Abstract
Objective
It is unclear how alterations in gray matter volume and white matter density affect elderly patients with irritable bowel syndrome (IBS). This study aimed to investigate the relationship between structural changes in the brain and psychological stress in elderly IBS patients.
Methods
Eighteen IBS patients and 12 healthy controls underwent structural magnetic resonance imaging. Voxel‐based morphometry and diffusion tensor imaging analysis were used to identify abnormalities in cortical regions and white matter, respectively.
Results
The IBS group showed a significant GMV reduction in the cingulate gyrus, occipital lobe, hippocampus, frontal lobe, medial frontal gyrus, superior frontal gyrus, and limbic lobe as well as a higher GMV in the insula, superior temporal gyrus, angular gyrus, and supramarginal gyrus. Diffusion tensor imaging indicated that the IBS group had lower fractional anisotropy in the corpus callosum, upper corona, fornix, internal capsule, and brainstem. Additionally, IBS patients showed higher mean diffusivity in the cingulate gyrus, corpus callosum, upper corona, internal capsule, external capsule, fornix, and superior longitudinal fasciculus.
Conclusion
Structural changes in the brain play a role in the condition of elderly IBS patients. Psychological stress is an important factor for developing IBS via the hypothalamic‐pituitary‐adrenal axis.
Keywords: diffusion tensor imaging, irritable bowel syndrome, microstructural changes, psychological stress, voxel‐based morphometry
1. INTRODUCTION
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder characterized by abdominal discomfort, cramps, or pain associated with alterations in bowel habits, affecting 11.2% of the global population.1, 2, 3 Many studies have shown that IBS patients have altered gastrointestinal motility, visceral and somatic hypersensitivity, and psychosocial factors, such as depression, somatization, and heightened anxiety.4, 5, 6 Previous studies have recognized a link between gastrointestinal sensory and psychological processes and motor functions; however, more and more studies suggest that the dysregulation of the brain‐gut axis, including abnormal central nervous systems and gastrointestinal dysfunction, is associated with IBS.7, 8 Additionally, stress can result in overactivity or underactivity of the autonomic nervous, metabolic, and even immune systems though the hypothalamic‐pituitary‐adrenal (HPA) axis, which could alter brain‐gut interactions, and ultimately affect different physiological functions of the gastrointestinal tract.
Recent brain imaging studies have indicated abnormal pain‐related activation in IBS patients. Structural magnetic resonance imaging (sMRI) has emerged as a tool with great potential to study brain anatomy.6 Recently, many studies have reported the abnormalities of gray matter density in various chronic pain populations; they found that a decreased gray matter density was associated with chronic pain. Geha et al9 showed that reduced gray matter density in the right insular cortex, nucleus accumbens, and ventromedial prefrontal cortex was related to chronic regional pain syndrome. Furthermore, evidence for central alterations in IBS chronic pelvic pain or vulvodynia was found in gray matter density or gray matter volume (GMV).10 Using diffusion tensor imaging (DTI), white matter abnormalities in the insula and anterior cingulate cortex were found in IBS patients.11
During brain growth and healthy aging, human brain morphology changes in a specific pattern of development and atrophy. Previous studies have reported that GMV significantly decreases with age from the 20s to the 70s; however, white matter volume changes only slightly.11 It is not clear whether age‐related GMV changes react to the development of IBS. In many cases, IBS is associated with mood swings, anxiety, or anger.12, 13 However, older adults may have less tendency for outbursts or feelings of anger and anxiety compared with younger adults. Overall, relations among stress, brain morphology, and IBS in older people have not been reported.
The present study attempted to compare network properties and morphological brain architecture between elderly IBS patients and healthy controls (HCs). It aimed to test whether: (a) abnormal cortical thickness in key brain regions, such as the limbic lobe, insula, and hippocampus, is associated with increased rectal sensitivity; (b) gray matter abnormalities are accompanied by white matter abnormalities in IBS patients; and (c) abnormal feelings, such as depression and anxiety, explain some of the structural differences in the brain between IBS patients and HCs.
2. MATERIALS AND METHODS
2.1. Subjects
Data of clinical and neuropsychological examinations were collected from elderly IBS patients who were admitted to the Huashan Hospital between September 2013 and February 2015. The inclusion criteria were as follows: (a) patients aged 60‐89 years who were right‐handed and lived in Shanghai with Chinese as their native language; (b) patients who were diagnosed with IBS using the Rome III criteria; and (c) patients who could understand and sign the informed consent.
