Abstract
Background
MicroRNAs (miRNAs), which modulate the expression of their target genes, are commonly involved in stimulating and adjusting of many processes that result in cardiovascular diseases, contain cardiac ischemia/reperfusion (I/R) damage. However, the expression and role of miR-149 in pyroptosis mediated myocardial I/R damage remains unclear.
Material/Methods
Real-time polymerase chain reaction was performed to measure the miR-149 and FoxO3 expression in I/R stimulated H9C2 cells. The cell proliferation, pyroptosis-related inflammatory genes in I/R-treated H9C2 cells transfected miR-149 mimics or miR-149 inhibitor were both explored. We predicted and confirmed miR-149 targets by using bioinformatics analyses and luciferase reporter assay. In addition, the potential relationship between miR-149 and FoxO3 in pyroptosis from I/R treated H9C2 cells was analyzed.
Results
Our results showed that miR-149 was upregulated, while FoxO3 was downregulated in I/R stimulated H9C2 cells. Over-expression of miR-149 inhibited cell viability and promote pyroptosis, however, down-expression of miR-149 had an opposite effect in I/R treated H9C2 cells. Furthermore, miR-149 could negatively regulate FoxO3 expression by binding 3′UTR, whereas silencing of FoxO3 attenuated the effect of miR-149-mimics on cell proliferation and pyroptosis in I/R treated H9C2 cells.
Conclusions
Our study found that miR-149 played a critical role in pyroptosis during cardiac I/R injury, and thus, might provide a novel therapeutic target.
MeSH Keywords: MicroRNAs, Myocardial Contraction, Reperfusion Injury
Background
Ischemia caused by the interruption of heart blood flow lead to significant damage to myocardial cells, contrary to this, subsequent reperfusion also activates various injury responses and further tissue damage; these injuries are referred to as ischemia-reperfusion (I/R) injury [1]. Ischemic heart disease has been one of the worldwide health problems accompanied by high morbidity and mortality in recent years [2]. After myocardial ischemia, myocardial I/R injury brings about adverse cardiovascular outcomes including myocardial systolic dysfunction, cardiac electrophysiology disorder, and myocardial necrosis [3], as well as leading to direct destruction of myocardial cells [4]. Therefore, it is essential to explore myocardial I/R injury in detail in order to provide effective treatment strategies.
MicroRNAs (miRNAs) are a group of small non-coding single-stranded RNA molecules with 18–25 nucleotides, acting as negative regulators of gene expression by restraining the translation of mRNA and/or encouraging mRNA degradation by complementary base-pairing with 3-untranslated regions (3-UTRs) [5]. Recently, miRNAs have been recognized as a key regulator in the occurrence and development of diversified cardiovascular diseases [6], such as myocardial hypertrophy [7], coronary atherosclerosis [8], and acute myocardial infarction [9]. Accordingly, numerous studies focus on miRNAs as having a pivotal role in the myocardial functions such as contraction and morphogenesis [10], as well as being involved in the progression of myocardial I/R damage [11]. For example, Wang et al. declared that miR-125 was able to protect the myocardium from I/R damage by preventing p53-mediated apoptotic signal pathway and inhibiting TRAF6-mediated NF-κB activation [12]. Liu et al. reported that knockdown of miR-27 promoted cardioprotective effect on relieving myocardial I/R damage via ABCA1 and NF-κB signaling pathway in both cellular and animal models [13]. MiR-149 has been well characterized in many cardiovascular diseases including myocardial infarction [14], congenital heart disease [15], and coronary heart disease [16], of which I/R injury played a key role in relevant pathological processes. Additionally, the miR-149 polymorphisms were previously verified to be associated with ischemic stroke pathogenesis, which also might be involved in progression of I/R damage [17]. However, the special role and the underlying mechanism of miR-149 during the development of cardiac I/R injury was still unclear.
Pyroptosis is a pro-inflammatory programmed cell death, associated with the release of cytokines such as interleukin-1β (IL-1β) and interleukin-18 (IL-18), which recruit inflammatory cells and expand the inflammatory response[18]. The abnormal inflammatory reactions can further lead to many pathological mechanisms including oxidative stress damage, ultimately leading to organ damages [19]. NLR family pyrin domain containing 3 (NLRP3) inflammasome is recently recognized as a multiprotein complex consisting of NLRP3, apoptosis-associated speck-like protein containing a caspase-1 recruitment domain (ASC) and caspase-1, and the latter also have been investigated as a vital enzyme to the biological process during pyroptosis [20]. The cell formation was simulated by caspase-1 activation, which subsequently resulted in production and releasing of pyroptosis related inflammatory factors [21]. Many studies reported that pyroptosis was participated in multiple pathophysiological processes and diseases, including atherosclerosis [21], epilepsy [22], and Alzheimer’s disease [23], however, only a few articles have reported its role in process of myocardial I/R injury.
In the present study, we tried to explore the function and possible mechanisms of miR-149 on regulation during myocardial I/R damage. As expected, our findings supposed that miR-149 aggravated cell pyroptosis by targeting forkhead box O3 (FoxO3), describing a new pathogenesis in myocardial I/R damage.
Material and Methods
Cell culture and I/R treatment
Myocardial cell line H9C2 was obtained from Institute of Biochemistry and Cell Biology of Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China) and incubated in RPMI-1640 medium (Gibco, USA) containing 10% heat-inactivated fetal calf bovine serum (FBS, Gibco, USA) in 5% CO2 at 37°C. The cells were first exposed to hypoxia for 2 hours and then aerobically cultured for 12 hours to establish the I/R cell model.
