Abstract
Background
Latent tuberculosis infection (LTBI) screening and treatment is a key component of the World Health Organization (WHO) EndTB Strategy, but the impact of LTBI screening and treatment at a population level is unclear. We aimed to estimate the impact of LTBI screening and treatment in a population of migrants to British Columbia (BC), Canada.
Methods
This retrospective cohort included all individuals (N = 1 080 908) who immigrated to Canada as permanent residents between 1985 and 2012 and were residents in BC at any time up to 2013. Multiple administrative databases were linked to identify people with risk factors who met the WHO strong recommendations for screening: people with tuberculosis (TB) contact, with human immunodeficiency virus, on dialysis, with tumor necrosis factor-alpha inhibitors, who had an organ/haematological transplant, or with silicosis. Additional TB risk factors included immunosuppressive medications, cancer, diabetes, and migration from a country with a high TB burden. We defined active TB as preventable if diagnosed ≥6 months after a risk factor diagnosis. We estimated the number of preventable TB cases, given optimal LTBI screening and treatment, based on these risk factors.
Results
There were 16 085 people (1.5%) identified with WHO strong risk factors. Of the 2814 people with active TB, 118 (4.2%) were considered preventable through screening with WHO risk factors. Less than half (49.4%) were considered preventable with expanded screening to include people migrating from countries with high TB burdens, people who had been prescribed immunosuppressive medications, or people with diabetes or cancer.
Conclusions
The application of WHO LTBI strong recommendations for screening would have minimally impacted the TB incidence in this population. Further high-risk groups must be identified to develop an effective LTBI screening and treatment strategy for low-incidence regions.
Keywords: latent tuberculosis infection, public health, epidemiology, immigration health
We modelled the impact of latent tuberculosis screening and treatment in a cohort of migrants to a low-incidence region. Screening and treatment, based on the World Health Organization’s recommendations, minimally impacted the tuberculosis incidence and would not achieve elimination goals.
(See the Editorial Commentary by Katrak and Barry on pages 2109–11.)
Tuberculosis (TB) epidemiology has shifted significantly over the past few decades in many countries with low TB incidences [1, 2]. In these settings, TB incidences appear to be driven largely by the reactivation of latent TB infections (LTBIs) in people migrating from regions with high TB incidences, rather than from local TB transmission [2]. TB elimination policies must effectively address LTBI screening in migrant populations to be effective in reducing TB rates in low-incidence regions [2]. Accordingly, numerous organizations have produced evidence-based LTBI screening guidelines that recommend the targeted testing of certain people who are migrating to low-incidence regions and are at high risk of developing TB [3, 4]. However, often these recommendations are based on limited evidence [3, 5].
In 2014, the World Health Organization (WHO) published TB elimination guidelines for regions with low TB incidences [1]. These guidelines identified the treatment of LTBI as a key strategy for achieving TB elimination [1]. Based on the Grading of Recommendations, Assessment, Development and Evaluations framework, the WHO LTBI screening guidelines made several strong recommendations for the systematic testing and treatment of LTBI, and several conditional recommendations based on weak evidence [5]. The strong recommendations include targeted screening based on close TB contact or specific medical risk factors, including human immunodeficiency virus (HIV) infection, use of dialysis, use of tumor necrosis factor (TNF)-alpha inhibitors, a history of a transplant, and silicosis [5]. Conditional recommendations for systematic LTBI testing included screening people migrating from countries with high TB burdens, healthcare workers, homeless persons, prisoners, and illicit drug users [5].
The epidemiological impact of targeted LTBI screening programs is unclear, in part because there have been no population-based evaluations of the impact of targeted screening in a region with a low TB incidence. In this study, we evaluated the potential impact of LTBI screening guidelines among people immigrating to British Columbia (BC), a Canadian province with a low TB incidence. We first evaluated the potential impact of a focused screening strategy based solely on the WHO strong recommendations. We further estimated the potential impact of additional LTBI screening and treatment of medical risk groups. Finally, we assessed how screening based on demographic factors alone could assist in achieving TB elimination in this low-incidence region.
METHODS
Study Population and Data Sources
This study is part of a larger project to describe TB epidemiology in foreign-born residents after immigration to BC, and descriptions of the databases and methods to identify the cohort have been described in detail in previous publications [6, 7]. Briefly, the data for this study were provided through Population Data BC, a multi-institution data resource that is among the world’s largest collections of administrative and disease registry data [7]. Data were extracted by Population Data BC from several linked databases, including the Immigration, Refugees, and Citizenship Canadian Permanent Residents Database, BC provincial health insurance registration (Medical Services Plan [MSP]), hospitalizations, physician billing, medication dispensation from community pharmacies, vital statistics, and the provincial TB, HIV, cancer, and renal disease registries [8–17]. All extracted data were then provided to the researchers as disidentifiable data sets, which could be linked together using unique, scrambled identification numbers.