Gender and education levels were not limited. In this study, brain MRI was performed to exclude brain tumors. This study was approved by the ethics committee of Huashan Hospital (2015‐165).
2.2. Questionnaire
Each participant completed the Zung Self‐Rating Anxiety Scale (SAS) and the Zung Self‐Rating Depression Scale (SDS), which comprise 20 statements of feelings related to anxiety and depression, respectively. Patients were asked to rate the degree to which they had these feelings using a numerical scale from 0 (not at all or rarely) to 4 (most or all of the time).
2.3. MRI protocol
A magnetic resonance scanner (GE Discovery MR 750 3.0 T, General Electric Company, Fairfield, CT, USA) with a 12‐channel head coil was used. Conventional scans included sagittal T1WI scan, axial T1WI and T2WI, as well as fluid‐attenuated inversion recovery (FLAIR) and diffusion‐weighted imaging (DWI) scans. Sagittal T1WI scanning (repetition time [TR] = 2000 ms, echo time [TE] = 18 ms, field of view [FOV] = 240 × 240 mm2, thickness of layers = 5 mm, and matrix size = 320 × 240) was performed after three‐plane positioning. The joint line with the front and rear connected was used as the baseline of scanning to perform oblique axis scanning, and axial T1WI (TR = 2000 ms, TE = 17 ms, FOV = 200 × 230 mm2, thickness of layers = 6 mm, and matrix size = 320 × 168) was performed. Axial T2WI was performed with 3500 ms TR, 95 ms TE, 200 × 230 mm2 FOV, 6‐mm thick layers, and a matrix of 256 × 256. Axial FLAIR (TR = 9000 ms, TE = 102 ms, FOV = 200 × 230 mm2, thickness of layers = 6 mm, and matrix size = 256 × 190) was conducted as well. DWI of axial magnetic resonance was carried out with 27‐ms TR and 20‐ms TE.
High‐resolution whole‐brain scan was performed with a cross‐sectional 3D T1BRAVO (General Electric, Fairfield, CT, USA) with 82‐ms TR, 3.2‐ms TE, 12° flip angle, and 1.0‐mm thick layers, while continuous scanning without interval (FOV = 256 × 256 mm2, matrix of 256 × 256, and voxel of 1 × 1 × 1 mm3) was also taken. A spin echo echo‐planar imaging of single excitation was used with a b value equal to 0. A diffusion‐sensitive gradient was applied in 30 different directions at 1000 mm/s, 8600‐ms TR, 84‐ms TE, and 1.5‐mm thick layer. Continuous scanning was performed with the FOV = 212 × 212 mm2, matrix = 128 × 128, and voxel = 1.65 × 1.65 × 1.5 mm3.
2.4. Voxel‐based morphometry data analyses
The voxel‐based morphometry (VBM) analysis was performed using SPM8 and VBM8 software (Wellcome Department of Cognitive Neurology, London, UK). Prior to the analysis, image segmentation was performed for 3D structural images of all the subjects using VBM8, which were divided into gray matter, white matter, and cerebrospinal fluid. All the images were normalized onto the template that came with SPM8. Voxels were multiplied by nonlinear components to modulate images. All the gray matter images were smoothed after normalization, with the full width at half maximum equal to 10 mm. The obtained gray matter images were averaged after smoothing, and the optimal threshold was selected as the gray matter mask of this study.
2.5. Voxel‐based DTI
To allow for voxel‐based analysis, the fractional anisotropy (FA) and mean diffusivity (MD) maps were normalized using SPM8, and these normalized FA and MD maps were smoothed with a 6‐mm isotropic Gaussian kernel, which was the same value in the VBM method.
2.6. Statistical analysis
A whole‐brain voxel‐based jackknife approach was used to assess the microstructural brain changes in 18 IBS patients and 12 HCs. An independent two‐sample t test was performed for each subject's final gray matter image and FA or MD maps between the IBS patients and the HCs. The statistical threshold for significance was P < 0.01 with an additional cluster extent threshold of 50 voxels. Subsequently, questionnaire data (SAS and SDS score) as a covariate were also conducted to assess the microstructural changes using 1‐factorial analysis of variance. Then, the Student's t test was used to examine the differences of age, Fazekas score, SAS score, and SDS score between the two groups to assess the statistical significance. P values and 95% confidence intervals from the t test were calculated and reported.