For cell transfection, miR-149 mimics, miR-149 inhibitor, scramble and si-FoxO3 was all obtained from Ribobio (Guangzhou, China). Then 50 nM miR-149 mimics, 50 nM miR-149 inhibitor, or 100 nM si-FoxO3 were transfected into H9C2 by employing Lipofectamine 2000 (Invitrogen, USA) following manufacturer’s specification. After 4 8-hour transfection, the cells were harvested for further analysis.
Cell viability assay
In briefly, the transfected H9C2 cells were first seeded in 96-well plates (5×103cells/well) and then incubated with Cell Counting Kit-8 (CCK8) solution (10 μL/well, Sigma, USA) for an additional 2 hours at the end of the indicated time. Next, a microplate reader (Molecular Devices, USA) was utilized to measure the absorbance at 450 nm.
Detection content of superoxide dismutase (SOD) activity and malondialdehyde (MDA)
The superoxide dismutase (SOD) activity and malondialdehyde (MDA) content were measured in I/R treated-H9C2 cells for evaluating oxidative stress. The concentration of MDA and SOD activity was detected with MDA assay kit (Beyotime, China) and Total Superoxide Dismutase Assay Kit (Beyotime, China) respectively.
Western blot
The cells were first lysed with ice-cold RIPA buffer (Beyotime, China). Next, the extracts was transferred to the polyvinylidene difluoride (PVDF) membranes (Millipore, USA) following by blocking 5% non-fat milk powder and then incubated with the primary antibodies as follow: anti-NLRP3 (1: 1000, Abcam, UK), anti-caspase 1 (1: 1000, Abcam, UK), anti-IL-1β (1: 1000, Abcam, UK), anti-IL-18 (1: 1000, Abcam, UK), anti-FoxO3 (1: 1000, Abcam, UK), and anti-GAPDH (1: 5000, Abcam, UK) overnight at 4°C. Thereafter, the secondary antibody conjugated with horseradish peroxidase (Abcam, UK) was added into the solutions and incubated for 1 hour. Chemiluminescent detection was performed using an enhanced chemiluminescence (ECL) system (GE Healthcare, USA) and analyzed by ImageJ software (Rawak, Germany). All the blot intensities were normalized with that of loading control GAPDH.
Quantitative real-time quantitative polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells using the TRIzol® Reagent (Invitrogen, USA) and reversed transcribed to cDNA using Transcriptor First Strand cDNA Synthesis Kit (Roche, Shanghai). The amplification consisted of a real-time polymerase chain reaction (RT-PCR) performed on a CFX96 RT-PCR system (Biorad, Hemel Hempstead, UK) using SYBR Green Realtime kit (Takara, Japan) according to manufacturer’s specification. U6 and GAPDH were used as an endogenous control. The primers are shown in Table 1.
Table 1.
Primer sequences for RT-PCR analysis.
| cDNA | Forward primer | Reverse primer |
|---|---|---|
| miR-149 | 5′-CCCTCACTTCTGTGCCAC-3′ | 5′- GTGCAGGGTCCGAGGT-3′ |
| FoxO3 | 5′- CTGGACGGAGTAGCTCCA AG-3′ | 5′ - TCAGCATCTCCTCGGTCTCT-3′ |
| NLRP3 | 5′-GAAAACGGATCCAGATGAAGATGGCA-3′ | 5′-TTCCACTCGAGCCAAGAAGGCTCAAAGACG-3′ |
| Caspase-1 | 5′- ATCCACGAGCAGAGTCAAAG-3′ | 5′- CCTGGTCAACTGTCACAAAAC-3′ |
| IL-1β | 5′-CCTTGTCGAGAATGGGCAGT- 3′ | 5′- TTCTGTCGACAATGCTGCCT- 3′ |
| IL-18 | 5′-TACTACCGAAACTTGGACC -3′ | 5′-CTCAAACTACCTTCTCCG- 3′ |
| U6 | 5′-CTCGCTTCGGCAGCACA-3′ | 5′-AACGCTTCACGAATTTGCGT-3′ |
| β-actin | 5′-ACAACTTTGGTATCGTGGAAGG-3′ | 5′-GCCATCACGCCACAGTTTC-3′ |
RT-PCR – real-time polymerase chain reaction; IL – interleukin.
Immunofluorescence staining
Briefly, the transfected H9C2 cells were rinsed with phosphate-buffered saline (PBS) and then fixed with 4% paraformaldehyde for 25 minutes at 37°C. After adding blocking solution (1% bovine serum albumin and 0.1% Triton-X in PBS), the cells were incubated with primary antibodies against FoxO3 (1: 500, Abcam, UK) and caspase-1 (1: 500, Abcam, UK) overnight at 4°C. Subsequently, the cells were inoculated with corresponding secondary antibodies (1: 200, Abcam, UK) at room temperature for 2 hours, whereas nuclei were detected by DAPI (4′,6-diamidino-2-phenylindole) stain (Sigma-Aldrich, USA). Immunofluorescence was captured by using fluorescence microscope (LSM 700; Carl Zeiss GmbH; Germany).
Luciferase assay
We first predicted that the miR-149 could act on the 3′-untranslated region (UTR) of FoxO3 and influence its biological activity using biochemical information database. Next, we cloned and amplified wild-type (WT) or mutant (MUT) segments of the into luciferase reporter plasmids (Promega, USA). Then, the cells were co-transfected with luciferase reporter plasmids and miR-149 mimics or miR-149 inhibitors using Lipofectamine 2000 (Invitrogen; USA). After 48-hour transfection, the luciferase activities of the cells were measured using a luciferase assay kit (Promega, USA) following manufacturer’s guidance.
