Abstract
Background
Following the conclusion of a human rotavirus vaccine (HRV) cluster-randomized, controlled trial (CRT) in Matlab, Bangladesh, HRV was included in Matlab’s routine immunization program. We describe the population-level impact of programmatic rotavirus vaccination in Bangladesh in children <2 years of age.
Methods
Interrupted time series were used to estimate the impact of HRV introduction. We used diarrheal surveillance collected between 2000 and 2014 within the 2 service delivery areas (International Centre for Diarrhoeal Disease Research, Bangladesh [icddr,b] service area [ISA] and government service area [GSA]) of the Matlab Health and Demographic Surveillance System, administered by icddr,b. Age group–specific incidence rates were calculated for both rotavirus-positive (RV+) and rotavirus-negative (RV–) diarrhea diagnoses of any severity presenting to the hospital. We used 2 models to assess the impact within each service area: Model 1 used the pre-vaccine time period in all villages (HRV– and control-only) and Model 2 combined the pre-vaccine time period and the CRT time period, using outcomes from control-only villages.
Results
Both models demonstrated a downward trend in RV+ diarrheal incidences in the ISA villages during 3.5 years of routine HRV use, though only Model 2 was statistically significant. Significant impacts of HRV on RV+ diarrhea incidences in GSA villages were not observed in either model. Differences in population-level impacts between the 2 delivery areas may be due to the varied rotavirus vaccine coverage and presentation rates to the hospital.
Conclusions
This study provides initial evidence of the population-level impact of rotavirus vaccines in children <2 years of age in Matlab, Bangladesh. Further studies are needed of the rotavirus vaccine impact after the nationwide introduction in Bangladesh.
Keywords: rotavirus vaccine, impact, time-series
A time-series analysis suggests a downward trend in rotavirus diarrhea in children following the rotavirus vaccine’s introduction in Matlab, Bangladesh. Further research is needed to confirm the findings and to increase support for the introduction of rotavirus vaccines in Asia.
(See the Editorial Commentary by Steele and Parashar on pages 2071–3.)
Globally, an estimated 13 000 deaths due to rotavirus diarrhea occur annually in children <5 years of age, with most of the burden in sub-Saharan Africa and Asia [1]. While diarrhea-associated mortality rates have decreased globally in the last decade, the burden of rotavirus diarrhea remains substantial in low-income settings [2]. In 2006, 2 rotavirus vaccines were introduced worldwide: GlaxoSmithKline’s human rotavirus vaccine (HRV; Rotarix) and Merck’s pentavalent rotavirus vaccine (PRV; RotaTeq). Large, multi-site, randomized, controlled trials (RCTs) of both vaccines in Africa demonstrated moderate vaccine efficacy (VE) against severe rotavirus diarrhea during the first year of life [3, 4]. As of August 2018, 96 countries, of which 46 are Gavi-eligible, have introduced rotavirus vaccines into their regional or national immunization programs [5]. In the World Health Organization (WHO) Africa region, 74% of countries have introduced rotavirus vaccination. Studies in sub-Saharan Africa have shown statistically significant rotavirus vaccine effectiveness and population-level impacts against all-cause and rotavirus diarrhea in children <5 years of age within 2–3 years of the initiation of routine use [6–14].
Despite the WHO recommendation for rotavirus vaccine use worldwide, only 18% of countries in the WHO southeast Asia region have introduced a rotavirus vaccine [5]. Limited data on vaccine effectiveness and population impacts may have slowed the introduction of rotavirus vaccines in Asia [15]. The only multi-site RCT of PRV in Asia demonstrated moderate vaccine efficacy against severe rotavirus gastroenteritis in the first 2 years of life (Bangladesh VE 42.7%, 95% confidence interval [CI] 10.4–63.9; Vietnam VE 63.9%, 95% CI 7.6–90.9; combined VE 51.0%, 95% CI 12.8–73.3) [16]. In Bangladesh, this RCT included half of the Matlab villages (International Centre for Diarrhoeal Disease Research, Bangladesh [icddr,b] service areas).
To evaluate the effectiveness of HRV on rotavirus diarrhea in Asia, a 2-year cluster-randomized trial (CRT) was conducted in all villages in Matlab, Bangladesh, beginning in 2008 [17]. The overall effectiveness, which assesses the overall reduction in the incidence of acute rotavirus diarrhea, regardless of vaccination status, was 29.0% (95% CI 11.3–43.1) in children <2 years of age. This study provided initial evidence of the potential population impact of routine rotavirus vaccine use in Bangladesh. After the CRT, HRV was provided for routine use among infants in all Matlab villages between March 2011 and September 2014.
To evaluate the population-level impact of HRV in Matlab, Bangladesh, during the 3.5 years of routine use following the CRT, we examined trends in the rotavirus-positive (RV+) and rotavirus-negative (RV–) diarrhea incidence rates of any severity presenting to Matlab Hospital between February 2000 and September 2014.
METHODS
Study Setting
The study utilized diarrheal surveillance data collected among children <2 years of age residing in villages of the Matlab Health and Demographic Surveillance System (HDSS), administered by the icddr,b, and presenting to Matlab Hospital [18]. The HDSS is divided into the icddr,b service area (ISA; 67 villages) and the government service area (GSA; 75 villages). The icddr,b provides ISA villages with child and maternal health intervention programs and the Bangladesh Ministry of Health and Family Welfare provides GSA villages with the government standard of care. The HDSS maintains a census and registration of vital events, including internal and external migration.
Immunization Records
The HDSS also maintains immunization records through a formal record-keeping system. In the ISA villages, community health workers maintain vaccination records, and in the GSA villages, community health workers check vaccination cards or ask mothers if the card is missing.
