Abstract
The in vitro MultiFlow® DNA Damage Assay multiplexes γH2AX, p53, phospho-histone H3, and polyploidization biomarkers into a single flow cytometric analysis. The current report describes a tiered sequential data analysis strategy based on data generated from exposure of human TK6 cells to a previously described 85 chemical training set and a new pharmaceutical-centric test set (n = 40). In each case, exposure was continuous over a range of closely spaced concentrations, and cell aliquots were removed for analysis following 4 and 24 hr of treatment. The first data analysis step focused on chemicals’ genotoxic potential, and for this purpose, we evaluated the performance of a machine learning (ML) ensemble, a rubric that considered fold increases in biomarkers against global evaluation factors (GEFs), and a hybrid strategy that considered ML and GEFs. This first tier further used ML output and/or GEFs to classify genotoxic activity as clastogenic and/or aneugenic. Test set results demonstrated the generalizability of the first tier, with particularly good performance from the ML ensemble: 35/40 (88%) concordance with a priori genotoxicity expectations and 21/24 (88%) agreement with expected mode of action (MoA). A second tier applied unsupervised hierarchical clustering to the biomarker response data, and these analyses were found to group certain chemicals, especially aneugens, according to their molecular targets. Finally, a third tier utilized benchmark dose analyses and MultiFlow biomarker responses to rank genotoxic potency. The relevance of these rankings is supported by the strong agreement found between benchmark dose values derived from MultiFlow biomarkers compared to those generated from parallel in vitro micronucleus analyses. Collectively, the results suggest that a tiered MultiFlow data analysis pipeline is capable of rapidly and effectively identifying genotoxic hazards while providing additional information that is useful for modern risk assessments—MoA, molecular targets, and potency.
Keywords: TK6 cells, mode of action, multiplexed, γH2AX, p53, clastogen, aneugen, benchmark dose
INTRODUCTION
Our laboratories have pursued the development and validation of a multiplexed flow cytometric assay that combines information from several biomarkers relevant to DNA damage response pathways and aneuploidy induction (Bryce et al. 2014, 2016, 2017, 2018; Bernacki et al. 2016). This so-called MultiFlow® DNA Damage Assay is formatted as an add-and-read test that efficiently prepares cells in microtiter plates for flow cytometric analysis. The biomarkers measured are: (1) phosphorylation of H2AX at serine 139 (γH2AX) to detect DNA double strand breaks, (2) phosphorylation of histone H3 at serine 10 (p-H3) to identify mitotic cells, (3) nuclear p53 content as an indicator of p53 activation in response to DNA damage, (4) frequency of 8n+ cells to monitor polyploidization, and (5) determination of nuclei counts to provide information about treatment-related cytotoxicity and cytostasis. Relative to individual standard in vitro genotoxicity assays, an advantage of the MultiFlow method and related high information content assays is that they go beyond genotoxic hazard identification by distinguishing between clastogenic and aneugenic modes of action (MoA) (Cheung et al., 2015; Khoury et al., 2016; Bryce et al. 2016).
Given the multiplexed nature of the MultiFlow assay, the data analysis procedures used to synthesize and interpret biomarker responses have resembled pattern-recognition tools as opposed to parametric and nonparametric pair-wise tests that are commonly applied to traditional single endpoint genotoxicity assays. One published example of a MultiFlow data analysis strategy makes use of a series of global evaluation factors (GEFs; Bryce et al. 2017). This approach is based on cutoff response values that were derived for each biomarker and time point from data collected by seven laboratories. To optimize agreement with a priori calls, a rubric was developed around the collection of cutoff values that categorizes chemicals as genotoxic or not, and if the former, whether the activity is clastogenic, aneugenic, or both. This approach was reported to exhibit good sensitivity and specificity across laboratories, and it provided reliable MoA information. However, an important caveat is that the initial report did not evaluate the method’s performance against chemicals that were outside of the training set, that is, with an external test set that was not used to develop the GEFs and associated rubric.
Other data analysis strategies have made use of supervised machine learning (ML) tools. In this paradigm, mathematical algorithms were developed based on training set data where genotoxic potential and MoA are known. The labeled data provided a means to create models that could then be used to make predictions based on new biomarker response data that were not part of the training set. For instance, most recently, an ensemble of three ML algorithms consisting of logistic regression (LR), random forest (RF), and an artificial neural network (ANN) has been described (Bryce et al. 2018). In this case, a majority vote was used to make a final prediction about genotoxicity and genotoxic MoA. As with GEFs, this ML strategy also demonstrated good performance characteristics, but in this case in a more convincing fashion, as performance was maintained with an external test set of 103 chemicals.
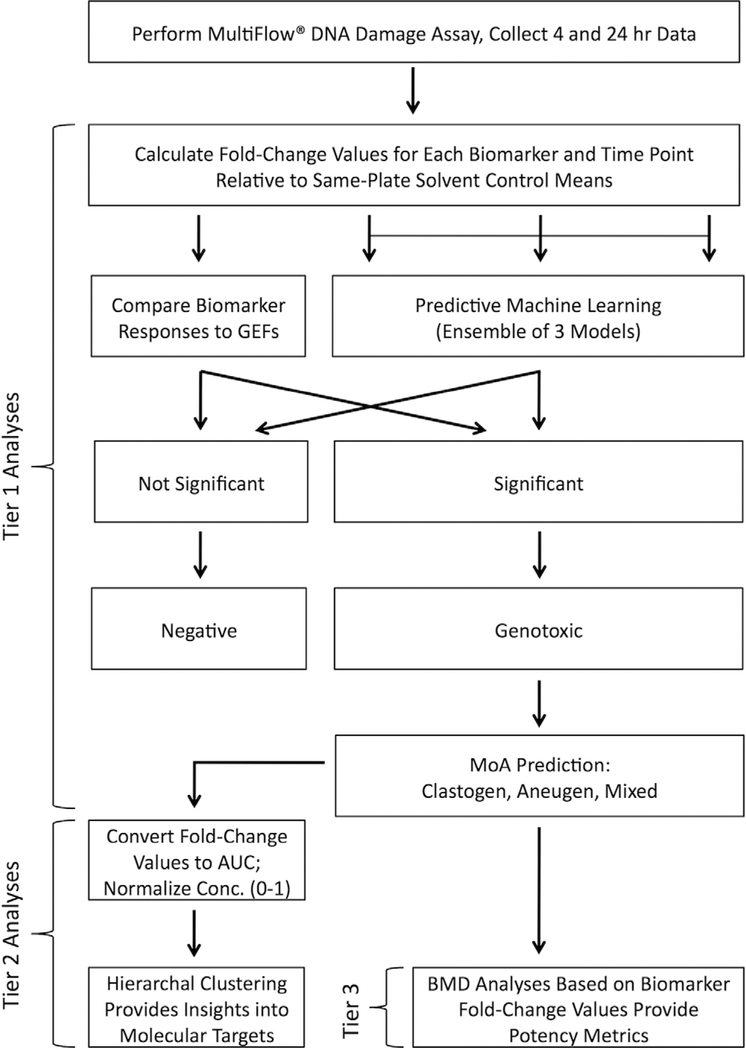
Although there are certain advantages and disadvantages to the GEF and ML data analysis strategies, their use is not mutually exclusive, so it was of interest to evaluate them further, both in isolation and together. The current experiments were therefore designed to extend our work with MultiFlow data analysis strategies by testing the performance of the GEF rubric and/or an ML ensemble using chemicals outside the training set. Furthermore, we investigated the utility of hierarchical clustering to group genotoxic chemicals with similar molecular targets, and evaluated the capacity of MultiFlow biomarker responses to provide genotoxicity potency ranking. For these investigations, MultiFlow data were generated from TK6 cells exposed to a diverse set of chemicals using a continuous treatment design (i.e., 24 hr), and in some cases these analyses were supplemented with in vitro micronucleus (MN) measurements. The results are discussed in terms of the performance and benefits of a sequential, tiered, high information content data analysis pipeline (see Fig. 1).
Fig. 1.
Flowchart representing a tiered MultiFlow assay data analysis pipeline. With this strategy, chemicals are evaluated for their genotoxic potential and genotoxic mode of action (Tier 1), insights into molecular target are provided by unsupervised clustering (Tier 2), and finally potency metrics are generated (Tier 3).
MATERIALS AND METHODS
Chemicals
The identities of 85 previously reported training set chemicals (Bryce et al. 2018) and a new set of pharmaceutical-centric test set chemicals (n = 40), the source, and other information, are provided in Table I. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ (MSD), supplied 20 of the 40 test chemicals (coded) to Litron, and these were stored at −20°C until they were solubilized in dimethyl sulfoxide (DMSO), at which point they were refrozen at −20°C. Additional test set chemicals (n = 20) were selected by Litron scientists largely from the list recommended by Kirkland and colleagues for evaluating new genotoxicity tests (Kirkland et al. 2016). Our a priori expectation regarding the in vitro mammalian cell genotoxicity potential for each of the 125 chemicals can be found in Table I. As explained in more detail below, the experiments reported herein occurred in the absence of an exogenous metabolic activation system. Thus, the a priori calls provided in Table I reflect expected genotoxicity assay results in the context of an S9-free mammalian assay system.
TABLE I.
