Abstract
ERG, an ETS family transcription factor, is known to be expressed in endothelial cells, and oncogenic ERG gene fusions occur in subsets of prostatic carcinoma, acute myeloid leukemia, and Ewing sarcoma. In this study, we immunohistochemically investigated nuclear ERG expression using a new monoclonal antibody CPDR ERG-MAb, highly specific for detecting ERG protein and ERG-expressing prostate carcinomas. A broad range of vascular endothelial (n=250), other mesenchymal (n=973), and epithelial tumors (n=657) were examined in order to determine the utility of ERG immunohistochemistry in surgical pathology. Only immunostains with ERG-positive normal endothelia (internal control) were considered valid, and only nuclear staining was considered positive. In adult tissues, ERG was restricted to endothelial cells and a subset of bone marrow precursors, but early fetal mesenchyme and subpopulations of fetal cartilage were also positive. In vascular tumors, ERG was expressed in endothelia of all hemangiomas and lymphangiomas and typically extensively in 96/100 angiosarcomas, 42/43 epithelioid hemangioendotheliomas and all 26 Kaposi sarcomas. Among non-vascular mesenchymal tumors, only blastic extramedullary myeloid tumors (7/10) and rare Ewing sarcomas (2/29) were positive. Among epithelial tumors, 30/66 prostatic adenocarcinomas showed focal to extensive ERG-positivity, with no immunoreactivity in normal prostate. Other carcinomas and epithelial tumors (n = 643) were negative, with the exception of 1/42 large cell undifferentiated pulmonary carcinomas and 1/27 mesotheliomas, each of which showed focal nuclear ERG-positivity. Based on the above observations, ERG is a highly specific new marker for benign and malignant vascular tumors. Among epithelial tumors, ERG shows a great promise as a marker to identify prostatic carcinoma in both primary and metastatic setting.
Keywords: ETS-family transcription factor, ERG, angiosarcoma, hemangioendothelioma, hemangioma, Immunohistochemistry, prostate carcinoma
INTRODUCTION
Specific identification of malignant vascular endothelial tumors - angiosarcoma, Kaposi sarcoma, and epithelioid hemangioendothelioma – can be challenging in view of their many histological mimics. Availability of tumor type-specific chemotherapy regimens that include paclitaxel for angiosarcoma has given accurate tumor typing new therapeutic significance. 7
Current markers for angiosarcoma include CD31 (primary marker) 4,13,18, CD34 18, 23,31,32, podoplanin (D2-40) 3, and vascular endothelial growth factor receptors (VEGFRs) 11,22 as other potential markers. None of these markers have ideal sensitivity and specificity. CD31 is also expressed in some hematopoietic cells, including tissue histiocytes and platelets. 13,17,18,26 CD34 is widely expressed in non-endothelial neoplasms, such as many fibroblastic tumors and gastrointestinal stromal tumors. 32 VEGFR3 and podoplanin preferentially identify lymphatic endothelia and some but not all angiosarcomas, and the latter is also expressed in other tumor types, for example in squamous cell carcinoma and seminoma. 22,28 Von Willebrand factor (factor VIII-related antigen) is an older marker with a lower detection sensitivity for angiosarcoma and frequent interpretation problems being a serum component.18
ETS-family transcription factors, including ERG, ETS-1, Fli-1, NERF-2, and TEL are expressed in vascular endothelial cells and are therefore also potential endothelial markers.27 Fli-1 (Freund’s leukemia integration site 1), also involved in the most common Ewing sarcoma translocation, is constitutionally expressed in endothelial cells and lymphoid cells. Although one immunohistochemical study suggested high specificity and sensitivity of a polyclonal antibody for vascular tumors in addition to Ewing sarcoma5, another series also showed common immunoreactivity in synovial sarcoma, melanoma, and pulmonary adenocarcinoma. 24
ERG (avian v-ets erythroblastosis virus E26 oncogene homolog), member of the ETS family transcription factors, is constitutionally expressed in endothelial cells regulating angiogenesis and endothelial apoptosis.2 ERG has been recently studied immunohisto-chemically in prostate carcinoma, subsets of which have oncogenic TMPRSS2-ERG gene fusions and express ERG protein, and in human tissues, vascular endothelial cells also consistently express ERG. 6,21,30
In this study, we explored ERG as an immunohistochemical marker in the study of vascular tumors, of which no data exist on ERG expression. We also examined a large number of non-epithelial and epithelial neoplasms to explore the specificity of ERG for vascular tumors and also for prostate carcinoma among carcinomas. Based on our observations, ERG has a high sensitivity and specificity for endothelial malignancies. Known other entities for ERG-gene fusions, acute myeloid leukemia and Ewing sarcoma, are among rare exceptions on positive non-endothelial neoplasms. On the other hand, ERG is highly specific for prostate carcinoma among epithelial tumors, and is expected to be useful in assessment of prostate carcinoma and prostatic origin of metastases.
MATERIALS AND METHODS
A wide variety of normal (n > 100) and neoplastic tissues of different lineages (n = 1880) were immunohistochemically examined for ERG expression. For these studies, we mainly used sections generated from multi-tissue blocks containing 5-50 different tissue samples. Additional cases of vascular tumors were examined on slides created from conventional tissue blocks. In order to create an understanding of ERG tissue distribution, adult and fetal human tissues from surgical specimens were also studied.