Diarrheal Surveillance
Matlab Hospital is the central diarrhea treatment facility for the Matlab HDSS population. This study includes data from children <2 years of age. The incidence rate for presentations to Matlab Hospital of all-cause diarrhea among children from GSA villages has historically been about half of the incidence rate for presentations from ISA villages [17]. Stool specimens are collected from all patients presenting with diarrhea (3 or more loose stools per 24 hours) to Matlab Hospital. The samples are tested for group A rotavirus VP6 antigens using a solid-phase, sandwich-type enzyme immunoassay (Prospect, Oxoid Diagnostics Ltd, Hampshire, United Kingdom).
Statistical Analysis
Interrupted time series, using segmented regression models, were used to estimate the impact of the rotavirus vaccine introduction in Matlab, Bangladesh, among children <2 years of age [19]. The monthly incidence rates of RV+ and RV– diarrhea were examined separately, by age group (0 to <12 months, 12 to <24 months, and combined [0 to <24 months]). The incidence rates were calculated for RV+ and RV– diarrhea with the number of events presenting to Matlab Hospital per month as the numerator and the monthly population at risk, using HDSS census estimates, as the denominator.
Due to varied rotavirus vaccine coverage and baseline diarrheal incidences, analyses were conducted separately for the ISA and GSA villages.
Among the ISA villages, the pre-vaccine time period was defined as February 2000–February 2007; the RCT period as March 2007–March 2009); the CRT period as April 2009–March 2011; and the HRV introduction period as April 2011–September 2014. Among the GSA villages, the pre-vaccine time period was defined as February 2000–October 2008; the CRT period as November 2008–March 2011; and the HRV introduction period as April 2011–September 2014. During the CRT periods, the villages were stratified by service area and then randomized to control-only (no placebo) or HRV.
We used 2 models to estimate the impact of HRV use on RV+ and RV– diarrhea incidence rates. Model 1 was defined a priori, while Model 2 was defined after examining the count data. Model 1 and Model 2 differ by both the baseline period used as the referent category and the types of villages included (HRV– and/or control-only). In both models, a generalized linear model was fit to the time-series data, assuming a negative, binomial distribution due to over-dispersion of the data [20]. Calendar months were included in each model to account for seasonality, and a sequential, monthly term for every month over the entire time period was included to account for secular trends. The natural log of the monthly population at risk was included in the model as the offset term. The Breusch-Godfrey test identified some autocorrelation; therefore, 95% CIs were estimated using Newey-West heteroskedastic- and autocorrelation-consistent variance estimators, with a lag of 2 [19, 21]. The estimates of the coefficients for each time period were exponentiated to estimate incidence rate ratios (IRRs), compared to the referent category.
In Model 1, within the ISA and GSA areas separately, the corresponding pre-vaccine time period was used as the referent category. Villages randomized as both HRV and control-only were included in the analysis. To estimate the IRRs and corresponding 95% CIs, the time periods corresponding to the RCT, CRT, and each of the 3.5 years of routine HRV use were modeled with separate indicator variables. This is a conservative model, which directly compares incidence rates in February 2000–February 2007 (ISA villages) and February 2000–October 2008 (GSA villages) to the years of routine HRV use, starting in April 2011 in all ISA and GSA villages.
In the secondary analysis (Model 2), only the villages randomized as control-only during the CRT were used. Within the ISA and GSA regions, the pre-vaccine and CRT time periods were combined in the referent category. The time period corresponding to the RCT was excluded. To estimate the IRRs and corresponding 95% CIs, each of the 3.5 years of routine HRV use were modeled with separate indicator variables. This approach directly compared incidence rates in February 2000–March 2011, excluding the RCT time period, to the years of routine HRV use, starting in April 2011 in those ISA and GSA villages randomized as controls.
The monthly vaccine coverage was estimated as the proportion of children 6 to <52 weeks old receiving each HRV dose within regions of Matlab, Bangladesh. Analyses were completed using Stata version 14 (Stata Corporation, College Station, TX). This study was approved by the ethical review committee of icddr,b in Bangladesh and the Fred Hutchinson Cancer Research Center.
RESULTS
Tables 1 and 2 and Figure 1 show RV+ and RV– counts and average incidence rates over time in the GSA and ISA villages, using the study populations used for Models 1 and 2.
Table 1.
Trends in Diarrhea Presenting to Matlab Hospital by Time Period and Model in International Centre for Diarrhoeal Disease Research, Bangladesh, Service Area
| Model 1 | Model 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ISA | February 2000– February 2007 (prevaccine) | March 2007– March 2009 (RCT) | April 2009– March 2011 (CRT) | April 2011– March 2012 (YR1) | April 2012– March 2013 (YR2) | April 2013– March 2014 (YR3) | April 2014– September 2014 (YR3.5) | Vaccine Years (April 2011– September 2014) | February 2000– March 2011 (prevaccine and CRT, exclude RCT) | April 2011– March 2012 (YR1) | April 2012– March 2013 (YR2) | April 2013– March 2014 (YR3) | April 2014– September 2014 (YR3.5) | Vaccine Years (April 2011– September 2014) |
| 0–12 months of age | ||||||||||||||
| Population | 18 281 | 5153 | 4936 | 2494 | 2683 | 2586 | 1286 | 9048 | 12 119 | 1327 | 1427 | 1342 | 681 | 4777 |
| RV+, count | 738 | 265 | 216 | 64 | 81 | 54 | 24 | 223 | 511 | 41 | 47 | 33 | 15 | 136 |
| RV–, count | 1258 | 823 | 380 | 179 | 208 | 166 | 79 | 632 | 865 | 92 | 122 | 97 | 44 | 355 |
| RV+ incidence | 40 | 51 | 44 | 26 | 30 | 21 | 19 | 25 | 42 | 31 | 33 | 25 | 22 | 28 |
| RV– incidence | 69 | 160 | 77 | 72 | 78 | 64 | 61 | 70 | 71 | 69 | 85 | 72 | 65 | 74 |
| 12–24 months of age | ||||||||||||||
| Population | 18 363 | 5124 | 5008 | 2437 | 2493 | 2649 | 1275 | 8853 | 12 243 | 1280 | 1325 | 1408 | 661 | 4674 |
| RV+, count | 502 | 200 | 145 | 43 | 41 | 49 | 9 | 142 | 351 | 27 | 20 | 28 | 4 | 79 |
| RV–, count | 844 | 432 | 185 | 87 | 90 | 89 | 44 | 310 | 558 | 46 | 47 | 53 | 24 | 170 |
| RV+ incidence | 27 | 39 | 29 | 18 | 16 | 19 | 7 | 16 | 29 | 21 | 15 | 20 | 6 | 17 |
| RV– incidence | 46 | 84 | 37 | 36 | 36 | 34 | 35 | 35 | 46 | 36 | 35 | 38 | 36 | 36 |
| 0–24 months of age | ||||||||||||||
| Population | 36 644 | 10 276 | 9945 | 4930 | 5176 | 5235 | 2561 | 17 901 | 24 363 | 2607 | 2752 | 2750 | 1342 | 9451 |
| RV+, count | 1240 | 465 | 361 | 107 | 122 | 103 | 33 | 365 | 862 | 68 | 67 | 61 | 19 | 215 |
| RV–, count | 2102 | 1255 | 565 | 266 | 298 | 255 | 123 | 942 | 1423 | 138 | 169 | 150 | 68 | 525 |
| RV+ incidence | 34 | 45 | 36 | 22 | 24 | 20 | 13 | 20 | 35 | 26 | 24 | 22 | 14 | 23 |
| RV– incidence | 57 | 122 | 57 | 54 | 58 | 49 | 48 | 53 | 58 | 53 | 61 | 55 | 51 | 56 |
Incidence data are per 1000 person-years.
