Abstract
Background
Trichomonas vaginalis virus (TVV) is a non-segmented, 4.5–5.5 kilo-base pair (kbp), double-stranded RNA virus infecting T. vaginalis. The objectives of this study were to examine the TVV prevalence in US Trichomonas vaginalis isolates and TVV’s associations with patient demographics, clinical outcomes, and metronidazole resistance.
Methods
Archived T. vaginalis isolates from the enrollment visits of 355 women participating in a T. vaginalis treatment trial in Birmingham, Alabama, were thawed and grown in culture. Their total RNA was extracted using a Trizol reagent. Contaminating, single-stranded RNA was precipitated using 4.0 M Lithium Chloride and centrifugation. The samples were analyzed by gel electrophoresis to visualize a 4.5 kbp band representative of TVV. In vitro testing for metronidazole resistance was also performed on 25/47 isolates obtained from the women’s test of cure visits.
Results
TVV was detected in 142/355 (40%) isolates at the enrollment visit. Women with TVV-positive (TVV+) isolates were significantly older (P = .01), more likely to smoke (P = .04), and less likely to report a history of gonorrhea (P = .04). There was no association between the presence of clinical symptoms or repeat T. vaginalis infections with TVV+ isolates (P = .14 and P = .44, respectively). Of 25 test of cure isolates tested for metronidazole resistance, 0/10 TVV+ isolates demonstrated resistance, while 2/15 TVV-negative isolates demonstrated mild to moderate resistance (P = .23).
Conclusions
Of 355 T. vaginalis isolates tested for TVV, T. vaginalis isolates tested for TVV, the prevalence was 40%. However, there was no association of TVV+ isolates with clinical symptoms, repeat infections, or metronidazole resistance. These results suggest that TVV may be commensal to T. vaginalis.
Keywords: Trichomonas vaginalis, Trichomonas vaginalis virus, double-stranded RNA virus, trichomoniasis
Among women with trichomoniasis, Trichomonas vaginalis virus (TVV) infected 142/355 (40%) of T. vaginalis isolates and was independently associated with older age and smoking. Women reporting a history of gonorrhea were less likely to be infected. Metronidazole drug resistance was not associated with TVV.
Trichomonas vaginalis (TV) is a parasitic protozoan responsible for the sexually transmitted infection (STI) trichomoniasis. Trichomoniasis affects an estimated 3.7 million people nationwide and is among the most common curable STIs [1]. While a majority of women with a TV infection are asymptomatic, trichomoniasis can present with genital signs and symptoms, including vaginal erythema, itching, vaginal discharge, an odor, and dysuria [1, 2]. TV infections have also been associated with older age [3]. There are various methods of testing for TV in women, the most common of which is microscopic examination of a wet mount of vaginal fluid [4, 5]. Until the availability of the highly sensitive TV nucleic acid amplification test (NAAT) [6–8], the gold standard for diagnosis in women had been a TV culture [5]. Metronidazole (MTZ) and tinidazole are the drugs currently recommended by the Centers for Disease Control and Prevention (CDC) and the World Health Organization for the treatment of trichomoniasis [9, 10].
Virus-like particles were first observed in trichomonads in 1985 and were later recognized as being components of a double-stranded RNA (dsRNA) virus infecting the protozoans [11, 12]. Trichomonas vaginalis virus (TVV) is a non-segmented, 4.5–5.5 kilo-base pair (kbp), dsRNA virus belonging to the viral family Totiviridae [13]. TVV is encased within an 85 kilodalton major viral protein capsid arranged in the shape of a 120-subunit icosahedral and is closely associated with the Golgi complex [14]. TVV uses a viral, RNA-dependent RNA polymerase to replicate its genetic material. TVV affects the total protein expression of TV, and especially the expression of cysteine proteinases and the immunogenic protein P270, which are linked to cytotoxicity, cytoadherence, and host immune evasion [15–17]. TVV can be divided into 4 different viral strains that have the ability to co-infect TV at the same time: TVV1, TVV2, TVV3, and TVV4 [18, 19]. Each TVV strain has different effects on various aspects of TV pathogenesis. TVV1 and TVV2 have been linked to genital symptom severity [20], while TVV2 and TVV3 are involved in the surface expression of P270 and other virulence factors of TV [21]. The role of TVV4 has yet to be elucidated.