Monoclonal antibody to ERG (CPDR ERG-MAb) created to a synthetic peptide of an N-terminal sequence of ERG protein was used in a final concentration of 3.7 μg/mL (1:1000 dilution of reconstituted purified antibody stock). This antibody was created at the Center for Prostate Disease Research (CPDR) of Walter Reed Army Medical Center and Uniformed Services University, Rockville, Maryland. It has been shown specific to ERG by immunoblotting and evaluation of ERG-negative and positive cell lines, with no cross-reaction with other Ets family proteins, such as Fli-1.20 Furthermore, it selectively identifies ERG gene-translocated subset of prostate carcinomas.6
Immunostaining was performed in Leica Bond-Max automatic immunostainer (Leica, Bannockburn, IL). Heat induced epitope retrieval (high pH, EDTA-based buffer, pH 9.0, Leica Bond-Max) for 25 minutes, followed by normal goat serum (Vector, Burlingame, CA, dilution 1:10) were applied for 10 minutes prior to the primary antibody. The primary antibody was incubated for 30 minutes at room temperature. Leica Bond-Max avidin-biotin free polymer system was used in the detection according to the company’s recommended procedure. Diaminobenzidine was used as the chromogen, following blocking of endogenous peroxidase with 3% hydrogen peroxide diluted in phosphate buffer. Finally, a light hematoxylin counterstain was applied.
The portion of positive tumor cell nuclei was semiquantitatively assessed. Only nuclear immunoreactivity was counted as positive, and only cases with positive normal endothelial staining (internal control) were scored. Based on this, approximately 5% of cases were excluded from the study. These cases especially included acid-decalcified tissue, tissues obtained from autopsies, and very old unstained slides. Monoclonal antibody JC70A to CD31 (Dako Cytomation, Carpinteria, CA), diluted 1:100, with similar epitope retrieval as employed for ERG, was used in the characterization of vascular tumors. Monoclonal antibody to smooth muscle actin (Clone 1A4, Sigma Chemicals, St. Louis, Missouri), diluted 1:32000, with no pretreatment, was used in the characterization of pericytic populations in vascular tumors. Von Willebrand factor (factor VIII-related antigen) was not used in this study as a marker due to its known lower sensitivity in the detection of angiosarcomas.
RESULTS
Normal human adult and developing tissues
In normal adult tissues: skin, breast, tonsil, spleen, thymus, lung, esophagus, stomach, small intestine, colon, liver, pancreas, kidney, urinary bladder, prostate, seminal vesicle, epididymis, testis, endo- and myometrium, (postmenopausal) ovary, adrenal gland, thyroid, parathyroid, brain, and mesothelia, ERG expression was restricted to vascular endothelial cells. Vascular pericytes and smooth muscle cells were negative. In adult bone marrow, a subpopulation of immature myeloid cells was positive. In these tissues, nuclei of endothelia of blood vessels of various calibers and lymphatics were equally highlighted. Pericytes and vascular smooth muscle were consistently negative. No adult epithelial, other mesenchymal, neuroectodermal, or lymphoid cells were positive.
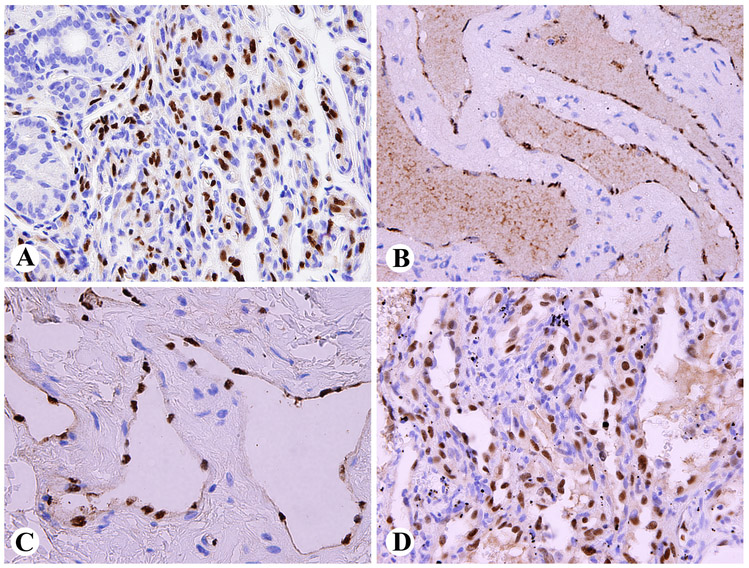
In an early first trimester human embryo, subepidermal and paraspinal mesenchyme showed nuclear positivity, in addition to endothelial cells (Fig. 1A). Spinal cord and differentiating epithelial and mesenchymal elements in single profiles of lung, heart, liver and gastrointestinal tract were negative. In a late first trimester fetus, nuclear ERG-positivity was detected in the periphery of cartilage, especially at the joints, as well as focally in perichondrial mesenchyme, but was otherwise restricted to endothelial cells in small intestine, kidney, liver, and spleen (Fig. 1B). In term, first and second trimester placenta, ERG was also restricted into endothelial cells, and yolk sac and placental trophoblast were negative.
Fig. 1.
