SUMMARY
Alcohol produces both stimulant and sedative effects in humans and rodents. In humans, alcohol abuse disorder is associated with a higher stimulant and lower sedative responses to alcohol. Here, we show that this association is conserved in mice and demonstrate a causal link with another liability factor: low expression of striatal dopamine D2 receptors (D2Rs). Using transgenic mouse lines, we find that the selective loss of D2Rs on striatal medium spiny neurons enhances sensitivity to ethanol stimulation and generates resilience to ethanol sedation. These mice also display higher preference and escalation of ethanol drinking, which continues despite adverse outcomes. We find that striatal D1R activation is required for ethanol stimulation and that this signaling is enhanced in mice with low striatal D2Rs. These data demonstrate a link between two vulnerability factors for alcohol abuse and offer evidence for a mechanism in which low striatal D2Rs trigger D1R hypersensitivity, ultimately leading to compulsive-like drinking.
In Brief
Phenotypes associated with alcohol abuse are well established. Bocarsly et al. identify the upregulation of D1R functioning as an underlying mechanism. We provide direct evidence that low levels of D2Rs on striatal projection neurons heighten ethanol stimulation and drinking, despite adverse outcomes contributing to abuse liability via enhanced D1R signaling.
Graphical Abstract

INTRODUCTION
Alcohol use disorder (AUD) is a chronic relapsing disease characterized by escalating alcohol drinking and loss of control over consumption, which leads to compulsive alcohol use (Koob and Volkow, 2010, 2016). The diagnosis of AUD requires that individuals meet 2 of the 11 criteria detailed in the Diagnostic and Statistical Manual of Mental Disorders-V (DSM-V; Grant et al., 2015). It is unclear why only a proportion of individuals who consume alcohol developed AUD. Genetic factors account for approximately half of the risk for developing AUD, and environmental interactions are thought to contribute the remainder of the risk (Reilly et al., 2017). While multiple genes and traits have been associated with AUD, the mechanisms underlying vulnerability are unknown and, as a consequence, AUD treatments are unreliable.
Two factors are well known to confer vulnerability for AUD. The quality and magnitude of the acute response to alcohol are predictive of alcohol abuse. High stimulation and low sedation in response to alcohol are known to predispose individuals toward abuse (Erblich and Earleywine, 2003; Holdstock et al., 2000; King et al., 2011, 2016). Rodents have proven to be good animal models for ethanol-induced stimulation and allow for cellular and molecular analysis of the underlying striatal mechanisms (Becker and Ron, 2014; Lovinger and Alvarez, 2017). While the perceived stimulant effects of alcohol are linked to striatal activity (Weafer et al., 2018), the neurobiology underlying ethanol stimulation and driving the association with abuse is poorly understood.
In addition to the stimulant effects of ethanol, low levels of dopamine D2 receptor (D2R) availability in the striatum is a common feature associated with addictive disorders, including AUD (Hietala et al., 1994; Tupala et al., 2001; Volkow et al., 2002; Volkow and Morales, 2015). This raises the possibility that a low level of striatal D2R is a predisposing factor for AUD. This hypothesis is further driven by findings that after months of alcohol abstinence, D2R availability is not recovered in individuals with AUD (Volkow et al., 2002). In animal models, the overexpression of striatal D2Rs was shown to reduce ethanol self-administration and preference (Thanos et al., 2001), but in a more recent study, D2R overexpression in the ventral striatum did not produce the same phenotype (Gallo et al., 2015).
Global Drd2 knockout mice with a ubiquitous deletion of D2Rs show enhanced ethanol-induced simulation when tested in a familiar environment and decreased sedation in a novel environment (Palmer et al., 2003; Phillips et al., 1998), suggesting a role for these receptors. However, Drd2 knockout mice have also been shown to self-administer less ethanol compared to controls (Risinger et al., 2000). While it is tempting to conclude that these data indicate that D2Rs are unrelated to the reinforcing effects of alcohol, the Drd2 knockout mice also self-administered less food and sucrose, indicating a more generalized reward deficit.
The reason for these confounding data is likely that D2Rs are located on a variety of cell types throughout the striatum, including GABAergic medium spiny projection neurons, cholinergic interneurons, and dopaminergic terminals emanating from midbrain dopamine (DA) neurons, where they are known to have differential effects. Fully understanding the role of D2Rs in alcohol-related circuitry demands the use of cell-type-specific analysis, rather than global knockouts. This allows us to identify which populations of striatal D2Rs are affected in instances of alcohol abuse and contribute to the abuse-vulnerable phenotype.
In the present study, we demonstrate a link between striatal D2Rs and the stimulant effects of ethanol, pointing to a critical role of D2Rs expressed on medium spiny neurons (MSNs) in generating the vulnerability and offer direct evidence for a mechanism driving higher ethanol preference and escalation of drinking.
We validate the use of mouse models in the study of the stimulant effects of alcohol. We then show that pre-existing low levels of striatal D2Rs in select cell types are sufficient in modulating ethanol stimulation and alcohol drinking behavior. The selective loss of D2Rs on medium spiny neurons left mice with a higher sensitivity to ethanol stimulation and resistance to the sedative effects. These mice also showed a higher ethanol preference and escalation of drinking. We further show that the activation of D1Rs in the dorsal striatum is required for ethanol stimulation and promotes ethanol preference. The present study then builds on our previous research, showing that low levels of D2Rs in medium spiny neurons are associated with an upregulation in DA D1 receptor (D1R) functioning in the direct-pathway medium spiny neurons. As such, here, we propose that the loss of striatal medium spiny neuron D2Rs triggers the hypersensitivity of striatal D1Rs, which in turn drives the stimulatory effects of ethanol and compulsive-like intake. This study offers a mechanistic understanding of how low levels of D2Rs confer vulnerability to alcohol abuse.
RESULTS
Assessment of Ethanol Stimulatory and Sedative Effects in Mice of C57BL/6J Background
We first characterized and validated the use of locomotor measurements in assessing the biphasic ethanol-induced stimulatory and sedative effects in Drd2loxP/loxP mice. These mice have loxP sites in the Drd2 gene, making them amenable to Cre-induced deletion of the start codon of Drd2 mRNA, but are otherwise identical to C57BL/6J wild-type mice (Bello et al., 2011). Male and female Drd2loxP/loxP mice were administered varying doses of ethanol (0.5–3 g/kg, intraperitoneally [i.p.]) using a Latin square design, and locomotion was quantified using infrared beam breaks. Consistent with the literature, ethanol produced transient, dose-dependent stimulatory effects that peak at 2 g/kg (Figure 1A, p < 0.05; Figure 1B, p < 0.01 for 2 g/kg ethanol) (Cohen et al., 1997; Liljequist et al., 1981; Read et al., 1960). There was a great deal of individual variability in ethanol stimulation, despite the fact that these mice are an inbred, genetically homogeneous strain (Figure 1C). While on average, the mice showed increased locomotion when administered ethanol compared to saline, some mice showed larger effects than others, and females showed higher stimulation (Figures S1A and S1B, p < 0.05) and larger individual variability compared to males (SD: 191 versus 93 for females and males, respectively). These sex differences are consistent with the literature (Crabbe et al., 1987).
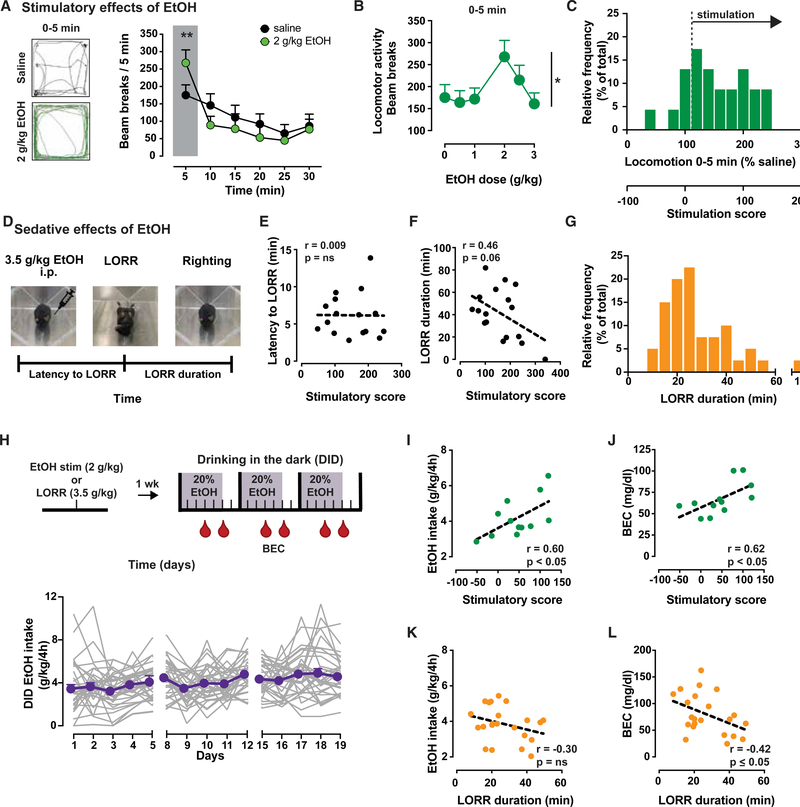
Figure 1. Inbred Mice Show Ethanol Stimulatory and Sedative Effects with Individual Variability Correlating with Intoxication.
A) Left, sample traces of locomotor activity for 5 min following injection of saline (black) or 2 g/kg ethanol (green) in Drd2loxP/loxP mice (n = 23). Right, time course of locomotor activity following saline (black) or 2 g/kg ethanol (green) in Drd2loxP/loxP mice (2-way repeated-measures [RMs] ANOVA: F5,110 = 4.3, p < 0.01). Shaded box highlights ethanol stimulation during first 5 min. **p < 0.01, Bonferroni’s multiple comparison.
(B) Dose-dependent ethanol stimulation plotted as locomotion 5 min after ethanol administration (n = 23; F3.59,78.89 = 2.8; *p < 0.05).
(C) Frequency histogram of individual mouse responses to 2 g/kg ethanol during first 5 min, presented as percentage of activity after saline (top × axis) and as stimulation score (bottom × axis).
(D) Schematic illustrating the loss of righting reflex (LORR) task.
(E and F) No significant correlation between stimulatory scores and latency to LORR (E) (n = 17; Pearson’s r = 0.01, p > 0.05), while an inverse trend is seen between stimulation scores and LORR duration in Drd2loxP/loxP mice (F) (n = 17; Pearson’s r = 0.46, p = 0.06).
(G) Frequency histogram of mouse LORR duration illustrates the range of individual variability.
(H) Top, outline of experimental protocol showing sequence of tests and days (ticks) in which tail blood samples (red drop) were taken and processed for BEC. Bottom, average ethanol intake (purple symbols) of individual mouse data (gray lines; n = 34).
(I and J) The mean individual ethanol intake (I) and BEC (J) achieved during DID show a significant positive correlation with the stimulation score of each mouse (n = 12; Pearson’s r = 0.6 and r = 0.62, respectively; p < 0.05).
(K and L) The mean individual ethanol intake showed no correlation with LORR duration (K) (n = 22; Pearson’s r = −0.42, p ≤ 0.05) but an inverse correlation with mean BEC achieved during DID (L) (Pearson’s r = −0.30, p > 0.05).
All of the symbols with error bars are means ± SEMs; symbols without error bars represent data from individual animals.
The transient ethanol-induced stimulation was followed by the onset of the sedative effects of ethanol that were longer lasting and maximal at higher doses (Figures S2A and S2B). The sedative effects of ethanol also showed significant individual variability (Figure S2C); however, no statistical difference was seen between males and females following 2.5 or 3 g/kg ethanol (Figures S1C and S1D).
Stimulatory and sedative scores were calculated for each mouse by normalizing locomotion following 2 g/kg ethanol to the value following saline and subtracting 100, so no change from saline corresponded to a zero score (Figures S2D and S2E). We identified an inverse correlation between the transient stimulatory score and the subsequent sedative score among individual animals (Figure S2F, p < 0.05), which parallels what is seen in humans (Martin et al., 1993). However, because the two measurements rely on the same locomotor task and are therefore not independent and dissociable, we considered it important to obtain an independent measurement of ethanol-induced sedation.
