3 late respiratory events: 2 (10) Bone fracture: 3 (15) |
|
|
[53] Multicentre 2010–2016 |
CRPC | ≤3 EC (bone or LN only) | n = 141 | n = 41 | ADT | SBRT: 41 | 11 (CI NR) | 22 (CI NR) | No grade 3/4 toxicity |
|
[55] Multicentre 2007–2015 |
RCC | ≤3 EC or CNS | n = 55 | n = 51 | Sunitinib, pazopanib, sorafenib |
XRT: 25 Surgery: 25 Cryo/thermo-ablation: 5 |
14 (95% CI 6.9–21) (EC-OPD and CNS) | No toxicity data reported |
ADT, androgen deprivation therapy; ALK, anaplastic lymphoma kinase; CI, confidence interval; CNS, central nervous system; CRPC, castrate-resistant prostate cancer; EC, extra-cranial; EGFR, epidermal growth factor receptor; LN, lymph node; NR, not recorded; NSCLC, non-small cell lung cancer; OPD, oligoprogressive disease; PD, progressive disease; PFS, progression-free survival; RCC, renal cell carcinoma; RFA, radiofrequency ablation; SACT, systemic anti-cancer therapy; SBRT, stereotactic body radiotherapy; SRS, stereotactic radiosurgery; TKI, tyrosine kinase inhibitor; XRT, external beam radiotherapy.
The definition of OPD varies among studies, with three or fewer sites of disease being the most common definition for OPD, but with some studies including up to five lesions. Patients with both intra-cranial OPD and EC-OPD were included in some series and patients were treated with varying local ablative or palliative radiotherapy for OPD with or without continuation of systemic treatments.
The one prospective trial published by Iyenger et al. [57] in 2014 included 24 NSCLC patients with six or fewer sites of EC disease progressing after chemotherapy on PET/CT scan. Patients received SBRT to all active sites and started erlotinib until further disease progression. This study found a median further PFS of 14.7 months and a median overall survival of 20.4 months. This study highlights a potential benefit for local ablative therapy in the metastatic setting, a new approach to treating these patients in combination with targeted treatments [57].
Stereotactic Body Radiotherapy for Oligoprogressive Disease and Current Studies
The SBRT technique allows non-invasive ablative doses to achieve high rates of local lesion control (70–90%) with a well-tolerated toxicity profile [17]. An example of the high conformity achieved with a SBRT plan, for a patient with metastatic prostate cancer with OPD in a solitary sternal metastasis on abiraterone, is shown in Figure 2. Currently, the main indication for radiotherapy in metastatic cancers is to palliate local symptoms. There are few prospective trials assessing the toxicity and tolerance of SBRT in combination with systemic therapies in patients with widespread disease. Although the prospective studies in OMD have tended to use SBRT doses with biological equivalent doses of over 100 Gy, lower doses may be appropriate in OPD, optimising the balance between achieving local lesion control and toxicity of SBRT, potentially to multiple lesions, combined with systemic therapy.
Fig 2.
Axial image of a stereotactic body radiotherapy plan for a patient with oligoprogressive disease in the sternum. The pink line indicates the planning target volume. The dose wash key is on the left.
To assess the true clinical benefit for patients of the addition of SBRT to systemic therapy in OPD, prospective trials are needed. Current open studies are summarised in Table 2 and are discussed below.
Table 2.
