Abstract
Background
Whether persistent low-level viremia (pLLV) predicts virologic failure (VF) is unclear. We used data from the US Military HIV Natural History Study (NHS), to examine the association of pLLV and VF.
Methods
NHS subjects who initiated combination antiretroviral therapy (ART) after 1996 were included if they had 2 or more VLs measured with a lower limit of detection of ≤50 copies/mL. VF was defined as a confirmed VL ≥200 copies/mL or any VL >1000 copies/mL. Participants were categorized into mutually exclusive virologic categories: intermittent LLV (iLLV) (VL of 50–199 copies/mL on <25% of measurements), pLLV (VL of 50–199 copies/mL on ≥25% of measurements), high-level viremia (hLV) (VL of 200–1000 copies/mL), and continuous suppression (all VL <50 copies/mL). Cox proportional hazards models were used to evaluate the association between VF and LLV; hazard ratios and 95% confidence interval (CI) are presented.
Results
Two thousand six subjects (median age 29.2 years, 93% male, 41% black) were included; 383 subjects (19%) experienced VF. After adjusting for demographics, VL, CD4 counts, ART regimen, prior use of mono or dual antiretrovirals, and time to ART start, pLLV (3.46 [2.42–4.93]), and hLV (2.29 [1.78–2.96]) were associated with VF. Other factors associated with VF include black ethnicity (1.33 [1.06–1.68]) and antiretroviral use prior to ART (1.79 [1.34–2.38]). Older age at ART initiation (0.71 [0.61–0.82]) and non-nucleoside reverse transcriptase inhibitor (0.68 [0.51–0.90]) or integrase strand transfer inhibitor use (0.26 [0.13–0.53]) were protective.
Conclusion
Our data add to the body of evidence that suggests persistent LLV is associated with deleterious virologic consequences.
Keywords: persistent low-level viremia, HIV, virologic failure
Results of a large systematic analysis of subjects infected with human immunodeficiency virus with persistent low-level viremia (pLLV) indicate that pLLV is an independent predictor of virologic failure; presence of pLLV should not be merely ascribed to laboratory variation and needs investigation.
The advent of antiretroviral therapy (ART) has revolutionized the management of individuals infected with human immunodeficiency virus (HIV). The goals of ART are immune reconstitution and virologic suppression (VS). Over time, the sensitivities of the viral load assays have improved, currently most assays used in resource rich settings have thresholds of detection that are <20 copies/mL. Changes in assay sensitivity has meant that the definition of virologic failure (VF) and virologic thresholds to switch ART have also varied with time. Further, definitions for VF and thresholds for switches vary by region and differ based on the organizations making these recommendations [1–3].
Although generally VF is defined as the inability to achieve a VL below 50 copies/mL within 6 months of ART initiation, or a failure to maintain the VL below this threshold while on ART, there are specific considerations by the recommending organization. The strictest definition of VF is the one adopted by the European AIDS Clinical Society; VF is defined as a VL > 50 copies/mL for 1 month, and the recommendation is to consider changing therapy at that time [1]. In the United States, the Department of Health and Human Services (DHHS) guidelines state that VF is the inability to maintain a VL below 200 copies/mL with a recommendation to switch regimens, based on the results of resistance testing, if the VL is confirmed as being ≥200 copies/mL [2]. However, the World Health Organization uses a less stringent definition of VF, requiring a VL of >1000 copies/mL on 2 occasions for recommendation to switch ART [3].
Conflicting data on the clinical and virologic consequences of low-level viremia (LLV) (ie, a detectable VL that is <200 copies/mL) is one of the reasons for this variable threshold [4–13]. Although some studies suggest a threshold of >200 copies/mL as being associated with VF, yet other studies suggest that a higher threshold (ie, VL >400 copies/mL) puts one at risk for VF [11, 13]. In contrast, other studies note an increased risk of VF with VL between 50 and 199 copies/mL [6, 7, 12]. In addition, viral blips, although common, have not been known to predict an increased risk of VF or viral evolution [4, 5, 8].
In light of the differences in guidelines and disparate evidence, we decided to retrospectively examine the US Military HIV Natural History Study (NHS), a well-characterized, racially diverse cohort of HIV-infected individuals with limited concomitant illicit drug use and open access to care and medications to determine if LLV predicts unfavorable virologic consequences [14].
METHODS
Study Population
The NHS is a prospective, multicenter, open cohort composed of HIV-positive Department of Defense beneficiaries. Due to the mandatory HIV screening policies of the US military, NHS participants are often diagnosed and treated early in their infection. NHS visits are conducted approximately every 6 months at 6 participating military treatment facilities [14]. During the visits, participants undergo blood draws, are examined by a physician, interviewed by research personnel, and undertake questionnaires including one on medication adherence. Research personnel collect clinical diagnoses, ART history, and results of laboratory testing to include CD4 counts and VLs. For this retrospective analysis, we included subjects who initiated ART after 1 January 1996 and had at least 2 documented VLs (using an assay with a lower limit of quantification of <50 copies/mL) 6 months after ART initiation, and while on ART. Follow-up for this report ended on 30 October 2017.
