Abstract
The mouse has been used in many medical fields as a powerful model to reveal the genetic basis of human physiology and disease. The past two decades have witnessed an enormous wealth of genetic and informatic resources dedicated to this humble organism. With the ongoing revolution in mapping neural circuitry governing behavior, the mouse is an ideal model organism poised to unravel the mysteries of general anesthetic action. This chapter will describe and provide guidelines for anesthetic phenotyping in the mouse including both motor-dependent and motor-independent assessments.
1. INTRODUCTION
The mouse has served as a valuable organism yielding insights into human health and disease. It remains the premier model for the genetic basis of physiology and pathophysiology because it is small, relatively inexpensive to maintain, and has a short gestation period of just under 3 weeks (Peters et al., 2007). Mice become sexually mature by the age of 6–8 weeks making the generation time roughly 3 months. It is commonly felt that the biochemical, cellular, developmental, and neuronal pathways in mice are highly conserved between mice and humans, further emphasizing the use of the murine model toward translational and reverse translational studies (Baker, 2011; Deussing, 2013). Within the field of anesthesiology, studies in genetically engineered mice have helped to validate the importance of putative molecular targets of anesthetic action and to offer critical glimpses into the translational promiscuity of general anesthetic drugs from the in vitro to the in vivo setting (Behlke et al., 2016; Chen, Shu, & Bayliss, 2009; Heurteaux et al., 2004; Jurd et al., 2003; Rudolph & Antkowiak, 2004). In this chapter, we will focus on the methods used to assess sedation and hypnosis in mice using both customary motor-dependent and motor-independent measures.
2. ANESTHETIC DOSING IN MICE
2.1. Volatile Anesthetics
Delivery of an anesthetic agent to mice merits consideration as every drug must ultimately reach its effect site to elicit its biological actions. Since it is uncommon to intubate or perform a tracheostomy on a mouse, studies using inhaled anesthetics in mice typically occur with the animal placed in a gastight chamber. Inhaled anesthetics are delivered using a calibrated vaporizer in an enriched stream of oxygen. In addition to the customary factors (Eger & Saidman, 2005), the kinetics of inhaled anesthetic uptake will also be dependent upon the fresh gas flow rate and the volume of the anesthetizing chamber. Relative to lower flow rates, increasing the fresh gas flow rate to the anesthetized chamber to achieve one volume turnover per minute will decrease the time required for the mouse to equilibrate with a stepwise increasing or decreasing series of inhaled anesthetic doses, but may also induce relative hypothermia in mice that can confound the interpretation of behavioral responses. Without an active warming, mice breathing inhaled anesthetic gases in fresh gas flow rates as little as 100 mL/min can approach room temperature in less than an hour (Sun et al., 2006). Anesthetic-induced hypothermia is often accompanied by other detrimental physiologic effects. Therefore, it is critical to measure and maintain normothermia in mice. Strategies to avoid hypothermia in mice include partially submerging the anesthetizing chamber in a heated water bath or using a servocontrolled radiant heat source such as a lamp or warmed floor that shuts off when continuous measures of core body temperature reach a normothermic range (Caro, Hankenson, & Marx, 2013).
2.2. Injectable Anesthetics
Due to the ease of administration, the most common route to deliver anesthetic drugs in mice is intraperitoneal (IP). Route of anesthetic delivery can dramatically affect experimental results. It is well known that lesions that impair cardiac output will pharmacokinetically slow anesthetic induction of intravenous agents while speeding induction with inhaled anesthetics (Eger & Saidman, 2005; Upton, Ludbrook, Grant, & Martinez, 1999). Similarly, pharmacokinetic factors can affect the apparent anesthetic phenotype of mice dosed with the same anesthetic drug if given by distinct routes. As shown in Fig. 1, intraperitoneal dosing of 400 μg/kg of dexmedetomidine was given to age-matched 4-to 6-month-old mice genetically engineered with a homozygous deletion of the dopamine β hydroxylase gene (Dβh−/−) and to their heterozygous siblings that inherit one wild-type copy of the dbh gene and consequently retain normal levels of catecholamines (Thomas, Marck, Palmiter, & Matsumoto, 1998). Using the loss of righting reflex as a behavioral measure of hypnosis (described in Section 3.1), neither the latency to loss of righting nor the duration of loss of righting differed between Dβh−/− and Dβh+/− with IP dexmedetomidine. This is in sharp contrast with the known hypersensitivity of Dβh−/− to a variety of anesthetic drugs, including dexmedetomidine when administered intravenously (Hu et al., 2012). Based upon the cardiac compensations in the Dβh−/− knockouts (Liles et al., 2007; Swoap, Weinshenker, Palmiter, & Garber, 2004), it is believed that the slower uptake of IP dexmedetomidine from the peritoneum masks the hypersensitivity phenotype of reduced latency to loss of righting and prolonged duration of loss of righting (Fig. 1) that is apparent with intravenous dosing. More recent studies of propofol given to wild-type C57BL/6J mice further highlight that even among intravenously dosed anesthetics, delivery via an indwelling tail vein IV catheter does not produce identical results to the same dose of propofol given intravenously through a central line (Alex Proekt, University of Pennsylvania, unpublished observations). Hence, when designing and reporting results in mice careful thought must be given to the route of anesthetic drug delivery.
Fig. 1.