Statistical analysis
All data are presented as the mean±standard deviation (SD) pattern and all statistical analyses were implemented using SPSS software, version 22.0 (IBM, Inc., USA). The statistical methods including Student t-test for comparison of 2 groups and one-way ANOVA test for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
The pyroptosis happened in I/R-treated H9C2 cells
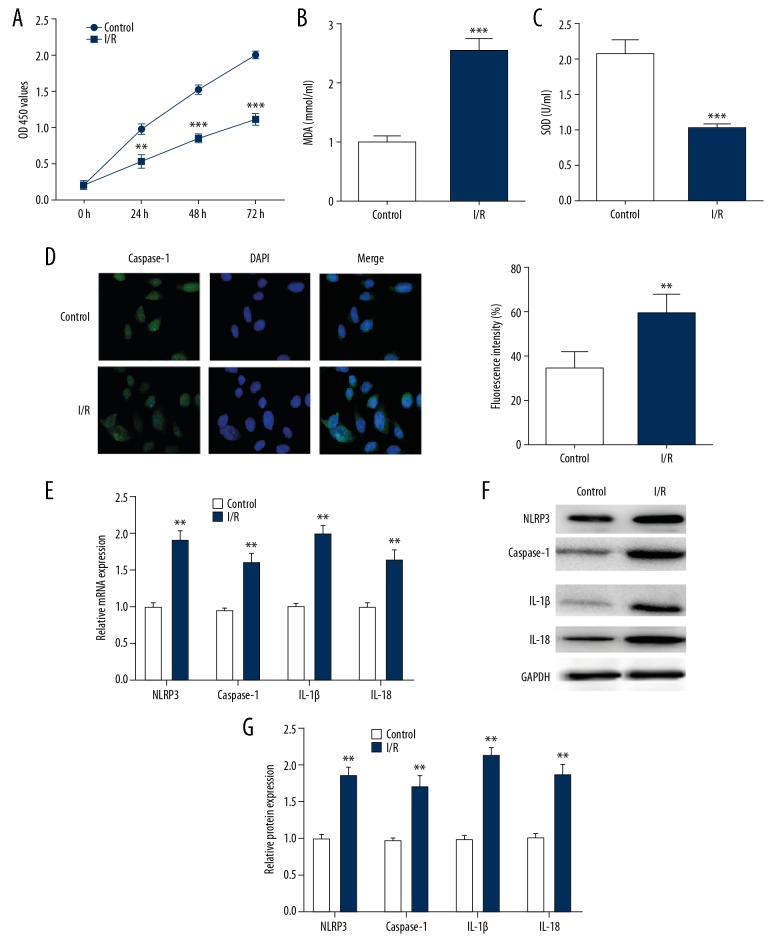
We investigated the influence of I/R damage on H9C2 cells. Our results showed cell viability and SOD were significantly decreased in H9C2 cells after I/R treatment compared with that of control cells (Figure 1A, 1C), whereas cell apoptosis marker MDA level was conspicuously increased in I/R group (Figure 1B). To further explore the effect of pyroptosis on myocardial I/R injury, we detected the caspase-1 expression in I/R treated H9C2 cells using immunofluorescence, which found the fluorescence intensity of caspase-1 was dramatically upregulated (Figure 1D). Additionally, the expression of NLRP3, caspase-1, IL-1β, and IL-18 were also remarkably ascending in I/R simulated H9C2 cells compared with controls (Figure 1E–1G). Collectively, our data indicated that pyroptosis might occur in H9C2 cells after I/R treatment.
Figure 1.
The pyroptosis happened in I/R-treated H9C2 cells. The cell proliferation (A), MDA content (B) and SOD activity (C) of the H9C2 cells were detected after I/R treatment. (D) The expression of caspase-1 in I/R-treated H9C2 cells which measured by immunofluorescence stain (E–G) The RT-PCR and western blotting were performed to detect mRNA and protein levels of NLRP3, caspase-1, IL-1β and IL-18. The data are presented as the mean±SD, n=3;** P<0.01 versus control. I/R – ischemia/reperfusion; MDA – malondialdehyde; SOD – superoxide dismutase; RT-PCR – real-time polymerase chain reaction; IL – interleukin; SD – standard deviation.
MiR-149 and FoxO3 were involved in myocardial I/R injure
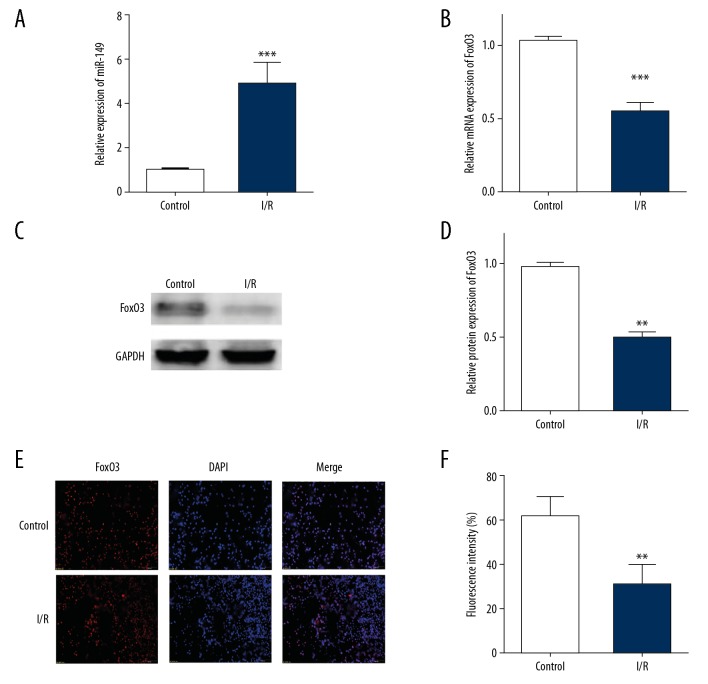
To investigate the effect of miR-149 and FoxO3 on the process of pyroptosis, we first measured their expression in I/R treated H9C2 cells. Compared to the control group, we found that miR-149 was significantly upregulated in I/R-treated H9C2 cells (Figure 2A). However, after I/R treatment in H9C2 cells, we observed an extreme decline of FoxO3a in mRNA and protein levels (Figure 2B–2D). Moreover, we affirmed the downregulation of FoxO3 in I/R-treated H9C2 cells by using immunofluorescence assay (Figure 2E). Therefore, we supposed that miR-149 and FoxO3 might participate in the process of myocardial I/R injury.