The loss of righting reflex (LORR) test was carried out to further examine sedation. Control mice were administered a high, anesthetic dose of ethanol (3.5 g/kg, i.p.), and the latency to lose and regain the ability to right from a supine position was recorded (Figure 1D). The latency to LORR was 4.5 ± 0.8 min, and the time to regain the righting reflex was 33 ± 3 min. We then looked at the correlation between parameters of the LORR task and the stimulatory response to ethanol. While the latency to lose the righting reflex was not correlated with the stimulatory response to ethanol (Figure 1E, p > 0.05), the time to regain the reflex showed a trend of negative correlation with ethanol-induced locomotion (Figure 1F, p = 0.06). This is further indicative of an inverse correlation between ethanol-induced sedation and stimulation in rodents, as assessed by two independent measures. Again, individual variation was observed in this congenic mouse population (Figure 1G). When examining sex differences, there was no difference in the latency to LORR in male and female mice (Figure S1E, p > 0.05). However, the LORR duration was longer and individual variability was larger in males compared to females, indicating that female mice are more resistant to ethanol sedation (Figure S1F; SD males: 11.6 versus SD females: 5.8).
Individual Variability in Ethanol Stimulation and Sedation Has Predictive Value on Voluntary Ethanol Intake
To further test whether individual variability in stimulatory response to ethanol had predictive value over ethanol consumption, as suggested in humans, we examined the relation between stimulatory and sedative responses and ethanol consumption.
Two separate groups of mice were tested for either ethanol stimulation (2 g/kg ethanol) or LORR (3.5 g/kg ethanol). All of the mice were then given access to 20% ethanol solution in the home cage for 4 h/day. This 3-week-long paradigm of voluntarily drinking is a modified version of the “drinking in the dark” (DID) paradigm (Figure 1H, upper panel). Tail blood samples were taken twice per week and analyzed for blood ethanol concentration (BEC). Again, individual variability was noticed during DID (Figure 1H, lower panel). We found that the degree of ethanol-induced stimulation for each mouse was positively correlated with voluntary ethanol intake and BECs (Figures 1I and 1J, p < 0.05 for both). Ethanol-induced sedation, on the contrary, showed a negative correlation with BECs (p = 0.05) and no association with voluntary intake (Figures 1K and 1L, p = 0.17). Consistent with the enhanced stimulation, female mice consumed more ethanol than did males during DID (Figure S1G, p < 0.05). While not significant, there was a trend toward higher BECs following voluntary drinking in females (Figure S1H, p > 0.05). These data are in agreement with previous reports showing higher ethanol intake in females on operant and non-operant paradigms (Blegen et al., 2018; Yoneyama et al., 2008).
In the present experiment, all of the mice were exposed to an acute dose of ethanol (either in stimulatory testing or LORR), which would have subsequent effects on drinking. However, despite the fact that all of the mice were similarly exposed, individual variability endured.
Taken together, the findings from the mouse models align with the clinical literature, showing that in mice, similar to humans, individual variability in the stimulant and sedative effects of ethanol has predictive value for voluntary intoxication. These findings validate the use of these behavioral approaches to study the mechanisms underlying AUD. Finally, the fact that significant individual variability in ethanol response persists in a congenic mouse strain reinforces the idea that the phenotypic variation is the result of gene-environment interactions and not genes alone.
Mouse Models of Cell-Specific Ablation of D2Rs in the Striatum
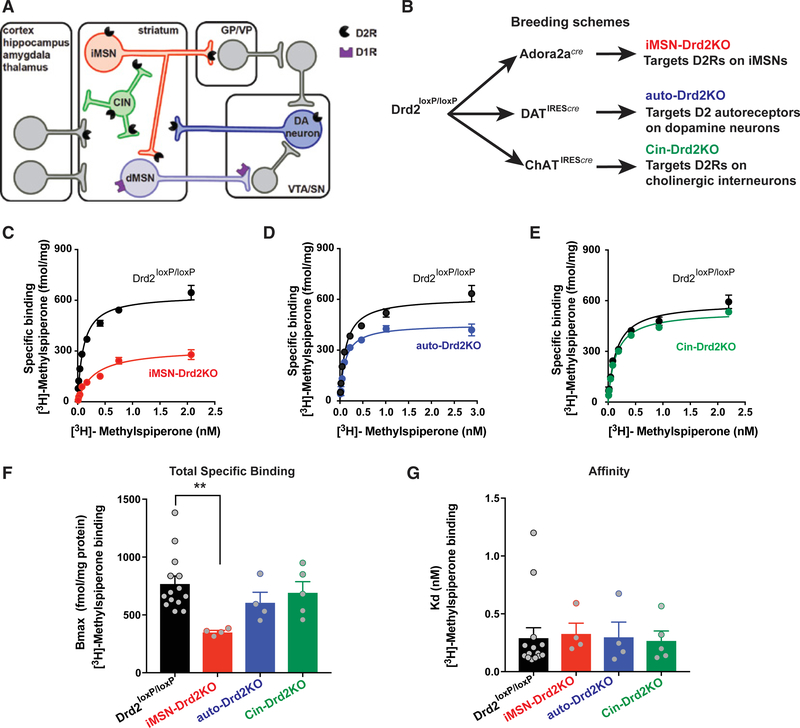
Three conditional mutant mouse lines with deletion of D2Rs in specific neuronal types were generated and used to test the link between low striatal D2R availability and ethanol behaviors. D2Rs were selectively removed from striatal GABAergic medium spiny neurons that form the indirect projection pathway (iMSNs), cholinergic interneurons (CINs), or the axonal projections originating from midbrain DA neurons (Figure 2A). To selectively remove the subpopulations of D2Rs, conditional mice for the Drd2 gene (Drd2loxP/loxP) were crossed with mice expressing Cre under different promoters to selectively target iMSNs, CINs, and DA neurons (adenosine 2a receptors [Adora2a], cholinergic acetyl transferase [ChAT], and DA transporter [DAT], respectively; Figure 2B) (Bello et al., 2011; Lemos et al., 2016). Striatal D2R availability was assessed using radioligand binding of the D2R-like antagonist [3H]-methylspiperone in homogenized striatal tissue. A robust decrease in D2R binding (54% ± 2%) was found in iMSN-Drd2KO (knockout) mice compared to littermate controls (Figures 2C–2G, p < 0.05). This is anatomically consistent in that 95% of striatal neurons are medium spiny neurons, half of which express D2Rs (Gerfen and Surmeier, 2011; Tepper et al., 2007). Auto-Drd2KO (mice lacking D2Rs in DA neurons) and Cin-Drd2KO (mice lacking D2Rs on cholinergic interneurons) showed moderate decreases in striatal D2R binding (21% ± 11% and 10% ± 12%, respectively), which reflect their portions of the total striatal D2R population. Low-expressing D3R or D4R does not significantly contribute to striatal D2-like radioligand binding, as D3R (Accili et al., 1996; Xu et al., 1997) or D4R (Rubinstein et al., 1997) global knockout mice do not show a decrease in D2R binding, and binding is completely ablated in global D2R-deficient animals (Baik et al., 1995).
Figure 2. Cell-Specific Deletion of Drd2 Gene Causes Selective Reduction of D2R Binding Availability.
(A) Diagram illustrating the main neuronal populations, with D2Rs localized in the striatum.
(B) Breeding scheme for the cell-specific knockout of Drd2.
(C–E) Representative saturation binding curves for [3H]-methylspiperone in striatal samples from iMSN-Drd2KO (C), auto-Drd2KO (D), and Cin-Drd2KO (E) and respective littermate controls. Lines represent fit from non-linear regression analyses.
(F and G) Total specific binding, Bmax, (F) and binding affinity, dissociation constant (G). **p < 0.01, Dunnett’s multiple comparison after 1-way ANOVA: F5,31 = 2.6, p < 0.05 for total specific binding. No difference was seen in Kd across genotypes (F5,31 = 0.13, p > 0.05; n = 4–5/group, 3 replicates).
All of the symbols with error bars are means ± SEMs; symbols without error bars represent data from individual animals.
Targeted Deletion of D2Rs on MSNs Enhances Ethanol-Induced Stimulation
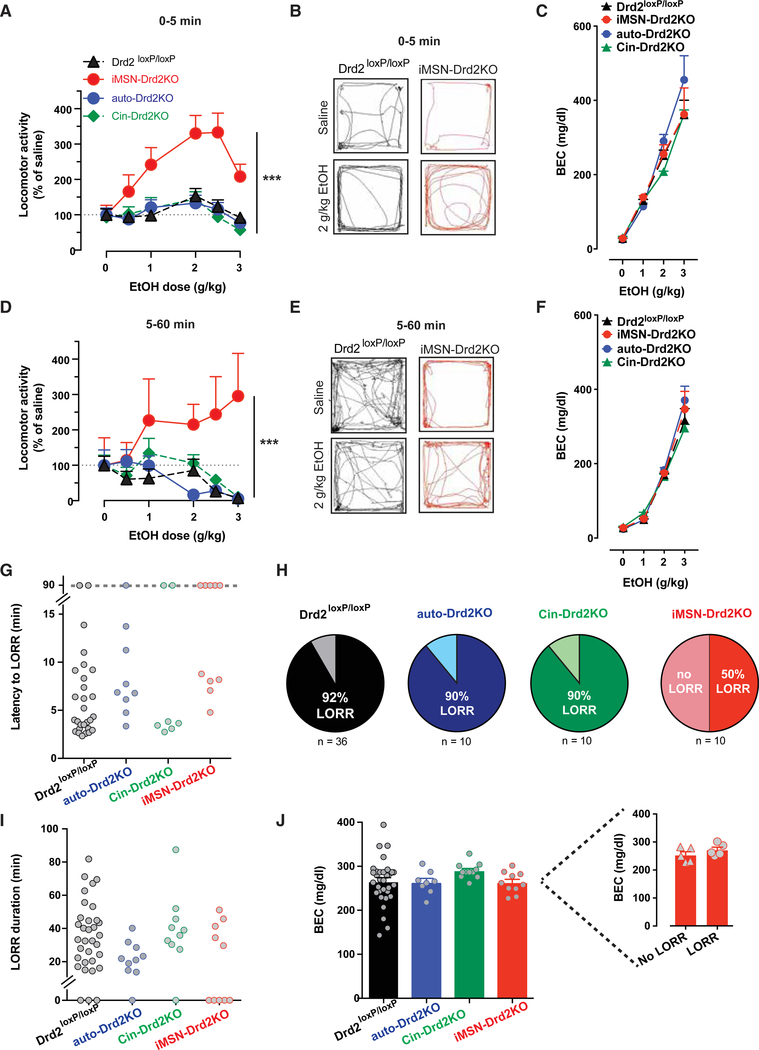
All three mouse lines were tested for ethanol-induced stimulation and sedation in comparison to littermate controls. No differences were found among the littermate controls, so all of the control data were pooled. iMSN-Drd2KO mice show an upward shift in the dose-specific stimulatory effects of ethanol. They also display a larger increase in locomotion normalized to baseline for all doses compared to control littermates, Cin-Drd2KO, and auto-Drd2KO mice (Figure 3A, corresponding raw data in Figure S3E, p < 0.05). Ethanol-induced stimulation was longer lasting in iMSN-Drd2KO mice compared to littermate controls. In agreement with our previous report, iMSN-Drd2KO mice are hypolocomotive at baseline compared to littermate controls and the other genotypes (Figure S3) (Lemos et al., 2016). As such, ethanol at 2 g/kg appears to restore locomotion to the levels seen in littermate controls (Figures 3B and S3E); however, locomotion is visually uncoordinated and impaired (see Video S1) and mice are intoxicated, as shown by similar levels of blood ethanol concentration (Figure 3C). Ethanol stimulation in Cin-Drd2KO and auto-Drd2KO mice was indistinguishable from littermate controls. At high ethanol doses (2 or 3 g/kg), littermate controls, Cin-Drd2KO, and auto-Drd2KO mice show ethanol-induced sedation, while iMSN-Drd2KO mice show a profound and persistent stimulatory effect (Figures S3C, S3D, and S3J).