The study cohort included all individuals who immigrated to Canada between 1 January 1985 and 31 December 2012 as permanent residents and who established BC residency by 2013. We identified individuals as BC residents when they registered in MSP. BC residents are required to enroll in MSP if they are Canadian citizens or lawfully admitted to Canada for permanent residence, and are physically present in BC ≥6 months per calendar year. Depending on an individual’s income, MSP coverage may be free or may require monthly premiums. We therefore considered MSP coverage a good proxy measure for BC residency.
Follow-up times for all individuals started at their index date, defined as 90 days before their first MSP registration date, to account for the mandatory waiting period for starting MSP after arrival to BC. We ended the follow-up at the participant’s active TB diagnosis, end of MSP coverage, or death or at the end of the study period (31 December 2013), whichever came first. We excluded people with a case of active TB (n = 93; 3.2% of TB cases) diagnosed in BC before their index date (median time from TB diagnosis to index date was 236 days, interquartile range 94–565). We identified TB diagnoses based on the province’s centralized TB Registry data (held at the BC Centre for Disease Control) and included all TB sites (ie, pulmonary and extrapulmonary), whether microbiologically or clinically confirmed.
Identification of LTBI Screening Factors and Active Tuberculosis Outcomes
We extracted demographic variables for participants, including age at index (in years), calendar year of index, gender, country and WHO region of origin, TB incidence in country of origin, and immigration classification. We defined immigration classification as the stream through which people immigrated as permanent residents to Canada: Economic, Family, Refugee, and Other [6, 18]. The TB incidence in the country of origin was derived from country-level WHO TB incidence data (all TB forms/100 000 population) [19]. We defined each individual’s country of origin as either their country of birth or country of last permanent residence, using whichever had the highest estimated TB incidence in the calendar year of their index date. We further grouped the TB incidences in countries of origin, based on Canadian TB Standards cut-points: 0–30/100 000 population, 31–100, 101–200, and >2003.
To determine the impact of targeted LTBI screening based on the WHO strong recommendations, we identified the dates when a person had known contact with active TB in BC or was first diagnosed with a medical risk factor targeted for LTBI screening by the WHO strong recommendations [5]. These risk factors included having HIV/acquired immunodeficiency syndrome, being on dialysis, using TNF-alpha inhibitors, having a solid organ or bone marrow transplant, and silicosis [5]. Wherever possible, medical risk factors were derived from multiple databases—including disease registries, drug dispensation records, and health administrative databases—using validated algorithms; our definitions of medical risk factors and demographic characteristics have been described previously [7].
To evaluate the impact of broader LTBI screening and treatment guidelines, we also identified when a person developed cancer (head and neck, lung, or hematologic) or used other medical immunosuppressive treatments (high-dose steroids or high-risk disease-modifying antirheumatic drugs [DMARDs]). We defined high-dose steroid use as receiving a daily dose of at least 20 mg of prednisone equivalents for a minimum of 14 days within 21 days and defined high-risk DMARD use as any drug dispensation record of cyclophosphamide, cyclosporine, leflunomide, mycophenolate, sirolimus, or tacrolimus. Our drug definitions were based on a previous study, which identified these drugs as creating a high risk for active TB development [20].
We also considered an expanded approach to LTBI screening and treatment that included people migrating from countries with high TB burdens, based on WHO conditional recommendations for LTBI screening. We defined a country of origin as having a high TB burden if it had a TB incidence >200/100 000 people, and limited this potential screening population to people migrating to BC at age ≤50 years. We selected these age and incidence cut-points based on the estimated threshold whereby LTBI treatment would benefit an individual in quality-adjusted life years [6, 21]. In other words, in this cohort, people at these cut-points had active TB incidences exceeding the threshold for an individual benefit of LTBI therapy. This was chosen as our threshold for mass, demographic-based screening.
Finally, we identified when a person was diagnosed with diabetes. Notably, the WHO recommends against the systematic screening of people with diabetes in the absence of other risk factors [5]. Similarly, the US Preventive Services Task Force does not recommend systematic LTBI screening of patients with diabetes [22]. However, screening among select individuals with diabetes is recommended by several other country-level LTBI screening guidelines (for example, the US American Thoracic Society/Centers for Disease Control and Prevention [23], Canadian TB Standards [3], and UK National Institute for Health and Care Excellence (NICE) [24] guidelines). Hence, we included diabetes in our evaluation.
Our primary outcomes were active TB and potentially preventable, active TB. Active TB was defined as being potentially preventable through targeted LTBI screening and treatment when the active TB diagnosis date occurred ≥6 months after a risk factor diagnosis. When active TB was diagnosed <6 months after a risk factor diagnosis, we did not consider this preventable by targeted LTBI screening and treatment.