Chemicals and a priori Classifications
| Chemical, abbreviation | CAS No., source if not Sigma-Aldrich | Chemical set | A priori mammalian cell genotoxicity and MoA classifications | Notes; References |
|---|---|---|---|---|
| 17β-Estradiol (est) | 50–28–2 | Training | Genotoxic; Aneugen | Steroid hormone; Hernández et al. (2013) |
| AMG-900 (amg) | 945595–80–2 | Training | Genotoxic; Aneugen | Pan-Aurora kinase inhibitor (A/B/C); Payton et al. (2010) |
| Carbendazim (car) | 10605–21–7 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Van Hummelen et al. (1995) |
| Colchicine (col) | 64–86–8 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Kirkland et al. (2016) |
| Crizotinib | 877399–52–5 | Training | Genotoxic; Aneugen | Tyrosine kinase inhibitor, potent activity against c-Met and ALK, with evidence of off-target Aurora kinase inhibition; Kong et al. (2018) |
| Diethylstilbestrol (des) | 56–53–1 | Training | Genotoxic; Aneugen | Synthetic estrogen; Parry et al. (2002) |
| Flubendazole (flu) | 3143015–6 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Tweats et al. (2016) |
| Griseofulvin (gli) | 126–07–8 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Oliver et al. (2006) |
| Mebendazole (meb) | 31431–39–7 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Van Hummelen et al. (1995) |
| Nocodazole (noc) | 31430–18–9 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Verdoodt et al. (1999) |
| Noscapine (nos) | 128–62–1 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Schuler et al. (1999) |
| Paclitaxel (pac) | 33069–62–4 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Kirkland et al. (2016) |
| Vinblastine sulfate (vin) | 143–67–9 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Kirkland et al. (2016) |
| Vincristine sulfate (vis) | 2068–78–2 | Training | Genotoxic; Aneugen | Mitotic spindle poison; Kondo et al. (1992) |
| 1,3-Propane sultone (psu) | 1120–71–4 | Training | Genotoxic; Clastogen | Alkylator; Dertinger et al. (2011) |
| 4-Nitroquinoline 1-oxide (nqo) | 56–57–5 | Training | Genotoxic; Clastogen | Likely several modes of clastogenic action that may include ROS; Kirkland et al. (2016) |
| 5-Fluorouracil | 51–21–8 | Training | Genotoxic; Clastogen | Anti-metabolite, thymidylate synthase inhibitor; Kirkland et al. (2016) |
| Aphidicolin | 38966–21–1 | Training | Genotoxic; Clastogen | DNA polymerase inhibitor; Glover et al. (1984) |
| Azathioprine | 446–86–6 | Training | Genotoxic; Clastogen | Prodrug of mercaptopurine, purine analog; Henderson et al. (1993) |
| Azidothymidine (azt) | 30516–87–1 | Training | Genotoxic; Clastogen | Nucleoside analog; Kirkland et al. (2016) |
| Bleomycin sulfate (bls) | 9041–93–4 | Training | Genotoxic; Clastogen | Radiomimetic; Rosefort et al. (2004) |
| Camptothecin (cam) | 7689–03–4 | Training | Genotoxic; Clastogen | Topoisomerase I inhibitor; Attia et al. (2009) |
| Chlorambucil (chl) | 305–03–3 | Training | Genotoxic; Clastogen | Nitrogen mustard-type alkylator; Dertinger et al. (2012) |
| Cisplatin (cis) | 15663–27–1 | Training | Genotoxic; Clastogen | Atypical alkylator; Kirkland et al. (2016) |
| Cytosine arabinoside (cya) | 147–94–4 | Training | Genotoxic; Clastogen | Anti-metabolite; Kirkland et al. (2016) |
| Doxorubicin | 23214–92–8 | Training | Genotoxic; Clastogen | Anthracycline, likely several modes of action that includes inhibition of topoisomerase II; Gewirtz (1999) |
| Emodin | 518–82–1 | Training | Genotoxic; Clastogen | Anthraquinone, topoisomerase II inhibitor; Li et al. (2010) |
| Ethyl methanesulfonate (ems) | 62–50–0 | Training | Genotoxic; Clastogen | Alkylator; Gocke et al. (2009) |
| Etoposide (etp) | 33419–42–0 | Training | Genotoxic; Clastogen | Topoisomerase II inhibitor; Kirkland et al. (2016) |
| Glycidamide (gly) | 5694–00–8 | Training | Genotoxic; Clastogen | Major in vivo metabolite of acrylamide; Paulsson et al. (2003) |
| Hydralazine HCl | 304–20–1 | Training | Genotoxic; Clastogen | Prepared in RPMI medium; Martelli et al. (1995) |
| Hydrogen peroxide (hyp) | 7722–84–1 | Training | Genotoxic; Clastogen | ROS, prepared in RPMI medium; Kimura et al. (2013) |
| Hydroxyurea (hyu) | 127–07–1 | Training | Genotoxic; Clastogen | Anti-metabolite, ribonucleotide reductase inhibitor; Dertinger et al. (2012) |
| Melphalan | 142–82–3 | Training | Genotoxic; Clastogen | Nitrogen mustard-type alkylator; Dertinger et al. (2012) |
| Menadione (men) | 58–27–5 | Training | Genotoxic; Clastogen | ROS implicated; Cojocel et al. (2006) |
| Methotrexate | 59–05–2 | Training | Genotoxic; Clastogen | Anti-metabolite; Keshava et al. (1998) |
| Methyl methanesulfonate | 66–27–3 | Training | Genotoxic; Clastogen | Alkylator; Kirkland et al. (2016) |
| N-Methyl-N′-nitro-N-nitrosoguanidine (MNNG) | 70–25–7 | Training | Genotoxic; Clastogen | Alkylator; Nikolova et al. (2014) |
| Mitomycin C (mmc) | 50–07–7 | Training | Genotoxic; Clastogen | DNA cross-linker; Kirkland et al. (2016) |
| N-Ethyl-N-nitrosourea | 759–73–9 | Training | Genotoxic; Clastogen | Alkylator; Kirkland et al. (2016) |
| Olaparib (ola) | 763113–22–0 | Training | Genotoxic; Clastogen | PARP inhibitor; FDA approved label (Lynparza™ 2014) |
| Propyl gallate | 121–79–9 | Training | Genotoxic; Clastogen | ROS likely; Tayama and Nakagawa (2001) |
| Resorcinol diglycidyl ether | 101–90–6 | Training | Genotoxic; Clastogen | Gulati et al. (1989) |
| Stavudine | 3056–17–5 | Training | Genotoxic; Clastogen | Nucleoside analog; FDA approved label (Zerit® 2002) |
| Temozolomide (tmz) | 85622–93–1 | Training | Genotoxic; Clastogen | Alkylator; Chinnasamy et al. (1997) |
| Thiotepa (thi) | 52–24–4 | Training | Genotoxic; Clastogen | Alkylator; Dertinger et al. (2012) |
| Topotecan (top) | 123948–87–8 | Training | Genotoxic; Clastogen | Topoisomerase I inhibitor; Aydemir and Bilaloğlu (2003) |
| Alosetron HCl | 122852–42–0 | Training | Non-genotoxic | 5-HT3 antagonist; Kirkland et al. (2016) |
| Amitrole | 61–82–5 | Training | Non-genotoxic | Kirkland et al. (2016) |
| Anthranilic acid | 118–92–3 | Training | Non-genotoxic | Kirkland et al. (2016) |
| Brefeldin A | 20350–15–6 | Training | Non-genotoxic | ER-golgi transporter inhibitor, ER stress-induced apoptosis; Moon et al. (2012) |
| Caffeine | 58–08–2 | Training | Non-genotoxic | Mitochondria-dependent apoptosis, ROS involvement likely; Lu et al. (2008) |
| Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) | 555–60–2 | Training | Non-genotoxic | Uncoupler of oxidative phosphorylation; de Graaf et al. (2004) |
| Clofibrate | 637–07–0 | Training | Non-genotoxic | Antilipidemic agent; IARC Monograph (2018) |
| Cyclohexanone | 108–94–1 | Training | Non-genotoxic | Industrial chemical; Kirkland et al. (2008) |
| Cycloheximide | 66–81–9 | Training | Non-genotoxic | Protein synthesis inhibitor; Youngblom et al. (1989) |
| D-Limonene | 5989–27–5 | Training | Non-genotoxic | Male rat kidney tumors due to α2μ-globulin nephropathy; Kirkland et al. (2016) |
| D-Mannitol | 69–65–8 | Training | Non-genotoxic | Polyol; Kirkland et al. (2016) |
| Dexamethasone | 50–02–2 | Training | Non-genotoxic | Glucocorticoid receptor agonist; Krishna et al. (1995) |
| Dextrose | 50–99–7 | Training | Non-genotoxic | Sugar; Lotz et al. (2009) |
| Di-(2-ethylhexyl) phthalate (DEHP) | 117–81–7 | Training | Non-genotoxic | Organic plasticizer; Kirkland et al. (2016) |
| Diethanolamine | 111–42–2 | Training | Non-genotoxic | Secondary amine; Kirkland et al. (2016) |
| Erythromycin | 114–07–8 | Training | Non-genotoxic | Antibiotic; Kirkland et al. (2016) |
| Famotidine | 76824–35–6 | Training | Non-genotoxic | Histamine H2 receptor antagonist; FDA approved label (Pepcid® 2011) |
| Imatinib mesylate | 152459–95–5 | Training | Non-genotoxic | Protein-tyrosine kinase inhibitor; FDA approved label (Gleevec® 2001) |
| Hexachloroethane | 67–72–1 | Training | Non-genotoxic | Industrial chemical; Kirkland et al. (2016) |
| Lidocaine | 137–58–6 | Training | Non-genotoxic | Amide, local anesthetic; FDA approved label (Lidoderm® 2004) |
| Lovastatin | 75330–75–5 | Training | Non-genotoxic | HMG-CoA reductase inhibitor; FDA approved label (Mevacor® 2012) |
| Melamine | 108–78–1 | Training | Non-genotoxic | Industrial organic base; Kirkland et al. (2016) |
| Methyl carbamate | 598–55–0 | Training | Non-genotoxic | Industrial intermediate; Kirkland et al. (2016) |
| N-Butyl chloride | 109–69–3 | Training | Non-genotoxic | Fumigant; Kirkland et al. (2016) |
| Ofloxacin | 82419–36–1 | Training | Non-genotoxic | Fluoroquinoline antibiotic; FDA approved label (Floxin® 2008) |
| Paroxetine | 61869–08–7 | Training | Non-genotoxic | SSRI antidepressant; FDA approved label (Paxil® 2011) |
| Phenanthrene | 85–01–8 | Training | Non-genotoxic | Polycyclic aromatic hydrocarbon; Kirkland et al. (2008) |
| Phenformin HCl | 834–28–6 | Training | Non-genotoxic | Biguanide antidiabetic; Kirkland et al. (2016) |
| Progesterone | 57–83–0 | Training | Non-genotoxic | Steroid hormone; Kirkland et al. (2008) |
| Pyridine | 110–86–1 | Training | Non-genotoxic | Heterocyclic organic compound; Kirkland et al. (2016) |
| Sodium chloride | 7647–14–5 | Training | Non-genotoxic | Prepared in RPMI medium; Matsushima et al. (1999) |
| Sodium dodecyl sulfate | 151–21–3 | Training | Non-genotoxic | Ionic detergent; National Toxicology Program Report (2018b) |
| Sucrose | 57–50–1 | Training | Non-genotoxic | Diaz et al. (2007) |
| Tert-butyl alcohol | 75–65–0 | Training | Non-genotoxic | Kirkland et al. (2016) |
| Thapsigargin | 6726–95–8 | Training | Non-genotoxic | ER stress-induced apoptosis; Futami et al. (2005) |
| Tolterodine L-tartrate | 124937–52–6 | Training | Non-genotoxic | Muscarinic receptor antagonist; Kirkland et al. (2016) |
| Tunicamycin | 11089–65–9 | Training | Non-genotoxic | Glycosylation inhibitor, ER stress-mediated apoptosis; Han et al. (2008) |
| Zonisamide | 68291–97–4 | Training | Non-genotoxic | Sulfonamide anticonvulsant; Kirkland et al. (2016) |
| 10j | MSD | Test | Genotoxic; Aneugen | Tyrosine kinase inhibitor; Ames neg., CHO MN pos., CHO ChromAb neg. but polyploidy evident; MSD in-house results |
| 13 m | MSD | Test | Genotoxic; assumed Aneugen | Tyrosine kinase inhibitor; CHO and TK6 MN pos.; MSD in-house results |
| 14n | MSD | Test | Genotoxic; Aneugen | Serine/threonine kinase inhibitor; Ames neg., CHO MN pos., CHO ChromAb neg. but polyploidy and endoreduplication evident; TK6 MN neg.; MSD in-house results |
| 16p | MSD | Test | Genotoxic; Mixed | Azobenzimidazole structure; Likely >1 MoA; Ames neg., CHO MN and ChromAb pos. with premature centromere separation at metaphase in addition to structural aberrations, Rat MN neg.; MSD in-house results |
| 17q | MSD | Test | Genotoxic; assumed Aneugen | Benzimidazole structure; Ames neg., CHO MN pos.; MSD in-house results |
| Phenolphthalein, supplied coded as 3c | 77–09–8; MSD | Test | Genotoxic; Mixed | Likely >1 MoA; Spindle poison, centromere amplification (Heard et al. 2013); CHO ChromAb pos.; MSD in-house results |
| 6f | MSD | Test | Genotoxic; Aneugen | Kinase inhibitor, leucine-rich repeat; Ames neg., CHO MN pos., CHO ChromAb neg., rat MN pos.; MSD in-house results |
| Hesperadin | 422513–13–1; Selleckchem | Test | Genotoxic; Aneugen | Aurora kinase inhibitor (B); in vitro MN pos., aberrant metaphases; Hauf et al. (2003), Kurihara et al. (2006) |
| Tozasertib | 639089–54–6; Selleckchem | Test | Genotoxic; Aneugen | Pan-Aurora kinase inhibitor (A/B/C); Gollapudi et al. (2014) |
| ZM-447439 | 331771–20–1; Selleckchem | Test | Genotoxic; Aneugen | Aurora kinase inhibitor (A/B); Gollapudi et al. (2014) |
| 7 g | MSD | Test | Genotoxic, MoA uncertain | Possibly >1 MoA; Ames pos., CHO MN pos., CHO ChromAb neg., TK6 MN pos., HPBL MN neg.; mechanism affects tubulin so suspected aneugen, but Ames pos. suggests primary DNA damage; MSD in-house results |
| 9i | MSD | Test | Genotoxic; Clastogen | Non-nucleoside antiviral; Ames neg., CHO MN pos., CHO ChromAb pos.; MSD in-house results |
| AZD2858 | 486424–20–8; Selleckchem | Test | Genotoxic; Clastogen | Glycogen synthase-3 inhibitor; in vitro MN and ChromAb pos., Ann Doherty, personal communication |
| Beta-Lapachone | 4707–32–8; Selleckchem | Test | Genotoxic; Clastogen | Topoisomerase I inhibitor; in vitro ChromAb and comet pos.; Degrassi et al. (1993) |
| Ciprofloxacin | 85721–33–1; Selleckchem | Test | Genotoxic; Clastogen | Topoisomerase II inhibitor; in vitro MN pos.; Curry et al. (1996) |
