Abstract
With aging, the pathogenesis processes of atrial fibrillation (AF) are heightened. In this article, we review the mechanisms that predispose elderly patients to AF. We also highlight the unique features in diagnosis, stroke prevention, and treatment strategies for the elderly patient with AF.
Keywords: aging, arrhythmia, atrial fibrillation
1. BACKGROUND
Atrial fibrillation (AF) is one of the most common diseases in elderly patients, and its prevalence increases with age.1 The prevalence is estimated to be 0.3% in subjects of 40 years old, 5%‐9% in those between 60 and 80 years old, and approximately 10% of patients more than 80 years old.2 There are two primary factors that contribute to the high prevalence of AF in the elderly, the degenerative changes of the aging heart itself and the accompanying structural heart and systemic diseases, most notably coronary artery disease and hypertension, whose incidences increase cumulatively over one's life.
2. ATRIAL REMODELING IN AGING HEARTS
The pathogenesis processes of AF are heightened by aging. The incidence of comorbidities such as ischemic heart disease and heart failure that are risk factors for AF is significantly increased in the elderly. Aging is associated with increased p‐wave duration and dispersion, reflecting intra‐atrial conduction abnormalities. Changes in p‐wave morphology may herald the onset of AF.3
Atrial structural remodeling predisposes aged atria to AF. Progressive atrial fibrosis is a hallmark of aging heart.4 Atrial fibrosis is known to occur as the result of aging‐related changes in the cardiac collagen matrix and predisposes patients to AF.5, 6 Aging was identified as the most significant factor in atrial fibrogenesis (Figure 1).7 An increase in fibrotic and a decrease in hypoxic signaling and microvessel density coupled with a differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases that favors fibrosis may contribute to age‐related atrial fibrogenesis.
Figure 1.
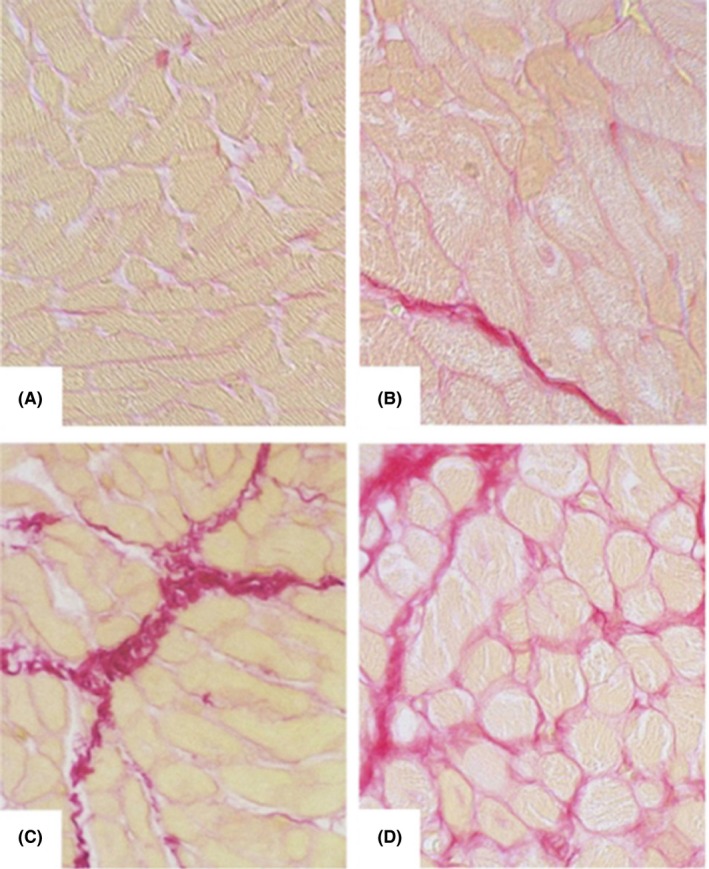
Histological images of Sirius red‐stained atrial myocardium from patients of different age groups. A, <50 y; B, 51‐60 y; C, 61‐70 y; D, >70 y. (Magnification ×40. Reproduced with kind permission of the American Aging Association and permission from the authors: Age‐related atrial fibrosis. AGE, 2009;31:27‐38.)
Cellular hypertrophy of atrial cardiomyocytes occurs as a compensatory response to aging‐related loss or apoptosis of atrial cardiomyocytes.8 Hypertrophic myocytes in the rat left atrium demonstrated an increased propensity to spontaneous Ca2+ transients with a potential to generate triggered arrhythmias.9
Electrical remodeling is an important pathophysiological mechanism that promotes the progression from paroxysmal AF to permanent AF. The shortening of the atrial action potential duration (APD), and thereby the atrial effective refractory period (AERP), potentiates the pathogenesis of AF and underlies the progression of AF as “AF begets AF.”10 However, there have been conflicting data in age‐related changes in AERP although APD seems to be shortened with aging.11, 12, 13, 14, 15 Such inconsistency in data could be a reflection of the heterogeneity in AERP distribution and nonlinear nature of the correlation between the APD and AERP, as well as the limited pacing sites and pacing cycle lengths.
