Abstract
Objective
Long‐term use of proton pump inhibitors (PPIs) has been associated with an increased risk of harm. There are few studies evaluating pharmacist‐led PPI deprescribing interventions within a long‐term care facility setting. The aim of this study was to describe the changes and influencing factors seen with a pharmacist‐led PPI deprescribing intervention in two Fraser Health Authority long‐term care facilities in British Columbia.
Methods
This 4‐month intervention involved lists of residents who had active PPI orders being handed out to physicians from two facilities. The pharmacist conducted weekly reviews of residents from Facility 1 and offered deprescribing recommendations. The number and methods of PPI deprescribing orders per facility were determined after the intervention.
Results
Out of 58 residents from the two facilities, 30 (62.5%) had a deprescribing order. Facility 1 had 83.3% (20/24) of residents with a PPI deprescribing order, in contrast to 41.7% (10/24) from Facility 2. Overall, 80.0% of residents had successfully completed PPI deprescribing orders by the end of the study period.
Conclusion
Clinical pharmacist intervention may increase the rate of initiation in PPI deprescribing orders within a long‐term care facility setting. Factors that influence success include intervention timing, active collaboration, having residents under direct care, and clear documentation of PPI indications.
Keywords: deprescribing, pharmacist, proton pump inhibitors
1. INTRODUCTION
Proton pump inhibitors (PPIs) are acid‐suppressant drugs that are widely prescribed for a number of gastrointestinal (GI) indications. In 2015, PPIs accounted for Can$253.3 million in public drug program spending in Canada, and were one of the top ten drug classes with the highest spending.1 Furthermore, pantoprazole was found to be the fifth most common drug prescribed in Canada, with more than 11 million prescriptions dispensed in 2012.2
With pantoprazole's high prevalence of use, there is a growing concern in the appropriateness and duration of its use. A Canadian prospective study found that 30.7% of patients were inappropriately prescribed with PPIs, in relation to the Quebec guidelines.3 In addition, a retrospective cross‐sectional study conducted in British Columbia found that the proportion of PPI orders without a documented common evidence‐based indication or broad evidence‐based indication were 43.7% and 16.2%, respectively.4 While PPIs are generally well‐tolerated, the long‐term safety profiles of PPIs are controversial, as they are often associated with an increased risk of Clostridium difficile infections, bone fractures, interstitial nephritis, and hypomagnesemia.5 Inappropriate PPI use can also contribute to polypharmacy, prescribing cascades, adverse events, and hospitalizations.6
Deprescribing is a complex process of tapering or stopping medications as a means of managing polypharmacy. A recent evidence‐based practice guideline recommends deprescribing PPIs in adults with resolved heartburn or gastroesophageal reflux disease after a minimum of 4 weeks’ treatment.6 Various methods of deprescribing PPIs can be utilized, such as complete discontinuation, reducing dose, changing to on‐demand (as needed) use, or switching to an H2 receptor antagonist.6 Yet, research is lacking in recommending optimal PPI tapering regimens and studying deprescribing effects in the elderly population.6
In order to minimize the risk of adverse events related to long‐term PPI use and reduce excess health care costs, interventions aimed at deprescribing PPIs have been trialed. Previous studies that involved pharmacists have demonstrated decreased pill burden, reduced annualized PPI cost, and increased documentation of PPI indications.7, 8, 9, 10, 11 Yet, fewer data exist for the factors that influence the success of a pharmacist‐led deprescribing intervention within long‐term care facilities in Canada. This retrospective observational study aims to contribute to existing knowledge by describing the changes of a simple intervention led by a clinical pharmacist in deprescribing PPIs for long‐term care facility residents. It also explores the possible factors that contribute to the rate of success in the initiation and completion of PPI deprescribing orders.
2. METHODS
This study was conducted from June 6 to November 12, 2018 in two Fraser Health Authority (FHA) long‐term care facilities affiliated in British Columbia. The study has been approved and granted an exemption from the Fraser Health Research Ethics Board as it qualifies as a quality‐improvement and evaluation study.
2.1. Intervention
The 4‐month intervention involved four strategies: (1) generation of drug use evaluation reports in Months 1 and 3; (2) in‐person discussions with physicians on‐site; (3) faxing physicians that were mostly off‐site; and (4) following up with prescribers and a second pharmacist.
