Abstract
The extracellular signal-regulated kinases (ERKs) are key transducers of the extracellular signals into intracellular responses and represent major molecular players in tumorigenesis. The aim of this study was to determine how curcumin (CRM) used as an adjuvant supports the apoptotic process induced by a single chemical agent treatment (cisplatin-CisPT) on two head and neck squamous cell carcinoma cell lines (FaDu and PE/CA-PJ49) and the involvement of ERK1/2 and/or p53 activation in this process. Data have shown that the CisPt effect is potentiated by CRM. CRM induced an increase of p53 protein phosphorylation in both cell lines. CisPt decreased p53 protein phosphorylation in FaDu cells, but increased it in PE/CA-PJ49 cells. Data showed that the constitutive expression of activated ERK1/2 protein-kinase was different in the two analyzed tumor cell lines. ERK1/2 activation status was essential for both cell processes, proliferation and apoptosis induced by CisPt and/or CRM treatment on squamous cell carcinoma cells. Our data suggest that p53 phosphorylation in the apoptotic process induced by CRM treatment might require the involvement of ERK1/2. In this regard the CisPt treatment suggested that p53 phosphorylation is ERK1/2 independent in FaDu cells having a p53 gene deletion and ERK1/2 dependent in PE/CA-PJ49 cells having a p53 gene amplification. Moreover, in both tumor cell lines our results support the involvement of p53 phosphorylation-ERK1/2 activation-dependent in the apoptosis induced by combined treatments (CisPt and CRM). The use of CRM as adjuvant could increase the efficiency of chemotherapy by modulating cellular activation processes of ERK1/2 signaling pathways. In conclusion, the particular mode of intervention by which ERK1/2 might influence cell proliferation and/or apoptosis processes depends on the type of therapeutic agent, the cells' particularities, and the activation status of the ERK1/2.
Keywords: head and neck tumor cells, cisplatin, curcumin, p53, apoptosis, ERK1/2
Introduction
Oral cavity cancer has shown an increased incidence in recent years in Romania (1) and also worldwide (2). The most commune type of oral cavity cancer is squamous cell carcinoma (commonly referred as ‘Head and Neck Squamous Cell Carcinoma’, HNSCC). The disease affects several anatomic structures: Oral cavity, oropharynx, nasopharynx, hypopharynx and larynx (3–5). Most of HNSCC patients are, unfortunately, diagnosed when the disease has already progressed to an advanced stage. In this instance the therapeutic approach is complex and involves the combination of chemo and radiotherapy either before or after the surgical procedure based on the protocol established for each patient (6–8). Thus, the protocols are somewhat personalized, hower, the development of resistance to conventional treatments and an increased recurrence rate of primary tumors lead to a 5-year decline of the survival rate of patients with HNSCC (9–12). Therefore, additional clinical approaches of this complex disease are needed. The new therapeutic strategies have to tackle at least two points, besides increasing the survival rate, it should reduce the adverse effects of chemotherapy. The use of natural compounds as adjuvants can be beneficial by increasing the efficacy of conventional treatments and very importantly to limit the adverse reactions. The disruption of normal cellular mechanisms such as proliferation or apoptosis, is accountable for the development of tumoral processes in different types of cancer, including HNSCC. Apoptosis, known as ‘programmed cell death’, is a mechanism that provides crucial control over the cell homeostasis. Apoptosis enables the removal of cells having DNA mutations, aberrant cellular cycle and that are prone to malignant transformation (13,14). Defects observed in the apoptotic pathway associated to cancer have an important role in conventional oncotherapies (radio- and chemotherapy) since apoptosis induction needs high dosages of therapeutic agents. Cisplatin (CisPt), one of the most used chemotherapeutic agent in HNSCC treatment, has the capacity to interact with DNA, RNA and different proteins, and by activation of specific mechanisms, some of them still incompletely known, can induce apoptosis (15–17).
The elucidation of the mechanisms pertaining to the apoptosis process is a key step. The restoration of the cellular mechanisms responsible for tumor cell apoptosis are of upmost importance in malignant transformation, tumor evasion and anticancer therapy (18–20). Factors that determine a damaged cell to go either through apoptosis or to repair the damage and continue the cell cycle, are still to be discovered. It is well known that one important apoptosis regulator is the tumor suppressor gene TP53 (TP53). TP53 tumor suppressor has mutations in 40–60% of the HNSCC cases (21). This is an untimely event, identified in precancerous lesions and associated with a poor prognosis. Some studies showed that the rehabilitation of the TP53 function in an early stage of the disease had no effect, but can lead to the regression of the tumor in advanced stages (22,23). This suggests that TP53 tumor suppressor is not activated in the early phases of the disease, but can be activated in later phases of tumorigenesis (24,25). Another important player in the regulation of cell tumorigenesis-related functions (proliferation, transformation, differentiation, apoptosis, angiogenesis) is activation of mitogen-activated protein kinases (MAPKs) pathways such as ERKs (26–30). There are studies showing that the activation of the MAPK pathway is correlated with the cancer prognosis influencing the therapeutic outcome in many types of cancer (31–33). Correlated once with the intimate deciphering of molecular pathways that regulate oncogenesis, new modern and specific therapies able to improve the current therapeutic methods will be developed. One of the approaches is to maximize the effectiveness of initial therapy by the use of a chemotherapeutic drug together with a supporting agent (34). Some studies have been focused on the discovery of new therapeutic agents obtained from natural compounds proving anticancer and anti-proliferation effects (35,36). One of these compounds is curcumin (CRM). CRM is the principal compound of turmeric extracted from the plant Curcuma longa and has many diverse properties - anti-inflammatory, anti-bacterial, anti-fungal, anti-viral and anti-carcinogenic (37). The mechanisms through which CRM exerts its antitumoral effects are complex and diverse; they appear to act in the processes of growth and apoptosis and also in different stages of carcinogenesis (38,39).
Acknowledging all the mentioned issues in the this type of carcinoma the focus of this study is to investigate how a natural adjuvant (CRM) supports the apoptotic process induced by a mono chemical standard agent (CisPt) in an in vitro experimental model using HNSCC standard cell lines. Moreover, in our study we investigated the ERK1/2 and/or p53 involvement in treatment response. The use of adjuvant might have a beneficial effect decreasing the CisPt doses, therefore reducing the adverse reactions induced by a chemotherapeutic agent.
Materials and methods
Cell lines culture
The squamous carcinoma cell line PE/CA-PJ49 was from European Collection of Authenticated Cell Cultures (ECACC cat. no. 0060606). The cell line was obtained from a 57-year old male patient with tongue carcinoma. The FaDu cell line was obtained from the American Type Culture Collection (ATCC-HTB-43 cat.). The cell line was derived from a 56-year-old male patient with pharyngeal squamous cell carcinoma. Both lines are showing adherent epithelial type morphology. The cell lines were grown and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 1% penicillin, and 1% streptomycin at 37°C in 5% CO2. The sub-confluent cultures (70–80%) were split 1:4-1:8 (i.e. seeding at 1–3×10,000 cells/cm2) using trypsin-EDTA (0.25% trypsin, 0.03% EDTA).