Although it is generally recognized that intra‐atrial conduction is slowed in the aging atria, the sodium channel current INa and expression of Nav1.5 are not significantly affected by aging.16 The effects of aging on atrial late sodium current (INa‐Late) have not been fully elucidated although INa‐Late may be increased in age‐related comorbidities, such as heart failure that promotes AF. Depressed ICa‐L contributes to the aging‐related deterioration of the normal pace making activity of the sinoatrial node with reduced expression of the Cav1.2 proteins, leading to sick sinus syndrome that is known to predicate AF.17 Aging‐related downregulation of connexin 43, not that of connexin 40 or 45, has been reported to increase the propensity to AF.18
The effects of aging on repolarization currents are more complex. The densities of the transient outward potassium current (Ito), and sustained potassium current (Isus), are significantly increased in the aged canine right atria,19 while such changes are not observed in aged left atrial cells.20 The balance between an aging‐related increase in Isus and a decrease in L‐type calcium current (ICa‐L) may dictate in either an unchanged or prolonged ERP in the right atria. The acetylcholine‐induced potassium current (IK‐ACh) plays a vital role in vagal‐induced AF and increases the risk of AF in elderly patients.
Aging is associated with deterioration of calcium homeostasis in the atrial cardiomyocytes.21 Cardiomyocytes in pulmonary veins (PV) of aged rabbits exhibit larger delayed afterdepolarization (DAD) and greater resting membrane potential and longer APDs with increased incidence of both APD alternans and contractile alternans.22 These focal mechanisms may underlie the increased incidence of PV‐related ectopy that initiates AF. Aged rabbit left atria tend to have a higher incidence of DAD that could serve as the source of non‐PV triggers for AF.22 Furthermore, the effects of aging on atrial arrhythmogenesis through dysregulation of sodium and calcium homeostasis can be aggravated in disease states, such as heart failure, that is, associated with calcium overload.23
Mechanical loading on myocardial tissue may modulate the electrical activities of the heart by altering the electrical function of the cells, a phenomenon termed mechanoelectric feedback. Mechanoelectric feedback could alter APD and generate ectopic beats through the activation of stretch‐activated channels. Patients with AF often have left atrial dilatation due to either volume or pressure overload which increases vulnerability to AF through mechanoelectric feedback.24 However, it is not clear whether aging itself impacts directly on the mechanoelectric feedback or simply increases AF vulnerability due to the co‐existing conditions commonly seen in elderly that trigger the mechanoelectric feedback.
The role of autonomic dysfunction in AF has been well documented, and many of these changes are age related.25 There are many other factors contributing to age‐related atrial remodeling, such as chronic activation of the renin–angiotensin–aldosterone axis, excessive beta‐adrenergic and endothelin signaling, activation of the TGF‐b1 pathway, recruitment of mononuclear cells, fibroblast progenitors, increased generation of reactive oxygen species and diminished antioxidant capacity.26, 27, 28, 29, 30, 31, 32 Aging is reportedly associated with oxidative damage in the atrium.12 Oxidative changes are present in the atrial tissue of patients with AF and are associated with upregulation of genes involved in the production of reactive oxygen species.33
3. DIAGNOSIS OF AF IN ELDERLY PATIENTS
AF is often asymptomatic and under‐diagnosed in the elderly even though the incidence of AF increases dramatically with age.34 The elderly patients with AF tend not to have a rapid ventricular response due to intrinsic atrioventricular conduction abnormalities, reduced physical activities, or antihypertensive medication that is also negatively dromotropic. The clinical presentation is frequently atypical with intermittent fatigue and sleepiness as the primary complaints. Such mild and nonspecific symptoms are commonly ascribed to “being old.” Tragically, many of them present with stroke as their first clinical manifestation of AF. On the other hand, incidence of heart failure is increased in the elderly patients with AF.35
Public awareness of the increased incidence of AF in elderly patients and its embolic consequences is crucial in meeting this major challenge of public health. Palpation of one's pulses for irregularity followed by electrocardiography (ECG) or event recorder in those with an irregular pulse is the simplest and most cost‐effective means of screening for silent or mildly symptomatic AF. It is also cost‐effective to perform intermittent screening, either by standard 12‐lead or by single‐lead ECG, for silent AF in elderly population (>65 years old, especially those with heart failure or other precipitating factors for AF) as well as in other at‐risk populations.36, 37, 38 However, the sensitivity of such screening is low for paroxysmal AF even with continuous Holter monitoring.38, 39
Elderly patients with a history of cryptogenic stroke or transient ischemic attacks (TIA) deserve special attention because the detection of AF in this patient subgroup fundamentally changes the treatment approach from antiplatelet therapy to anticoagulation. CRYSTAL‐AF trial showed that, with an implantable recording device (Figure 2), AF detection rate was reported to be 8.9% at 6 months, 12.4% at 12 months, and 30% at 3 years in those with cryptogenic stroke. This finding is collaborated by other studies.40, 41
Figure 2.