Abbreviations: CRT, cluster-randomized controlled trial; ISA, International Centre for Diarrhoeal Disease Research, Bangladesh, service area; RCT, randomized, controlled trial; RV–, rotavirus negative; RV+, rotavirus positive; YR, year.
Table 2.
Trends in Diarrhea Presenting to Matlab Hospital by Time Period and Model in Government Service Area
| Model 1 | Model 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSA | February 2000- October 2008 (prevaccine) | November 2008-March 2011 (CRT) | April 2011– March 2012 (YR1) | April 2012– March 2013 (YR2) | April 2013– March 2014 (YR3) | April 2014– September 2014 (YR3.5) | Vaccine Years (April 2011–September 2014) | February 2000– March 2011 (prevaccine and CRT) | April 2011– March 2012 (YR1) | April 2012– March 2013 (YR2) | April 2013– March 2014 (YR3) | April 2014– September 2014 (YR3.5) | Vaccine Years (April 2011–September 2014) |
| 0–12 months of age | |||||||||||||
| Population | 22 777 | 5359 | 2317 | 2306 | 2201 | 1162 | 7987 | 12 163 | 962 | 988 | 934 | 483 | 3368 |
| RV+, count | 542 | 144 | 35 | 51 | 37 | 15 | 138 | 285 | 21 | 18 | 14 | 5 | 58 |
| RV–, count | 671 | 142 | 59 | 80 | 64 | 26 | 229 | 310 | 27 | 32 | 24 | 11 | 94 |
| RV+ incidence | 24 | 27 | 15 | 22 | 17 | 13 | 17 | 23 | 22 | 18 | 15 | 10 | 17 |
| RV– incidence | 29 | 26 | 25 | 35 | 29 | 22 | 29 | 25 | 28 | 32 | 26 | 23 | 28 |
| 12–24 months of age | |||||||||||||
| Population | 23 168 | 5641 | 2224 | 2312 | 2305 | 1111 | 7951 | 12 507 | 939 | 970 | 993 | 479 | 3381 |
| RV+, count | 365 | 90 | 46 | 28 | 20 | 8 | 102 | 196 | 25 | 12 | 12 | 1 | 50 |
| RV–, count | 459 | 94 | 40 | 44 | 24 | 13 | 121 | 249 | 15 | 11 | 11 | 5 | 42 |
| RV+ incidence | 16 | 16 | 21 | 12 | 9 | 7 | 13 | 16 | 27 | 12 | 12 | 2 | 15 |
| RV– incidence | 20 | 17 | 18 | 19 | 10 | 12 | 15 | 20 | 16 | 11 | 11 | 10 | 12 |
| 0–24 months of age | |||||||||||||
| Population | 45 945 | 10 999 | 4541 | 4618 | 4506 | 2273 | 15 938 | 24 670 | 1901 | 1958 | 1927 | 963 | 6748 |
| RV+, count | 907 | 234 | 81 | 79 | 57 | 23 | 240 | 481 | 46 | 30 | 26 | 6 | 108 |
| RV–, count | 1130 | 236 | 99 | 124 | 88 | 39 | 350 | 559 | 42 | 43 | 35 | 16 | 136 |
| RV+ incidence | 20 | 21 | 18 | 17 | 13 | 10 | 15 | 19 | 24 | 15 | 13 | 6 | 16 |
| RV– incidence | 25 | 21 | 22 | 27 | 20 | 17 | 22 | 23 | 22 | 22 | 18 | 17 | 20 |
Incidence data are per 1000 person-years.
Abbreviations: CRT, cluster-randomized controlled trial; GSA, government service area; RV–, rotavirus negative; RV+, rotavirus positive; YR, year.