The study protocol was approved by the Ethics Committee of ‘Stefan S. Nicolau’ Virology Institute.
Drugs and treatments
CisPt and CRM (97% purity), were obtained from Sigma-Aldrich. They were initially dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at a concentration of 5 mM. Further, milli-Q water was used to generate 1 mM stock solutions. The stock solutions were filtered using a cellulose acetate hydrophilic filter (0.20 µm) (Sigma-Aldrich). Dilutions used in the experimental model were done in DMEM to generate the following concentration ranges: 2–160 µM for CisPt and 5–100 µM for CRM. Tumor cells were incubated for 6, 24 or 48 h either in the presence of the drugs (CisPt and/or CRM) or vehicle control (DMSO ≤0.1%). For inhibition studies of ERK1/2 function, the cells were pre-incubated for 2 h with 25 µM PD98059 as previously reported (40). The treated tumor cells were used to determine cell proliferation, FISH, apoptosis and conserved as cell pellets at −80°C in order to obtain cell lysates used in ELISA assays. Non-treated cells were used as controls throughout the experiments.
Cell viability assay
Tumor cells (1–2×103 cell/well) were seeded in 96-microwell plates, incubated at 37°C for 24 h to accomplish full adherence and then treated with different concentrations of CisPt (2–160 µM) or CRM (5–100 µM). The cell viability was assessed by the ability of metabolically active cells to reduce the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich) to colored formazan compounds. The absorbance was measured with an enzyme-linked immunosorbent assay reader (Dynex plate reader; wavelength 450 nm) (41). The data are presented as the mean values from at least three different experiments. Untreated cells served as control having 100% viability. Viability % = (T-B)/(U-B) ×100, (where T, absorbance of treated cells; U, absorbance of untreated cells, iar B, absorbance of blank).
Cell proliferation assay
CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) was used. The test is based on the reduction of yellow MTS tetrazolium salt by the viable cells and generation of colored formazan soluble in the culture medium. The product was spectrophotometrically quantified by measuring the absorbance at λ=490 nm (42) using a Dynex plate reader (DYNEX Technologies-MRS). Results were expressed as mean values of three determinations ± standard deviation (SD). Untreated cells served as control and considered to have proliferation index (PI) equal 1. PI = absorbance of treated cells/absorbance of untreated cells.
Fluorescence in situ hybridization
FISH technique was performed after optimization of the protocol using commercially available probe from Abbott/Vysis (Vysis) (43,44). According with the manufacturer's protocol Locus specific identifier LSA TP53/CEP17 FISH Probe Kit detects the LSI TP53 probe Spectrum Orange target located on chromosome 17p13.1 and CEP17 (17p11.1-q11.1 Alpha Satellite) probe Spectrum Green Dual Colour target located on the centromere of chromosome 17.
Cells and slide preparation: The slides were cleaned in an ice-cold mixture of 40% methanol and 60% distilled water, then air dryed and stored at 4°C. Cells were pelleted and suspended in 0.075 M KCl hypotonic solution for 20 min at 37°C. Tumor cells were pelleted by centrifugation at 300 × g for 5 min at 4°C and resuspended in 0.5 ml fixative (3:1 methyl alcohol and glacial acetic acid). Afterwards the cells were diluted at the appropriate density and distributed on several locations on the slide. The air dried slides were incubated at −20°C for 30 min.
FISH probe preparation and hybridization from cell culture: The slides were denatured for 5 min in 70% formamide/2X SSC at 73°C. Then the slides were dehydrated by immersion in 70, 80 and 100% cold ethanol solution, 5 min for each step. The slides were air-dried and 10 µl of the probe LSI TP53/CEP17 was added in the selected hybridization area. The smears were covered with a 22×22 mm coverslip, sealed and incubated overnight in a humid chamber at 37°C. Two washes were performed post hybridization, using washing solutions: 0.4X SSC/0.3% NP-40 at 73°C for 2 min, and 2X SSC/0.1% NP-40 for 2 min. The slides were air-dried and 4′,6-diamidino-2-phenylindole (DAPI II) was added for counterstaining. The slides were analyzed using a Zeiss Axio Imager M1 epifluorescence microscope (Zeiss) equipped with filters for DAPI, SpectrumOrange and SpectrumGreen and a triple filter (simultaneous DAPI/Orange/Green). Images were acquired at a magnification of ×1,000 and captured using MetaSystems digital camera. Images were analyzed using Isis version 5.2, MetaSystems software for quantitative analysis of samples generated by FISH technique (Altlussheim). For each sample hybridized signals were counted in 100 nuclei.
ELISA assay
ELISA assays were used to measure both total and phosphorylated p53 and ERK1/2proteins in cell lysates. Briefly, untreated, CisPt and/or CRM treated tumor cells were lysed in PBS (pH 7.2–7.4) containing 1 mM EDTA, 0.5% Triton X-100, 5 mM NaF, 6 M urea, 10 µg/ml leupeptin, 10 µg/ml pepstatin, 100 µM PMSF, 3 µg/ml aprotinin, 2.5 mM sodium pyrophosphate, 1 mM sodium orthovanadate. The lysate was kept 30 min on ice with stirring every 5 min. The lysate was centrifuged at 2,000 × g for 5 min at room temperature. Furthermore, the obtained supernatant was centrifuged at 14,000 × g for 15 min at 4°C. Protein concentration of the lysates was measured using Bradford assay. DuoSet_IC Human Total p53 ELISA [cat. no. DYC1043]; DuoSet_IC Human Phospho-p53 (S15) ELISA [cat. no. DYC1839]; DuoSet_IC Human/Mouse/Rat Total ERK1/2 [cat. no. DYC1940] DuoSet_IC Human/Mouse/Rat Phospho- ERK1/2 ELISA [cat. no. DYC1018B] were purchased from R&D Systems Inc. The protein of interest was measured using a standard Streptavidin-HRP system (45). All experiments were performed in triplicates and sample O.D. was measured at λ=450 nm using Dynex plate reader.
Analysis of apoptosis
The apoptosis assay was carried out with the Annexin V-FITC kit using the manufacturer's protocol (BD Pharmingen) (46). Treated and untreated 1×106 cells/ml were resuspended in cold binding buffer and staining simultaneously with 5 µl FITC-Annexin V (green fluorescence) and 5 µl propidium iodide (PI) in the dark at room temperature for 15 min. Then 400 µl of Annexin V binding buffer was added and 10,000 cells/sample were acquired using BD Canto II flow cytometer. The analysis was performed using DIVA 6.2 software in order to discriminate viable cells (FITC−PI−) from necrotic cells (FITC+PI+) and early apoptosis (FITC+PI−) from late apoptosis.
Statistical analysis
Data were analyzed using Student's t-test (paired type and one tailed distibution). One-way analysis of variation with P-value of <0.05 was considered statistically significant.