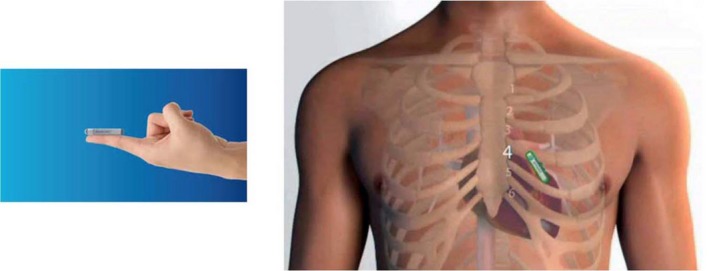
Implantable cardiac monitor. Implantable cardiac monitor is smaller than a pacemaker and capable of continuously monitoring the patient's electrocardiography for up to several years
Implantation ECG monitoring device was shown to dramatically increase the detection rate of previously undiagnosed paroxysmal AF (up to 40% over 30 months of monitoring) in an older population (mean age ± standard deviation: 71.5 ± 9.9) with CHADS2 score of 3 or higher.42 Although it was a small‐sized study involving only 446 patients, its implication is tremendous. It suggests the incidence of AF has been grossly underestimated, and the ramification on public healthcare delivery and costs almost reaches an unimaginable proportion. Of important note, the very risk factors that constitute the CHADS2 score, such as age, hypertension, diabetes, and heart failure, also predispose patients to AF.
4. STROKE PREVENTION IN ELDERLY PATIENTS
Aging increases the risks of embolic stroke from AF and hemorrhage from anticoagulation. Stroke is one of the most serious complications in patients with AF. The risk of stroke increases with age: it is 1.5% at age 50‐59 years and 23.5% at age 80‐89 years.43 AF appears to increase the incidence of dementia in patients with a history of stroke,42 AF is responsible for around 10%‐20% of all strokes, and, in patients age 80 to 89 years, AF is accountable for over 25% of strokes.44 Anticoagulation is the most effective treatment for stroke prevention in elderly patients. The CHADS2 and CHA2DS2‐VASc scores emphasize the importance of aging in the evaluation of thromboembolic risk.45, 46 Elderly patients with AF will benefit from using the anticoagulation therapy.2, 47 However, age is also a risk factor for major bleeding and elderly patients are more likely to suffer from untoward side effects of anticoagulation due to their tendency to fall, especially those with dementia and Parkinson's disease (Table 1).48
Table 1.
Stroke and bleeding risk stratification with the CHA2DS2VASc and HAS‐BLED schemas
| CHA2DS2‐VASc | Score | HAS‐BLED | Score |
|---|---|---|---|
| Congestive heart failure/LV dysfunction | 1 | Hypertension, that is, uncontrolled BP | 1 |
| Hypertension | 1 | Abnormal renal/liver function | 1 or 2 |
| Aged ≥ 75 | 2 | Stroke | 1 |
| Diabetes mellitus | 1 | Bleeding tendency or predisposition | 1 |
| Stroke/TIA/TE | 2 | Labile INR | 1 |
| Vascular disease [prior MI, PAD, or aortic plaque] | 1 | Age (eg, > 65) | 1 |
| Aged 65‐74 y | 1 | Drugs (eg, concomitant aspirin or NSAIDSs) or alcohol | 1 |
| Sex category [ie, female gender] | 1 | ||
| Maximum score | 9 | 9 |
4.1. Warfarin
Warfarin is vitamin K antagonist and the oral anticoagulant with the longest history of clinical use. The daily maintenance dose of warfarin declines with the increasing of age; therefore, lower initiation and maintenance dose should be considered in elderly to reduce the risk of bleeding. The American Heart Association guidelines for AF management suggests that the target of INR is 1.6‐2.5 in patients over 70 years old who were using warfarin.49 The primary difficulty in the clinical use of warfarin is its striking heterogeneity in pharmacological response among different races and age groups as well as its significant interaction with a wide range of common drugs and vitamin K containing foods. Even though warfarin use can be monitored by a standardized test for prothrombin time (international normalized ratio or INR), maintenance of appropriate levels of INR requires frequent blood tests. Instead of routine laboratory measurements of prothrombin time, fingerstick‐based equipment is now available that can be used at a point of care such as patients’ homes and clinics and were shown to be accurate.50
4.2. New oral anticoagulants
New or novel oral anticoagulants (NOACs) are now available for patients with nonvalvular AF. NOACs have few interactions with other drugs and food, and do not require INR monitoring. NOACS are currently used in clinical practice including direct thrombin inhibitor, such as dabigatran, and direct factor Xa inhibitors, such as rivaroxaban, apixaban, and edoxaban. A recent meta‐analysis showed that NOACs, compared with warfarin, lead to a significant reduction in strokes, intracranial hemorrhages, mortality, and comparable major bleeding events.51 But, NOACs are associated with a greater risk of gastrointestinal bleedings. With the exception of dabigatran whose effect can be reversed by idarucizumab, there is no effective antagonist to reverse the effects of other NOAGs yet.52 Active research is ongoing to develop such antidotes. In addition, the primary barrier to the wide use of NOAGs is their costs which can be as high as $3000 a year per patient. A recent meta‐analysis study indicates the incremental cost per quality‐adjusted life‐year versus warfarin amounts to over $20 000.53
The recommended dose of dabigatran is 150 mg twice daily for adults with CrCl at least 30 mL/min, but in elderly patients, dabigatran 110 mg twice a day is recommended.54 The rivaroxaban 20 mg daily can be used in most patients, but in patients with high bleeding risk or renal dysfunction, 15 mg daily is recommended.55 Apixaban has shown a significant reduction in stroke, systemic embolism, major bleeding, and all‐cause mortality compared with warfarin, and apixaban 5 mg twice daily is recommended, but in patients aged 80 years or older, the dosage should be adjusted to 2.5 mg twice daily.56
