Abstract
Summary
Objective:
GABAA receptor subunit gene mutations are significant causes of epilepsy, which are often accompanied with various neuropsychiatric comorbidities, but the underlying mechanisms are unclear. It has been suggested that the comorbidities are caused by seizures as they often present in severe epilepsies. However, findings from both humans and animal models argue against this conclusion. Mutations in the GABAA receptor γ2 subunit gene GABRG2 have been associated with anxiety alone or with severe epilepsy syndromes and comorbid anxiety, suggesting a core molecular defect gives rise to the phenotypical spectrum. Here we determined the pathophysiology of comorbid anxiety in epilepsy and identified the central nucleus of the amygdala (CeA) as a primary neurosubstrate and a potential rescue via neuromodulation of CeA neurons.
Methods:
We used brain slice recordings, subcellular fractionation and western blot, immunohistochemistry, confocal microscopy and a battery of behavior tests in combination with a chemogenetic approach to characterize anxiety and its underlying mechanisms in a Gabrg2+/Q390X knockin mouse and a Gabrg2+/− knockout mouse, each associated with a different epilepsy syndrome.
Results:
We found that impaired GABAergic neurotransmission in CeA underlies anxiety in epilepsy, which is due to reduced GABAA receptor subunit expression resulting from the mutations. Impaired GABAA receptor expression reduced GABAergic neurotransmission in CeA, but not in BLA. Activation or inactivation of inhibitory neurons using a chemogenetic approach in CeA alone modulated anxiety-like behaviors. Similarly, pharmacological enhancement of GABAergic signaling via γ2 subunit-containing receptors rescued the anxiety.
Significance:
Together, these data demonstrate the molecular basis for a comorbidity of epilepsy and suggest that impaired GABAA receptor function in CeA due to mutation per se could at least contribute to anxiety. Modulation of CeA neurons could cause or suppress anxiety, suggesting a potential use of CeA neurons as therapeutic targets for treatment of anxiety in addition to traditional pharmacological approaches.
Keywords: GABAA receptors, epilepsy, anxiety, amygdala, comorbidity, chemogenetic
Introduction (<4200)
Epilepsy is a common neurological disorder with various comorbidities including anxiety1, autism and others. The underlying mechanisms for comorbidities in epilepsy are often viewed as consequence of seizures but this is debatable. For example, anxiety is often present before the onset of epilepsy, suggesting a common pathophysiological basis of epilepsy and anxiety in a brain developmental disorder2 rather than a causal relationship3. Likewise, animal seizure models induced with chemoconvulsants also develop anxiety, suggesting co-occurrence of anxiety and seizures by blockade of a common pathway4 and a shared mechanism for both conditions3. Given the fact that multiple comorbidities coexist in severe epilepsy syndromes without known pathophysiology, we here take anxiety as an example to explore the molecular basis for comorbidities associated with genetic epilepsies.
Altered GABAergic signaling has been established as a major cause for epilepsy and has also implicated in the pathogenesis of anxiety5–8. The comorbidity of anxiety and seizures has been observed in both human and rodent epilepsies regardless of seizure severity9. Reduced brain concentration of the inhibitory neurotransmitter γ-aminobutyric acid (GABA)10;11 and reduced expression of GABAA receptors (GABRs) have both been implicated in anxiety7. In depression/anxiety patients, the density of somatostatin-positive inhibitory interneurons in the amygdala was reported to be reduced12. This is consistent with the circuitry study that the central lateral amygdala (CeL), in particular the somatostatin-expressing neurons in CeL is critical for both learning and the expression of defensive responses 13 and validates the importance of the central amygdala in emotion processing.
Mutations in GABRG2 are a major cause of genetic epilepsy. Two mouse models with GABRG2 loss-of-function mutations display anxiety7,14. This suggests that mouse models harboring GABRG2 epilepsy mutations could be repurposed for studying anxiety. Gabrg2+/Q390X knockin (KI) mice show spontaneous generalized tonic clonic seizures (GTCS), myoclonic jerks, sudden unexplained death in epilepsy (SUDEP), anxiety, impaired social activity and cognition, representing a mouse model of severe epilepsy15. Gabrg2+/− knockout (KO) mice have been reported to have anxiety without seizures or with the mild epilepsy syndrome, generalized absence epilepsy 16;17, representing a mouse model of mild epilepsy6 or anxiety14.
In this study, we compared the anxiety phenotype of the two mouse models but focused on the severe epilepsy Gabrg2+/Q390X mouse because our purpose is to understand the molecular basis of comorbidity in severe epilepsy. We used immunohistochemistry, biochemistry and slice electrophysiology in combination with a chemogenetic approach to study the amygdala of control and Gabrg2+/Q390X mice. The amygdala is a brain region that is known to be involved in generating anxiety. We demonstrated that impaired GABAergic neurotransmission due to the loss of functional GABAA receptors in the central nucleus of the amygdala (CeA), including both central lateral (CeL) and central medial (CeM) nuclei18, underlies the anxiety phenotype in the het Gabrg2+/Q390X mice. Since the amygdala/limbic circuitry is highly conserved across species19 and CeA has been recently identified as a major neurosubstrate for fear conditioning20, this study in rodents should provide critical insights into therapeutic development for anxiety alone or for comorbidity in epilepsy in humans.
Materials and Methods
Mice
The Gabrg2+/Q390X KI mouse line was recently developed15, and the Gabrg2+/− KO mouse line was reported before21. Mice used in the study were crossed with C57BL/6J mice for at least 8 generations and were between 2–4 months old. Both genders were included. All experimental procedures were approved by Vanderbilt University Division of Animal Care.
The details for antibodies and experimental procedures were provided in Supplementary Method section.
EEG surgery and recordings and behavioral tests
The surgery to implant the EEG headmount and the video-monitoring synchronized EEG recordings and behavioral tests were conducted as previously described 15;17.
Subcellular fractionation and isolation of synaptosomes.
The experimental procedures were as based on previous protocols from previous studies15;17;22.
Brain slice immunohistochemistry and related quantifications
The experimental procedures were as previously described15. For all γ2, α1 and β2/3 subunit intensity measurements on western blots, values of the subunit intensity in the somatic and neuropil regions were measured by subtracting background from the raw values in each field with ImageJ. The colocalization of GABAA receptor subunits and Map2 was measured by MetaMorph.
Brain slice preparation and recording
Recordings of GABAergic mIPSCs were obtained from CeA neurons in brain slices as previously described13. Whole-cell patch clamp recordings from neurons were made at room temperature from CeA visualized with an upright Nikon eclipse FN-1 IR-DIC microscope. Data were collected using a MultiClamp 700B amplifier, filtered at 2 kHz and digitized at 20 kHz using a Digidata 1440A analog to digital converter (Molecular Devices Inc.). The GABAergic mIPSCs were identified automatically off-line using Minianalysis 6.0 (Synaptosoft, CA) and were confirmed visually. The details for mIPSCs analysis are included in Supplementary method section.