Results
Culture parameters of the standardized cell lines used in in vitro experiments
In the present study two human HNSCC cell lines were used, FaDu derived from pharyngeal squamous carcinoma and PE/CA-PJ49 obtained from a tongue squamous carcinoma, both cell lines presenting adherent and epithelial-type morphology. To achieve the successive passages of cells kept in culture, the density of cells in the culture plates were carefully supervised in order to avoid over-population and any sign of aging. At the end of each experiment, samples from used cells were frozen to create a batch of cells that can be used anytime to repeat or continue the experiments. Some studies have shown that the number of cells that present with polysomy at the level of the 17th chromosome is raised in oral cavity squamous carcinomas and this may be correlated with the process of carcinogenesis (47). The exact role of genetic modifications of TP53 in different stages of the tumorigenesis process is not completely established, but it is known that the gene has the ability to induce the restoration of damaged DNA by activating certain proteins and by stopping the cellular cycle and induction of apoptosis. Thereby, it has been tried to identify some possible numeric aberrations of chromosome 17, such as deletion or amplification of TP53 gene in the FaDu and PE/CA-PJ49 cells. This genetic endeavor was done in order to discover possible explications between these abnormalities and the cellular response to therapy. Using the FISH technique, the intention was to obtain information on the TP53 gene status in the FaDu and PE/CA-PJ49 tumor cells before starting the experiments. The PE/CA-PJ49 tumor line showed an amplification of the TP53 gene since all analyzed cells had 4 signals for both TP53 (17p13) (red dots on Fig. 1A) and 17(D17Z1) centromere probe (green dots on Fig. 1A). FaDu did not show the amplification of TP53 gene on chromosome 17. In contrast to this, the cells had only one signal for TP53 (17p13) (red dot on Fig. 1B) suggesting a deletion of the TP53 gene, without modification at the level of chromosome 17 which presented only two signals for the 17(D17Z1) centromere (green dots Fig. 1B).
Figure 1.
Fluorescent in situ hybridization analysis of TP53 gene performed on PE-CA/PJ49 (A) and FaDu (B) tumor cells. Two targets, LSI TP53 probe Spectrum Orange target located at chromosome 17p13.1 and CEP17 (17p11.1-q11.1 Alpha Satellite) probe Spectrum Green Dual Colour target located at the centromere of chromosome 17, were detected by locus specific identifier LSA TP53/CEP17 FISH Probe. The data were aquired at a magnification of ×1,000. The hybridized signals were counted in 100 nuclei for each sample. (A) PE-CA/PJ49 cells showed 4 red signals for both targets TP53 (amplified gene) and CEP17 (tetrasomy). (B) FaDu cells showed 1 red signal for TP53 (gene deletion) and 2 green signals for CEP17 (normal chromosome number).
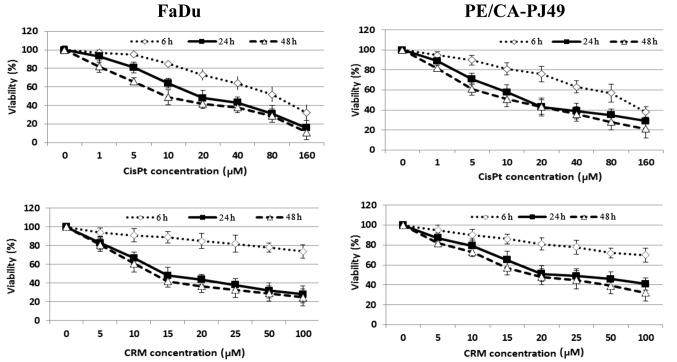
Effects of cisplatin and/or curcumin treatment on the cellular viability of HNSCC
Cisplatin is one of the most utilized cytostatic drugs in the treatment of HNSCC. Unfortunately many patients develop relapses or cisplatin resistance. A natural compound such as curcumin might improve and maintain the antitumoral effect of cisplatin. In order to determine the optimal concentration of the drug needed to inhibit half of the maximum biological response (IC50) both tumor cell lines were treated with the following concentrations 0, 1, 5, 10, 20, 40, 80, 160 µM CisPt or 0, 5, 10, 15, 20, 25, 50, 100 µM CRM. To determine the optimal treatment time, cells were treated for different time periods (6, 24 or 48 h). The inhibitory effect of CisPt or CRM on the cellular viability was dose- and time-dependent (Fig. 2). The results showed that 24 h CisPt treatment had an IC50=11.25 µM for FaDu cells and IC50=10.55 µM for PE/CA-PJ49 cells. CRM treatment for 24 h reduced the cellular viability with an IC50=13.72 µM for FaDu cells and IC50=15.20 µM for PE/CA-PJ49 tumor cells. Based on the obtained results the optimal time of treatment was 24 h and the optimal concentration was 10 µM for CisPt and 15 µM for CRM (Fig. 2). We tested concentrations less than 2 µM (data not shown) and they did not have any effect on the treated FaDu or PE/CA-PJ49 cells.
Figure 2.
Effect of CisPt or CRM on HNSCC cells viability. FaDu and PE/CA-PJ49 cells were either left untreated or treated with different concentrations of CisPt or CRM for 6, 24 or 48 h. FaDu. Viability (%) vs. untreated cells (100%) was determined. Data shown are representative of three independent experiments and are expressed as mean of three replicates ± SD (n=3). Untreated cells were considered to have 100% viability. Viability % = (T-B)/(U-B) ×100, (where T, absorbance of treated cells; U, absorbance of untreated cells; and B, absorbance of blank).
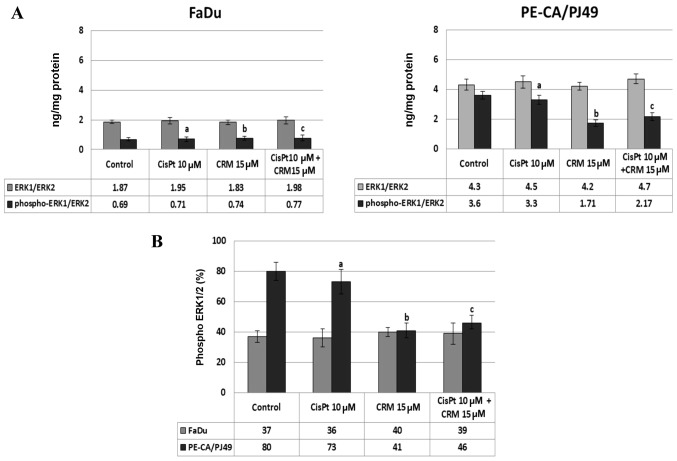
The effect of the CisPt and/or CRM treatment on the protein-kinase ERK1/2 expression
MAPKs transmit and amplify the signals involved in proliferation, as well as in cellular death. Using ELISA assay, we evaluated the expression and activation of protein-kinase ERK1/2 induced by CisPt and/or CRM treatment of FaDu and PE-CA/PJ49 tumor cells. The data show that in the tumor cells treated with CisPt and/or CRM for different time-points, the ERK1/2 protein-kinase is highly expressed after 24 h treatment. The untreated PE-CA/PJ49 cells (control) have a statistically significant higher expression of total ERK1/2 (4.3 ng/mg protein) compared to untreated FaDu cells (1.87 ng/mg protein) (Fig. 3). The CisPt and/or CRM treatment applied individually or in combination did not significantly modify the expression of total ERK1/2 form in the two analyzed tumor cell lines (Fig. 3A). In order to analyze the effect of CisPt and/or CRM treatment on the activation of ERK1/2, the level of ERK1/2 phosphorylation (% phospho-ERK1/ERK2) was quantified. The results show that FaDu cells responded differently to treatment compared to PE-CA/PJ49 cells.