4.3. Percutaneous left atrial appendage closure
For patients with AF at high stroke risk and have concerns over long‐term anticoagulation, left atrial appendage (LAA) closure should be considered.57 There are several clinical trials enrolled elderly patients over 75 years of age and proved the efficacy of percutaneous LAA closure devices.58, 59, 60, 61, 62, 63 The 5‐year outcomes of the PREVAIL trial, combined with the 5‐year outcomes of the PROTECT AF trial, demonstrate that LAA closure using Watchman™ device provides stroke prevention in nonvalvular AF comparable to warfarin, with additional reductions in major bleeding, particularly hemorrhagic stroke, and mortality.60 Therefore, LAA closure has been proposed as a reasonable alternative to warfarin therapy for stroke prevention in patients with nonvalvular AF who do not have an absolute contraindication to short‐term warfarin therapy.61 The ASAP trial (ASA Plavix Feasibility Study with Watchman Left Atrial Appendage Closure Technology) suggests that LAA closure with the Watchman device can be safely performed without a warfarin transition and is a reasonable alternative to consider for patients at high risk for stroke but with contraindications to systemic oral anticoagulation.62 However, ASAP trial was a nonrandomized study and confirmation with multicenter randomized trials is warranted. The question also remains how LAA closure will stand against the NOAGs as NOAGs outperform warfarin in stroke prevention and intracranial bleeding.51 On the other hand, LAA closure represents a rapidly expanding field of research and technology innovation with newer devices under clinical investigation that may offer better outcomes than the currently available devices.63, 64
5. CHOICE OF RATE VS RHYTHM CONTROL IN ELDERLY AF PATIENTS
The primary objectives in AF management are (i) prevention of stroke or embolism (see above: Stroke prevention in elderly patients) and (ii) improvement of the quality of life in symptomatic patients that could include those with tachycardia‐induced heart failure and existing heart failure exacerbated by AF. AF could worsen heart failure, not only through uncontrolled ventricular response but also by lack of atrial contribution (“atrial kick”) and irregularity in heart rate, both of which compromise ventricular filling, particularly in patients with diastolic dysfunction. There are two general approaches to achieve the second objective: rate control and rhythm control.
5.1. Rate control
There are several studies indicating that primary rate control is not inferior to rhythm control in many patients, especially in elderly without symptoms.65, 66, 67, 68, 69, 70 In these patients, rate control is considered as the first‐line therapy. The most commonly used drugs for rate control include beta‐blockers, nondihydropyridine calcium channel blockers, and digoxin. Beta‐blockers are the most effective drugs of choice for rate control. Calcium channel blockers can be used as an alternative, especially in patients with intolerance to beta‐blockers. Digoxin is recommended to patients with left ventricular systolic dysfunction when other drugs are not tolerated or contraindicated with preexisting borderline blood pressure. However, the potential drug toxicity, especially in elderly patients with renal dysfunction, should be weighed against the benefit. Furthermore, digoxin primarily affects the resting heart rate and is of much less effect in heart rate during exertion. More importantly, recent randomized trial showed no clinical benefit of a strict rate control, and it did not improve the morbidity and mortality.69 One must be conscientious about the potential risk of postconversion pause and bradycardia, especially those patients with a complaint of syncope or near syncope. For elderly patients, rate control targeting resting heart rates about 115 beats per minute is acceptable.65
The ultimate means of rate control is ablation of the atrioventricular node with the implantation of permanent pacemakers. This is reserved for patients with refractory rapid ventricular response despite maximum doses of rate control drugs that the patients can tolerate. However, RV pacing could lead to left ventricular systolic dysfunction.71 Therefore, in patients with the preexisting left ventricular systolic dysfunction, re‐synchronization therapy with a bi‐ventricular pacemaker should be considered.
5.2. Rhythm control
Antiarrhythmic drugs are used to maintain sinus rhythm, but the conversion of AF to sinus rhythm often requires electrical cardioversion. It is difficult to control rhythm pharmacologically in elderly patients with risk of AF recurrence.65 Moreover, the rhythm control groups had more adverse drug effects, such as pro‐arrhythmia, drug interactions, and age‐related comorbidities, without clear mortality benefit.66, 67, 68, 69, 70 Therefore, antiarrhythmic drugs are only commonly used in patients with symptoms.