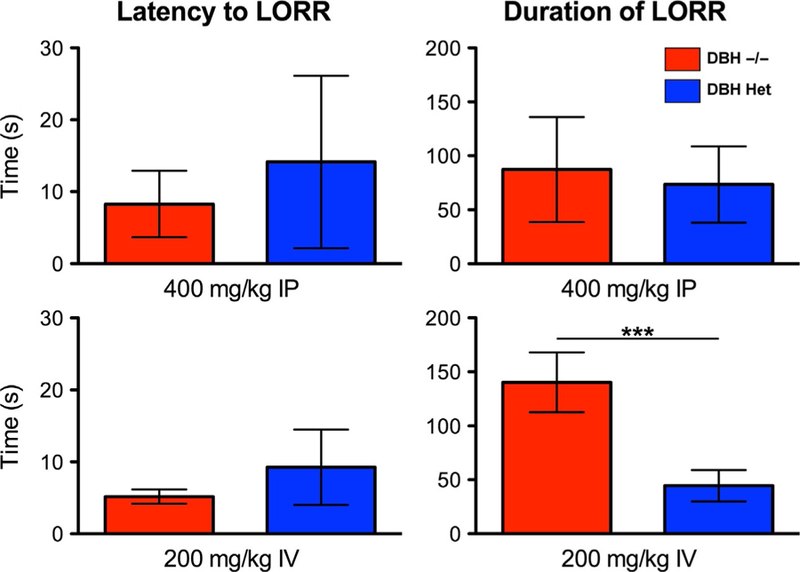
Route of drug administration plays an integral role in the apparent anesthetic efficacy. When delivered intraperitoneally (IP), dexmedetomidine produces a statistically indistinguishable (mean ± SD) latency to loss of righting (top left) and duration of loss of righting (top right) in dopamine β hydroxylase (DβH) knockout mice that are devoid of both epinephrine and norepinephrine (n=10) and DβH heterozygous control mice that retain a single wild-type allele and consequently have normal levels of adrenergic ligands (n=11). However, when dexmedetomidine is given intravenously to DβH knockout mice and heterozygous control mice, knockouts show a significant dose-dependent increase both the latency and duration of loss of righting. Shown in the lower panel are data from an equipotent intravenous dose of 200mcg/kg (n=5–6/group) in which knockouts show a significantly longer duration of loss of righting. ***P < 0.001. From Hu, F. Y., Hanna, G. M., Han, W., Mardini, F., Thomas, S. A., Wyner, A. J., et al. (2012). Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine beta-hydroxylase knockout mice. Anesthesiology, 117(5), 1006–1017.
3. DETERMINATION OF HYPNOTIC SENSITIVITY IN MICE
The term anesthesia was first coined by Oliver Wendell Holmes in the 1846 to describe the state that we recognize today as encompassing amnesia, analgesia, motor atonia, and most critically, unconsciousness, which is typically referred to as hypnosis in animals. For a more comprehensive overview of these endpoints, we refer the interested reader to several excellent reviews (Campagna, Miller, & Forman, 2003; Franks, 2008; Hemmings et al., 2005; Mashour, Orser, & Avidan, 2011; Sonner et al., 2003). Each of the anesthetic endpoints, as well as the undesirable autonomic side effects that accompany the anesthetic state, is experimentally approachable in the laboratory mouse.
3.1. Behavioral Testing
The loss and subsequent return of the righting reflex is an established behavioral means to assess behavioral responsiveness in rodents (Franks, 2006). With exposure to hypnotic doses of anesthetic drugs, mice that have been tipped onto their backs in a position of vulnerable dorsal recumbency, lose the protective response to flip back onto all four paws. The loss of the righting reflex is convenient and reliable means to indicate the accompanying loss of consciousness. As rolling a mouse onto its back is sufficient to awaken a sleeping animal but the constant vestibular bombarding inputs of being upside down are not sufficient to antagonize an anesthetic state, investigators regularly use this nonnoxious behavioral paradigm to assess whether an individual animal retains its perceptive awareness of its surroundings or whether the drug delivery has produced a state of hypnosis. By determining the fraction of mice that lose (regain) their righting reflex as a function of increasing (decreasing) anesthetic concentrations, population response curves are easily generated by plotting the fraction of the population that remains responsive vs the log of the anesthetic concentration. A sigmoidal fit yields the population’s ED50 concentrations for hypnosis as well as its hill slope. The Hill slope term describes the uniformity of the population’s state transition from awake to anesthetized. Shallower Hill slopes dictate increased variability, which could be due either to environmental or genetic factors (Sonner, 2002). In the extreme case of an infinite Hill slope, variability would be zero and each member would undergo the state transition at an identical anesthetic dose.
A second means of determining when a mouse has entered a behavioral state of hypnosis can be determined with the tape test. This test has been applied to mice as a sensitive measure of sensorimotor deficits in mice (Bouet et al., 2009). However, as shown in Fig. 2, the test can also be utilized as a sensitive measure of anesthetic hypnosis in mice. Before anesthetic challenge, mice are trained on the tape test by transiently restraining each animal and placing a round, 0.5-cm diameter sticker on its nose. Each mouse is then transferred into an empty cage. The time to contact and to remove the tape is recorded. These corresponding latencies are measures of intact sensation and motor coordination (Bouet et al., 2009). Mice are considered trained when they remove the tape in fewer than 10s. Typically, training takes less than four trials. Following a 2-h exposure to 2.3% sevoflurane, 1.2% isoflurane, or 1.0% halothane as previously described (Sun et al., 2006), and after regaining their righting reflexes, mice, respectively, took an additional 25.7±5.1, 30.9±13.1, and 128±54.5 s to first contact the tape and 74.4±21.6, 57.7±25.1, and 286.4±130.6 s to successfully remove the tape. The tape test can be a useful adjunct to testing the righting reflex or a behavioral alternative for assessing the loss of perceptive awareness that defines the hypnotic state in electroencephalography (EEG)-tethered mice in whom testing the righting reflex is not possible.