It is currently unclear whether TVV alters trichomonal virulence or affects its clinical response to therapy. The prevalence of TVV in US TV isolates is also not well known. With this in mind, the objectives of this study were to examine the prevalence of TVV in archived TV isolates from a randomized, controlled trial (RCT) treating TV in human immunodeficiency virus–negative women [22] and to examine the association of TVV-positive (TVV+) isolates with patient demographics, clinical characteristics, and MTZ resistance.
METHODS
This study was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board (protocol number F130425010) and the Tulane University Institutional Review Board (reference number 17-1065480E). Written informed consent for the TV trial was obtained from participants and included permission to use stored TV isolates for additional research. The study was also reviewed by the CDC, which deemed CDC personnel to be non-engaged, as they had no contact with the study participants or access to personal identifiers.
Randomized, Controlled Trichomonas vaginalis Treatment Trial
Stored TV isolates from 355 human immunodeficiency virus–negative women enrolled in a TV treatment trial [22] at UAB were used for the purposes of this study. The TV treatment trial consisted of an enrollment visit and a test of cure (TOC) visit 4 weeks post–treatment completion, as previously described [22]. Participants completed audio, computer-assisted, self-administered interviews at both the enrollment and TOC visits. Survey data obtained at the time of enrollment and at TOC included participant demographics, tobacco use, contraceptive use, sexual history data, sexual behavior data, and vaginal symptoms (vaginal discharge, odor, itching, irritation, dysuria, and pelvic pain). Participants in the RCT were randomized to receive oral MTZ, either as a single, 2-gram dose or at 500 mg twice daily for 7 days. Women who were TV positive by culture at TOC had their TOC visit isolates tested for MTZ resistance.
Trichomonas vaginalis Nucleic Acid Amplification Testing
Women underwent TV testing by physician-collected NAATs at both the enrollment and TOC visits. The vaginal swabs were tested for TV using the transcription-mediated amplification/hybridization protection assay assay from Hologic, Inc. (Bedford, MA). All NAATs were done at the laboratory of the Louisiana State University Health Sciences Center, according to the manufacturer’s instructions.
Trichomonas vaginalis Culture
Women also underwent TV testing by physician-collected TV culture at both the enrollment and TOC visits. The vaginal swabs were placed into a TV culture InPouch medium (BioMed Diagnostics, White City, OR) that was incubated at 37°C. Trained personnel read the pouches, following the manufacturer’s protocol. The personnel performed 3 pouch readings over 7 days. The detection of any live trichomonads was considered a positive result. The specimen was considered TV negative after 3 negative readings [23].
Trichomonas vaginalis Isolate Growth
There were 400 participants enrolled into the RCT trial at UAB. However, 23 participants did not give informed consent to the use of their TV isolates for further testing. An additional 22 isolates were either contaminated with yeast or failed to grow once collected. Thus, 355 stored TV isolates from the enrollment visit of the RCT were subsequently thawed and grown in 15 ml polystyrene conical tubes containing 9 ml of Diamond’s trypticase, yeast extract, maltose growth media and an additional 220 μl of 100X penicillin-streptomycin-amphotericin B. The isolates were allowed to incubate for 3 to 5 days at 37°C and were routinely checked for optimal cell growth. Once the isolates reached optimal growth, they were centrifuged at high speed for 10 minutes. The supernatant was removed using a pipette, leaving behind the pellet of TV cells. The pellet was transferred into a 1.5 mL polypropylene centrifuge vial for total RNA extraction.
RNA Extraction
Total RNA was extracted from the cell pellets using Trizol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. The cells were mixed with 1 ml of Trizol using a pipette and were incubated at room temperature for 5 minutes. Next, 200 μl of chloroform was added to the vial, which was shaken for 15 seconds and incubated at room temperature for 10 minutes. The samples were centrifuged at 12 000 g for 15 minutes, and the aqueous layer was transferred to a new, 1.5 ml centrifuge vial, mixed with 500 μl of isopropanol, and incubated for 10 minutes at room temperature. The vials were centrifuged for 8 minutes at 12 000 g and the supernatant was carefully removed to avoid disrupting the RNA pellet at the bottom of the vial. The pellet was then washed using 1 ml of 75% ethanol and centrifuged for 5 minutes at 12 000 g. The ethanol was removed and the RNA pellet was allowed to air dry for 5 minutes before 30 μl of distilled water was added to dissolve the RNA pellet. The final step consisted of the vial being heated at 55°C in a heating block for 15 minutes for further RNA solubilization.