Figure 3.
Effect of CisPt and/or CRM treatment on ERK1/2 protein-kinase expression in HNSCC tumor cell lines. (A) Total or phosphorylated ERK1/ERK2 protein concentrations in the cellular lysates are expressed as protein of interest/total protein (ng/mg). (B) The percent of phospho-ERK1/2 is calculated by formula: % phospho-ERK1/2 = [(ng phospho ERK1/2/ng ERK1/2 total) ×100]. The experiments were done in triplicates, data shown are representative of three independent experiments and were expressed as mean ± SD. a, CisPt vs. Control; b, CRM vs. Control; c, CisPt+CRM vs. Control; P>0.05, not significant.
In untreated FaDu cells 37% of ERK1/2 protein was phosphorylated and under the 10 µM CisPt and/or 15 µM CRM treatment the phosphorylation was not significantly modified compared to the untreated cells (Fig. 3A and B). In untreated PE-CA/PJ49 cells 80% of ERK1/2 was phosphorylated (Fig. 3B), 10 µM CisPt alone slightly reduced the level of phosphorylation of ERK1/2 to 73% (a; P=0.03), and 15 µM CRM per se induced a significant inhibition of ERK1/2 phosphorylation (41%, b; P=0.003) (Fig. 3B).
The combined treatment, CisPt and CRM, led to a significant reduction (42%) of the ERK1/2 phosphorylation compared to the control (c; P=0.004) (Fig. 3B). The data show that PE-CA/PJ49 cells having TP53 amplification are more sensitive to CRM treatment. CRM individually or in combination with CisPt can inhibit phosphorylation of protein ERK1/2 (Fig. 3B). FaDu having a TP53 deletion and constitutively a lower expression of phospho ERK1/2, does not respond to any of the treatments (Fig. 3B).
ERK1/2 activation correlates with the proliferation of tumor cells in HNSCC treated with cisplatin and/or curcumin
PE/CA-PJ49 and FaDu cells were treated with CisPt and/or CRM for 6, 24 and 48 h. The cell proliferation index (PI) was not significantly changed during 6 h treatments. Moreover, PI for 48 h treatments was not significantly different compared to the 24 h of treatment. CRM treated cells also had a decreased PI (b; P=0.0004) for FaDu and for PE/CA-PJ49 (b; P=0.0006) compared to the control (Fig. 4). CisPt treatment for 24 h determined a significant decrease of the proliferation process compared to the untreated cells, both in the case of tumoral cells FaDu (a; P=0.003) and in the cells PE/CA-PJ49 (a; P=0.0009). CRM treated cells also had a decreased PI (b; P=0.0004) for FaDu and for PE/CA-PJ49 (b; P=0.0006) compared to the control. A marked PI inhibition was observed in both cell lines when the cells were treated simultaneously with CisPt and CRM (Fig. 4). The results are statistically significant when compared to the control (FaDu cells (c); P=0.0006 and PE/CA-PJ49 (c) P=0.0005) (Fig. 4). Based on the obtained data, CRM had the capacity to potentiate the effect induced by CisPt treatment on human head and neck cancer cell lines (Fig. 4).
Figure 4.
Downstream effect of phospho ERK1/2 inhibition. The proliferation index (PI) of FaDu and PE/CA-PJ49 cells treated with CisPt and/or CRM in the presence or absence of PD98059 was calculated. PI, absorbance of treated cells/absorbance of untreated cells. Results are expressed as mean values of three determinations ± standard deviation (SD). Untreated cells were considered to have PI equal 1. a, CisPt vs. Control; b, CRM vs. Control; c, CisPt +CRM vs. Control; d, CisPt PD98059(−) vs. CisPt PD98059(+); e, CRM PD98059(−) vs. CRM PD98059(+); f, CisPt+CRM PD98059(−) vs. CisPt+CRM PD98059.
Since the basal activation status of protein-kinase ERK1/2 in the analyzed tumor cell lines is different in the investigated cell lines, we studied the effect of ERK1/2 activation on the proliferation process as response to the drug treatment. Thus, the tumor cells were pretreated for 2 h with a specific ERK1/2 inhibitor, 25 µM PD98059 (concentration determined after sketching the dose-effect curve) was used for pretreatments, and then the cells were treated with CisPt and/or CRM for another 24 h. FaDu cells have a TP53 deletion (Fig. 1) and a constitutively lower expression of phospho ERK1/2 (Fig. 3B). The presence of the specific inhibitor PD98059 did not influence significantly the proliferation process compared to control cells (no. PD98059) (P>0.05) (Fig. 4).
PE-CA/PJ49 cells have TP53 amplification (Fig. 1) and a constitutively high expression of phospho ERK1/2 (Fig. 3B). Pretreating with the PD98059 inhibitor of the PE-CA/PJ49 cells induced an increase of the proliferative activity in the case of treated cells with CisPt (d; P=0.005) or CRM (e; P=0.007). Similar effect was obtained in the case of the combined treatment of CRM and CisPt (f; P=0.003), compared to the cells that were subject to the same treatment, but in the absence of the PD98059 inhibitor (Fig. 4). These results show that the inhibition of the ERK1/2 activity facilitates the restoration of the proliferative process of the CisPt and/or CRM treated PE-CA/PJ49 cells. These observations lead to the hypothesis that proliferation of tumor cells depends on the level of protein-kinase ERK1/2 activation.
The role of ERK1/2 in the activation of p53 in HNSCC cells treated with CisPt and/or CRM
FaDu and PE/CA-PJ49 were treated for 24 h as described, 10 µM CisPt (a; P>0.05) did not affect the expression of total p53 protein in FaDu cells (Fig. 5A). On the contrary, 15 µM CRM alone (b; P=0.003) or combined with CisPt (c; P=0.0001) induced a significant increase of total p53 expression compared to untreated cells (Fig. 5A). In the same cell line, the expression of phospho-p53 protein was inhibited by CisPt treatment compared to untreated cells (a; P=0.003) (Fig. 5A). The expression of phospho-p53 showed a significant increase in the presence of either CRM alone (b; P=0.005) or combined with CisPt (c; P=0.0007) compared to untreated cells (Fig. 5A).
Figure 5.