Fig. 2.
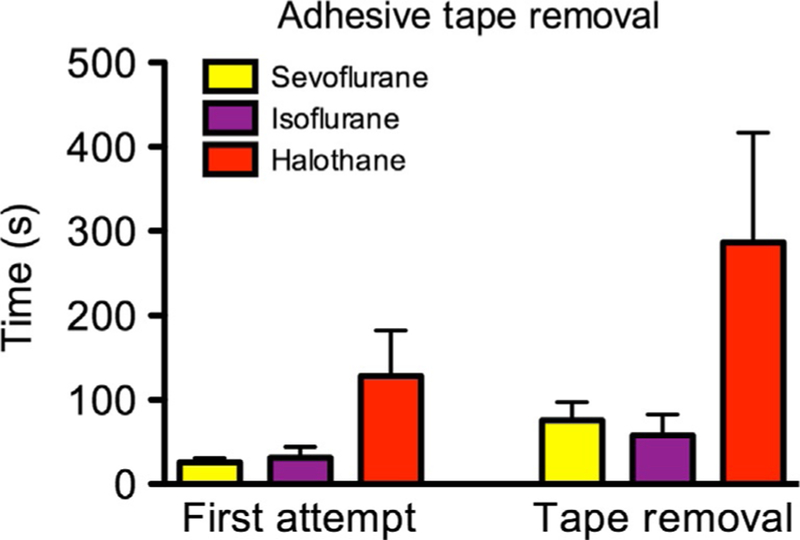
Adhesive tape removal test. The adhesive tape removal test can be used as a complementary marker for the hypnotic component of anesthesia. When fully trained mice will remove a tape sticker from their nose in under 10s but show a significant anesthetic-induced impairment in time (mean±SEM) to their first attempt to removing the tape and in the time to remove the tape following exposure to hypnotic doses of sevoflurane, isoflurane, and halothane (n=9/group).
A third behavioral marker for determining anesthetic-induced loss of consciousness is the forced walking test (Hwang, Kim, Shin, & Choi, 2010). It has the chief advantage of not having to handle the mouse during testing. In this paradigm, mice are placed on a treadmill that moves at 5cm/s to mimic a spontaneous, comfortable walking pace. Prior to testing, mice are habituated by being suspended in a nonweight bearing position above the treadmill for 15min and then allowed to walk for another 5min at 5cm/s. Through indwelling lines, anesthetic drugs can be remotely infused to induce general anesthesia. Another major advantage of this test is that the precise instant in which gait fails can be continuously measured and determined as the loss of movement. This test consequently yields significant temporal advantages over intermittent assessment of the righting reflex which is impractical to simultaneously assess more frequently than every 30s in large cohorts of mice. Another advantage of the forced walking test is that it can be simultaneously acquired with continuous recording of EEG in mice (Hwang et al., 2010).
3.2. Motor-Independent Measures
One theoretical problem with assessing hypnotic sensitivity of anesthetics with all behavioral criteria is that these depend upon a motor output. Therefore, a drug that intrinsically affects muscle tone as anesthetics do, could in principle elicit hypnosis, impair movement, or both. For this reason, acquisition of a motor-independent measure of hypnosis is desirable. Experiments in mice typically turn to EEG, electrocorticography, or local field potentials acquired from chronically implanted depth electrodes.
As is true for humans, processed EEG measures can be useful for phenotyping the state of anesthetic hypnosis. Issues in EEG signal acquisition and processing that are true in humans (Proekt, 2018) also apply to murine EEG. One common manipulation often applied to the EEG is a Fourier transformation (Hjorth, 1991). This converts the EEG from a voltage as a function of time into the sum of a series of sine waves of varying amplitudes and frequencies. Such spectral decomposition of the EEG with the Fourier technique has been applied both in humans (Bruhn et al., 2003) and mice (Hwang et al., 2010) to characterize anesthetic-induced waveform changes that reflect varying depth of hypnosis. However, a major advantage of conducting studies of anesthetic hypnosis in mice is the ability to test hypotheses about the contribution of putative molecular targets and neuronal of anesthetic action. For example, positive allosteric modulation of GABAA receptor signaling by the general anesthetic, etomidate, occurs preferentially at β2-and β3-containing GABAA receptors and is entirely dependent on the presence of an asparagine at the 265th residue. Reynolds and colleagues used knock-in mice with an N265S point mutation that renders β2 subunits insensitive to etomidate and then dosed β2 N265S knock-in or control animals with intravenous etomidate and assessed both behavioral and EEG measures of sensitivity (Reynolds et al., 2003). They demonstrated that hypnotic actions of etomidate as measured by righting reflex and EEG indices of deep anesthesia, characterized by the appearance of burst suppression, did not differ as a function of genotype. However, etomidate-induced sedation (discussed in Section 4) does indeed depend upon β2-containing GABAA receptors. In an elegant and complementary study, Jurd and colleagues used β3 N265M GABAA receptor knock-in mice to demonstrate that these latter GABAA receptors that are also insensitive to etomidate, do indeed serve as a molecular target that contributes to etomidate-induced hypnosis and immobility (Jurd et al., 2003).