Current studies open in stereotactic body radiotherapy for oligoprogressive disease (OPD)
| Study | Estimated recruitment | Tumour type | No. OPD sites | Systemic treatment | Oligoprogression eligibility criteria | End points |
|---|---|---|---|---|---|---|
| Stereotactic radiotherapy for metastatic kidney cancer being treated with sunitinib Phase II multicentre single-arm trial |
n = 68 | RCC | ≤5 OPD metastases Maximum 3 lesions in soft tissue (EC or CNS metastases) |
First-line sunitinib | PD of individual lesion by RECIST v1.1, ≥5 mm increase in size New metastatic lesion Progressive enlargement of a known metastasis on 2 consecutive imaging studies 2–3 months apart, with ≥5 mm increase |
Primary end point: LC rate at 1 year Secondary end points: PFS, late and acute toxicity |
| STOP-NSCLC (Stereotactic radiotherapy for oligoprogressive NSCLC) Phase II randomised trial |
n = 54 | NSCLC | ≤5 OPD metastases, maximum 3 in any single organ (EC or CNS metastases) |
Any cytotoxic or targeted treatment for NSCLC | PD of individual lesion by RECIST v1.1 New metastatic lesion ≥5 mm Progressive enlargement of a known metastasis on 2 consecutive imaging studies 2–3 months apart, with ≥5 mm increase |
Primary end point: PFS Secondary end points: OS, QoL, toxicity, LC rate, total time on chemotherapy, duration of systemic treatment after SBRT, location of sites of further progression |
| HALT (Stereotactic body radiotherapy for the treatment of OPD) Multicentre, phase II/III randomised trial |
n = 110 | NSCLC with an actionable mutation receiving TKI treatment | ≤3 EC-OPD | TKI | Visible OPD on imaging suitable for SBRT as determined by HALT virtual MDT | Primary end point: PFS Key secondary and exploratory end points: OS, time to change in systemic treatment, patterns of progression, acute and late toxicity, QoL, ctDNA deep sequence analysis, PET/CT findings in relation to outcome, time to failure of next treatment |
| TRAP (Targeted radiotherapy in androgen-suppressed prostate cancer patients) Multicentre, phase II single-arm trial |
n = 84 | CRPC | ≤2 EC-OPD in lymph nodes, bone, prostate or lung only | Abiraterone/enzalutamide | Visible OPD suitable for SBRT or clinical progression | Primary end point: PFS Key secondary and exploratory end points: LC, proportion of patients with detectable ctDNA and ctDNA response to SBRT, development of a biomarker panel predicting for PFS, OS, acute and late toxicity, QoL, time to delay of next treatment, subgroup analysis of PFS of local prostate OPD to metastatic OPD, ctDNA kinetics to predict time to progression |
CNS, central nervous system; CRPC, castrate-resistant prostate cancer; ctDNA, circulating DNA; EC, extra-cranial; LC, local control; MDT, multidisciplinary team; NSCLC, non-small cell lung cancer; OS, overall survival; PD, progressive disease; PET/CT, positron emission tomography/computed tomography; PFS, progression-free survival; QoL, quality of life; RCC, renal cell carcinoma; RECIST, Response Evaluation Criteria in Solid Tumours; SBRT, stereotactic body radiotherapy; TKI, tyrosine kinase inhibitor.
A phase II, multicentre single-arm study of treatment with SBRT or stereotactic radiosurgery for oligoprogression (EC- and intra-cranial OPD) in metastatic renal cell cancer patients on first-line sunitinib is aiming to recruit 68 patients. The primary end point is local control rate at 1 year, with secondary end points including PFS, as per RECIST v1.1, and toxicity. Radiotherapy doses vary from single fraction treatments to 30 Gy in five fractions depending on the site of disease (NCT 02019576).
The STOP-NSCLC trial (NCT 02756793) is a phase II multicentre randomised trial of SBRT for OPD in patients with NSCLC on any systemic anti-cancer therapy. The study aims to recruit 54 patients. Patients are randomised to SBRT and continuation of systemic therapy versus standard of care. Standard of care includes continuation of systemic therapy, observation or switch to the next line of treatment based on physician preference and patients may also receive palliative radiotherapy. The primary end point is PFS with patients allowed to have further SBRT at subsequent oligoprogression.
The HALT trial (NCT03256981) is a phase II–III multicentre randomised trial of SBRT for OPD in patients with advanced NSCLC on a TKI therapy for a targeted mutation and oligoprogression in three or fewer sites of EC-OPD. The randomisation is 2:1 for SBRT and continuation of TKI versus continuation of TKI alone and the trial aims to recruit 110 patients. The primary end point is PFS, defined as lesions with a 20% increase in size no longer suitable for SBRT or clinically symptomatic progression or more than three lesions with progression. Secondary outcomes include overall survival, toxicity and quality of life. Radiotherapy doses range from 30 to 52 Gy in three to eight fractions to OPD lesions; a lower dose range is used to minimise toxicity in the palliative setting. Circulating DNA analysis and deep sequencing analysis of tumour tissue at baseline and at progression, where available, will also be explored. The trial utilises a virtual multidisciplinary team (MDT) meeting for the discussion of all potential trial patients to allow real-time centralised radiology review by MDT members. In the context of no clear definition of OPD, the virtual MDT scrutinises entry criteria, which is informative for recruiting centres.