Figure 2.
MiR-149 and FoxO3 were involved in myocardial I/R injure. (A) Relative miRNA levels of miR-149a in H9C2 cells. (B–D) mRNA and protein levels of FoxO3 in H9C2 cells subjected to I/R by using RT-PCR and western blotting (E) The expression of FoxO3 in I/R-treated H9C2 cells measured by immunofluorescence stain. The data are presented as the mean±SD, n=3; ** P<0.01 versus control. I/R – ischemia/reperfusion; RT-PCR – real-time polymerase chain reaction; SD – standard deviation.
MiR-149 regulated pyroptosis in I/R-treated H9C2 cells
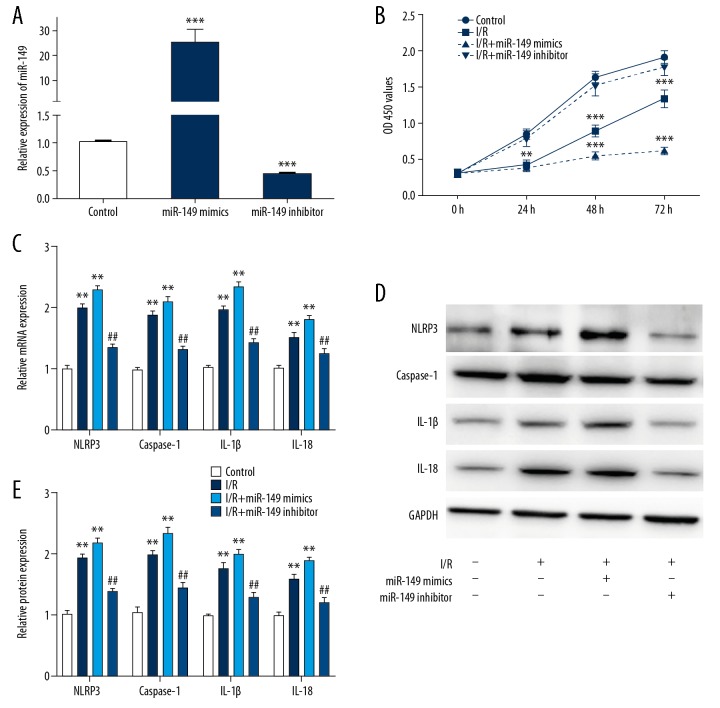
We verified the transfection efficiency of the miR-149 mimics and miR-149 inhibitor (Figure. 3A). Then we investigated cell proliferation in I/R-treated H9C2 cells transfected with miR-149 mimics or miR-149 inhibitor. As illustrated in Figure 3B, over-expression of miR-149 significantly suppressed cell growth, while knockdown of miR-149 had an opposite effect. Furthermore, we detected the pyroptosis and inflammatory related genes as NLRP3, caspase-1, IL-1β, and IL-18 in I/R-treated H9C2 cells transfected with miR-149 mimics or miR-149 inhibitor. Our results implied that NLRP3, caspase-1, IL-1β, and IL-18 were greatly upregulated by miR-149 mimics and downregulated by miR-149 inhibitor in both mRNA and protein levels (Figure 3C–3E). Taken together, we suggested that miR-149 might regulate pyroptosis in I/R-treated H9C2 cells.
Figure 3.
MiR-149 regulated pyroptosis in I/R-treated H9C2 cells. (A) The expression of miR-149 in cells transfected with miR-149-5p mimics or miR-149-5p inhibitor. (B) CCK-8 assay showing the cell proliferation in H9C2 cells during I/R treatment when transfected with miR-149mimics or miR-149 inhibitor. (C–E) The mRNA and protein levels of NLRP3, caspase-1, IL-1β, and IL-18 were detected by western blotting and RT-PCR in H9C2 cells transfected with miR-149mimics or miR-149 inhibitor. The data are presented as the mean±SD, n=3;** P<0.01 vs. control. ## P<0.01 versus I/R group. NC – negative control; I/R – ischemia/reperfusion; CCK-8 – Cell Counting Kit-8; RT-PCR – real-time polymerase chain reaction; IL – interleukin; SD – standard deviation.