Additional insights into the hypnotic actions of anesthetics have also arisen from studying mice. While it has been commonly assumed that entry into and exit from states of general anesthesia are mirror opposite processes, results obtained from mice with a genetic lesion that ablates all orexin/ hypocretin neurons and causes narcolepsy have demonstrated that afflicted animals have a specific deficit exiting states of isoflurane and sevoflurane anesthesia that is not explained by pharmacokinetic factors (Kelz et al., 2008). In behavioral tests of righting, mice that inherited the gene causing narcolepsy exhibited significantly slower return of righting. As shown in Fig. 3, spectral analysis of EEG recorded before, during, and after isoflurane exposure from narcoleptic mice and their wild-type sibling controls reveal similar loss of EEG power with induction of isoflurane, but consistent with delayed return of righting, narcoleptic orexin–ataxin-3 mice exhibit a slower return of EEG power, confirming a motor-independent delayed emergence.
Fig. 3.
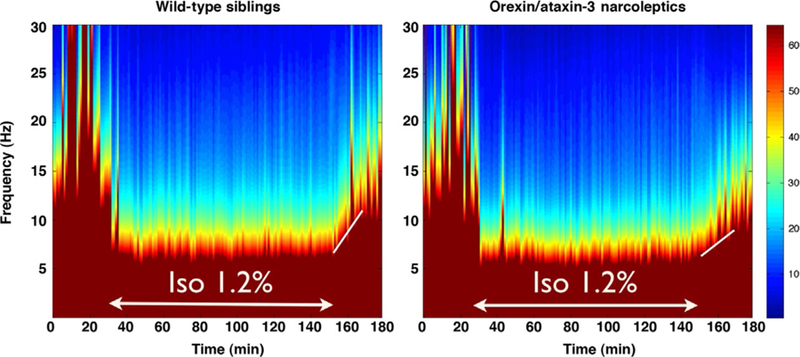
Spectral analysis of murine EEG shows delayed emergence in narcoleptic orexin/ ataxin-3 mice. Compared to their wild-type siblings (n=4, left), narcoleptic orexin/ ataxin-3 (n=4, right) shows similar baseline spectral power in the 30min prior to a 2-h 1.2% isoflurane exposure, a similar decrease in higher frequency power during the isoflurane anesthetic, but a delayed return of power in the 30min after isoflurane is discontinued. Note the shallower slope of the white line in the narcoleptic mice to the right.
4. ASSESSING SEDATION IN MICE AS A MEASURE OF ANESTHETIC SENSITIVITY
Sedative effects of anesthetic drugs are also easily modeled in mice. Most commonly, behavioral measures to evaluate the sedative effects of anesthetics depend upon a loss of motor coordination, such as with a reduced ability of mice to remain on a rotating bar in the “rotorod” test (Lakhlani et al., 1997) or with reduced locomotor activity of mice (Drexler, Antkowiak, Engin, & Rudolph, 2011). One could also envision the application of postural sway with a force plate (Zumwalt, Hamrick, & Schmitt, 2006) as another sensitive means of measuring the sedative effects of anesthetic drugs. Given that the molecular targets underlying sedation are dissociable from those underlying hypnosis (Jurd et al., 2003; Reynolds et al., 2003), molecular genetic studies in intact mice have provided valuable insights. More recently, sophisticated hijacking of neuronal activity using viral transformation of discrete populations in mouse brain has pointed to neuronal targets that differentially impact anesthetic sedation from hypnosis (Zhang et al., 2015). In the following sections, we detail affordable strategies to measure anesthetic sedation in mice using video tracking, accelerometry, and electromyographic tone.
4.1. Videographic Analysis of Anesthetic-Induced Reductions in Movement
Video recording of the mouse model is generally considered to be the paragon methodology toward assessment of immobility (Baker, 2011). Video tracking is commonly used as a direct measure of movement and inactivity, where minor image processing allows for automated frame-by-frame detection of a mouse about its home cage or experimental enclosure. Video recording for social and behavioral analysis is extensively used in pharmacological, transgenic, and sleep research studies to track movement and can be applied to classify differences within a variety of behaviors, such as rearing or grooming (Noldus, Spink, & Tegelenbosch, 2001; Ou-Yang, Tsai, Yen, & Lin, 2011). Moreover, video analysis has been used to distinguish wakefulness from NREM and REM sleep (McShane et al., 2012, 2010; Pack et al., 2007). Hence, it should not be surprising that video tracking is also useful in the context of studying anesthetics (Fig. 4). Our generalized protocol for creating video tracking of individual mice requires the Image Processing Toolbox within MATLAB® (Mathworks®, Natick, MA) and is described in brief later. Optimal mouse detection occurs with uniform lighting and high color contrast between the mouse and its environment, such as black mouse against a light-colored box. With a recording area that is uniformly lit and a camera positioned in a top-down view, ensure that the entire recording area is in full view. Measure the recording area’s length and width, which will establish the physical features of the frames for subsequent distance tracking. Let the mouse habituate to the novel environment and then proceed to record a period of movement activity. Five frames per second afford sufficient temporal resolution while minimizing the movie’s file sizes. Load the video file into MATLAB® using a videoreader function and create a structure array in which each individual frame color information will be included in the array. Each frames’ physical height and width can be converted into pixel units. The number of frames in the video based upon the movie’s duration and frame rate must also be specified to preallocate sufficient memory for the MATLAB® structure array. A “for” loop can be used to load the video data frame-by-frame into the video structure.
Fig. 4.