3. RESULTS
3.1. Patient characteristics
Study participants—18 IBS patients and 12 HCs—were confirmed to be right‐handed by the Edinburgh Handedness Inventory.14 The study included 24 (80%) men and six (20%) women with a mean age of 77.6 ± 7.8 years (range, 61‐89 years). The mean age ± SD of the IBS group was 79.3 ± 5.0 years, and that of the HC group was 76.2 ± 7.7 years (P = 0.23). The mean SAS in IBS patients (48.6 ± 6.8) was higher than that in HCs (37.3 ± 6.8; P < 0.001). Similarly, the mean SDS in IBS patients (49.5 ± 10.8) was higher than that in HCs (34.9 ± 5.7; P < 0.001; Table 1).
Table 1.
The characteristics of IBS patients and HCs
| IBS patients | HCs | P | |
|---|---|---|---|
| Age, years (mean ± SD) | 79.3 ± 5.0 | 76.2 ± 7.7 | 0.23 |
| Male (n, %) | 14, 77.8% | 10, 83.3% | 0.85 |
| SAS | 48.6 ± 6.8 | 37.3 ± 6.8 | <0.001 |
| SDS | 49.5 ± 10.8 | 34.9 ± 5.7 | <0.001 |
HCs, healthy controls; IBS, irritable bowel syndrome; SAS, Zung Self‐Rating Anxiety Scale; SDS, Zung Self‐Rating Depression Scale.
3.2. Cortical thinning in IBS and HCs
The present study showed a significantly decreased GMV in IBS patients compared with HCs in some brain regions, including the limbic lobe, occipital lobe, inferior occipital gyrus, precentral gyrus, hippocampus, frontal lobe, middle frontal gyrus, anterior cerebellum lobe, posterior cerebellar lobe, and cerebellum nodules (P < 0.01). However, a greater GMV in the insula, left supramarginal gyrus, and middle temporal gyrus was associated with more abdominal pain in those patients (P < 0.01; Table 2 and Figure 1). When SAS and SDS were taken into consideration, a significant reduction in GMV occurred in the cingulate gyrus, occipital lobe, hippocampus, frontal lobe, medial frontal gyrus, superior frontal gyrus (affective and interoceptive processing), and limbic lobe in IBS patients compared with HCs. However, a greater GMV in the insula, superior temporal gyrus, middle temporal gyrus, angular gyrus, and supramarginal gyrus (pain modulation) was found in those people.
Table 2.
Alteration region of gray matter volume in IBS patients and HCs
| Location | Regions | P value | Voxels (mm3) | t value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 31.5 | −57 | −36 | Anterior cerebellum lobe, posterior cerebellar lobe, cerebellum nodules | <0.01 | 605 | 3.3454 |
| 21 | −100.5 | −7.5 | Occipital lobe, precentral gyrus, inferior occipital gyrus | <0.01 | 243 | 3.5038 |
| −15 | −25.5 | −9 | Limbic lobe, hippocampus, insula, left supramarginal gyrus | <0.01 | 77 | 3.4123 |
| 49.5 | −66 | 10.5 | Middle temporal gyrus | <0.01 | 402 | 3.0391 |
| 34.5 | 48 | 7.5 | Frontal lobe, middle frontal gyrus | <0.01 | 77 | 2.7774 |
HCs, healthy controls; IBS, irritable bowel syndrome.
Figure 1.
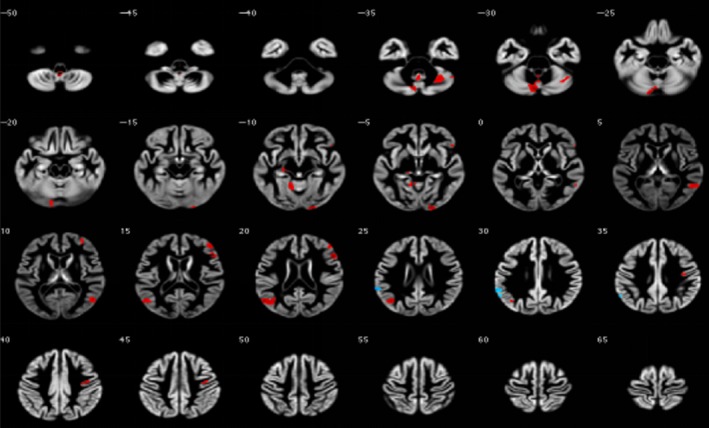
Alteration region of gray matter volume in irritable bowel syndrome patients and healthy controls
Next, the relation between psychological stress and cortical thickness was tested. The region of interest (ROI) analysis indicated a strong negative correlation between psychological stress and cortical thickness in IBS patients. No significant difference was seen in the HC group. A decreased gray matter density volume was more often found in the frontal and temporal lobes in patients with higher SAS and SDS scores (Table 3 and Figure 2).