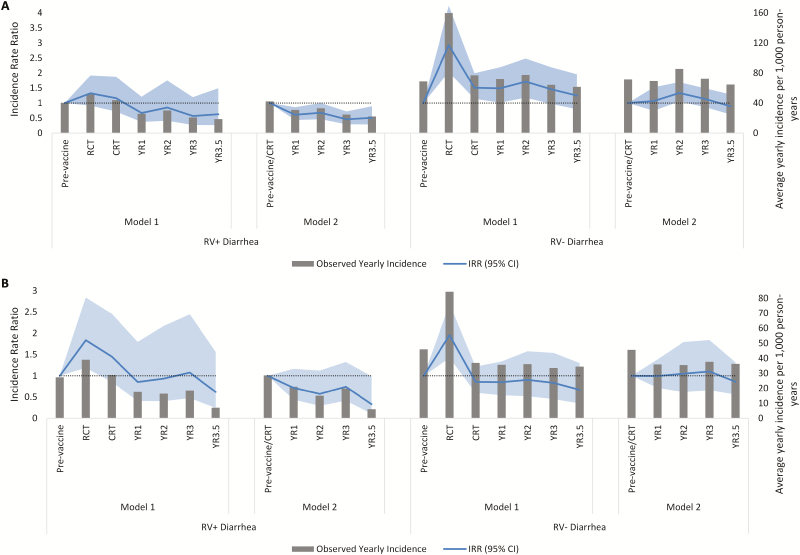
Figure 1.
Observed counts of rotavirus-positive (RV+) and rotavirus-negative (RV–) diarrhea of any severity, presenting to Matlab Hospital in (A) ISA and (B) GSA areas. Abbreviations: CRT, cluster-randomized controlled trial; GSA, government service area; ISA, International Centre for Diarrhoeal Disease Research, Bangladesh, service area; RCT, randomized, controlled trial; YR, year.
Rotavirus Vaccine Coverage and Timing
Rotavirus vaccine was not available in Matlab between February 2000 and March 2007. Between April 2007 and March 2009, 568 infants in ISA villages were randomized to receive PRV and 568 infants were randomized to placebo as part of a multi-site RCT [16]. In the stratified HRV CRT in both ISA and GSA areas, villages were randomized to 2 doses of HRV at 6 and 10 weeks of age or randomized as observed, control-only villages [17]. In the GSA villages, the CRT started in November 2008, and in the ISA villages, the CRT started in April 2009. Follow-ups and vaccinations during the CRT occurred in both the ISA and GSA villages through March 2011. Through a donation of vaccines post-CRT, HRV was provided routinely starting in April 2011. After September 2014, the rotavirus vaccine was unavailable.
HRV vaccine coverage levels among children <1 year of age changed during the study period (Figure 2). During the CRT, both the ISA and GSA villages showed similar vaccine coverage levels. Following the CRT, the coverage level among age-eligible children in ISA villages was maintained at between 65–80%, while GSA villages decreased to 42% at the end of the study period.
Figure 2.
Timing of HRV coverage (dose 1) over time by ISA and GSA villages randomized to HRV or control only in <1-year-olds. Abbreviations: GSA, government service area; HRV, human rotavirus vaccine; ISA, International Centre for Diarrhoeal Disease Research, Bangladesh, service area. ISA, HRV: icddr, b service areas randomized to HRV during the CRT; ISA, Control: icddr, b service areas randomized as control-only villages during the CRT; GSA, HRV: Government service areas randomized to HRV during the CRT; GSA, Control: icddr, b service areas randomized as control-only villages during the CRT; *23 children were vaccinated in GSA Villages in September-October 2008 before the start of the cluster-randomized trial (CRT). This time period is still considered prevaccine due to the small number of children vaccinated.
Observed and predicted RV+ diarrhea counts in ISA and GSA villages for both models demonstrated a satisfactory model fit (Supplementary Figures 1–2).
Diarrhea Incidence Trends: International Centre for Diarrhoeal Disease Research, Bangladesh, Service Area Villages
Using Model 1, with the pre-vaccine time period as the referent category, RV+ diarrhea rates increased during the RCT period and the CRT period in both age groups in ISA villages (Table 3; Figure 3). During periods of routine HRV use, there was a downward trend that was not statistically significant in RV+ diarrhea incidences after each additional year of vaccine use. During the entire 3.5 years of routine use, there was no meaningful decrease in RV+ diarrhea rates in 0- to <12-month-old children (IRR 0.72, 95% CI 0.39–1.33) or 12- to <24-month-old children (IRR 0.91, 95% CI 0.46–1.83). Using Model 2, combining the pre-vaccine time period and the CRT time period in the reference category and using control-only villages, there was a downward trend in the RV+ diarrhea incidence rates after each additional year of routine HRV use in both age groups (Table 4; Figure 3). During 3.5 years of routine HRV use, there was a statistically significant, 41% decrease in RV+ diarrhea rates in 0- to <12-month-old children (IRR 0.59, 95% CI 0.43–0.80), a 35% decrease in 12- to <24-month-old children (IRR 0.65, 95% CI 0.42–1.02), and a statistically significant, 39% decrease in children 0 to <24 months of age (IRR 0.61, 95% CI 0.45–0.82).
Table 3.