Statistical Analysis
We described demographic characteristics at the time of cohort entry (index date) and calculated person-years of follow-up, stratified by each characteristic. To assess the frequency of specific risk factors among active TB cases, we calculated the number of people who developed active TB during follow-up, stratified by the number of years after the index date and by the proportion with risk factors present at the time of an active TB diagnosis.
To assess the potential resource implications and impacts of targeted LTBI screening and treatment strategies, we calculated the number of people with specific risk factors diagnosed during the study follow-up, the number of people developing active TB after a specific risk factor was diagnosed, and the number of potentially preventable, active TB diagnoses with this screening strategy. We also estimated the number of people needed to screen annually and the number needed to screen and treat, when indicated, to prevent 1 active TB case. We then compared the cumulative increases in the proportion of preventable active TB cases with the application of different LTBI screening strategies: (1) screening based on WHO strong recommendations only; (2) screening based on WHO strong recommendations plus broader medical risk factors (ie, adding cancer and steroids/DMARDs); (3) screening based on WHO strong recommendations and broader medical risk factors, plus screening people ≤50 years at cohort entry and who immigrated from countries with TB incidences >200/100 000 people (high-incidence countries); and (4) screening based on all of the above plus diabetes. Finally, we estimated the cumulative number of active TB cases that could have been prevented using different LTBI screening and treatment strategies, and compared these to the WHO TB elimination benchmark (ie, <1 case/million population annually). For sensitivity analyses, we limited the time period for estimating the impact of immunosuppressive medications to 1996 onwards (as we did not have medications data before this year); lowered the threshold for defining high-dose steroids; limited the analysis of the impact of diabetes screening to people ≤65 years at the time of the diabetes diagnosis (as recommended by Canadian TB Standards); expanded mass screening thresholds to ≤65 years and ≥100/100 000; and excluded people who were documented as having been screened and/or treated for LTBI at any point in BC.
Analyses were conducted in SAS version 9.4 (SAS Institute Inc., Cary, NC). This study received ethics approval by the University of BC Clinical Ethics Review Board (H16-00265).
RESULTS
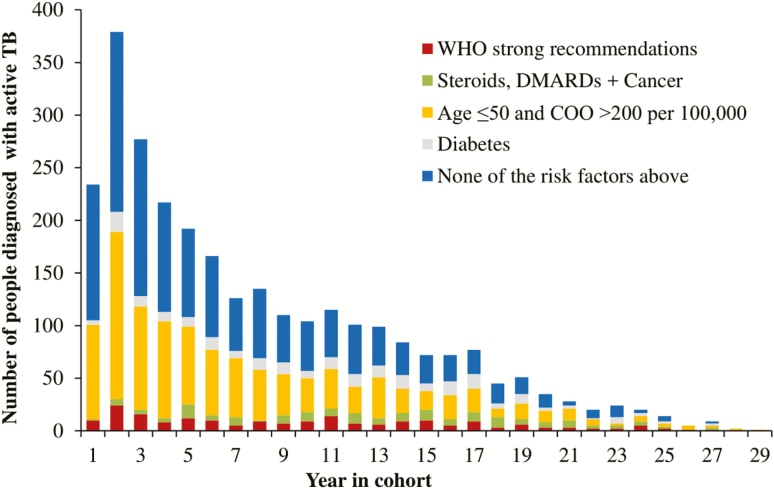
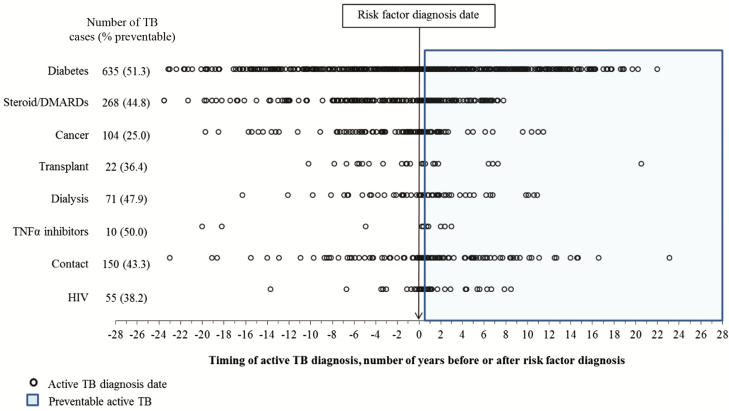
The cohort included 1 080 908 people, with a median study follow-up of 10.1 years per person (Table 1). The median age of the cohort at their index dates was 30 years (interquartile range 19–41). There were 2814 people (0.3%) diagnosed with active TB during the study period, with almost half (46.2%) diagnosed in the first 5 years after their index date (Figure 1). The proportion of people with medical risk factors at the time of their active TB diagnosis increased with the time since their index date (Figure 1). Many active TB diagnoses predated the risk factor diagnosis date or fell within the first 6 months after a risk factor diagnosis date (Figure 2). For example, almost half of the 635 people with active TB and diabetes were diagnosed with active TB before they were diagnosed with diabetes (Figure 2). There were 3028 people with 2 or more comorbidity risk factors (including WHO strong recommendations, cancer, and steroids/DMARDs; data not shown).