Chemogenetic modulation of CeA
Designer receptors exclusively activated by designer drugs (DREADDs) are G protein coupled receptors that are engineered to only respond to a synthetic and otherwise biologically inactive ligand. Expression of different types of DREADDs in neurons and then exposure of them to DREADD agonist allows produces temporary de- or hyperpolarization and thus chemogenetic activation or inactivation 23;24 in neurons. We stereotactically delivered a solution (0.15 μl) of AAV (UNC Vector core) expressing the inhibitory DREADD, hSyn-HA-HM4D(GI)-IRES-mcitrine (titer 2.2 X 10^12) (M4D), the excitatory DREADD (pAAV-hSyn-HA-hM3D(Gq)-mCherry) (M3D) (titer 3.48 X 10^12) or a control (hSyn-EGFP) (titer 3.0 X 10^12) into CeA bilaterally. Higher volumes (from 3 μl to 0.5 μl) were tested and found to have spill-over. A 0.5 mm burr hole was drilled in the skull, and a Hamilton syringe was stereotactically positioned (1.2 mm AP, ± 3.0 mm ML, 5.0 mm DV) and 0.15 μl of AAV-containing solution was delivered slowly. Mice were tested 3 weeks after viral injection. The DREADD agonist, clozapine-N-oxide (CNO) (Tocris) was tested at doses from 0.1 to 1 mg/kg i.p. in initial experiments. A dose of 0.3 mg/kg i.p. was used in the experiments reported in this study. CNO was administered 20 min before testing.
Experimental design and Statistical analysis
Statistical analysis was performed using Prism 5 software (GraphPad Software). Details on statistical analysis and experimental design, including tests performed, exact p values, and sample sizes are provided in the result section describing each figure, or within the legend of each figure. For biochemistry experiment, subunit integrated density values (IDVs) were quantified on western blots by using the Quantity One or Odyssey fluorescence imaging system (Li-Cor). The fluorescence intensity values were quantified by using ImageJ. All data were expressed as mean ± S.E.M values. Analysis of variance (ANOVA), including one-way and two-way ANOVA, and unpaired Student t tests were used. Post hoc and a priori Bonferroni comparisons or Newman-Keuls Multiple Comparison test were conducted to evaluate individual mean comparisons where appropriate. All analyses used an alpha level of 0.05 to determine statistical significance.
Results
Loss- or reduction-of-function is a common phenomenon for GABRG2 mutations and two GABRG2 loss-of-function mouse epilepsy models, Gabrg2+/Q390X KI and Gabrg2+/− KO mice, associated with severe and mild epilepsy syndromes, displayed an anxiety phenotype.
Many mutations in GABRG2 have been associated with various epilepsy syndromes and with multiple comorbidities25 (https://www.ncbi.nlm.nih.gov/clinvar/) (Figure 1A), but studies often focus on the epilepsy phenotype alone, thus precluding a more integrated view of the disease. To characterize the impact of GABRG2 mutations, we coexpressed mutant γ2 subunit cDNAs associated with a wide spectrum of epilepsy syndromes with the human wildtype α1 and β2 subunits to form α1β2γ2 receptors. We evaluated the subunit protein expression at the cell surface, which represents surface trafficking of assembled receptors. We compared the effects of multiple GABRG2 mutations on γ2 subunit function and determined that loss or reduction of mutant γ2 subunit-containing receptors is a common phenomenon across GABRG2 mutations (Figure 1B).
Figure 1. Mouse models of both mild and severe epilepsy with Gabrg2 deficiency had anxiety.
A. GABRG2 encodes the human GABAA receptor γ2 subunit and is an established epilepsy gene. Over 200 variations/mutations in GABRG2 have been associated with various epilepsy syndromes. These mutations are distributed in various locations and domains of the γ2 subunit. The red dots represent the relative locations of the epilepsy related mutations.
B. The flow cytometry histograms depict surface γ2 subunit expression levels on HEK293T cells coexpressing the human wildtype or mutant γ2 subunit cDNAs with the α1 and β2 subunit cDNAs for 48 hrs. The γ2 subunit protein was immunostained with a rabbit anti-γ2 subunit antibody. The relative surface levels of each mutant subunit were normalized to the wildtype subunit in α1β2γ2 receptors, which was arbitrarily taken as 1. Purple arrows designate the recombinant receptors equivalent to mouse models used in the study (*** p < 0.001 vs wt, n = 4–5).
C. Somatosensory cortex is associated with epilepsy while amygdala, including the central medial (CeM) and central lateral (CeL) amygdala, is a neural substrate for anxiety. A coronal brain image was generated using the Allen Mouse Brain Atlas. GABA positive neurons in amygdala in the wildtype and Gabrg2+/Q390X mice were shown. The frozen brain sections from mouse littermates at 4 months old were immunostained with rabbit anti-GABA antibody and visualized with Alexa 488 under confocal microscopy.
D. Gabrg2+/Q390X mice associated with severe epilepsy have spontaneous generalized tonic clonic seizures (GTCS) and myoclonic seizures. Gabrg2+/− mice in a C57BL/6J background associated with mild epilepsy only have infrequent absence seizure-like activity. E, F. Both Gabrg2+/Q390X and Gabrg2+/− mice spent less time (sec) in the open arms (E) and had reduced total number of entry into open arms (F). G-J. Both Gabrg2+/Q390X and Gabrg2+/− mice had increased distance travelled (cm) (G) and reduced time in the center vs periphery (H). Epilepsy mice spent more time (sec) in the periphery resting (I) but less time in center resting (J). (n = 24 for wt KO and 30 for other groups from E to J. For Gabrg2+/Q390X and Gabrg2+/− mice, the het mice were only compared with their own wt littermates (*p < 0.05; ** p < 0.01; *** p < 0.001 vs wt). Data were presented as mean as mean ± S.E.M.
Since the patients all carry het GABRG2 mutation, we used the het Gabrg2+/− and Gabrg2+/Q390X mouse models. We have used anxiety, for example, to address the pathophysiology of a comorbidity. Comorbidities like anxiety and their neuro-substrates like CeA and basal lateral amygdala (BLA) (Figure 1C), have not been studied adequately. We first identified the GABA positive neurons in CeA and found that there were no differences in the number of GABA positive neurons between wildtype (wt) and mutant Gabrg2+/Q390X mice. The Gabrg2+/Q390X heterozygous (het) KI mice had spontaneous generalized tonic clonic seizures and myoclonic jerks, while the Gabrg2+/− KO mice had only infrequent absence seizures, which were very brief and often without behavioral correlation in the C57/BL/6J mouse background (Figure 1D).