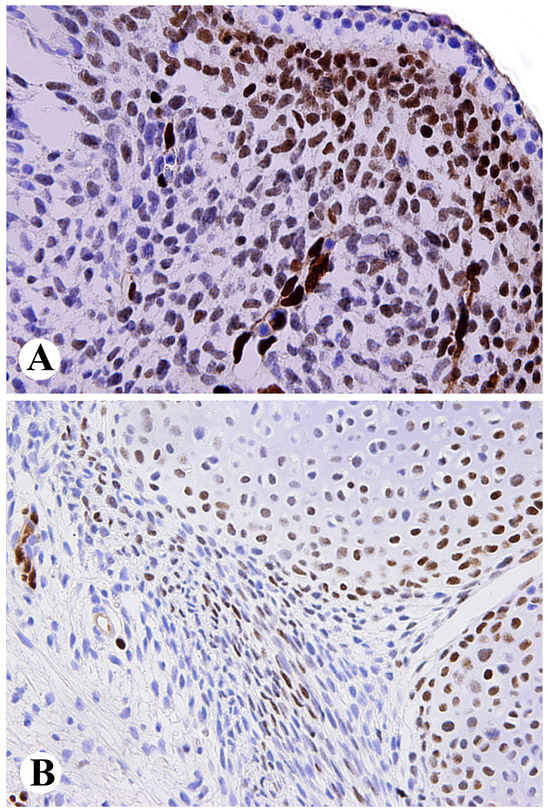
A. Nuclear ERG-expression in primitive subepidermal mesenchyme and vascular endothelial cells in early first trimester human embryo. B. Developing cartilage, especially peripherally at the joints and foci of perichondrial mesenchyme and capillary endothelia show nuclear ERG-positivity in late first trimester fetus.
Vascular endothelial tumors
Nuclear positivity was uniformly detected in hemangiomas of different types, as well as in lymphangiomas and lymphangioendotheliomas (Fig. 2). Pericytic elements, also detected by smooth muscle actin immunostaining and especially prominent in cellular hemangiomas, such as juvenile capillary hemangioma, were negative. (Fig. 2A). In spindle cell hemangioma, the nuclei of vascular lumen-lining endothelial cells were positive, whereas the interstitial spindle cells were negative (Table 1, Fig. 2D).
Fig. 2.
Nuclear ERG-expression in benign vascular tumors. A. Juvenile capillary hemangioma involving lacrimal gland contains both ERG-positive endothelial cells and ERG-negative pericytes. B. Cavernous hemangioma vascular lumens are lined by ERG-positive endothelial cells. C. Lymphangioma endothelia are ERG-positive. D. In spindle cell hemangioma, ERG is restricted to the endothelial component, and the interstitial spindle cells are negative.
Table 1.
Endothelial nuclear ERG expression in vascular tumors
| Hemangioma, total | 61/61 |
| Juvenile | 8/8 |
| Lobular capillary | 8/8 |
| Cavernous | 10/10 |
| Miscellaneous capillary | 12/12 |
| Papillary endothelial hyperplasia | 5/5 |
| Epithelioid hemangioma | 4/4 |
| Spindle cell hemangioma | 14/14 |
| Lymphangioma | 11/11 |
| Lymphangioendothelioma | 2/2 |
| Kaposiform hemangioendothelioma | 3/3 |
| Retiform/Dabska hemangioendothelioma (3+1) | 4/4 |
| Epithelioid hemangioendothelioma | 42/43 |
| Angiosarcoma, total | 96/100 |
| Angiosarcoma of scalp or face | 21/21 |
| Other cutaneous angiosarcoma (non radiation-associated) | 6/6 |
| Post-radiation angiosarcoma of breast/chest wall | 6/6 |
| Lymphedema-associated angiosarcoma | 5/5 |
| Angiosarcoma of deep soft tissue, peripheral | 15/16 |
| Angiosarcoma, intra-abdominal/mediastinal | 12/13 |
| Angiosarcoma of breast parenchyma | 1/1 |
| Hepatic angiosarcoma | 1/1 |
| Splenic angiosarcoma | 5/5 |
| Angiosarcoma of bone | 2/2 |
| Other visceral angiosarcomas | 22/24 |
| Angiosarcoma of epithelioid cell type (included above) | 15/15 |
| Kaposi sarcoma (including 3 AIDS-associated) | 26/26 |
| Total for vascular tumors | 250 |
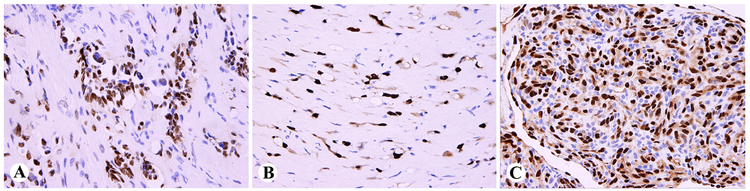
In retiform hemangioendothelioma, the neoplastic endothelial cells were uniformly ERG-positive (Fig. 3A). In epithelioid hemangioendothelioma, virtually all tumor cell nuclei in the infiltrative cords were highlighted as ERG-positive, and cytoplasmic staining was also present (Table 1, Fig. 3B). In kaposiform hemangioendothelioma, the endothelial cells were ERG-positive, but the abundant pericytic component (also smooth muscle actin-positive), was ERG-negative (Fig. 3C). A similar situation was observed in one Dabska-type papillary intravascular lymphangioendothelioma.
Fig. 3.
ERG-expression in hemangioendotheliomas. A. Retiform hemangioendo-thelioma with ERG-positive endothelial nuclei. B. Epithelioid hemangioendothelioma cords show both nuclear and cytoplasmic ERG-expression. C. Kaposiform hemangioendothelioma contains an ERG-positive endothelial component and an ERG-negative pericytic element.