Table 3.
Alteration region of gray matter volume in IBS patients and HCs when SAS and SDS were taken into consideration
| Location | Regions | P value | Voxels (mm3) | t value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| −34.5 | −84 | −16.5 | Occipital lobe, angular gyrus hippocampus | <0.01 | 770 | 3.9421 |
| −34.5 | 12 | −13.5 | Limbic lobe, insula cingulate gyrus | <0.01 | 335 | 3.1116 |
| 52.5 | 21 | 21 | Superior frontal gyrus, medial frontal gyrus, frontal lobe | <0.01 | 227 | 4.0585 |
| 39 | 16.5 | −7.5 | Supramarginal gyrus, superior temporal gyrus, middle temporal gyrus | <0.01 | 63 | 2.7957 |
HCs, healthy controls; IBS, irritable bowel syndrome; SAS, Zung Self‐Rating Anxiety Scale; SDS, Zung Self‐Rating Depression Scale.
Figure 2.
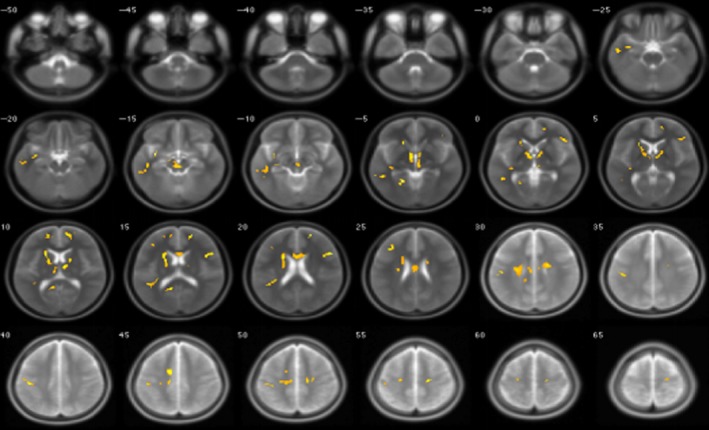
Alteration region of gray matter volume in irritable bowel syndrome patients and healthy controls when the Zung Self‐Rating Anxiety Scale and Zung Self‐Rating Depression Scale were taken into consideration
3.3. ROI‐based FA and MD
We tested the hypothesis that white matter abnormalities were related to IBS, and the deep white matter was evaluated according to the Fazekas score. No significant differences in the Fazekas score were found between IBS patients (1.67 ± 1.30) and HCs (1.83 ± 1.72; P = 0.78). The FA (a measure of the anisotropic component of the diffusion tensor) and the MD (a measure of mean water mobility) were used as surrogate measures for white matter microstructural integrity within the human brain. MRI DTI images indicated that the IBS group had lower FA in the callosum, upper corona, fornix, internal capsule, and caudex cerebri (Table 4 and Figure 3). Additionally, IBS patients had a higher mean MD in the cingulate gyrus, callosum, upper corona, internal capsule, external capsule, caudex cerebri, fornix, front corona, and superior longitudinal fasciculus (Table 5 and Figure 4).
Table 4.
Abnormal fractional anisotropy in IBS patients and HCs
| Location | Regions | P value | Voxels (mm3) | t value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| −4 | −14 | 28 | Callosum | <0.01 | 215 | 3.01 |
| −10 | 6 | 2 | Internal capsule, caudex cerebri, fornix | <0.01 | 159 | 3.26 |
| 20 | −10 | 32 | Upper corona | <0.01 | 50 | 50 |
HCs, healthy controls; IBS, irritable bowel syndrome.
Figure 3.