Adverse events with flecainide and propafenone are increased elderly, probably due to age‐related pharmacokinetic changes and comorbidities.72 Amiodarone is the most effective drug and safe in heart failure patients, but its side effects on thyroid, hepatic, and pulmonary function should be carefully monitored during the follow‐up. In patients using sotalol and dofetilide, abnormal QT prolongation and torsades de pointes should be observed carefully. In patients with significant renal dysfunction, administration of sotalol and dofetilide should be used with extreme caution or avoided altogether.73 Dronedarone has a similar structure to amiodarone without the iodine component, but it is contraindicated in heart failure patients, patients with permanent AF, and those with severe hepatic impairment. Overall, dronedarone has fewer thyroid, neurologic, dermatologic, and other side effects than amiodarone and can be used in elderly patients.74 However, the clinical efficacy of dronedarone may be quite limited.
Catheter ablation has been accepted in selected elderly patients with symptomatic paroxysmal or persistent AF, and it was recommended as Class IIa indications as in young patients.49 Risk of thromboembolism may persist in the elderly population over 75 years of age undergoing AF ablation regardless of whether sinus rhythm is apparently maintained.75 A number of studies that have specifically focused on reporting the outcomes of AF ablation in older individuals76, 77, 78, 79, 80, 81, 82, 83, 84 and several randomized trials have included patients who were >75 years of age.85, 86, 87, 88 The results of these studies provide evidence that catheter ablation of AF has an acceptable safety and efficacy profile in the elderly over the age of 75 or 80 years. Age has the significant impact on the long‐term outcomes of AF ablation; for every 10‐year increase in age, there was a higher multivariate‐adjusted risk of AF recurrence, death, and major cardiac events.83 Furthermore, the complications of the procedure are somewhat increased in older individuals, the need for concomitant antiarrhythmic therapy postablation is greater, and the efficacy is somewhat reduced.76, 89 It is important to emphasize that apparent success in rhythm control does not eliminate the risk of stroke in patients with AF, no matter whether the success in rhythm control is achieved pharmacologically or through ablation.
6. CONCLUSION
The prevalence of AF increases with aging because of age‐related changes that affect the underlying pathogenesis of AF as well as the increased incidence of accompanying structural heart disease in elderly patients. The reported incidence of asymptomatic AF likely represents a significant underestimate, particularly paroxysmal AF of short durations. However, new strategies with implantable cardiac monitoring devices are under investigation for early identification that could lead to improved prevention of stroke. Because of the age‐related difference in comorbidity, impaired hepatic and renal functions, and the heightened risk of thromboembolism, choosing AF treatment options in the elderly patient requires careful balancing between benefits and risks. In particular, older age is not only the risk factor of AF but also predispose patients to both stroke and major bleeding at the same time that frequently represents a challenging dilemma. NOAGs provide effective alternative anticoagulation with less bleeding side effects and without the need for regular dose monitoring in comparison with warfarin. Nonpharmacological approaches in AF management, including left atrial appendage occlusion and AF ablation, offer new and innovative therapy to elderly patients as well as to the patients with AF at large. Public awareness, as well as technological breakthrough in both detection and treatment, will be critical in combating AF that has emerged as one of the major health issues in the world.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
Chen Q, Yi Z, Cheng J. Atrial fibrillation in aging population. Aging Med. 2018;1:67‐74. 10.1002/agm2.12015
REFERENCES
- 1. Lakshminarayan K, Solid CA, Collins AJ, et al. Atrial fibrillation and stroke in the general Medicare population: a 10‐year perspective (1992 to 2002). Stroke. 2006;37:1969‐1974. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370‐2375. [DOI] [PubMed] [Google Scholar]
- 3. Yoshizawa T, Niwano S, Niwano H, et al. Prediction of new onset atrial fibrillation through P wave analysis in 12 lead ECG. Int Heart J. 2014;55:422‐427. [DOI] [PubMed] [Google Scholar]
- 4. Li Q, Liu X, Wei J. Ageing related periostin expression increase from cardiac fibroblasts promotes cardiomyocytes senescent. Biochem Biophys Res Comm. 2014;452:497‐502. [DOI] [PubMed] [Google Scholar]
- 5. Horn MA, Trafford AW. Aging and the cardiac collagen matrix: novel mediators of fibrotic remodeling. J Mol Cell Cardiol. 2016;93:175‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802‐809. [DOI] [PubMed] [Google Scholar]