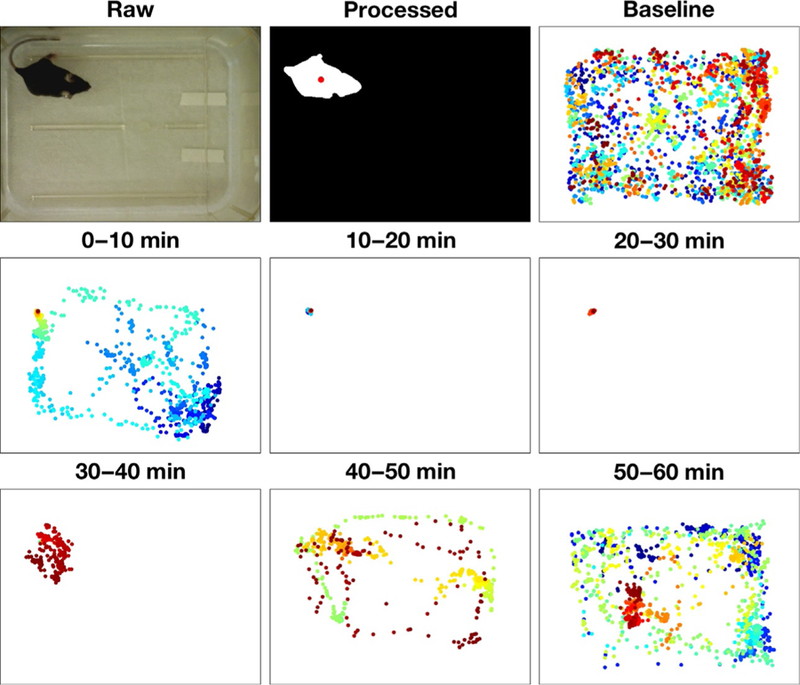
Video tracking of movement reveals duration of anesthetic sedation. Using the mouse tracking methodology described, a representative experimental paradigm is seen in this figure for a 100mg/kg IP injection of propofol for a single mouse. Video was captured at 5fps and was digitally processed to highlight the body of the mouse, with the central point of the mouse being featured in the processed image. Ten minutes epochs are shown for baseline activity, where no drug intervention is present, and for each subsequent 10min epoch following the 100mg/kg IP propofol injection up to 60min after injection. Every point represents a single frame from the video, where time within each epoch is represented by color (0min=Blue → 10min=red).
Create a temporary variable that contains color information as a matrix for the first frame. Upload this first frame into the Color Thresholder App found within MATLAB® and generate a color threshold mask using. Highlight a portion of the mouse to choose the color of interest. Force a binary output for color thresholding, and export the Color Thresholding parameters using the internal export function feature. Next, produce a region of interest mask using the roipoly function, marking areas of the image that are to be included in the analysis. The entire cage can be selected to be the region of interest. Note that the creation of the color and region of interest masks only need to be created once for a given experiment, as lighting conditions and cage position should remain unchanged between frames.
Filter the first frame with a two-dimensional Gaussian smoothing kernel. This creates a blurred image that helps in the removal of noise in the image acquisition. Proceed by applying both the Color Thresholding mask and the region of interest mask. The resulting image should be binary.
Continue image processing by dilating the image using a morphological structuring element to overlap any coarse features between the foreground and background in the processed image, and to remove fine structures such as the tail and whiskers, since the aim is to determine the central point of the mouse body. Use of imdilate and strel functions is recommended. Subsequently, fill image holes using the imfill function. At this point, image processing is complete. The resulting binary image should convey an isolated mouse.
Determine the centroid point of the mouse using the regionprops function. It is recommended to use the “area” property name when determining the centroid point, due to the irregular shape of the mouse body. The resulting point is the pixel at which the center of the mouse is located as determined using the described image analysis. Repeat the procedure for each frame of the loaded video using a “for” loop. A preallocated matrix can be created where the x–y pixel coordinates for the mouse centroid point can be stored for each frame. Once centroid points are established for each frame, periods of immobility can be defined by total distance traveled or by occupation of a single area over an extended period of time (McShane et al., 2012; Nelson et al., 2002; Noldus et al., 2001; Ou-Yang et al., 2011; Pack et al., 2007). This centroid tracking approach can be applied to both inhalational and intravenous anesthetic challenges.
4.2. Accelerometric Analysis of Anesthetic-Induced Reductions in Movement
The use of accelerometer to directly measure mouse movement can be applied to assess both the sedating and immobilizing properties of anesthetic drugs. Darting is a phenotyping assay routinely used in murine open-field testing, which highlights periods of increased acceleration as a defining feature of mouse behavior (Kafkafi et al., 2003). Due to the physical constraints of experimental space and to the inherent unpredictability in mouse movement, it is improbable that a mouse will be able to sustain a constant velocity for a prolonged period of time (Proekt, Banavar, Maritan, & Pfaff, 2012). Changes in acceleration are a result of fluctuations in velocity, therefore, acceleration can be used as a direct measure of movement. One way to use accelerometer data to determine immobility in anesthetic challenges is by determining prolonged periods where net acceleration is zero. Since it is not possible to establish tilt or orientation on a 3-axis accelerometer without use of a gyroscope, periods of net acceleration equivalent to 1g (unit of gravity) are interpreted as periods of immobility. During instances of quiescence, the square root of the sum of squares with respect to the 3-axis accelerometer data resembles 1g. A simple subtraction of 1g from this cumulative sensor data resulting from periods of motionlessness would yield values near zero. Tri-axis accelerometer voltage data are converted to acceleration in units of gravity by first obtaining the zero-g bias voltage and voltage sensitivity per unit-g. Static accelerometer calibration methods are described in brief later for a RHD-2000 amplifier chip with accelerometer (Intan Technologies, Los Angeles, CA) (Godfrey, Conway, Meagher, & O’Laighin, 2008; Steele et al., 2000; Yang & Hsu, 2010).