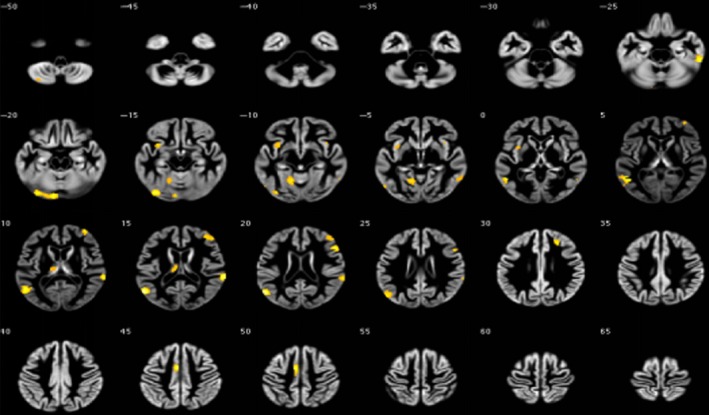
Abnormal fractional anisotropy in irritable bowel syndrome patients and healthy controls
Table 5.
Abnormal mean diffusivity in IBS patients and HCs
| Location | Regions | P value | Voxels (mm3) | t value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| −20 | 16 | 12 | Upper corona, internal capsule, callosum | <0.01 | 659 | 4.02 |
| −4 | −24 | −14 | Caudex cerebri | <0.01 | 256 | 4.11 |
| −42 | −42 | 20 | Superior longitudinal fasciculus | <0.01 | 93 | 3.83 |
| −34 | 2 | −14 | External capsule | <0.01 | 63 | 4.26 |
| −10 | −50 | 10 | Cingulate gyrus | <0.01 | 56 | 3.52 |
| −34 | −26 | 0 | Fornix | <0.01 | 55 | 3.05 |
HCs, healthy controls; IBS, irritable bowel syndrome.
Figure 4.
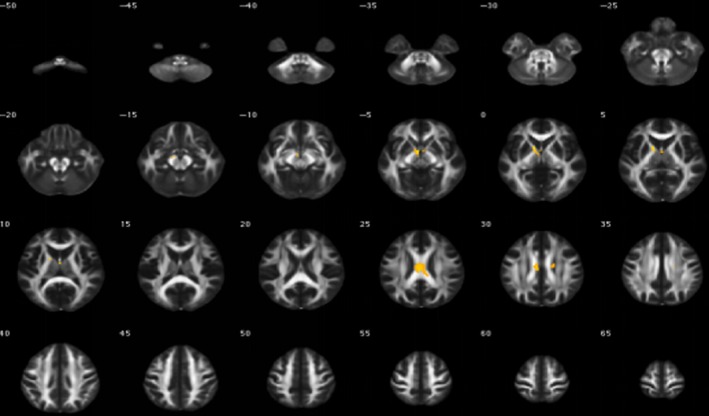
Abnormal mean diffusivity in irritable bowel syndrome patients and healthy controls
4. DISCUSSION
Structural differences in the brain were evaluated between 18 IBS patients and 12 HCs in this study in terms of gray matter density and white matter volume. The present data provided novel evidence that: (a) abnormal GMV and white matter abnormalities occurred in some key brain regions in IBS patients; and (b) abnormal feelings, such as depression and anxiety, were related to the structural differences in the brain between IBS patients and HCs. Although some similarities with previously published results from other patient populations were observed, the present data give additional support to the view that the brain plays a crucial interactive role in the condition of IBS in elderly patients.
Many previous studies have demonstrated that abnormal rectal sensitivity in IBS patients is significantly associated with reduced GMV in the bilateral amygdala, bilateral insula, bilateral superior frontal gyrus, bilateral hippocampus, left gyrus rectus, left cingulate gyrus, left putamen, and brainstem.6, 8, 15, 16 In other studies, however, a greater GMV was observed in several brain regions in IBS patients, including the hypothalamus, anterior cingulate cortex, hippocampus, basal ganglia, and primary somatosensory cortex (S1).17, 18, 19, 20 Together, these studies support the concept that altered regional brain morphology is related to chronic abdominal pain and visceral hyperalgesia in IBS patients. Although various molecular mechanisms underlying the observed GMV alterations have been proposed, the relation between GMV alterations and IBS is still unclear. In patients with IBS, Seminowicz et al16 reported decreased GMV more often found in regions involved in increased responsiveness to rectal distension and its expectation, while an increased GMV in these regions was related to cognitive and attentional modulation of interoceptive information. However, it remains ambiguous as to whether changes in brain regions are a consequence of IBS symptoms or possibly the determined risk factors.