To study comorbid anxiety, we first assayed both mouse epilepsy models with EZM and OF tests. In the EZM test, both Gabrg2+/Q390X KI and Gabrg2+/− KO mice spent less time in the open arms compared with the wildtype littermates (Figure 1E). The KI and KO mice also had reduced number of entries into the open arm (Figure 1F). In the OF test, both het KI and het KO mice travelled longer distances (Figure 1G) and spent less time in the center (Figure 1H). Consistently, both het mice spent more time resting in the periphery (Figure 1I) but less time resting in the center (Figure 1J, Supplementary table 1). The findings from both EZM and OF tests indicated that both the het Gabrg2+/Q390X KI and het Gabrg2+/− KO mice had anxiety phenotypes. Since the Gabrg2+/− KO mouse, the hemizygous (hemi) condition, which represents a very mild epilepsy, displayed anxiety, it is likely all the other GABRG2 mutations cause a similar phenotype or produce a similar susceptibility to anxiety in patients. The follow up study thus has mainly focused on the Gabrg2+/Q390X KI mouse associated with a more severe epilepsy, the Dravet syndrome.
The amplitude of GABAergic mIPSCs in CeA, but not BLA, in Gabrg2+/Q390X KI mice was reduced.
We then determined the function of GABAergic signaling in amygdala, which plays a central role in CNS processing of afferent and efferent connections related to emotional functioning and has been suggested to be the site of anxiety perception. We recorded GABAergic mIPSCs in both BLA and CeA as indicated by the pink or green circle and arrow in an image of the live brain slice (Figure 2A). The relative locations of CeA and BLA were identified based on a previous study26. It has been demonstrated that BLA is comprised of ~90% glutamatergic neurons while CeM contains 95% GABAergic medium sized neurons27. A primary output sub-region of the amygdala is CeM. We demonstrated that the amplitudes of GABAergic mIPSCs were reduced in CeA but were unchanged in BLA (34.86 ± 3.64 pA for wt and 20.97 ± 2.57 pA for CeA, p = 0.020) (Figure 2B, C). The frequencies of GABAergic mIPSCs in CeA and BLA were unchanged (Figure 2D).
Figure 2. CeA including CeM and CeL, but not BLA, had reduced GABAergic neurotransmission in Gabrg2+/Q390X mice.
A. The image represents the relative locations of CeA (purple circle) and BLA (green circle) in a coronal mouse brain slice.
B. Representative traces of GABAergic mIPSCs from the neurons in CeA and BLA in the wildtype (wt) and the Gabrg2+/Q390X het mice at 2 months of age.
C, D. The amplitude (C) or frequency (D) of GABAergic mIPSCs in CeA and BLA in wt or het Gabrg2+/Q390X mice. Data were presented as mean ± S.E.M (n = 19 for wt and n = 9 for het for CeA; n = 9 for wt and n = 8 for het for BLA, *p < 0.05 vs wt).
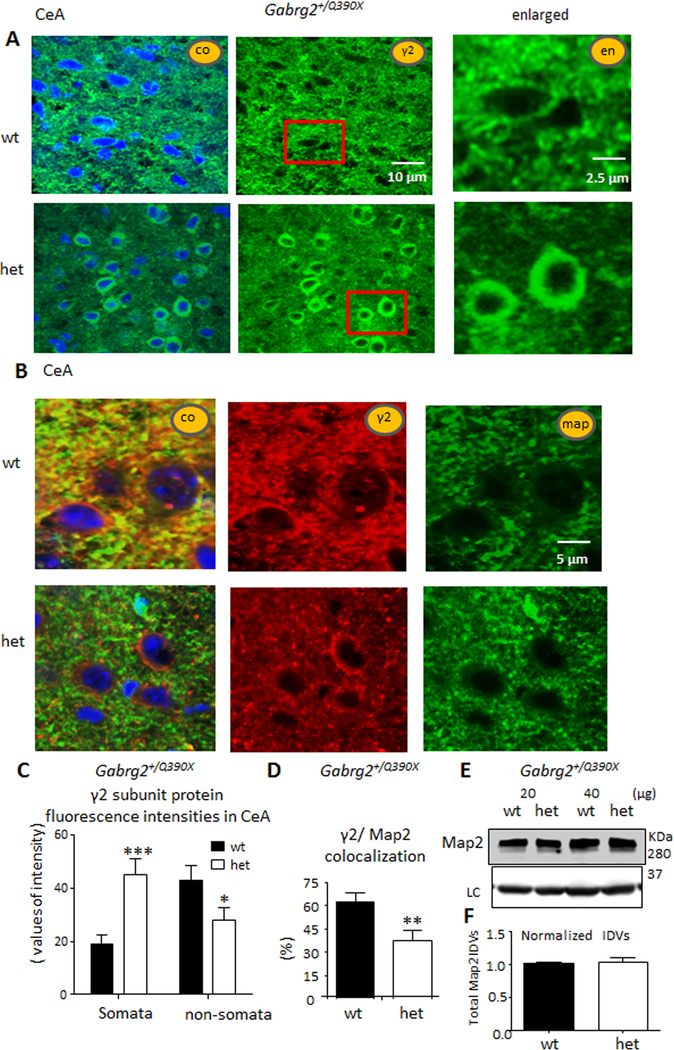
The γ2 subunits accumulated in the somata and were reduced in the dendrites of CeA neurons in Gabrg2+/Q390X KI mice, suggesting that functional GABAA receptors were reduced in inhibitory synapses.
We then determined the expression of γ2 subunits in CeA neurons from wt and het KI mice. The cell nuclei were stained with TO-PRO-3 (blue) and overlaid with γ2 subunits (green) (Figure 3A) which showed the γ2 subunit in puncta on cell bodies and processes. To further understand the location of γ2 subunits, we colabeled the neurons from wt and het KI mice with anti-γ2 subunit and anti-Map2 antibodies (Figure 3B), the neuronal dendrite marker, and measured the overlap of γ2 subunit and Map2 protein fluorescence. We measured the fluorescence intensity of γ2 subunits in the somata and in the non-somata regions in all neurons in CeA (Figure 3C). Consistent with previous findings in cortical neurons17, γ2 subunits were increased in the somata of neurons (19.1 ± 3.5 vs 45.2 ± 5.9, p = 0.0004) but reduced in the non-somata region (43.3 ± 5.6 vs 28.6 ± 4.6, p = 0.0475, unpaired t test) in CeA. Consistent with the reduced γ2 subunit distribution in non-somata regions, γ2 subunit/Map2 colocalization was higher in wt than in het KI mice (62.5 ± 5.5 vs 37.8 ± 6.6, p = 0.0088) (Figure 3D). We noticed the labeling of Map2 was more puncta-like in wildtype than in mutant mice. We thus determined if the total protein level of Map2 was altered in the mutant mice using western blotting (Figure 3E, top). However, the total protein IDVs of Map2 were unchanged (Figure 3F, bottom). We also surveyed the BLA in wt and het KI mice where no change of γ2 subunit was observed. This was not surprising since most neurons in BLA are glutamatergic neurons while majority of the neurons in CeA are GABAergic interneurons26.