Table 1.
Characteristics of Cohort at Time of Index Date, Total Follow-up in Person-years, and Number of Active Tuberculosis Cases Diagnosed in British Columbia
| Characteristic | Number of People (%) | Total Person-years in BC, in 1000s | Number of Active TB Cases |
|---|---|---|---|
| All | 1 080 908 | 11 640 | 2814 |
| Age group, years | |||
| 0–5 | 59 070 (5.5) | 670 | 31 |
| 6–20 | 241 145 (22.3) | 2695 | 368 |
| 21–35 | 377 275 (34.9) | 4038 | 929 |
| 36–50 | 271 122 (25.1) | 2828 | 586 |
| 51–65 | 95 025 (8.8) | 1056 | 566 |
| >65 | 37 271 (3.6) | 353 | 334 |
| Gender | |||
| Female | 553 976 (51.3) | 6071 | 1391 |
| Male | 526 932 (48.8) | 5569 | 1423 |
| Immigration class | |||
| Economic | 637 775 (59.0) | 6489 | 942 |
| Refugee | 86 803 (8.0) | 1069 | 362 |
| Family | 326 106 (30.2) | 3723 | 1387 |
| Other | 30 224 (2.8) | 359 | 123 |
| WHO region, country of origina | |||
| Southeast Asia | 153 061 (14.2) | 1631 | 698 |
| Africa | 31 161 (2.9) | 320 | 101 |
| Western Pacific | 586 669 (54.3) | 6476 | 1773 |
| Eastern Mediterranean | 84 057 (7.8) | 778 | 134 |
| The Americas | 80 297 (7.4) | 839 | 49 |
| Europe | 145 661 (13.5) | 1595 | 59 |
| TB rate, country of origina,b | |||
| 0–30 | 186 165 (17.2) | 1733 | 37 |
| 31–100 | 278 081 (25.7) | 2582 | 249 |
| 101–200 | 340 901 (31.5) | 4067 | 952 |
| >200 | 275 759 (25.5) | 3257 | 1576 |
| Cohort entry (index year) | |||
| 1985–1994 | 299 501 (27.7) | 5051 | 1386 |
| 1995–2004 | 443 941 (41.1) | 5032 | 1084 |
| 2005–2013 | 337 466 (31.2) | 1556 | 344 |
Abbreviations: BC, British Columbia; TB, tuberculosis; WHO, World Health Organization.
aCountry of birth missing (n = 2).
bWHO-estimated annual number of active TB cases, per 100 000 population, in year of index date.
Figure 1.
Number of people diagnosed with active TB, by number of years after index date and presence of risk factors. WHO strong recommendations are indicated for patients with HIV, TB contacts, TNF-alpha inhibitors, dialysis, transplant, and silicosis. A COO’s TB incidence was defined as the WHO incidence in the year of index. Abbreviations: COO, country of origin; DMARD, disease-modifying antirheumatic drugs; TB, tuberculosis; TNF, tumor necrosis factor; WHO, World Health Organization.
Figure 2.
Number of years to active TB diagnosis, before or after identification of target risk factor. Preventable active TB was defined as TB diagnosed ≥6 months after a risk factor diagnosis (2814 TB cases in the population in total). Abbreviations: DMARD, disease-modifying antirheumatic drugs; HIV, human immunodeficiency virus; TB, tuberculosis; TNF, tumor necrosis factor.
Comparing different screening strategies, a total of 16 085 people (1.5% of 1 080 908 people) had at least 1 WHO strong risk factor diagnosed during the study period (Table 2). Only 118 active TB cases (4.2% of 2814 cases) were potentially preventable if screening and treatment had been based solely on the WHO strong recommendations (Strategy 1). Adding the use of steroids/DMARDs and/or a cancer diagnosis (Strategy 2) nearly doubled the proportion of preventable, active TB cases in the cohort (8.3%), but required screening 3.8% of the total population (Table 2). Meanwhile, screening people migrating from high-burden countries at age ≤50 years (Strategy 3) increased the proportion of potentially preventable, active TB cases to 42.4%, but required screening 9152 people annually (24.6% of the total population). Adding diabetes to the screening strategy (Strategy 4) increased the proportion of active TB cases that would be potentially preventable to 49.4%, while requiring an estimated 11 363 people be screened annually (30.5% of the total population; Table 2).
Table 2.