MiR-149 targeted FoxO3 in I/R-treated H9C2 cells
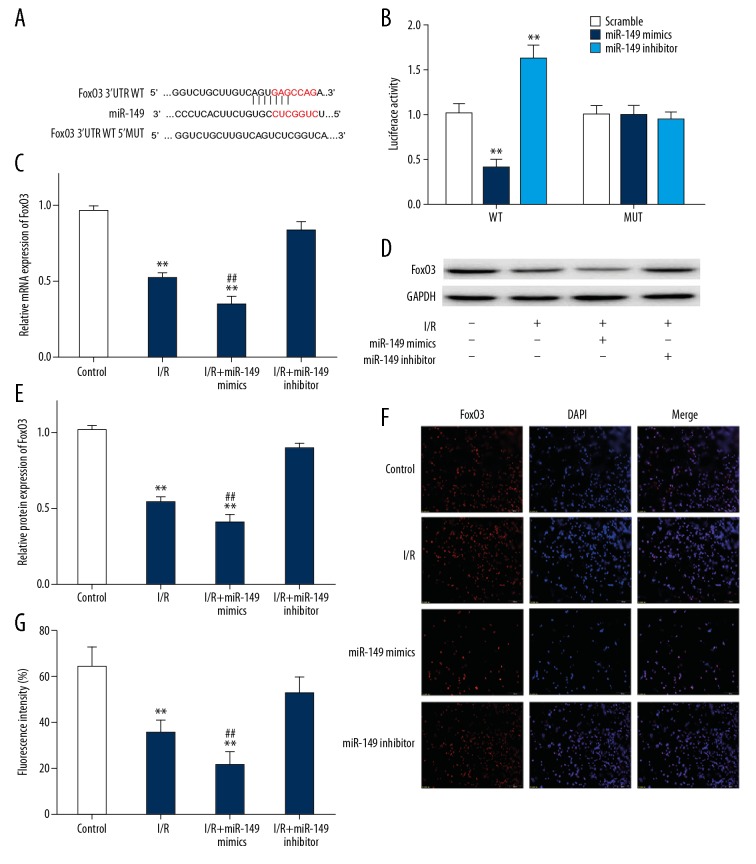
In order to detect the relationship between miR-149a and FoxO3a, we next carried out a series of experiments. We had predicted a conserved binding site for miR-149 at the 3′-UTR of the FoxO3 using bioinformatics analysis (Figure 4A). As illustrated in Figure 4B, luciferase assays showed that FoxO3 was a target gene of miR-149. To determine the effect of changes in miR-149 expression on FoxO3, we further measured the expression of FoxO3 in I/R-treated H9C2 cells which transfected miR-149 mimics or miR-149 inhibitor. Our data revealed that expression of FoxO3 was dramatically downregulated in I/R-treated H9C2 cells transfected miR-149 mimics, while the protein and mRNA levels of FoxO3 were increased in miR-149 inhibitor group (Figure 4C–4E). Furthermore, we also validated the FoxO3 expression was evidently downregulated in I/R-treated H9C2 cells with transfection of miR-149 mimics and upregulated in the miR-149 inhibitor group by using immunofluorescence (Figure 4F, 4G). Hence, these data indicated that FoxO3 was a directly target for miR-149.
Figure 4.
MiR-149 targeted FoxO3 in I/R-treated H9C2 cells. (A) Schematic representation of FoxO3 3′UTR showing the putative miR-149 target site; (B) Luciferase reporter activities of reporter plasmid carrying the wild or mutant FoxO3 3′-UTR containing the miR-149 binding sites; (C–E) The RT-PCR and western blotting were performed to detect mRNA and protein levels of FoxO3 in NC group, I/R group, I/R+ miR-149 mimics group and I/R+ miR-149 inhibitor group; (F, G) The expression of FoxO3 was measured by immunofluorescence stain. The data are presented as the mean±SD, n=3; ** P<0.01, *** P<0.001 versus control. ## P<0.01 versus I/R group. I/R – ischemia/reperfusion; RT-PCR – real-time polymerase chain reaction; NC – negative control; SD – standard deviation.
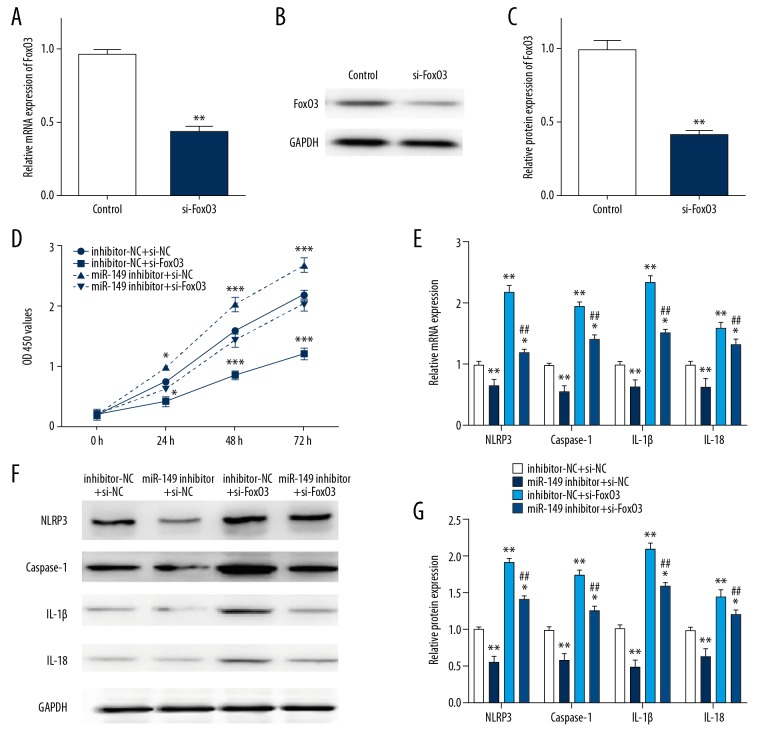
MiR-149 regulated I/R-treated H9C2 cells pyroptosis via FoxO3
We confirmed a successfully effect on knockdown FoxO3 by using si-FoxO3 (Figure 5A–5C). Next, we performed CCK8 assay to test cell proliferation in I/R-treated H9C2 cells co-transfected with either miR-149 inhibitor or inhibitor-NC and si-FoxO3 or si-NC. As shown in Figure 5D, knockdown of FoxO3 suppressed H9C2 cell proliferation, which could be rescued by co-transfection of miR-149. Besides, FoxO3 knockdown upregulated the expression level of pyroptotic and inflammatory related genes as NLRP3, caspase-1, IL-1β, and IL-18, which also could be attenuated by down-expression of miR-149 (Figure 5E–5G). Our findings suggested that miR-149 might exert its effects on pyroptosis in I/R-treated H9C2 cells via FoxO3a.
Figure 5.