Diarrhea Trends in International Centre for Diarrhoeal Disease Research, Bangladesh, Service Area Region (Model 1)
| ISA | Feb 2000- Feb 2007 (prevaccine) | March 2007– March 2009 (RCT) | April 2009– March 2011 (CRT) | April 2011– March 2012 (YR1) | April 2012– March 2013 (YR2) | April 2013– March 2014 (YR3) | April 2014– September 2014 (YR3.5) | April 2011– September 2014 (vaccine years) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | ||
| 0–12 months of age | ||||||||||||||||||||||
| RV+ | REF | 1.32 | 0.91 | 1.92 | 1.16 | 0.72 | 1.88 | 0.67 | 0.37 | 1.21 | 0.85 | 0.41 | 1.75 | 0.57 | 0.27 | 1.20 | 0.63 | 0.26 | 1.49 | 0.72 | 0.39 | 1.33 |
| RV– | REF | 2.93 | 2.03 | 4.24 | 1.51 | 1.14 | 2.00 | 1.49 | 1.01 | 2.19 | 1.71 | 1.18 | 2.49 | 1.46 | 0.97 | 2.19 | 1.25 | 0.79 | 1.96 | 1.59 | 1.09 | 2.31 |
| 12–24 months of age | ||||||||||||||||||||||
| RV+ | REF | 1.84 | 1.19 | 2.84 | 1.45 | 0.86 | 2.46 | 0.86 | 0.41 | 1.80 | 0.94 | 0.40 | 2.17 | 1.08 | 0.47 | 2.45 | 0.62 | 0.25 | 1.56 | 0.91 | 0.46 | 1.83 |
| RV– | REF | 1.95 | 1.42 | 2.69 | 0.86 | 0.60 | 1.22 | 0.85 | 0.54 | 1.34 | 0.91 | 0.52 | 1.58 | 0.83 | 0.45 | 1.54 | 0.67 | 0.35 | 1.30 | 0.88 | 0.56 | 1.38 |
| 0–24 months of age | ||||||||||||||||||||||
| RV+ | REF | 1.50 | 1.03 | 2.19 | 1.26 | 0.80 | 2.00 | 0.72 | 0.40 | 1.31 | 0.90 | 0.44 | 1.84 | 0.74 | 0.36 | 1.52 | 0.66 | 0.29 | 1.51 | 0.79 | 0.43 | 1.43 |
| RV– | REF | 2.55 | 1.91 | 3.41 | 1.24 | 0.98 | 1.55 | 1.23 | 0.89 | 1.70 | 1.40 | 0.99 | 1.97 | 1.19 | 0.82 | 1.73 | 1.01 | 0.68 | 1.49 | 1.31 | 0.95 | 1.79 |
Abbreviations: CI, confidence interval; CRT, cluster-randomized controlled trial; IRR, incidence rate ratio; ISA, International Centre for Diarrhoeal Disease Research, Bangladesh, service area; RCT, randomized, controlled trial; REF, referent; RV–, rotavirus negative; RV+, rotavirus positive; YR, year.
Figure 3.
Observed incidences and IRRs of RV+ and RV– diarrhea of any severity presenting to Matlab Hospital in ISA villages using Models 1 and 2 in (A) 0– to <12-month-old children and (B) 12 to <24-month-old children. Abbreviations: CI, confidence interval; CRT, cluster-randomized controlled trial; IRR, incidence rate ratio; ISA, International Centre for Diarrhoeal Disease Research, Bangladesh, service area; RV–, rotavirus negative; RV+, rotavirus positive; RCT, randomized, controlled trial; YR, year.
Table 4.
Diarrhea Trends, International Centre for Diarrhoeal Disease Research, Bangladesh, Service Area Region (Model 2)
| ISA | Feb 2000-March 2011 (prevaccine and CRT) | April 2011– March 2012 (YR1) | April 2012– March 2013 (YR2) | April 2013– March 2014 (YR3) | April 2014– September 2014 (YR3.5) | April 2011– September 2014 (vaccine years) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | ||
| 0–12 months of age | ||||||||||||||||
| RV+ | REF | 0.61 | 0.43 | 0.86 | 0.68 | 0.46 | 1.00 | 0.46 | 0.29 | 0.72 | 0.51 | 0.29 | 0.90 | 0.59 | 0.43 | 0.80 |
| RV– | REF | 1.07 | 0.74 | 1.52 | 1.33 | 1.05 | 1.69 | 1.14 | 0.87 | 1.49 | 0.89 | 0.62 | 1.28 | 1.15 | 0.91 | 1.47 |
| 12–24 months of age | ||||||||||||||||
| RV+ | REF | 0.72 | 0.44 | 1.17 | 0.58 | 0.29 | 1.12 | 0.74 | 0.41 | 1.33 | 0.33 | 0.11 | 0.99 | 0.65 | 0.42 | 1.02 |
| RV– | REF | 1.00 | 0.72 | 1.39 | 1.05 | 0.62 | 1.79 | 1.10 | 0.66 | 1.84 | 0.86 | 0.56 | 1.32 | 1.03 | 0.74 | 1.43 |
| 0–24 months of age | ||||||||||||||||
| RV+ | REF | 0.64 | 0.46 | 0.89 | 0.66 | 0.44 | 0.98 | 0.55 | 0.35 | 0.85 | 0.48 | 0.25 | 0.91 | 0.61 | 0.45 | 0.82 |
| RV– | REF | 1.05 | 0.80 | 1.38 | 1.27 | 0.98 | 1.64 | 1.12 | 0.85 | 1.46 | 0.89 | 0.70 | 1.13 | 1.12 | 0.91 | 1.37 |
Abbreviations: CI, confidence interval; CRT, cluster-randomized controlled trial; IRR, incidence rate ratio; ISA, International Centre for Diarrhoeal Disease Research, Bangladesh, service area; RV–, rotavirus negative; RV+, rotavirus positive; YR, year.
In Model 1, RV– diarrhea rates increased during the RCT period and the CRT period in both age groups. During periods of routine HRV use, there was an increased risk of RV– diarrhea in 0- to <12-month-old children (IRR 1.59, 95% CI 1.09–2.31) and no meaningful change in 12- to <24-month-old children. In Model 2, there were no statistically significant changes in RV– diarrhea rates during periods of HRV routine use.
Diarrhea Incidence Trends: Government Service Area Villages
Using Model 1, with the pre-vaccine time period as the referent category, the incidence of RV+ diarrhea increased during the CRT period in 0- to <12-month-old children, but did not meaningfully change in 12- to <24-month-old children (Table 5; Figure 4). During periods of routine HRV use, there was an upward trend in the RV+ diarrhea incidence after each additional year of vaccine use in 0- to <12-month-old children, but no clear trends in 12- to <24-month-old children. During 3.5 years of routine use, there was no statistically significant change in the incidences of RV+ diarrhea in 0- to <12-month-old children (IRR 1.25, 95% CI 0.78–2.01) or in 12- to <24-month-old children (IRR 1.00, 95% CI 0.52–1.92). Using Model 2, there was a downward trend in the RV+ diarrhea incidence after each additional year of routine HRV use in both age groups (Table 6; Figure 4). However, during 3.5 years of routine HRV use, there was no meaningful change in the RV+ diarrhea rate in either age group. In Models 1 and 2, there were no statistically significant changes in RV– diarrhea rates during periods of HRV routine use.