- 7. Gramley F, Lorenzen J, Knackstedt C, et al. Age‐related atrial fibrosis. Age (Dordr). 2009;31:27‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheydina A, Riordon DR, Boheler KR. Molecular mechanisms of cardiomyocyte aging. Clin Sci (Lond). 2011;121:315‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H, Cannell MB, Kim SJ, et al. Cellular hypertrophy and increased susceptibility to spontaneous calcium‐release of rat left atrial myocytes due to elevated afterload. PLoS ONE. 2015;10:e0144309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954‐1968. [DOI] [PubMed] [Google Scholar]
- 11. Hayashi H, Wang C, Miyauchi Y, et al. Aging‐related increase to inducible atrial fibrillation in the rat model. J Cardiovasc Electrophysiol. 2002;13:801‐808. [DOI] [PubMed] [Google Scholar]
- 12. Kavanagh KM, Wyse DG, Mitchell LB, Du HJ. Cardiac refractoriness: age‐dependence in normal subjects. J Electrocardiol. 1989;22:221‐225. [DOI] [PubMed] [Google Scholar]
- 13. Michelucci A, Padeletti L, Fradella GA, et al. Aging and atrial electrophysiological properties in man. Int J Cardiol. 1984;5:75‐81. [DOI] [PubMed] [Google Scholar]
- 14. Taneja T, Mahnert BW, Passman R, et al. Effects of sex and age on electrocardiographic and cardiac electrophysiological properties in adults. Pacing Clin Electrophysiol. 2001;24:16‐21. [DOI] [PubMed] [Google Scholar]
- 15. Kistler PM, Sanders P, Fynn SP, et al. Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol. 2004;44:109‐116. [DOI] [PubMed] [Google Scholar]
- 16. Baba S, Dun W, Hirose M, Boyden PA. Sodium current function in adult and aged canine atrial cells. Am J Physiol Heart Circ Physiol. 2006;291:H756‐H761. [DOI] [PubMed] [Google Scholar]
- 17. Jones SA, Boyett MR, Lancaster MK. Declining into failure: the age‐dependent loss of the L‐type calcium channel within the sinoatrial node. Circulation. 2007;115:1183‐1190. [DOI] [PubMed] [Google Scholar]
- 18. Nagibin V, Egan Benova T, Viczenczova C, et al. Ageing related down‐regulation of myocardial connexin‐43 and up‐regulation of MMP‐2 may predict propensity to atrial fibrillation in experimental animals. Physiol Res. 2016;65(Suppl 1):S91‐S100. [DOI] [PubMed] [Google Scholar]
- 19. Dun W, Yagi T, Rosen MR, Boyden PA. Calcium and potassium currents in cells from adult and aged canine right atria. Cardiovasc Res. 2003;58:526‐534. [DOI] [PubMed] [Google Scholar]
- 20. Dun W, Baba S, Boyden PA. Aged‐related changes in transient outward and sustained currents in canine right atrial free wall cells are not seen in left atrial cells. In: Proceedings of Keystone Symposia for Molecular Pathology of Cardiac Arrhythmias, 2003.
- 21. Herraiz‐Martínez A, Álvarez‐García J, Llach A, et al. Ageing is associated with deterioration of calcium homeostasis in isolated human right atrial myocytes. Cardiovasc Res. 2015;106:76‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wongcharoen W, Chen YC, Chen YJ, et al. Aging increases pulmonary veins arrhythmogenesis and susceptibility to calcium regulation agents. Heart Rhythm. 2007;4:1338‐1349. [DOI] [PubMed] [Google Scholar]
- 23. Chang SL, Chen YC, Yeh YH, et al. Heart failure enhanced pulmonary vein arrhythmogenesis and dysregulated sodium and calcium homeostasis with increased calcium sparks. J Cardiovasc Electrophysiol. 2011;22:1378‐1386. [DOI] [PubMed] [Google Scholar]
- 24. Bode F, Katchman A, Woosley RL, Franz MR. Gadolinium decreases stretch‐induced vulnerability to atrial fibrillation. Circulation. 2000;101:2200‐2205. [DOI] [PubMed] [Google Scholar]
- 25. Parashar R, Amir M, Pakhare A, et al. Age related changes in autonomic functions. J Clin Diagn Res. 2016;10:CC11‐CC15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Disertori M, Quintarelli S. Renin‐angiotensin system and atrial fibrillation: understanding the connection. J Atr Fibrillation. 2011;4:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. González de la Fuente M, Barana A, Gómez R, et al. Chronic atrial fibrillation up‐regulates β1‐Adrenoceptors affecting repolarizing currents and action potential duration. Cardiovasc Res. 2013;97:379‐388. [DOI] [PubMed] [Google Scholar]
- 28. Baltogiannis GG, Kolettis TM, Chierchia GB, et al. Contribution of the endothelin system to the genesis and maintenance of atrial fibrillation: review of the literature and clinical implications. Hellenic J Cardiol. 2015;56:279‐284. [PubMed] [Google Scholar]
- 29. Behnes M, Hoffmann U, Lang S, et al. Transforming growth factor beta 1 (TGF‐beta 1) in atrial fibrillation and acute congestive heart failure. Clin Res Cardiol. 2011;100:335‐342. [DOI] [PubMed] [Google Scholar]
- 30. Kim SK, Pak HN, Park JH, et al. Non‐ischaemic titrated cardiac injury caused by radiofrequency catheter ablation of atrial fibrillation mobilizes CD34‐positive mononuclear cells by non‐stromal cell‐derived factor‐1alpha mechanism. Europace. 2009;11:1024‐1031. [DOI] [PubMed] [Google Scholar]