A clinical pharmacist requested a drug use evaluation report of residents who had active orders of any dose of PPI to be generated from Meditech, an FHA electronic health records system. As pantoprazole and esomeprazole are the only two PPIs available on the FHA formulary, residents on the list were seen to be taking one of the two. We included all residents in two long‐term care facilities who were presently on a PPI at the time of the first report generated.
In the first and second months, copies of the list were handed out to a total of seven physicians in both Facilities 1 and 2, with residents under their care highlighted. Information on the lists included the names of residents, the facility they were in, and the type of PPI and dosing regimen they were on. The pharmacist then discussed strategies of PPI deprescribing with each physician in person, which included abrupt discontinuation with monitoring, tapering the dose, switching to as‐needed ranitidine, or switching to as‐scheduled ranitidine.
In the third month, the pharmacist took several verbal orders for any PPI changes and provided updated copies of the list to physicians, highlighting patients that were yet to be reviewed. Three physicians who had five or fewer residents under their care and were mostly off‐site had the updated lists faxed to them as a reminder. Since the pharmacist that led the intervention was mostly responsible for Facility 1, a reminder was provided to the other clinical pharmacist responsible for residents at Facility 2 to hold a discussion with the medical director there. In the fourth month, the first pharmacist followed up with the three faxed physicians, as well as the second pharmacist from the other facility.
2.2. Data collection
The investigators created a data extraction form that consisted of patient demographic information, details of PPI regimen, documented indications for PPI use, and changes observed after intervention. From October to November 2018, one reviewer extracted relevant data from electronic health records on Meditech. If the required information could not be found through online records, physical charts on the wards were consulted. Two quality‐assurance checks were also conducted: (1) A second investigator repeated the data collection process for the first five patients that were reviewed, before comparing it to the information extracted by the first reviewer; and (2) once data were extracted for all residents by the first reviewer, the second reviewer collected data for ten patients at random, excluding the first five that were reviewed previously. Any discrepancies were resolved by consensus between the two reviewers, or mediated by a third reviewer.
2.3. Outcomes
The primary outcome was the proportion of residents who had a PPI deprescribing order after the intervention. Secondary outcomes included the rate of successful deprescribing by the end of the study period, reasons for deprescribing failures, and deprescribing methods that were utilized most often or had the highest success rate.
2.4. Data analysis
Descriptive statistics were used to summarize and report the data collected in terms of measures of frequency, proportion, and variance.
3. RESULTS
Baseline demographic characteristics are presented in Table 1. A total of 58 residents were eligible for inclusion in the study period: 29 (50%) from Facility 1 and 29 (50%) from Facility 2. The mean age (± SD) of all residents was 80 ± 12.1 years. A higher proportion of residents were female (n = 44, 75.9%) and aged between 90 and 99 years old (n = 29, 50.0%). The top two documented PPI indications included gastroesophageal reflux disease (n = 6, 10.3%) and GI bleeding (n = 6, 10.3%). However, the latter might have been a remote history for some residents. There were likely indications for PPI use in 21 residents (36.2%); however, they were not clearly documented in their charts and only inferred from supporting chart notes. There were neither documented nor likely PPI indications found for 14 residents (24.1%). With regards to dosing, most residents were receiving pantoprazole 40 mg once daily or esomeprazole 40 mg once daily.
Table 1.
Baseline characteristics of included patients in residential care facilities (n = 58)
| Demographics and baseline parameters | No. of residents (%) |
|---|---|
| Sex | |
| Female | 44 (75.9) |
| Male | 14 (24.1) |
| Age (years) | |
| 40‐49 | 1 (1.7) |
| 50‐59 | 2 (3.4) |
| 60‐69 | 4 (6.9) |
| 70‐79 | 6 (10.3) |
| 80‐89 | 16 (27.6) |
| 90‐99 | 29 (50.0) |
| PPI indication | |
| GI bleed | 6 (10.3) |
| Gastroesophageal reflux disease | 6 (10.3) |
| Dysphagia/nausea/emesis | 5 (8.6) |
| GI condition (e.g, diverticulitis, ulcerations) | 4 (6.9) |
| Stomach protectant for concomitant drug use (e.g, NSAIDs, DOACs) | 2 (3.4) |
| Likely indication | 21 (36.2) |
| Unknown | 14 (24.1) |
| PPI use (total daily dose)* | |
| Pantoprazole | |
| 20 mg once daily | 7 (12.1) |
| 20 mg twice daily | 1 (1.7) |
| 40 mg once daily | 25 (43.1) |
| 40 mg twice daily | 6 (10.3) |
| 40 mg as needed | 1 (1.7) |
| Esomeprazole | |
| 20 mg once daily | 2 (3.4) |
| 20 mg twice daily | 1 (1.7) |
| 40 mg once daily | 15 (25.9) |
*Correction added on 16 May 2019, after first online publication: The subheading has been changed from 'totally daily base' to 'total daily dose'.
