Highlight
-
•
This study adds information about forecasting demand, procurement, storage, for rabies biologics in Vietnam, a canine rabies endemic country.
Abbreviations: DPMC, District Preventative Medicine Centers; eRIG, equine rabies immunoglobulin; Gavi, Gavi the Vaccine Alliance; ID, intramuscular; IM, intradermal; PEP, post-exposure prophylaxis; PPMC, provincial preventative medicine centers; WHO, World Health Organization
Keywords: Rabies, Canine, Human, Vaccine, Post-exposure prophylaxis, Immunoglobulin, Vietnam
Abstract
Background
Canine-mediated human rabies deaths typically occur in poor and rural populations with limited access to rabies biologics: vaccine and immunoglobulin. A critical aspect of reducing rabies deaths is understanding how these countries procure, deliver, and forecast rabies biologics. Vietnam is one of the few endemic countries where biologics is widely available. However, a formal evaluation of its current rabies biologics distribution system has not been conducted.
Methods
In 2017, we conducted a formal evaluation of Vietnam’s rabies biologics distribution system. Our goals were (1) to identify centers providing rabies biologics (2) identify costs to the patient and centers and (3) assess the rabies biologic procurement and delivery system at eligible district and provincial centers (provides and orders biologics for itself and other centers directly from the manufacture). To conduct the formal evaluation, we developed a standardized survey that was distributed to centers.
Results
Of the 780 designated rabies biologics centers in Vietnam, 659 (84%) of them provide rabies immunoglobulin (eRIG), vaccine, or both. Of the 177 eligible centers, 90% (160) responded to the survey. The average costs to patients were $8.45 (range: 5.43–12.77) for one dose of IM injection, $13.90 (range: 11.86–16.71) for domestic eRIG, and $23 (21.11–27.11) for imported eRIG. Respondents reported experiencing delays in receiving vaccine in 50 centers and eRIG in 14 centers within the past year. Respondents stated their top three challenges in providing biologics were: delays or shortages from manufactures, lack of funds to pay for biologics, and the high cost of biologics.
Conclusions and relevance
Despite the wide availability of biologics in Vietnam, more work is needed to provide affordable and reliable supply of biologics to patients. This includes the expansion of ID injection use throughout the country to lower vaccine demand, and decrease the costs to centers and patients. Furthermore, a more coordinated effort to share biologics among centers, possibly through a more centralized system at the provincial level may alleviate delays and shortages.
1. Introduction
Each year, an estimated 59,000 people die from rabies worldwide [1]. Canine-endemic countries in Africa and Asia account for 96% of these deaths [2]. Deaths are preventable with post-exposure prophylaxis (PEP) consisting of wound treatment and biologics (immunoglobulin, vaccine) [2]. Unfortunately, these deaths typically occur in poor and rural populations with limited access to biologics [3], [4], [5]. Victims often encounter high out of pocket cost, long travel distances to PEP centers, or stock-outs when at a health facility [2], [6], [7], [8].
Many of these barriers could be addressed in low-income countries if rabies biologics were included into Gavi, the Vaccine Alliance’s investment portfolio. Attempts have been made for this inclusion in 2008 and 2013, however an investment was not approved due to the limited published data available for their decision making process [6], [9]. To rectify this, Gavi commissioned studies in low-income and canine-endemic countries to fill these data gaps [10]. Some of the major gaps in knowledge were how countries procure, distribute, and deliver PEP [10], [11]. Understanding this process will allow better global prediction of biologics needed to fulfill requests from countries [11].
Through the Gavi Learning Agenda and in conjunction with the World Health Organization’s (WHO) Neglected Tropical Diseases Department, some of this work was conducted in Vietnam, one of the few rabies endemic countries in Southeast Asia where PEP is widely available. Currently, animal-bite victims can receive care at any commune, district or provincial health center but are responsible for PEP-associated costs. Investment by the government and international partners for rabies prevention and control has played a substantial role in reducing human rabies deaths from 404 cases in 1992 to 91 cases in 2016 [12]. Since 1992, the country vaccinates an average of 439,692 patients per year [12]. However, while Vietnam has demonstrated its ability to provide biologics, a formal evaluation of the current biologics distribution system has not been conducted. In this report, we share findings from a formal evaluation conducted in 2017. The aims for the evaluation were (1) to identify centers providing rabies biologics and (2) assess the rabies biologic procurement and delivery system at eligible district and provincial centers (provides and orders biologics for itself and other centers directly from the manufacturer).