| Dasatinib | 302962–49–8; Selleckchem | Test | Genotoxic; Clastogen | Tyrosine kinase inhibitor, especially Ber-Abl, Scr, c-Kit; Ames neg., clastogenic in CHO, in vivo MN neg.; Sprycel® (dasatinib) (2010) |
| Genistein | 446–72–0 | Test | Genotoxic; Clastogen | Topoisomerase II inhibitor; in vitro MN pos.; Klein and King (2007) |
| Irinotecan | 57852–57–0; Selleckchem | Test | Genotoxic; Clastogen | Topoisomerase I inhibitor; Ames neg., in vitro ChromAb pos., in vivo MN pos.; Camptosar (2014) |
| Mitoxantrone 2HCl | 70476–82–3; Selleckchem | Test | Genotoxic; Clastogen | Topoisomerase II inhibitor; in vitro MN pos., γH2AX pos.; Smart et al. (2008) |
| Teniposide | 29767–20–2; Selleckchem | Test | Genotoxic; Clastogen | Topoisomerase II inhibitor; in vitro ChromAb pos., in vitro MLA pos.; DeMarini et al. (1987) |
| Entecavir, supplied coded as 19 s | MSD | Test | Genotoxic; Clastogen | Guanine nucleoside analog; CHO MN pos., CHO ChromAb pos.; MSD in-house data |
| Hydroquinone, supplied coded as 1a | 123–31–9; MSD | Test | Genotoxic; Mixed | Likely >1 MoA; Kirkland et al. (2016) |
| 20 t | MSD | Test | Genotoxic; Clastogen | Adenosine nucleoside analog; Ames neg., CHO MN pos., CHO ChromAb pos.; MSD in-house results |
| Tetrahydroxydiboron, supplied coded as 4d | MSD | Test | Genotoxic; Clastogen | Ames pos., CHO MN pos., CHO ChromAb pos.; MSD in-house results |
| 6-Thioguanine | 154–42–7 | Test | Genotoxic; Clastogen | Antimetabolite, purine analog; Ames pos., in vitro ChromAb pos., in vivo MN pos.; National Toxicology Program Report (2018a) |
| 11 k | MSD | Test | Non-genotoxic | Tryrosine kinase inhibitor; Ames neg., CHO MN neg., Rat MN neg.; MSD in-house data |
| 12 L | MSD | Test | Non-genotoxic | Drug candidate; Ames neg., CHO MN neg.; MSD in-house results |
| 15o | MSD | Test | Non-genotoxic (in TK6 cells) | Tryrosine kinase inhibitor; CHO MN weak pos. only at 24 hr; CHO ChromAb neg., TK6 MN neg., Rat MN neg.; MSD in-house results |
| 18r | MSD | Test | Non-genotoxic | Benzimidazole structure; Ames neg., CHO MN neg.; MSD in-house results |
| 2b | 20624–25–3; MSD | Test | Non-genotoxic | Sodium diethyldicarbamate trihydrate; CHO MN pos., TK6 MN pos., but cited authors attribute results to cytotoxicity; Hilliard et al. (1998), Galloway et al. (1998), Greenwood et al. (2004); Cu and Zn chelator, superoxide dismutase inhibitor; Heikkila et al. (1976), Nicotera et al. (1989) |
| 5e | MSD | Test | Non-genotoxic (in mammalian cells) | Aryl boronic acid; Ames pos., CHO MN neg.; MSD in-house results |
| 8 h | MSD | Test | Non-genotoxic | HDAC inhibitor; Ames neg., CHO MN neg.; CHO ChromAb neg.; MSD in-house results |
| Ampicillin trihydrate | 7177–48–2 | Test | Non-genotoxic | Ames neg., in vitro ChromAb neg., in vivo MN neg.; Kirkland et al. (2016) |
| Anisomycin | 22862–76–6 | Test | Non-genotoxic | Protein biosynthesis inhibitor; in vitro MN neg. with high levels of apoptosis; personal communication, Maik Schuler |
| Chlorocholine chloride | 999–81–5 | Test | Non-genotoxic | Ames neg., in vitro and in vivo ChromAb neg.; Kirkland et al. (2016) |
| Menthol | 89–78–1 | Test | Non-genotoxic | Ames neg., in vitro MN neg. in p53 competent cell lines, in vivo MN and comet neg.; Kirkland et al. (2016) |
| Osimertinib | 1421373–65–0 | Test | Non-genotoxic | EGFR kinase inhibitor; in vitro and in vivo genetox neg.; Tagrisso™ (Osimertinib) (2012) |
| Topiramate | 97240–79–4 | Test | Non-genotoxic | Ames, in vitro ChromAb and MLA neg., in vivo ChromAb Neg.; Kirkland et al. (2016) |
| Tris (2-ethylhexyl) phosphate | 78–42–2 | Test | Non-genotoxic | Ames neg., in vitro ChromAb neg., in vivo ChromAb and MN neg.; Kirkland et al. (2016) |
| Zafirlukast | 107753–78–6 | Test | Non-genotoxic | Ames, in vitro ChromAb, MLA, and Hprt neg.; Kirkland et al. (2016) |
Abbreviations: CHO, Chinese hamster ovary cells; ChromAb, chromosome aberration; EGFR, epithelial growth factor receptor; ER, endoplasmic reticulum; FDA, US Food and Drug Administration; Hprt, hypoxanthine guanine phosphoribosyltransferase; MDS, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.; MLA, mouse lymphoma assay; MN, micronuclei; MoA, mode of action; NTP, National Toxicology Program; PARP, poly ADP ribose polymerase; ROS, reactive oxygen species.
Cell Culture and Treatments
TK6 cells were purchased from ATCC® (cat. no. CRL-8015). Cells were grown in a humidified atmosphere at 37°C with 5% CO2, and were maintained at or below 1 × 106 cells/mL. The culture medium consisted of RPMI 1640 with 200 μg/mL sodium pyruvate (both from Sigma-Aldrich, St. Louis, MO), 200 μM L-glutamine, 50 units/mL penicillin and 50 μg/mL streptomycin (from Mediatech Inc., Manassas, VA), and 10% v/v heat-inactivated horse serum (Gibco®, a Thermo Fisher Scientific Company, Waltham, MA).
Chemicals selected by Litron scientists were tested using the same experimental design described previously (Bryce et al. 2016, 2017). Briefly, treatments occurred in U-bottom 96 well plates, with 198 μL TK6 cell suspension (2 × 105/mL) combined with 2 μL of DMSO-solubilized test chemical per well. The highest concentration tested was 1 mM, and the 19 additional concentrations were tested using a square root dilution scheme—that is, each concentration differed from the one above by a factor of 70.71%. In this manner, a wide range of concentrations were evaluated (i.e., nearly three orders of magnitude, 0.0014 to 1 mM). Each of the 20 concentrations was tested in a single well, whereas solvent was evaluated in four replicate wells. Upon addition of test chemical, the plates were immediately incubated in a humidified atmosphere at 37°C with 5% CO2 for 24 hr.
MSD-supplied chemicals were tested similarly, with the following exceptions. Preliminary dose-range finding experiments were used to generate 24 hr relative nuclei count (RNC) data for each chemical provided (via Multi-Flow®—Cleaved PARP Kit, Litron Laboratories, Rochester, NY). Concentrations for the definitive experiment were chosen based on the RNC results with the intention to test at least one concentration that approached or slightly exceeded the MultiFlow assay’s cytotoxicity limit, which is 80% reduction to RNC at 24 hr (Bryce et al. 2016). There were two exceptions, 14n and 16p, compounds that were tested up to maximal feasible concentrations due to the low quantity of chemical that could be supplied (4.41 and 100 μM, respectively). For the definitive experiments, 10 concentrations of each chemical were tested in duplicate wells of a 96 well plate. As described above, the majority of chemicals were tested using a square root 2 dilution scheme. Based on data from preliminary dose-range finding experiments, some chemicals were tested using finer dilution schemes.
MultiFlow Assay
TK6 cells were prepared for analysis using reagents and instructions included in the MultiFlow® DNA Damage Kit—p53, γH2AX, Phospho-Histone H3 (Litron Laboratories). Components and preparation of the MultiFlow working solution have been described in detail previously (Bryce et al. 2016, 2017). At the 4 and 24 hr sampling times, cells were resuspended with pipetting, then 25 μL were removed from each well and added to a new 96-well plate containing 50 μL/well of pre-aliquoted working MultiFlow reagent solution. Mixing was accomplished by pipetting the contents of each well several times. After incubation at room temperature for 30 min, samples were analyzed via flow cytometry.
Flow cytometric analysis was carried out using either a FACSCanto™ II flow cytometer equipped with a BD™ High Throughput Sampler or a Miltenyi Biotec MACSQuant® Analyzer 10 flow cytometer with integrated 96-well MiniSampler device. Stock photomultiplier tube detectors and associated optical filter sets were used to detect fluorescence emissions associated with the fluorochromes: FITC (detected in the FITC channel, to use BD instrument parlance), PE (PE channel), propidium iodide (PerCP-Cy5.5 channel), and Alexa Fluor® 647 (APC channel).
Representative bivariate graphs, gating logic, and position of regions were described in detail in earlier reports (Bernacki et al. 2016; Bryce et al. 2016, 2017). Briefly, two biomarker measurements, γH2AX and p53, were based on the shift in median channel fluorescence intensity relative to same-plate solvent controls. Polyploidy and p-H3 biomarker measurements were based on their frequency among other nuclei. Nuclei to counting bead ratios were calculated for each sample, and these ratios were used to determine absolute nuclei counts (those with 2n and greater DNA-associated propidium iodide fluorescence). Nuclei counts were used to derive RNC, and percentage of cytotoxicity was calculated as 100% minus %RNC at 24 hr.
MultiFlow Data Analysis: Preprocessing
Data analyses described herein were restricted to those concentrations that did not exceed the MultiFlow assay’s cytotoxicity limit, that is, the top concentration of each chemical had to exhibit ≤80% reduction to RNC at the 24 hr time point. This has been described previously by Bryce et al. (2016, 2017). The present report differs slightly, such that in addition to the 80% maximum cytotoxicity limit noted above, only two concentrations within the cytotoxicity range 70–80% were permitted. Finally, except for 14n and 16p as noted above, in the absence of excessive cytotoxicity the top concentration was 1 mM or the lowest precipitating concentration, whichever was lower.
For the GEF, ML, and benchmark dose analyses described below, 4 and 24 hr γH2AX, p53, and p-H3 measurements, and 24 hr polyploidy frequencies, were converted to fold-change values by dividing them by the mean value associated with solvent-exposed cultures on the same plate (Microsoft Excel 2008, v12.3.6). This was performed for every test article concentration that was not excluded due to excessive cytotoxicity or other limits described above.
Unsupervised clustering analyses benefitted from several transformations. First, feature scaling (also known as unity-based normalization) was applied to every test article concentration to bring the values into the range 0 to 1 (Jayalakshmi and Santhakumaran 2011). Second, for each biomarker response and time point combination, fold-change values vs. normalized concentration curves were used to generate an area under the curve (AUC) value. AUC provided a means of converting each biomarker dose–response relationship for every chemical into a single value. This was accomplished using Microsoft Excel via the trapezoidal rule as described at https://calculushowto.com/find-the-area-under-the-curve-inexcel. One (1) was subtracted from every biomarker’s fold-change value before AUC calculations were made in order to set the no effect (baseline) value to zero. With this offset in place, AUC values were zero or nearly so in the case of no response, positive in the case of an increase, and negative in the case of a reduction. Also note that polyploid fold-change values were transformed with the square root function, a processing step that converted this biomarker’s dynamic range to one that more closely approximated that of the other biomarkers (found to be advantageous for ANN models, see Bryce et al. 2018).
MultiFlow Data Analysis: GEFs
MultiFlow biomarker/time point combinations were compared to GEFs reported by Bryce et al. (2017). GEFs for the three clastogen-responsive biomarkers 4 hr γH2AX, 4 hr p53, and 24 hr γH2AX were 1.51-, 1.40-, and 2.11-fold, respectively; GEFs for the three aneugen-responsive biomarkers 4 hr p-H3, 24 hr p-H3, and 24 hr polyploidy were 1.71-, 1.52-, and 5.86-fold, respectively; and the GEF for the pan-genotoxicant (clastogen- and aneugen-responsive) biomarker, 24 hr p53 was 1.45-fold. Meeting or exceeding these interlaboratory-derived values identified a significant biomarker response at a particular time point. To synthesize the results of these multiple comparisons and to make judgments about genotoxic potential and MoA, the following rubric was applied. A genotoxic call with a clastogenic MoA required two successive concentrations to meet or exceed the GEF for at least two out of four clastogen-sensitive biomarkers: 4 hr γH2AX, 4 hr p53, 24 hr γH2AX, and 24 hr p53. A genotoxic call with an aneugenic MoA required two successive concentrations to meet or exceed the GEF for at least two out of four aneugen-sensitive biomarkers: 4 hr p-H3, 24 hr p-H3, 24 hr polyploidy, and 24 hr p53. In cases where both clastogen and aneugen call criteria were met, the call was genotoxic with a “mixed” MoA. When the above criteria were not met, the call was non-genotoxic under the test conditions.