Lithium Chloride RNA Clean-up
The protocol used for LiCl RNA purification was modified from another protocol (The Springer Lab, University of Minnesota, St. Paul, MN). Contaminating, single-stranded RNA was precipitated by adding 30 μl of 2.0 M LiCl to the 30 μl RNA solution and incubating at −20°C for 30 minutes. After incubation, the vial was centrifuged at 12 000 g for 30 minutes. Then, 50 μl of the supernatant was removed from the vial, leaving 10 μl. An addition of 50 μl of 4.0 M LiCl was added, and the vial was incubated at -20°C for 30 minutes, followed by centrifugation at 12 000 g for an additional 30 minutes. Finally, 50 μl of supernatant was removed and replaced by 20 μl of distilled water. This brought the final volume to 30 μl of purified dsRNA.
Gel Electrophoresis
The TV isolates were analyzed for the presence of the representative, 4.5–5.5 kbp TVV band by gel electrophoresis on a 1% agarose gel in 1X(Concentration) Tris-borate-EDTA buffer with ethidium bromide staining, as previously described [12]. A 1 kilo-base DNA ladder (New England Biolabs, Ipswich, MA) was used to compare the band size and extracts of TV strain ATCC 30001 (American Type Culture Collection, Manassas, VA) [12], which was used as a TVV+ control. The other lanes contained the dsRNA solutions of the TV isolates. The TVV+ bands were detected under ultraviolet light.
Drug Susceptibility Testing
MTZ resistance testing on the TOC TV isolates was performed at the CDC, using a protocol previously described [24]. Briefly, MTZ was solubilized in dimethyl sulfoxide and used to prepare 2-fold serial dilutions (400 μg/ml to 0.1 μg/ml) in Diamond’s media in round-bottom microtiter plates. The drug concentration was tested in triplicate; duplicate serial dilutions of dimethyl sulfoxide were tested to control for parasite viability. Trichomonads (104/well) were added to each well and the plates were incubated at 37°C in 5% CO2. After 48 hours, the plates were examined using an inverted microscope. The lowest concentration of MTZ at which no viable parasites were observed was recorded as the minimal lethal concentration. Resistance was defined as a minimal lethal concentration ≥ 50 μg/ml.
Statistical Analysis
Associations between TVV and selected demographic and clinical characteristics and MTZ resistance were examined using Chi-square and t-test statistics. Those variables associated with TVV at a P value of 0.10 or less in bivariate analyses were examined in multivariable logistic regression. The association between the variables and TVV were also examined by TV-positive status at TOC and by MTZ resistance. The association of the TVV status and the TOC status was examined in cross tabulations. All analyses were conducted using Statistical Package for the Social Sciences statistical software version 19.0 (IBM SPSS, New York).
RESULTS
Trichomonas vaginalis Virus Prevalence
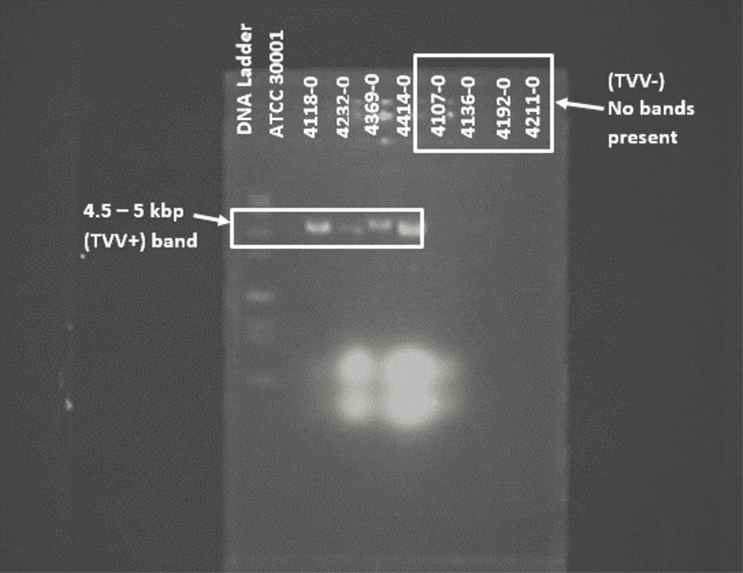
Of the 355 RCT enrollment visit TV isolates screened, 142 (40.0%) were positive for TVV. Figure 1 is a representative gel of 8 TV isolates screened for the presence of TVV.