Abbreviations: DOACs, direct oral anticoagulants; GI, gastrointestinal; PPI, proton pump inhibitor.
Table 2.
Outcome 4 months after intervention based on each deprescribing method (n = 30)
| Outcome | Number of residents (%) | |||
|---|---|---|---|---|
| Taper dose before stopping (n = 19) | Decrease dose only (n = 4) | Hard stop (n = 5) | Switch to ranitidine (n = 2) | |
| Completed deprescribing successfully | 14 (73.7) | 4 (100.0) | 4 (80.0) | 2 (100.0) |
| Restarted on PPI | 1 (5.3) | 0 | 0 | 0 |
| Patient moved after intervention | 1 (5.3) | 0 | 0 | 0 |
| Patient died after intervention | 3 (15.8) | 0 | 1 (20.0) | 0 |
Abbreviations: PPI, proton pump inhibitor.
In addition, we examined other medications associated with PPI use, such as NSAIDs, anticoagulants, and antiplatelets. Eight residents were taking one associated medication concurrently with their PPI, while seven residents were taking more than one associated medication. Interestingly, nine of these 15 residents had a likely PPI indication that was not clearly documented. Of those taking one associated medication, acetylsalicylic acid was the most common (n = 4), followed by ibuprofen (n = 2), warfarin (n = 1) and diclofenac (n = 1).
Figure 1 depicts the overall process of the intervention, grouping residents according to facility. There were two physicians mainly in charge of the residents from each facility. The first physician was responsible for 20 residents (34.4%) in Facility 1, while the second physician was responsible for 21 residents (36.2%) in Facility 2. The remaining five physicians had eight or fewer residents under their care. Five residents from each facility moved or died after the report was generated, but before deprescribing could be initiated for them; thus, they were excluded from any subsequent data analysis.
Figure 1.
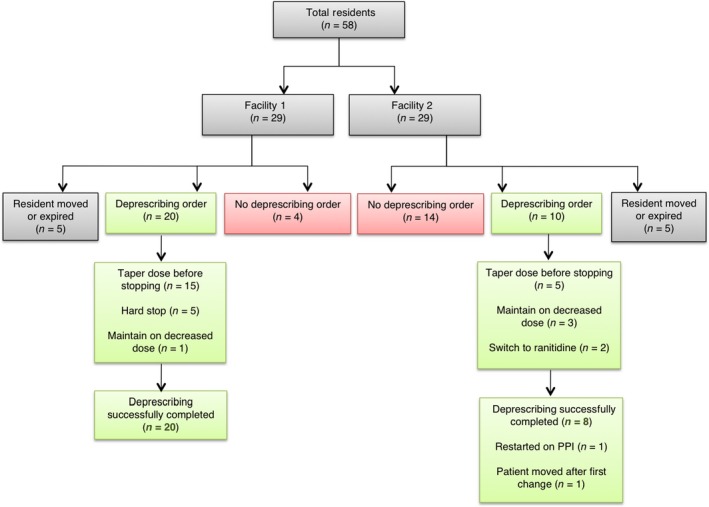
Flowchart of study process and main outcomes. PPI, proton pump inhibitor
3.1. Primary outcome
A total of 62.5% (30/48) of residents from both facilities had a deprescribing order. In Facility 1, 83.3% (20/24) of residents had a PPI deprescribing order, in contrast to 41.7% (10/24) of residents from Facility 2.