Definitions
For this study, we categorized subjects into 4 mutually exclusive exposure categories. Subjects with 1 or more unconfirmed or nonconsecutive VL measurements between 200 and 1000 copies/mL that did not meet the definition of VF were classified as having high-level viremia (hLV). LLV was defined as having VLs between 50 and 199 copies/mL. Because this phenomenon can be intermittent or persistent, we grouped subjects into 2 groups; if LLV occurred in <25% of measurements, it was categorized as intermittent LLV (iLLV) and otherwise persistent LLV (pLLV). If all measured VLs were <50 copies/mL, then subjects were classified as continuously suppressed (CS). VF was defined as having a VL of ≥200 copies/mL on 2 consecutive measurements or any VL of >1000 copies/mL, 6 months after initiation of ART and while on ART. Any ART interruption of ≥2 weeks was considered a treatment interruption. The NHS definition of ART was used for the study. This is defined as the use of 3 active agents, usually 2 nucleoside reverse transcriptase inhibitors (NRTIs) with a third active antiretroviral (ARV) that is either an integrase strand transfer inhibitor (INSTI), non-nucleoside reverse transcriptase inhibitor (NNRTI), or a protease inhibitor (PI), or a triple NRTI regimen.
Statistical Analysis
Descriptive statistics are presented as medians with interquartile ranges (IQRs) for continuous variables and counts with proportions for categorical variables. For group comparisons, Kruskal-Wallis test and χ2/Fisher exact test were used to calculate 2-sided P values for continuous and categorical variables, respectively. Adjusted Cox proportional hazards models with time-varying covariates were utilized to assess the association between the time to VF and LLVs. The time to VF was defined as the time to either the first VL being measured at ≥1000 copies/mL or the first of 2 consecutive VLs ≥ 200 copies/mL. Subjects without VF were censored at their last study visit. The models were adjusted for sex, race, VL at ART initiation, use of mono or dual ARVs prior to ART initiation, and time from HIV diagnosis to ART initiation as time-invariant covariates; age, CD4 counts, and ART regimen types were evaluated as time-varying covariates. Unadjusted and adjusted hazard ratios are reported with its 95% confidence intervals (CIs) and P values. We also performed a subgroup analysis restricted to subjects initiating ART after 1 January 2007. The date was chosen as the “1 pill once a day regimen” of tenofovir/emtricitabine, and efavirenz was approved by the Food and Drug Administration in mid-2006. All reported P values are 2-sided with a P value <.05 considered to indicate statistical significance. All analyses were conducted using SAS, version 9.4 (SAS Institute).
RESULTS
Baseline Characteristics
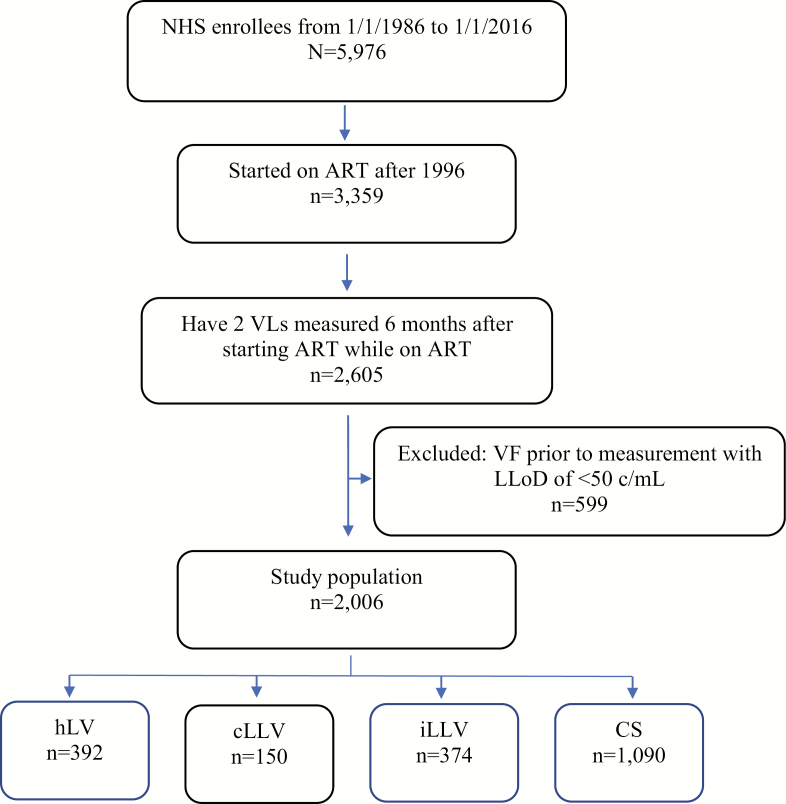
Of the total 5976 subjects ever enrolled in the NHS, 3359 initiated ART after 1996. Over three-quarters of the NHS participants initiating ART (n = 2605) had ≥2 VL measurements 6 months after starting ART and while on ART. We excluded 599 participants because they met criteria for VF before ever having a VL measured using an assay with a lower limit of detection <50 copies/mL, leaving us with 2006 participants eligible for this analysis (Figure 1). The study population was predominantly male (93%), racially diverse (42% white, 41% black, and 18% Hispanic/Other), and young (median age at HIV diagnosis was 29.2 years [24.6–36.2]). The median CD4 count at HIV diagnosis was 454 cells/uL (328–605). Most subjects (64%) were diagnosed with HIV after the calendar year 2000. The median age, CD4 count, and VL at ART initiation were 32.8 years (26.8–39.4), 372 cells/uL (268–495), and 4.5 log10 c/mL (3.9–5.0), respectively. Subjects most often initiated ART with a NNRTI-based regimen (49%); other regimens used include unboosted PI (24%), integrase inhibitors (11%), or a boosted PI-based regimen (10%). A total of 408 patients (20%) had received mono or dual antiretroviral therapy (ARV) prior to ART initiation. Baseline characteristics are summarized in Table 1. The median follow-up time was 7.8 years (4.0–14.0) after HIV diagnosis and 5.3 years (3.0–9.3) after ART initiation. The median number of VL measurements per subject using the lower limit of quantitation of 50 copies/mL was 9 (4.0–17.0), with a median of 2 measurements per year for each subject. Forty-six percent of subjects had detectable viremia that did not meet criteria for VF; about 1 in 5 had either hLV (n = 392) or iLLV (n = 374), and ~7% had pLLV (n = 150) (Table 2).