Figure 3. iMSN-Drd2KO Mice Display High Ethanol Stimulation and Resiliency to Ethanol Sedation.
(A and D) Dose-response curve of ethanol-induced locomotion for iMSN-Drd2KO (red), auto-Drd2KO (blue), Cin-Drd2KO (green), and littermate control (black) mice 5 min following ethanol administration (A); n = 12–23/group; 2-way RMs ANOVA ethanol dose × genotype: F15,250 = 2.69, p < 0.001; ***p < 0.001. Main effect of dose: F5,250 = 8.15, p < 0.0001. Main effect of genotype: F3,50 = 13.8, p < 0.0001, and 5–60 min following ethanol injection (D); ethanol dose × genotype: F15,250 = 2.27, p < 0.001; ***p < 0.001. Main effect of genotype: F3,50 = 4.06, p < 0.05.
(B and E) Representative traces of locomotor activity in Drd2loxP/loxP (black) and iMSN-Drd2KO (red) mice during first 5 min (B) and 5–60 min (E) following saline injection (left) or 2 g/kg ethanol (right).
(C and F) BEC measured 5 min (C); n = 8–12/group; 2-way RMs ANOVA ethanol dose × genotype: F9,100 = 0.89, p > 0.05 and 60 min (F); 2-way RMs ANOVA ethanol dose × genotype: F9,96 = 0.97, p > 0.05 following injection of 0, 1, 2, and 3 g/kg ethanol.
(G and I) Time to LORR (G) (n = 7–10/group; 1-way ANOVA: F2,32 = 0.25, p > 0.05) and duration of LORR (I); 1-way ANOVA: F2,32 = 2.42, p > 0.05. Data points above dotted line (G) indicate mice that did not lose the righting reflex by the 90-min cutoff time.
(H) Percentage of mice from each genotype that lost the righting reflex for each genotype.
(J) BEC measured at regaining righting reflex or at the cutoff time (1-way ANOVA: F3,58 = 1.19, p > 0.05). Inset shows BEC for iMSN-Drd2KO mice that lost the righting reflex (LORR) compared to those that did not (no LORR; independent sample t test: t(8) = 0.1, p > 0.05).
All of the symbols with error bars are means ± SEMs; symbols without error bars represent data from individual animals.
Experiments were counterbalanced for sex, so observed genotype differences are not likely driven by sex differences. While underpowered, the sex-sorted data presented in Figures S4A and S4B show that both male and female iMSN-Drd2KO mice have a 3-fold increase in locomotion in response to ethanol, which is higher than the 50% increase seen in controls (Figures S1B and S4B). Similar to the findings in Figure S1G, female iMSN-Drd2KO mice show a higher daily intake of ethanol on DID compared to males (Figure S4C). While some sex differences seen in the control mice are similarly preserved in the iMSN-Drd2KO mice, these effects do not appear to be driving the observed genotype differences.
Of note, on the locomotor stimulatory task, auto-Drd2KO mice were more sensitive to the sedative properties of ethanol, with mice showing lower locomotor activity 1 h after 2 g/kg ethanol, compared to littermate controls (Figure 3D). Across genotypes, there were no differences in BECs measured 5 or 60 min post-ethanol administration (p > 0.05 for both time points), indicating that the differences in ethanol-induced stimulation and sedation are not likely due to differences in drug metabolism (Figures 3C and 3F). Furthermore, we tested whether Cre expression in medium spiny neurons could account for the hypersensitive ethanol stimulation seen in iMSN-Drd2KO mice. Adora2a-Cre+ mice showed locomotor responses that were indistinguishable from Cre− littermate controls when given 3 g/kg ethanol (Figures S4D and S4E, p > 0.05 for both). These results indicate that the phenotypic difference seen in iMSN-Drd2KO mice are not merely due to the expression of Cre.
Selective Loss of D2Rs on Medium Spiny Neurons Creates Resilience to Ethanol Sedation
iMSN-Drd2KO mice were also resilient to the sedative effects of ethanol on the LORR task. Only 50% of iMSN-Drd2KO mice lost the righting reflex after 3.5 g/kg ethanol, while >90% of auto-Drd2KO, Cin-Drd2Kos, and littermate controls did (Figures 3G and 3H, p < 0.05). A similar mean latency to loss and duration of the effect were seen across genotypes among the mice that lost their righting reflex (Figure 3G). Again, there were no differences in BECs across genotypes at the time of righting regain (Figure 3J). Furthermore, BECs were no different in iMSN-Drd2KO mice that lost their righting reflex and those that did not (Figure 3J, inset). Consistent with our previous finding, these results support the interpretation that iMSN-Drd2KO mice are less sensitive to the sedative properties of ethanol, despite achieving similar BECs.
Higher Preference for Ethanol Consumption in Mice with Enhanced Ethanol Stimulation
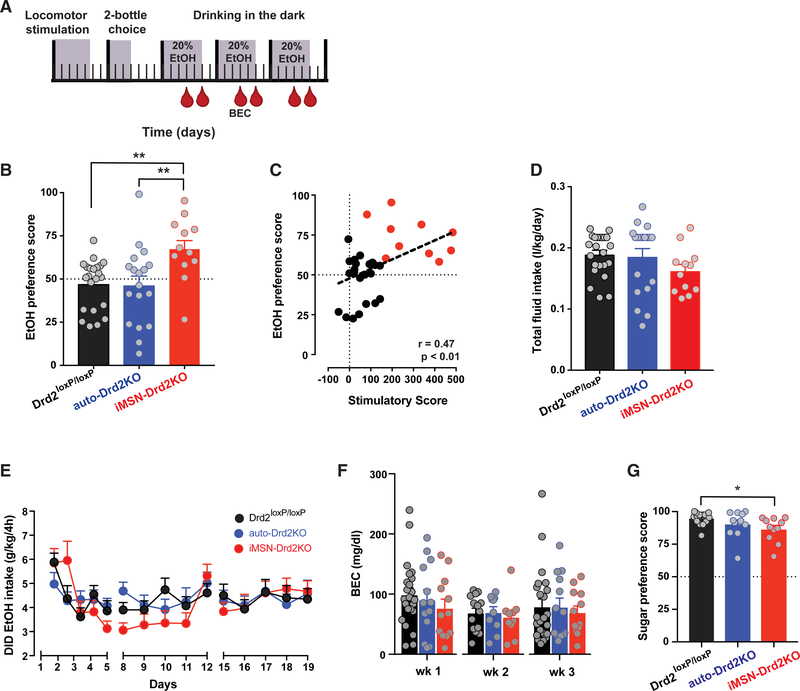
Mice were run on the ethanol locomotor test (2 g/kg ethanol) and then assessed in a 2-bottle choice test, where they were given ad libitum access to both ethanol and water for 3 days (Figure 4A). Preference was determined as the ratio of ethanol consumed to total fluid intake. Because CIN-Drd2KO mice showed no differential response to ethanol stimulation or sedation, they were not tested further. iMSN-Drd2KO mice showed a higher ethanol preference compared to littermate controls and auto-Drd2KO mice (Figure 4B, p < 0.01). Across iMSN-Drd2KO mice and littermate controls, ethanol preference scores were positively correlated with ethanol-induced stimulation (Figure 4C, p < 0.01). No significant differences were found in overall ethanol consumption or fluid intake across genotypes (Figure 4D). Furthermore, when ethanol intake was assessed using the DID paradigm, all of the genotypes consumed similar amounts of ethanol and showed no differences in BECs (Figures 4E and 4F). Thus, while mice with low D2Rs in medium spiny neurons show a higher preference for ethanol, they do not consume more ethanol under the parameters tested here.
Figure 4. Higher Preference for Ethanol Drinking in Mice Lacking D2Rs in iMSNs.
(A) Schematic of experimental design; mice were tested for ethanol-induced locomotion followed by 2-bottle ethanol preference test and 3 weeks of DID. Blood sampling for BEC indicated by red drop. Shaded areas indicate days of experimental testing.
(B) Average ethanol preference scores on 2-bottle choice (n = 12–23/group; 1-way ANOVA: F2,49 = 5.87, p < 0.01) followed by Tukey tests; **p < 0.01.
(C) Individual preference scores plotted as a function of stimulatory score for each Drd2loxP/loxP (black) and iMSN-Drd2KO (red) mouse (Pearson’s r = 0.47, p < 0.01).
(D) No differences in total daily fluid intake during 2-bottle choice test (1-way ANOVA: F2,51 = 0.19, p > 0.05).
(E) Average daily ethanol intake during DID (n = 12–23/group; 2-way RMs ANOVA time × genotype: F2,28 = 0.7, p > 0.05).
(F) Weekly mean BECs achieved during DID (2-way RMs ANOVA time × genotype, F1,2 = 8.44, p > 0.05).
(G) Sucrose preference scores (n = 9–11/group; 1-way ANOVA: F2,37 = 4.25 followed by Tukey tests; *p < 0.05).
All of the symbols with error bars are means ± SEMs; symbols without error bars represent data from individual animals.
The higher ethanol preference scores observed in iMSN-Drd2KO mice compared to littermate controls were selective for ethanol, as these mice did not show a higher preference for 1% sucrose solution (Figure 4G; iMSN-Drd2KO mice: 87% ± 3% preference, Drd2loxP/loxP mice: 95% ± 1% preference, auto-Drd2KO mice: 91% ± 3%).
Mice with Low Striatal D2Rs Show More Escalation of Ethanol Intake in an Operant Paradigm
iMSN-Drd2KO mice, auto-Drd2KO mice, and littermate controls were tested in an operant paradigm for ethanol self-administration. Mice were pre-exposed to 20% ethanol solution using the DID paradigm (data previously described; Figure 4E). After 3 weeks, mice were given access to the same solution in an operant chamber with 2 levers (active and inactive) and a retractable sipper tube (Figure 5A). The first 3 training sessions were 6 h long and the remainder of the training sessions were 2 h. No sucrose fading was used during operant training. No differences were found across the genotypes in the rate of response for active and inactive levers during the 2-h training sessions (Figures S5A and S5B).
Figure 5. More Escalation of Ethanol Drinking and Higher Resistance to Quinine Adulteration in Mice Lacking iMSN D2Rs during Operant Self-Administration Task.
(A) Schematic of experimental design for operant ethanol self-administration (SA). Following 3 weeks of DID, mice were trained to self-administer ethanol (FR1,FR3). After training, mice were tested for ethanol adulteration with quinine, ethanol seeking after abstinence, and ethanol relapse (n = 6–13/group).
(B) Ethanol intake during the 2-h SA sessions for iMSN-Drd2KO (red), auto-Drd2KO (blue), and littermate control (black) mice.
(C) Ethanol intake during early training (open bars/round symbols) and later training (filled bars/square symbols). The connected symbols represent data from individual mice.
(D) Rate of sipper licking during training sessions for iMSN-Drd2KO (red), auto-Drd2KO (blue), and littermate control (black) mice (n = 18/group).
(E) Percentage of mice from each genotype displaying escalating drinking pattern.
(F) Ethanol intake during first 3 training sessions (open bars/round symbols) and the relapse session (filled bars/square symbols). The connected symbols represent data from individual mice (paired t test t(5) = 3.25; *p < 0.05 for iMSN-Drd2KO).
(G) Ethanol intake during quinine adulteration sessions (2-way ANOVA genotype × dose: F4,44 = 3.01, p < 0.001; followed by Dunnett’s multiple comparison; ***p < 0.001).
(H) Two-bottle choice test for water and water adulterated with quinine. No differences in preference scores (n = 14/group; 2-way ANOVA quinine dose × genotype: F1,51 = 0.83, p > 0.05) for iMSN-Drd2KO (red) and littermate controls (black).
All of the symbols with error bars are means ± SEMs; symbols without error bars represent data from individual animals.