3.2. Secondary outcomes
Four months post‐intervention, 100.0% (20/20) of residents from Facility 1 underwent successful PPI deprescribing, whereas Facility 2 saw a lower proportion of 80.0% (8/10). Of the two that were not considered successful in deprescribing, one resident was restarted on a PPI due to a recurrence of heartburn symptoms 89 days after PPI discontinuation, while another resident moved after the first PPI taper; thus, it was not clear if deprescribing was completed.
We then analyzed the type of deprescribing method first initiated in residents from both Facilities 1 and 2 combined (Figure 2). The most common deprescribing method was tapering PPI dose before discontinuation, seen in 63.3% of residents (23/30). Other methods seen included discontinuing PPI without tapering (5/30, 16.7%), maintaining a decreased PPI dose (4/30, 13.3%), and switching to ranitidine (2/30, 6.7%).
Figure 2.
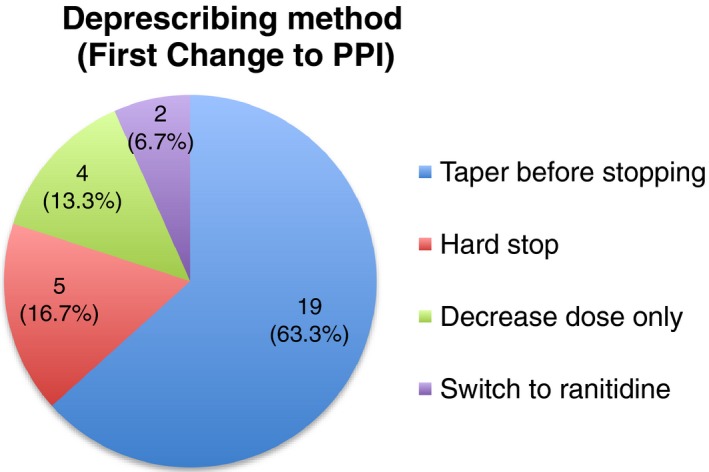
Type of deprescribing method observed in residents (n = 30)
The outcomes 4 months after the start of the intervention are presented in Table 2, grouped according to each deprescribing method. We defined a successful completion of deprescribing as residents who remained off of their PPIs after discontinuation or switching to ranitidine, and residents who continued to be maintained on a decreased PPI dose by the end of the study period. There was a 100.0% success rate seen in the two groups: maintenance on decreased PPI dose (n = 4) and switching to ranitidine (n = 2); and 73.7% (n = 14) of residents were successfully tapered off of their PPIs. Within this group, one resident moved and three died after the intervention. Notably, one resident had to be restarted on their PPI after the initial taper. As for residents who had their PPI completely discontinued as a first change, four out of five residents continued to stay off of their PPIs, as one resident died after the intervention. Overall, 80.0% of 30 residents who were initiated PPI deprescribing orders had completed them successfully by the end of the study period.
4. DISCUSSION
4.1. Main findings
In our study, the simple intervention led by the clinical pharmacist resulted in a high rate of PPI deprescribing (62.5%). Tapering the PPI was the most common method of deprescribing. Four months after the intervention, 24 (80.0%) of these residents did not require reinitiation or an increased dose of their PPIs. Of the remaining six residents, four died after the intervention, one resident was restarted on a PPI, and one resident moved to another facility in the midst of tapering. All four residents who died were actually tapered off of their PPIs successfully; however, we considered them as part of incomplete deprescribing based on our definition of success.
This 80% success rate in our study is similar to a project conducted by Lee et al., with a PPI discontinuation success rate of 70%.7 Both studies assessed the elderly population in a residential care site situated in British Columbia and had a single pharmacist leading the intervention. The slightly higher success rate in our study could be attributed to the greater number of deprescribing strategies we included, in contrast to Lee et al., who only had PPI discontinuation without tapering as their sole recommendation.7 Lee et al. also saw three residents restarted on PPIs and one resident initiated on an H2 receptor antagonist, with a mean time of 23.5 days before reinitiation.7 Reasons included reflux, heartburn, and coffee ground emesis.7 The higher incidence of symptom recurrence over a shorter mean time to PPI reinitiation compared to our study might be due to the close monitoring of residents with weekly follow‐ups by three pharmacists as the primary objective of their study was to determine the rate of GI symptom recurrence.7
Two other studies with closer success rates of 81%‐83% have also been observed; however, there were notable differences in study design and setting compared to our study.11, 12 The study by Ramser et al. took place in an internal medicine clinic that included patient telephone interviews to ascertain PPI indications that could not be determined through chart reviews.11 In addition, Murie et al. utilized an intervention led by a specialist nurse not limited to the elderly population, involving verbal and written educational information to patients regarding their condition and alternatives to PPI use.12 With a high prevalence of dementia seen in the residents in our study, this poses a barrier to determine PPI indications through direct questioning and educational interventions.