Figure 1.
Study population. Abbreviations: ART, antiretroviral therapy; cLLV, continuous low-level viremia; CS, continuous suppression; hLV, high-level viremia; iLLV, intermittent low-level viremia; LLoD, lower limit of detection; NHS, Natural History Study; VF, virologic failure.
Table 1.
Baseline Characteristics of US Military HIV Natural History Study Subjects With and Without Virologic Failure
| Total | Virologic Failure | No Virologic Failure | P Value | |
|---|---|---|---|---|
| (N = 2006) | (n = 383) | (n = 1623) | ||
| Malea | 1872 (93.3) | 352 (91.9) | 1520 (93.7) | .20 |
| Racea | <.001 | |||
| White | 833 (41.5) | 154 (40.2) | 679 (41.8) | |
| Black | 815 (40.6) | 182 (47.5) | 633 (39.0) | |
| Hispanic/other | 358 (17.8) | 47 (12.3) | 311 (19.2) | |
| Age at HIV diagnosisb (years) | 29.2 (24.6–36.2) | 28.9 (24.4–35.5) | 29.3 (24.7–36.4) | .29 |
| HIV diagnosis eraa | <.0001 | |||
| Prior to 1996 | 451 (22.5) | 179 (46.7) | 272 (16.8) | |
| 1996–2000 | 278 (13.9) | 87 (22.7) | 191 (11.8) | |
| After 2000 | 1277 (63.7) | 117 (30.5) | 1160 (71.5) | |
| Time from HIV diagnosis to ART initiation (years)b | 0.8 (0.2–3.8) | 2.4 (0.3–7.4) | 0.6 (0.2–3.1) | <.0001 |
| Initial ART regimena | <.0001 | |||
| Unboosted PI | 475 (23.7) | 182 (47.5) | 293 (18.1) | |
| Boosted PI | 208 (10.4) | 29 (7.6) | 179 (11.0) | |
| NNRTI | 980 (48.9) | 127 (33.2) | 853 (52.6) | |
| INSTI | 220 (11.0) | 5 (1.3) | 215 (13.2) | |
| Other | 123 (6.1) | 40 (10.4) | 83 (5.1) | |
| Antiretroviral use prior to ARTa | 408 (20.3) | 176 (46.0) | 232 (14.3) | <.0001 |
| CD4 count at HIV diagnosis (cells/uL)b,c | 454.0 (328.0–604.5) | 444.0 (326.0–617.0) | 456.0 (328.0–602.0) | .91 |
| CD4 count at ART initiation (cells/uL)b,c | 372.0 (267.5–494.5) | 370.5 (246.5–483.5) | 372.0 (274.0–498.5) | .09 |
| CD4 count <200 cells/uL at ART initiationa,c | 233 (12.6) | 66 (19.4) | 167 (11.1) | <.0001 |
| Nadir CD4 count (cells/uL)b | 324.0 (238.0–429.0) | 300.0 (196.0–400.0) | 331.5 (246.0–437.0) | <.0001 |
| Log viral load at HIV diagnosis (copies/mL)b,c | 4.5 (3.9–4.9) | 4.5 (3.9–4.9) | 4.5 (3.9–4.9) | .99 |
| Log viral load at ART initiation (copies/mL)b,c | 4.5 (3.9–5.0) | 4.4 (3.7–4.9) | 4.5 (3.9–5.0) | .008 |
| Follow-up time after HIV diagnosis (years)b | 7.8 (4.0–14.0) | 7.7 (4.0–13.2) | 7.9 (4.0–14.3) | .21 |
| Follow up time after ART initiation (years)b | 5.3 (3.0–9.3) | 3.9 (2.3–6.4) | 5.8 (3.2–10.3) | <.0001 |
| Number of VL measurements/yearb | 2.0 (2.0-2.0) | 2.0 (2.0–3.0) | 2.0 (1.5–2.0) | <.0001 |
P values were calculated by Kruskal-Wallis test and χ2/Fisher exact test for continuous and categorical variables, respectively.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; VL, viral load.
an (%).
bMedian (1st quartile to 3rd quartile).
cSubjects with missing values were not included in the computation of percentages and statistics.
Table 2.