Proportion of Active Tuberculosis Cases Considered Potentially Preventable by Latent Tuberculosis Infection Screening and Treatment Strategies
| LTBI Screening Strategy | # People to Screen | # of Active TB Cases Potentially Preventable | % of Total Active TB Cases Potentially Preventablea | Estimated # of People to Screen Annually | Estimated NNSTb |
|---|---|---|---|---|---|
| Strategy 1 | 16 085 | 118 | 4.2% | 555 | 136 |
| ▪ WHO strong recommendationsc | |||||
| Strategy 2 | 41 255 | 234 | 8.3% | 1423 | 176 |
| ▪ WHO strong recommendations | |||||
| ▪ Steroids, DMARDs, cancerd | |||||
| Strategy 3 | 265 405 | 1192 | 42.4% | 9152 | 223 |
| ▪ WHO strong recommendations | |||||
| ▪ Steroids, DMARDs, cancer | |||||
| ▪ Age ≤50 and COO >200 per 105 e | |||||
| Strategy 4 | 329 533 | 1389 | 49.4% | 11 363 | 237 |
| ▪ WHO strong recommendations | |||||
| ▪ Steroids, DMARDS, cancer | |||||
| ▪ Age ≤50 and COO >200 per 105 | |||||
| ▪ Diabetes | |||||
| Strategy 5 | 292 029 | 1430 | 50.8% | 10 070 | 204 |
| ▪ WHO strong recommendations | |||||
| ▪ Steroids, DMARDS, cancer | |||||
| ▪ Age ≤65 and COO >200 per 105 | |||||
| Strategy 6 | 552 378 | 1674 | 59.5% | 19 048 | 330 |
| ▪ WHO strong recommendations | |||||
| ▪ Steroids, DMARDs, cancer | |||||
| ▪ Age ≤50 and COO >100 per 105 | |||||
| Individual risk factor–based screening | |||||
| HIV | 1445 | 21 | 0.7% | 50 | 69 |
| TB contacts | 11 877 | 65 | 2.3% | 410 | 183 |
| TNF-alpha inhibitor | 843 | 5 | 0.2% | 29 | 169 |
| Dialysis | 1832 | 34 | 1.2% | 63 | 54 |
| Transplantf | 653 | 8 | 0.3% | 23 | 82 |
| Silicosis | 12 | 0 | 0 | <1 | n/a |
| Steroids, DMARDs | 21 709 | 120 | 4.3% | 749 | 181 |
| Cancer | 6508 | 26 | 0.9% | 224 | 250 |
| Age ≤50 and COO >200 per 105 | 236 785 | 1045 | 37.1% | 8165 | 226 |
| Diabetes | 95 739 | 326 | 11.6% | 3301 | 294 |
Abbreviations: COO, country of origin; DMARD, disease-modifying antirheumatic drugs; HIV, human immunodeficiency virus; LTBI, latent tuberculosis infection; NNST, number needed to screen and treat; TB, tuberculosis; TNF, tumor necrosis factor; WHO, World Health Organization.
aThere were 2814 TB cases among 1 080 908 people in total. Preventable active TB was defined as TB diagnosed ≥6 months after a risk factor diagnosis.
bNNST to prevent 1 active TB case, assuming optimal screening and treatment.
cWHO strong recommendations are indicated for patients with HIV, TB contacts, TNF-alpha inhibitors, dialysis, transplant, and silicosis.
dCancer criteria were diagnoses of head and neck, lung, or hematologic cancers.
eA COO’s TB incidence was defined as the WHO incidence in the year of index.
fTransplants of solid organs and bone marrow.
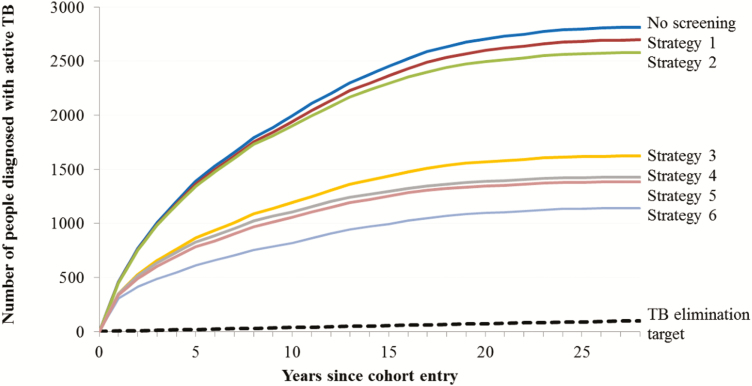
In sensitivity analyses, we increased the age threshold to ≤65 (Strategy 5) and lowered the threshold for a high-burden country to >100/100 000 (Strategy 6); these variants increased the proportions of active TB cases that were preventable to 50.8% and 59.5%, respectively, while requiring screening of 27.0% and 51.1% of the total population, respectively (Table 2). Additionally, we lowered the high-dose steroid threshold; for Strategy 4, at a 15 mg cut-off, 1400 cases could be preventable with screening 331 996 people (data not shown). Further, we excluded the 36 185 people with documented LTBI screenings before or after a screening factor diagnosis. This reduced the estimated annual number needed to screen in Strategy 4 to 10 115 (data not shown). The cumulative number of active TB cases occurring over the study follow-up period, assuming optimal screening and treatment according to the different strategies, is summarized in Figure 3 and the Supplementary Material.