Figure 3. γ2 subunits accumulated in the somata but were depleted in dendrites in CeA in Gabrg2+/Q390X mice.
A, B. Images from CeA regions of brain sections that were stained with rabbit anti-γ2 subunit (green) antibody alone (A), or rabbit anti-γ2 subunit (red) and mouse anti-Map2 (green) antibodies (B). The nuclei were stained with TO-PRO-3.
C. The γ2 subunit fluorescence intensities were analyzed by ImageJ. The γ2 subunit fluorescence signals in somata or non-somata regions in the whole section were measured by subtracting the background value in the nuclei region from the total raw value (n = 28, 28 sections from 4 pairs of mice).
D. γ2 subunit fluorescence puncta colocalized with Map2 in the whole field of each sampled region (n = 15) were measured. The fluorescence signal from the whole section were measured (n = 15, 15 sections from 4 pairs of mice).
E. Total lysates of CeA were analyzed by western blot, and the membranes were immunoblotted with anti-mouse Map2 antibody (upper panel).
F. The normalized integrated density values (IDVs) of Map2 were plotted. The IDVs of Map2 were normalized to its internal control. Data were presented as mean as mean ± S.E.M (*p < 0.05, **p < 0.01, ***p < 0.001 vs wt).
Partnering α1 subunits and remaining wildtype γ2 subunits were reduced in CeA in Gabrg2+/Q390X KI mice.
We demonstrated previously in vitro that mutant γ2 subunits could interfere with assembly and trafficking of wt partnering subunits, resulting in endoplasmic reticulum retention28. We thus compared the subcellular localization of partnering α1 and β2/3 subunits in CeA of wt and het KI mice (Figure 4A). Like γ2 subunits, α1 subunits had increased expression on neuronal somata of CeA neurons (16 ± 2.4 vs 26 ± 3.8, p = 0.03), but decreased expression in the non-somata region (39 ± 2.8 vs 29 ± 4.0, p = 0.0454) in the CeA (Figure 4B). However, there was a trend for similar differences for β2/3 subunit expression that were not statistically significant in both somata and non-somata regions in the CeA of wt and het KI mice (Figure 4C). Using western blotting, we found that total expression of γ2 and α1 subunits in the het KI mice was reduced to 50–60% of that of the wt in CeA (Figure 4D–4E, 4G–4H).The findings were further confirmed by the reduced expression level of γ2 subunits in isolated synaptosomes from het mice with subcellular fractionation from CeA (0.448 ± 0.026 for het vs wt which was arbitrarily taken as 1) (Figure 4F and 4I). This thus suggested that functional α1β2/3γ2 receptors were reduced in the CeA of het KI mice due to intracellular retention. The accumulated subunits inside the somata were largely nonfunctional, and the pool of functional receptor was reduced in CeA neurons.
Figure 4. Wildtype α1 subunits were reduced in CeA in Gabrg2+/Q390X mice.
A. Images of CeA from wildtype (wt) or heterozygous Gabrg2+/Q390X (het) mouse brain sections that were stained with rabbit anti-α1 (green) and mouse anti-β2/3 (red) subunit antibodies. The nuclei were stained with TO-PRO-3.
B, C. The α1 or β2/3 subunit fluorescence signals in somata or non-somata regions in the whole sampled field were measured subtracting the background value in the nuclei region from the total raw value of fluorescence (n = 28 neurons or sampled fields).
D-I. The total lysates of CeA from 2, 3 and 4 m old mice respectively (D-E) or isolated synaptosomes (Spm) by subcellular fractionation from 2–4 months old mice combined (F) were analyzed by western blot. The membranes were blotted with rabbit anti-γ2 subunit that only recognized the wt γ2 subunit (D, F) or mouse anti-α1 subunit (E) antibody. The normalized integrated density values (IDVs) of γ2 (E) or α1 (G) subunit protein were measured by Odyssey system (n = 6). The γ2 subunit (n = 6) (G, I) or α1 subunit (n = 6) (H) IDVs were normalized to internal control ATPase IDVs (LC) first and then normalized to wt subunit IDVs, which were arbitrarily taken as 1 (*p < 0.05, **p < 0.01, *** p<0.001 vs wt). Data were presented as mean as mean ± S.E.M.
Chemogenetic DREADD activation or inactivation of inhibitory neurons in CeA alone modulated anxiety-like behaviors.