Categorization of Subjects by Virologic Status
| Total | Virologic Failure | No Virologic Failure | P Value | |
|---|---|---|---|---|
| (N = 2006) | (n = 383) | (n = 1623) | ||
| hLV, n (%) | 392 (19.5) | 182 (47.5) | 210 (12.9) | <.0001 |
| pLLV, n (%) | 150 (7.5) | 52 (13.6) | 98 (6.0) | <.0001 |
| iLLV, n (%) | 374 (18.6) | 22 (5.7) | 352 (21.7) | <.0001 |
| CS, n (%) | 1090 (54.3) | 127 (33.2) | 963 (59.3) | <.0001 |
P values were calculated by χ2 test.
Abbreviations: CS, continuous suppression; hLV, high-level viremia; iLLV, intermittent low-level viremia; pLLV, persistent low-level viremia.
Comparison of Subjects With and Without VF
Subjects with and without VF were similar in terms of sex, age, and VLs at HIV diagnosis. Those with VF were more likely to be black (47.5% vs 39.0%), be diagnosed with HIV before the calendar year 2000 (69.5% vs 28.5%), have a longer median time from HIV diagnosis to ART initiation (median of 2.4 years (0.3–7.4) vs 0.6 years (0.2–3.1), a lower nadir CD4 count (median 300 cells/uL [196–400] vs 332 cells/uL [246–437], more likely to have been treated with mono or dual ARV prior to ART [46.0% vs. 14.3%], and have initiated an ART regimen containing an unboosted PI [47.5% vs. 18.1%]) (Table 1).
Comparison of Subjects With and Without iLLV
A total of 374 subjects had iLLV. A majority of the subjects (63%) had just one VL that was between 50 and 199 copies/mL. Compared to those without iLLV, those with iLLV were more likely to be white (46% vs 41%), older at ART initiation (median age 34.3 years vs 32.6 years), more likely to initiate ART with an NNRTI containing regimen (55% vs 48%), and were less likely to have received mono or dual ARV before ART initiation (15% vs 22%). However, they were more likely to have a lower nadir CD4 count (median 302 cells/uL vs 328 cells/uL), a lower CD4 count at ART initiation (median 344 cells/uL vs 378 cells/uL), and a higher VL at ART initiation (median 4.7 log log10 c/mL vs 4.5 log log10 c/mL). Further, they had longer follow-up (median 10.5 years vs 7.2 years), and more VL determinations per subject (median of 17.0 vs 7.0) (Table 3).
Table 3.
Comparison of Subjects With and Without Intermittent Low-level Viremia
| iLLV | Others | P Value | |
|---|---|---|---|
| (n = 374) | (n = 1632) | ||
| Malea | 354 (94.7) | 1518 (93.0) | .27 |
| Racea | .004 | ||
| White | 170 (45.5) | 663 (40.6) | |
| Black | 124 (33.2) | 691 (42.3) | |
| Hispanic/other | 80 (21.4) | 278 (17.0) | |
| Age at HIV diagnosis (years)b | 31.0 (25.5–37.6) | 29.0 (24.4–35.9) | .003 |
| HIV diagnosis eraa | .17 | ||
| Prior to 1996 | 71 (19.0) | 380 (23.3) | |
| 1996–2000 | 51 (13.6) | 227 (13.9) | |
| After 2000 | 252 (67.4) | 1025 (62.8) | |
| Time from HIV diagnosis to ART initiation (years)b | 0.7 (0.2–3.3) | 0.8 (0.2–3.8) | .68 |
| Initial ART regimena | <.0001 | ||
| Unboosted PI | 76 (20.3) | 399 (24.4) | |
| Boosted PI | 51 (13.6) | 157 (9.6) | |
| NNRTI | 204 (54.5) | 776 (47.5) | |
| INSTI | 15 (4.0) | 205 (12.6) | |
| Other | 28 (7.5) | 95 (5.8) | |
| Antiretroviral use prior to ARTa | 55 (14.7) | 353 (21.6) | .003 |
| CD4 count at HIV diagnosisb,c | 434.5 (298.0–610.0) | 459.5 (336.0–604.0) | .05 |
| CD4 count at ART initiationb,c | 344.0 (245.0–464.0) | 378.0 (276.0–507.0) | <.001 |
| Nadir CD4 countb | 301.5 (228.0–392.0) | 328.0 (243.0–436.0) | .001 |
| Log viral load at ART initiation (copies/mL)b,c | 4.7 (4.1–5.0) | 4.5 (3.8–4.9) | <.0001 |
| Follow-up time after HIV diagnosis (years)b | 10.5 (6.7–16.4) | 7.2 (3.6–13.2) | <.0001 |
| Follow-up time after ART initiation (years)b | 8.3 (5.5–13.7) | 4.7 (2.6–8.2) | <.0001 |
| Number of VL measurements/yearb | 2.0 (2.0–2.5) | 2.0 (1.5–2.0) | <.0001 |
| Number of VL measurements/subject with a LoQ of ≤50 copies/mLb | 17.0 (11.0–25.0) | 7.0 (4.0–14.0) | <.0001 |
| Number of VL measurements/subject with any LoQb | 18.0 (11.0–28.0) | 11.0 (6.0–22.0) | <.0001 |
P values were calculated by Kruskal-Wallis test and χ2/Fisher exact test for continuous and categorical variables, respectively.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; LoQ, limit of quantification; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; VL, viral load.
an (%).
bMedian (1st quartile to 3rd quartile).
cSubjects with missing values were not included in the computation of percentages and statistics.