ERK1/2 in the activation of p53 in HNSCC cells treated with CisPt and/or CRM. (A) The total and phospho-p53 protein expression (ng/mg total protein lysate) in FaDu, PE/CA-PJ49 cells treated with CisPt and/or CRM. a, CisPt vs. Control; b, CRM vs. Control; c, CisPt+CRM vs. Control. (B) Inhibitory effect of PD98059 on the total and phospho-p53 expression. The experiments were performed in triplicates. Results are expressed as mean values of three determinations ± standard deviation (SD). [PD98059 effect (%) = (protein expression+PD98059/protein expression-PD98059) ×100]. a, CisPt vs. Control; b, CRM vs. Control; c, CisPt +CRM vs. Control.
The analysis of total p53 expression in PE/CA-PJ49 cells showed that either CisPt (a; P=0.00006) or CRM treatment applied alone (b; P=0.002) induced a significant increase compared to control cells (Fig. 5A). The same result was obtained when cells were treated with the combined treatment (c;P=0.0001) (Fig. 5A). The p53 phosphorylation in PE/CA-PJ49 cells was amplified by either CisPt (a; P=0.001) or CRM (b; P=0.0005) treatment compared to untreated cells. The combined treatment of CisPt and CRM amplified the expression of phospho-p53 (c; P=0.0002) compared to control, but the effect of the two agents was not additive (Fig. 5A).
In order to analyze the role of protein kinase ERK1/2 in the process of p53 phosphorylation, the HNSCC tumor cells were pretreated with 25 µM PD98059 (specific inhibitor of ERK1/2) for 2 h. Then, the cells were treated with CisPt and/or CRM for another 24 h.
As Fig. 5B depicts the presence of PD98059 inhibitor did not significantly influence the level of total p53 expression (P>0.05). Untreated FaDu cells in the presence of PD98059 inhibitor expressed phospho-p53 in 22%, while in the cells treated with CRM in 16% (b; P=0.03). In the case of cells treated with CisPt alone the process of phosphorylation seems to be less affected by the presence of PD98059, the phosphorylated form of protein p53 being expressed in 65% (a; P=0.0007). The combined CisPt and CRM treatment in the presence of ERK1/2 inhibitor led to the decrease of the expression of phosphorylated p53 to 42% (c; P=0.0004) (Fig. 5B).
In the PE/CA-PJ49 untreated cells, the presence of PD98059 reduced the phospho-p53 expression to 53% (Fig. 5B). The presence of the specific ERK1/2 inhibitor significantly affected the level of p53 phosphorylation induced by either CisPt (30%) (a; P=0.007) or CRM (27%) (b; P=0.005) treatment. The combined CisPt and CRM treatment in the presence of PD98059 kept the phosphorylated form of protein p53 to 30% (c; P=0.007) since the effect of the two agents was not additive (Fig. 5B).
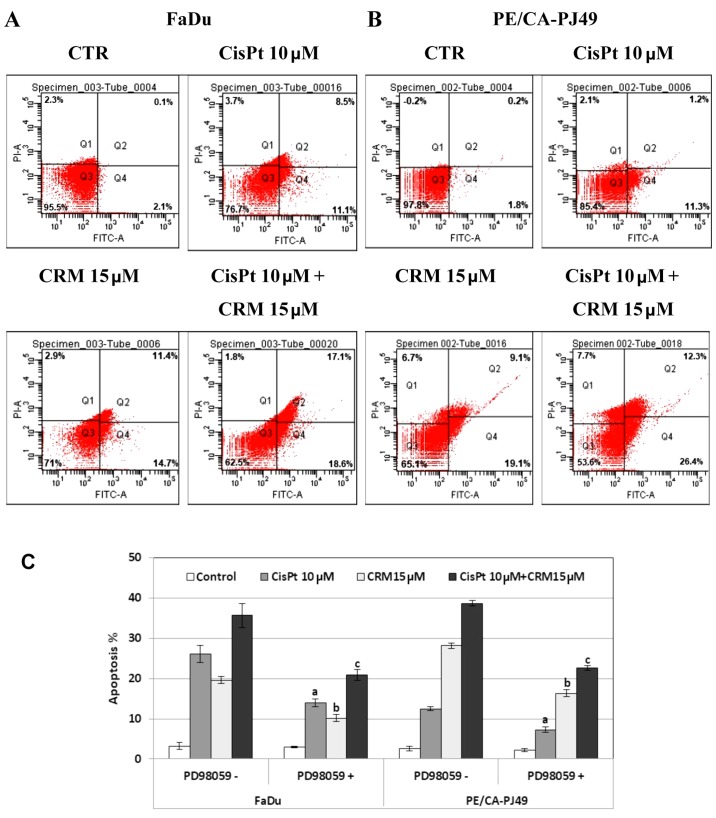
ERK1/2 in the modulation of HNSCC apoptosis induced by CisPt and/or CRM treatment
FaDu and PE/CA-PJ49 were treated for 24 h with 10 µM CisPt, 15 µM CRM, combined treatment and compared to control cells (untreated). CisPt treated FaDu cells had 9 times enhancement of the apoptotic events (19.6%) compared to untreated cells (2.2%). CRM treated FaDu cells had a 12 times higher apoptosis (26.1%) than untreated cells. The combined treatment of CisPt and CRM induced 35.7% apoptosis. This indicates a 16 times higher apoptosis than in the untreated cells. Moreover, apoptosis was almost twice higher (1.8×) than the value obtained for CisPt treatment alone. This indicates that CRM amplified the apoptotic process and supported the tumoricidal effect of CisPt (Fig. 6A).
Figure 6.
Apoptosis of HNSCC cells treated with CisPt and/or CRM. Apoptosis of FaDu (A) and PE/CA-PJ49 (B) cells treated with CisPt and/or CRM in the presence or absence of the PD98059 inhibitor (C). a, CisPt PD98059(−) vs. CisPt PD98059(+); b, CRM PD98059(−) vs. CRM PD98059(+); c, CisPt+CRM PD98059(−) vs. CisPt+CRM PD98059(+).
CisPt treatment of PE/CA-PJ49 cells induced a 6× higher apoptosis (12.5%) compared to untreated cells (2.0%). In the same manner, apoptosis induced by CRM alone (28.8%) or combined treatment of CisPt and CRM (38.7%) was 14× and respectively 19× higher compared to untreated cells. CRM significantly amplified the effect induced by CisPt treatment on the PE/CA-PJ49 apoptotic process since apoptosis was 3× higher than the value obtained for CisPt treatment (Fig. 6B).
Our data support the CRM apoptosis inducer capabilities considering that CRM had the capacity to induce apoptosis in both tumor lines. Also, CRM potentiated the effect induced by CisPt treatment.