Position the accelerometer such that one axis is parallel to vertical and acquire approximately 10s of acceleration data from the accelerometer in this orientation. Use modeling clay to secure the accelerometer in a fixed position. Reverse the orientation of the accelerometer and record a small amount of acceleration data again. The voltages seen from each orientation correlate to ±1g. Open-source Open-Ephys hardware (http://www.open-ephys.org) can be used for data acquisition. Take the average of voltage of the two orientations to obtain the zero-g voltage bias, and calculate half of the voltage difference between the two orientations to attain the voltage sensitivity of the vertically oriented axis. Repeat this procedure for the remaining two axes. This concludes calibration of the accelerometer chip. With actual accelerometery data, subtract zero-g voltage bias with respect to each axis for all data points, and then divide by the respective voltage sensitivity per axis to convert raw voltages into units of gravity. Perform a square root sum of squares with the converted axes data per time point to obtain cumulative sensor acceleration. Subtract 1g from this time series data vector to readjust the data such that the value of zero coincides with no movement. Smooth the time series data with a nonoverlapping 100ms root mean square (RMS) moving window. Fig. 5 shows prototypic results of experiments in mice in which an RHD-2000 amplifier chip is used in conjunction with mice that have been surgically implanted with EEG and electromyography (EMG) electrodes (Wasilczuk, Proekt, Kelz, & McKinstry-Wu, 2016).
Fig. 5.
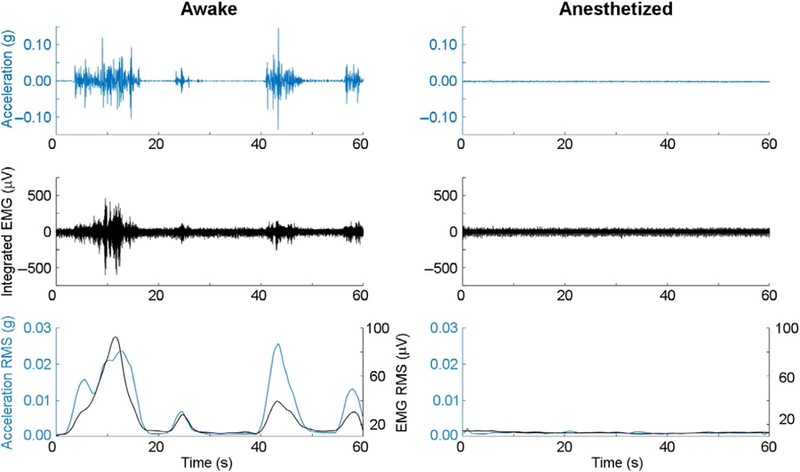
Analysis of acceleration and EMG are useful modalities to determine duration of anesthetic sedation. One-minute raw acceleration (Row 1) and electromyographic traces (Row 2) are shown before (Left) and after (Right) a 1.2% isoflurane anesthetic exposure for an individual mouse. RMS moving average filtering of the raw data shows high correlation (>0.7) between accelometry and electromyography for movement detection (Row 3).
4.3. Electromyographic Analysis of Anesthetic-Induced Reductions in Motor Tone
Another methodology that can be used to determine anesthetic reductions in movement is through EMG analysis (Fig. 5). This technique delivers information about the electrical activity of skeletal muscles. Typically for EMG recordings, intramuscular electrodes are used to create muscle contraction; however, passive recording of EMG reveals spontaneous muscle tone and contractility characteristics that are correlated to muscle activity (De Luca, Gilmore, Kuznetsov, & Roy, 2010). Biopotentials are generated when electrical activity is recorded between two electrodes. Strategic placement of EMG electrodes in cervical muscles provides insight into murine polysomnography, discerning wakefulness, nonREM, and REM sleep (Fisher et al., 2012; Pack et al., 2007). Similarly, EMG can provide a gradient for anesthetic phenotyping, ranging from the high motor tone seen in active waking state to the profound drop in tone that commonly characterizes the unconscious state. A succinct protocol for the analysis of EMG activity can be found below, highlighting two common methods of signal processing, rectified and integrated EMG (Oishi et al., 2016). The aim of these methodologies is to combat the ephemeral oscillations of the raw EMG signal, where smoothing would provide uninterpretable data.
Implant intramuscular electrodes into nuchal muscle as previously described, and allow 2 weeks of recovery time before the animal is used in experimental studies (Oishi et al., 2016; Wasilczuk et al., 2016). For the integrated EMG analysis method, apply a high-pass filter on the EMG biopotential with cutoff frequency of 20Hz. This removes any DC offset in the signal and movement artifact that may be present (van Boxtel, 2001). If the raw data sampling frequency is greater than 1000Hz, additional low-pass filtering should be performed, as the optimal low-pass cutoff frequency for muscle activity is approximately 500Hz (van Boxtel, 2001). After filtering the data, apply a nonoverlapping 100ms window RMS moving average filter to the filtered data to provide data informing muscle tone and activation about the head of the animal. If the rectified analysis approach is desired, simply take the mean-removed absolute value of the raw signal. Then apply a similar 100ms window moving average filter onto the rectified data allowing for indirect evidence of muscle tone and activity. As shown in Fig. 5, nuchal EMG biopotentials and accelerometry data are highly correlated signals.
5. CONCLUSIONS
In this chapter, we highlight the uses of mice as a model organism for assessing the effects of anesthetic drugs with the ultimate goal of determining both molecular and neuronal sites of sedation and hypnosis. Given the explosive technical advances in recent years (Bucan & Abel, 2002; Rossant & McMahon, 1999; Singh, Schimenti, & Bolcun-Filas, 2015; Susaki, Ukai, & Ueda, 2017) and new found ability to manipulate the activity of discrete populations of neurons with optogenetic and chemogenetic approaches (Ressler, 2017), it is increasingly likely that anesthetic mechanisms research in mice will continue to provide novel insights into drug action.