MultiFlow Data Analysis: ML Ensemble
The development and use of three ML models, multinomial LR, ANN, and RF, was described in detail previously (Bryce et al. 2018). Briefly, these various models utilize 4 and 24 hr MultiFlow data fold-change values and predict whether a chemical exhibits genotoxic activity or not, and if present whether the genotoxicity occurs via a clastogenic, aneugenic, or clastogenic and aneugenic MoA. Each model’s output was synthesized into genotoxicity and MoA calls as follows. Genotoxic, with evidence for a clastogenic MoA, required two successive concentrations to exhibit clastogen probability scores ≥80%, or one concentration to exhibit a clastogen probability score ≥90%. Genotoxic, with evidence for an aneugen MoA, required two successive concentrations to exhibit aneugen probability scores ≥80%, or one concentration to exhibit an aneugen probability score ≥90%. Non-genotoxic was defined as the absence of two successive concentrations exhibiting clastogen or aneugen probability scores ≥80%, and no one concentration exhibiting a clastogen or aneugen probability score ≥90%.
A majority vote ensemble considered the genotoxicity calls from each of the three modeling approaches as described above. A simple majority (two out of three) was necessary for a summary genotoxic call. For most chemicals, MoA predictions were found to be in agreement across models. In instances when models showed significant clastogen and aneugen probabilities, the chemical was considered genotoxic with evidence for a mixed MoA.
MultiFlow Performance Assessments
Training and test set chemicals were evaluated against a priori genotoxicity and MoA expectations. This was accomplished by evaluating the performance of the GEF rubric and ML ensemble on their own. Furthermore, we investigated a hybrid strategy that made use of both GEFs and ML predictions. With this approach, an overall genotoxic call was made when either the GEF or ML ensemble was positive.
For each strategy described above, performance was assessed by determining the level of agreement between expected and observed genotoxicity calls. This was accomplished by calculating the percentage of chemicals correctly identified as being genotoxic or non-genotoxic. Furthermore, for those agents that were identified as genotoxic, the level of agreement between MoA calls was also made by calculating the percentage of compounds that showed expected MoA. In the several instances where a priori MoA was either difficult to define or hypothesized to be a mixed MoA, any genotoxic MoA prediction was considered correct. In cases where a presumably non-genotoxic chemical was identified as genotoxic, any/all associated MoA calls were considered incorrect.
Unsupervised Clustering
Chemicals that were identified as aneugens by the GEF and/or ML approach were evaluated using JMP software’s unsupervised clustering platform (JMP, v12.0.1). As described above, the biomarker response data were first converted to AUC values, and when clustering aneugens, the following seven biomarkers were used as variables: 4 hr γH2AX, p-H3 and p53, and 24 hr γH2AX, p-H3, p53 and 24 hr polyploidy. The analysis options were set as follows: clustering method = hierarchical; method for calculating distances between clusters = “Ward”; data as usual = “Standardize Data”; data visualization = “Dendrogram,” with “two way clustering.”
Chemicals identified as clastogens by the GEF and/or ML approach were evaluated in a similar manner. However, in this case, the four variables were utilized: 4 hr γH2AX, 4 hr p53, 24 hr γH2AX, and 24 hr p53.
Benchmark Dose Analyses
A subset of the reference genotoxic chemicals (n = 34) was evaluated for in vitro MN formation using TK6 cells from the same treated cultures used in the MultiFlow assay. These analyses were conducted at the 24 hr time point, and were accomplished via flow cytometric analysis using In Vitro MicroFlow® Kit reagents (Litron Laboratories). These methods have been reported in detail elsewhere (Avlasevich et al. 2006). For the MN endpoint, concentrations were limited to those that resulted in ≤55% reduction to RNCs.
The Benchmark Dose (BMD) for continuous data is defined as the dose or exposure that results in a predetermined percent change (benchmark response, BMR) in the response rate of an adverse effect relative to the response in the concurrent controls, generally in the range of 1–10% increase in the background (MacGregor et al. 2015). Traditionally, the BMD approach is utilized to estimate a Point of Departure (PoD) value from in vivo studies and thereby derive compound-specific reference values in human health safety assessment; in these cases, the choice of BMR value should be justified. There is much debate over the appropriate BMR value to be selected, with several expert groups suggesting that an endpoint-specific effect size approach is necessary (Slob 2016; Zeller et al. 2016, 2017). Briefly, a “one-size-fits-all” BMR in the range of 1–10% is suggested to be inappropriate to apply to all endpoints. One theory favors scaling the BMR in percent change to the maximum response observed for the endpoint, which takes into account natural variation (Slob 2016). Based on a similar principle, work by Zeller et al. (2016) examined data from multiple genotoxicity endpoints to calculate endpoint-specific “response quotients” by dividing the assay’s response at a non-genotoxic dose level by the response of the concurrent vehicle control. The resulting fold-increase values over control were converted into BMR values in percent. Results of applying the approach to datasets derived from multiple in vivo genotoxicity studies range from 20% to 110%.
Importantly, it is not the purpose of this study to derive PoD metrics to infer reference values. Rather, we chose BMRs to minimize variation at the respective point in the dose response curve and thereby generate more precise PoD estimates (Slob and Setzer 2014; Wills et al. 2015; Bemis et al. 2016). Furthermore, the choice of BMR is not critical for in vitro endpoint comparisons, providing that it is the same value for all chemicals in the group. This has been demonstrated by Bemis et al. (2016), who produced BMD confidence intervals for various BMR values and showed similar correlations.
BMD analyses were performed for the subset of 34 chemicals with concurrent MultiFlow and MicroFlow data. Specifically, γH2AX, p-H3, p53, and in vitro MN dose responses were evaluated using PROAST (v63.3). Values for Critical Effect Size (CES, in PROAST notation) of 0.5 (BMR 50%), or 1.0 (BMR 100%, in the case of in vitro MN compounds mitomycin C, 4-nitroquinoline 1-oxide, and topotecan—which failed to yield a dose response with BMR 50%) were used for BMD analysis for the compounds. Compound was selected as covariate per endpoint and CES. The resulting 95% confidence intervals (CIs) were used to represent the relative potency of the compound for the endpoint under study. After ranking the in vitro MN induction potency of each compound, the data were compared with 24 hr γH2AX and 24 hr p53 endpoints for the clastogen group of compounds, and 24 hr p-H3 and 24 hr p53 endpoints for the aneugen group of compounds. These correlations are represented in cross system plots on a double log scale (Bemis et al. 2016; Soeteman-Hernández et al. 2016). The analyses were conducted separately for clastogens (n = 21) and aneugens (n = 13).
Figure 1 is a schematic representation of the overall data analysis strategy that was configured into three tiers and applied to MultiFlow data as described in detail above.
RESULTS AND DISCUSSION
Tier 1 Analyses: Training Set
The 85 reference chemicals that comprise the training set were given a priori classifications in regard to their genotoxic potential, as well as their predominant genotoxic MoA, clastogenicity or aneugenicity (Table I). Results for several of these agents are presented in detail in order to describe prototypical response profiles, and to introduce a new data visualization tool. These examples should provide a useful background for interpreting the aggregate chemical results that are presented hereafter.
Thapsigargin is an inhibitor of the sarco/endoplasmic reticulum Ca++ ATPase (Rogers et al. 1995). A radar plot portrays each biomarker response and time point combination as a function of concentration (Fig. 2a). As expected for a non-genotoxicant, no substantial increases in γH2AX, p-H3, p53, or polyploidization biomarkers were observed, despite that fact that it was tested to cytotoxic concentrations (71% cytotoxicity). Thus, it is not surprising that neither the GEF rubric or any of the three ML models predicted genotoxicity (Table II).
Fig. 2.
Radar plots show MultiFlow assay data for seven biomarker/time point combinations and for each of four chemicals: (a) thapsigargin, (b) 4-nitroquinoline 1-oxide (4NQO), (c) mebendazole, and (d) crizotinib. The biomarker data are expressed as fold-increase over mean solvent control on the same plate, and each chemical concentration appears as a different colored line. The topmost endpoint (24 hr p53, at 12 o’clock) is a pan-genotoxic biomarker, whereas the biomarkers arranged on the right side of the graph are responsive to clastogens and those arranged on the left are responsive to aneugens.
TABLE II.
Training Set Chemicals, N = 85
| Chemical | Group | a priori Genotox Expectation | Machine Learning (ML) Ensemble | Global Evaluation Factor (GEF) Rubric | ML + GEF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aneugen Calls | Clastogen Calls | Overall ML Genotoxicity Call | Overall ML MoA Call | Aneugen Calls | Clastogen Calls | Overall GEF Genotoxicity Calls | Overall GEF MoA Calls | Overall ML + GEF Genotoxicity Calls | Overall MF + GEF MoA Calls | |||||||
| RF | LR | ANN | RF | FR | ANN | |||||||||||
| 17B-Estradiol | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| AMG-900 | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Carbendazim | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Colchicine | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Crizotinib | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Diethylstilbestrol | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Flubendazole | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Griseofulvin | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Mebendazole | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Nocodazole | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Noscapine | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Paclitaxel | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Vinblastine sulfate | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| Vincristine sulfate | Training | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| 1,3-Propane sultone | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| 4-Nitroquinoline N-oxide | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| 5-Fluorouracil | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Aphidicolin | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Azathioprine | Training | Clastogen | – | – | – | + | – | + | + | C | – | – | – | + | C | |
| Azidothymidine | Training | Clastogen | – | – | – | + | + | + | + | C | – | – | – | + | C | |
| Bleomycin sulfate | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Camptothecin | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Chlorambucil | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Cisplatin | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Cytosine Arabinoside | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Doxorubicin | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Emodin | Training | Clastogen | – | – | – | + | + | + | + | C | – | – | – | + | C | |
| Ethyl methanesulfonate | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Etoposide | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Glycidamide | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Hydralazine | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Hydrogen peroxide | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Hydroxyurea | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Melphalan | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Menadione | Training | Clastogen | – | – | – | + | + | – | + | C | – | – | – | + | C | |
| Methotrexate | Training | Clastogen | – | – | – | + | + | + | + | C | – | – | – | + | C | |
| Methyl methanesulfonate | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Mitomycin C | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| N-Ethyl-N-nitrosourea | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| N-Methyl-N’-nitro-N-nitrosoguanidine | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Olaparib | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Propyl gallate | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Resorcinol diglycidyl ether | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Stavudine | Training | Clastogen | – | – | – | + | + | + | + | C | – | – | – | + | C | |
| Temozolomide | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Thiotepa | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Topotecan | Training | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Alosetron | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Amitrole | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Anthranillic acid | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Brefeldin A | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Caffeine | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| CCCP | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Clofibrate | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Cyclohexanone | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Cycloheximide | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| D-Limonene | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| D-Mannitol | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Dexamethasone | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Dextrose | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Di-(2-ethylhexyl) phthalate | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Diethanolamine | Training | Non-genotoxicant | – | – | – | – | – | + | – | – | – | – | – | |||
| Erythromycin | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Famotidine | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Imatinib mesylate | Training | Non-genotoxicant | – | – | – | – | + | + | + | C | – | – | – | + | C | |
| Hexachloroethane | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Lidocaine | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Lovastatin | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Melamine | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Methyl carbamate | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| N-Butyl chloride | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Ofloxacin | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Paroxetine | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Phenanthrene | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Phenformin HCl | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Progesterone | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Pyridine | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Sodium chloride | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Sodium dodecyl sulfate | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Sucrose | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Tert-butyl alcohol | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Thapsigargin | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Tolterodine l-tartrate | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Tunicamycin | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Zonisamide | Training | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | |||
| Concordance with a priori Expectation, Training Set: | 84/85 | 47/48 | 79/85 | 41/41 | 84/85 | 47/48 | ||||||||||
| 99% | 98% | 93% | 100% | 99% | 98% | |||||||||||
Column 3 is color coded as to “a priori Genotox Expectation”, where aneugen = blue, clastogen = yellow, non-genotoxicant = white, and likely ≥ 1 MoA = green.