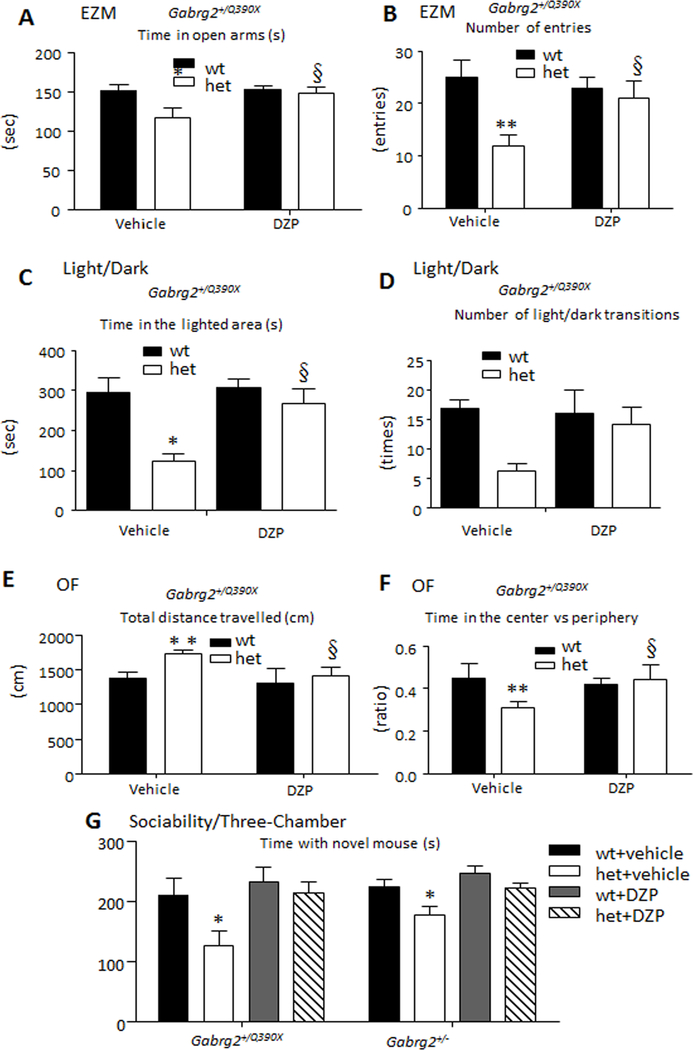
We next utilized a chemogenetic approach to determine if reversibly activating or inactivating CeA neurons would modulate anxiety-like behavior24;29. A solution containing the excitatory DREADD M3D virus, the inhibitory DREADD M4D virus or a control virus was injected into CeA using digital stereotaxis at the desired coordinates (Figure 5A). A small volume of virus or control solution (0.15 μl) was injected to avoid spread, and behavioral tests were carried out three weeks after virus injection (Figure 5B). Both M3D and M4D DREADDs were activated by CNO (0.3 mg/kg). Activation of M3D DREADDs enhances neuronal firing and should enhance anxiety, while activation of M4D DREADDs reduces neuronal firing and should reduce anxiety. We chose a CNO dose of 0.3 mg/kg based on a previous study in which activation of M3D DREADDs in CA1 of the hippocampus by a CNO dose of 0.5 mg/kg reliably evoked limbic seizures, while no seizures were detected after administration of lower CNO doses from 0.03 to 0.3 mg/kg24. We used three different neurobehavioral tests to evaluate the effect of M3D or M4D DREADDs on anxiety (Figure 5C–E). There was a 2-day interval between each test, and the sequence of tests was EZM, light-dark choice and open field (OF). Diazepam (0.3 mg/kg ip, DZP) was administered 30 min before testing7. The time spent in the open arms in EZM (Figure 5C), time spent in the lighted area (light/dark choice) (Figure 5D) and total distance travelled (cm) in the OF test (Figure 5E) were measured. The results of the three tests were provided below.
Figure 5. Activation or inactivation of GABAergic neurons in the CeA alone via a chemogenetic approach using a DREADD modulated anxiety-like behavior.
A. A sample image showing virus injection site as purple circled region in mouse central nucleus of amygdala (CeA). B. Mice were administered vehicle (normal saline), pAAV-hSyn-HA-hM3D(Gq)-mCherry (M3D) or hSyn-HA-HM4D(GI)-IRES-mcitrine (M4D) and testing occurred three weeks after administration. The DREADD agonist, clozapine-N-oxide CNO (0.3mg/kg ip) was administered 20 min before testing. Diazepam (0.3 mg/kg ip, DZP) was administered 30 min before testing. All mice were subject to three behavioral tests. C, In the elevated zero maze (EZM) test, the time spent in open arms was measured. D, In the light/dark choice, the time spent in the lighted area was measured. E. In Open Field (OF), the total travelled distance (cm) (D) was measured. In C-E, * p < 0.05, ** p < 0.01, *** p < 0.001 vs wt treated with saline in the same group; § p<0.05, §§§p<0.001 vs wt treated with saline in the same group; † p < 0.05, ††p < 0.01, ††† p<0.001 vs het treated with saline in the same group. n = 9–18 mice for C-E. Data were presented as mean as mean ± S.E.M). M3D stands for all mice treated with M3D DREADD while M4D stands for all mice treated with M4D DREADDs. Purple boxed groups represent mice treated with saline. The treatment conditions in M3D were applied to M4D. The comparisons were within either M3D or M4D group and no cross comparisons were made.
The mice injected with M3D or M4D DREADDs and treated with vehicle (normal saline) displayed a phenotype similar to those of untreated wt and het mice with EZM and OF tests (Figure 1C–E), suggesting M3D or M4D DREADDs alone without CNO treatment had no effect on mouse anxiety.
In mice expressing M3D DREADDs, activation of CeA neuronal activity with CNO treatment caused an anxiety-like phenotype in wt mice in OF and light/dark, but not EZM, tests (EZM: 120.1 ± 18.3 for saline vs 92.3 ± 15.3 for M3D with CNO treatment, p = 0.1340); OF: 1408 ± 125 cm for saline treated vs 2102 ± 302 cm for M3D with CNO treatment, p = 0.0411); light/dark: 298.3 ± 31 sec for saline treated vs 157.8 ± 22 sec for M3D with CNO treatment (p = 0.0008). CNO treatment did not alter the behavioral phenotype of the het mice expressing M3D DREADDs. DZP treatment rescued the anxiety phenotype in het mice treated with CNO in light/dark test (136.5 ± 32 sec for het M3D treated with saline vs 289.6 vs ± 17.8 sec for het mice expressing M3D DREADDs treated with CNO+DZP, p=0.0034). Because CNO treatment alone did not rescue the phenotype in het mice expressing M3D DREADDs, this suggested that the rescue in het mice expressing M3D DREADDs was due to DZP administration.
In contrast, in mice injected with M4D DREADDs, inhibiting CeA neuronal activity with CNO treatment decreased the anxiety-like phenotype in het mice in the light/dark, but not EZM or OF, tests ((EZM: 95.3 ± 12.1 sec for saline treated vs 121.7 ± 8.5 sec for M4D with CNO treatment (CNO), p = 0.088; OF: 2509.3 ± 421 cm for saline treated vs 1876.8 ± 235.8 cm for M4D with CNO, p = 0.2035; light/dark: 141.1 ± 12 sec for saline vs 288.3 ± 25.2 sec for M4D with CNO), p < 0.0001)). DZP administration normalized the anxiety behavior response in the het mice in CNO+DZP group as treated with CNO alone (Figure 5C, saline vs CNO+ DZP, p = 0.0221, two-way ANOVA; wt vs het, p = 0.04; Figure 5D, Saline vs CNO+DZP, Figure 5E, saline vs CNO +DZP, two-way ANOVA, p < 0.0001). Similar findings of rescue by M4D DREADDs with CNO treatment were obtained from the Gabrg2+/− mice (data not shown), suggesting that chemogenetic inactivation or activation of neurons in CeA modulated anxiety-like behavior. The findings also suggest that CeA is a specific target for anxiety treatment because viral injection in dentate gyrus did not have the similar effect). There was no obvious change in EEGs recorded from mice treated with DREADDs (Supplementary Figure 1). However, there was increased distance travelled in the EZM test in wildtype mice expressing DREADDs with CNO treatment, which was normalized by DZP. The detailed mechanism may require further study of the normal fear response and DZP as well as of chemogenetic modulation.
Pharmacological enhancement of current from γ2 subunit-containing GABAA receptors rescued anxiety-like behavior in Gabrg2 deficient mice.