In addition, 15 (25.9%) residents were observed to be taking at least one medication associated with PPI use. The only drug‐related indication for long‐term PPI use includes chronic NSAID use in patients with bleeding risk. While 14 of these residents were taking at least one NSAID, nine of them did not have clear documentation of the reason for their PPI use. This led to our assumption that these residents had likely PPI indications due to the concomitant use of NSAIDs seen in their medical records. As recommended in other studies, including indications in the directions for use can be a possible solution to minimize assumptions about indications and improve the quality of patient care.4, 13
Our study also suggests four main factors that influence the rate of successful initiation of PPI deprescribing. First, the timing of the intervention might play a role in facilitating communication between clinicians. Our study occurred over the summer months, with many physicians away on vacation during the 4‐month intervention. Thus, this might have contributed to a delay in the review of residents for appropriate PPI deprescribing and hinder ongoing, efficient communication between the pharmacist and physicians for some of the residents.
Second, active collaboration between pharmacists and physicians increases the chances of deprescribing success. In our study, the clinical pharmacist was involved in weekly in‐person care conference meetings and had stronger ties with the physicians working in Facility 1, compared to Facility 2. A systematic review on deprescribing medications for chronic diseases in primary care settings supports this as it found that out of five outpatient drug‐specific studies, two showed a statistically significant reduction in medication burden, which required intense pharmacist‐physician collaboration.14 The review also revealed that interventions with intense pharmacist involvement in clinician education and patient‐specific drug recommendations led to the greatest success in reducing polypharmacy.14
Third, residents directly under the care of the pharmacist who led the intervention had a higher chance of success in the initiation of PPI deprescribing. The primary pharmacist regularly followed up with the residents mainly in Facility 1 and might have been more familiar with the residents’ GI conditions and symptoms. This could have contributed to a greater confidence in recommending PPI deprescriptions to physicians.
Lastly, a lack of documented PPI indications could lead to a sense of uneasiness with deprescribing PPIs. This study showed that 36.2% of residents had a likely PPI indication that was not clearly documented, and 24.1% had no PPI documentation at all. Interestingly, a systematic review conducted by Dills et al. found that PPIs were one of the four drug classes that were resistant to deprescription despite intense intervention.14 With insufficient documentation on PPI indications, weighing the benefits and risks of keeping a patient on PPIs becomes challenging as PPIs are generally harmless with few acute adverse events, although increasing evidence shows harms associated with long‐term use.5
4.2. Limitations
As a small‐scale quality‐improvement project, our study had a smaller sample size compared to other interventional studies, thus limiting its generalizability. Participants were included in the study at the time when the list of residents was first generated; thus, we might have missed newly admitted residents with active orders for PPIs after that point in time. Most preadmission and outpatient data were not available, which made it difficult to ascertain documented PPI indications. Deprescribing orders also occurred throughout the 4 months of intervention and we did not have a standardized follow‐up period to monitor the recurrence of GI symptoms in all residents with initiated changes. Symptoms were only closely examined from medical records after residents were seen to have their PPI therapy reinitiated.
5. CONCLUSION
A simple, pharmacist‐led intervention saw an initiation of PPI deprescribing in 62.5% of residents in a long‐term care facility setting; 80.0% of these residents completed deprescribing successfully. Factors that influence success include the timing of the intervention, active collaboration between pharmacists and physicians, having residents under the direct care of the pharmacist, and clear documentation of PPI indications.
CONFLICT OF INTEREST
There are no conflicts of interest to be reported by the authors of this study.