Columns 4+ are color coded as to Machine Learning and Global Evaluation Factor predictions, where aneugen = blue, clastogen = yellow, non-genotoxicant = white, and likely ≥ 1 MoA = green.
Treatment of TK6 cells with the reference genotoxicant 4-nitroquinoline 1-oxide resulted in a prototypical clastogenic response profile (Fig. 2b). The γH2AX biomarker was increased at 4 and 24 hr. Although p53 activation at the 24 hr time point is a pan-genotoxicity signal, activation at 4 hr, as observed here, is quite specific for clastogens (Bryce et al. 2014, 2016). Additionally, 4-nitroquinoline 1-oxide did not increase polyploidization, and the p-H3 biomarker was reduced in a dose-dependent manner. Both of these observations provide additional evidence of clastogenic as opposed to aneugenic activity. As shown in Table II, the GEF rubric and all three of the ML models predicted genotoxicity, with a clastogenic MoA.
Mebendazole’s aneugenicity has been attributed to microtubule binding (Laclette et al. 1980). MultiFlow response data illustrate a typical tubulin binder-induced aneugenic response profile (Fig. 2c). While anti-γH2AX-associated fluorescence did not increase at either time point and p53 translocation was not apparent at 4 hr, marked p53 responses were observed at 24 hr. Furthermore, robust increases in p-H3 positive events were induced by mebendazole, and this was accompanied by polyploidization. GEFs as well as the ML ensemble identified this compound as genotoxic, with evidence for an aneugenic MoA.
Crizotinib is another aneugen that is instructive for several reasons. Crizotinib is a potent inhibitor of c-Met and ALK (anaplastic lymphoma kinase), with cell-based assay IC50 values in the low nM range (Awad and Shaw 2014). Even so, there is evidence that the agent’s in vitro aneugenic activity may be related to off-target effects on aurora kinase(s) (Kong et al. 2018). Data presented in Figure 2d support this view, as it generated response profiles that are similar to several confirmed aurora kinase inhibitors tested in the MultiFlow assay (e.g., ZM-447439 and tozasertib). As with many tubulin binders, p53 activation and polyploidization were observed at the 24 hr time point. In the case of this kinase inhibitor, polyploidization was especially robust, and was evident well before the assay’s cytotoxicity limit was reached (i.e., eightfold increase in polyploidy at 59% cytotoxicity). Unlike tubulin binders, the proportion of p-H3-positive events was not elevated. Rather, at the highest concentrations tested, severe decreases were observed. These observations are consistent with aurora kinase inhibition, as this activity would be expected to repress serine 10 phosphorylation of histone H3 on mitotic chromosomes (Crosio et al. 2002). Despite the response profile being quite different than spindle poisons, both the GEF rubric as well as all three of the ML models identified crizotinib as genotoxic, with evidence for an aneugenic MoA (Table II).
Results from the Tier 1 data analyses are presented for all 85 training set chemicals in Table II. For the ML ensemble, the concordance between a priori expected and observed genotoxicity calls was 99%. For those agents with a genotoxic call, the agreement with expected MoA was 98%. In both cases, the one mischaracterized agent was imatinib mesylate (identified as a clastogen). Supporting Information Figure S1a–c provides Manhattan-type plots that show ML probabilities for each of the 85 chemicals at every concentration tested.
Table II also provides performance metrics for GEFs. The most obvious difference between GEFs and ML is that the latter was effective for both genotoxicity calls and MoA predictions (at least with a training set size of 85 chemicals), while the GEF rubric showed a lower level of agreement between expected and observed genotoxic activity calls (i.e., 93% concordance), especially for clastogens. As shown in Table II, the hybrid strategy, GEF + ML, did not outperform ML on its own.
Tier 1 Analyses: Test Set
With promising results evident for 85 training set chemicals, work with compounds that were not used to devise the GEF rubric or the ML models were tested in the MultiFlow assay. The results from Tier 1 analyses are presented in Table III. For this set of 40 diverse chemicals, the ML ensemble provided the best performance: 88% agreement between expected and observed genotoxicity calls. As with the training set, GEFs alone did not perform as well, as it resulted in 75% agreement with a priori classifications. Furthermore, the hybrid GEF + ML strategy did not outperform the ML-only approach (88%).
TABLE III.
Test Set Chemicals, N = 40
| Chemical | Group | a priori Genotox Expectation | Machine Learning (ML) Ensemble | Global Evaluation Factor (GEF) Rubric | ML + GEF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aneugen Calls | Clastogen Calls | Overall ML Genotoxicity Call | Overall ML MoA Call | Aneugen Calls | Clastogen Calls | Overall GEF Genotoxicity Calls | Overall GEF MoA Calls | Overall ML + GEF Genotoxicity Calls | Overall ML + GEF MoA Calls | |||||||
| RF | LR | ANN | RF | LR | ANN | |||||||||||
| 10j | Test | Aneugen | – | + | + | – | – | – | + | A | + | – | + | A | + | A |
| 13m | Test | Aneugen | – | – | + | – | – | + | – | – | – | – | – | |||
| 14n | Test | Aneugen | – | – | – | – | – | – | – | – | – | – | – | |||
| 17q | Test | Aneugen | + | + | + | – | – | – | + | A | + | – | + | A | + | A |
| 6f | Test | Aneugen | – | – | – | – | – | + | – | – | – | – | – | |||
| Hesperadin | Test | Aneugen | – | + | + | – | + | – | + | A | + | – | + | A | + | A |
| Tozasertib | Test | Aneugen | – | + | + | – | – | – | + | A | + | – | + | A | + | A |
| ZM-447439 | Test | Aneugen | – | + | + | – | – | – | + | A | + | – | + | A | + | A |
| 3c | Test | Aneugen, possibly >1 MoA | + | + | + | – | – | + | + | A | + | – | + | A | + | A |
| 16p | Test | Aneugen, likely >1 MoA | + | + | + | – | + | + | + | A / C | + | – | + | A / C | + | A / C |
| 1a | Test | Genotoxic, likely > 1 MoA | – | – | – | + | + | + | + | C | – | – | – | + | C | |
| 7g | Test | Genotoxic, MoA uncertain | – | – | – | + | + | – | + | C | – | – | – | + | C | |
| 9i | Test | Clastogen | – | – | – | + | + | + | + | C | – | – | – | + | C | |
| Beta-Lapachone | Test | Clastogen | – | – | – | + | + | + | + | C | – | – | – | + | C | |
| Ciprofloxacin | Test | Clastogen | – | + | + | – | – | – | + | A | – | – | – | + | A | |
| Dasatinib | Test | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Genistein | Test | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Irinotecan | Test | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| Mitoxantrone | Test | Clastogen | – | – | + | + | + | + | + | C | – | + | + | C | + | C |
| Teniposide | Test | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| 19s | Test | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| 20t | Test | Clastogen | – | – | – | – | + | + | + | C | – | – | – | + | C | |
| 4d | Test | Clastogen | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| 6-Thioguanine | Test | Clastogen | – | – | – | – | + | + | + | C | – | + | + | C | + | C |
| AZD2858 | Test | Clastogen, possibly >1 MoA | – | – | – | + | – | + | + | C | – | – | – | + | C | |
| 11k | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| 12l | Test | Non-genotoxicant | – | – | – | + | + | + | + | C | – | + | + | C | + | C |
| 15o | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| 18r | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| 2b | Test | Non-genotoxicant | – | – | – | + | + | + | + | C | – | – | – | + | C | |
| 5e | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| 8h | Test | Non-genotoxicant | – | – | – | – | – | + | – | – | – | – | – | – | ||
| Ampicillin | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Anisomycin | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Chlorocholine | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Menthol | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Osmertinib | Test | Non-genotoxicant | – | – | – | + | – | – | – | – | – | – | – | – | ||
| Topiramate | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Tris (2-ethylhexyl) phosphate | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Zafirlukast | Test | Non-genotoxicant | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Concordance with a priori Expectation, Test Set: | 35/40 | 21/24 | 30/40 | 15/17 | 35/40 | 21/24 | ||||||||||
| 88% | 88% | 75% | 88% | 88% | 88% | |||||||||||
Column 3 is color coded as to “a priori Genotox Expectation”, where aneugen = blue, clastogen = yellow, non-genotoxicant = white, and likely ≥ 1 MoA = green.
Columns 4+ are color coded as to Machine Learning and Global Evaluation Factor predictions, where aneugen = blue, clastogen = yellow, non-genotoxicant = white, and likely ≥ 1 MoA = green.
Three suspected genotoxicants were not identified as such by any of the Tier 1 analysis strategies that were evaluated: 6f, 13m, and 14n. While 6f was an anticipated aneugen, it was not observed to affect any of the aneugen-sensitive biomarkers, despite the fact that analyses included concentrations that induced up to 63.8% cytotoxicity. Compound 14n was also classified a priori as aneugenic, and in this case only one aneugen biomarker was slightly induced: 4 hr p-H3 was increased by 1.39-fold at the highest concentration tested, 4.41 μM. This false negative result for 14n should be qualified, since cytotoxicity at the highest feasible concentration tested was 48.7%, well below the assay’s cytotoxicity limit of 80%. The third false negative result, 13m, is also noteworthy. Whereas 6f and 14n showed slight to nil biomarker responses, 13m caused robust increases that exceeded biomarker GEFs for 4 hr p-H3 and 4 hr γH2AX across several consecutive concentrations, as well as 24 hr polyploidy at the highest concentration (Fig. 3a). This response profile was not observed in the 85 chemical training set, and consequentially the GEF rubric was not developed with this in mind, and the ML models have no experience with this pattern.
Fig. 3.
Radar plots show MultiFlow assay data for seven biomarker/time point combinations and for each of two chemicals: MSD-supplied test compounds (a) 13m and (b) 16p. The biomarker data are expressed as fold-increase over mean solvent control on the same plate, and each chemical concentration appears as a different colored line. Same format as Figure 2.
Tier 1 mischaracterized two non-genotoxicants as genotoxic: 2b and 12L. In the case of 2b (a.k.a., sodium diethyldithiocarbamate trihydrate), it should be noted that this compound has been shown to induce cytogenetic damage in both CHO and TK6 cells (Galloway et al. 1998; Hilliard et al. 1998; Greenwood et al. 2004), and DNA double strand breaks in rat hepatocytes (Storer et al. 1996), but genotoxicity was seen only at concentrations deemed overly cytotoxic by current testing standards. There are at least two biologically plausible causes for indirect effects leading to in vitro DNA damage: diethyldithiocarbamate chelates copper and zinc, and it is a potent inhibitor of superoxide dismutase (Heikkila et al. 1976; Nicotera et al. 1989).
Of the chemicals identified as genotoxic, Tier 1 analyses were also used to predict their genotoxic MoA. As shown in Table III, ML resulted in 88% agreement between expected and observed calls. GEF alone and the hybrid GEF + ML approaches performed similarly (88%). One compound, 16p, showed mixed activities, as both clastogen and aneugen biomarker responses were detected. This was an expected result, as 16p has an azobenzimidazole structure that was previously observed to induce premature centromere separation at metaphase in addition to induction of micronuclei and structural aberrations. MultiFlow biomarker results for this atypical agent are shown in Figure 3b. The three chemicals with misidentified MoA included the aneugen call for ciprofloxacin, a fluoroquinoline class antibiotic that was expected to exhibit clastogenic activity based on its reported topoisomerase II inhibitor activity, and the two a priori non-genotoxicants discussed above (i.e., 2b and 12L; both identified as clastogens).
Supporting Information Figure S2a–c provides Manhattan-type plots that show ML probabilities for each of the 40 test set chemicals at every concentration evaluated. Overall, the high concordance values speak to the generalizability of the ML ensemble to detect genotoxicants, and to furthermore provide an indication of genotoxic MoA.