We determined if DZP could reduce the anxiety in both mouse models. In the EZM test, the treated het KI mice had reduced time in the open arm (Figure 6A) and reduced number of entries into the open arm (Figure 7B), while the mice treated with DZP spent similar times in the open arm and had a number of entries similar to those of wt mice. In the light-dark choice, the het KI mice spent less time in the lighted area and had a reduced number of light/dark transitions while the DZP treatment increased the time in the lighted area in the het mice (Figure 6C, D). In the OF test (Figure 6E, F), the het KI mice treated with normal saline spent a longer travelling but travelled a similar distance compared to those with DZP treatment. Similar rescue with DZP was observed with Gabrg2+/− mice (data not shown). In the Three-Chamber test for sociability (Figure 6G), both het KI and KO mice treated with DZP spent similar amounts of time with the novel mouse, indicating a rescue from social anxiety (Supplementary table 2).
Figure 6. Pharmacologic enhancement of γ2 subunit containing GABAA receptor function rescued anxiety-like behavior in Gabrg2 deficient epilepsy mice.
A, B. Either vehicle (normal saline) or diazepam (0.3 mg/kg ip, DZP) was administered 30 min before testing. In the elevated zero maze (EZM), the time spent in open arms (A) and the total number of entry into open arms (B) were measured.
C, D. In OF test, the total distance travelled (C) and the ratio of time in center vs periphery (D) were measured.
E, F. In light/dark choice, the time spent in the lit area and the number of light/dark transitions were measured.
G. In three-chamber test, the time spent with novel mouse was measured. (*p < 0.05; **p < 0.01 vs wt, § p<0.05 vs het with vehicle. n = 8 mice for het = vehicle and n = 9 mice for other groups. Data were presented as mean as mean ± S.E.M).
Discussion
Neuropsychiatric comorbidities are common in epilepsy, but their underlying mechanisms have not been addressed adequately, thus preventing a comprehensive view of epilepsy syndromes. Although memory deficits have long been known to be associated with temporal lobe epilepsy 30;31, and autistic traits were rescued by enhancing GABAergic signaling in epilepsy with an Scn1a mutation 8, there is no study characterizing the mechanisms underlying comorbidities in epilepsy with GABR mutations. This study took anxiety, a common comorbidity of epilepsy as an example, to address the molecular basis for comorbidities in epilepsy associated with GABRG2 mutations. We propose that functional impairment in other neuroanatomical substrates could give rise to corresponding comorbid phenotypes via mechanisms similar to those for epilepsy, depending on specific neurocircuits involved. In Gabrg2 deficient mice, anxiety was caused by reduction of functional γ2 subunit-containing receptors while enhancing synaptic GABAA receptor function rescued anxiety, similar to epilepsy32. Findings from this study suggest that the pathophysiology from the mutation itself may directly contribute to anxiety (Supplementary Figure 2).
It is commonly thought that CeA output neurons use GABA, and not glutamate, as a neurotransmitter17. The vast majority of neurons in CeA contain glutamic acid decarboxylase 65 (GAD65) and GAD67 mRNA33, key enzymes for the synthesis of GABA. It was found that central amygdala stimulation enhances, whereas central amygdala inactivation or lesion suppresses, conditioned fear responses. As illustrated in Figure 5B, activation of CeA neurons by M3D DREADDs enhanced anxiety while inactivation of CeA neurons by M4D DREADDs reduced anxiety. This is consistent with our findings that GABAergic mIPSCs were impaired in the neurons in CeA, but not in the neurons in BLA because majority of the neurons in CeA are GABAergic while the most of neurons in BLA are not GABAergic. This also explains why activation of CeA by M3D DREADDs resulted in anxiety while inhibition of CeA by M4D DREADDs suppressed normal fear. Gabrg2+/Q390X mice treated with M4D DREADDs spent more time in the open arms and lighted areas in EZM and Light/dark choice tests compared with saline treated mice, suggesting reduced anxiety with M4D DREADDs treatment (Figure 5C). This indicates that neuromodulation of CeA neurons could be a potential treatment option for anxiety. However other neuronal circuits, such as those in hippocampus and frontal cortex may be also involved in anxiety. For example, it has been demonstrated that memory of fear extinction requires mGluR5-mediated activation of infralimbic neurons34. Nevertheless, we propose that CeA is a major neuroanatomical substrate for anxiety in epilepsy associated with Gabrg2 deficiency as modulation of hippocampal circuitry by either M3D or M4D DREADDs injection in dentate gyrus did not elicit the same phenotypes (Supplementary Figure 1).
This study indicates that modulation of neurons in CeA alone alters the anxiety phenotype. It is likely due to the fact that the majority of targeted neurons are GABAergic in CeA27, Functional circuits in CeA and amygdala have been elegantly characterized with optogenetic and chemogenetic tools20. Using a Cre-dependent virus to express the inhibitory DREADD receptor of M4D, the somatostatin-positive neurons in which Cre recombinase is expressed could be reversibly and selectively inhibited. This inhibition prevented fear acquisition and excitatory synaptic enhancement. CeA has emerged as an active participant in fear conditioning instead of being a passive relay between the amygdala complex and downstream fear effectors20. This finding is not surprising given that GABAA receptors are the prime targets for anxiolytic therapies, and CeA neurons are primarily GABAergic.
The data suggest that CeA could be a therapeutic target for anxiety alone or for being a comorbidity with other diseases such as epilepsy. In Gabrg2+/Q390X mice, impaired GABAergic neurotransmission in CeA is likely the key mechanism because activation or inactivation of neurons in CeA alone by DREADDs modulated the anxiety-like phenotype. However, any neuro-behavior may involve a network and multiple circuits, which could be functionally overlapping. Given the ubiquitous distribution of γ2 subunits in the brain, it is possible that reduction of γ2 subunits in other brain regions may also modify the anxiety phenotype. These brain regions may include prefrontal cortex, hippocampus and hypothalamus, which are implicated in anxiety5;14;35;36. Nevertheless, CeA is a primary target as activation or inactivation of neurons in hippocampus with dentate gyrus viral injection did not elicit similar response as in CeA in Gabrg2+/Q390X mice. It is not feasible to have a thorough study of protein expression and GABAergic function as well as circuits for all involved regions. This study on CeA only serves as an example for understanding epilepsy comorbidities. It is likely that similar changes are present in other regions with corresponding phenotypes, but there may be variation due to specific temporal and spatial subunit expression.