ACKNOWLEDGMENTS
This study received no specific funding. A.M. is funded by the Lower Mainland Pharmacy Services and Therapeutic Initiative (University of British Columbia [UBC]), in addition to receiving honoraria from other lawyers for advice on drug evidence, Divisions of Family Practice, Ministry of Health BC, MEDS Conference Winnipeg, BSMC Conference Vancouver, PEIP Conference Edmonton, and UBC. C.B. is funded by the Fraser Health Authority and White Rock South Surrey Division of Family Practice.
Tandun R, Bubbar C, Tejani AM. Who has the guts to deprescribe proton pump inhibitors? A pharmacist‐led intervention in a long‐term care facility setting. Aging Med. 2019;2:112–117. 10.1002/agm2.12063
[Correction added on 16 May 2019, after first online publication: The corresponding author’s email address has been corrected.]
REFERENCES
- 1. Canadian Institute for Health Information . Prescribed drugs spending in Canada, 2016. Canadian Institute for Health Information website. https://secure.cihi.ca/free_products/Prescribed%20Drug%20Spending%20in%20Canada_2016_EN_web.pdf. Published 2016. Accessed 2018.
- 2. Canadian Healthcare Network: Pharmacy Practice. Top 100 drugs. Canadian Healthcare Network website. http://www.canadianhealthcarenetwork.ca/pharmacists/magazines/pharmacypractice/February-2013. Published March 4, 2013. Accessed 2018.
- 3. Nguyen PV, Tamaz R. Inappropriate prescription of proton pump inhibitors in a community setting. Can J Hosp Pharm. 2018;71(4):267‐271. [PMC free article] [PubMed] [Google Scholar]
- 4. Chan A, Liang L, Tung ACH, Kinkade A, Tejani AM. Is there a reason for the proton pump inhibitor? An assessment of prescribing for residential care patients in British Columbia. Can J Hosp Pharm. 2018;71(5):295‐301. [PMC free article] [PubMed] [Google Scholar]
- 5. Schoenfeld A, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med. 2016;176(2):172‐174. [DOI] [PubMed] [Google Scholar]
- 6. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors. Evidence‐based clinical practice guideline. Can Fam Physician. 2017;63(5):354‐364. [PMC free article] [PubMed] [Google Scholar]
- 7. Lee C, Lo A, Ubhi K, Milewski M. Outcome after discontinuation of proton pump inhibitors at a residential care site: quality improvement project. Can J Hosp Pharm. 2017;70(3):215‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bundeff AW, Zaiken K. Impact of clinical pharmacists’ recommendations on a proton pump inhibitor taper protocol in ambulatory care practice. J Manag Care Pharm. 2013;19(4):325‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clyne B, Smith SM, Hughes CM. Effectiveness of a multifaceted intervention for potentially inappropriate prescribing in older patients in primary care: a cluster‐randomized controlled trial (OPTI‐SCRIPT Study). Ann Fam Med. 2015;13(6):545‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lucas LM, Gerrity MS, Anderson T. A practice‐based approach for converting from proton pump inhibitors to less costly therapy. Eff Clin Pract. 2001;4(6):263‐270. [PubMed] [Google Scholar]
- 11. Ramser KL, Sprabery LR, Hamann GL, George CM, Will A. Results of an intervention in an academic internal medicine clinic to continue, step down, or discontinue proton pump inhibitor therapy related to a Tennessee Medicaid formulary change. J Manag Care Pharm. 2009;15(4):344‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murie J, Allen J, Simmonds R, de Wet C. Glad you brought it up: a patient‐centred programme to reduce proton‐pump inhibitor prescribing in general practice. Qual Prim Care. 2012;20(2):141‐148. [PubMed] [Google Scholar]
- 13. Schiff GD, Seoane‐Vazquez E, Wright A. Incorporating indications into medication ordering‐time to enter the age of reason. N Engl J Med. 2016;375(4):306‐309. [DOI] [PubMed] [Google Scholar]
- 14. Dills H, Shah K, Messinger‐Rapport B, Bradford K, Syed Q. Deprescribing medications for chronic diseases management in primary care settings: a systematic review of randomized controlled trials. J Post Acute Long Term Care Med. 2018;19(11):923‐935. [DOI] [PubMed] [Google Scholar]