The TRAP trial (NCT03644303) is a phase II multicentre single-arm trial aiming to recruit 84 metastatic CRPC patients with two or fewer sites of OPD in bone, lymph node, lung or prostate while on abiraterone or enzalutamide. Patients will receive SBRT at 30 Gy in five fractions to the OPD site(s) and continue abiraterone or enzalutamide. Alternatively, patients with prostate progression may receive 36 Gy in six fractions. The primary end point is PFS and secondary end points include local control rates, prostate-specific antigen response, overall survival and toxicity. This study also has an exploratory end point to identify a biomarker panel predicting for PFS after SBRT, including diffusion-weighted whole-body MRI scans performed at baseline and circulating tumour DNA.
Conclusions
OPD is a recently recognised pattern of disease progression on systemic anti-cancer therapy. The optimal approach to the diagnosis and management of this disease state is not yet established. As observed in prospective studies of OMD, local ablative therapies, such as SBRT, may be of clinical benefit. Given the lack of randomised data to date, local therapy for OPD should ideally be delivered within a clinical trial in order to gain further insight into the patient population that may benefit from such an approach.
Conflict of Interest
A.C. Tree reports a grant from PCUK as research funding for the TRAP trial, testing SBRT for oligoprogression in prostate cancer. P.H. Patel reports receiving funding from the NIHR Biomedical Research Centre and PCUK for employment as a clinical research fellow for the TRAP and HALT trials, testing SBRT for oligoprogression in prostate and lung cancer, respectively. F. McDonald reports a grant from CRUK as research funding for the HALT trial, testing SBRT for oligoprogression in lung cancer.
Acknowledgements
We acknowledge NHS funding to the NIHR Biomedical Research Centre at The Royal Marsden and the ICR. We are grateful to Prostate Cancer UK who fund the TRAP trial and CRUK (CRUK/16/020) who fund the HALT trial.
References
- 1.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Hughes K., Simon R., Songhorabodi S., Adson M., Ilstrup D., Fortner J. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- 3.Casiraghi M., De Pas T., Maisonneuve P., Brambilla D., Ciprandi B., Galetta D. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the “international registry of lung metastases”. J Thorac Oncol. 2011;6:1373–1378. doi: 10.1097/JTO.0b013e3182208e58. [DOI] [PubMed] [Google Scholar]
- 4.Simmonds P.C., Primrose J.N., Colquitt J.L., Garden O.J., Poston G.J., Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Primrose J.N. Surgery for colorectal liver metastases. Br J Cancer. 2010;102:1313–1318. doi: 10.1038/sj.bjc.6605659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Geel A.N., Van Der Sijp J.R., Schmitz P.I. Which soft tissue sarcoma patients with lung metastases should undergo pulmonary resection? Sarcoma. 2002;6:57–60. doi: 10.1155/S1357714X02000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaifi J.T., Gusani N.J., Deshaies I., Kimchi E.T., Reed M.F., Mahraj R.P. Indications and approach to surgical resection of lung metastases. J Surg Oncol. 2010;102:187–195. doi: 10.1002/jso.21596. [DOI] [PubMed] [Google Scholar]
- 8.Da Silva Sardenberg R.A., De Figueiredo L.P., Haddad F.J., Luiz Gross J., Younes R.N. Pulmonary metastasectomy from soft tissue sarcomas. Clin Sao Paulo Brazil. 2010;65:871–876. doi: 10.1590/S1807-59322010000900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aitken K., Tree A., Thomas K., Nutting C., Hawkins M., Tait D. Initial UK experience of stereotactic body radiotherapy for extracranial oligometastases: can we change the therapeutic paradigm? Clin Oncol. 2015;27:411–419. doi: 10.1016/j.clon.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Milano M.T., Katz A.W., Zhang H., Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 11.Gomez D.R., Blumenschein G.R., Jack Lee J., Hernandez M., Ye R., Ross Camidge D. Local consolidative therapy versus maintenance therapy/observation for patients with oligometastatic non-small cell lung cancer without progression after front-line systemic therapy: results of a multi-institutional phase II randomized study. Lancet Oncol. 2016;17:1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iyengar P., Wardak Z., Gerber D.E., Tumati V., Ahn C., Hughes R.S. Consolidative radiotherapy for limited non-small-cell lung cancer. A phase 2 radomized clinical trial. JAMA Oncol. 2018;4:1–8. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palma D.A., Olson R.A., Harrow S., Gaede S., Louie A.V., Haasbeek C. Stereotactic Ablative Radiation Therapy for the Comprehensive Treatment of Oligometastatic Tumors (SABR-COMET): results of a randomized trial. Int J Radiat Oncol Biol Phys. 2018;102:S3–S4. [Google Scholar]