In order to analyze the role of ERK1/2 protein kinase in the modulation of the apoptotic process, HNSCC tumor cells were pretreated for 2 h with 25 µM ERK1/2 specific inhibitor, PD98059. Then the cells were treated for 24 h with CisPt and/or CRM, and subjected to flow cytometric analysis of the apoptotic process. As shown in Fig. 6C, the PD98059 inhibition of ERK1/2 protein kinase led to a decrease of the apoptotic cell percentage in both cell lines compared to untreated cells. The decreased was statistically significant for all treatments as follows: FaDu cells (a) CisPt treated P=0.007; (b) CRM treated P=4.4E-05; (c) combined treatment; P=0.004; PE/CA-PJ49 cells (a) CisPt treated P=0.006; (b) CRM treated P=5.1E-05; (c) combined treatment P=0.0003. These results demonstrate the involvement of ERK1/2 protein kinase in the apoptotic mechanisms induced by CisPt and/or CRM on the analyzed tumor cells.
Discussion
The essential step during carcinogenesis of oral squamous cell carcinomas is the acquisition of genetic instability that occurs at the nucleotide or the chromosome level (48). The genotypic abnormalities such as polysomy of chromosome 17 may be associated with the development of an early recurrence or second primary tumors and can influence the therapy response (49). In this study the effect of CisPt and/or CRM treatment on two HNSCC tumor cell lines (FaDu and PE/CA-PJ49) were analyzed. One of the most frequent genetic abnormalities associated with HNSCC affects p53 onco-supressor gene. Abnormalities in the p53 gene cause an inefficient checkpoint system for the repair and destruction of mutant cells. The cell lines were analyzed using FISH analysis to detect the possible alterations of chromosome 17 involving the TP53 gene. PE/CA-PJ49 cell line had an amplification of the TP53 gene associated with polysomy in chromosome 17. FaDu tumor line presented a deletion in gene TP53, without chromosome 17 modifications. This data can explain the different response of each cell line to CisPt and/or CRM treatment.
Genetic modifications, as well as p53 protein expression alterations could influence the activation of several intracellular signaling pathways, such as ERK1/2 and therefore it could contribute to the modulation of the response to therapy. In many studies, the ERK signaling pathway is associated with proliferative (50) and cellular differentiation processes (51) on one hand, and on the other hand, there are studies showing that this pathway has a role in the apoptotic process (52–54). This suggests its importance in the modulation of the response to antitumor therapy (55). The activation status of ERK1/2 in HNSCC cell lines was evaluated to establish if the cellular response to CisPt and/or CRM treatment is influenced by the level of ERK1/2 activation. Expression of the total and phosphorylated ERK1/2 protein was quantified in treated and untreated cells. The data show that the total and phosphorylated ERK1/2 protein expression is much higher in untreated PE/CA-PJ49 than in FaDu cells. FaDu cell line treated with CisPt and/or CRM did not show a significant change of ERK1/2 phosphorylation. A significant decrease of phospho ERK1/2 was observed in PE/CA-PJ49 cells treated with CisPt and CRM. The constitutively expressed activated ERK1/2 protein-kinase was different in the two tumor cell lines. This was reflected in downstream events such as the cell proliferation process. The inhibition of ERK1/2 activity using PD98059 did not significantly affect the proliferation of the cells (e.g. FaDu) having a low expression of the protein. Cells with a constitutively high expression of ERK1/2 protein-kinase (PE/CA-PJ49) responded to the presence of the inhibitor by reversing the proliferative process. This suggests the existence of an ERK1/2 protein-kinase activation threshold which modulates the proliferative process of tumor cells.
How p53 responds to the drug treatment of tumor cells having a different expression pattern of ERK1/2 was evaluated. In untreated cells, the expression of total and phosphorylated p53 was higher in FaDu compared to PE/CA-PJ49 cells (Fig. 5A). This difference can be associated with the amplification of p53 gene in PE/CA-PJ49 cells and the p53 deletion in tumor cells FaDu (Fig. 1). CRM treatment induced an increase of the total p53 protein expression associated with an increase of the phosphorylation process in both cell lines. The response of FaDu cells to CisPt treatment led to a decrease of the phosphorylation process without affecting the expression of total p53 protein, while CisP induced a significant increase of p53 phosphorylation in PE/CA-PJ49 tumor cells (Fig. 5A and B).
The response of FaDu CRM treated cells in the presence of ERK1/2 inhibitor PD98059 suggest that the ERK1/2 might be involved in the p53 phosphorylation. The response of FaDu CisPt treated cells to the presence of the same inhibitor suggests that p53 upstream events might follow a different pathway than ERK1/2. Regardless of the treatment of PE/CA-PJ49 cells, phosphorylation of p53 involved ERK1/2 activation. Moreover, the results support the involvement of ERK1/2 and phospho p53 in CisPt and/or CRM induced apoptosis. Many studies support the antitumor and pro-apoptotic effect of CRM (56–58). It is not well known if the effect of CRM is due to the existence of a link between the activation level of ERK1/2 and the apoptotic process. The results of our study show that CRM increased the apoptotic process in both tumor cell lines. CRM acts as an apoptosis-inducer factor and more importantly potentiates the effect induced by CisPt treatment (Fig. 6).
Conclusions and future directions. The results showed that the tumor cell line FaDu presented a deletion of the TP53 gene, while cell line PE-CA/PJ49 presented polysomy. Both modifications are associated with cell proliferation and response to therapy. The use of an adjuvant (CRM) can increase the efficiency of chemotherapy (CisPt) effect by modulating cell activation processes such as ERK1/2 phosphorylation. The presence of the adjuvant can decrease the required dose of drug, therefore reducing the chemotherapeutic adverse reactions of the agent.
The data show that the ERK1/2 way of action either on cell proliferation or apoptosis depends on the type of cell characteristics and therapeutic agents. In order to achieve an efficient personalized therapy, more investigations are necessary for a better functional interpretation of the intracellular signaling pathways.
In conclusion, evaluating the level of ERK1/2 activation in tumor cells can be a useful tool for an individualized treatment plan, but in order to achieve this goal extended investigations on patient tumor specimens are needed.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CisPt
cisplatin
- CRM
curcumin
- ERK1/2
extracellular signal-regulated kinase
- FISH
fluorescence in situ hibridization
- PD98059
(2′-amino-3′-methoxyflavone)
- MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
- DMSO
dimethyl sulfoxide
- EDTA
etylenediaminetetraacetic acid
- PBS
phosphate-buffered saline
- FaDu
human pharynx squamous cell carcinoma
- PE/CA-PJ49
human tongue squamous cell carcinoma
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- TP53
the tumor suppressor gene TP53
- DAPI II
4′,6-Diamidino-2-phenylindole
- SSC buffer
the saline-sodium citrate buffer
- NP-40
detergent solution
- FITC
fluorescein isothiocyanate
- PI
propidium iodide
- PMSF
phenylmethylsulfonyl fluoride
- ELISA
enzyme-linked immunosorbent assay.
Funding
This study was supported by Grants PN-III-P1-1.2-PCCDI-2017-0341/2018, PN-III-P1-1.2-PCCDI-2017-0782/2018, PN 19.29.01.01, and by Ministry of Research and Innovation in Romania, under Program 1 - The Improvement of the National System of Research and Development, Subprogram 1.2 - Institutional Excellence - Projects of Excellence Funding in RDI, contract no. 7PFE/16.10.2018.