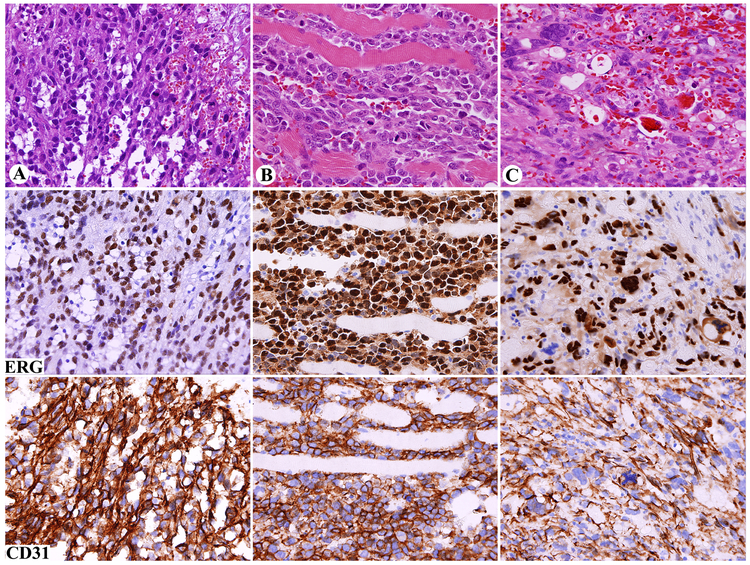
All but 4 of 100 angiosarcomas (96%) of different types, clinicopathologic subgroups, and different sites, confirmed as CD31-positive in this study, showed nuclear ERG-immunoreactivity (Table 1). This varied from 5% to 100% (median, 100%). In addition to the 4 negative cases, only 4 cases showed ERG-immunoreactivity in < 30% of the tumor cells, and only 8 cases in 30-49% of tumor cells. Nuclear ERG staining was equally seen in differentiated, vasoformative areas, undifferentiated solid, and rare pleomorphic areas (Fig. 4 A-C). In contrast to hemangioma, a distinct ERG-negative (pericytic) cell population was generally absent. In angiosarcoma, cytoplasmic ERG-positivity was variably present, but less prominent than observed in epithelioid hemangioendothelioma. In mitotic cells, nuclear ERG-positivity was exceptionally undetectable (Fig. 4C). In Kaposi sarcoma, both endothelial cells and neoplastic spindle cells showed nuclear ERG-positivity.
Fig. 4.
Nuclear ERG expression in angiosarcoma in comparison with cytoplasmic and membranous CD31 staining. Each vertical group represents one case and horizontal line one marker. A. Vasoformative pericardial angiosarcoma in a man with a history of mediastinal radiation. B. Solid, poorly differentiated angiosarcoma involving skeletal muscle. C. Pleomorphic splenic angiosarcoma; mitotic cells lack distinct nuclear ERG-staining. Note that all tumors are positive for both markers.
All vascular-related, non-endothelial tumors, such as hemangioperiocytoma (both peripheral and central nervous system meningeal examples), cerebellar hemangioblastoma, and glomus tumor cells were negative, with endothelial cells only being ERG-positive. Also negative were highly vascular or hemorrhagic angiosarcoma or Kaposi sarcoma mimics, such as angiomatoid (n = 28) and aneurysmal fibrous histiocytoma (n = 3).
Other non-epithelial and epithelial tumors
A wide array of mesenchymal, neuroectodermal, and hematopoietic tumors were negative for ERG with no nuclear positivity in the neoplastic elements (Table 2). This included epithelioid hemangioendothelioma mimics with corded patterns and myxohyaline stroma such as primary and metastatic carcinomas, sclerosing perineurioma, chordoma, and extraskeletal myxoid chondrosarcoma. None of the metastatic melanomas showed non-endothelial ERG-immunoreactivity.
Table 2.
Nuclear ERG-immunoreactivity in non-epithelial mesenchymal, neuroectodermal, and hematopoietic tumors, other than vascular endothelial tumors.
SFT = solitary fibrous tumor.
| Alveolar soft part sarcoma | 0/13 |
| Angioleiomyoma | 0/4 |
| Angiomyolipoma | 0/9 |
| Astrocytoma, cerebellar | 0/7 |
| Chondrosarcoma | 0/8 |
| Chondrosarcoma, extraskeletal myxoid | 0/17 |
| Chondrosarcoma, dedifferentiated | 0/6 |
| Chondrosarcoma, mesenchymal | 0/7 |
| Chordoma | 0/22 |
| Desmoid fibromatosis | 0/19 |
| Dermatofibrosarcoma protuberans | 0/32 |
| Endometrial stromal sarcoma | 0/6 |
| Ependymoma | 0/4 |
| Epithelioid sarcoma | 0/8 |
| Ewing sarcoma | 2/29 |
| Extramedullary myeloid tumor, blastic | 7/10 |
| Fibrous histiocytoma, angiomatoid | 0/28 |
| Fibrous histiocytoma, benign, cutaneous | 0/18 |
| GIST (50 gastric, 38 small intestinal) | 0/88 |
| Glioblastoma multiforme | 0/17 |
| Glomus tumor | 0/18 |
| Granular cell tumor | 0/20 |
| Granulosa cell tumor of ovary | 0/9 |
| Hemangioblastoma of cerebellum | 0/6 |
| Hemangiopericytoma, meningeal | 0/24 |
| Hemangiopericytoma/SFT, soft tissue | 0/51 |
| Leiomyosarcoma | 0/26 |
| Liposarcoma, well-differentiated | 0/9 |
| Liposarcoma, dedifferentiated | 0/14 |
| Liposarcoma, myxoid/round cell | 0/25 |
| Liposarcoma, pleomorphic | 0/17 |
| Low-grade fibromyxoid sarcoma | 0/12 |
| Lymphoma, anaplastic large cell | 0/7 |
| Lymphoma, diffuse large B-cell | 0/9 |