- 14.Al-Halabi H., Sayegh K., Digamurthy S.R., Niemierko A., Piotrowska Z., Willers H. Pattern of failure analysis in metastatic EGFR-mutant lung cancer treated with tyrosine kinase inhibitors to identify candidates for consolidation stereotactic body radiation therapy. J Thorac Oncol. 2015;10:1601–1607. doi: 10.1097/JTO.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 15.Kelly P., Ma Z., Baidas S., Moroose R., Shah N., Dagan R. Patterns of progression in metastatic estrogen receptor positive breast cancer: an argument for local therapy. Int J Breast Cancer. 2017 doi: 10.1155/2017/1367159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung P. Stereotactic body radiotherapy for oligoprogressive cancer. BJR. 2016;89(1066):20160251. doi: 10.1259/bjr.20160251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tree A.C., Khoo V.S., Eeles R.A., Ahmed M., Dearnaley D.P., Hawkins M.A. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14(1):PE28–PE37. doi: 10.1016/S1470-2045(12)70510-7. [DOI] [PubMed] [Google Scholar]
- 18.Ost P., Jereczek-Fossa B.A., Van As N., Zilli T., Muacevic A., Olivier K. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2016;69:9–12. doi: 10.1016/j.eururo.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita H., Niibe Y., Yamamoto T., Katsui K., Jingu K., Kanazawa S. Lung stereotactic radiotherapy for oligometastases: comparison of oligo-recurrence and sync-oligometastases. Jpn J Clin Oncol. 2016;46:687–691. doi: 10.1093/jjco/hyw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weickhardt A.J., Scheier B., Burke J.M., Gan G., Lu X., Bunn P.A. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta M. The dandelion effect: treat the whole lawn or weed selectively? J Clin Oncol. 2011;29:121–124. doi: 10.1200/JCO.2010.33.3294. [DOI] [PubMed] [Google Scholar]
- 22.James N., de Bono J., Spears M., Clarke N., Dearnaley D., Ritchie A. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fizazi K., Tran N., Fein L., Matsubara N., Rodriguez-Antolin A., Alekseev B.Y. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 24.de Bono J.S., Logothetis C.J., Molina A., Fizazi K., North S., Chu L. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beer T.M., Armstrong A.J., Rathkopf D.E., Loriot Y., Sternberg C.N., Higano C.S. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swain S.M., Baselga J., Kim S.-B., Ro J., Semiglazov V., Campone M. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 28.Mok T.S., Wu Y.-L., Thongprasert S., Yang C.-H., Chu D.-T., Saijo N. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 29.Shaw A.T., Kim D.-W., Nakagawa K., Seto T., Crinó L., Ahn M.-J. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 30.Solomon B.J., Mok T., Kim D.-W., Wu Y.-L., Nakagawa K., Mekhail T. First-line crizotinib versus chemotherapy in ALK-postive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 31.Sequist L.V., Waltman B.A., Dias-Santagata D., Digumarthy S., Turke A.B., Fidias P. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002003. 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H.A., Arcila M.E., Rekhtman N., Sima C.S., Zakowski M.F., Pao W. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottesman M.M. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 34.Gerlinger M., Rowan A.J., Horswell S., Larkin J., Endesfelder D., Gronroos E. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soucheray M., Capelletti M., Pulido I., Kuang Y., Paweletz C.P., Becker J.H. Intratumoral heterogeneity in EGFR-mutant NSCLC results in divergent resistance mechanisms in response to EGFR tyrosine kinase inhibition. Cancer Res. 2015;75:4372–4383. doi: 10.1158/0008-5472.CAN-15-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karantanos T., Evans C., Tombal B., Thompson T.C., Montironi R., Isaacs W.B. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur Urol. 2015;67:470–479. doi: 10.1016/j.eururo.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barok M., Joensuu H., Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:3378. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramos P., Bentires-Alj M. Mechanism-based cancer therapy: resistance to therapy, therapy for resistance. Oncogene. 2015;34:3617–3626. doi: 10.1038/onc.2014.314. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama R., Wataya H., Seto T., Ichinose Y. Treatment after the failure of gefitinib in patients with advanced or recurrent non-small cell lung cancer. Anticancer Res. 2009;29:4217–4221. [PubMed] [Google Scholar]