2. Methods
2.1. Vietnam PEP delivery system
In Vietnam, animal-bite treatment is divided across two health systems: curative and preventative. The curative health system provides wound treatment at district and provincial hospitals [13]. Patients receiving treatment requiring complex procedures such as major reconstructive surgery are referred to regional or national referral hospitals. Patients with a rabies risk are then referred to centers that fall under the preventive health system for rabies biologics. In 2017, there were 780 centers: 4 Regional Institutes (RIs), 63 Provincial Preventative Medicine Centers (PPMCs), and 713 District Preventative Medicine Centers (DPMCs). In Vietnam, national guidelines stipulate that vaccine must be available in every district, equine immunoglobulin (eRIG) does not fall under these guidelines [13]. eRIG is typically offered at RIs and PPMCs; however, some DPMCs offer eRIG as well. To reduce duplication of efforts, not all centers located in the same district offer biologics. For example, a PPMC may not provide eRIG or vaccine because a RI located in the same district is already providing biologics. Prior to this evaluation, it was unclear how many of the 780 centers provided biologics, and the type of biologics (vaccine, eRIG, or both) they provided.
2.2. Biologics
Vietnam adheres to WHO’s recommended PEP regimen and currently offers three imported cell culture vaccines and eRIG. Two are WHO prequalified vaccines (Verorab® and Rabipur®) and one is not (Abhayrab®) [14]. The majority of centers provide intramuscular regimen (IM; Essen 5-dose regimen schedule). A small minority of centers provide both IM and intradermal regimens (ID; Updated Thai Red Cross schedule).
At the time of this evaluation, both imported and domestically produced eRIG were available in country. The four RIs and a minority of PPMCs order and secure funding for biologics for their own centers. The majority of PPMCs order biologics for their own centers and for the DPMCs located in their province. Each center responsible for securing their own funding. In Vietnam, patients are responsible for cost associated with PEP, therefore, they are able to choose any center (at any administrative level) to initiate and continue the PEP regimen.
2.3. Sampling frame
This evaluation focused on biologics (eRIG and vaccine) therefore we only surveyed PPMCs and DPMCs in the preventative health system. We did not survey RIs because they function autonomously (do not order biologics for other administrative levels).
To assess the procurement and availability of rabies biologics, we contacted all PPMCs to ask whether they provided biologics. Among centers that provided biologics, we requested the eligible PPMCs (those that ordered biologics for itself or for itself and other centers) to complete our survey. We also asked the PPMCs to provide the names of DPMCs in their province that ordered directly from manufacturers. Second, we requested DPMCs that ordered directly from the manufacturer (we will refer to them as “independent DPMCs” in the remainder of this study) to complete the survey.
2.4. Survey, data, and data analysis
We mailed and emailed the same surveys to the PPMCs and independent DPMCs. Our survey collected qualitative and quantitative data regarding: (1) rabies biologics used at the centers and their associated costs to the centers and patients; (2) procurement and forecasting of biologics; (3) delivery and storage of biologics; and (4) challenges the centers may have experienced within the past year and initiatives implemented to address them (free text response). Free text responses were transformed into quantitative data by grouping them into themes. We attempted to address questionable or missing responses by phoning the person filling out the survey directly. Despite our attempts to contact all respondents, some centers had missing data.
Epi Info 7 and SPSS version 16.0 were used to collect and manage data. Data were computed as numbers, percentages, and mean ± standard deviation.