- 31. Sovari AA, Dudley SC Jr. Reactive oxygen species‐targeted therapeutic interventions for atrial fibrillation. Front Physiol. 2012;3:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ali‐Hassan‐Sayegh S, Mirhosseini SJ, Rezaeisadrabadi M, et al. Antioxidant supplementations for prevention of atrial fibrillation after cardiac surgery: an updated comprehensive systematic review and meta‐analysis of 23 randomized controlled trials. Interact Cardiovasc Thorac Surg. 2014;18:646‐654. [DOI] [PubMed] [Google Scholar]
- 33. Dudley SC Jr, Hoch NE, McCann LA, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112:1266‐1273. [DOI] [PubMed] [Google Scholar]
- 34. Davis RC, Hobbs FD, Kenkre JE, et al. Prevalence of atrial fibrillation in the general population and in high‐risk groups: the ECHOES study. Europace. 2012;14:1553‐1559. [DOI] [PubMed] [Google Scholar]
- 35. Kazemian P, Oudit G, Jugdutt BI. Atrial fibrillation and heart failure in the elderly. Heart Fail Rev. 2012;17:597‐613. [DOI] [PubMed] [Google Scholar]
- 36. Hobbs FD, Fitzmaurice DA, Mant J, et al. A randomised controlled trial and cost‐effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess. 2005;9:iii‐iv, ix‐x, 1‐74. [DOI] [PubMed] [Google Scholar]
- 37. Aronsson M, Svennberg E, Rosenqvist M, et al. Cost‐effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Europace. 2015;17:1023‐1029. [DOI] [PubMed] [Google Scholar]
- 38. Levin LÅ, Husberg M, Sobocinski PD, et al. A cost‐effectiveness analysis of screening for silent atrial fibrillation after ischaemic stroke. Europace. 2015;17:207‐214. [DOI] [PubMed] [Google Scholar]
- 39. Wachter R, Gröschel K, Gelbrich G, et al. Holter‐electrocardiogram‐monitoring in patients with acute ischemic stroke (Find‐AF(RANDOMISED)): an open‐label randomized controlled trial. Lancet Neurol. 2017;16:282‐290. [DOI] [PubMed] [Google Scholar]
- 40. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478‐2486. [DOI] [PubMed] [Google Scholar]
- 41. Higgins P, MacFarlane PW, Dawson J, et al. Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial. Stroke. 2013;44:2525‐2531. [DOI] [PubMed] [Google Scholar]
- 42. Reiffel JA, Verma A, Kowey PR, et al. Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high‐risk population: the REVEAL AF study. JAMA Cardiol. 2017;2:1120‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. 1991;22:983‐988. [DOI] [PubMed] [Google Scholar]
- 44. Kwok CS, Loke YK, Hale R, et al. Atrial fibrillation and incidence of dementia: a systematic review and meta‐analysis. Neurology. 2011;76:914‐922. [DOI] [PubMed] [Google Scholar]
- 45. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace. 2006;8:651‐745. [DOI] [PubMed] [Google Scholar]
- 46. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach. Chest. 2010;137:263‐272. [DOI] [PubMed] [Google Scholar]
- 47. Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomized controlled trial. Lancet. 2007;370:493‐503. [DOI] [PubMed] [Google Scholar]
- 48. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093‐1100. [DOI] [PubMed] [Google Scholar]
- 49. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1‐e76. [DOI] [PubMed] [Google Scholar]
- 50. Bussey HI, Chiquette E, Bianco TM, et al. A statistical and clinical evaluation of fingerstick and routine laboratory prothrombin time measurements. Pharmacotherapy. 1997;17:861‐866. [PubMed] [Google Scholar]
- 51. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955‐962. [DOI] [PubMed] [Google Scholar]
- 52. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal ‐ full cohort analysis. N Engl J Med. 2017;377:431‐441. [DOI] [PubMed] [Google Scholar]
- 53. Coyle D, Coyle K, Cameron C, et al. Cost‐effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value Health. 2013;16:498‐506. [DOI] [PubMed] [Google Scholar]
- 54. Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long‐term anticoagulant therapy (RE‐LY) trial. Circulation. 2011;123:2363‐2372. [DOI] [PubMed] [Google Scholar]
- 55. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719‐2747. [DOI] [PubMed] [Google Scholar]
- 56. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981‐992. [DOI] [PubMed] [Google Scholar]
- 57. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360‐1420. [DOI] [PubMed] [Google Scholar]
- 58. Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomized non‐inferiority trial. Lancet. 2009;374:534‐542. [DOI] [PubMed] [Google Scholar]
- 59. Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988‐1998. [DOI] [PubMed] [Google Scholar]
- 60. Reddy VY, Doshi SK, Kar S, et al. 5‐Year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964‐2975. [DOI] [PubMed] [Google Scholar]
- 61. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long‐term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1‐12. [DOI] [PubMed] [Google Scholar]
- 62. Reddy VY, Möbius‐Winkler S, Miller MA, et al. Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA Plavix Feasibility Study with Watchman Left Atrial Appendage Closure Technology). J Am Coll Cardiol. 2013;61:2551‐2556. [DOI] [PubMed] [Google Scholar]