This study suggests a novel treatment option for comorbidities in epilepsy. Neuromodulation of a specific group of neurons to control seizures or comorbidities such as anxiety would be an ideal choice for epilepsy as it would avoid side effect from broad alteration of brain activity in drug treatment which globally decreases excitation37. Despite lack of precise temporal control afforded by optogenetics, DREADDs have proven to be well suited for translation due to the slow temporal control and chronic modulation as well as its feasibility38;39. It is highly attractive for treatment development using DREADD as CNO can be delivered orally40;41 or via other safe routes such as topical administration42. This thus suggests that in addition to pharmacological approaches, selectively increased inhibition in CeA via neuromodulation could be a feasible treatment option for anxiety.
Supplementary Material
A sample image showing virus injection site and positive labeling cells in mouse amygdala.
A. A sample image from mouse injected with GFP virus (0.15μl) and the brain tissue was harvested 3 weeks later. The purple circle showing virus injection site and localized positive labeling cells in mouse amygdala.
B. The enlarged view showing minimal spread of virus in the amygdala.
C. Baseline EEG traces from mice treated with DREADDS (pAAV-hSyn-HA-hM3D(Gq)-mCherry (M3D)). Purple boxed regions showing spike-wave discharges in a 4 months old mouse treated with M3D for 3 weeks.
D. Mice were administered vehicle (normal saline) pAAV-hSyn-HA-hM3D(Gq)-mCherry (M3D) or hSyn-HA-HM4D(GI)-IRES-mcitrine (M4D) and testing occurred three weeks after administration. The DREADD agonist, clozapine-N-oxide CNO (0.3 mg/kg ip) was administered 20 min before testing for the elevated zero maze (EZM). Diazepam (0.3 mg/kg ip, DZP) was administered 30 min before testing. The time spent in open arms in EZM was measured and shown as example. (*p < 0.05; **p < 0.01 vs wt. Data were presented as mean ± S.E.M).
Detailed measurements for Figure 1E-J.
Reduced GABAA receptor function results in impaired GABAergic neurotransmission and contributes to comorbidities as well as to seizures in GABRG2 deficient epilepsy while specific neuromodulation of CeA would be a treatment option for anxiety
A. The reduced functional GABAA receptors due to either impaired trafficking as in GABRG2(Q390X) mutation or impaired channel kinetics as in other GABRG2 mutations would compromise net GABAA receptor function and GABAergic neurotransmission. Impaired GABAergic neurotransmission in CeA underlies anxiety in GABRG2 deficient epilepsy. Neuromodulation could be a promising treatment option for anxiety for GABRG2 deficient epilepsy.
B. GABRG2 mutation is a common cause for genetic epilepsy. The impaired GABAA receptor function due to various mechanisms would result in impaired GABAergic neurotransmission in various brain regions which may give rise to different behavioral output such as seizures and anxiety depending on specifically involved neuronal network and circuitries. Additionally, the presence of mutant proteins may cause other less characterized molecular pathologies, which may give rise to uncharacterized phenotypes either at molecular or behavioral levels. Neuromodulation to interrupt specifically involved circuitry could be a good treatment option in addition to pharmacological approach via enhancing remaining GABAA receptor function.
Detailed measurements for Figure 6 A-G.
Key Points.
Epilepsy mouse models of Gabrg2 haploinsufficiency either with or without dominant negative suppression displayed anxiety.
GABAA receptor γ2 subunits were reduced in the central nucleus of amygdala (CeA) with or without additional loss of partnering subunits.
GABAergic neurotransmission was impaired in CeA in Gabrg2+/Q390X knockin epilepsy mice.
Activation or inactivation of inhibitory neurons using a chemogenetic approach in CeA alone modulated anxiety-like behaviors.
Neuromodulation of neurons in CeA could be a potential therapeutic approach for anxiety in epilepsy.
Acknowledgements:
Research was supported by grants from Citizen United for Research in Epilepsy (CURE), Dravet syndrome foundation (DSF), Vanderbilt Brain Institute Award and Vanderbilt Clinical and Translation Science Award and NINDS R01 NS082635 to J.Q.K., NINDS R01 NS51590 to R.L.M and R01 NS064286 to MJG. Special thanks to Dr. Rita Baldi for the advice on identifying the central nucleus of the amygdala in mouse brain slices and Dr. Sachin Patel for constructive discussion. We are also grateful to Dr. Liping in Vanderbilt Center of Quantitative Sciences for her consultation on statistics in this study. All the behavioral tests were done in Vanderbilt University Medical Center (VUMC) Murine Neurobehavioral Core with great assistance from Dr. John Allison. Imaging data were performed in part through the VUMC Cell Imaging Shared Resource.
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
Conflict of Interest statement: None of authors declared any conflict of interest.
Reference List
- 1.Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a nationally representative population-based study. Epilepsia 2012;53:1095–103. [DOI] [PubMed] [Google Scholar]
- 2.Berg AT, Tarquinio D, Koh S. Early Life Epilepsies are a Comorbidity of Developmental Brain Disorders. Semin Pediatr Neurol 2017;24:251–63. [DOI] [PubMed] [Google Scholar]
- 3.Jones NC, Salzberg MR, Kumar G, Couper A, Morris MJ, O’Brien TJ. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Exp Neurol 2008;209:254–60. [DOI] [PubMed] [Google Scholar]
- 4.Hoeller AA, Duzzioni M, Duarte FS et al. GABA-A receptor modulators alter emotionality and hippocampal theta rhythm in an animal model of long-lasting anxiety. Brain Res 2013;1532:21–31. [DOI] [PubMed] [Google Scholar]
- 5.Ren Z, Sahir N, Murakami S et al. Defects in dendrite and spine maturation and synaptogenesis associated with an anxious-depressive-like phenotype of GABAA receptor-deficient mice. Neuropharmacology 2015;88:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen Q, Lal R, Luellen BA, Earnheart JC, Andrews AM, Luscher B. gamma-Aminobutyric acid-type A receptor deficits cause hypothalamic-pituitary-adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Biol Psychiatry 2010;68:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crestani F, Lorez M, Baer K et al. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci 1999;2:833–9. [DOI] [PubMed] [Google Scholar]
- 8.Han S, Tai C, Westenbroek RE et al. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 2012;489:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung ME, Lal H, Gatch MB. The discriminative stimulus effects of pentylenetetrazol as a model of anxiety: recent developments. Neurosci Biobehav Rev 2002;26:429–39. [DOI] [PubMed] [Google Scholar]
- 10.Sanacora G, Gueorguieva R, Epperson CN et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry 2004;61:705–13. [DOI] [PubMed] [Google Scholar]
- 11.Sanacora G, Mason GF, Rothman DL et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1999;56:1043–7. [DOI] [PubMed] [Google Scholar]