MiR-149 regulated I/R-treated H9C2 cells pyroptosis via FoxO3. (A–C) The mRNA and protein expression of FoxO3 in H9C2 cells transfected with si-FoxO3 or control. (D) CCK-8 assay showing the cell proliferation in H9C2 cells during I/R treatment when co-transfected with miR-149 inhibitor, inhibitor-NC, si-FoxO3 and/or si-NC groups. (E–G) Relative mRNA and protein level of NLRP3, caspase-1, IL-1β and IL-18 in H9C2 cells during I/R treatment when co-transfected with miR-149 inhibitor, inhibitor-NC, si-FoxO3 and/or si-NC groups. The data are presented as the mean±SD, n=3;* P<0.05, ** P<0.01 versus control, ## P<0.01 versus I/R group. NC – negative control; I/R – ischemia/reperfusion; CCK-8 – Cell Counting Kit-8; RT-PCR – real-time polymerase chain reaction; SD – standard deviation.
Discussion
Despite advances in prevention and treatment of ischemic heart disease, it still maintains higher morbidity and mortality rates [24]. Thrombolytic therapy or percutaneous coronary intervention is the only standard therapy for myocardial reperfusion. However, in the first year after infarction, the patient might develop complications [25], such as cardiogenic shock, hospital mortality, unstable angina, left ventricular remodeling, recurrent myocardial infarction, or heart failure rehospitalization [26]. Myocardial I/R injury is currently recognized as a main cause of such complications, which contain multiple molecular mechanisms [27,28], such as intracellular pH changes, opening of the mitochondrial permeability transition pore (mPTP), intracellular calcium overload, production of the oxidative stress by reactive oxygen species (ROS), and inflammation modulated by the various cytokines and the complement system through the ROS [29], resulting in diversified forms of myocardial cell death [30]. Therefore, multitudinous therapeutic interventions were frequently proposed to prevent myocardial I/R injury, however, their translation to the clinical practices was rather limited due to the unexpected absence of most ischemic events [31].
Currently, increasing evident indicated that pyroptosis, morphologically characterized by inflammatory related intracellular programmed death, might be involved in cardiac 1/R injury [32], for example, Ye et al. highlighted that inhibition of TLR4/MyD88/NF-κB/NLRP3 inflammasome pathway mediated pyroptosis could alleviate myocardial I/R injury in both cellular and animal models [33]. The causes of pyroptosis in I/R damage progression considered that I/R might offer a combination of priming and triggering which subsequently activated the inflammasome pathway [34]. Indeed, the expression of pyroptotic indicator as NLRP3 was found to be remarkable increased after reperfusion resulting in cell death, a process which contributed to activate caspase-1 in response to endogenous and exogenous danger signals, leading to product and maturate pro-inflammatory cytokines IL-1β and IL-18 during innate immunity process [33]. Meanwhile, it has been shown that caspase-1 could form pores in the cytomembrane, allowing water to enter the cell, causing cell swelling and eventual lysis [32]. The currently views are that the factors associated with the inflammasome NLRP3 release during myocardial I/R injury include ROS, mitochondrial dysfunction, K+ efflux and cell volume regulation [34]. Hence, pyroptosis gradually has come to be considered a crucial modulator of the inflammatory response to I/R injury, whilst a variety of incentives generated from I/R could enhance pyroptotic process. Similarly, our results indicated that the level of NLRP3 and its downstream genes (caspase-1, IL-1β, and IL-18) were prominently upregulated in I/R–stimulated H9C2 cells, representing pyroptosis which might be involved in the process of cardiomyocyte 1/R injury.
MiRNAs, which act as an important regulator, have been reported to played pivotal roles in various diseases and participated in a lot of biological activity, which accounting for up to 30% of all human genes [35]. Upregulation of miRNAs are common in various diseases and abnormal expression of miRNAs are usually involved in specific diseases, such as ischemic disease [36]. Recently, accumulative evidence supposed that dysfunction of miRNAs might be involved in multifactorial and complex processes of I/R impairment of heart’s function [37]. For example, a good deal of miRNAs were shown to have contributed to cardioprotective effects against ischemia-reperfusion damage as miRNA-145-5p [38] and miR-24-3p [39], the others exacerbated I/R-induced cardiomyocyte injury as miR-181c-5p [40] and miR-208a [41]. At the moment, a number of studies rationally speculated that dysregulated miRNAs in the early phase of reperfusion might be relative to oxidative stress, whist miRNAs occurring in the later phase of reperfusion might result in ischemic postconditioning or function as compensatory mechanisms [42]. Accordingly, myocardial I/R-related miRNAs have been recognized in modulating several important segments in cardiomyocyte function such as programmed cell death, inflammation response, fibrosis, and neoangiogenesis, which could lead to ischemic heart disease, cardiac fibrosis, or hypertrophy [12,17,38].
MiR-149 was previously implicated in tumor progression through the regulation of the proliferation, migration, and even the invasion in cells [43]. However, the effect of miR-149 on I/R injury was still not fully explained; Wan et al. reported that miR-149-5p might function as a potential target for treatment of blood-brain barrier disruption after ischemic stroke [17]. In our study, we first revealed that the expression of miR-149 was upregulated in H9C2 cells when subjected to I/R treatment, which was supposed to function as a vital role in cardiac I/R injury. To our knowledge, only a handful of studies have reported the role of miRNAs in pyroptotic mediated I/R damage until now. For instance, Wu et al. found a new signaling pathway of miR-155 regulated renal pyroptosis under I/R injury conditions via suppressing FoxO3a expression [44]. Wan et al. claimed miR-21 engaged in microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury [45]. Consistent with these study results, our data showed that miR-149 could regulate pyroptosis and inflammation in I/R-treated H9C2 cells and served as a pyroptotic promoter in I/R injury.