| Lymphoma, T-cell lymphoblastic | 0/7 |
| Lymphoma, mantle cell | 0/9 |
| Malignant peripheral nerve sheath tumor | 0/12 |
| Medulloblastoma | 0/19 |
| Melanoma, metastatic | 0/34 |
| Meningioma | 0/65 |
| MFH, pleomorphic | 0/35 |
| Myofibroma | 0/9 |
| Neuroblastoma | 0/19 |
| Neurofibroma | 0/6 |
| Nodular fasciitis | 0/29 |
| Oligodendroglioma | 0/17 |
| Osteosarcoma | 0/12 |
| Paraganglioma | 0/14 |
| Perineurioma, sclerosing | 0/6 |
| Rhabdomyosarcoma, alveolar | 0/16 |
| Rhabdomyosarcoma, embryonal | 0/15 |
| Schwannoma | 0/14 |
| Synovial sarcoma | 0/36 |
| Total | 9/973 |
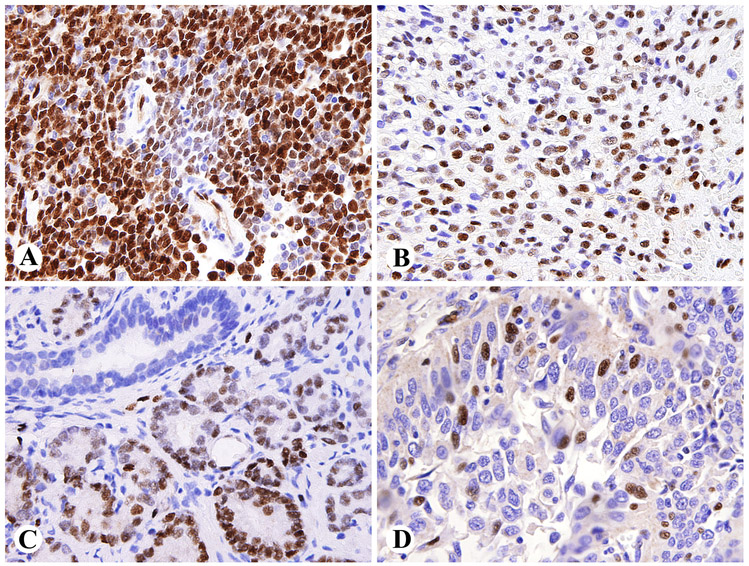
The exceptions were blastic extramedullary myeloid tumors (acute myeloid leukemia tissue infiltrates), of which 7 of 10 were ERG-positive, usually with uniform nuclear labeling (Fig. 5A). Also, 2 of 29 Ewing sarcomas showed nuclear ERG-immunoreactivity (Fig 5B). None of the 25 myxoid liposarcomas were ERG-positive in our studies. Chondrosarcomas of different types were tested in view of primitive mesenchymal and perichondrial ERG-positivity in embryonal tissue. However, all conventional, dedifferentiated, and mesenchymal chondrosarcomas were negative.
Fig 5.
Nuclear ERG-expression in non-vascular tumors. A. Intestinal relapse of acute myeloid leukemia (blastic extramedullary myeloid tumor). B. Ewing sarcoma. C. ERG-positive prostatic adenocarcinoma with a negative normal duct. D. Large cell anaplastic pulmonary carcinoma with focal nuclear ERG-expression.
Epithelial neoplasms
ERG-positivity was present in nearly half of prostate carcinomas: 30/66 (45.4%), most of which were old transurethral electroresection specimens (Table 3). Positivity was encountered in well-differentiated and poorly differentiated high-grade tumors, and intraepithelial neoplasia components, in some cases only as a focal finding (Fig. 5C). However, there was some tendency for higher frequency in high-grade tumors. In all these specimens, normal prostatic epithelia were negative. 3 of 6 metastatic prostate carcinomas to abdominal and neck lymph nodes were also positive.
Table 3.
Results on ERG-immunoreactivity in epithelial neoplasms. Carcinoma is abbreviated as Ca.
| Adenocarcinoma, ductal, breast | 0/26 |
| Adenocarcinoma, colon | 0/45 |
| Adenocarcinoma, endometrium, differentiated | 0/14 |
| Adenocarcinoma, endometrium, sarcomatous (MMMT) | 0/9 |
| Adenocarcinoma, lung | 0/27 |
| Adenocarcinoma, pancreas | 0/13 |
| Adenocarcinoma, prostate | 30/66 |
| Adenocarcinoma, stomach (14 signet ring cell) | 0/24 |
| Adenoid cystic carcinoma, major/minor salivary glands | 0/16 |
| Ca basal cell, skin | 0/5 |
| Ca cholangiocarcinoma, hepatic | 0/6 |
| Ca hepatocellular | 0/7 |
| Ca renal cell | 0/52 |
| Ca serous papillary, ovary or peritoneum | 0/29 |
| Ca small cell, lung | 0/26 |
| Ca squamous cell, lung | 0/20 |
| Ca squamous cell, esophagus | 0/8 |
| Ca squamous cell, larynx | 0/24 |
| Ca squamous cell, uterine cervix | 0/12 |
| Ca transitional cell, urinary bladder or renal pelvis | 0/21 |
| Ca undifferentiated large cell, lung | 1/42 |
| Ca, Merkel cell | 0/4 |
| Ca thyroid, anaplastic | 0/13 |
| Ca thyroid, follicular | 0/12 |
| Ca thyroid, medullary | 0/7 |
| Ca thyroid, papillary | 0/14 |
| Carcinoid, intestinal/Islet cell tumor | 0/14 |
| Ca, undifferentiated, small intestine | 0/9 |
| Malignant mesothelioma, pleura/peritoneum | 1/27 |
| Mixed tumor/myoepithelioma | 0/31 |
| Embryonal carcinoma, testis | 0/8 |
| Seminoma, testis | 0/10 |
| Wilms tumor | 0/16 |
| Total | 32/657 |
Among the exceptional non-prostatic epithelial malignancies showing ERG-immunoreactivity were one pulmonary large cell undifferentiated carcinoma (1/42) and one pleural epithelial, tubulopapillary mesothelioma (1/27), each of which showed nuclear ERG-immunoreactivity in sporadic tumor cells (Fig. 5D).