Risk Factors Associated With VF
In this analysis, about 1 in 5 NHS subjects experienced VF (n = 383), and 207 (54.1%) subjects were on their first ART regimen when they failed. The median time to VF from ART initiation was 3.9 years (2.3–6.4). The proportion of subjects experiencing VF varied by exposure category. About half of the subjects with documented hLV (n = 182), a third with pLLV (n = 52), 1 in 10 subjects with CS (n = 127), and 1 in 20 subjects with iLLV (n = 22) met criteria for VF (Table 2). Risk of VF varied by HIV diagnosis era and was highest in those diagnosed prior to 1996 (40%), declining in those diagnosed between 1996 and 2000 (31%) and was lowest in those diagnosed after 2000 (9%) (Table 1).
In the adjusted Cox regression models, subjects with hLV (adjusted hazard ratio [aHR] 2.29, 95% CI 1.78–2.96) and pLLV (aHR 3.46, 95% CI 2.42–4.93) had a greater hazard for VF, whereas iLLV was protective (aHR 0.33, 95% CI .21–.52). Other factors associated with VF include black ethnicity (aHR 1.33, 95% CI 1.06–1.68), ARV use prior to ART (aHR 1.79, 95% CI 1.34–2.38), and a higher VL at ART initiation (aHR 1.15, per log increase, 95% CI 1.02–1.30). Older age at ART initiation (aHR 0.71, per 10-year increase, 95% CI .61–.82) and use of a NNRTI (aHR 0.68, 95% CI .51–.90) or an INSTI-based regimen (aHR 0.26, 95% CI .13–.53) were protective (Table 4).
Table 4.
Factors Associated With Virologic Failure
| Unadjusted HR | Adjusted HR | |||
|---|---|---|---|---|
| Risk Factor | HR (95% CI) | P Value | HR (95% CI) | P Value |
| LLV (Ref. CS) | ||||
| hLV | 3.08 (2.42–3.93) | <.0001 | 2.29 (1.78–2.96) | <.0001 |
| pLLV | 3.89 (2.74–5.52) | <.0001 | 3.46 (2.42–4.93) | <.0001 |
| iLLV | 0.34 (.22–.54) | <.0001 | 0.33 (.21–.52) | <.0001 |
| Male | 1.01 (.67–1.53) | .96 | 1.18 (.78–1.80) | .43 |
| Race (Ref. white) | ||||
| Black | 1.31 (1.05–1.65) | .02 | 1.33 (1.06–1.68) | .02 |
| Hispanic/Other | 0.81 (.57–1.15) | .24 | 1.03 (.72–1.47) | .87 |
| Age at ART initiationa (per 10 year increase) | 0.88 (.78–1.00) | .04 | 0.70 (.61–.82) | <.0001 |
| Log viral load at ART initiation (copies/mL) (per log increase) | 0.95 (.85–1.05) | .31 | 1.15 (1.02–1.30) | .02 |
| Antiretroviral use prior to ART | 2.64 (2.12–3.27) | <.0001 | 1.79 (1.34–2.38) | <.0001 |
| Time from HIV diagnosis to ART initiation (per year increase) | 1.05 (1.03–1.07) | <.0001 | 1.03 (1.00–1.06) | .06 |
| CD4 countsa (per 100 cells/uL increase) | 0.94 (.90–.98) | .002 | 0.97 (.93–1.01) | .14 |
| ART regimena (Ref. Unboosted PI) | ||||
| Boosted PI | 0.70 (.50–1.00) | .05 | 0.96 (.67–1.37) | .81 |
| INSTI | 0.16 (.08–.31) | <.0001 | 0.26 (.13–.53) | <.001 |
| NNRTI | 0.45 (.35–.58) | <.0001 | 0.68 (.51–.90) | .007 |
| Other combinations | 0.95 (.68–1.34) | .78 | 1.18 (.83–1.66) | .36 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CS, continuous suppression; hLV, high-level viremia; HR, hazard ratio; iLLV, intermittent low-level viremia; INSTI, integrase strand transfer inhibitor; LLV, low-level viremia; NNRT, non-nucleoside reverse transcriptase inhibitor; OR, odds ratio; PI, protease inhibitor; pLLV, persistent low-level viremia; Ref., reference.
aAge, CD4 counts, and ART regimen class were time-updated covariates.
The subgroup analysis, restricted to 934 subjects initiating ART after 2007, showed similar association with pLLV and VF (aHR 7.33, 95% CI 3.22–16.70; Supplementary Table 1). Of note, ~1% of the subjects in this group were receiving an unboosted PI (Supplementary Table 2).