A strong correlation exists between the severity of IBS and psychological stress, especially depression and anxiety. Surdea‐Blaga et al21 reported a significant increase in the stressor score between normal individuals and IBS patients. Previous studies have strongly suggested that psychological or psychosocial stressors are related to the development of IBS. In this study, a significant reduction in GMV occurred in the cingulate gyrus, occipital lobe, hippocampus, frontal lobe, medial frontal gyrus, superior frontal gyrus, and limbic lobe in IBS patients when SAS and SDS were taken into consideration. Recently, HPA axis dysregulation has been considered as a key mechanism in IBS pathophysiology, including an abnormal function in the autonomic and enteric nervous systems. Systemic or interoceptive stressors trigger the HPA to release corticotropin‐releasing hormone, which mediates the stimulation of inflammatory cytokines.22, 23 Additionally, the corticotropin‐releasing factor system can also regulate autonomic nervous system activity through modulation of the forebrain, hindbrain, and spinal sites, which can stimulate the sympathetic nervous system and induce the release of catecholamines.22 In summary, HPA axis responses vary in different disease states with different impacts on the duration of stress response and on peripheral and central cortisol levels. Therefore, among the diverse factors related to IBS, the pharmacological and non‐pharmacological approaches for stress‐related disorders are important and effective treatments.24, 25, 26
Although many studies have reported abnormal gray matter regions in patients with IBS or other chronic pain conditions, whether white matter abnormalities occur in IBS patients needs further exploration. The present study found that IBS patients had lower FA in the callosum, upper corona, fornix, internal capsule, and caudex cerebri, indicating that white matter abnormalities occurred in IBS patients having gray matter abnormalities. The value of FA is affected by the consistency of the white matter fiber, the uniformity of the arrangement direction, and the integrity of the nerve fiber myelin sheath. Chen et al11 reported that IBS patients had higher FA in the fornix and external capsule adjacent to the right posterior insula. Ellingson et al27 demonstrated that patients had lower FA in the thalamic regions, basal ganglia, and sensory/motor association/integration regions, as well as higher FA in the frontal lobe regions and corpus callosum. They also found that IBS patients had higher MD in the thalamus, internal capsule, and coronal radiata projecting to sensory/motor regions, and reduced MD in the globus pallidus.27 The sample size and distinctive sample characteristic of the present study might have contributed to the difference. Most of the elderly patients in the present study had experienced exogenous stressors and direct effects of intestinal interoceptive stressors for a long time. These stimuli induce neurotoxicity on white matter. The correlation between the severity and durability of the stress and the severity of the clinical symptoms indicated that the duration of disease is an important factor for IBS.
The brain, as well as other organs, presents some structural and functional changes during the process of healthy aging. Previous longitudinal and cross‐sectional studies using MRI have demonstrated age‐related brain changes, including smaller brain volume, GMV and white matter atrophy, and enlarged ventricular system. As structural anatomical changes in the brain affect functional changes, motor function, sensory abilities, and cognitive and learning function decline in elderly adults. The present comparative study showed no significant difference in the ages of patients in the two groups, indicating that differences in GMV and white matter between the groups were correlated with IBS, not healthy aging.
The present study had some limitations. First, it did not confirm whether the observed changes in the brain in regional GMV and white matter volume preceded or resulted from IBS, owing to its cross‐sectional nature. Second, although only older adults were included in the present study, hormone levels were not measured, and therefore the study could not address the possible influence of hormones on the findings. Third, the small sample size may not have been adequately powered to detect smaller group differences.
5. CONCLUSIONS
The results of this study support the view that changes in regional GMV and white matter volume play a role in IBS symptoms. The observed changes (increases and decreases) in GMV identified in this study may reflect different pathophysiological processes in IBS, including chronic pain‐related mechanisms (decreased gray matter in the insula), associated increase in emotional arousal (decreased gray matter in the hippocampus), and increased sensitivity to somatic and visceral stimuli (increased GMV in S1). Specifically, psychological stress is an important factor for the development of IBS, through stimulating the HPA axis and then triggering adrenocorticotropic hormone and cortisol, hence influencing gut function. Despite its limited sample size, this study indicates that structural changes in the brain help to provide insight into the mechanisms of IBS.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
L.Z. and Y.W. conceived and coordinated the study, designed, performed, and analyzed the experiments, and wrote the paper. L.Z., Y.W., and Y.Z. carried out the data collection, data analysis, and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Zhao L, Wang Y, Zhang Y. Microstructural changes in the brain in elderly patients with irritable bowel syndrome. Aging Med. 2018;1:141–148. 10.1002/agm2.12034
Zhao and Wang contributed equally to this work.