Figure 1.
Representative gel of TV isolates screened for TVV. The first 2 lanes of the gel contain the DNA ladder and ATCC 30001 TVV+ control, respectively. The next 4 lanes were TVV+ isolates that clearly showed the expected dsRNA bands representative of the virus presence, at 4.5 kbp. The last 4 lanes were of TVV− isolates, and no bands were present. Abbreviations: ATCC, American Type Culture Collection; dsRNA, double-stranded RNA; kbp, kilo-base pair; TV, Trichomonas vaginalis; TVV, Trichomonas vaginalis virus; TVV+, TVV-positive; TVV−, TVV-negative.
Associations Between Trichomonas vaginalis Virus, Patient Demographics, and Clinical Characteristics
Table 1 displays the patient demographics and clinical characteristics at RCT enrollment, stratified by the presence of TVV. In a univariate analysis, women with TVV+ isolates were significantly older (age > 30 years, 65/142 [45.8%]) compared to those with TVV-negative (TVV-) isolates (age > 30 years, 70/213 [32.9%]; P = .01). Women with TVV+ isolates were also significantly more likely to be current cigarette smokers, compared to women with TVV− isolates (54.9% vs 43.7%; P = .04). In contrast, women who reported a history of gonorrhea were significantly more likely to have TVV− isolates (38.2% vs 27.7%; P = .04). There was no association between the presence of self-reported genital symptoms (vaginal itching, odor, discharge, and/or dysuria) at enrollment with the presence of TVV+ isolates (P = .14). Age, smoking, and history of gonorrhea were subsequently included in a multivariable logistic regression model (Table 2). In the multivariable analysis, older age (odds ratio [OR] 1.63, 95% confidence interval [CI] 1.05–2.55; P = .03) and smoking (OR 1.47, 95% CI 0.95–2.55; P = .08) remained independently associated with TVV+ isolates. In contrast, TV-infected women reporting a history of gonorrhea were independently more likely to have TVV− isolates (OR 0.60, 95% CI 0.38–0.96; P = .03).
Table 1.
Patient Demographics and Clinical Characteristics at Randomized, Controlled Trial Enrollment, Stratified by Trichomonas vaginalis Virus Presence (N = 355)
| TVV+ n/N (%) |
TVV− n/N (%) |
P Value | |
|---|---|---|---|
| Age 30+ years and older | 65/142 (45.8%) | 70/213 (32.9%) | .01 |
| % African American | 134/142 (94.4%) | 205/213 (96.2%) | .40 |
| % Self-reported genital symptomsa | 88/142 (62.0%) | 148/213 (69.5%) | .14 |
| Vaginal douching ≥ monthly | 45/142 (31.7%) | 56/213 (26.3%) | .27 |
| Binge drinking in last week | 30/142 (21.1%) | 37/213 (17.4%) | .38 |
| Current cigarette smoking | 78/142 (54.9%) | 93/213 (43.7%) | .04 |
| Multiple male sexual partners | 56/142 (39.4%) | 73/213 (34.3%) | .32 |
| Sex with women | 24/142 (16.9%) | 30/213 (14.1%) | .47 |
| History of BV | 71/142 (44.3%) | 94/212 (50.0%) | .30 |
| BV at RCT enrollment | 69/139 (49.6%) | 99/210 (47.1%) | .65 |
| History of TV | 81/142 (57.0%) | 117/212 (55.2%) | .73 |
| History of chlamydia | 72/142 (50.7%) | 123/213 (57.7%) | .19 |
| History of gonorrhea | 39/142 (27.7%) | 81/212 (38.2%) | .04 |
| History of syphilis | 11/142 (7.7%) | 10/213 (4.7%) | .23 |
| History of genital herpes | 13/142 (9.2%) | 21/212 (9.9%) | .81 |
| History of yeast infection | 105/142 (74.5%) | 150/212 (70.8%) | .45 |
Bolded values are significant at a P value < .05.