Tier 2 Analyses
A set of 21 a priori aneugens and mixed MoA chemicals that were identified as such in Tier 1 ML analyses were evaluated via unsupervised hierarchical clustering using 4 and 24 hr MultiFlow biomarker data that were each converted to a single AUC value. The resulting groupings are presented in Figure 4 in the form of a two dimensional dendrogram. The clade denoted by “TB” was entirely comprised of tubulin binders. Note that although the exact mechanism of test agent 17q is not known, it is a benzimidazole-containing structure and therefore expected to have tubulin-binding properties. The other clear grouping is denoted by “KI,” a clade that included each of the presumptive mitotic kinase inhibitors that were tested: AMG 900, crizotinib, tozasertib, hesperadin, ZM-447439, and 10j.
Fig. 4.
Unsupervised clustering results are shown as a two-dimensional dendrogram for 21 chemicals that were identified as exhibiting aneugenic activity. As described in Materials and Methods section, each biomarker dose response was converted to an area under the curve for this analysis. The abbreviations TB (tubulin binder) and KI (kinase inhibitor) are used to denote clades with chemicals that are known to exhibit these activities. The bottommost graph shows the horizontal distances between join points.
The set of 46 a priori clastogens that were identified as such in Tier 1 analyses were also evaluated via unsupervised clustering using the four clastogen-responsive biomarkers. The results are shown in Figure 5. For this set of diverse clastogens, it is less obvious that clusters formed around different molecular targets. That said, the clade identified as “TI” was highly enriched for topoisomerase inhibitors (6/8), and the “C-L” grouping was enriched for DNA cross-linking agents (5/9).
Fig. 5.
Unsupervised clustering results are shown as a two-dimensional dendrogram for 46 chemicals that were identified as exhibiting clastogenic activity. As described in Materials and Methods section, each biomarker dose response was converted to an area under the curve for this analysis. The abbreviations TI (topoisomerase inhibitor) and C-L (cross-linker) are used to denote clades that are enriched for chemicals known to exhibit these activities. The bottommost graph shows the horizontal distances between join points.
Taken together, a second tier that consists of unsupervised hierarchical clustering appears to complement genotoxic potential and MoA analyses, as it provides useful information about likely molecular targets. This is especially true in the case of delineating aneugens that target mitotic kinases vs. those that interfere with tubulin polymerization.
Tier 3 Analyses
BMD metrics served as a basis for Tier 3 analyses that were conducted to determine whether MultiFlow biomarker(s) could provide a reliable indication of chemicals’ genotoxic potency as measured by the in vitro MN assay. The advantage of using BMD-derived potency metrics has been previously discussed by Soeteman-Hernández et al. (2015, 2016), and here we have expanded the utility to in vitro datasets. As shown in Figures 6–8, the BMDs in the MultiFlow endpoints were plotted against MN response BMDs on a double-log scale. As opposed to representing correlation with a numerical coefficient value, a linear relationship with intercept zero equals a straight line in a double-log plot. Therefore, two lines with unity slope were drawn on each correlation plot in such a manner that the majority of the BMD confidence intervals are encompassed between the lines. The distribution of BMD positions within the two lines show approximate linearity, differing by a proportionality constant. Furthermore, the vertical distance between the two lines translates into an uncertainty margin given by the estimation of a BMD on the y axis based on a specified BMD on the x axis, and vice versa. The uncertainty margin is used as a measure of correlation between two endpoints.
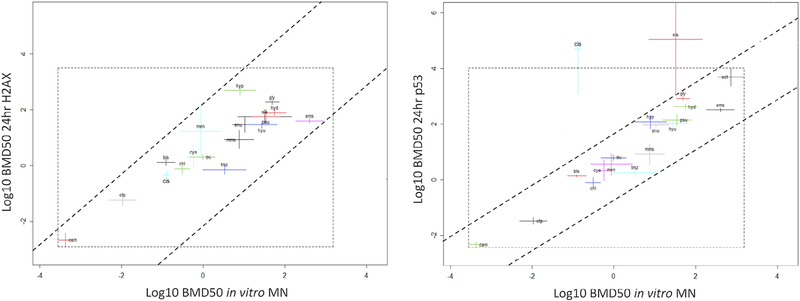
Fig. 6.
Left panel: BMD analyses of aneugen compounds represented in cross system plots with BMD50 CIs for in vitro MN against BMD50 CIs 24 hr p-H3 responses in TK6 cells, with both x and y axes representing Log10 concentration of compounds in μM. The dashed parallel lines are drawn in such a way that encompasses most of the CIs. Dashed square box represents default PROAST output encompassing finite BMD CIs. Compound “car” falls outside the trend with unbound CI in the 24 hr p-H3 endpoint. Right panel: BMD50 CIs for in vitro MN against BMD50 24 hr p53 responses in TK6 cells, with both x and y axes representing Log10 concentration of compounds in μM. Dashed parallel lines encompass most of the BMD CIs, similarly to the left panel correlation plot. Dashed square box represents default PROAST output encompassing finite BMD CIs. Compounds “gli” and “des” lie outside the general observed trend, with unbound upper CI in the 24 hr p53 endpoint. Dashed horizontal lines obtain the uncertainty range with corresponding circles intercept with the x axis predicting the BMD50 for in vitro MN response. See Table I for compound abbreviations (BMD, Benchmark Dose; CI, confidence interval; MN, micronucleus).
Fig. 8.
Left panel: BMD analyses of clastogen compounds represented in cross system plots with BMD100 CIs for in vitro MN vs. BMD50 24 hr γH2AX responses in TK6 cells, with both x and y axes representing Log10 concentration of compounds in μM. The dashed parallel lines are drawn in such a way that encompasses all of the CIs. Dashed square box represents default PROAST output encompassing finite BMD CIs. Right Panel: BMD100 CIs for in vitro MN vs. BMD50 24 hr p53 responses in TK6 cells, with both x and y axes representing Log10 concentration of compounds in μM. Dashed parallel lines encompass most of the BMD CIs. Dashed square box represents default PROAST output encompassing finite BMD CIs. See Table I for compound abbreviations (BMD, Benchmark Dose; CI, confidence interval; MN, micronucleus).
For the aneugens, when comparing MN induction to p53 responses, the cross system plots show good correlation, with the majority of the compounds located between the two lines (Fig. 6). Taking the microtubule binder nocodazole as an example, the horizontal dashed line intersections with the sloped dashed lines may be considered as the respective upper and lower bounds of the uncertainty range for the in vitro MN endpoint. The intercepts of approximately −3 and −1 on the log scale correspond to lower and upper bounds of 10−3 = 0.001 and 10−1 = 0.1 μM, respectively. Hence, the in vitro MN BMD for nocodazole is estimated to lie between 0.001 and 0.1 μM considering an uncertainty margin of approximately 1 log. In fact, the in vitro MN potency for nocodazole in the dataset represented in Figure 6 has both BMDL and BMDU either side of −2 log, and hence within the estimated potency of −3 and −1 log estimated from the p53 response. The MN vs. 24 hr p-H3 system plot also indicates the BMDs for the majority of compounds in both systems are proportionally related (Fig. 6), however the two lines are drawn further apart than the MN vs. 24 hr p53 system (i.e., 2 logs vs. 1 log). In both cases, MN vs. p53 and MN vs. p-H3, the data are randomly scattered with good correlation.
For the clastogens, good correlation is observed for MN vs. γH2AX and MN vs. p53, with data randomly scattered between the two diagonal lines of the unity slopes, with distances of approximately 3 and 2 log, respectively, for each system (Fig. 7). The in vitro MN BMD100 CI for compounds mmc, nqo, and top, plotted against BMD50 γH2AX and p53 endpoints show similar correlation with BMD CIs lying within approximately 2 log for both systems (Fig. 8).
Fig. 7.
Left panel: BMD analyses of clastogen compounds represented in cross system plots with BMD50 CIs for in vitro MN vs. BMD50 CIs 24 hr γH2AX responses in TK6 cells, with both x and y axes representing Log10 concentration of compounds in μM. The dashed parallel lines are drawn in such a way that encompasses all of the CIs. Dashed square box represents default PROAST output encompassing finite BMD CIs. Right panel: BMD50 CIs for in vitro MN vs. BMD50 24 hr p53 responses in TK6 cells, with both x and y axes representing Log10 concentration of compounds in μM. Dashed parallel lines encompass most of the BMD CIs. Dashed square box represents default PROAST output encompassing finite BMD CIs except outliers. Compound ola lies outside the general observed trend, with an unbound upper CI in the p53 endpoint. Compound cis displays an unbound upper CI in the p53 endpoint. See Table I for compound abbreviations (BMD, Benchmark Dose; CI, confidence interval; MN, micronucleus).
The correlations observed here are consistent with those of other genotoxicity endpoints which have been compared using similar methodologies. Bemis et al. (2016) obtained an uncertainty margin of approximately 1.5 log when comparing the in vitro MN responses against in vivo MN responses for a group of seven clastogens. Similarly, Soeteman-Hernández et al. (2015) assessed the ability to predict in vivo MN potency from in vitro MN data. BMD confidence intervals spanned two orders of magnitude, with in vivo BMD confidence intervals generally showing smaller than those from in vitro studies.
Conclusions
The MultiFlow DNA Damage Assay’s ability to predict chemicals’ in vitro genotoxic potential and MoA was demonstrated with an external test set of 40 largely pharmaceutical-centric compounds. Although the GEF and associated rubric exhibited high specificity and accurate MoA predictions, it provided lower sensitivity to detect genotoxicants relative to an ML ensemble. Indeed, ML exhibited a good balance between genotoxic potential predictions and MoA information. Interestingly, a hybrid strategy whereby GEFs and ML were used to make calls was no better than ML on its own. That being said, from a practical perspective, GEFs may serve a useful function at novice laboratories. GEFs can be used right away, even as training set data are being generated. Furthermore, while early ML models are being built and tested, concurrent use of the GEF rubric represents a safety net of sorts, as it is capable of highlighting biomarker response patterns that the ML model(s) may not have encountered. On a related point, additional practical advice for new adopters of this assay is provided in Supporting Information, that is, a shorter list of diverse training set chemicals (n = 24) that can be used to build base learner(s).
Unsupervised clustering is able to group certain genotoxicants with the same or similar molecular targets based on multifactorial biomarker response patterns. This was especially successful with aneugens that were clustered into tubulin binder and kinase inhibitor groups. While these analyses do not offer proof of molecular targets, they do represent a powerful hypothesis-generating tool, one that could be used to efficiently design the necessary follow-up test(s) aimed at directly and conclusively identifying molecular target(s) responsible for in vitro genotoxicity.
With respect to the BMD analyses reported herein, the strong correlation of MultiFlow biomarkers to bona fide genomic damage in the form of MN provides assurances of the relevance of the new assay’s endpoints. Furthermore, the correlations suggest that potency determinations based on MultiFlow endpoints, at least on a rank-order basis, are likely comparable to those derived from the MN assay. This bolsters the use case whereby the constellation of MultiFlow assay biomarkers serves as a reliable genotoxicity screening tool that is predictive of in vitro MN formation, with the benefit of providing more mechanistic information. Finally, dose–response analyses such as these are worth pursuing further because they reflect the paradigm shift that has been transitioning genotoxicity away from a simple binary yes/no characteristic to a quantitative metric that has the potential to better inform risk assessments as margin of exposure and other toxicological principles can be considered (Pottenger and Gollapudi 2009, 2010; Gollapudi et al. 2013; Johnson et al. 2014; MacGregor et al. 2015a, 2015b; Dearfield et al. 2017).
Supplementary Material
ACKNOWLEDGMENTS
This work was funded in part by a grant from the National Institute of Health/National Institute of Environmental Health Sciences (NIEHS; grant no. R44ES029014). The contents are solely the responsibility of the authors, and do not necessarily represent the official views of the NIEHS.
Grant sponsor: National Institute of Health/National Institute of Environmental Health Sciences; Grant number: R44ES029014.
Footnotes
CONFLICT OF INTEREST
DTB, SMB, NH, JCB, and SDD are employed by Litron Laboratories. Litron has a patent covering the flow cytometry-based assay described in this manuscript and sells a commercial kit based on these procedures: MultiFlow® DNA Damage Kit—p53, γH2AX, Phospho-Histone H3.