DISCUSSION
Our results add to the body of literature that suggests persistent viral loads between 50 and 199 c/mL is associated with deleterious consequences [6, 7, 11, 12, 15]. Variations in assay characteristics resulting in artefactual associations with intermittent viremia and lack of viral evolution during episodes of LLV have been suggested as possible explanations for why LLV is not associated with overt treatment failure [8, 16–18]. We believe persistent VL elevation, between 51 and 199 c/mL, when observed, should not be ascribed merely to assay variation and at the very least should prompt a thorough assessment focused on understanding a given individual’s compliance with the ART regimen and evaluating pharmacokinetic interactions that could influence bioavailability. In keeping with our observation, a study demonstrated subtherapeutic drug levels during episodes of LLV and other studies have indicated that episodes of LLV are associated with suboptimal adherence to ART and selection of resistance mutations predisposing one to VF [19–22]. Further, our results suggest higher viral loads between 200 and 999 c/mL, even on one determination, increase the risk of VF. Given that about 1 in 2 subjects with hLV experienced VF, we believe that the World Health Organization cutoff of >1000 copies/mL for regimen switch is lenient and should be reconsidered [3].
In our study, iLLV conveyed lower risk of VF. Most of the episodes of iLLV were blips of low magnitude (78%, data not shown). Most authors believe blips represent a random variation around a mean, and when low in magnitude (as in our case) are unlikely to be clinically significant [8, 18, 23–25]. Although we cannot be certain as to why those with iLLV had a lower incidence of VF, there are differences in group characteristics that are hypothesis generating. Participants with iLLV had more VL measurements than others in this study, possibly due to having iLLV, and were followed for longer duration since ART initiation. Taken together, these findings imply that these participants had more contact with our healthcare system, perhaps affording greater opportunities for adherence counseling, or perhaps this is a proxy for a group of individuals who were likely to have a better outcome. Engagement and retention in healthcare have been associated with improved virologic outcomes, and this is likely true even in a setting with universal access to treatment and medications [26]. It is also possible that more frequent monitoring led to the identification of episodes of detectable viremia due to release of nonreplicating virus detected inadvertently due to the frequent monitoring they underwent. These episodes could also include transient viremia due to concomitant illness for which they sought care and may have been captured. It is important to note that in other studies that have examined the association of VF in subjects with LLV, those with infrequent LLV (equivalent to iLLV in our study) were classified as suppressed rather than broken out separately, which could have masked a potential beneficial association, as seen in the present study. For example, in the study by Laprise et al, those with VLs between 51 and 200 copies/mL for <6 months were classified in the undetectable class and were not analyzed separately [6]. Future studies should consider examination of this category separately. Beyond this, an immunologic basis may have contributed to our observations. Although this is an area of debate, some studies suggest an immunologic benefit to episodes of intermittent viremia with improved magnitude and breadth of immune responses, which hypothetically could have contributed to the improved virologic outcome observed in this group [27–29].
The lack of consistency between definitions of VF and persistent LLV makes comparisons between studies challenging. Most studies examining the issue have used a virologic threshold ranging from 400 to 1000 copies/mL to define VF; few use the DHHS proposed cutoff of 200 copies/mL. We used the more stringent threshold of >200 copies/mL and demonstrate that persistent LLV is deleterious even when this cutoff is used. In our study we used a proportion (quarter of the measurements) to define persistent LLV; others have used duration (such as >6 months), and yet other studies have merely used a cross-sectional determination. Hence, it is important that future studies use consistent definitions. We believe our definition of persistent LLV is a comprehensive way of capturing an individual’s virologic history. In our cohort LLV was common with approximately 1 in 4 participants affected, so it is very likely that a clinician taking care of HIV-infected patients will encounter the situation and should know how to treat these individuals. The use of varying definitions of VF and LLV in the literature adds a layer of difficulty when interpreting these results for the treating clinician; hence, we recommend consideration for the use of a consistent definition of VF and pLLV, such as ours. Other independent risk factors for VF found in this study are similar to those described in the literature to include younger age at ART initiation, black ethnicity, higher viral loads, and ARV use prior to ART [14, 30–32]. Additionally, use of INSTI and NNRTI was beneficial and is reflected in the DHHS guidelines, which recommend against the use of unboosted PIs as initial regimens and favor the use of INSTIs and NNRTIs [2]. Because ~25% of the participants initiated an unboosted PI, to evaluate the relevance of pLLV in the modern ART era, we performed a subgroup analysis restricted to participants initiating ART after 2007. Participants in this group primarily initiated a NNRTI (61%), INSTI (24%), or a boosted PI (13%) regimen. Even in this group, pLLV was associated with VF.
The strengths of the study are the large sample size and long follow-up interval (median of about 8 years since HIV diagnosis and 5 years since ART initiation), which allowed for the collection of several data points on a regular basis (median of 2 viral loads per year for each subject and 9 per subject during follow-up), thereby improving the validity of our observations. Further, there is limited confounding, as NHS subjects have unrestricted access to healthcare and medications and low drug use factors known to influence virologic outcomes. Additionally, due to the mandatory active duty HIV screening policies, many NHS subjects are dated seroconverters who initiate ART relatively early in the course of the illness (median of 0.8 years since HIV diagnosis), reducing the impact of these variables on HIV outcomes. Finally, our analysis used time updated variables and accounted for treatment interruptions. The limitations are that the cohort is predominantly male, limiting generalizability to females. Adherence data have not been collected since the inception of the cohort and hence could not be examined in the multivariate analysis. Since 2006, the NHS has captured participant reported adherence; our evaluation suggests that self-reported adherence is very similar among those with and without VF (data not shown), consistent with known limitations of self-report. Further, clinical consequences were not examined. Few studies have reported on the clinical consequences of pLLV, future studies should examine whether the presence pLLV is associated with adverse clinical outcomes [33, 34].