Ethanol consumption was quantified in three ways: intake (volume consumed per body weight), rate of sipper licking, and BECs at the end of weeks 2 and 3. When compared across genotypes, iMSN-Drd2KO mice consumed less ethanol during the first 2-h training sessions, but by the end of the third week of training, the overall ethanol intake was similar for all of the genotypes (Figures 5B and 5C; Drd2loxP/loxP: 2.0 ± 0.2 g/kg/2 h, auto-Drd2KO: 1.5 ± 0.6 g/kg/2 h, and iMSN-Drd2KO: 1.6 ± 0.6 g/kg/2 h; p > 0.05). Licking behavior mirrored ethanol intake for iMSN-Drd2KO mice (Figure 5D). Thus, iMSN-Drd2KO mice show an escalating pattern of drinking behavior, which has been suggested to be a hallmark of addiction liability (Edwards and Koob, 2013). iMSN-Drd2KO mice did not drink more during the time frame tested here; however, if this escalation pattern were to continue, then higher levels of intake could be predicted over time.
Unbiased unsupervised cluster analysis of daily intake on self-administration for all of the mice allowed us to discriminate three patterns of ethanol intake: stable drinkers, escalators, and non-drinkers (Figure S6A). Non-drinkers and stable drinkers showed consistent intake patterns throughout training (Figures S6B–S6D; early sessions [4 and 5]: 1.5 ± 0.3 g/kg/day, late sessions [17 and 18]: 1.5 ± 0.2 g/kg/day), but non-drinkers consumed very little ethanol (early sessions: 0.15 ± 0.08 g/kg/day, late sessions: 0.04 ± 0.02 g/kg/day). Escalators, however, started by consuming low amounts of ethanol in the beginning (0.25 ± 0.16 g/kg/day) and increased their intake over the course of training (2.5 ± 0.4 g/kg/day; Figure S6D). iMSN-Drd2KO mice were more likely to be classified as escalators compared to control and auto-Drd2KO mice (Figures 5E and S6E). The escalation of intake and licking was also apparent during the relapse test, in which access to ethanol was reintroduced after 40 days of abstinence. iMSN-Drd2KO mice showed a 5-fold increase in intake during the 6-h relapse sessions compared to the 6-h training sessions (490% ± 89%), while auto-Drd2KO showed a 3-fold increase (312% ± 78%) and controls barely double intake (183% ± 35%; Figure 5F). The rate of licking was also higher during relapse selectively for iMSN-drd2KO (Figures S5C and S5D). When mice were tested for alcohol seeking in the absence of ethanol, iMSN-Drd2KO mice showed a lower rate of licking than baseline, suggesting that the high rate of licking during relapse was driven by ethanol availability and not just the presence of conditioned cues, such as sipper presentation (Figures S5E and S5F).
Resistance to Quinine Adulteration in Mice with Low Striatal D2Rs
Ethanol drinking despite adverse consequences is a hallmark of AUD. Using the operant paradigm, we tested whether the adulteration of ethanol with an unpleasant bitter tastant, quinine, would deter mice from drinking or seeking ethanol. As anticipated, littermate controls showed a concentration-dependent decrease in ethanol intake during adulteration sessions (0.5 mM: 73% ± 8% reduction and 1 mM: 54% ± 11% reduction) as did mice lacking D2Rs in DA neurons (0.5 mM: 47% ± 14% and 1 mM: 28% ± 11%). iMSN-Drd2KO mice were undeterred by quinine adulteration and showed no significant decrease in ethanol intake (Figure 5G). When iMSN-Drd2KO mice and littermate controls were given access to water with 0.5 or 1 mM quinine, all of the mice decreased their intake of the adulterated water (Figure 5H), indicating that all of the mice are able to taste the quinine and do not prefer it. Furthermore, it is unlikely that these results are due to generalized taste impairment, because iMSN-Drd2KO mice show a preference for sucrose over water (Figure 4G). These data show that while iMSN-Drd2KO mice do not drink more ethanol overall, they show qualitative changes in drinking patterns, including escalation of intake and insensitivity to aversive pairing.
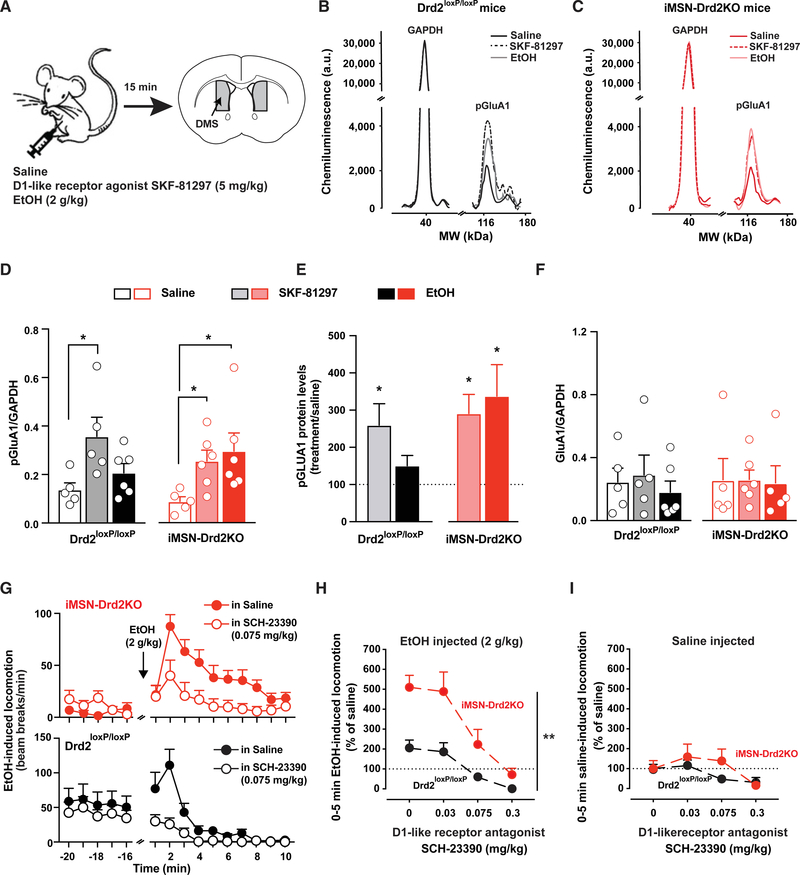
Ethanol Activates D1R-Mediated Intracellular Signaling in the Striatum
We used pharmacological tools to further understand the mechanism by which low expression of medium spiny neuron D2Rs causes enhanced ethanol stimulation and preference. There is substantial evidence that chronic ethanol drives the activation of D1R signaling in the dorsomedial striatum (Wang et al., 2012, 2015). We hypothesized that acute ethanol exposure would also activate D1R activity and lead to protein kinase A (PKA)-dependent phosphorylation of downstream targets, such as pGLUA1 at serine-845. Furthermore, we predicted that iMSN-Drd2KO mice would display enhanced activation of targets downstream of D1R based on our recent findings that mice lacking medium spiny neuron D2Rs display D1R hypersensitivity at both the cellular/signaling and behavioral levels (Dobbs et al., 2019).
Mice were administered either saline, the D1-like agonist SKF-81297 (5 mg/kg), or ethanol (2 g/kg). The dorsomedial striatum (DMS) was processed for quantitative protein analysis (Figures 6A–6C). SKF-81297 enhanced normalized pGluA1 levels in the DMS of both genotypes, which is consistent with previous findings. At the time point tested, following 2 g/kg ethanol, a small, statistically insignificant increase in pGluA1 levels was seen in Drd2loxP/loxP mice compared to when saline was administered (Figures 6D and 6E). However, in iMSN-Drd2KO mice, acute ethanol robustly increased pGluA1 to levels similar to those seen following the D1-like receptor agonist (Figures 6D and 6E). When normalized to total GluA1 levels, the effect of ethanol in iMSN-Drd2KO mice on DMS pGluA1 levels was further pronounced (Figure S7A). No changes were observed in total protein levels for GluA1 (Figure 6F) and glyceraldehyde 3-phosphate dehydrogenase GAPDH (Figure S7B) in the DMS across the genotypes and treatments.
Figure 6. Ethanol-Induced PKA-Dependent Signaling and D1R Activation Are Enhanced in Striatum of iMSN-Drd2KO Mice, and Blockade of D1R Attenuates Ethanol Stimulation.
(A) Schematic describing experimental procedure: iMSN-Drd2KO and littermate control mice were administered saline, SKF-81297, or ethanol. The dorsomedial striatum was dissected for protein level analysis (n = 5–6/group).
(B and C) Representative chemiluminescence traces for phosphorylated GluA1 (pGluA1) and control protein GAPDH in littermate (B) and iMSN-Drd2KO (C) mice following saline (black), SKF-81297 (dash), or ethanol (gray).
(D and F) Protein level quantification as pGluA1 and GAPDH ratio (D) and total GluA1 over GAPDH ratio (F) after saline (open), SKF-81297 (shaded), and ethanol (solid) for control littermate (black) and iMSN-Drd2KO mice (red). (D) pGLUA1 ratio 1-way ANOVA for control littermate mice: F2,13 = 4.45 and iMSN-Drd2KO mice: F2,14 = 3.89, followed by Tukey multiple comparison tests; *p < 0.05. For total GLUA1 ratio (F), 1-way ANOVA for littermate mice: F2,13 = 0.35 and iMSN-Drd2KO: F2,14 = 0.01, p > 0.05.
(E) pGluA1 protein levels after SKF-81297 (shaded) and ethanol (solid) expressed as percentage of saline in littermate (black) and iMSN-Drd2KO (red) mice. *p < 0.05, one-sample t test examining divergence from saline baseline.
(G) Locomotor activity measured in beam breaks/min before and after 2 g/kg ethanol in iMSN-Drd2KO (top, red) and littermate control (bottom, black) mice pretreated with either saline (solid) or SCH-23390 (open; n = 12/group).
(H) SCH-23390 blocks ethanol-induced locomotion in a dose-dependent manner. Two-way RMs ANOVA drug dose × genotype: F3,66 = 3.72; **p < 0.01; main effect of drug: F3,66 = 27.72 and genotype F1,22 = 15.01, p < 0.001 for both.
(I) No or little effect of SCH-23390 on locomotion after saline (2-way RMs ANOVA drug × genotype: F3,66 = 1.29, p > 0.05).
All of the symbols with error bars are means ± SEMs; symbols without error bars represent data from individual animals.
DA D1R Activation Is Required for Ethanol-Induced Stimulation
The protein analysis data show that ethanol exposure mimics exposure to a D1-like agonist in mice lacking D2Rs on medium spiny neurons. Previous work suggests a positive correlation between ethanol-induced stimulation and the sensitivity of D1Rs in the striatal pathway (Abrahao et al., 2014). We speculated that the D1R hypersensitivity in iMSN-Drd2KO mice could be the underlying mechanism driving the enhanced ethanol stimulation and ethanol preference.
We directly examined the effect of a D1-like receptor antagonist, SCH-23390, on ethanol-induced stimulation. Pretreatment with SCH-23390 dose-dependently blocked ethanol-induced locomotion in iMSN-Drd2KO mice and littermate controls (Figure 6G). The inhibition was significant with the pretreatment of 0.075 mg/kg SCH-23390 for both genotypes (Figure 6H; 71% ± 9% inhibition for Drd2loxP/loxP; 56% ± 15% inhibition in iMSN-Drd2KO) and was even larger at 0.3 mg/kg SCH-23390. SCH-23390 alone also decreased locomotion in littermate controls and iMSN-Drd2KO mice (Figures 6I, S7C, and S7D). Mice lacking D2Rs on medium spiny neurons were less sensitive to the acute effects of the D1-like receptor antagonist. In littermate controls, 0.075 g/kg SCH-23390 alone produced a 54% ± 12% suppression of locomotion compared to saline, but the same dose had no effect on locomotion in iMSN-Drd2KO mice (Figure 6I). A larger dose of SCH-23390 (0.3 g/kg) alone suppressed locomotion in both controls and iMSN-Drd2KO mice (Figure 6I), indicating a rightward shift in the dose response.
When measuring locomotion at later time points after ethanol administration, pretreatment with the D1-like receptor antagonist suppressed the long-lasting ethanol-induced stimulatory effects in iMSN-Drd2KO mice (Figures S7E and S7F). In summary, D1R activation is required for the stimulant but not the sedative effects of ethanol in both control mice and mice lacking medium spiny neuron D2Rs.