- 12.Douillard-Guilloux G, Lewis D, Seney ML, Sibille E. Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress Anxiety 2017;34:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu K, Garcia da SP, Albeanu DF, Li B. Central Amygdala Somatostatin Neurons Gate Passive and Active Defensive Behaviors. J Neurosci 2016;36:6488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B. Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang JQ, Shen W, Zhou C, Xu D, Macdonald RL. The human epilepsy mutation GABRG2(Q390X) causes chronic subunit accumulation and neurodegeneration. Nat Neurosci 2015;18:988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reid CA, Kim T, Phillips AM et al. Multiple molecular mechanisms for a single GABAA mutation in epilepsy. Neurology 2013;80:1003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warner TA, Shen W, Huang X, Liu Z, Macdonald RL, Kang JQ. DIfferential molecular and behavioral alterations in mouse models of GABRG2 haploinsufficiency versus dominant negative mutations associated with human epilepsy. Hum Mol Genet 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol 1982;208:401–18. [DOI] [PubMed] [Google Scholar]
- 19.Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature 2015;517:284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 2013;16:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunther U, Benson J, Benke D et al. Benzodiazepine-insensitive mice generated by targeted disruption of the gamma 2 subunit gene of gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A 1995;92:7749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamat PK, Kalani A, Tyagi N. Method and validation of synaptosomal preparation for isolation of synaptic membrane proteins from rat brain. MethodsX 2014;1:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coward P, Wada HG, Falk MS et al. Controlling signaling with a specifically designed Gi-coupled receptor. Proc Natl Acad Sci U S A 1998;95:352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander GM, Rogan SC, Abbas AI et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 2009;63:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JQ, Macdonald RL. Molecular Pathogenic Basis for GABRG2 Mutations Associated With a Spectrum of Epilepsy Syndromes, From Generalized Absence Epilepsy to Dravet Syndrome. JAMA Neurol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duvarci S, Popa D, Pare D. Central amygdala activity during fear conditioning. J Neurosci 2011;31:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marek R, Strobel C, Bredy TW, Sah P. The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol 2013;591:2381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang JQ, Shen W, Macdonald RL. The GABRG2 mutation, Q351X, associated with generalized epilepsy with febrile seizures plus, has both loss of function and dominant-negative suppression. J Neurosci 2009;29:2845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 2007;104:5163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol 2003;54:425–32. [DOI] [PubMed] [Google Scholar]
- 31.Jokeit H, Ebner A. Long term effects of refractory temporal lobe epilepsy on cognitive abilities: a cross sectional study. J Neurol Neurosurg Psychiatry 1999;67:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X, Zhou C, Tian M et al. Overexpressing wild-type gamma2 subunits rescued the seizure phenotype in Gabrg2+/Q390X Dravet syndrome mice. Epilepsia 2017;58:1451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitkanen A, Amaral DG. The distribution of GABAergic cells, fibers, and terminals in the monkey amygdaloid complex: an immunohistochemical and in situ hybridization study. J Neurosci 1994;14:2200–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb Cortex 2011;21:727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez-Jimenez B, Bisby JA, Horner AJ, King JA, Pine DS, Burgess N. Linked networks for learning and expressing location-specific threat. Proc Natl Acad Sci U S A 2018;115:E1032-E1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moscarello JM, Maren S. Flexibility in the face of fear: Hippocampal-prefrontal regulation of fear and avoidance. Curr Opin Behav Sci 2018;19:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lillis KP, Staley KJ. Optogenetic dissection of ictogenesis: in search of a targeted anti-epileptic therapy. J Neural Eng 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell EJ, Marchant NJ. The use of chemogenetics in behavioural neuroscience: receptor variants, targeting approaches and caveats. Br J Pharmacol 2018;175:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol 2015;55:399–417. [DOI] [PubMed] [Google Scholar]
- 40.Urban DJ, Zhu H, Marcinkiewcz CA et al. Elucidation of The Behavioral Program and Neuronal Network Encoded by Dorsal Raphe Serotonergic Neurons. Neuropsychopharmacology 2016;41:1404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain S, Ruiz dA I, Lu H, White MF, Guettier JM, Wess J. Chronic activation of a designer G(q)-coupled receptor improves beta cell function. J Clin Invest 2013;123:1750–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keenan WT, Fernandez DC, Shumway LJ, Zhao H, Hattar S. Eye-Drops for Activation of DREADDs. Front Neural Circuits 2017;11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A sample image showing virus injection site and positive labeling cells in mouse amygdala.
A. A sample image from mouse injected with GFP virus (0.15μl) and the brain tissue was harvested 3 weeks later. The purple circle showing virus injection site and localized positive labeling cells in mouse amygdala.
B. The enlarged view showing minimal spread of virus in the amygdala.
C. Baseline EEG traces from mice treated with DREADDS (pAAV-hSyn-HA-hM3D(Gq)-mCherry (M3D)). Purple boxed regions showing spike-wave discharges in a 4 months old mouse treated with M3D for 3 weeks.
D. Mice were administered vehicle (normal saline) pAAV-hSyn-HA-hM3D(Gq)-mCherry (M3D) or hSyn-HA-HM4D(GI)-IRES-mcitrine (M4D) and testing occurred three weeks after administration. The DREADD agonist, clozapine-N-oxide CNO (0.3 mg/kg ip) was administered 20 min before testing for the elevated zero maze (EZM). Diazepam (0.3 mg/kg ip, DZP) was administered 30 min before testing. The time spent in open arms in EZM was measured and shown as example. (*p < 0.05; **p < 0.01 vs wt. Data were presented as mean ± S.E.M).
Detailed measurements for Figure 1E-J.
Reduced GABAA receptor function results in impaired GABAergic neurotransmission and contributes to comorbidities as well as to seizures in GABRG2 deficient epilepsy while specific neuromodulation of CeA would be a treatment option for anxiety
A. The reduced functional GABAA receptors due to either impaired trafficking as in GABRG2(Q390X) mutation or impaired channel kinetics as in other GABRG2 mutations would compromise net GABAA receptor function and GABAergic neurotransmission. Impaired GABAergic neurotransmission in CeA underlies anxiety in GABRG2 deficient epilepsy. Neuromodulation could be a promising treatment option for anxiety for GABRG2 deficient epilepsy.
B. GABRG2 mutation is a common cause for genetic epilepsy. The impaired GABAA receptor function due to various mechanisms would result in impaired GABAergic neurotransmission in various brain regions which may give rise to different behavioral output such as seizures and anxiety depending on specifically involved neuronal network and circuitries. Additionally, the presence of mutant proteins may cause other less characterized molecular pathologies, which may give rise to uncharacterized phenotypes either at molecular or behavioral levels. Neuromodulation to interrupt specifically involved circuitry could be a good treatment option in addition to pharmacological approach via enhancing remaining GABAA receptor function.
Detailed measurements for Figure 6 A-G.