In conclusion, our results suggest that persistent LLV is likely clinically meaningful and should always be investigated and not merely attributed to variation in assay characteristics.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Presented in part: Annual Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 13–16 February 2017. Abstract 932.
Notes
Author contributions.A. G. and C. J. conceived the idea for this analysis. A. G., C. J., C. S., T. L., R. M., K. K., R. D., J. O., and B. A. implemented the study, collected data, and oversaw the individual participating sites. A. G. and C. J. drafted the article. S. H. W. performed the statistical analysis. All authors provided critical reads that helped shape the article.
Acknowledgments.The authors thank the participants and their caregivers without whom none of this work would be possible. They also thank the research coordinators and support staff who have diligently worked on the Department of Defense HIV Natural History Study, as well as the members of the Infectious Disease Clinical Research Program HIV Working Group, listed as follows: W. Bradley; S. Merritt; T. Merritt; C. Olsen; C. Rhodes; T. Sjoberg; C. Baker; S. Chambers; R. Colombo; T. Ferguson; LTC A. Kunz; C. Schofield; M. Stein; J. Metcalf; J. Powers; S. Siddiqui; E. Tramont; S. Banks; R. Tant; S. Cammarata, CDR J. Curry; N. Kirkland; G. Utz; M. Price; N. Aronson; T. Burgess; X. Chu; W. Horton; A. Noiman; E. Parmelee; D. Tribble; X. Wang; S. Won; LTC J. Ake; T. Crowell; L. Jagodzinski; N. Michael; S. Peel; M. Robb; I. Barahona; J. Blaylock; C. Decker; T. Gleeson; R. Ressner; D. Wallace; T. Whitman.
Disclaimer.The views expressed are those of the authors and do not reflect the official views and/or policy of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the National Institutes of Health or the Department of Health and Human Services, Brooke Army Medical Center, Walter Reed National Military Medical Center, Naval Medical Center San Diego, Madigan Army Medical Center, Naval Medical Center Portsmouth, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of Defense, the Departments of the Army, Navy or Air Force, or the US Government. Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45CRF46.
Some authors on this paper are military service members and/or employees of the US Government. As such, this work was prepared as part of official duties. Title 17 U.S.C. 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
Financial support.Support for this work (IDCRP-000-03) was provided by the Infectious Disease Clinical Research Program, a Department of Defense program executed through the Uniformed Services University of the Health Sciences. This project has been funded in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Inter-Agency Agreement Y1-AI-5072.
Potential conflicts of interest.All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. European AIDS Clinical Society. European AIDS clinical society guidelines 2017; Version 9.0 October 2017. Available at: http://www.eacsociety.org/files/guidelines_9.0-english.pdf. Accessed 10 September 2018.
- 2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services, 2018:H1–H9.1. Available at: https://aidsinfo.nih.gov/guidelines. Accessed 10 September 2018. [Google Scholar]
- 3. World Health Organization. Consolidated strategic information guidelines for HIV in the health sector. 2015. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing hiv infection 2016 recommendations for a public health approach second edition. Available at: https://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf Accessed 4 March 2019. [Google Scholar]
- 4. Havlir DV, Bassett R, Levitan D, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA 2001; 286:171–9. [DOI] [PubMed] [Google Scholar]
- 5. Kanapathipillai R, McManus H, Kamarulzaman A, et al. The significance of HIV ‘blips’ in resource-limited settings: is it the same? analysis of the treat Asia HIV Observational Database (TAHOD) and the Australian HIV Observational Database (AHOD). PLoS One 2014; 9:e86122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 2013; 57:1489–96. [DOI] [PubMed] [Google Scholar]
- 7. Leierer G, Grabmeier-Pfistershammer K, Steuer A, et al. A single quantifiable viral load is predictive of virological failure in human immunodeficiency virus (HIV)-infected patients on combination antiretroviral therapy: The Austrian HIV cohort study. Open Forum Infect Dis 2016; 3:ofw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (blips) and drug resistance in patients receiving HAART. JAMA 2005; 293:817–29. [DOI] [PubMed] [Google Scholar]
- 9. Silva J, Pereira K, Rijo J, et al. A retrospective observational study of low-level viraemia and its immunological and virological significance: which outcome to expect. J Int AIDS Soc 2014; 17:19668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. García-Gascó P, Maida I, Blanco F, et al. Episodes of low-level viral rebound in HIV-infected patients on antiretroviral therapy: frequency, predictors, and outcome. J Antimicrob Chemother 2008; 61:699–704. [DOI] [PubMed] [Google Scholar]
- 11. Navarro J, Caballero E, Curran A, et al. Impact of low-level viraemia on virological failure in HIV-1-infected patients with stable antiretroviral treatment. Antivir Ther 2016; 21:345–52. [DOI] [PubMed] [Google Scholar]