Abbreviations: BV, bacterial vaginosis; RCT, randomized, controlled trial; TV, Trichomonas vaginalis; TVV, Trichomonas vaginalis virus; TVV+, TVV-positive; TVV−, TVV-negative.
aCombination of vaginal itching, an odor, discharge, and/or dysuria.
Table 2.
Multivariable Logistic Regression of Variables Associated With Trichomonas vaginalis Virus–positive Isolates (N = 353)
| OR (95% CI) | P Value | |
|---|---|---|
| Age | ||
| 30+ years | 1.63 (1.05–2.55) | .03 |
| <30 years | 1.00 | |
| Current cigarette smoking | ||
| Yes | 1.47 (0.95–2.28) | .08 |
| No | 1.00 | |
| History of gonorrheaa | ||
| Yes | 0.60 (0.38–0.96) | .03 |
| No | 1.00 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aThere were 2 participants who had missing data for a self-reported history of gonorrhea.
Associations Between Trichomonas vaginalis Virus Status and Test of Cure
Women with TVV+ isolates at enrollment were not significantly more likely to be positive for TV at their TOC visit, compared to women with TVV− isolates (17/133 [12.8%] vs 30/189 [15.9%], respectively; risk ratio [RR] 0.97, 95% CI 0.88–1.06; P = .44). None of the tested variables were associated with a repeat TV infection. Of the 322 women who returned for their RCT TOC visit, 47 were TV-positive by NAAT: 17 (36.2%) of these women were TVV+ at enrollment and 30 (63.8%) were TVV− (P = .44). Subsequent in vitro testing for MTZ resistance was performed for 25 of 47 TOC isolates from patients that were TV-positive at the TOC visit; 10 of these isolates were TVV+. None of the TVV+ isolates demonstrated MTZ resistance, while 2/15 TVV− isolates demonstrated mild to moderate MTZ resistance (P = .23). Not all of the TV isolates from the TOC visit could be grown in vitro for MTZ resistance testing, because of excessive yeast growth. The relative risks and 95% CIs are presented in Table 3.
Table 3.
Trichomonas vaginalis Virus Status at Test of Cure Follow-up Visit (N = 322)a
| TVV+ n/N (%) |
TVV− n/N (%) |
RR (95% CI) | P Value | |
|---|---|---|---|---|
| Positive for TV at TOCb | 17/133 (12.8%) | 30/189 (15.9%) | 0.97 (0.88–1.06) | .44 |
| In vitro testing for MTZ resistancec | 10/133 (7.5%) | 15/189 (7.9%) | ||
| TV isolates showing MTZ resistance | 0/10 (0%) | 2/15 (13.3%) | .23 |
Abbreviations: CI, confidence interval; MTZ, metronidazole; RR, risk ratio; TOC, test of cure; TV, Trichomonas vaginalis; TVV, Trichomonas vaginalis virus; TVV+, TVV-positive; TVV−, TVV-negative.
aOf 355 women, 322 returned for a TOC follow-up visit (after drug treatment).
bAt TOC by nucleic acid amplification test, 47 women remained persistently positive for TV.
cOnly 25/47 isolates were tested for MTZ resistance, due to difficulty growing in vitro.
DISCUSSION
We tested a large number of US TV isolates for the presence of TVV, and evaluated the association of TVV with demographics, clinical outcomes, and MTZ resistance. The percentage of TV isolates positive for TVV was 40%. Among the 19 studies previously performed in the United States that were related to TVV positivity, the majority of which only included a small number of TV isolates [25], the percentage of TVV+ TV isolates ranged from a low of 30% [11] to a high of 100% [26]. Only 1 study had a larger sample size than this study, with 500 TV isolates, of which 250 (50%) were TVV+ [16].
Unlike other STIs, TV infection rates are higher among women 25 years or older [27], compared to other age groups [3]. In this study, we found that TVV positivity was also associated with older age. This finding was corroborated by Wendel et al [28] in a study of 28 TV-infected women at a Baltimore sexually transmitted disease clinic, which found that those TV isolates positive for TVV were significantly associated with older age (median age 38 years). It could be hypothesized that older women with TV have less immune surveillance towards TVV [28]. However, Heidary et al [29] did not find an association between TVV+ isolates and older age among 46 TV-infected women in Iran. Thus, the relationship between TVV positivity and older age remains controversial, and additional studies are needed.