Availability of data and materials
The data sets used and/or analysed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
MB and MTN, designed the research, data acquisition, analysis and interpretation of data, and wrote the manuscript. MB, MM and GGPM performed the experiments, data acquisition, analysis and interpretation of data, statistical analysis and manuscript drafting. VR, GI, LIB, RH, NR and CC contributed to data collection and interpretation, statistical analysis, critical revision of the manuscript for important intellectual content. All the authors read and approved the final manuscript, and had equal contribution.
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of ‘Stefan S. Nicolau’ Virology Institute.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ursu RG, Danciu M, Spiridon IA, Ridder R, Rehm S, Maffini F, McKay-Chopin S, Carreira C, Lucas E, Costan VV, et al. Role of mucosal high-risk human papillomavirus types in head and neck cancers in Romania. PLoS One. 2018;13:e0199663. doi: 10.1371/journal.pone.0199663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R, Ferlay J, editors. X. International Agency for Research on Cancer; Lyon: 2014. Cancer Incidence in Five Continents Vol. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Kumar M, Nanavati R, Modi TG, Dobariya C. Oral cancer: Etiology and risk factors: A review. J Cancer Res Ther. 2016;12:458–463. doi: 10.4103/0973-1482.186696. [DOI] [PubMed] [Google Scholar]
- 5.Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123–141. doi: 10.1016/j.coms.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, et al. EORTC 24971/TAX 323 Study Group Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez L, Jayakar SK, Ow TJ, Segall JE. Mechanisms of invasion in head and neck cancer. Arch Pathol Lab Med. 2015;139:1334–1348. doi: 10.5858/arpa.2014-0498-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voiculescu VM, Caruntu C, Solomon I, Lupu M, Ilie MA, Boda D, Constantin C, Neagu M. Squamous cell carcinoma: Biomarkers and potential therapeutic targets. In: Blumenberg M, editor. Human Skin Cancers - Pathways, Mechanisms, Targets and Treatments. IntechOpen; London: 2018. pp. 135–159. [Google Scholar]
- 9.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 10.Zhai TT, van Dijk LV, Huang BT, Lin ZX, Ribeiro CO, Brouwer CL, Oosting SF, Halmos GB, Witjes MJH, Langendijk JA, et al. Improving the prediction of overall survival for head and neck cancer patients using image biomarkers in combination with clinical parameters. Radiother Oncol. 2017;124:256–262. doi: 10.1016/j.radonc.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Lupu M, Caruntu A, Caruntu C, Boda D, Moraru L, Voiculescu V, Bastian A. Non-invasive imaging of actinic cheilitis and squamous cell carcinoma of the lip. Mol Clin Oncol. 2018;8:640–646. doi: 10.3892/mco.2018.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boda D, Docea AO, Calina D, Ilie MA, Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE, Voiculescu V, et al. Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review) Int J Oncol. 2018;52:637–655. doi: 10.3892/ijo.2018.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. BioMed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Neagu M, Constantin C, Popescu ID, Zipeto D, Tzanakakis G, Nikitovic D, Fenga C, Stratakis CA, Spandidos DA, Tsatsakis AM. Inflammation and metabolism in cancer cell - mitochondria key player. Front Oncol. 2019;9:348. doi: 10.3389/fonc.2019.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cagnol S, Chambard JC. ERK and cell death: Mechanisms of ERK-induced cell death - apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Zhang J, Shi C, Liu L, Wei Y. Survival, recurrence and toxicity of HNSCC in comparison of a radiotherapy combination with cisplatin versus cetuximab: A meta-analysis. BMC Cancer. 2016;16:689. doi: 10.1186/s12885-016-2706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neagu M, Caruntu C, Constantin C, Boda D, Zurac S, Spandidos DA, Tsatsakis AM. Chemically induced skin carcinogenesis: Updates in experimental models (Review) Oncol Rep. 2016;35:2516–2528. doi: 10.3892/or.2016.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kass JI, Moskowitz HS, Grandis JR. Multimodality Management. 2nd. Springer International Publishing; New York, NY: 2016. Oncogenomics/Proteomics of Head and Neck Cancers. Head and Neck Cancer; pp. 101–114. [Google Scholar]
- 19.Chen L, Tweddle DA. p53, SKP2, DKK3 as MYCN target genes and their potential therapeutic significance. Cancer Molecular Targets and Therapeutic. Front Oncol. 2012;2:173. doi: 10.3389/fonc.2012.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boda D. Cellomics as integrative omics for cancer. Curr Proteomics. 2013;10:237–245. doi: 10.2174/1570164611310030006. [DOI] [Google Scholar]
- 21.Powell E, Piwnica-Worms D, Piwnica-Worms H. Contribution of p53 to metastasis. Cancer Discov. 2014;4:405–414. doi: 10.1158/2159-8290.CD-13-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat Rev Drug Discov. 2014;13:928–942. doi: 10.1038/nrd4281. [DOI] [PubMed] [Google Scholar]
- 23.Solomon I, Voiculescu VM, Caruntu C, Lupu M, Popa A, Ilie MA, Albulescu R, Caruntu A, Tanase C, Constantin C, et al. Neuroendocrine factors and head and neck squamous cell carcinoma: An affair to remember. Dis Markers. 2018;2018:9787831. doi: 10.1155/2018/9787831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang SH, Sharrocks AD, Whitmarsh AJ. MAP kinase signalling cascades and transcriptional regulation. Gene. 2013;513:1–13. doi: 10.1016/j.gene.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Lupu M, Caruntu A, Caruntu C, Papagheorghe LM, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki M, et al. Neuroendocrine factors: The missing link in non melanoma skin cancer (Review) Oncol Rep. 2017;38:1327–1340. doi: 10.3892/or.2017.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: A new perspective. Cancer. 2014;120:3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urosevic J, Nebreda AR, Gomis RR. MAPK signaling control of colon cancer metastasis. Cell Cycle. 2014;13:2641–2642. doi: 10.4161/15384101.2014.946374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanase CP, Neagu M, Albulescu R. Key signaling molecules in pituitary tumors. Expert Rev Mol Diagn. 2009;9:859–877. doi: 10.1586/erm.09.60. [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Huang CZ. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol. 2015;21:11673–11679. doi: 10.3748/wjg.v21.i41.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng Q, Deng Z, Pan H, Gu L, Liu O, Tang Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol Lett. 2018;15:1379–1388. doi: 10.3892/ol.2017.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumari R, Chouhan S, Singh S, Chhipa RR, Ajay AK, Bhat MK. Constitutively activated ERK sensitizes cancer cells to doxorubicin: Involvement of p53-EGFR-ERK pathway. J Biosci. 2017;42:31–41. doi: 10.1007/s12038-017-9667-8. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Luo L, Wang D, Guo B, Li J, Yang Z, Tang D. Combination adjuvant chemotherapy with targeted drugs for treatment of colorectal cancer: A network meta-analysis. J Cell Biochem. 2018;119:1521–1537. doi: 10.1002/jcb.26312. [DOI] [PubMed] [Google Scholar]