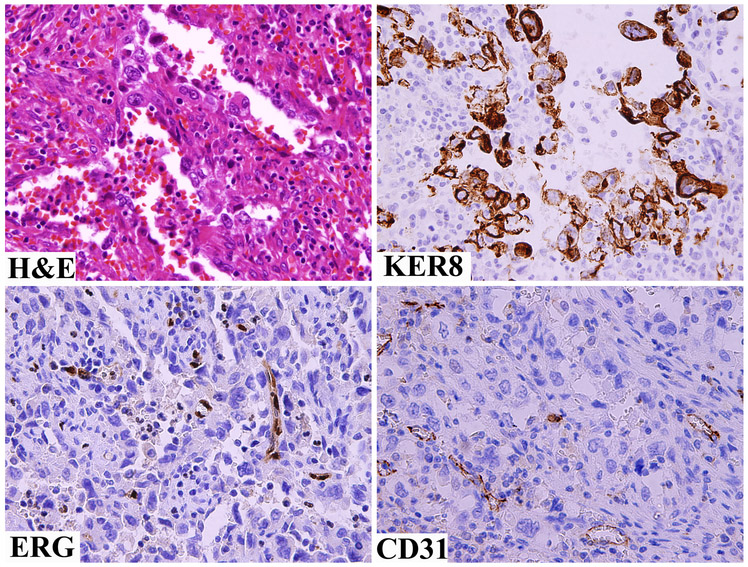
Cytoplasmic ERG-immunoreactivity without nuclear positivity was also seen in some non-endothelial tumors. Most commonly this was observed in gastrointestinal stromal tumors, and occasionally in carcinomas, especially ductal carcinoma of breast and thyroid papillary carcinoma with the latter showing membrane staining. ERG-expression was absent in the neoplastic cells of hemorrhagic poorly differentiated or neuroendocrine carcinomas that histologically sometimes simulate angiosarcoma (Fig 6).
Fig 6.
A hemorrhagic poorly differentiated carcinoma simulating an angiosarcoma is negative for both ERG and CD31, but is positive for keratin 8.
DISCUSSION
In this study we evaluated a large number of normal tissues and vascular and non-vascular mesenchymal tumors, other non-epithelial tumors, and various carcinomas in order to examine the potential of ERG transcription factor as an immunohistochemical marker, based on its expression in endothelial cells, as noted in animal models (sea urchin1 and mouse20,33), and human endothelial cell lines10, and tissues. 6,21 We also explored ERG as a tissue type-specific marker for prostate carcinoma among carcinomas, because nearly 50% of prostate carcinomas are known to harbor TMPRSS2-ERG gene fusion translocations and express ERG. 6,21
Our findings demonstrate ERG as a highly specific vascular endothelial marker in normal adult tissues. However, in adult bone marrow it is also expressed in some myeloid precursor cells, probably including marrow stem cells known to express ERG.15 In addition, in human fetal tissue, ERG is additionally expressed in subsets of primitive mesenchymal cells subsequently being restricted to peripheral portions of cartilage and perichondrial mesenchyme in the late first trimester. This seems to mirror ERG distribution observed in mouse embryos by RNA in-situ hybridization33 and immunohistochemical studies. 20 Nevertheless, we were not able to find ERG-expression in neoplastic cartilage in conventional, mesenchymal, dedifferentiated, or extraskeletal myxoid chondrosarcomas.
Based on our observations, ERG-immunoreactivity is consistently present in endothelial components of various hemangiomas. The presence of a dual cell population, ERG positive endothelial cells and ERG-negative non-endothelial components (especially, pericytes), could be helpful to support the diagnosis of hemangioma over angiosarcoma in some instances, especially very cellular hemangiomas. In contrast, angiosarcoma generally has ERG-positive endothelial components only. This is similar to application of smooth muscle actin immunohistochemistry to detect complete, pericyte-positive vascular differentiation in hemangioma vs. angiosarcoma; the latter does not typically contain a smooth muscle actin-positive pericytic component. 9
ERG is also conserved in malignant vascular endothelial neoplasms (hemangioendotheliomas, angiosarcomas, and Kaposi sarcoma) to be a new promising marker for these tumors. The staining is usually seen in a great majority of tumor cells indicating that expression of ERG transcription factor in endothelial cells and angiosarcomas is an all or none phenomenon. ERG is also useful in separating angiosarcomas and epithelioid hemangioendotheliomas from their histologic mimics, such as non-endothelial tumors with corded, myxohyaline, and hemorrhagic, highly vascular patterns. In angiosarcomas and related tumors, ERG-expression may be a constitutional phenotypic feature unrelated to ERG gene rearrangements, although a possible oncogenic role or ERG in vascular tumors cannot be ruled out. The ubiquitous expression of ERG in endothelial tumors necessitates the use of other markers to differentiate between various tumor types, such as angiosarcoma and Kaposi sarcoma.