FoxO3, which belongs to the transcriptional adjustment factor of the fork head family, has been convinced of express in developmental and adult cardiomyocytes [46]. FoxOs plays a vital role in cardiomyocyte size regulation by inhibiting hypertrophy and inducing autophagy, which also can facilitate cell cycle withdrawal by activating the p27KIP1 and p21CIP1 which act as cell cycle inhibitor genes during the neonatal period [46]. Numerous studies have reported that FoxOs is able to fight oxidative stress by regulating SOD2, and acts as an antioxidant gene in a variety of cell types [47]. FoxO3 has been identified in co-expression in cardiomyocytes and coordinately regulated under starvation conditions or during neonatal cell cycle withdrawal [47]. Current studies have reported that the deficiency of FoxO3 was specifically response for increasing oxidative damage and decreasing myocardial function after acute I/R injury [48]. The lack of FoxO3 resulted in the development of premature ovarian failure and mild hemolytic anemia as well as cardiac hypertrophy in a mouse model [49]. It has been reported that FoxO3 takes part in the promotion of cellular growth and the suppression of cellular death in I/R diseases [49]. In our present study, we also found that miR-149 participated in cardiac I/R injury via targeting FoxO3 directly and regulating cardiac cell pyroptosis and inflammation. Likewise, we further predicted that miR-149 might stimulate the FoxO3 mediated antioxidative stress signaling pathway, which could feedback to regulate the pyroptosis process in I/R-induced cardiac cells; the dysfunction of FoxO3 would destruct its subcellular distribution between the nucleus and the cytoplasm, giving rise to obstruct cardiovascular and metabolic homeostasis.
Conclusions
Our findings offered the adequate evidence that pyroptosis of cardiomyocyte in I/R injury was regulated by miR-149 via directly targeting of FoxO3, providing a novel therapeutic target for cardiac I/R injury.
Footnotes
Source of support: Departmental sources
Reference
- 1.Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hearse DJ. Myocardial protection during ischemia and reperfusion. Mol Cell Biochem. 1998;186:177–84. [PubMed] [Google Scholar]
- 4.Han J, Wang D, Ye L, et al. Rosmarinic acid protects against inflammation and cardiomyocyte apoptosis during myocardial ischemia/reperfusion injury by activating peroxisome proliferator-activated receptor gamma. Front Pharmacol. 2017;8:456. doi: 10.3389/fphar.2017.00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lui JC. Regulation of body growth by microRNAs. Mol Cell Endocrinol. 2017;456:2–8. doi: 10.1016/j.mce.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thum T, Condorelli G. Long noncoding RNAs and microRNAs in cardiovascular pathophysiology. Circ Res. 2015;116:751–62. doi: 10.1161/CIRCRESAHA.116.303549. [DOI] [PubMed] [Google Scholar]
- 7.Boureima Oumarou D, Ji H, Xu J, et al. Involvement of microRNA-23b-5p in the promotion of cardiac hypertrophy and dysfunction via the HMGB2 signaling pathway. Biomed Pharmacother. 2019;116:108977. doi: 10.1016/j.biopha.2019.108977. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan JF, Neylon A, Fahy EF, et al. MiR-93-5p is a novel predictor of coronary in-stent restenosis. Heart Asia. 2019;11:e011134. doi: 10.1136/heartasia-2018-011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin S, Choi JW, Moon H, et al. Simultaneous suppression of multiple programmed cell death pathways by miRNA-105 in cardiac ischemic injury. Mol Ther Nucleic Acids. 2019;14:438–49. doi: 10.1016/j.omtn.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye Y, Perez-Polo JR, Qian J, Birnbaum Y. The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol Genomics. 2011;43:534–42. doi: 10.1152/physiolgenomics.00130.2010. [DOI] [PubMed] [Google Scholar]
- 11.Diaz I, Calderon-Sanchez E, Toro RD, et al. miR-125a, miR-139 and miR-324 contribute to Urocortin protection against myocardial ischemia-reperfusion injury. Sci Rep. 2017;7:8898. doi: 10.1038/s41598-017-09198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Ha T, Zou J, et al. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102:385–95. doi: 10.1093/cvr/cvu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu JY, Shang J, Mu XD, Gao ZY. Protective effect of down-regulated microRNA-27a mediating high thoracic epidural block on myocardial ischemia-reperfusion injury in mice through regulating ABCA1 and NF-kappaB signaling pathway. Biomed Pharmacother. 2019;112:108606. doi: 10.1016/j.biopha.2019.108606. [DOI] [PubMed] [Google Scholar]
- 14.Ma H, Chen P, Sang C, et al. Modulation of apoptosis-related microRNAs following myocardial infarction in fat-1 transgenic mice vs. wild-type mice. J Cell Mol Med. 2018;22:5698–707. doi: 10.1111/jcmm.13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu J, Hu Z, Xu Z, et al. Functional variant in microRNA-196a2 contributes to the susceptibility of congenital heart disease in a Chinese population. Hum Mutat. 2009;30:1231–36. doi: 10.1002/humu.21044. [DOI] [PubMed] [Google Scholar]
- 16.Ghaffarzadeh M, Ghaedi H, Alipoor B, et al. Association of MiR-149 (RS2292832) variant with the risk of coronary artery disease. J Med Biochem. 2017;36:251–58. doi: 10.1515/jomb-2017-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Y, Jin HJ, Zhu YY, et al. MicroRNA-149-5p regulates blood-brain barrier permeability after transient middle cerebral artery occlusion in rats by targeting S1PR2 of pericytes. FASEB J. 2018;32:3133–48. doi: 10.1096/fj.201701121R. [DOI] [PubMed] [Google Scholar]