REFERENCES
- Baker M. (2011). Animal models: Inside the minds of mice and men. Nature, 475(7354), 123–128. [DOI] [PubMed] [Google Scholar]
- Behlke LM, Foster RA, Liu J, Benke D, Benham RS, Nathanson AJ, et al. (2016). A pharmacogenetic ‘restriction-of-function’ approach reveals evidence for anxiolytic-like actions mediated by alpha5-containing GABAA receptors in mice. Neuropsychopharmacology, 41(10), 2492–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, et al. (2009). The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nature Protocols, 4(10), 1560–1564. [DOI] [PubMed] [Google Scholar]
- Bruhn J, Bouillon TW, Radulescu L, Hoeft A, Bertaccini E, & Shafer SL. (2003). Correlation of approximate entropy, bispectral index, and spectral edge frequency 95 (SEF95) with clinical signs of “anesthetic depth” during coadministration of propofol and remifentanil. Anesthesiology, 98(3), 621–627. [DOI] [PubMed] [Google Scholar]
- Bucan M, & Abel T. (2002). The mouse: Genetics meets behaviour. Nature Reviews Genetics, 3(2), 114–123. [DOI] [PubMed] [Google Scholar]
- Campagna JA, Miller KW, & Forman SA. (2003). Mechanisms of actions of inhaled anesthetics. The New England Journal of Medicine, 348(21), 2110–2124. [DOI] [PubMed] [Google Scholar]
- Caro AC, Hankenson FC, & Marx JO. (2013). Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. Journal of the American Association for Laboratory Animal Science, 52(5), 577–583. [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shu S, & Bayliss DA. (2009). HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. The Journal of Neuroscience, 29(3), 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Gilmore LD, Kuznetsov M, & Roy SH. (2010). Filtering the surface EMG signal: Movement artifact and baseline noise contamination. Journal of Biomechanics, 43(8), 1573–1579. [DOI] [PubMed] [Google Scholar]
- Deussing JM. (2013). Targeted mutagenesis tools for modelling psychiatric disorders. Cell and Tissue Research, 354(1), 9–25. [DOI] [PubMed] [Google Scholar]
- Drexler B, Antkowiak B, Engin E, & Rudolph U. (2011). Identification and characterization of anesthetic targets by mouse molecular genetics approaches. Canadian Journal of Anaesthesia, 58(2), 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eger EI 2nd, & Saidman LJ. (2005). Illustrations of inhaled anesthetic uptake, including intertissue diffusion to and from fat. Anesthesia and Analgesia, 100(4), 1020–1033. [DOI] [PubMed] [Google Scholar]
- Fisher SP, Godinho SI, Pothecary CA, Hankins MW, Foster RG, & Peirson SN. (2012). Rapid assessment of sleep-wake behavior in mice. Journal of Biological Rhythms, 27(1), 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP. (2006). Molecular targets underlying general anaesthesia. British Journal of Pharmacology, 147(Suppl. 1), S72–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP. (2008). General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nature Reviews Neuroscience, 9(5), 370–386. [DOI] [PubMed] [Google Scholar]
- Godfrey A, Conway R, Meagher D, & O’Laighin G. (2008). Direct measurement of human movement by accelerometry. Medical Engineering & Physics, 30(10), 1364–1386. [DOI] [PubMed] [Google Scholar]
- Hemmings HC Jr., Akabas MH, Goldstein PA, Trudell JR, Orser BA, & Harrison NL. (2005). Emerging molecular mechanisms of general anesthetic action. Trends in Pharmacological Sciences, 26(10), 503–510. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, et al. (2004). TREK-1, a K+ channel involved in neuroprotection and general anesthesia. The EMBO Journal, 23(13), 2684–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth B. (1991). Principles for transformation of scalp EEG from potential field into source distribution. Journal of Clinical Neurophysiology, 8(4), 391–396. [DOI] [PubMed] [Google Scholar]
- Hu FY, Hanna GM, Han W, Mardini F, Thomas SA, Wyner AJ, et al. (2012). Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine beta-hydroxylase knockout mice. Anesthesiology, 117(5), 1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E, Kim S, Shin HS, & Choi JH. (2010). The forced walking test: A novel test for pinpointing the anesthetic-induced transition in consciousness in mouse. Journal of Neuroscience Methods, 188(1), 14–23. [DOI] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, et al. (2003). General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. The FASEB Journal, 17(2), 250–252. [DOI] [PubMed] [Google Scholar]
- Kafkafi N, Pagis M, Lipkind D, Mayo CL, Bemjamini Y, Golani I, et al. (2003). Darting behavior: A quantitative movement pattern designed for discrimination and replicability in mouse locomotor behavior. Behavioural Brain Research, 142(1–2), 193–205. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, et al. (2008). An essential role for orexins in emergence from general anesthesia. Proceedings of the National Academy of Sciences of the United States of America, 105(4), 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, et al. (1997). Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proceedings of the National Academy of Sciences of the United States of America, 94(18), 9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles JT, Baber SR, Deng W, Porter JR, Corll C, Murthy SN, et al. (2007). Pressor responses to ephedrine are not impaired in dopamine beta-hydroxylase knockout mice. British Journal of Pharmacology, 150(1), 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour GA, Orser BA, & Avidan MS. (2011). Intraoperative awareness: From neurobiology to clinical practice. Anesthesiology, 114(5), 1218–1233. [DOI] [PubMed] [Google Scholar]