REFERENCES
- 1. Undseth R, Berstad A, Valeur J. Systemic symptoms in irritable bowel syndrome: an investigative study on the role of enterocyte disintegrity, endotoxemia and inflammation. Mol Med Rep. 2016;14:5072‐5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roohafza H, Keshteli AH, Daghaghzadeh H, Afshar H, Erfani Z, Adibi P. Life stressors, coping strategies, and social supports in patients with irritable bowel syndrome. Adv Biomed Res. 2016;5:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Popa SL, Brate AT, Leucuta DC, Baban A. Impaired pressure management in the irritable bowel syndrome. J Gastrointestin Liver Dis. 2016;25:566‐567. [PubMed] [Google Scholar]
- 4. Duerden EG, Albanese MC. Localization of pain‐related brain activation: a meta‐analysis of neuroimaging data. Hum Brain Mapp. 2013;34:109‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caldarella MP, Giamberardino MA, Sacco F, et al. Sensitivity disturbances in patients with irritable bowel syndrome and fibromyalgia. Am J Gastroenterol. 2006;101:2782‐2789. [DOI] [PubMed] [Google Scholar]
- 6. Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre‐existing and disease‐driven factors. Gastroenterology. 2010;138:1783‐1789. [DOI] [PubMed] [Google Scholar]
- 7. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050‐11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70:153‐154. [DOI] [PubMed] [Google Scholar]
- 9. Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray‐white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elsenbruch S, Schmid J, Kullmann JS, et al. Visceral sensitivity correlates with decreased regional gray matter volume in healthy volunteers: a voxel‐based morphometry study. Pain. 2014;155:244‐249. [DOI] [PubMed] [Google Scholar]
- 11. Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;1392:121‐131. [DOI] [PubMed] [Google Scholar]
- 12. Porcelli P, De Carne M, Leandro G. The role of alexithymia and gastrointestinal‐specific anxiety as predictors of treatment outcome in irritable bowel syndrome. Compr Psychiatry. 2017;73:127‐135. [DOI] [PubMed] [Google Scholar]
- 13. Mather M. The affective neuroscience of aging. Annu Rev Psychol. 2016;67:213‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milenkovic S, Dragovic M. Modification of the Edinburgh Handedness Inventory: a replication study. Laterality. 2013;18:340‐348. [DOI] [PubMed] [Google Scholar]
- 15. Labus JS, Dinov ID, Jiang Z, et al. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155:137‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seminowicz DA, Labus JS, Bueller JA, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69:1990‐1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA. Different pain, different brain: thalamic anatomy in neuropathic and non‐neuropathic chronic pain syndromes. J Neurosci. 2011;31:5956‐5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gustin SM, Peck CC, Cheney LB, Macey PM, Murray GM, Henderson LA. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J Neurosci. 2012;32:14874‐14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teutsch S, Herken W, Bingel U, Schoell E, May A. Changes in brain gray matter due to repetitive painful stimulation. NeuroImage. 2008;42:845‐849. [DOI] [PubMed] [Google Scholar]
- 21. Surdea‐Blaga T, Baban A, Dumitrascu DL. Psychosocial determinants of irritable bowel syndrome. World J Gastroenterol. 2012;18:616‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larauche M, Mulak A, Tache Y. Stress‐related alterations of visceral sensation: animal models for irritable bowel syndrome study. J Neurogastroenterol Motil. 2011;17:213‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol. 2012;233:49‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van den Wijngaard RM, Stanisor OI, van Diest SA, et al. Peripheral alpha‐helical CRF (9‐41) does not reverse stress‐induced mast cell dependent visceral hypersensitivity in maternally separated rats. Neurogastroenterol Motil. 2012;24:274‐282. [DOI] [PubMed] [Google Scholar]
- 25. Larauche M. Novel insights in the role of peripheral corticotropin‐releasing factor and mast cells in stress‐induced visceral hypersensitivity. Neurogastroenterol Motil. 2012;24:201‐205. [DOI] [PubMed] [Google Scholar]
- 26. Barreau F, Salvador‐Cartier C, Houdeau E, Bueno L, Fioramonti J. Long‐term alterations of colonic nerve‐mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57:582‐590. [DOI] [PubMed] [Google Scholar]
- 27. Ellingson BM, Mayer E, Harris RJ, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013;154:1528‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]