- 33.Bailon-Moscoso N, Cevallos-Solorzano G, Romero-Benavides JC, Orellana MI. Natural compounds as modulators of cell cycle arrest: Application for anticancer chemotherapies. Curr Genomics. 2017;18:106–131. doi: 10.2174/1389202917666160808125645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seca AM, Pinto DC. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int J Mol Sci. 2018;19:E263. doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP, Sethi G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules. 2015;20:2728–2769. doi: 10.3390/molecules20022728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miao Y, Zhao S, Gao Y, Wang R, Wu Q, Wu H, Luo T. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res Bull. 2016;121:9–15. doi: 10.1016/j.brainresbull.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Shi D, Xu Y, Du X, Chen X, Zhang X, Lou J, Li M, Zhuo J. Co-treatment of THP-1 cells with naringenin and curcumin induces cell cycle arrest and apoptosis via numerous pathways. Mol Med Rep. 2015;12:8223–8228. doi: 10.3892/mmr.2015.4480. [DOI] [PubMed] [Google Scholar]
- 38.Patel PB, Thakkar VR, Patel JS. Cellular effect of curcumin and citral combination on breast cancer cells: Induction of apoptosis and cell cycle arrest. J Breast Cancer. 2015;18:225–234. doi: 10.4048/jbc.2015.18.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neagu M, Constantin C, Tanase C, Boda D. Patented biomarker panels in early detection of cancer. Recent Pat Biomark. 2011;1:10–24. doi: 10.2174/2210310411101010010. [DOI] [Google Scholar]
- 40.Chen XY, Cai HZ, Wang XY, Chen QY, Yang H, Chen YJ, Tang YP. Application of the ERK signaling pathway inhibitor PD98059 in long-term in vivo experiments. Genet Mol Res. 2015;14:18325–33. doi: 10.4238/2015.December.23.20. [DOI] [PubMed] [Google Scholar]
- 41.Barltrop JA, Owen TC, Cory AH, Cory JG. 5-(3-carboxymethoxyphenyl)-2-(4,5-dimenthylthiazoly)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg Med Chem Lett. 1991;1:611–614. doi: 10.1016/S0960-894X(01)81162-8. [DOI] [Google Scholar]
- 42.Petrică-Matei GG, Iordache F, Hainăroşie R, Bostan M. Characterization of the tumor cells from human head and neck cancer. Rom J Morphol Embryol. 2016;57(Suppl 2):791–799. [PubMed] [Google Scholar]
- 43.Hirshberg A, Yarom N, Amariglio N, Yahalom R, Adam I, Stanchescu R, Ben-Dov I, Taicher S, Rechavi G, Trakhtenbrot L. Detection of non-diploid cells in premalignant and malignant oral lesions using combined morphological and FISH analysis - a new method for early detection of suspicious oral lesions. Cancer Lett. 2007;253:282–290. doi: 10.1016/j.canlet.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Salido M, Tusquets I, Corominas JM, Suarez M, Espinet B, Corzo C, Bellet M, Fabregat X, Serrano S, Solé F. Genetic alterations of chromosome 17 in human breast carcinoma studied by fluorescence in situ hybridization and molecular DNA techniques. Breast Cancer Res. 2005;7:267–273. doi: 10.1186/bcr996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sunahori K, Nagpal K, Hedrich CM, Mizui M, Fitzgerald LM, Tsokos GC. The catalytic subunit of protein phosphatase 2A (PP2Ac) promotes DNA hypomethylation by suppressing the phosphorylated mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)/phosphorylated ERK/DNMT1 protein pathway in T-cells from controls and systemic lupus erythematosus patients. J Biol Chem. 2013;288:21936–21944. doi: 10.1074/jbc.M113.467266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bundscherer A, Malsy M, Lange R, Hofmann P, Metterlein T, Graf BM, Gruber M. Cell harvesting method influences results of apoptosis analysis by Annexin V staining. Anticancer Res. 2013;33:3201–3204. [PubMed] [Google Scholar]
- 47.Zedan W, Mourad MI, El-Aziz SMA, Salamaa NM, Shalaby AA. Cytogenetic significance of chromosome 17 aberrations and P53 gene mutations as prognostic markers in oral squamous cell carcinoma. Diagn Pathol. 2015;10:2. doi: 10.1186/s13000-015-0232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin C, Jin Y, Wennerberg J, Akervall J, Dictor M, Mertens F. Karyotypic heterogeneity and clonal evolution in squamous cell carcinomas of the head and neck. Cancer Genet Cytogenet. 2002;132:85–96. doi: 10.1016/S0165-4608(01)00535-0. [DOI] [PubMed] [Google Scholar]
- 49.Papavasileiou D, Tosios K, Christopoulos P, Goutas N, Vlachodimitropoulos D. Her-2 immunohistochemical expression in oral squamous cell carcinomas is associated with polysomy of chromosome 17, not Her-2 amplification. Head Neck Pathol. 2009;3:263–270. doi: 10.1007/s12105-009-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L, Liu T, Nishioka M, Aguirre RL, Win SS, Okada N. Activation of ERK1/2 and cyclin D1 expression in oral tongue squamous cell carcinomas: Relationship between clinicopathological appearances and cell proliferation. Oral Oncol. 2006;42:625–631. doi: 10.1016/j.oraloncology.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Chambard JC, Lefloch R, Pouysségur J, Lenormand P. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Aguzzi A, Maggioni D, Nicolini G, Tredici G, Gaini RM, Garavello W. MAP kinase modulation in squamous cell carcinoma of the oral cavity. Anticancer Res. 2009;29:303–308. [PubMed] [Google Scholar]
- 53.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Engelbrecht AM, Gebhardt S, Louw L. Ex vivo study of MAPK profiles correlated with parameters of apoptosis during cervical carcinogenesis. Cancer Lett. 2006;235:93–99. doi: 10.1016/j.canlet.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Squatrito M, Brennan CW, Helmy K, Huse JT, Petrini JH, Holland EC. Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell. 2010;18:619–629. doi: 10.1016/j.ccr.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasima N, Aggarwal BB. Cancer-linked targets modulated by curcumin. Int J Biochem Mol Biol. 2012;3:328–351. [PMC free article] [PubMed] [Google Scholar]
- 57.Baharuddin P, Satar N, Fakiruddin KS, Zakaria N, Lim MN, Yusoff NM, Zakaria Z, Yahaya BH. Curcumin improves the efficacy of cisplatin by targeting cancer stem-like cells through p21 and cyclin D1-mediated tumour cell inhibition in non-small cell lung cancer cell lines. Oncol Rep. 2016;35:13–25. doi: 10.3892/or.2015.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borges GÁ, Rêgo DF, Assad DX, Coletta RD, De Luca Canto G, Guerra EN. In vivo and in vitro effects of curcumin on head and neck carcinoma: A systematic review. J Oral Pathol Med. 2017;46:3–20. doi: 10.1111/jop.12455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analysed during the present study are available from the corresponding author on reasonable request.