Figure 3.
Estimated cumulative number of active TB cases, with different targeted latent tuberculosis infection screening and treatment strategies. Abbreviation: TB, tuberculosis.
DISCUSSION
Our results suggest that TB elimination targets for low-incidence regions will not be achieved using current WHO LTBI screening and treatment recommendations alone. Targeted LTBI screening at the time of medical risk factor diagnoses had a very limited potential impact on active TB incidences in people migrating to a region with a low TB incidence. Expanding screening to include individuals migrating by age 50 from high-burden countries could potentially reduce TB incidences in this cohort by close to 50%. When combined with the TB incidence in the general population of BC, this could potentially achieve the pre-elimination targets (<10 cases per million population).
This analysis, however, assumes near perfect LTBI screening and treatment conditions, which are not experienced in the real world [25]. Even with optimally expanded LTBI screening and treatment, the elimination of TB in low-incidence regions appears unlikely if it relies solely on LTBI screening and treatment. Further investments in new research and technologies, along with building the capacity for TB elimination in high-resource regions, will be required for TB elimination in low-incidence regions, as described in the WHO EndTB strategy.
The limited impact of the current targeted LTBI screening and treatment guidelines in reducing TB incidences and the need for a scale-up of LTBI screening programs have been previously recognized by researchers and policymakers [26–28]. In the United States, for example, Walter et al [29] found that the strict application of US LTBI screening guidelines, which included screening people from high-incidence countries ≤5 years postmigration, would have missed 43% of active TB cases in San Francisco, with no indications for LTBI testing. In a recent Canadian study in the province of Alberta, expanding LTBI screening to all people migrating from countries with high TB incidences was suggested, given that over two-thirds of culture-positive pulmonary TB cases and 100% of secondary TB cases occurred in people migrating from countries with high TB incidences [26]. Similarly, the WHO LTBI guidelines conditionally recommend targeting screening to individuals from countries with high TB burdens [5], while the UK NICE guidelines recommend targeting LTBI screening to migrants from countries with a TB incidence >150/100 000 population or from sub-Saharan Africa.
To move closer to eliminating TB in low-incidence regions, there is a need for a scale-up of LTBI screening and treatment. Our study results suggest a favorable number needed to screen and treat, compared to other public health programs targeting prevention [30]. The calculation of individual risks and benefits could aid clinical decision making, and further delineate those at the highest risk of developing TB. However, these results represent a good starting point for designing evidence-based LTBI screening and treatment programs with a population-level impact. The logistics and feasibility of broad LTBI screening programs require careful consideration.
We recognize that the expansion of LTBI screening programs will require the investment of more resources. Efficiency cannot be ignored, and cost-effectiveness analyses will need to be performed to best understand which screening strategies will maximize impacts while minimizing costs [6]. But there is always an equity versus efficiency trade-off in resource allocation decisions, and maximizing efficiency does not always achieve equity [31, 32]. If our goal is to achieve health equity for residents of low-incidence regions, then we need to ensure better access to LTBI screening and treatment programs for those most at risk for developing active TB. And importantly, LTBI screening and treatment programs need to be developed and implemented in an equitable, nonpunitive, and nonstigmatizing way [33, 34].
A major strength of our study is that it represents a near-complete capture, over a 29-year period, of the demographic, immigration, and healthcare service utilization of more than a million people who immigrated to a region with a low TB incidence. This makes it one of the largest cohorts used to investigate TB epidemiology with individual-level data, and one of the first to capture medical risk factors to such an extent.
A limitation of our study is that individuals required contact with the BC healthcare system for comorbid diagnoses to be identified; therefore, it is possible that the diagnosis dates and prevalences of some risk factors were incorrectly estimated. In addition, we did not exclude people that received LTBI screening and/or treatment outside of BC; thus, it is possible that we overestimated how many people required screening. LTBI screening and treatment, however, are uncommon in high-burden regions, so we feel that it is unlikely that prior LTBI treatments impacted our results. We did not have medication data before 1996; thus, we may have underestimated the number of active TB cases prevented in people initiating immunosuppressive medications. Additionally, the Immigration, Refugees, and Citizenship Canada database only includes individuals with permanent resident status in Canada, so it necessarily excludes temporary visitors and workers, some refugee applicants, and undocumented migrants. Finally, as is typical with health administrative database studies, misclassification errors due to inaccurate data are possible, since the data were not collected for research purposes.