ERG compares favorably with presently widely used endothelial markers, including CD31, the current gold standard in the definition of angiosarcoma. Due to its global but highly endothelium-restricted expression, ERG immunoreactivity is straightforward to interpret. In contrast, interpretation of CD31 can be problematic considering its presence in hematopoietic-derived cells, especially tissue histiocytes and plasma cells, which can be diagnostic pitfall leading into overdiagnosis of angiosarcoma. 17,18 Also, the presence of CD31 in platelets can cause diffuse immunoreactivity in areas of hemorrhage and necrosis, complicating the interpretation of CD31 immunostaining in tumor cells. 26
In view of immunohistochemical complexity of many angiosarcomas, including common expression of some simple epithelial (“low molecular weight”) keratins19, ERG is helpful in the distinction of hemorrhagic, poorly differentiated and neuroendocrine carcinomas from angiosarcomas. In our experience, these tumors were occasionally incorrectly diagnosed as angiosarcomas prior to use of current immunohistochemical markers. Non-endothelial vascular related tumors, such as glomus tumor, hemangiopericytoma/solitary fibrous tumor, and cerebellar hemangioblastoma were uniformly negative for ERG, except for endothelial expression, supporting non-endothelial phenotype of these tumors. In some cases, highly vascular hemangiopericytomas can resemble angiosarcomas, so that ERG-immunohistochemistry can be useful in this distinction.
With a few exceptions, ERG is highly specific for vascular endothelial neoplasms. We tested a wide range of tumors, including entities with known ERG-fusion translocatons: Ewing sarcoma, acute myeloid leukemia, and myxoid liposarcomas. Blastic extramedullary myeloid tumors (acute myeloid leukemia infiltrates/tissue relapses) showed a high frequency of immunohistochemical ERG-positivity (7/10). A subset of acute myeloid leukemias (AML) contains ERG-involving gene fusions12 and express ERG (as measured by mRNA content), and these especially include poor prognosis AMLs. 16 Such aggressive tumors could be overrepresented among the ERG-positive blastic extramedullary myeloid tumors. The high frequency of ERG-expression in blastic extramedullary myeloid tumors (AML tissue infiltrates) may alternatively indicate that such expression is not restricted to ERG-involving translocation cases. The expression of ERG in normal hematopoietic stem cells also suggests this possibility.15 In our study, genetic correlation for blastic extramedullary myeloid tumors was not available.
Among Ewing sarcomas, 2 of 29 cases were positive. This frequency matches with the known approximately 10% frequency of EWSR1-ERG gene fusion in Ewing sarcoma. 8,14 , although ERG-fusion status was not known in our cases. ERG involving gene fusions have been also detected in a rare subset of myxoid liposarcoma. 25 Thus, ERG immunoreactivity would be expected to be detectable in some examples, although it was not found in our relatively limited number of cases studied.
The most notable ERG-expressing non-vascular, non-mesenchymal tumor is prostate carcinoma. In this study: 30/66 cases were positive, including regional and distant nodal metastases. This mirrors two recent studies, in which nearly 50% of prostate cancers were found to contain ERG-immunoreactivity, specifically including prostate carcinomas with TMPRSS2-ERG gene fusions that lead to overexpression of ERG. 6,21
Based on our observations of ERG-immunoreactivity in 2/549 (0.4%) non-prostatic epithelial malignancies of different types and sites, ERG-expression is highly restricted to prostate carcinoma among epithelial cancers. It is conserved in metastases, as previously shown in regional lymph nodes6 and sufficiently prevalent (approximately 50%) to serve as a useful immunohistochemical marker to explore the possible prostatic origin of a metastatic carcinoma, in a manner similar to other transcription factors, TTF1 for pulmonary carcinoma and CDX2 in gastrointestinal carcinoma. Our findings also support previous observations of restriction of ERG-immunoreactivity to prostatic carcinoma vs. non-neoplastic epithelia6, so that ERG could also serve as a “malignancy marker” in prostate biopsies; additional studies are warranted in this respect.
The very occasional non-prostatic, ERG-positive epithelial malignancies included one pulmonary large cell carcinoma and one epithelial mesothelioma, each with only focal ERG expression. The significance of these observations remains unclear, but possible mechanisms could include sporadic ERG-involving translocations or other gene rearrangements inducing ERG overexpression in non-prostatic carcinomas. ERG-involving translocations have also been detected in uterine cervical carcinoma lines.29 However, in this study, we were not able to find ERG immunoreactivity in cervical squamous cell carcinomas, suggesting that ERG expression is not a common finding in these tumors in vivo.