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Attia SM, Aleisa AM, Bakheet SA, Al-Yahya AA, Al-Rejaie SS, Ashour AE, Al-Shabanah OA. 2009. Molecular cytogenetic evaluation of the mechanism of micronucleus formation induced by camptothecin, topotecan, and irinotecan. Environ Mol Mutagen 50: 145–151. [DOI] [PubMed] [Google Scholar]
- Avlasevich SL, Bryce SM, Cairns SE, Dertinger SD. 2006. In vitro micronucleus scoring by flow cytometry: differential staining of micronuclei versus apoptotic and necrotic chromatin enhances assay reliability. Environ Mol Mutagen 47:56–66. [DOI] [PubMed] [Google Scholar]
- Awad MM, Shaw AT. 2014. ALK inhibitors in non-small cell lung cancer: crizotinib and beyond. Clin Adv Hematol Oncol 12:429–439. [PMC free article] [PubMed] [Google Scholar]
- Aydemir N, Bilaloğlu R. 2003. Genotoxicity of two anticancer drugs, gemcitabine and topotecan, in mouse bone marrow in vivo. Mutat Res 537:43–51. [DOI] [PubMed] [Google Scholar]
- Bemis JC, Willis JW, Bryce SM, Torous DK, Dertinger SD, Slob W. 2016. Comparison of in vitro and in vivo clastogenic potency based on benchmark dose analysis of flow cytometric micronucleus data. Mutagenesis 31:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacki DT, Bryce SM, Bemis JC, Kirkland D, Dertinger SD. 2016. γH2AX and p53 responses in TK6 cells discriminate promutagens and nongenotoxicant in the presence of rat liver S9. Environ Mol Mutagen 57:546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce SM, Bemis JC, Mereness JA, Spellman RA, Moss J, Dickinson D, Schuler MJ, Dertinger SD. 2014. Interpreting in vitro micronucleus positive results: simple biomarker matrix discriminates clastogens, aneugens, and misleading positive agents. Environ Mol Mutagen 55:542–555. [DOI] [PubMed] [Google Scholar]
- Bryce SM, Bernacki DT, Bemis JC, Dertinger SD. 2016. Genotoxic mode of action predictions from a multiplexed flow cytometric assay and a machine learning approach. Environ Mol Mutagen 57:171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce SM, Bernacki DT, Bemis JC, Spellman RA, Engel ME, Schuler M, Lorge E, Heikkinen PT, Hemmann U, Thybaud V, Wilde S, Queisser N, Sutter A, Zeller A, Guérard M, Kirkland D, Dertinger SD. 2017. Interlaboratory evaluation of a multiplexed high information content in vitro genotoxicity assay. Environ Mol Mutagen 58:146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce SM, Bernacki DT, Smith-Roe SL, Witt KL, Bemis JC, Dertinger SD. 2018. Investigating the generalizability of the MultiFlow® DNA damage assay and several machine learning models with a set of 103 diverse test chemicals. Toxicol Sci 162:146–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JR, Dickinson DA, Moss J, Schuler MJ, Spellman RA, Heard PL. 2015. Histone markers identify the mode of action for compounds positive in the TK6 micronucleus assay. Mutat Res 777:7–16. [DOI] [PubMed] [Google Scholar]
- Chinnasamy N, Rafferty JA, Hickson I, Ashby J, Tinwell H, Margison GP, Dexter TM, Fairbairn LJ. 1997. O6-benzylguanine potentiates the in vivo toxicity and clastogenicity of temozolomide and BCNU in mouse bone marrow. Blood 89:1566–1573. [PubMed] [Google Scholar]
- Cojocel C, Novotny L, Vachalkova A. 2006. Mutagenic and carcinogenic potential of menadione. Neoplasma 53:316–323. [PubMed] [Google Scholar]
- Crosio C, Fimia GM, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis CD, Sassone-Corsi P. 2002. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol 22:874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry PT, Kropko ML, Garvin JR, Fiedler RD, Theiss JC. 1996. In vitro induction of micronuclei and chromosome aberrations by quinolones: possible mechanisms. Mutat Res 352:143–150. [DOI] [PubMed] [Google Scholar]
- Dearfield KL, Gollapudi BB, Bemis JC, Benz RD, Douglas GR, Elespuru RK, Johnson GE, Kirkland DJ, LeBaron MJ, Li AP, Marchetti F, Pottenger LH, Rorije E, Tanir JY, Thybaud V, van Benthem J, Yauk CL, Zeiger E, Luijten M. 2017. Next generation testing strategy for assessment of genomic damage: a conceptual framework and considerations. Environ Mol Mutagen 58: 264–283. [DOI] [PubMed] [Google Scholar]
- Degrassi F, De Salvia R, Berghella L. 1993. The production of chromosomal alterations by β-lapachone, an activator of topoisomerase I. Mutat Res 288:263–267. [DOI] [PubMed] [Google Scholar]
- DeMarini DM, Brock KH, Doerr CL, Moore MM. 1987. Mutagenicity and clastogenicity of teniposide (VM-26) in L5178Y/TK+/− −3.7.2C mouse lymphoma cells. Mutat Res 187:141–149. [DOI] [PubMed] [Google Scholar]
- Dertinger SD, Phonethepswath S, Avlasevich SL, Torous DK, Mereness J, Bryce SM, Bemis JC, Bell S, Weller P, MacGregor JT. 2012. Efficient monitoring of in vivo Pig-a gene mutation and chromosomal damage: summary of 7 published studies and results from 11 new reference compounds. Toxicol Sci 130:328–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertinger SD, Phonethepswath S, Weller P, Avlasevich S, Torous DK, Mereness JA, Bryce SM, Bemis JC, Bell S, Portugal S, Aylott M, MacGregor JT. 2011. Interlaboratory Pig-a gene mutation assay trial: studies of 1,3-propane sultone with immunomagnetic enrichment of mutant erythrocytes. Environ Mol Mutagen 52:748–755. [DOI] [PubMed] [Google Scholar]
- Diaz D, Scott A, Carmichael P, Shi W, Costales C. 2007. Evaluation of an automated in vitro micronucleus assay in CHO-K1 cells. Mutat Res. 630:1–13. [DOI] [PubMed] [Google Scholar]
- Floxin® Raritan, NJ: Ortho-McNeil; 2008. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/019735s059lbl.pdf. Accessed October 12, 2018. [Google Scholar]
- Futami T, Miyagishi M, Taira K. 2005. Identification of a network involved in thapsigargin-induced apoptosis using a library of small interfering RNA expression vectors. J Biol Chem 280:826–831. [DOI] [PubMed] [Google Scholar]
- Galloway SM, Miller JE, Armstrong MJ, Bean CL, Skopek TR, Nichols WW. 1998. DNA synthesis inhibition as an indirect mechanism of chromosome aberrations: comparison of DNA-reactive and non-DNA-reactive clastogens. Mutat Res 400:169–186. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. 1999. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57: 727–741. [DOI] [PubMed] [Google Scholar]
- Gleevec®. East Hanover, NJ: Novartis; 2001. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021588s024lbl.pdf. Accessed October 12, 2018. [Google Scholar]
- Glover TW, Berger C, Coyle J, Echo B. 1984. DNA polymerase alpha inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum Genet 67:136–142. [DOI] [PubMed] [Google Scholar]
- Gocke E, Bürgin H, Müller L, Pfister T. 2009. Literature review on the genotoxicity, reproductive toxicity, and carcinogenicity of ethyl methanesulfonate. Toxicol Lett 190:254–265. [DOI] [PubMed] [Google Scholar]
- Gollapudi BB, Johnson GE, Hernández LG, et al. 2013. Quantitative approaches for assessing dose-response relationships in genetic toxicology studies. Environ Mol Mutagen 54:8–18. [DOI] [PubMed] [Google Scholar]
- Gollapudi P, Hasegawa LS, Eastmond DA. 2014. A comparative study of the aneugenic and polyploidy-inducing effects of fisetin and two model Aurora kinase inhibitors. Mutat Res 767:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf AO, van den Heuvel LP, Dijkman HB, de Abreu RA, Birkenkamp KU, de White T, van der Reijden BA, Smeitink JA, Jansen JH. 2004. Bcl-2 prevents loss of mitochondria in CCCP-induced apoptosis. Exp Cell Res 299:533–540. [DOI] [PubMed] [Google Scholar]
- Greenwood SK, Hill RB, Sun JT, Armstrong MJ, Johnson TE, Gara JP, Galloway SM. 2004. Population doubling: a simple and more accurate estimation of cell growth suppression in the in vitro assay for chromosomal aberrations that reduces irrelevant positive results. Environ Mol Mutagen 43:36–44. [DOI] [PubMed] [Google Scholar]
- Gulati DK, Witt K, Anderson B, Zeiger E, Shelby MD. 1989. Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in vitro III: results with 27 chemicals. Environ Mol Mutagen 13:133–193. [DOI] [PubMed] [Google Scholar]
- Han C, Nam MK, Park HJ, Seong YM, Kang S, Rhim H. 2008. Tunicamycin-induced ER stress upregulates the expression of mitochondrial HtrA2 and promotes apoptosis through the cytosolic release of HtrA2. J Microbiol Biotechnol 18:1197–1202. [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. 2003. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol 161:281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard PL, Rubitski EE, Spellman RA, Schuler MJ. 2013. Phenolphthalein induces centrosome amplification and tubulin depolymerization in vitro. Environ Mol Mutagen 54:308–316. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Cabbat FS, Cohen G. 1976. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem 251: 2182–2185. [PubMed] [Google Scholar]
- Henderson L, Fedyk J, Windebank S, Smith M. 1993. Induction of micronuclei in rat bone marrow and peripheral blood following acute and subchronic administrate of azathioprine. Mutat Res 291:79–85. [DOI] [PubMed] [Google Scholar]
- Hernández LG, van Benthem J, Johnson GE. 2013. A mode-of action approach for the identification of genotoxic carcinogens. PLoS One 8:e64532 10.1371/journal.pone.0064532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard C, Armstrong M, Bradt C, Hill R, Greenwood S, Galloway S. 1998. Chromosome aberrations in vitro related to cytotoxicity of non-mutagenic chemicals. Environ Mol Mutagen 31:316–326. [PubMed] [Google Scholar]
- IARC Monograph, Clofibrate. Available at: http://monographs.iarc.fr/ENG/Monographs/vol66/mono66-17.pdf. Accessed October 12, 2018.