3. Results
3.1. Centers providing biologics
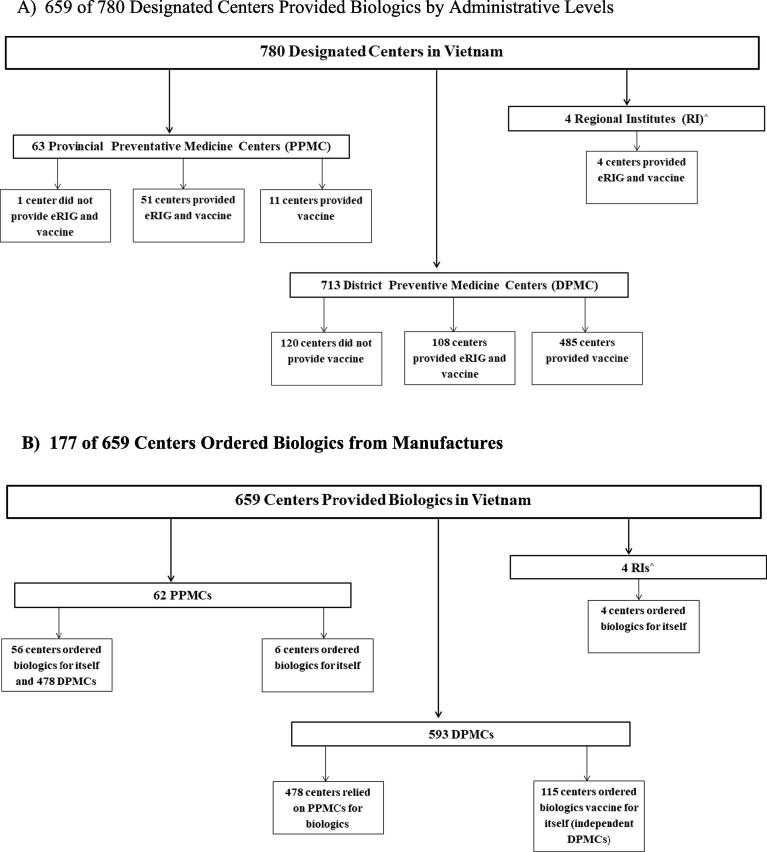
There were 780 designated centers in Vietnam: four RIs, 63 PPMCs, and 713 DPMCs (Fig. 1, Panel A). In total, 659 (84.4%) centers in Vietnam provide eRIG, vaccine, or both. Of the 63 PPMCs, 51 (80.9%) provided vaccine and eRIG, eleven (17.5%) provided vaccine, and one did not provide either vaccine or eRIG. (This DPMC is located in the same district as a RI where biologics are already available.) Of the 713 DPMCs, 108 (15.1%) provided vaccine and eRIG, 485 (68%) provided vaccine, and 120 (16.8%) did not provide either vaccine or eRIG. We identified 51 of 713 districts that did not have a center that provide vaccines. Centers ordering biologics
Fig. 1.
Designated Centers Providing and Ordering Rabies Biologics in Vietnam, 2017. (A) 659 of 780 Designated Centers Provided Biologics by Administrative Levels. (B) 177 of 659 Centers Ordered Biologics from Manufactures.
Among the 659 centers that provided biologics in Vietnam, six PPMCs and four RIs ordered biologics for their centers (Fig. 1, Panel B). Fifty-six (90.3%) PPMCs ordered biologics for their centers and 478 (88.5%) DPMCs located in their provinces. Based on these findings, we identified 115 independent DPMCs responsible for ordering their own biologics directly from the manufacturers.
3.2. Survey participation and representativeness
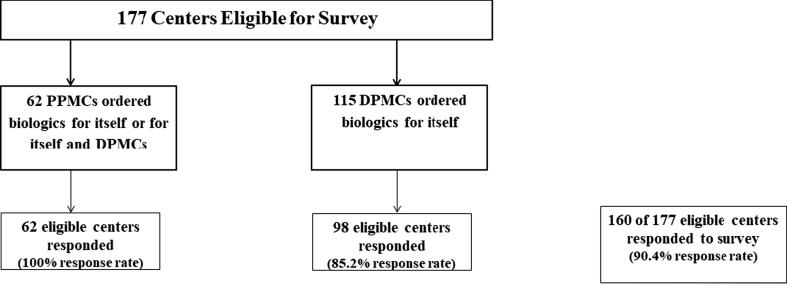
We achieved a 90.4% (160) survey response rate among eligible centers: 62 PPMCs and 98 of 115 independent DPMCs (Fig. 2). Overall, our evaluation represented information for 96.8% (638) of the eligible centers nationwide; these 638 centers covered approximately 66 million (71%) people in Vietnam. This included 62 PPMCs and 478 DPMCs that relied on PPMCs for biologics and 98 independent DPMCs.
Fig. 2.
Survey Response of Eligible Centers Providing and Ordering Biologics.
3.3. Biologics and their associated cost
Of the 160 centers that participated in the survey, all of them provided vaccines and 78 provided eRIG. Among centers that provided vaccine, 95 (59.4%) provided two or more brands of rabies vaccine (Table 1). Forty-one (25.6%) centers provided both IM and ID regimens. Among the 78 centers, 61.5% (48) provided domestically produced eRIG.
Table 1.