CONCLUSION
This study examined the potential yields of different targeted screening approaches among residents of Canada who were born in other countries and reside in a province with a low TB incidence. We found a limited impact of LTBI screening and treatment, based on medical and TB contact risk factors alone. Screening at the time of entry to a low-incidence region, based on demographic factors, appears to be a more effective but resource-intensive strategy. To achieve the WHO targets of TB elimination in low-incidence countries, investments in new technologies and efforts to reduce TB incidences in high-burden countries will also be required.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. J. C. J. initiated the project and is principal investigator for the grant. J. C. J. and V. J. C. are tuberculosis research team leaders. L. A. R. conducted the analyses and drafted the manuscript. J. C. J., L. A. R., J. R. C., C. R., R. B., K. R., D. Z. R., F. M., K. S., and V. J. C. were investigators on the project, who shared in the study design, data interpretation, and manuscript editing. All authors provided important intellectual content and gave their final approval of the version submitted for publication.
Acknowledgments. The authors thank Fay Hutton and Leslie Chiang for assistance with data cleaning and Andrew Basham, Dr Div Kumar, and Dr Jennifer Gardy for input on data interpretation and presentation. The authors acknowledge all the people included in the cohort.
Disclaimer. The study sponsors had no role in the study design, collection, analysis, or interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication. The partners in research are Population Data British Columbia (BC); BC Centre for Disease Control; BC Ministry of Health; BC Vital Statistics Agency; Immigration, Refugees, and Citizenship Canada; BC Cancer Agency; and BC Renal Agency. All inferences, opinions, and conclusions drawn in this manuscript are those of the authors, and do not reflect the opinions or policies of the Data Steward(s).
Financial support. This work was supported by the Michael Smith Foundation for Health Research Scholar Award (to J. C. J.) and the Canadian Institutes for Health Research Grant (grant number 377364).
Potential conflicts of interest.All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Towards tuberculosis elimination: an action framework for low-incidence countries. WHO/HTM/TB/2014.13. 2014:1–63. WHO; Geneva, Switzerland: Available at: https://apps.who.int/iris/bitstream/handle/10665/132231/9789241507707_eng.pdf;jsessionid=2716FEC791707DDB0C30D12D7E66B377?sequence=1. Accessed 31 March 2019. [Google Scholar]
- 2. Lönnroth K, Mor Z, Erkens C, et al. Tuberculosis in migrants in low-incidence countries: epidemiology and intervention entry points. Int J Tuberc Lung Dis 2017; 21:624–37. [DOI] [PubMed] [Google Scholar]
- 3. Canadian Thoracic Society and Public Health Agency of Canada. Canadian Tuberculosis Standards. 7th ed. Available at: http://www.respiratoryguidelines.ca/tb-standards-2013. Accessed 30 March 2017. [Google Scholar]
- 4. Greenaway C, Sandoe A, Vissandjee B, et al. ; Canadian Collaboration for Immigrant and Refugee Health Tuberculosis: evidence review for newly arriving immigrants and refugees. CMAJ 2011; 183:E939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Latent tuberculosis infection: Updated and consolidated guidelines for programmatic management [WHO reference number: WHO/CDS/TB/2018.4]. Available at: http://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf;jsessionid=6366262D5B881690FDC629FC6E9F1660?sequence=1. Accessed 1 October 2018. [PubMed] [Google Scholar]
- 6. Ronald LA, Campbell JR, Balshaw RF, et al. Demographic predictors of active tuberculosis in people migrating to British Columbia, Canada: a retrospective cohort study. CMAJ 2018; 190:E209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ronald LA, Campbell JR, Balshaw RF, et al. Predicting tuberculosis risk in the foreign-born population of British Columbia, Canada: study protocol for a retrospective population-based cohort study. BMJ Open 2016; 6:e013488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Immigration, Refugees, and Citizenship Canada [creator] (2014). Permanent Resident Database Population BC [publisher]. Data Extract. IRCC; 2015. Available at: http://www.popdata.bc.ca/data. Accessed 31 March 2019. [Google Scholar]
- 9. BC Vital Statistics Agency [creator] (2015): Vital Statistics Deaths. V2 Population Data BC [publisher]. Data Extract. BC Vital Statistics Agency, 2014. Available at: http://www.popdata.bc.ca/data [Google Scholar]
- 10. British Columbia Ministry of Health [creator] (2015): Consolidation File (MSP Registration & Premium Billing) Population Data BC [publisher]. Data Extract. MOH, 2014. Available at: http://www.popdata.bc.ca/data [Google Scholar]