- Jayalakshmi T, Santhakumaran A. 2011. Statistical normalization and back propagation for classification. Int J Comput Theory Eng 3: 89–93. [Google Scholar]
- Johnson GE, Soeteman-Hernández LG, Gollapudi BB, Bodger OG, Dearfield KL, Heflich RH, Hixon JG, Lovell DP, MacGregor JT, Pottenger LH, Thompson CM, Abraham L, Thybaud V, Tanir JY, Zeiger E, van Benthem J, White PA. 2014. Derivation of point of departure (PoD) estimates in genetic toxicology studies and their potential applications in risk assessment. Environ Mol Mutagen 55:609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshava C, Keshava N, Whong WZ, Nath J, Ong TM. 1998. Inhibition of methotrexate-induced chromosomal damage by folinic acid in V79 cells. Mutat Res 397:221–228. [DOI] [PubMed] [Google Scholar]
- Khoury L, Zalko D, Audebert M. 2016. Complementarity of phosphorylated histones H2AX and H3 quantification in different cell lines for genotoxicity screening. Arch Toxicol 90:1983–1995. [DOI] [PubMed] [Google Scholar]
- Kimura A, Miyata A, Honma M. 2013. A combination of in vitro comet assay and micronucleus test using human lymphoblastoid TK6 cells. Mutagenesis 28:583–590. [DOI] [PubMed] [Google Scholar]
- Kirkland D, Kasper P, Martus H-J, Müller L, van Benthem J, Madia F, Corvi R. 2016. Updated recommended lists of genotoxic and non-genotoxic chemicals for assessment of the performance of new or improved genotoxicity tests. Mutat Res 795:7–30. [DOI] [PubMed] [Google Scholar]
- Kirkland D, Kasper P, Müller L, Corvi R, Speit G. 2008. Recommended lists of genotoxic and non-genotoxic chemicals for assessment of the performance of new or improved genotoxicity tests: a followup to an ECVAM workshop. Mutat Res 653:99–108. [DOI] [PubMed] [Google Scholar]
- Klein CB, King AA. 2007. Genistein genotoxicity: critical considerations of in vitro exposure dose. Toxicol Appl Pharmacol 224:1–11. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Honda S, Nakajima M, Miyahana K, Hayashi M, Shinagawa Y, Sato S, Inoue K, Nito S, Ariyuki F. 1992. Micronucleus test with vincristine sulfate and colchicine in peripheral blood reticulocytes of mice using acridine orange supravital staining. Mutat Res 278:187–191. [PubMed] [Google Scholar]
- Kong Y, Bender A, Yan A. 2018. Identification of novel aurora kinase A (AURKA) inhibitors via hierarchical ligand-based virtual screening. J Chem Inf Model 58:36–47. [DOI] [PubMed] [Google Scholar]
- Krishna G, Urda G, Tefera W, Lalwani ND, Theiss J. 1995. Simultaneous evaluation of dexamethasone-induced apoptosis and micronuclei in rat primary spleen cell cultures. Mutat Res. 332:1–8. [DOI] [PubMed] [Google Scholar]
- Kurihara D, Matsunaga S, Kawabe A, Fujimoto S, Noda M, Uchiyama S, Fukui K. 2006. Aurora kinase is required for chromosome segregation in tobacco BY-2 cells. Plant J 48:572–580. [DOI] [PubMed] [Google Scholar]
- Laclette JP, Guerra G, Zetina C. 1980. Inhibition of tubulin polymerization by mebendazole. Biochem Biophys Res Commun 92:417–423. [DOI] [PubMed] [Google Scholar]
- Li Y, Luan Y, Qi X, Li M, Gong L, Xue X, Wu X, Wu Y, Chen M, Xing G, Yao J, Ren J. 2010. Emodin triggers DNA double-strand breaks by stabilizing topoisomerase II-DNA cleavage complexes and by inhibiting ATP hydrolysis of topoisomerase II. Toxicol Sci 118:435–443. [DOI] [PubMed] [Google Scholar]
- Lidoderm® Chad’s Ford, PA: Endo Pharmaceuticals; 2004. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020612s007lbl.pdf. Accessed October 12, 2018. [Google Scholar]
- Lotz AS, Havla JB, Richter E, Frölich K, Staudenmaier R, Hagen R, Kleinsasser NH. 2009. Cytotoxic and genotoxic effects of matrices for cartilage tissue engineering. Toxicol Lett 190:128–133. [DOI] [PubMed] [Google Scholar]
- Lu P-Z, Lai C-Y, Chan W-H. 2008. Caffeine induces cell death via activation of apoptotic signal and inactivation of survival signal in human osteoblasts. Int J Mol Sci 9:698–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynparza™. Wilmington, DE: AstraZeneca Pharmaceuticals LP, 2014. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206162lbl.pdf. Accessed October 12, 2018. [Google Scholar]
- MacGregor JT, Frötschl R, White PA, et al. 2015a. IWGT report on quantitative approaches to genotoxicity risk assessment I. Methods and metrics for defining exposure-response relationships and points of departure (PoDs). Mutat Res 783:55–65. [DOI] [PubMed] [Google Scholar]
- MacGregor JT, Frötschl R, White PA, et al. 2015b. IWGT report on quantitative approaches to genotoxicity risk assessment II. Use of point-of-departure (PoD) metrics in defining acceptable exposure limits and assessing human risk. Mutat Res 783:66–78. [DOI] [PubMed] [Google Scholar]
- Martelli A, Allavena A, Campart GB, Canonero R, Ghia M, Mattioli F, Mereto E, Robbiano L, Brambilla G. 1995. In vitro and in vivo testing of hydralazine genotoxicity. J Pharmacol Exp Ther 273: 113–120. [PubMed] [Google Scholar]
- Matsushima T, Hayashi M, Matsuoka A, Ishidate M Jr, Miura KF, Shimizu H, Suzuki Y, Morimoto K, Ogura H, Mure K, Koshi K, Sofuni T. 1999. Validation study of the in vitro micronucleus test in a Chinese hamster lung cell line (CHL/IU). Mutagenesis 14:569–580. [DOI] [PubMed] [Google Scholar]
- Mevacor®. Whitehouse Station, NJ, USA: Merck Sharp & Dohme Corp; 2012. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019643s085lbl.pdf. Accessed October 12, 2018. [Google Scholar]
- Moon JL, Kim SY, Shin SW, Park J-W. 2012. Regulation of brefeldin Ainduced ER stress and apoptosis by mitochondrial NADP+ -dependent isocitrate dehydrogenase. Biochem Biophys Res Commun 417:760–764. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program Report, 6-Thioguanine. Available at: https://ntp.niehs.nih.gov/testing/status/agents/ts-m890081.html. Accessed October 12, 2018a.
- National Toxicology Program Report, Sodium Dodecyl Sulfate. Available at: http://ntp.niehs.nih.gov/testing/status/agents/ts-10604-g.html. Accessed October 12, 2018b.
- Nicotera TM, Notaro J, Notaro S, Schumer J, Sandberg AA. 1989. Elevated superoxide dismutase in bloom syndrome: a genetic condition of oxidative stress. Cancer Res 49:5239–5243. [PubMed] [Google Scholar]
- Nikolova T, Dvorak M, Jung F, Adam I, Krämer E, Gerhold-Ay A, Kaina B. 2014. γH2AX assay for genotoxic and nongenotoxic agents: comparison of H2AX phosphorylation with cell death response. Toxicol Sci 140:103–117. [DOI] [PubMed] [Google Scholar]
- Oliver J, Meunier JR, Awogi T, Elhajouji A, Ouldelhkim MC, Bichet N, Thybaud V, Lorenzon G, Marzin D, Lorge E. 2006. SFTG international collaborative study on in vitro micronucleus test V. Using L5178Y cells. Mutat Res 607:125–152. [DOI] [PubMed] [Google Scholar]
- Parry EM, Parry JM, Corso C, Doherty A, Haddad F, Hermine TF, Johnson G, Kayani M, Quick E, Warr T, Williamson J. 2002. Detection and characterization of mechanism of action of aneugenic chemicals. Mutagenesis 17:509–521. [DOI] [PubMed] [Google Scholar]
- Paulsson B, Kotova N, Grawé J, Henderson A, Granath F, Golding B, Törnqvist M. 2003. Induction of micronuclei in mouse and rat by glycidamide, genotoxic metabolite of acrylamide. Mutat Res 535:15–24. [DOI] [PubMed] [Google Scholar]
- Paxil®. Research Triangle Park, NC: GlaxoSmithKline; 2011. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020031s058s066,020710s022s030lbl.pdf. Accessed October 12, 2018. [Google Scholar]
- Payton M, Bush TL, Chung G, Ziegler B, Eden P, McElroy P, Ross S, Cee VJ, Deak HL, Hodous BL, Nguyen HN, Olivieri PR, Romero K, Schenkel LB, Bak A, Stanton M, Dussault I, Patel VF, Geuns-Meyer S, Radinsky R, Kendall RL. 2010. Preclinical evaluation of AMG 900, a novel potent and highly selective pan-aurora kinase inhibitor with activity in taxane-resistant tumor cell lines. Cancer Res 70:9846–9854. [DOI] [PubMed] [Google Scholar]
- Pepcid®. Whitehouse Station, NJ, USA: Merck Sharp & Dohme Corp; 2011. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019462s037lbl.pdf. Accessed October 12, 2018. [Google Scholar]
- Pottenger LH, Gollapudi BB. 2009. A case for a new paradigm in genetic toxicology testing. Mutat Res 678:148–151. [DOI] [PubMed] [Google Scholar]
- Pottenger LH, Gollapudi BB. 2010. Genotoxicity testing: moving beyond qualitative “screen and bin” approach towards characterization of dose-response and thresholds. Environ Molec Mutagen 51: 792–799. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Inesi G, Wade R, Lederer WJ. 1995. Use of thapsigargin to study Ca2+ homeostasis in cardiac cells. Biosci Rep 15: 341–349. [DOI] [PubMed] [Google Scholar]
- Rosefort C, Fauth E, Zanki H. 2004. Micronuclei induced by aneugens and clastogens in mononucleate and binucleate cells using the cytokinesis block assay. Mutagenesis 19:277–284. [DOI] [PubMed] [Google Scholar]
- Schuler M, Muehlbauer P, Guzzie P, Eastmond DA. 1999. Noscapine hydrochloride disrupts the mitotic spindle in mammalian cells and induces aneuploidy as well as polyploidy in cultured human lymphocytes. Mutagenesis 14:51–56. [DOI] [PubMed] [Google Scholar]
- Slob W 2016. A general theory of effect size, and its consequences for defining the benchmark response (BMR) for continuous endpoints. Crit Rev Toxicol 47:342–351. [DOI] [PubMed] [Google Scholar]
- Slob W, Setzer RW. 2014. Shape and steepness of toxicological dose–response relationships of continuous endpoints. Critical Reviews in Toxicology 44:270–297. [DOI] [PubMed] [Google Scholar]
- Smart DJ, Halicka HD, Schmuck G, Traganos F, Darzynkiewicz Z, Williams GM. 2008. Assessment of DNA double-strand breaks and gammaH2AX induced by the topoisomerase II poisons etoposide and mitoxantrone. Mutat Res 641:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeteman-Hernández LG, Fellows MD, Johnson GE, Slob W. 2015. Correlation of in vivo versus in vitro benchmark doses (BMDs) derived from micronucleus test data: proof of concept study. Toxicol Sci 148:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeteman-Hernández LG, Johnson GE, Slob W. 2016. Estimating the carcinogenic potency of chemicals from the in vivo micronucleus test. Mutagenesis 31:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprycel® (dasatinib) Bristol-Myers Squibb Co, Princeton, NJ: 2010. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021986s7s8lbl.pdf. Accessed October 12, 2018. [Google Scholar]
- Storer RD, McKelvey TW, Kraynaka AR, Elia MC, Barnum JE, Harmon LS, Nichols WW, DeLuca JG. 1996. Revalidation of the in vitro alkaline elution/hepatocyte assay for DNA damage: improved criteria for assessment of cytotoxicity and genotoxicity and results for 81 compounds. Mutat Res 368:59–101. [DOI] [PubMed] [Google Scholar]
- Tagrisso™ (Osimertinib). AstraZeneca Pharmaceuticals LP, Wilmington, DE: 2012. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/208065s000lbl.pdf. Accessed October 12, 2018. [Google Scholar]
- Tayama S, Nakagawa Y. 2001. Cytogenetic effects of propyl gallate in CHO-K1 cells. Mutat Res 498:117–127. [DOI] [PubMed] [Google Scholar]
- Tweats DJ, Johnson GE, Scandale I, Whitwell J, Evans DB. 2016. Genotoxicity of flubendazole and its metabolites in vitro and the impact of a new formulation on in vivo aneugenicity. Mutagenesis 31:309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hummelen P, Elhajouji A, Kirsch-Volders M. 1995. Clastogenic and aneugenic effects of three benzimidazole derivates in the in vitro micronucleus test using human lymphocytes. Mutagenesis 10: 23–29. [DOI] [PubMed] [Google Scholar]
- Verdoodt B, Decordier I, Geleyns K, Cunha M, Cundari E, Kirsch-Volders M. 1999. Induction of polyploidy and apoptosis after exposure to high concentrations of the spindle poison nocodazole. Mutagenesis 14:513–520. [DOI] [PubMed] [Google Scholar]
- Wills JW, Johnson GE, Doak SH, Soeteman-Hernández LG, Slob W, White PA. 2015. Empirical analysis of BMD metrics in genetic toxicology part I: in vitro analyses to provide robust potency rankings and support MOA determinations. Mutagenesis 31:255–263. [DOI] [PubMed] [Google Scholar]
- Youngblom JH, Wiencke JK, Wolff S. 1989. Inhibition of the adaptive response of human lymphocytes to very low doses of ionizing radiation by the protein synthesis inhibitor cycloheximide. Mutat Res 227: 257–261. [DOI] [PubMed] [Google Scholar]
- Zeller A, Duran-Pacheco G, Guerard M. 2017. An appraisal of critical effect sizes for the benchmark dose response approach to assess dose-response relationships in genetic toxicology. Arch Toxicol 91:3799–3807. [DOI] [PubMed] [Google Scholar]
- Zeller A, Tang L, Dertinger SD, Funk J, Duran-Pacheco G, Guerard M. 2016. A proposal for a novel rationale for critical effect size in dose-response analysis based on a multi-endpoint in vivo study with methyl methanesulfonate. Mutagenesis 31:239–253. [DOI] [PubMed] [Google Scholar]
- Zerit® Bristol-Myers Squibb Virology, Princeton, NJ: 2002. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/20412S017.pdf. Accessed October 12, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.