There was no association between self-reported genital symptoms or repeat TV infections among women with TVV+ isolates in our study. These results are similar to a study of TV-infected women in India, in which TVV+ isolates were not associated with genital symptoms [30]. A Kenyan study of TV-infected women also found no association between genital symptoms and TVV+ isolates [31]. In contrast, a Cuban study of 37 TV isolates found an association between the presence of genital symptoms and signs and TVV positivity among 21 isolates that were TVV+ (P < .01) [32]. When the 21 TVV+ isolates were typed [20], the TV isolates from patients with mild genital symptoms were found to be infected with TVV-1, while TVV-2 was present in the isolates of patients with moderate to severe genital symptoms. The authors suggested that TVV may have a role in the pathogenesis of trichomoniasis. El-Gayar et al [27] also found a significant association between TVV positivity and clinical manifestations of trichomoniasis among 40 TV-infected women in Egypt. However, the sample sizes of these studies were small and the associations between genital symptoms and TVV+ isolates have not been seen in larger studies, including our present study [28, 30, 31].
TVV positivity was associated with smoking in our study. Smoking is known to contribute to a reduction in vaginal lactobacilli (particularly Lactobacillus crispatus) [33], an altered vaginal tract metabolomics profile [34], and increased susceptibility to bacterial vaginosis and other STIs, including trichomoniasis [35–37]. TVV upregulates the expression of certain proteins involved in cytoadhesion and cytotoxicity, such as the immunogenic P270 and cysteine proteinases in TV parasites [15–17]. Hypothetically, this could help TV better adhere to and compete for space in the vaginal epithelium. It has been suggested that smoking cessation should be investigated as an adjunct to reducing bacterial vaginosis [33]. Whether or not smoking cessation could also reduce TV acquisition is unknown and could be an area of future study.
TV infections have been previously associated with concurrent gonorrhea infections [36, 38, 39]. However, in this study, women with TVV− isolates were significantly more likely to report a history of gonorrhea than women with TVV+ isolates. The clinical significance, if any, of this finding is unknown. Self-reported STI histories are subject to social desirability and recall biases, and this result should be interpreted with caution. In addition, we did not have consistent gonococcal NAAT data for all patients at the time of RCT enrollment. Thus, we could not determine whether an association exists between TVV+ TV and a concurrent gonorrhea diagnosis. These findings should be further explored in future studies.
TV isolates positive for TVV have been negatively associated with MTZ resistance in prior studies [30, 40]. Malla et al [30] found that TVV+ TV isolates were initially sensitive to MTZ; however, 1 isolate developed borderline or increased resistance with the loss of TVV after long-term cultivation. In our study, we found that TVV+ isolates were not associated with MTZ resistance. None of the TV isolates containing TVV (0/10) showed in vitro resistance, while 2/15 TVV− isolates did. Additional research is needed to determine the specific mechanisms by which TVV affects TV susceptibility to MTZ.
This study has several limitations. Trichomonas vaginalis isolates obtained from this study were from primarily African American women, as this is the demographic most commonly seen at the Jefferson County Department of Health sexually transmitted disease clinic in Birmingham, Alabama. Thus, the results of this study may not be generalizable to other populations or other geographic areas of the United States. Second, we were not able to type the TVV+ isolates in this study, due to budgetary constraints. This would have provided additional data to explore associations between specific TVV types and demographic variables, clinical outcomes, and MTZ resistance. We continue to preserve the specimens, which can be typed in the future once additional funding becomes available. Additionally, genital symptoms were self-reported by participants and prone to social desirability biases. Provider-documented reports of patient signs and symptoms in future studies may yield more accurate data. MTZ resistance testing on a larger number of TV isolates could provide a more complete picture on the effects of TVV on MTZ resistance. Finally, tinidazole resistance testing was not performed on the TV isolates in this study, but should be done in future studies.