Rabies Vaccine and Equine Immunoglobulin in Vietnam, 2017.
| Vaccine | Equine Immunoglobulin (eRIG) | ||
|---|---|---|---|
| Characteristics | Characteristic | ||
| Brand N = 160 | No. (%) | Type N = 78 | No. (%) |
| Abhayrab only | 50 (31.3) | Domestic only | 48 (61.5) |
| Verorab only | 15 (9.4) | Imported only | 6 (7.7) |
| Abhayrab, Verorab | 78 (48.8) | Both Domestic and imported | 24 (30.8) |
| Abhayrab, Verorab and Rabipur | 17 (10.6) | ||
| Route of administration N = 160 | |||
| Intramuscular only | 119 (74.4) | ||
| Intramuscular and intradermal | 41 (25.6) | ||
| Abhayrab*n = 125 | Average (Range) | Domestic eRIG+ n = 53 | Average (Range) |
| Purchase price from manufactures | $7.01 (6.24–8.88) | Purchase price from manufactures | $13.90 (11.86–16.71) |
| Cost to patient at centers for IM injection | $8.16 (6.46–12.77) | Cost to patient at centers | $16.37 (13.46–21.99) |
| Cost difference | $1.17 (0.28–3.89) | Cost difference | $ 2.46 (0.22–10.13) |
| Rabipur*n = 6 | Imported eRIG+ n = 18 | ||
| Purchase price from manufactures | $7.60 (6.16–9.23) | Purchase price from manufactures | $23.00 (21.11–27.11) |
| Cost to patient at centers for IM injection | $8.85 (5.43–11.43) | Cost to patient at centers | $25.64 (23.34–30.78) |
| Cost difference | $1.28 (0.31–3.87) | Cost difference | $ 2.64 (0.44–7.92) |
| Verorab*n = 96 | |||
| Purchase price from manufactures | $6.89 (6.46–7.04) | ||
| Cost to patient at centers for IM injection | $8.25 (7.10–9.10) | ||
| Cost difference | $1.36 (0.64–2.22) | ||
Notes: N = 160 medical centers that participated in the survey and provided eRIG, vaccine, or both. Due to rounding, percentages may not equal 100%. $1 USD = 22,740VND.
Price per vial or IM injection. +Price per 5 mL vial, patients pay for entire vial.
The average costs to patients (in USD) was $8.45 (range: 5.43–12.77) for one dose of IM regimen, $13.90 (range: 11.86–16.71) for domestic eRIG, and $23 (21.11–27.11) for imported eRIG. The average cost of the 5-dose IM regimen to the patients was $40.08 (32.30–63.85) for Abhayrab®, $44.25 (27.15–57.15) for Rapipur®, and $41.25 (35.50–45.50) for Verorab® (Table 1). The average price difference between the vaccine purchase price from manufacturer and cost to the patient for a complete course of vaccination were $5.85 (1.40–19.45) for Abhayrab®, $6.40 (1.55–19.35) for Rapipur®, and $6.80 (3.2–11.10) for Verorab®. The average cost for the ID regimen was not collected. The average cost of a 5 mL vial to the patients were $16.37 (13.46–21.99) for domestically produced eRIG and $25.64 (23.34–30.78) for imported eRIG. The difference between the eRIG purchase price from the manufacturer and cost to the patient per 5 mL vial were $2.46 (0.22–10.13) for domestically produced eRIG, and $2.64 (0.44–7.92) for imported eRIG. In Vietnam, patients are required to purchase the entire vial. On average, a patient in Vietnam requires two vials.
3.4. Procurement and forecasting
Of the 160 centers providing vaccine, 146 (91.3%) used internal operational funds to pay for vaccine, nine (5.6%) used funding from the local government, and five (3.1%) used loans from the manufacturer or center staff (manufacturers provided loans to the centers for the procurement of biologics or staff pooled their personal funds to procure biologics and were reimbursed by the center at the end of the month.) (Table 2). Seventy-nine (69.3%) of 114 centers reported to have ≥ four weeks to pay for the vaccine after it was delivered. Eight-seven (54.4%) of 160 centers reported to have ordered vaccine as needed and 21(13.1%) reported to have ordered weekly. Ninety-four (60.2%) of 156 centers reported to have received their vaccine the same day or next day. Ninety-five (59.4%) of 160 of centers reported the demand of the vaccine has increase over the past three years. Ninety-four (58.8%) of 160 centers forecasted their vaccine by the number of patients they see monthly or yearly. Factors that affect their purchase and forecasting were demand by other centers (31.3%) and vaccine shortage (26%). Given that some PPMCs order for their DPMCs, their forecast is based other center’s demands for biologics.
Table 2.