Table 5.
Diarrhea Trends, Government Service Area Region (Model 1)
| GSA | Feb 2000– Oct 2008 (prevaccine) | Nov 2008– March 2011 (CRT) | April 2011– March 2012 (YR1) | April 2012– March 2013 (YR2) | April 2013– March 2014 (YR3) | April 2014– September 2014 (YR3.5) | April 2011– September 2014 (vaccine years) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | ||
| 0–12 months of age | |||||||||||||||||||
| RV+ | REF | 1.46 | 1.02 | 2.09 | 0.99 | 0.63 | 1.54 | 1.58 | 0.90 | 2.77 | 1.27 | 0.75 | 2.16 | 1.55 | 0.66 | 3.61 | 1.25 | 0.78 | 2.01 |
| RV– | REF | 1.01 | 0.75 | 1.37 | 0.96 | 0.62 | 1.47 | 1.33 | 0.87 | 2.04 | 1.13 | 0.70 | 1.82 | 0.79 | 0.43 | 1.43 | 1.10 | 0.73 | 1.65 |
| RV+ | REF | 0.98 | 0.63 | 1.53 | 1.31 | 0.73 | 2.33 | 0.82 | 0.38 | 1.77 | 0.60 | 0.28 | 1.25 | 0.89 | 0.36 | 2.22 | 1.00 | 0.52 | 1.92 |
| RV– | REF | 0.97 | 0.72 | 1.32 | 1.06 | 0.70 | 1.62 | 1.16 | 0.76 | 1.75 | 0.65 | 0.37 | 1.13 | 0.67 | 0.40 | 1.14 | 0.98 | 0.64 | 1.50 |
| 0–24 months of age | |||||||||||||||||||
| RV+ | REF | 1.26 | 0.89 | 1.77 | 1.18 | 0.76 | 1.85 | 1.24 | 0.72 | 2.12 | 0.96 | 0.57 | 1.63 | 1.34 | 0.61 | 2.95 | 1.16 | 0.73 | 1.85 |
| RV– | REF | 0.98 | 0.77 | 1.26 | 0.99 | 0.71 | 1.38 | 1.25 | 0.88 | 1.78 | 0.92 | 0.61 | 1.40 | 0.75 | 0.47 | 1.19 | 1.04 | 0.74 | 1.47 |
Abbreviations: CI, confidence interval; CRT, cluster-randomized controlled trial; GSA, government service area; IRR, incidence rate ratio; RV–, rotavirus negative; RV+, rotavirus positive; YR, year.
Figure 4.
Observed incidence and IRRs of RV+ and RV– diarrhea of any severity presenting to Matlab Hospital in GSA villages using Models 1 and 2 in (A) 0 to <12-month-old children and (B) 12 to <24-month-old children. Abbreviations: CI, confidence interval; CRT, cluster-randomized controlled trial; GSA, government service area; IRR, incidence rate ratio; RV–, rotavirus negative; RV+, rotavirus positive; RCT, randomized, controlled trial; YR, year.
Table 6.
Diarrhea Trends, Government Service Area Region (Model 2)
| GSA | Feb 2000–March 2011 (prevaccine and CRT) | April 2011–March 2012 (YR1) | April 2012–March 2013 (YR2) | April 2013–March 2014 (YR3) | April 2014–September 2014 (YR3.5) | April 2011–September 2014 (vaccine years) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | IRR | 95% | CI | ||
| 0–12 months of age | ||||||||||||||||
| RV+ | REF | 0.91 | 0.58 | 1.43 | 0.79 | 0.39 | 1.61 | 0.62 | 0.34 | 1.14 | 0.65 | 0.29 | 1.46 | 0.78 | 0.48 | 1.27 |
| RV– | REF | 1.38 | 0.93 | 2.06 | 1.66 | 1.00 | 2.75 | 1.36 | 0.85 | 2.17 | 1.06 | 0.60 | 1.85 | 1.43 | 0.97 | 2.12 |
| 12–24 months of age | ||||||||||||||||
| RV+ | REF | 1.38 | 0.91 | 2.07 | 0.63 | 0.34 | 1.16 | 0.61 | 0.36 | 1.03 | 0.23 | 0.03 | 1.53 | 0.87 | 0.53 | 1.43 |
| RV– | REF | 0.98 | 0.56 | 1.72 | 0.72 | 0.43 | 1.22 | 0.72 | 0.40 | 1.30 | 0.61 | 0.33 | 1.10 | 0.79 | 0.51 | 1.23 |
| 0–24 months of age | ||||||||||||||||
| RV+ | REF | 1.12 | 0.80 | 1.55 | 0.72 | 0.48 | 1.08 | 0.62 | 0.41 | 0.94 | 0.50 | 0.24 | 1.06 | 0.82 | 0.57 | 1.19 |
| RV– | REF | 1.20 | 0.87 | 1.67 | 1.24 | 0.84 | 1.84 | 1.06 | 0.71 | 1.58 | 0.86 | 0.56 | 1.31 | 1.15 | 0.86 | 1.53 |
Abbreviations: CI, confidence interval; CRT, cluster-randomized controlled trial; GSA, government service area; IRR, incidence rate ratio; RV–, rotavirus negative; RV+, rotavirus positive; YR, year.