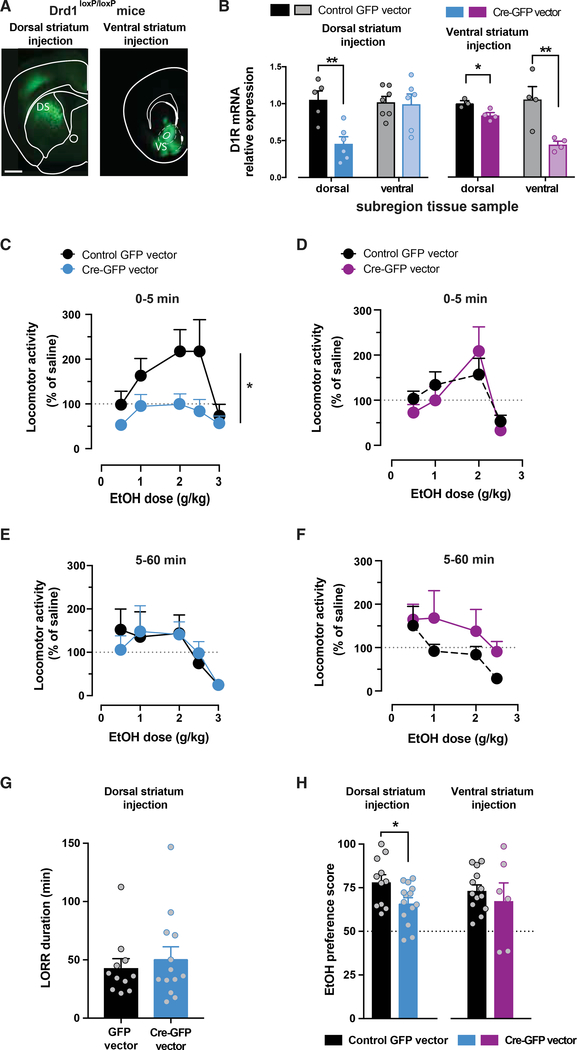
D1Rs Expressed in the Dorsal Striatum Are Required for Ethanol-Induced Stimulation
To determine the regional specificity of the role of D1R in mediating the stimulant effects of ethanol, we selectively knocked down the Drd1 gene in the ventral or the dorsal subregions of the striatum. A viral vector expressing Cre recombinase and GFP, or a control vector expressing only GFP, was injected intracranially in these areas in transgenic mice homozygous for the conditional Drd1 allele (Drd1loxP/loxP; Figure 7A; Sariñana et al., 2014). The degree of Drd1 knockdown was assessed using qPCR analysis of tissue. Targeting the Cre vector to the dorsal subregion resulted in a 54% ± 9% reduction in Drd1 mRNA levels selectively in the dorsal striatum 8 weeks after viral vector injection (Figure 7B). It did not affect Drd1 mRNA levels in the ventral striatum compared to littermate mice that received the control GFP vector. When targeting the ventral striatum, Drd1 mRNA levels were reduced by 56% ± 4% in the ventral region (Figure 7B), and a modest decrease (16% ± 4%) was noted in the dorsal striatum after 8 weeks.
Figure 7. Selective Knockdown of D1R Gene in Dorsal Striatum Ablates Ethanol-Induced Stimulation.
(A) Fluorescent images of coronal brain sections showing the expression site of GFP-Cre in the dorsal (left; n = 12–14/group) and ventral striatum (right; n = 8/group) of Drd1loxP/loxP mice. Scale bar, 1 mm.
(B) Levels of Drd1 mRNA normalized to the control gene in samples from dorsal and ventral striatum of Drd1loxP/loxP mice that received intracranial injection in dorsal (left; n = 5–6/group) or ventral striatum (right; n = 3–4/group) of control viral vector (black) or Cre-expressing vector (blue or purple). Independent sample t test t(9) = 4.01; **p < 0.01 for dorsal samples of dorsal injections, t(6) = 3.43 for ventral samples of ventral injections, and t(5) = 3.21 for dorsal samples of ventral injections; *p < 0.05; all of the samples were run in duplicate.
(C and D) Dose-dependent ethanol stimulation 5 min after ethanol administration in Drd1loxP/loxP mice with Cre-GFP (blue/purple) or control GFP (black) expression targeted to dorsal (C) or ventral (D) striatum. Two-way RMs ANOVA ethanol dose × viral injection: F4,92 = 2.63, *p < 0.05 for dorsal striatum injections. Main effect of ethanol dose: F4,92 = 7.36, p < 0.0001, and viral injection: F1,23 = 5.07, p < 0.05. Ventral striatum injections 2-way RMs ANOVA ethanol dose × injection: F4,64 = 0.51, p > 0.05.
(E and F) Dose-response curve for ethanol sedation, 5–60 min following ethanol administration, in Drd1loxP/loxP mice with Cre-GFP (blue/purple) or control GFP (black) expression targeted to dorsal (E) or ventral striatum (F). Two-way RMs ANOVA ethanol dose × viral injection: F4,88 = 0.39 for dorsal injections and F4,64 = 0.68 for ventral injections, p > 0.05 for both.
(G) LORR duration in Drd1loxP/loxP mice that received control GFP or Cre-GFP viral vectors in dorsal striatum. Independent sample t test: t(21) = 1.2, p > 0.05. (H) Ethanol preference score in 2-bottle choice test for Drd1loxP/loxP mice, with Drd1 knockdown targeted to dorsal (blue, left) or ventral striatum (purple, right) and mice that received a control vector (black). Independent sample t test: t(23) = 2.4; *p < 0.05 for dorsal injections and t(17) = 0.7, p > 0.05 for ventral injections; n = 6–14/group.
All of the symbols with error bars are means ± SEMs; symbols without error bars represent data from individual animals.
The degree of functional loss of D1R activation was assessed by measuring the locomotor response to the D1-like receptor agonist SKF-81297 (7.5 mg/kg) 6 weeks after surgery (data not shown). In the case of the ventral striatum-targeted deletion, the agonist induced robust locomotor activation to a similar degree in both GFP-expressing control mice and Cre-expressing mice (522% ± 64% and 657% ± 90% increase over saline injection for control GFP and Cre, respectively; saline versus SKF; F1,9 = 126.1, p < 0.01; no effect of virus group). However, mice with targeted knockdown of Drd1 in the dorsal striatum showed >50% reduction in SKF 81297-induced locomotion compared to GFP controls (1,020% ± 124% and 489% ± 54% increase over saline injection for control GFP-expressing and Cre-expressing mice; F1,14 = 16.3, p < 0.01). Baseline locomotion was not altered by these manipulations (1,303 ± 673 and 1,616 ± 593 counts per hour for Cre and GFP-control vector, respectively; t(24) = 0.35, p > 0.05). Thus, this combined viral and genetic approach can effectively reduce Drd1 mRNA in select striatal subregions and produce a functional impairment in the behavioral response to the D1-like agonist.
Ethanol-induced stimulation was tested. Selective knockdown of Drd1 mRNA expression in the dorsal striatum dramatically suppressed the acute ethanol stimulation at all doses, causing a downward shift in the dose-response curve (Figure 7C). Littermate mice that received the control GFP vector showed an unaltered stimulatory response to ethanol that peaked at 2 g/kg (Figure 7C). No differences were found between Cre-injected and GFP-injected mice on locomotion at 5–60 min after ethanol administration (Figure 7E) or on the LORR test (Figure 7G), indicating that the sedative effects of ethanol were intact after selective knockdown of Drd1 in the dorsal striatum.
In the ventral striatum, knockdown of Drd1 mRNA expression had no effect on ethanol stimulation as the dose-response curves overlapped (Figure 7D). Ethanol sedation was also intact, as there were no statistical differences in the dose response 5–60 min following ethanol administration (Figure 7F).
These data indicate that activation of the dorsal striatum D1Rs is required for ethanol stimulation and may play a role in ethanol preference and drinking. Ethanol preference was tested using a two-bottle choice test. Mice with decreased dorsal D1Rs demonstrated a decreased preference for ethanol (Figure 7G), while no differences in preference were noted in mice with a knockdown of D1Rs in the ventral striatum. These data indicate the importance of the dorsal striatal D1R in selectively modulating the ethanol-induced stimulation and ethanol preference.
DISCUSSION
This study identifies a link between two well-known risk factors for alcohol abuse in humans: low striatal D2R availability and high sensitivity to the stimulatory effects of ethanol. We propose that striatal D1R hypersensitivity is the substrate linking these vulnerability factors. In a recent publication, we showed D1R hypersensitivity under conditions of low striatal D2Rs in medium spiny neurons (Dobbs et al., 2019). Here, we demonstrate that this upregulation in D1Rs is functionally relevant, with D1Rs driving ethanol stimulation. We also offer direct evidence that low-D2R-expression iMSNs contribute to the vulnerability for alcohol abuse by triggering D1R hypersensitivity, which in turn drives the stimulatory effects of ethanol and renders mice insensitive to the sedative effects of ethanol. It also promotes the preference for ethanol drinking and is associated with the escalation in intake and compulsive-like drinking.
Mouse Models of Ethanol Stimulation Have Clinical Relevance
Clinical studies indicate that individuals who drink heavily are more sensitive to the stimulatory effects of ethanol and consequently more prone to alcohol abuse (Erblich and Earleywine, 2003; Hendler et al., 2013; King et al., 2011, 2016). In mice, locomotor activity is used to examine the stimulatory properties of drugs of abuse, including ethanol. Consistent with the literature, our study finds that ethanol stimulation is short-lived and followed by the onset of sedative effects (Pohorecky, 1977). Furthermore, we found that mice with a high ethanol stimulatory response show an enhanced ethanol preference. This, too, is similar to the clinical condition in which higher perceived ethanol stimulation is associated with a greater propensity toward ethanol intake and abuse liability (Erblich and Earleywine, 2003; Holdstock et al., 2000; King et al., 2011, 2016).
We observed a great deal of individual variability in the stimulatory and sedative responses to ethanol within these inbred mice, further indicating that genetics alone cannot account for the differences. This suggests that environmental and epigenetic factors play also an important role in this phenotype variability.
We also noted sex differences, with female mice showing higher ethanol stimulation and increased ethanol intake when normalized to body weight. These findings could be interpreted as increased vulnerability for alcohol abuse in females. However, in the human literature more men than women report using alcohol and suffering from AUD (Substance Abuse and Mental Health Services Administration, 2016). Non-biological factors, such as reporting bias, differential access to alcohol, or age of drinking onset, may be responsible for the opposing trends between humans and rodents.
There is a possibility that the sex differences could account for the enhanced ethanol stimulation seen in iMSN-Drd2KO mice. However, this seems unlikely because all of the experiments were counterbalanced with sex as a variable, and female iMSN-Drd2KO mice did not show higher stimulation than males.
Role of DA D2R in Vulnerability for Alcohol Abuse
Lower levels of striatal D2R availability have been associated with addictive behaviors, including AUD. In humans, positron emission tomography (PET) imaging studies show lower D2R availability in individuals who abuse alcohol (Volkow et al., 1996, 2002), and striatal D2R availability predicts how healthy subjects perceive intoxication following alcohol exposure (Yoder et al., 2005). In rodents, chronic alcohol consumption reduces striatal D2R availability (Feltmann et al., 2018), and the overexpression of D2Rs in alcohol-preferring rats causes a transient reduction in ethanol preference and intake (Thanos et al., 2001). Thus, decades of clinical and preclinical work support a link between low striatal D2R levels, alcohol drinking, and preference.
In a transgenic mouse line with an extreme manipulation of D2R levels (full D2RKO), ethanol stimulation is enhanced, while sedation is absent (Palmer et al., 2003; Phillips et al., 1998; Risinger et al., 2000). These findings suggested a causal link between D2R downregulation and ethanol-induced stimulation, but the mechanism remained unknown, as a full knockout does not allow for the discrimination of specific subpopulations of striatal D2Rs contributing to the association/vulnerability.
By using transgenic mouse models with cell-type-specific knockout of D2Rs, we were able to dissect out the contributions of each population of D2Rs in ethanol-related behaviors and vulnerability factors. This specific circuitry dissection is a benefit of transgenic mouse lines. However, the use of transgenic mice also adds the complexity of disentangling the acute effects of the gene knockout and the developmental compensations.