In conclusion, ERG transcription factor shows a conserved expression and narrow tissue distribution in both benign and malignant vascular endothelial cells, and is therefore a promising new marker in the identification of angiosarcoma, Kaposi sarcoma, and hemangioendotheliomas. On the other hand, the common presence of ERG in prostatic carcinoma and its extremely rare expression in other epithelial malignancies makes it a suitable marker in the search of possible prostatic origin for a metastatic carcinoma. On the other hand, absence of ERG in non-neoplastic prostate tissue indicates potential for ERG as a marker for prostatic malignancy. ERG expression in other tumors, such as blastic extramedullary myeloid tumor/acute myeloid leukemia, Ewing sarcoma and occasional carcinomas and mesothelioma, must be recognized and mitigated by the use of appropriate immunohistochemical panels in the differential diagnosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Baltzinger M, Mager-Heckel AM, Remy P. XI erg: Expression pattern and overexpression during development plead for a role in endothelial cell differentiation. Dev Dyn 1999;216:420–433. [DOI] [PubMed] [Google Scholar]
- 2.Birdsey GM, Dryden NH, Amsellem V, et al. Transcription factor erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood 2008;111:3498–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999;154:385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Young BR, Wick MR, Fitzgibbon JF, et al. CD31: An immunospecific marker for endothelial differentiation in human neoplasms. Appl Immunohistochem 1993;1:97–100. [Google Scholar]
- 5.Folpe AL, Chand EM, Goldblum JR, et al. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol 2001;25:1061–1066. [DOI] [PubMed] [Google Scholar]
- 6.Furusato B, Tan SH, Young D, et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate cancer Prostatic Dis 2010;13:228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fury MG, Antonescu CR, van Zee KJ, et al. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J 2005;11:241–247. [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg JP, de Alava E, Ladanyi M, et al. EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing’s sarcoma. J Clin Oncol 1999;17;1809–1814. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Crussi F, Reyes-Mugica M. Cellular hemangiomas (“hemangio-endotheliomas”) in infants. Light microscopic, immunohistochemical, and ultrastructural observations. Am J Surg Pathol 1991;15:769–778. [PubMed] [Google Scholar]
- 10.Hewett PW, Nishi K, Daft EL, et al. Selective expression of erg isoforms in human endothelial cells. Int J Biochem Cell Biol 2001;33:347–355. [DOI] [PubMed] [Google Scholar]
- 11.Itakura E, Yamamoto H, Oda Y, et al. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol 2008;97:74–81. [DOI] [PubMed] [Google Scholar]
- 12.Kong XT, Ida K, Ichikawa H, et al. Consistent detection of TLS/FUS-ERG chimeric transcripts in acute myeloid leukemia with t(16;21)(p11;q22) and identification of a novel transcript. Blood 1997;90:1192–1199. [PubMed] [Google Scholar]
- 13.Kuzu I, Bicknell R, Harris AL, et al. Heterogeneity of vascular endothelial cells with relevance to diagnosis of vascular tumours. J Clin Pathol 1992;45:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladanyi M The emerging molecular genetics of sarcoma translocations. Diagn Mol Pathol 1995;4:162–173. [DOI] [PubMed] [Google Scholar]
- 15.Loughran SJ, Kruse EA, Hacking DF, et al. The transcription factor Erg is essential for definitive hematopoiesis and the function of adult hematopoietic stem cells. Nat Immunol 2008;9:810–819. [DOI] [PubMed] [Google Scholar]
- 16.Marcucci G, Baldus CD, Ruppert AS, et al. Overexpression of the ETS-related gene, ERG, predicts a worse outcome in acute myeloid leukemia with normal karyotype: A cancer and leukemia group B study. J Clin Oncol 2008;23:9234–9242. [DOI] [PubMed] [Google Scholar]
- 17.McKenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol 2001;25:1167–1173. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens - Evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand’s factor. Mod Pathol 1994; 7:82–90. [PubMed] [Google Scholar]
- 19.Miettinen M, Fetsch JF. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: implications in tumor diagnosis. Hum Pathol 2000;31:1062–1067. [DOI] [PubMed] [Google Scholar]
- 20.Mohamed AA, Tan SH, Mikhalkevich N, et al. Ets family protein, Erg expression in developing and adult mouse tissues by a highly specific monoclonal antibody. J Cancer 2010;1:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park K, Tomlins SA, Mudaliar KM, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia 2010;12:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer 1999;86:2406–2412. [PubMed] [Google Scholar]
- 23.Ramani P, Bradley NJ, Fletcher CDM. QBEND/10, a new monoclonal antibody to endothelium: assessment of its diagnostic utility in paraffin sections. Histopathology 1990;17:237–242. [DOI] [PubMed] [Google Scholar]
- 24.Rossi S, Orvieto E, Furlanetto A, et al. Utility of the immunohistochemical detection of Fli-1 expression in round cell and vascular neoplasms using a monoclonal antibody. Mod Pathol 2004;17:547–552. [DOI] [PubMed] [Google Scholar]
- 25.Panagopoulos I, Lassen C, Isaksson M, et al. Characteristic sequence motifs at the breakpoints of the hybrid genes FUS/CHOP, EWS/CHOP, and FUS/ERG in myxoid liposarcoma and acute myeloid leukemia. Oncogene 1997;15:1357–1362. [DOI] [PubMed] [Google Scholar]
- 26.Pustztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 2006;54:385–395 [DOI] [PubMed] [Google Scholar]
- 27.Sato Y Role of ETS family transcription factors in vascular development and angiogenesis. Cell Structure and Function 2001;26:19–24. [DOI] [PubMed] [Google Scholar]
- 28.Schacht V, Dadras SS, Johnson LA, et al. Up-regulation of the lymphatic marker podoplainin, a mucin-type transmembrane glycoprotein in human squamous cell carcinomas and germ cell tumors. Am J Pathol 2005;166:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson S, Woodworth CD, DiPaolo JA. Altered expression of Erg and Ets-2 transcription factors is associated with genetic changes at 21q22.2-22.3 in immortal and cervical carcinoma cell lines. Oncogene 1997;14:2149–2157. [DOI] [PubMed] [Google Scholar]
- 30.Sun S, Dobi A, Mohamed A, et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene 2008;27:5348–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traweek ST, Kandalaft P, Mehta P, et al. The human progenitor cell antigen (CD34) in vascular neoplasia. Am J Clin Pathol 1991;96:25–31. [DOI] [PubMed] [Google Scholar]
- 32.van de Rijn M, Rouse RV. CD34 - A review. Appl Immunohistochem 1994;2:71–80. [Google Scholar]
- 33.Vlaeminck-Guillem V, Carrere S, Dewitte F, et al. The Ets family member Erg gene is expressed in mesodermal tissues and neural crests as fundamental steps in mouse embryogenesis. Mech Dev 2000;91:331–335. [DOI] [PubMed] [Google Scholar]