- 18.Xu YJ, Zheng L, Hu YW, Wang Q. Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta. 2018;476:28–37. doi: 10.1016/j.cca.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhou W, Chen C, Chen Z, et al. NLRP3: A novel mediator in cardiovascular disease. J Immunol Res. 2018;2018 doi: 10.1155/2018/5702103. 5702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–25. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 21.Bergsbaken T, Cookson BT. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan CC, Zhang JG, Tan MS, et al. NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling-induced rat model. J Neuroinflammation. 2015;12:18. doi: 10.1186/s12974-014-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terada T, Yokokura M, Obi T, et al. In vivo direct relation of tau pathology with neuroinflammation in early Alzheimer’s disease. J Neurol. 2019;266(9):2186–96. doi: 10.1007/s00415-019-09400-2. [DOI] [PubMed] [Google Scholar]
- 24.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–41. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Cung TT, Morel O, Cayla G, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–31. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 26.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 27.Bishopric NH, Andreka P, Slepak T, Webster KA. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr Opin Pharmacol. 2001;1:141–50. doi: 10.1016/s1471-4892(01)00032-7. [DOI] [PubMed] [Google Scholar]
- 28.Hajnoczky G, Csordas G, Madesh M, Pacher P. Control of apoptosis by IP(3) and ryanodine receptor driven calcium signals. Cell Calcium. 2000;28:349–63. doi: 10.1054/ceca.2000.0169. [DOI] [PubMed] [Google Scholar]
- 29.Frohlich GM, Meier P, White SK, et al. Myocardial reperfusion injury: Looking beyond primary PCI. Eur Heart J. 2013;34:1714–22. doi: 10.1093/eurheartj/eht090. [DOI] [PubMed] [Google Scholar]
- 30.Arumugam TV, Selvaraj PK, Woodruff TM, Mattson MP. Targeting ischemic brain injury with intravenous immunoglobulin. Expert Opin Ther Targets. 2008;12:19–29. doi: 10.1517/14728222.12.1.19. [DOI] [PubMed] [Google Scholar]
- 31.Jiang YQ, Chang GL, Wang Y, et al. Geniposide prevents hypoxia/reoxygenation-induced apoptosis in H9c2 cells: Improvement of mitochondrial dysfunction and activation of GLP-1R and the PI3K/AKT signaling pathway. Cell Physiol Biochem. 2016;39:407–21. doi: 10.1159/000445634. [DOI] [PubMed] [Google Scholar]
- 32.Audia JP, Yang XM, Crockett ES, et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018;113:32. doi: 10.1007/s00395-018-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye B, Chen X, Dai S, et al. Emodin alleviates myocardial ischemia/reperfusion injury by inhibiting gasdermin D-mediated pyroptosis in cardiomyocytes. Drug Des Devel Ther. 2019;13:975–90. doi: 10.2147/DDDT.S195412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315:H1553–68. doi: 10.1152/ajpheart.00158.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada H, Suzuki K, Ichino N, et al. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta. 2013;424:99–103. doi: 10.1016/j.cca.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Ma N, Bai J, Zhang W, et al. Trimetazidine protects against cardiac ischemia/reperfusion injury via effects on cardiac miRNA21 expression, Akt and the Bcl2/Bax pathway. Mol Med Rep. 2016;14:4216–22. doi: 10.3892/mmr.2016.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zampetaki A, Willeit P, Tilling L, et al. Prospective study on circulating MicroRNAs and risk of myocardial infarction. J Am Coll Cardiol. 2012;60:290–99. doi: 10.1016/j.jacc.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 38.Wu G, Tan J, Li J, et al. miRNA-145-5p induces apoptosis after ischemia-reperfusion by targeting dual specificity phosphatase 6. J Cell Physiol. 2019 doi: 10.1002/jcp.28291. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Wei W, Peng J, Shen T. Rosuvastatin alleviates ischemia/reperfusion injury in cardiomyocytes by downregulating Hsa-miR-24-3p to target upregulated uncoupling protein 2. Cell Reprogram. 2019;21(2):99–107. doi: 10.1089/cell.2018.0039. [DOI] [PubMed] [Google Scholar]
- 40.Ge L, Cai Y, Ying F, et al. MiR-181c-5p exacerbates hypoxia/reoxygenation-induced cardiomyocyte apoptosis via targeting PTPN4. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/1957920. 1957920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang F, Yuan Y, Yang P, Li X. Extracellular vesicles-mediated transfer of miR-208a/b exaggerates hypoxia/reoxygenation injury in cardiomyocytes by reducing QKI expression. Mol Cell Biochem. 2017;431:187–95. doi: 10.1007/s11010-017-2990-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhu H, Fan GC. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res. 2012;94:284–92. doi: 10.1093/cvr/cvr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ow SH, Chua PJ, Bay BH. miR-149 as a potential molecular target for cancer. Curr Med Chem. 2018;25(9):1046–54. doi: 10.2174/0929867324666170718102738. [DOI] [PubMed] [Google Scholar]
- 44.Wu H, Huang T, Ying L, et al. MiR-155 is involved in renal ischemia-reperfusion injury via direct targeting of FoxO3a and regulating renal tubular cell pyroptosis. Cell Physiol Biochem. 2016;40:1692–705. doi: 10.1159/000453218. [DOI] [PubMed] [Google Scholar]
- 45.Wan P, Su W, Zhang Y, et al. LncRNA H19 initiates microglial pyroptosis and neuronal death in retinal ischemia/reperfusion injury. Cell Death Differ. 2019 doi: 10.1038/s41418-019-0351-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284:28319–31. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 48.Sengupta A, Molkentin JD, Paik JH, et al. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–78. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosaka T, Biggs WH, 3rd, Tieu D, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA. 2004;101:2975–80. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]