- 40.Nishie K., Kawaguchi T., Tamiya A., Mimori T., Takeuchi N., Matsuda Y. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol. 2012;7:1722–1727. doi: 10.1097/JTO.0b013e31826913f7. [DOI] [PubMed] [Google Scholar]
- 41.Yap T.A., Macklin-Doherty A., Popat S. Continuing EGFR inhibition beyond progression in advanced non-small cell lung cancer. Eur J Cancer. 2017;70:12–21. doi: 10.1016/j.ejca.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Park K., Yu C.-J., Kim S.-W., Lin M.-C., Sriuranpong V., Tsai C.-M. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer. JAMA Oncol. 2016;2:305–312. doi: 10.1001/jamaoncol.2015.4921. [DOI] [PubMed] [Google Scholar]
- 43.Scher H.I., Morris M.J., Stadler W.M., Higano C., Basch E., Fizazi K. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz L.H., Litière S., De Vries E., Ford R., Gwyther S., Mandrekar S. RECIST 1.1 – update and clarification: from the RECIST Committee HHS Public Access. Eur J Cancer. 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishino M., Cardarella S., Dahlberg S.E., Jackman D.M., Ramaiya N.H., Hatabu H. Radiographic assessment and therapeutic decisions at RECIST progression in EGFR-mutant NSCLC treated with EGFR tyrosine kinase inhibitors. Lung Cancer. 2013;79:283–288. doi: 10.1016/j.lungcan.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan R.L., Camidge D.R. Reviewing RECIST in the era of prolonged and targeted therapy. J Thorac Oncol. 2018;13:154–164. doi: 10.1016/j.jtho.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 47.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida T., Yoh K., Niho S., Umemura S., Matsumoto S., Ohmatsu H. RECIST progression patterns during EGFR tyrosine kinase inhibitor treatment of advanced non-small cell lung cancer patients harboring an EGFR mutation. Lung Cancer. 2015;90:477–483. doi: 10.1016/j.lungcan.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 49.McDonald E., Cheng S., Arciero V.S., Saluja R., Zukotynski K.A., Cheung P. Prevalence of oligoprogressive, metastatic castration-resistant prostate cancer (mCRPC) amenable to stereotactic ablative radiotherapy (SABR) in men undergoing abiraterone acetate (AA) therapy. J Clin Oncol. 2017;35:e587. [Google Scholar]
- 50.Aparici C.M., Seo Y. NIH Public Access. Semin Nucl Med. 2012;42:328–342. doi: 10.1053/j.semnuclmed.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiu B., Liang Y., Li Q.W., Liu G.H., Wang F., Chen Z.L. Local therapy for oligoprogressive disease in patients with advanced stage non-small-cell lung cancer harboring epidermal growth factor receptor mutation. Clin Lung Cancer. 2017;18:e369–e373. doi: 10.1016/j.cllc.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Chan O.S.H., Lee V.H.F., Mok T.S.K., Mo F., Chang A.T.Y., Yeung R.M.W. The role of radiotherapy in epidermal growth factor receptor mutation-positive patients with oligoprogression: a matched-cohort analysis. Clin Oncol. 2017;29:568–575. doi: 10.1016/j.clon.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 53.Triggiani L., Alongi F., Buglione M., Detti B., Santoni R., Bruni A. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer. 2017;116:1520–1525. doi: 10.1038/bjc.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merino Lara T., Helou J., Poon I., Sahgal A., Chung H.T., Chu W. Multisite stereotactic body radiotherapy for metastatic non-small-cell lung cancer: delaying the need to start or change systemic therapy? Lung Cancer. 2018;124:219–226. doi: 10.1016/j.lungcan.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Santini D., Ratta R., Pantano F., Lisi D., Maruzzo M., Galli L. Outcome of oligoprogressing metastatic renal cell carcinoma patients treated with locoregional therapy: a multicenter retrospective analysis. Oncotarget. 2017;8:100708–100716. doi: 10.18632/oncotarget.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu H.A., Sima C.S., Huang J., Solomon S.B., Rimner A., Paik P. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iyengar P., Kavanagh B.D., Wardak Z., Smith I., Ahn C., Gerber D.E. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol. 2014;32:3824–3830. doi: 10.1200/JCO.2014.56.7412. [DOI] [PubMed] [Google Scholar]
- 58.Palma D.A., Olson R., Harrow S., Gaede S., Louie A.V., Haasbeek C. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019 May 18;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]