- 12. Vandenhende MA, Perrier A, Bonnet F, et al. ; AIDS Clinical Epidemiology Group of Aquitaine Risk of virological failure in HIV-1-infected patients experiencing low-level viraemia under active antiretroviral therapy (ANRS C03 cohort study). Antivir Ther 2015; 20:655–60. [DOI] [PubMed] [Google Scholar]
- 13. Ryscavage P, Kelly S, Li JZ, Harrigan PR, Taiwo B. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother 2014; 58:3585–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weintrob AC, Fieberg AM, Agan BK, et al. Increasing age at HIV seroconversion from 18 to 40 years is associated with favorable virologic and immunologic responses to HAART. J Acquir Immune Defic Syndr 2008; 49:40–7. [DOI] [PubMed] [Google Scholar]
- 15. Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 2018; 18:188–97. [DOI] [PubMed] [Google Scholar]
- 16. Kran AM, Jonassen TØ, Sannes M, et al. Overestimation of human immunodeficiency virus type 1 load caused by the presence of cells in plasma from plasma preparation tubes. J Clin Microbiol 2009; 47:2170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stosor V, Palella FJ Jr, Berzins B, et al. Transient viremia in HIV-infected patients and use of plasma preparation tubes. Clin Infect Dis 2005; 41:1671–4. [DOI] [PubMed] [Google Scholar]
- 18. Grennan JT, Loutfy MR, Su D, et al. ; CANOC Collaboration Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis 2012; 205:1230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez-Serna A, Swenson LC, Watson B, et al. A single untimed plasma drug concentration measurement during low-level HIV viremia predicts virologic failure. Clin Microbiol Infect 2016; 22:1004.e9–1004.e16. [DOI] [PubMed] [Google Scholar]
- 20. Mackie NE, Phillips AN, Kaye S, Booth C, Geretti AM. Antiretroviral drug resistance in HIV-1-infected patients with low-level viremia. J Infect Dis 2010; 201:1303–7. [DOI] [PubMed] [Google Scholar]
- 21. Leierer G, Grabmeier-Pfistershammer K, Steuer A, et al. ; Austrian HIV Cohort Study Group Factors associated with low-level viraemia and virological failure: results from the Austrian HIV cohort study. PLoS One 2015; 10:e0142923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. Decreased adherence to antiretroviral therapy observed prior to transient human immunodeficiency virus type 1 viremia. J Infect Dis 2007; 196:1773–8. [DOI] [PubMed] [Google Scholar]
- 23. Lee PK, Kieffer TL, Siliciano RF, Nettles RE. HIV-1 viral load blips are of limited clinical significance. J Antimicrob Chemother 2006; 57:803–5. [DOI] [PubMed] [Google Scholar]
- 24. Young J, Rickenbach M, Calmy A, et al. ; Swiss HIV Cohort Study Transient detectable viremia and the risk of viral rebound in patients from the Swiss HIV Cohort Study. BMC Infect Dis 2015; 15:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garner W, White K, Szwarcberg J, McCallister S, Zhong L, Wulfsohn M. Concordance of HIV-1 RNA values by amplicor and TaqMan 2.0 in patients with confirmed suppression in clinical trials. Clin Infect Dis 2016; 62:929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giordano TP, Gifford AL, White AC Jr, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis 2007; 44:1493–9. [DOI] [PubMed] [Google Scholar]
- 27. Papasavvas E, Kostman JR, Thiel B, et al. HIV-1-specific CD4+ T cell responses in chronically HIV-1 infected blippers on antiretroviral therapy in relation to viral replication following treatment interruption. J Clin Immunol 2006; 26:40–54. [DOI] [PubMed] [Google Scholar]
- 28. Ortiz GM, Hu J, Goldwitz JA, et al. Residual viral replication during antiretroviral therapy boosts human immunodeficiency virus type 1-specific CD8+ T-cell responses in subjects treated early after infection. J Virol 2002; 76:411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Castro P, Plana M, González R, et al. Influence of episodes of intermittent viremia (“blips”) on immune responses and viral load rebound in successfully treated HIV-infected patients. AIDS Res Hum Retroviruses 2013; 29:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chao C, Tang B, Hurley L, et al. Risk factors for short-term virologic outcomes among HIV-infected patients undergoing regimen switch of combination antiretroviral therapy. AIDS Res Hum Retroviruses 2012; 28:1630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bayu B, Tariku A, Bulti AB, Habitu YA, Derso T, Teshome DF. Determinants of virological failure among patients on highly active antiretroviral therapy in University of Gondar Referral Hospital, Northwest Ethiopia: a case-control study. HIV AIDS (Auckl) 2017; 9:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weintrob AC, Grandits GA, Agan BK, et al. ; IDCRP HIV Working Group Virologic response differences between African Americans and European Americans initiating highly active antiretroviral therapy with equal access to care. J Acquir Immune Defic Syndr 2009; 52:574–80. [DOI] [PubMed] [Google Scholar]
- 33. Chao C, Tang B, Towner W, Silverberg MJ, Hurley L, Horberg M. Short-term clinical outcomes among treatment-experienced HIV-positive patients with early low level viremia. AIDS Patient Care STDS 2012; 26:253–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elvstam O, Medstrand P, Yilmaz A, Isberg PE, Gisslén M, Björkman P. Virological failure and all-cause mortality in HIV-positive adults with low-level viremia during antiretroviral treatment. PLoS One 2017; 12:e0180761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.