In the present study, we found that mice with selective deletion of D2Rs on striatal medium spiny neurons (iMSN-Drd2KO) showed the greatest reduction in striatal D2R binding, have a heightened stimulant response to ethanol, and are the most resistant to the sedative effects of ethanol. When normalized, iMSN-Drd2KO mice show a dramatic upward shift in ethanol stimulatory response. When consulting the raw data, ethanol restores movement in these mice, which have a consistent hypolocomotive phenotype. A valid interpretation of these findings is that the actions of ethanol on D1R signaling are restoring locomotion to normal levels. iMSN-Drd2KO mice also exhibited an increased preference for ethanol, higher rates of escalation of ethanol drinking, and persistent drinking, despite adverse consequences. Meanwhile, mice with targeted deletion of D2Rs on striatal cholinergic interneurons or midbrain DA neurons showed a modest reduction in striatal D2R binding and no significant alterations in the stimulant or sedative effects of ethanol and related behaviors. Because iMSN-Drd2KO mice show an ethanol-induced stimulatory and sedative phenotype similar to that of the global D2R knockout, we conclude that D2Rs specifically on medium spiny neurons are driving the behavior.
While our findings provide direct evidence for a link between low striatal D2Rs, ethanol stimulation, ethanol preference, and escalation, iMSN-Drd2KO mice do not show a higher ethanol intake in DID or self-administration compared to controls. It is unclear why this is the case, but it is possible that mice lacking medium spiny neuron D2Rs are more sensitive to the reinforcing properties of ethanol and therefore do not need to consume as much to achieve the same intoxication state. The reference to “intoxication state” refers to mechanisms downstream of blood ethanol levels, because we demonstrated that BECs are not different across genotypes. An alternative explanation is that impairments in learning are limiting the initial level of ethanol intake in the operant self-administration task. The data indicate that these mice can learn to self-administer ethanol and suggest that given longer access periods, they could surpass littermate controls in ethanol intake.
iMSN-Drd2KO mice also show higher resistance to the adulteration of ethanol with quinine and continue to drink despite the averse taste. In ruling out a taste impairment and confirming that the resistance to taste adulteration was selective for ethanol, these findings could be interpreted as a sign of compulsive-like drinking.
Changing the Balance of Striatal Circuitry Drives Aberrant Ethanol Intake
Decades of research on basal ganglia circuit function and models of Parkinson disease indicate that the balance between the activity and signaling of D2R and D1R is critical for striatal circuitry function (Dobbs et al., 2017; Freeze et al., 2013; Gerfen et al., 2002; Lobo and Nestler, 2011). Our previous findings indicate that a selective reduction of D2Rs on iMSNs does not affect striatal Drd1 mRNA levels but rather leads to a functional upregulation and signaling shift of striatal D1R (Dobbs et al., 2019). Administration of a D1-like receptor agonist known to increase downstream D1R targets, such as pGluA1 at serine-845 in the DMS, was confirmed in the present study. iMSN-Drd2KO mice also show a robust increase in pGluA1 levels following ethanol, indicating enhanced D1R activation following ethanol. Using a viral knockdown approach, we further illustrated that D1Rs in the DMS are necessary for driving the stimulatory actions of ethanol.
D1R hypersensitivity is likely an adaptive response to the loss of striatal D2Rs, which we previously showed causes an increase in the GABAergic inhibition of D1R-expressing medium spiny neurons in the striatum (Lemos et al., 2016). At the same time, D1R hypersensitivity further disrupts the balance of D1R and D2R activity. We propose here that this imbalance in striatal D1R-D2R control of the circuitry generates abuse-vulnerable circuitry in which individuals are more sensitive to ethanol stimulation, and show ethanol preference, escalation, and compulsive-like use.
Concluding Remarks
These findings serve to settle a long-standing debate as to whether low striatal D2Rs are a cause of alcohol consumption (Everitt et al., 2008; Volkow and Baler, 2014). The present study demonstrates that the manipulation of striatal D2R levels on medium spiny neurons promotes ethanol-related phenotypes associated with abuse in humans, suggesting that it is a key predisposing factor for disordered alcohol intake.
The present study offers direct evidence that low levels of striatal D2Rs promote ethanol stimulation via the functional upregulation of D1R, which in turn drives maladaptive behaviors toward alcohol. These mechanistic findings suggest that an alteration of the balance of D1R-D2R activity promotes a state of striatal circuitry that predisposes individuals to alcohol abuse.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Veronica Alvarez (alvarezva@mail.nih.gov). This study did not generate new unique reagents.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All procedures were performed in accordance with guidelines from the NIAAA Animal Care and Use Committee. For all experiments, we use male and female mice (C57Bl6J background, p45–150 at start of experiment) that were group-housed unless otherwise specified on 12h:12h light cycle (6:30am on, 6:30pm off) with standard rodent chow and water available ad libitum, unless otherwise stated. All mouse lines used for breeding are commercially available and listed in Key Resources Table. Homozygous iMSN-Drd2KO (Adora2a-Cre+/−; Drd2loxP/loxP) mice were generated by crossing Drd2loxP/loxP mice, which carry the conditional allele for Drd2, with Adora2a-Cre+/− mice, which express Cre recombinase under the adenosine 2a receptor promoter. Auto-Drd2KO (DatiresCre+/−; Drd2loxP/loxP) mice were generated by crossing Drd2loxP/loxP mice with DatiresCre+/− mice, which express Cre recombinase under the DAT promoter. Cin-Drd2KO mice were generated by crossing Drd2loxP/loxP mice with ChatiresCre+/− mice, which express Cre recombinase under the Chat promoter. For all experiments, Cre negative Drd2loxP/loxP littermates were used as controls. Drd1loxP/loxP mice were used in viral vector injection studies. All mice were genotyped at weaning using real-time PCR with their respective probes by Transnetyx (Cordova, TN).
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit anti-glutamate receptor 1, phosphoSer 845 | Millipore | AB5849; RRID: AB_92079 |
| rabbit anti-glutamate receptor 1 | Abcam | 109450; RRID: AB_10860361 |
| mouse anti-GAPDH | Ambion | AM4300; RRID: AB_437392 |
| HRP-conjugated secondary anti-mouse antibody | ProteinSimple | DM-002 |
| HRP-conjugated secondary anti-rabbit antibody | ProteinSimple | DM-001 |
| Bacterial and Virus Strains | ||
| AAV9.CMV.HI.eGFP-Cre.WPRE.SV40 | Penn Vector Core | AV-9-PV2004, lot #CS1247-RP |
| AAV2/9.CB7.CI.EGFP.RBG | Penn Vector Core | AV-2-PV1046, lot #V1420 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Ethanol 190 Proof | Decon Laboratories | 2801G |
| Saline | Hospira | NDC 0409–4888–06 |
| Sucrose | Sigma-Aldrich | S7903 |
| SCH-23390 | abcam | ab120597 |
| SKF-81297 | Tocris | 1447 |
| Quinine hemisulfate monohydrate | Sigma-Aldrich | Q1250 |
| RIPA Lysis and Extraction Buffer | ThermoScientific | 89901 |
| Halt Protease and Phosphatase Inhibitor Cocktail (100X) | ThermoScientific | 78446 |
| iScript Reverse Transcription Supermix | BioRad | 1708841 |
| RNAlater Stabilization Solution | Ambion | AM7020 |
| Actb TaqMan Gene Expression Assays | Applied Biosystems | Mm01205647 |
| Drd1 TaqMan Gene Expression Assays | Applied Biosystems | Mm02620146_s1 |
| [3H]-methylspiperone | PerkinElmer | NET856250UC |
| ScintiVerse BD Cocktail | Fisher Scientific | SX18–4 |
| Ketanserin (+)-tartrate salt | Millipore Sigma | S006 |
| (+)-Butaclamol hydrochloride | Millipore Sigma | D033 |
| Polyethyleneimine | Millipore Sigma | 181978 |
| Critical Commercial Assays | ||
| BEC Assay kit for Analox GM7 Micro-stat analyzer | Analox | GMRD-113 |
| 12–230 kDa Wes Separation Module | ProteinSimple | SM-W004 |
| Pierce BCA Protein Assay Kit | ThermoScientific | 23227 |
| RNeasy Plus Mini kit | QIAGEN | 74136 |
| Experimental Models: Organisms/Strains | ||
| Mouse: Drd2loxP/loxP: B6.129S4(FVB)-Drd2tm1.1Mrub/J | JAX | 020631 |
| Mouse: Cin-Cre: B6;129S6-Chattm2(cre)Lowl/J | JAX | 006410 |
| Mouse: Adora2a-Cre: Tg(Adora2a-cre)KG139Gsat/Mmucd | MMRC | 031168-UCD |
| Mouse: DAT-Cre: B6.SJL-Slc6a3tm1.1(cre)Bkmn/J | JAX | 006660 |
| Mouse: Drd1loxP/loxP: Drd1tm2.1Stl/J | JAX | 025700 |
METHOD DETAILS
Outline of Experimental Design
Experiment 1: Control mice of the Drd2loxP/loxP line were tested on ethanol-induced locomotion using beam breaks. In order to examine dose dependence mice were administered either saline or ethanol (0.5, 1.0, 2.0, 2.5 or 3.0 g/kg; 10 mL/kg) i.p. using a Latin-square design over 6 consecutive days (Figures 1A–1C, S1A–S1D, and S2A–S2D). A subgroup of mice was also tested on the LORR test 1 week after locomotion test ended (data presented in Figures 1D and 1E).
Experiment 2: Adora2a-Cre−/− control mice from the Adora2a-Cre line were tested on the LORR, and then DID protocol (Figures 1E–1H, 1K, 1L, and S1E).
Experiment 3: A naive group of Drd2loxP/loxP mice were first tested on ethanol-induced locomotion and received either saline or ethanol (2 g/kg). Mice were then tested for ethanol drinking preference for 3 days using the 2-bottle choice test and given access to ethanol for 3 weeks using the DID protocol (Figures 1H–1J).
Experiment 4: Adora2a-Cre+/− mice and Adora2a-Cre−/− littermate controls were tested on ethanol-induced locomotion and mice received either saline or ethanol (2.5 g/kg; Figures S4A and S4B).
Experiment 5: Drd2 availability was analyzed in striatal tissue samples from naive Cin-Drd2KO, auto-Drd2KO and iMSN-Drd2KO mice and littermate controls (n = 4–5/group) using radioligand binding (Figure 2).
Experiment 6: Cin-Drd2KO, auto-Drd2KO and iMSN-Drd2KO mice and their littermate controls were tested on the ethanol-induced locomotion and mice were administered either saline or varying doses of ethanol (0.5, 1.0, 2.0, 2.5 or 3.0 g/kg, 10 mL/kg) i.p. using a Latin-square design (Figures 1A–1F and S3). The week following the locomotor testing, mice were tested on LORR (Figures 3A, 3B, 3D, 3E, and 3G–3J). To measure ethanol metabolism, a separate group of Cin-Drd2KO, auto-Drd2KO and iMSN-Drd2KO mice and littermate controls were injected with saline and ethanol, at varying doses (1.0, 2.0, or 3.0 g/kg; 10 mL/kg) i.p. using a Latin-square design and tail blood was obtained 5- and 60-minutes following injections and analyzed for BEC (Figures 3C and 3F).
Experiment 7: Auto-Drd2KO and iMSN-Drd2KO mice and littermate controls were tested on ethanol-induced locomotion and mice received either saline or ethanol (2 g/kg). Following locomotor testing, mice were tested for ethanol preference in a two-bottle choice paradigm, and then allow access to ethanol for 3 weeks on a DID protocol (Figures 4A–4D, 4F, and 4G). Following DID, mice were trained on the ethanol self-administration task (Figures 5A–5G). A separate group of auto-Drd2KO and iMSN-Drd2KO mice and littermate controls were tested for sucrose preference using a two-bottle choice paradigm for 3 days (data in Figure 4G).
Experiment 8: control mice (Drd2loxP/loxP) were run on ethanol-induced locomotion test and mice were pretreated with either saline or naloxone (1 mg/kg) 15 min before receiving saline or ethanol (2 g/kg) using a counterbalanced design (Figure S7).