Despite these limitations, this is one of the largest US studies of TV isolates screened for TVV. The prevalence of TVV+ isolates was relatively high, at 40%. The presence of TVV was significantly associated with smoking and older age, while the absence of TVV was associated with a reported history of gonorrhea infection. TVV was not associated with clinical symptoms, repeat TV infections, or MTZ resistance. These results suggest that TVV and TV may have a commensal relationship.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number R01 AI097080).
Potential conflicts of interest. J. R. S. reports grants and personal fees from Hologic, Symbiomix, BD Diagnostics, and Toltec and personal fees from Lupin and Talis, outside the submitted work. C. A. M. reports grants from the National Institutes of Health/ National Institute of Allergy and Infectious Diseases, as well as personal fees from Lupin Pharmaceuticals, Cepheid, BioFire Diagnostics, and Roche Diagnostics, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society of Microbiology Annual Meeting, Atlanta, Georgia, 8 June 2018. Poster presentation #697.
References
- 1. Centers for Disease Control and Prevention; Trichomoniasis [Web page]. Available at: www.cdc.gov/std/trichomonas. Accessed 24 March 2017. [Google Scholar]
- 2. Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev 2004; 17:794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel EU, Gaydos CA, Packman ZR, Quinn TC, Tobian AAR. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis 2018; 67:211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krieger JN, Tam MR, Stevens CE, et al. Diagnosis of trichomoniasis. Comparison of conventional wet-mount examination with cytologic studies, cultures, and monoclonal antibody staining of direct specimens. JAMA 1988; 259:1223–7. [DOI] [PubMed] [Google Scholar]
- 5. Hobbs MM, Seña AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infect 2013; 89:434–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwebke JR, Hobbs MM, Taylor SN, et al. Molecular testing for Trichomonas vaginalis in women: results from a prospective U.S. clinical trial. J Clin Microbiol 2011; 49:4106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper system. J Clin Microbiol 2014; 52:885–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwebke JR, Gaydos CA, Davis T, et al. Clinical evaluation of the cepheid Xpert TV assay for detection of Trichomonas vaginalis with prospectively collected specimens from men and women. J Clin Microbiol 2018; 56:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirkcaldy RD, Augostini P, Asbel LE, et al. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD Surveillance Network, 2009-2010. Emerg Infect Dis 2012; 18:939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Essential medicines and health products information portal. Available at: http://apps.who.int/medicinedocs/en/d/Jh2942e/4.9.html#Jh2942e.4.9. Accessed March 2017. [Google Scholar]
- 11. Wang AL, Wang CC. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci USA 1986; 83:7956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang AL, Wang CC. A linear double-stranded RNA in Trichomonas vaginalis. J Biol Chem 1985; 260:3697–702. [PubMed] [Google Scholar]
- 13. Goodman RP, Ghabrial SA, Fichorova RN, Nibert ML. Trichomonasvirus: a new genus of protozoan viruses in the family Totiviridae. Arch Virol 2011; 156:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parent KN, Takagi Y, Cardone G, et al. Structure of a protozoan virus from the human genitourinary parasite Trichomonas vaginalis. MBio 2013; 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khoshnan A, Alderete JF. Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J Virol 1994; 68:4035–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Provenzano D, Khoshnan A, Alderete JF. Involvement of dsRNA virus in the protein composition and growth kinetics of host Trichomonas vaginalis. Arch Virol 1997; 142:939–52. [DOI] [PubMed] [Google Scholar]
- 17. He D, Pengtao G, Ju Y, et al. Differential protein expressions in virus-infected and uninfected Trichomonas vaginalis. Korean J Parasitol 2017; 55:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benchimol M, Chang TH, Alderete JF. Trichomonas vaginalis: observation of coexistence of multiple viruses in the same isolate. FEMS Microbiol Lett 2002; 215:197–201. [DOI] [PubMed] [Google Scholar]