- 11. British Columbia Ministry of Health [creator] (2015): Medical Services Plan (MSP) Payment Information File Population Data BC [publisher]. Data Extract. MOH, 2014. Available at: http://www.popdata.bc.ca/data [Google Scholar]
- 12. Canadian Institute for Health Information [creator] (2015): Discharge Abstract Database (Hospital Separations) Population Data BC [publisher]. Data Extract. MOH, 2014. Available at: http://www.popdata.bc.ca/data [Google Scholar]
- 13. BC Ministry of Health [creator] (2015): PharmaNet BC Ministry of Health [publisher]. Data Extract. Data Stewardship Committee, 2014. Available at: http://www.popdata.bc.ca/data [Google Scholar]
- 14. BC Centre for Disease Control [creator] (2015): BC Provincial TB Registry (BCCDC-iPHIS) Population Data BC [publisher]. Data Extract. BCCDC, 2014. Available at: http://www.popdata.bc.ca/data [Google Scholar]
- 15. BC Renal Agency [creator] (2015): BC Provincial Renal Agency Database (PROMIS) Population Data BC [publisher]. Data Extract. PROMIS, 2014. Available at: http://www.popdata.bc.ca/data [Google Scholar]
- 16. BC Centre for Disease Control [creator] (2015): BC Provincial HIV/AIDS Surveillance Database (BCCDC-HAISIS) Population Data BC [publisher]. Data Extract. BCCDC, 2014. Available at: http://www.popdata.bc.ca/data [Google Scholar]
- 17. BC Cancer Agency Registry Data [creator] (2015): Population Data BC [publisher]. Data Extract. BC Cancer Agency, 2014. Available at: http://www.popdata.bc.ca/data [Google Scholar]
- 18. Citizenship and Immigration Canada. Facts and figures 2014—immigration overview: permanent residents. Glossary of Terms and Concepts Available at: http://www.cic.gc.ca/english/resources/statistics/facts2014/glossary.asp. Accessed 26 March 2017.
- 19.World Health Organization. WHO world TB database Available at: http://www.who.int/tb/country/data/download/en/. Accessed 22 April 2016.
- 20. Brode SK, Jamieson FB, Ng R, et al. Increased risk of mycobacterial infections associated with anti-rheumatic medications. Thorax 2015; 70:677–82. [DOI] [PubMed] [Google Scholar]
- 21. Dobler CC, Martin A, Marks GB. Benefit of treatment of latent tuberculosis infection in individual patients. Eur Respir J 2015; 46:1397–406. [DOI] [PubMed] [Google Scholar]
- 22. Bibbins-Domingo K, Grossman DC, Curry SJ, et al. ; US Preventive Services Task Force Screening for latent tuberculosis infection in adults: US preventive services task force recommendation statement. JAMA 2016; 316:962–9. [DOI] [PubMed] [Google Scholar]
- 23. American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep 2000; 49:1–51. [PubMed] [Google Scholar]
- 24. NICE. Tuberculosis (NICE guideline NG33) Available at: https://www.nice.org.uk/guidance/ng33/chapter/recommendations. Accessed 5 January 2018.
- 25. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16:1269–78. [DOI] [PubMed] [Google Scholar]
- 26. Asadi L, Heffernan C, Menzies D, Long R. Effectiveness of Canada’s tuberculosis surveillance strategy in identifying immigrants at risk of developing and transmitting tuberculosis: a population-based retrospective cohort study. Lancet Public Health 2017; 2:e450–7. [DOI] [PubMed] [Google Scholar]
- 27. Fojo AT, Stennis NL, Azman AS, et al. Current and future trends in tuberculosis incidence in New York City: a dynamic modelling analysis. Lancet Public Health 2017; 2:e323–e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walters E, Cotton MF, Rabie H, Schaaf HS, Walters LO, Marais BJ. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr 2008; 8:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walter ND, Jasmer RM, Grinsdale J, Kawamura LM, Hopewell PC, Nahid P. Reaching the limits of tuberculosis prevention among foreign-born individuals: a tuberculosis-control program perspective. Clin Infect Dis 2008; 46:103–6. [DOI] [PubMed] [Google Scholar]
- 30. Brisson M, Van de Velde N, De Wals P, Boily MC. Estimating the number needed to vaccinate to prevent diseases and death related to human papillomavirus infection. CMAJ 2007; 177:464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cleary SM. Commentary: trade-offs in scaling up HIV treatment in South Africa. Health Policy Plan 2010; 25:99–101. [DOI] [PubMed] [Google Scholar]
- 32. Mangham LJ, Hanson K. Scaling up in international health: what are the key issues? Health Policy Plan 2010; 25:85–96. [DOI] [PubMed] [Google Scholar]
- 33. Denholm JT, Matteelli A, Reis A. Latent tuberculous infection: ethical considerations in formulating public health policy. Int J Tuberc Lung Dis 2015; 19:137–40. [DOI] [PubMed] [Google Scholar]
- 34. Silva DS, Smith MJ, Upshur RE. Disadvantaging the disadvantaged: when public health policies and practices negatively affect marginalized populations. Can J Public Health 2013; 104:e410–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.