Experiment 9: iMSN-Drd2KO mice and littermate controls were run on ethanol-induced locomotion and mice were pretreated with either saline or 3 different doses of SCH-23390 (0.03, 0.075, 0.3 mg/kg) 15 min before receiving saline or ethanol (2 g/kg) using a counterbalanced design (Figure 6).
Experiment 10: iMSN-Drd2KO and littermate control mice (n = 5–6/group) were administered i.p. saline, SKF-81297 (5 mg/kg) or ethanol (2 mg/kg). Brains were harvested 15 min later and the dorsomedial striatum (DMS) was dissected for protein level analysis using the WES method (Figure 6).
Experiment 11: Drd1loxP/loxP mice were injected in the dorsal or ventral striatum with Cre-expressing or GFP-expressing control viral vectors using stereotaxic methods. After 4 weeks, mice were tested on ethanol-induced locomotion and mice were administered either saline or ethanol (0.5, 1.0, 2.0, 2.5 or 3.0 g/kg, 10 mL/kg) i.p. using a Latin-square design (data presented in Figures 7C–7F). Mice were then tested on the ethanol drinking preference using a two-bottle choice paradigm (Figure 7G). Mice with viral injections in the dorsal striatum were further tested on the LORR (Fig H-1). At the completion of all behavioral studies, striatia were dissected for confirmation of viral injection targeting using fluorescence visualization of viral expression. Drd1 expression was quantified in a subgroup of mice using qPCR (Figure 7B).
Behavioral Testing
Ethanol-Induced Locomotion
Naive mice (6–20 weeks old) were used for all experiments. Sample size determination sufficient for the detection of between-group differences was based on previous data from our laboratory. On average, 6 mice per sex/per group/ genotype were used. Testing was done during the light phase of the cycle. Mice were transferred to the experimental room and acclimated for 1 h before they were placed in polycarbonate chambers (20 cm H × 17 cm W × 28 cm D) equipped with infrared photobeam detectors (Columbus Instruments). Beam breaks were recorded for 1 h for habituation to locomotor chambers before mice received i.p. injections and beam breaks were recorded for an additional hour. Mice received saline 10 mL/kg, i.p. for days 1–3 in order to habituate to handling and injections. Subsequent days, during the testing phase, mice were administered either saline or ethanol (0.5, 1.0, 2.0, 2.5 or 3.0 g/kg, 10 mL/kg) i.p. using a Latin-square design over 6 consecutive days. When testing antagonists, mice were habituated to the chambers for 1 h as before. They received pretreatment with either saline or antagonist (10 mL/kg, i.p.). 15 min before receiving either ethanol (2 g/kg) or saline and beam breaks were recorded for an additional hour.
Loss of Righting Reflex
Mice were transferred to the experimental room and acclimated for 1 h before they were injected with ethanol (3.5 g/kg, 10 mL/kg, i.p.) and placed supine in a v-shaped trough. The time to lose the righting-reflex was defined as the time from injection to when the mouse showed an inability to right itself within a 30 s time interval, was recorded. The mice were then left undisturbed until they began to regain the righting reflex (flip themselves over unaided). Once the mouse self-rights, it is again placed supine. When the mouse can self-right three times within 30 s, the time of righting is recorded. The LORR time was defined as the time between losing the reflex and regaining it. Tail blood samples were immediately obtained following righting or at the termination of the study for BEC measurements. After 90 minutes, if a mouse did not lose its righting reflex, the experiment was terminated, and blood samples were obtained for BEC measurements.
Two-Bottle Preference Test for Ethanol-, Sucrose-, or Quinine-Adulterated Water
Mice were singly housed and for three days had ad libitum access to rodent chow, water, and test solution (either 1% sucrose (w/v), 20% ethanol (v/v) or water adulterated with quinine (0.5 mM or 1 mM)). Concentrations were selected based on the literature and previous studies (Hwa et al., 2011; Lewis et al., 2005). The test solution was prepared by diluting sucrose (Sigma-Aldrich) or 95% alcohol (190 proof, stored in glass, Deacon Labs) in tap water. Solutions were presented in glass tubes (25 × 100 mm, Pyrex®) fitted with straight, open-tipped metal sippers. Glass tubes were weighed every 24 hours to determine intake, and the position of the water and test solution tubes were switched. Procedure was repeated daily for 4 days. Preference was calculated as the ratio of the volume of test solution consumed divided by the total volume consumed in 24 hours and averaged for the final 3 days.
Drinking-in-the-Dark (DID) Procedure
Mice were acclimated to being singly-housed in a reversed-light-cycle room for at least one week before the start of the experiment. Three hours into the dark cycle, water bottles were replaced with bottles containing 20% ethanol (v/v). Four hours later, alcohol bottles were removed, and water bottles were returned to the cage. Intake was determined by change in bottle weight. Food was available ad libitum. This procedure was repeated 5 days per week, for 3 weeks. Blood ethanol concentrations (BECs) were determined twice a week.
Blood Ethanol Concentration (BEC) Measurements
Blood samples were collected twice a week, immediately following DID sessions. The tail vein was nicked with a razor blade and 15–50 μL of blood was collected in heparinized capillary tubes. Tubes were centrifuged for 5 min, and plasma was isolated. BECs in plasma samples were analyzed in duplicate using the Analox analyzer GM7 MicroStat (Analox Instruments, Lunenburg, MA).
Operant Self-Administration
The SIPPER training procedure was adapted from Blegen et al. (2018). Mice were pre-exposed to 3 weeks of modified DID in their home cages as described above. Mice were then trained to self-administer ethanol (20% v/v) in operant chambers (Med Associates). Each chamber is fitted with two levers (active and inactive), a cue light and a retractable lixit sipper and is housed in a sound-attenuating box with a ventilation fan. The retractable sipper is attached to a 5ml glass pipette containing the ethanol solution, which allows for precise measurements of the volume consumed. A lickometer attached to the sipper records contacts. Presses on the active lever result in sipper extension into the chamber, providing 1 min of access to the ethanol solution. Presses on the inactive lever are recorded but have no consequence. The cue light located above the active lever is illuminated as default and it turns off after lever press and while the sipper is extended. Acquisition of operant behavior began with 3 intermittently spaced days of 6h training sessions during which each active lever press resulted in an alcohol access period (FR1). As training progressed, session length is shortened to 2 h, 5 days a week, at FR1. After 12 sessions at these parameters, holding all other variables constant, the fixed ratio was increased to 3 (FR3) for the final 3 days of training. A food pellet was made available in the operant chamber. Because of no significant sex differences in operant behaviors, the data from males and females were collapsed. Data are presented as responding or intake per session.
Quinine Adulteration
Ethanol consumption despite negative consequences was measured as persistence of drinking after ethanol was adulterated with bitter tasting quinine. Following the third day of the FR3 training session, ethanol was adulterated during two consecutive days with increasing doses of quinine (0.5 and 1mM) and operant responses and ethanol consumption were recorded. Quinine adulteration sessions were 2 h long at FR3.
Ethanol-Seeking Test
After quinine adulteration, mice were kept in their home cages for 23 days in a forced ethanol abstinence period with ad libitum access to water and food. After abstinence, mice were brought back to the operant chambers and tested for ethanol seeking behavior. The ethanol seeking session was similar to the late operant training sessions (lasting 2h, FR3), with one change. When the active lever was pressed, the sipper was presented for 1 min, but no solution was made available. The number of presses and number of licks were recorded.
Relapse Test
Following the seeking test, mice were housed in their home cages for 18 days with ad libitum access to water and food. After a total of 41 days of forced ethanol abstinence, mice were tested on a relapse test. They were allowed to self-administer ethanol for 6h at FR3. Alcohol intake, lever presses and licks were recorded.
Classification Criteria
Using unsupervised hierarchical cluster analysis, mice were classified into distinct groups based on their daily alcohol intake during the 2-hour training sessions (16 sessions in total). A pairwise distance matrix of the square of the difference of the daily alcohol intake was calculated and used as the input data to generate an unsupervised hierarchical cluster. Agglomerative hierarchical clustering was generated according to the Ward’s minimum variance criterion using the function hclust from the basic stats package of the R software (version 3.4.2) and the optimal number of clusters was defined according to multiscale bootstrap resampling (n = 1000, alpha = 95%) using the pv.clust function from the pv.clust package. The best-fit tree was plotted using the ggplot2 package.
Stereotaxic Viral Injection
Mice were placed in a stereotaxic frame under isoflurane anesthesia and bilaterally infused with a viral vector expressing Cre (AAV9.CMV.HI.eGFP-Cre.WPRE.SV40; U Penn) or a control vector (AAV2/9.CB7.CI.EGFP.RBG, U Penn) into the ventral or dorsal striatum. Viral injections (200 nL/injection) were delivered at a rate of 100 nL/min and the injection needle was kept in place for 4 min following the infusion to promote proper diffusion and prevent backflow. Stereotaxic coordinates for the ventral striatum were (mm from bregma): +1.4 AP, ± 1.2 ML, −5.0 DV. Stereotaxic coordinates for the dorsal striatum were: +0.5, +1.0 AP, ± 1.5, ± 1.75 ML, −3.75 DV. Behavioral experiments began 4 weeks after viral injections. Viral expression was confirmed by fluorescence visualization and qPCR.
Quantitative Polymerase Chain Reaction
Mice were anesthetized with pentobarbital and decapitated. Brains were removed, and the striatum was dissected on ice using a 1mm coronal matrix, placed in RNAlater, homogenized, and total RNA was purified using RNeasy Plus Mini kit (QIAGEN). cDNA was synthesized using iScript Reverse Transcription Supermix (Biorad). Actb (Mm01205647) and Drd1 (Mm02620146_s1) TaqMan Gene Expression Assays (Applied Biosystems) were used to determine relative mRNA expression of the endogenous control gene β-actin and DA D1Rs, respectively. Samples were run in triplicate and in parallel with negative controls using the StepOnePlus Real-Time PCR system (Applied Biosystems). The cycling conditions were: initial hold at 95°C (20 s), 40 cycles of 95°C (1 s) and 60°C (20 s). Relative D1 receptor expression was calculated using the ΔΔCt method.
Radioligand Binding Assays
D2 receptor expression in mouse striata was measured using radioligand binding saturation assays. To decrease variability, striata from littermate-paired male mice were used. Mice were deeply anesthetized with isoflurane, brains were extracted, the striatum was rapidly dissected on ice and immediately frozen until further processed. Striata were lysed in Dounce homogenizers using 5 mM Tris-HCl and 5 mM MgCl2 at pH 7.4 and 4°C. The resulting membranes were pelleted by centrifugation at 30,000 × g for 30 minutes and then were resuspended in 50 mM Trizma at pH 7.4 and 4°C. Membrane preparations (400 μL) were added to 500 μL of the indicated concentrations of [3H]-methylspiperone (PerkinElmer, Waltham, MA) plus 100 μL of buffer or drugs for a final reaction volume of 1 mL. Binding was carried out in the presence of 50 nM ketanserin to block binding to 5-HT2-like receptors (Hamblin et al., 1984). Non-specific binding was determined in the presence of 4 μM (+)-butaclamol. After a 90 min incubation at room temperature, bound ligand was separated from free by filtration through Whatman GF/B filter paper pretreated with 0.3% polyethyleneimine using a Brandel Harvester (Brandel, Gaithersburg, MD). Filter paper was washed 3 times in ice-cold 50 mM Trizma buffer. After drying, filter paper samples were punched in to scintillation vials and mixed with 3 mL ScintiVerse BD Cocktail. Radioactivity bound to the filters was counted with a Beckman LS6500 scintillation counter.