DISCUSSION
Our study demonstrates a decreasing trend in RV+ diarrhea incidences among children <2 years of age from ISA villages presenting to Matlab Hospital during 3.5 years of routine HRV use. Using a conservative model to estimate pre-vaccination rotavirus diarrhea trends (Model 1), the results were not statistically significant. However, by restricting the analysis to control-only villages, we gained an additional 2 years of recent, pre-vaccine time to model baseline trends (Model 2), and found a statistically significant, 39% reduction in RV+ diarrhea rates in children 0 to <24 months of age. No significant impact of HRV on the RV+ diarrhea incidence among children from GSA villages was observed using either model. Differences in the population-level impacts between ISA and GSA villages are likely due to lower HRV coverage and lower reported diarrhea incidences in GSA areas, compared to ISA villages.
Our study also examined changes in the rate of RV– diarrhea as a control outcome, with the assumption that HRV introduction should have no significant impact on RV– diarrhea [22]. In Model 1, using only the pre-vaccine period in the referent category, we observed an increasing trend in both RV+ and RV– diarrhea rates in children 0 to <24 months of age in ISA villages during the RCT and CRT time periods. While other interventions or unmeasured biases may have influenced the all-cause gastroenteritis incidence, we believe this increase was due to changes in health-care–seeking behaviors due to the RCT. During the RCT, field staff visited the homes of infants enrolled in the study to remind parents to bring their child to the hospital for episodes of diarrhea [16]. A change in community health-care–seeking behavior is the most likely explanation, as there was no significant change in all-cause diarrhea in the corresponding time period in the GSA villages, where no RCT took place (Figure 1), and no specific pathogen was identified as a cause of the increase in all-cause diarrhea. The most conservative model to estimate the HRV impact (Model 1) modelled the RCT and CRT time periods separately and directly compared the pre-vaccine time period to the years of routine HRV use in both ISA and GSA villages. However, if increased health-care–seeking behaviors were sustained, results from Model 1 would underestimate the population-level impact of HRV.
In the secondary analysis (Model 2), both to increase power and to include relevant health-care–seeking behaviors to estimate the baseline incidence, we restricted the analysis to those villages randomized as control-only during the CRT period, and assessed the impact of routine HRV use on diarrhea over time. The referent category combined the pre-vaccine time period and the CRT time period. These models showed a significant impact of routine HRV use on RV+ diarrhea rates in 0- to <24-month-old children in ISA villages, but not in GSA villages. RV– diarrhea rates did not significantly change over time using this model. Notably, both models showed a decreasing trend in RV+ diarrhea in ISA villages during sustained HRV coverage. This analysis demonstrates the importance of using the appropriate baseline incidences and underlying trends in time-series analyses.
Despite the potential differences in health-care–seeking behavior over time, our results are similar to the RCT and CRT conducted in Matlab, Bangladesh, with the greatest impact of rotavirus vaccine on children 0 to <12 months of age. To our knowledge, no other population-level impact analyses have been reported in Asia with rotavirus diarrhea as the outcome, though a study in the Philippines saw a 60% (95% CI 55–64%) reduction in all-cause diarrhea hospitalizations within 4 years after rotavirus vaccine introduction [23]. Similar time-series analyses conducted 2–3 years after rotavirus introduction found a 49% (95% CI 32–63%) decrease in rotavirus diarrhea in <5-year-old children in Ghana [12], a 54% (95% CI 33–69%) decrease in rotavirus diarrhea in <1-year-old children in Malawi [11], a 33% (95% CI 25–41%) reduction in rotavirus diarrhea in <5-year-old children in Botswana [14], and a 38% reduction in rotavirus positivity among children <5 years old in Zambia [10]. Long-term impacts were also observed in Ghana [24] and Zambia [25]. Importantly, in these studies, >90% vaccine coverage for 1 or 2 doses of rotavirus vaccine were reported within 1 year of vaccine introduction. In our study, the maximum, 2-dose HRV coverage of 68% was attained in the ISA villages during the second year of routine use.
Our study has limitations. As in any time-series analysis, our study may have been confounded by other interventions or other unmeasured factors associated with RV+ diarrhea and the timing of the vaccine introduction. However, our confidence in the impact of HRV is increased, because no meaningful changes in RV– diarrhea incidences were observed. Second, while the Matlab HDSS database shows lower vaccine coverage in GSA areas, coverage may be underestimated or inaccurate due to the lack of recording on health cards in this region and potential reliance on maternal reports. Though measured with the same potential biases, during the study period, the average coverage for 3 doses of Diphtheria-Pertussis-Tetanus (DTP3) was 97% in ISA villages and 91% in GSA villages [26]. Third, with the available data, we were unable to assess the impact of the rotavirus vaccine on severe rotavirus diarrhea, as indicated by a Vesikari score ≥11, which is the outcome used in rotavirus vaccine clinical trials.
This study provides initial evidence of the population-level impact of rotavirus vaccines in children <2 years of age in regions of high vaccine coverage in Matlab, Bangladesh. Pecenka et al [27] estimated that, with a Gavi subsidy in Bangladesh, the averted cost/disability adjusted life year (DALY) ratio ranged between $58/DALY and $142/DALY, indicating a highly cost-effective vaccine, given 94% coverage of DTP3 in Bangladesh [27, 28] In our study, during the pre-vaccine period, rotavirus was detected in 34.5% of diarrhea cases in children <5 years of age presenting to Matlab Hospital. Other regions of Bangladesh show an average of 64% of diarrhea instances being due to rotavirus in children <5 years of age [29]. With sustained vaccine coverage and a considerable nationwide burden of rotavirus diarrhea, larger impacts of HRV on rotavirus gastroenteritis are likely to be observed long-term in Bangladesh. This may provide additional evidence to influence other countries in the region to introduce the rotavirus vaccine.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments.The authors thank PATH for its commitment to their research efforts and for the negotiation of vaccines for Matlab, Bangladesh, following the cluster-randomized study. They thank the governments of Bangladesh, Canada, Sweden, and the United Kingdom for providing core/unrestricted support. They thank the families of the Matlab field area who participated in this study, and the field staff of the International Centre for Diarrhoeal Disease Research, Bangladesh, without whom they could not have completed this research. They thank PATH and GlaxoSmithKline for the donation of vaccines to the study and the study population, both during the cluster-randomized trial and for 3 years following the study.