- McShane BB, Galante RJ, Biber M, Jensen ST, Wyner AJ, & Pack AI. (2012). Assessing REM sleep in mice using video data. Sleep, 35(3), 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane BB, Galante RJ, Jensen ST, Naidoo N, Pack AI, & Wyner A. (2010). Characterization of the bout durations of sleep and wakefulness. Journal of Neuroscience Methods, 193(2), 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, & Maze M. (2002). The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nature Neuroscience, 5(10), 979–984. [DOI] [PubMed] [Google Scholar]
- Noldus LP, Spink AJ, & Tegelenbosch RA. (2001). Ethovision: A versatile video tracking system for automation of behavioral experiments. Behavior Research Methods, Instruments, & Computers, 33(3), 398–414. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Takata Y, Taguchi Y, Kohtoh S, Urade Y, & Lazarus M. (2016). Polygraphic recording procedure for measuring sleep in mice. Journal of Visualized Experiments(107), e53678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou-Yang TH, Tsai ML, Yen CT, & Lin TT. (2011). An infrared range camera-based approach for three-dimensional locomotion tracking and pose reconstruction in a rodent. Journal of Neuroscience Methods, 201(1), 116–123. [DOI] [PubMed] [Google Scholar]
- Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, et al. (2007). Novel method for high-throughput phenotyping of sleep in mice. Physiological Genomics, 28(2), 232–238. [DOI] [PubMed] [Google Scholar]
- Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, & Svenson KL. (2007). The mouse as a model for human biology: A resource guide for complex trait analysis. Nature Reviews Genetics, 8(1), 58–69. [DOI] [PubMed] [Google Scholar]
- Proekt A. (2018). Brief introduction to electroencephalography. Methods in Enzymology, 603 (in press). [DOI] [PubMed] [Google Scholar]
- Proekt A, Banavar JR, Maritan A, & Pfaff DW. (2012). Scale invariance in the dynamics of spontaneous behavior. Proceedings of the National Academy of Sciences of the United States of America, 109(26), 10564–10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ. (2017). Neurocircuits to behavior: The new revolution. Harvard Review of Psychiatry, 25(2), 47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O’Meara GF, Haythornthwaite A, Newman RJ, et al. (2003). Sedation and anesthesia mediated by distinct GABA(A) receptor isoforms. The Journal of Neuroscience, 23(24), 8608–8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, & McMahon A. (1999). “Cre”-ating mouse mutants-a meeting review on conditional mouse genetics. Genes & Development, 13(2), 142–145. [DOI] [PubMed] [Google Scholar]
- Rudolph U, & Antkowiak B. (2004). Molecular and neuronal substrates for general anaesthetics. Nature Reviews Neuroscience, 5(9), 709–720. [DOI] [PubMed] [Google Scholar]
- Singh P, Schimenti JC, & Bolcun-Filas E. (2015). A mouse geneticist’s practical guide to CRISPR applications. Genetics, 199(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonner JM. (2002). Issues in the design and interpretation of minimum alveolar anesthetic concentration (MAC) studies. Anesthesia and Analgesia, 95(3), 609–614. [DOI] [PubMed] [Google Scholar]
- Sonner JM, Antognini JF, Dutton RC, Flood P, Gray AT, Harris RA, et al. (2003). Inhaled anesthetics and immobility: Mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesthesia and Analgesia, 97(3), 718–740. [DOI] [PubMed] [Google Scholar]
- Steele BG, Holt L, Belza B, Ferris S, Lakshminaryan S, & Buchner DM. (2000). Quantitating physical activity in COPD using a triaxial accelerometer. Chest, 117(5), 1359–1367. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen J, Pruckmayr G, Baumgardner JE, Eckmann DM, Eckenhoff RG, et al. (2006). High throughput modular chambers for rapid evaluation of anesthetic sensitivity. BMC Anesthesiology, 6(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki EA, Ukai H, & Ueda HR. (2017). Next-generation mammalian genetics toward organism-level systems biology. NPJ Systems Biology and Applications, 3, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Weinshenker D, Palmiter RD, & Garber G. (2004). Dbh(−/−) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 286(1), R108–R113. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Marck BT, Palmiter RD, & Matsumoto AM. (1998). Restoration of norepinephrine and reversal of phenotypes in mice lacking dopamine beta-hydroxylase. Journal of Neurochemistry, 70(6), 2468–2476. [DOI] [PubMed] [Google Scholar]
- Upton RN, Ludbrook GL, Grant C, & Martinez AM. (1999). Cardiac output is a determinant of the initial concentrations of propofol after short-infusion administration. Anesthesia and Analgesia, 89(3), 545–552. [DOI] [PubMed] [Google Scholar]
- van Boxtel A. (2001). Optimal signal bandwidth for the recording of surface EMG activity of facial, jaw, oral, and neck muscles. Psychophysiology, 38(1), 22–34. [PubMed] [Google Scholar]
- Wasilczuk AZ, Proekt A, Kelz MB, & McKinstry-Wu AR. (2016). High-density electroencephalographic acquisition in a rodent model using low-cost and open-source resources. Journal of Visualized Experiments, (117), e54908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, & Hsu YL. (2010). A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors (Basel), 10(8), 7772–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ferretti V, Guntan I, Moro A, Steinberg EA, Ye Z, et al. (2015). Neuronal ensembles sufficient for recovery sleep and the sedative actions of alpha2 adrenergic agonists. Nature Neuroscience, 18(4), 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumwalt AC, Hamrick M, & Schmitt D. (2006). Force plate for measuring the ground reaction forces in small animal locomotion. Journal of Biomechanics, 39(15), 2877–2881. [DOI] [PubMed] [Google Scholar]