Capillary Electrophoresis
Mice were administered either SKF-81297 (5 mg/kg), saline or ethanol (2 g/kg) i.p. 15 minutes later they were anesthetized with pentobarbital and the dorsal medial striatum was rapidly dissected on ice. The DMS was dissected between +1.18 and + 0.74 mm anterior from bregma, −2.25 to −3.75 mm below brain surface and ± 0.75 to 1.25 mm medial from midline. Tissue was flash frozen in liquid nitrogen and stored at −80°C until homogenized in ice cold RIPA buffer (ThermoScientific) spiked with Halt protease and phosphatase inhibitor cocktail (ThermoScientific). Protein amounts in the supernatant were quantified using the Pierce BCA assay (ThermoScientific), aliquoted, and frozen at –80°C. Capillary electrophoresis was performed using the fully automated Wes system (ProteinSimple) following the manufacturer’s recommendations. Briefly, 2 mg/mL of protein lysate were mixed with the fluorescent master mix. The samples and protein standard were denatured for 5 min at 95°C. Samples were loaded into 12- to 250-kDa separation matrix microplates (ProteinSimple) containing blocking buffer, primary and secondary antibodies, and wash buffer for automated, sequential processing. The chemiluminescence based electrophoretogram was auto generated and area under the chemiluminescent peaks was determined using Compass software (ProteinSimple). All samples were run in duplicate across microplates. Primary antibodies were rabbit anti-glutamate receptor 1, phosphoSer 845 (1:25; Millipore #AB5849), rabbit anti-glutamate receptor 1 (1:50; Abcam #109450) and mouse anti-GAPDH (1:2000; Ambion #AM4300). HRP-conjugated secondary anti-mouse and anti-rabbit antibodies (ProteinSimple) were used at the predefined concentrations provided by the manufacturer.
Drugs
Ethanol (Decon Laboratories, 190 proof, in glass container) was dissolved in tap water at 20% v/v. Quinine (Sigma-Aldrich) was dissolved in 20% alcohol. SCH-23390 (abcam) and SKF-81297 (hello bio) were dissolved in saline. Intraperitoneal (i.p.) drug administration was delivered at 10 ml/kg body weight.
QUANTIFICATION AND STATISTICAL ANALYSIS
Analyses were performed in Prism 7 (GraphPad). All statistical analysis is presented in the figure legends. Data from ethanol induced locomotion, blood ethanol concentrations, loss of righting reflex, preference testing, drinking in the dark, ethanol self-administration, and dopamine radioligand binding were analyzed using two-way ANOVA, with the addition of repeated-measures (RM) as appropriate. Significant main effects or interactions were followed-up with pairwise tests corrected for multiple comparisons. Paired or independent sample t test was used to analyze remainder of data. Results were considered significant at an alpha ≤ 0.05. All data are presented as mean ± SEM. Sample sizes were chosen based on previous research employing similar approaches and are sufficient for detecting strong effect sizes while using the minimal number of animals.
DATA AND CODE AVAILABILITY
This study did not generate code. The datasets supporting the current are available from the corresponding author on request.
Supplementary Material
Highlights.
Heightened ethanol stimulation has predictive value for ethanol abuse
Low D2 receptors on striatal projection neurons heightens ethanol stimulation
D2 receptors are linked to ethanol preference and drinking, despite adverse outcomes
Enhanced D1 receptor signaling is a key mechanism underlying abuse liability
ACKNOWLEDGMENTS
This study was funded by the Intramural Research Programs of the NIAAA and NINDS (ZIA-AA000421 to V.A.A.); a postdoctoral research associate training fellowship from NIGMS (Fi2GM117604–01 to M.E.B.); an AMGEN fellowship (NIH-OD to V.K. and M.E.B.); and the Center on Compulsive Behaviors, NIH, Director’s Challenge Award and DDIR Innovation Award (to V.A.A.). Special thanks to Dr. Christina Gremel (UCSD) for feedback on the manuscript and to Katie Teresi for designing the graphical abstract.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.09.059.
REFERENCES
- Abrahao KP, Goeldner FO, and Souza-Formigoni MLO (2014). Individual differences in ethanol locomotor sensitization are associated with dopamine D1 receptor intra-cellular signaling of DARPP-32 in the nucleus accumbens. PLoS One 9, e98296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accili D, Fishburn CS, Drago J, Steiner H, Lachowicz JE, Park BH, Gauda EB, Lee EJ, Cool MH, Sibley DR, et al. (1996). A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc. Natl. Acad. Sci. USA 93, 1945–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, and Borrelli E (1995). Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377, 424–428. [DOI] [PubMed] [Google Scholar]
- Becker HC, and Ron D (2014). Animal models of excessive alcohol consumption: recent advances and future challenges. Alcohol 48, 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, and Rubinstein M (2011). Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci 14, 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blegen MB, da Silva E Silva D, Bock R, Morisot N, Ron D, and Alvarez VA (2018). Alcohol operant self-administration: investigating how alcoholseeking behaviors predict drinking in mice using two operant approaches. Alcohol 67, 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Perrault G, and Sanger DJ (1997). Evidence for the involvement of dopamine receptors in ethanol-induced hyperactivity in mice. Neuropharmacology 36, 1099–1108. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Young ER, Deutsch CM, Tam BR, and Kosobud A (1987). Mice genetically selected for differences in open-field activity after ethanol. Pharmacol. Biochem. Behav 27, 577–581. [DOI] [PubMed] [Google Scholar]
- Dobbs LK, Lemos JC, and Alvarez VA (2017). Restructuring of basal ganglia circuitry and associated behaviors triggered by low striatal D2 receptor expression: implications for substance use disorders. Genes Brain Behav. 16, 56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LK, Kaplan AR, Bock R, Phamluong K, Shin JH, Bocarsly ME, Eberhart L, Ron D, and Alvarez VA (2019). D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors. Neuropsychopharmacology 44, 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, and Koob GF (2013). Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav. Pharmacol 24, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, and Earleywine M (2003). Behavioral undercontrol and subjective stimulant and sedative effects of alcohol intoxication: independent predictors of drinking habits? Alcohol. Clin. Exp. Res 27, 44–50. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, and Robbins TW (2008). Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. B Biol. Sci 363, 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltmann K, Borroto-Escuela DO, Rüegg J, Pinton L, de Oliveira Sergio T, Narváez M, Jiminez-Beristain A, Ekström, Fuxe K, and Steensland P (2018). Effects of long-term alcohol drinking on the dopamine D2 receptor: gene expression and heteroreceptor complexes in the striatum in rats. Alcohol Clin. Exp. Res 42, 338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze BS, Kravitz AV, Hammack N, Berke JD, and Kreitzer AC (2013). Control of basal ganglia output by direct and indirect pathway projection neurons. J. Neurosci 33, 18531–18539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo EF, Salling MC, Feng B, Morón JA, Harrison NL, Javitch JA, and Kellendonk C (2015). Upregulation of dopamine D2 receptors in the nucleus accumbens indirect pathway increases locomotion but does not reduce alcohol consumption. Neuropsychopharmacology 40, 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, and Surmeier DJ (2011). Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci 34, 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, and Brown P (2002). D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J. Neurosci 22, 5042–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, and Hasin DS (2015). Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin MW, Leff SE, and Creese I (1984). Interactions of agonists with D-2 dopamine receptors: evidence for a single receptor population existing in multiple agonist affinity-states in rat striatal membranes. Biochem. Pharmacol 33, 877–887. [DOI] [PubMed] [Google Scholar]
- Hendler RA, Ramchandani VA, Gilman J, and Hommer DW (2013). Stimulant and sedative effects of alcohol. Curr. Top. Behav. Neurosci 13, 489–509. [DOI] [PubMed] [Google Scholar]
- Hietala J, West C, Syvälahti E, Någren K, Lehikoinen P, Sonninen P, and Ruotsalainen U (1994). Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl.) 116, 285–290. [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, and de Wit H (2000). Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol. Clin. Exp. Res 24, 789–794. [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, and Miczek KA (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol. Clin. Exp. Res 35, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, and Cao D (2011). Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch. Gen. Psychiatry 68, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Hasin D, O’Connor SJ, McNamara PJ, and Cao D (2016). A prospective 5-year re-examination of alcohol response in heavy drinkers progressing in alcohol use disorder. Biol. Psychiatry 79, 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, and Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, and Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Friend DM, Kaplan AR, Shin JH, Rubinstein M, Kravitz AV, and Alvarez VA (2016). Enhanced GABA Transmission Drives Bradykinesia Following Loss of Dopamine D2 Receptor Signaling. Neuron 90, 824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, and Bodnar RJ (2005). Inbred mouse strain survey of sucrose intake. Physiol. Behav 85, 546–556. [DOI] [PubMed] [Google Scholar]
- Liljequist S, Berggren U, and Engel J (1981). The effect of catecholamine receptor antagonists on ethanol-induced locomotor stimulation. J. Neural Transm. (Vienna) 50, 57–67. [DOI] [PubMed] [Google Scholar]
- Lobo MK, and Nestler EJ (2011). The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front. Neuroanat 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, and Alvarez VA (2017). Alcohol and basal ganglia circuitry: animal models. Neuropharmacology 122, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, and Swift RM (1993). Development and validation of the Biphasic Alcohol Effects Scale. Alcohol. Clin. Exp. Res 17, 140–146. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Low MJ, Grandy DK, and Phillips TJ (2003). Effects of a Drd2 deletion mutation on ethanol-induced locomotor stimulation and sensitization suggest a role for epistasis. Behav. Genet 33, 311–324. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Brown KJ, Burkhart-Kasch S, Wenger CD, Kelly MA, Rubinstein M, Grandy DK, and Low MJ (1998). Alcohol preference and sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat. Neurosci 1, 610–615. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA (1977). Biphasic action of ethanol. Biobehav. Rev 1, 231–240. [Google Scholar]
- Read GW, Cutting W, and Furst A (1960). Comparison of excited phases after sedatives and tranquilizers. Psychopharmacology (Berl.) 1, 346–350. [DOI] [PubMed] [Google Scholar]
- Reilly MT, Noronha A, Goldman D, and Koob GF (2017). Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology 122, 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Rubinstein M, Low MJ, and Grandy DK (2000). Lack of operant ethanol self-administration in dopamine D2 receptor knockout mice. Psychopharmacology (Berl.) 152, 343–350. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, et al. (1997). Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90, 991–1001. [DOI] [PubMed] [Google Scholar]
- Sariñana J, Kitamura T, Künzler P, Sultzman L, and Tonegawa S (2014). Differential roles of the dopamine 1-class receptors, D1R and D5R, in hippocampal dependent memory. Proc. Natl. Acad. Sci. USA 111, 8245–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2016). 2015 National Survey on Drug Use and Health (Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services; ). [PubMed] [Google Scholar]
- Tepper JM, Abercrombie ED, and Bolam JP (2007). Basal ganglia macrocircuits. Prog. Brain Res 160, 3–7. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, and Hitzemann R (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. J. Neurochem 78, 1094–1103. [DOI] [PubMed] [Google Scholar]
- Tupala E, Hall H, Bergström K, Särkioja T, Räsänen P, Mantere T, Callaway J, Hiltunen J, and Tiihonen J (2001). Dopamine D(2)/D(3)-receptor and transporter densities in nucleus accumbens and amygdala of type 1 and 2 alcoholics. Mol. Psychiatry 6, 261–267. [DOI] [PubMed] [Google Scholar]
- Volkow ND, and Baler RD (2014). Addiction science: Uncovering neurobiological complexity. Neuropharmacology 76 (Pt B), 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, and Morales M (2015). The Brain on Drugs: From Reward to Addiction. Cell 162, 712–725. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, and Piscani K (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin. Exp. Res 20, 1594–1598. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, et al. (2002). Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 116, 163–172. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamida S, Ben, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, and Ron D (2012). Ethanol-Mediated Facilitation of AMPA Receptor Function in the Dorsomedial Striatum: Implications for Alcohol Drinking Behavior. J. Neurosci 32, 15124–15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, Ben Hamida S, and Ron D (2015). Alcohol Elicits Functional and Structural Plasticity Selectively in Dopamine D1 Receptor-Expressing Neurons of the Dorsomedial Striatum. J. Neurosci 35, 11634–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Ross TJ, O’Connor S, Stein EA, de Wit H, and Childs E (2018). Striatal activity correlates with stimulant-like effects of alcohol in healthy volunteers. Neuropsychopharmacology 43, 2532–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, and Tonegawa S (1997). Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 19, 837–848. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O’connor SJ, Wang C, Zheng QH, Mock B, and Morris ED (2005). Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol. Clin. Exp. Res 29, 965–970. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, and Finn DA (2008). Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate code. The datasets supporting the current are available from the corresponding author on request.