- 19. Goodman RP, Freret TS, Kula T, et al. Clinical isolates of Trichomonas vaginalis concurrently infected by strains of up to four Trichomonasvirus species (Family Totiviridae). J Virol 2011; 85:4258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fraga J, Rojas L, Sariego I, Fernández-Calienes A, Nuñez FA. Species typing of Cuban Trichomonas vaginalis virus by RT-PCR, and association of TVV-2 with high parasite adhesion levels and high pathogenicity in patients. Arch Virol 2012; 157:1789–95. [DOI] [PubMed] [Google Scholar]
- 21. Bessarab IN, Nakajima R, Liu HW, Tai JH. Identification and characterization of a type III Trichomonas vaginalis virus in the protozoan pathogen Trichomonas vaginalis. Arch Virol 2011; 156:285–94. [DOI] [PubMed] [Google Scholar]
- 22. Kissinger P, Muzny CA, Mena L, et al. A randomized trial of metronidazole in a single 2 g dose versus 500 mg twice daily for 7 days for the treatment of trichomoniasis in women. Lancet Infect Dis 2018; 18(11):1251–9. doi: 10.1016/S1473-3099(18)30423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biomed Diagnostics, Incorporated. InPouch™ TV - Trichomonas vaginalis test. 2015. Accessed March 2017. [Google Scholar]
- 24. Narcisi EM, Secor WE. In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob Agents Chemother 1996; 40:1121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Graves KJ, Ghosh AP, Kissinger PJ, Muzny CA. Trichomonas vaginalis virus: a review of the literature. Int J STD AIDS 2019; 956462418809767. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Khoshnan A, Alderete JF. Characterization of double-stranded RNA satellites associated with the Trichomonas vaginalis virus. J Virol 1995; 69:6892–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El-Gayar EK, Mokhtar AB, Hassan WA. Molecular characterization of double-stranded RNA virus in Trichomonas vaginalis Egyptian isolates and its association with pathogenicity. Parasitol Res 2016; 115:4027–36. [DOI] [PubMed] [Google Scholar]
- 28. Wendel KA, Rompalo AM, Erbelding EJ, Chang TH, Alderete JF. Double-stranded RNA viral infection of Trichomonas vaginalis infecting patients attending a sexually transmitted diseases clinic. J Infect Dis 2002; 186:558–61. [DOI] [PubMed] [Google Scholar]
- 29. Heidary S, Bandehpour M, Valadkhani Z, et al. Double-stranded RNA viral infection in Tehran Trichomonas vaginalis isolates. Iran J Parasitol 2013; 8:60–4. [PMC free article] [PubMed] [Google Scholar]
- 30. Malla N, Kaul P, Sehgal R, Gupta I. The presence of dsRNA virus in Trichomonas vaginalis isolates from symptomatic and asymptomatic Indian women and its correlation with in vitro metronidazole sensitivity. Indian J Med Microbiol 2011; 29:152–7. [DOI] [PubMed] [Google Scholar]
- 31. Masha SC, Cools P, Crucitti T, Sanders EJ, Vaneechoutte M. Molecular typing of Trichomonas vaginalis isolates by actin gene sequence analysis and carriage of T. vaginalis viruses. Parasit Vectors 2017; 10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fraga J, Rojas L, Sariego I, Fernández-Calienes A. Double-stranded RNA viral infection of Trichomonas vaginalis and correlation with genetic polymorphism of isolates. Exp Parasitol 2011; 127:593–9. [DOI] [PubMed] [Google Scholar]
- 33. Brotman RM, He X, Gajer P, et al. Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect Dis 2014; 14:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nelson TM, Borgogna JC, Michalek RD, et al. Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Sci Rep 2018; 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis 2008; 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Swartzendruber A, Sales JM, Brown JL, Diclemente RJ, Rose ES. Correlates of incident Trichomonas vaginalis infections among African American female adolescents. Sex Transm Dis 2014; 41:240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brotman RM, Bradford LL, Conrad M, et al. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis 2012; 39:807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muzny CA, Blackburn RJ, Sinsky RJ, Austin EL, Schwebke JR. Added benefit of nucleic acid amplification testing for the diagnosis of Trichomonas vaginalis among men and women attending a sexually transmitted diseases clinic. Clin Infect Dis 2014; 59:834–41. [DOI] [PubMed] [Google Scholar]
- 39. Lazenby GB, Soper DE, Nolte FS. Correlation of leukorrhea and Trichomonas vaginalis infection. J Clin Microbiol 2013; 51:2323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Snipes LJ, Gamard PM, Narcisi EM, Beard CB, Lehmann T, Secor WE. Molecular epidemiology of metronidazole resistance in a population of Trichomonas vaginalis clinical isolates. J Clin Microbiol 2000; 38:3004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]