Disclaimer.The findings and conclusions contained within are those of the authors and do not necessarily reflect the positions or policies of the Bill & Melinda Gates Foundation or the National Institutes of Health. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
Financial support.This work was supported by the Bill & Melinda Gates Foundation (grant number OPP1097672) and the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) (grant number MERIT R37 AI032042).
Potential conflicts of Interest.All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Troeger C, Khalil IA, Rao PC, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr 2018;172:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Troeger C, Forouzanfar M, Rao PC, et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the global burden of disease study 2015. Lancet Infect Dis 2017; 17:909–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. [DOI] [PubMed] [Google Scholar]
- 4. Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. [DOI] [PubMed] [Google Scholar]
- 5. International Vaccine Access Center Johns Hopkins Bloomberg School of Public Health. VIEW-hub global vaccine introduction and implementation report. Available at: www.jhsph.edu/ivac/view-hub. Accessed 23 March 2018.
- 6. Mujuru HA, Yen C, Nathoo KJ, et al. Reduction in diarrhea- and rotavirus-related healthcare visits among children <5 years of age after national rotavirus vaccine introduction in Zimbabwe. Pediatr Infect Dis J 2017; 36:995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maphalala G, Phungwayo N, Masona G, et al. Early impact of rotavirus vaccine in under 5 year old children hospitalized due to diarrhea, Swaziland. Vaccine 2017;36:7210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wandera EA, Mohammad S, Bundi M, et al. Impact of rotavirus vaccination on rotavirus and all-cause gastroenteritis in peri-urban Kenyan children. Vaccine 2017; 35:5217–23. [DOI] [PubMed] [Google Scholar]
- 9. Abeid KA, Jani B, Cortese MM, et al. Monovalent rotavirus vaccine effectiveness and impact on rotavirus hospitalizations in Zanzibar, Tanzania: data from the first 3 years after introduction. J Infect Dis 2016; 215:jiw524. [DOI] [PubMed] [Google Scholar]
- 10. Mpabalwani EM, Simwaka CJ, Mwenda JM, et al. Impact of rotavirus vaccination on diarrheal hospitalizations in children aged <5 years in Lusaka, Zambia. Clin Infect Dis. 2016; 62:S183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bar-Zeev N, Jere KC, Bennett A, et al. Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin Infect Dis 2016; 62:S213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin Infect Dis 2016; 62:S200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beres LK, Tate JE, Njobvu L, et al. A preliminary assessment of rotavirus vaccine effectiveness in Zambia. Clin Infect Dis 2016; 62:S175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enane La, Gastañaduy Pa, Goldfarb DM, et al. Impact of rotavirus vaccination on hospitalizations and deaths from childhood gastroenteritis in Botswana. Clin Infect Dis 2016; 62:S168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nelson EAS, de Quadros CA, Santosham M, Parashar UD, Steele D. Overcoming perceptions of financial barriers to rotavirus vaccine introduction in Asia. Hum Vaccin Immunother 2013; 9:2418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615–23. [DOI] [PubMed] [Google Scholar]
- 17. Zaman K, Sack DA, Neuzil KM, et al. Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial. PLOS Med 2017; 14:e1002282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alam N, Ali T, Razzaque A, et al. Health and demographic surveillance system (HDSS) in Matlab, Bangladesh. Int J Epidemiol 2017;46:809–16. [DOI] [PubMed] [Google Scholar]
- 19. Lopez Bernal J, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2016;46:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002; 27:299–309. [DOI] [PubMed] [Google Scholar]
- 21. Becketti S. Introduction to time series using stata. College Station, Texas: Stata Press, 2013. [Google Scholar]
- 22. Schwartz LM, Halloran ME, Rowhani-Rahbar A, Neuzil KM, Victor JC. Rotavirus vaccine effectiveness in low-income settings: an evaluation of the test-negative design. Vaccine 2017; 35:184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez AL, Raguindin PF, Esparagoza J, et al. Impact of rotavirus vaccine on diarrheal hospitalization and outpatient consultations in the Philippines: first evidence from a middle-income Asian country. Vaccine 2018; 36:3308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Enweronu-Laryea CC, Armah G, Sagoe KW, et al. Sustained impact of rotavirus vaccine introduction on rotavirus gastroenteritis hospitalizations in children <5 years of age, Ghana, 2009–2016. Vaccine 2018;36:7131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mpabalwani EM, Simwaka JC, Mwenda JM, Matapo B, Parashar UD, Tate JE. Sustained impact of rotavirus vaccine on rotavirus hospitalisations in Lusaka, Zambia, 2009–2016. Vaccine 2018;36:7165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. icddr,b. Health and Demographic Surveillance System–Matlab, v. 51. Registration of health and demographic events 2016, Scientific Report No. 138. Dhaka: icddr,b, 2018. Available at: http://dspace.icddrb.org/jspui/bitstream/123456789/9061/2/SR 138_HDSS AR2016 Final_April23_2018.pdf. Accessed 1 December 2018.
- 27. Pecenka C, Parashar U, Tate JE, et al. Impact and cost-effectiveness of rotavirus vaccination in Bangladesh. Vaccine 2017;35:3982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization/United Nations Children’s Fund (WHO-UNICEF) Coverage Estimates WHO World Health Organization: Immunization, Vaccines And Biologicals. Vaccine preventable diseases vaccines monitoring system 2018 global summary reference time series: DTP3 Available at: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragedtp3.html. Accessed 1 December 2018.
- 29. Satter SM, Aliabadi N, Gastañaduy PA, et al. An update from hospital-based surveillance for rotavirus gastroenteritis among young children in Bangladesh, July 2012 to June 2017. Vaccine 2018;36:7811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.