- 63. Huang H, Liu Y, Xu Y, et al. Percutaneous left atrial appendage closure with the LAmbre device for stroke prevention in atrial fibrillation: a prospective, Multicenter Clinical Study. JACC Cardiovasc Interv. 2017;10:2188‐2194. [DOI] [PubMed] [Google Scholar]
- 64. Cruz‐Gonzalez I, Fuertes‐Barahona M, Moreno‐Samos JC, et al. Left atrial appendage occlusion: the current device landscape and future perspectives. Interv Cardiol Clin. 2018;7:253‐265. [DOI] [PubMed] [Google Scholar]
- 65. Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834‐1840. [DOI] [PubMed] [Google Scholar]
- 66. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825‐1833. [DOI] [PubMed] [Google Scholar]
- 67. Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate‐control versus rhythm‐control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41:1690‐1696. [DOI] [PubMed] [Google Scholar]
- 68. Opolski G, Torbicki A, Kosior DA, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126:476‐486. [DOI] [PubMed] [Google Scholar]
- 69. Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667‐2677. [DOI] [PubMed] [Google Scholar]
- 70. Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation‐Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomized trial. Lancet. 2000;356:1789‐1794. [DOI] [PubMed] [Google Scholar]
- 71. Kiehl EL, Makki T, Kumar R, et al. Incidence and predictors of right ventricular pacing‐induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13:2272‐2278. [DOI] [PubMed] [Google Scholar]
- 72. Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781‐788. [DOI] [PubMed] [Google Scholar]
- 73. Akiyama T, Pawitan Y, Campbell WB, et al. Effects of advancing age on the efficacy and side effects of antiarrhythmic drugs in post‐myocardial infarction patients with ventricular arrhythmias. The CAST Investigators. J Am Geriatr Soc. 1992. Jul;40:666‐672. [DOI] [PubMed] [Google Scholar]
- 74. Le Heuzey JY, De Ferrari GM, Radzik D, et al. A short‐term, randomized, double‐blind, parallel‐group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 2010;21:597‐605. [DOI] [PubMed] [Google Scholar]
- 75. Kennedy R, Oral H. Catheter ablation of atrial fibrillation in the elderly: does the benefit outweigh the risk? Expert Rev Cardiovasc Ther. 2013;11:697‐704. [DOI] [PubMed] [Google Scholar]
- 76. Spragg DD, Dalal D, Cheema A, et al. Complications of catheter ablation for atrial fibrillation: incidence and predictors. J Cardiovasc Electrophysiol. 2008;19:627‐631. [DOI] [PubMed] [Google Scholar]
- 77. Kusumoto F, Prussak K, Wiesinger M, et al. Radiofrequency catheter ablation of atrial fibrillation in older patients: outcomes and complications. J Interv Card Electrophysiol. 2009;25:31‐35. [DOI] [PubMed] [Google Scholar]
- 78. Bunch TJ, Weiss JP, Crandall BG, et al. Long‐term clinical efficacy and risk of catheter ablation for atrial fibrillation in octogenarians. Pacing Clin Electrophysiol. 2010;33:146‐152. [DOI] [PubMed] [Google Scholar]
- 79. Santangeli P, Di Biase L, Al‐Ahmad A, et al. Ablation for atrial fibrillation: termination of atrial fibrillation is not the end point. Card Electrophysiol Clin. 2012;4:343‐352. [DOI] [PubMed] [Google Scholar]
- 80. Santangeli P, Di Biase L, Horton R, et al. Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications: evidence from a meta‐analysis. Circ Arrhythm Electrophysiol. 2012;5:302‐311. [DOI] [PubMed] [Google Scholar]
- 81. Santangeli P, Di Biase L, Mohanty P, et al. Catheter ablation of atrial fibrillation in octogenarians: safety and outcomes. J Cardiovasc Electrophysiol. 2012;23:687‐693. [DOI] [PubMed] [Google Scholar]
- 82. Nademanee K, Amnueypol M, Lee F, et al. Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm. 2015;12:44‐51. [DOI] [PubMed] [Google Scholar]
- 83. Bunch TJ, May HT, Bair TL, et al. The impact of age on 5‐year outcomes after atrial fibrillation catheter ablation. J Cardiovasc Electrophysiol. 2016;27:141‐146. [DOI] [PubMed] [Google Scholar]
- 84. Metzner I, Wissner E, Tilz RR, et al. Ablation of atrial fibrillation in patients ≥ 75 years: long‐term clinical outcome and safety. Europace. 2016;18:543‐549. [DOI] [PubMed] [Google Scholar]
- 85. Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug‐refractory atrial fibrillation: a prospective, multi‐centre, randomized, controlled study (Catheter Ablation for The Cure of Atrial Fibrillation Study). Eur Heart J. 2006;27:216‐221. [DOI] [PubMed] [Google Scholar]
- 86. Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010;303:333‐340. [DOI] [PubMed] [Google Scholar]
- 87. Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498‐2505. [DOI] [PubMed] [Google Scholar]
- 88. MacDonald MR, Connelly DT, Hawkins NM, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. 2011;97:740‐747. [DOI] [PubMed] [Google Scholar]
- 89. Leong‐Sit P, Zado E, Callans DJ, et al. Efficacy and risk of atrial fibrillation ablation before 45 years of age. Circ Arrhythm Electrophysiol. 2010;3:452‐457. [DOI] [PubMed] [Google Scholar]