Rabies Biologics Procurement and Forecasting in Vietnam, 2017.
| Vaccine | Equine Immunoglobulin (eRIG) | |
|---|---|---|
| Characteristics | n (%) | n (%) |
| Funding for biologics | N = 160 | N = 78 |
| Internal funds | 146 (91.3) | 62 (79.5) |
| From local government funds | 9 (5.6) | 0 (0.0) |
| Internal and local government funds | 0 (0.0) | 6 (7.7) |
| Loan from company or health center staff | 5 (3.1) | 10 (12.8) |
| Payment after biologics are received | n = 114 | n = 67 |
| 1 week | 18 (15.8) | 17 (25.4) |
| 2 weeks | 6 (5.3) | 4 (6.0) |
| 3 weeks | 11 (9.6) | 7 (10.5) |
| 4 weeks | 55 (48.2) | 27 (40.3) |
| > 4 weeks | 24 (21.1) | 12 (17.9) |
| Order frequency | n = 160 | n = 78 |
| As needed | 87 (54.4) | 45 (57.7) |
| Weekly | 21 (13.1) | 10 (12.8) |
| Monthly | 42 (26.3) | 15 (19.2) |
| Quarterly or Yearly | 10 (6.25) | 8 (10.3) |
| Number of days from order placement to arrival of biologics | n = 156 | n = 78 |
| Same day | 29 (18.6) | 17 (21.8) |
| 1 day | 65 (41.7) | 26 (33.3) |
| 2 days | 27 (17.3) | 13 (16.7) |
| 3 days | 15 (9.6) | 7 (9.0) |
| 4–7 days | 18 (11.5) | 13 (16.7) |
| >7 days | 2 (1.3) | 2 (2.6) |
| Vaccine demand over the past 3 years | n = 160 | n = 78 |
| Increase | 95 (59.4) | 46 (59.0) |
| Decrease | 14 (8.8) | 7 (9.0) |
| No change | 45 (28.1) | 25 (32.1) |
| Do not know | 6 (3.8) | 0 (0.0) |
| Forecasting considerations | n = 160 | n = 78 |
| The number of patients seen monthly | 68 (42.5) | 35 (44.9) |
| The number of patients yearly | 26 (16.3) | 14 (17.9) |
| The number of patients seen monthly and yearly | 7 (4.4) | 3 (3.8) |
| Seasonality | 13 (8.1) | 0 (0.0) |
| Outbreak period | 7 (4.4) | 3 (3.8) |
| Centers order a set amount each time | 12 (7.5) | 11 (14.1) |
| Combination of the number of patients, outbreak period, seasonality | 27 (16.9) | 12 (15.4) |
| Factors affecting purchase and forecasting | n = 160 | n = 78 |
| Demand by other centers | 50 (31.2) | 24 (30.8) |
| Shortage | 39 (24.4) | 16 (20.5) |
| Price | 25 (15.6) | 14 (17.9) |
| Payment method | 6 (3.8) | 2 (2.6) |
| Price and payment method | 1 (0.6) | 0 (0.0) |
| Vaccine shortage and price or payment method | 19 (11.9) | 11 (14.1) |
| Vaccine shortage and demand by other centers | 9 (5.6) | 5 (6.4) |
| Combination of vaccine shortage, price, payment methods, and demand by other centers | 11 (6.9) | 6 (7.7) |
Notes: Due to rounding, percentages may not equal 100%. N = 160 eligible centers participated in the survey. All 160 centers provided vaccine and 78 of the 160 centers provided eRIG. Some centers did not respond to all of the questions, therefore denominators are provided. Seasonality is defined as the months with more patients seeking biologics (typically in the summer months). Outbreak period is defined as when a human rabies case is reported in that district.
Of the 78 centers providing eRIG, 62 (79.5%) centers used internal funds to pay for eRIG and 10 (12.8%) used loans from the manufacturer or health facility staff and 39 (58.2%) centers reported to have at least four weeks to pay for the eRIG after it was delivered (Table 2). There were some delays in procurement among centers; however, 55.1% (41) of centers reported receiving eRIG the same or next day. Majority of centers providing eRIG (59% {46}), reported that the demand for eRIG had increase over the past three years, and 49 (62.8%) centers forecasted their need for eRIG by the number of patients they saw on a monthly or yearly basis. When examining factors that affected purchasing and forecasting of eRIG demand, 30.8% (24) of centers reported demand by other centers and 20.5% (16) reported eRIG shortages.
3.4.1. Delivery and storage
The primary mode of delivery for the biologics was by refrigerated vehicle by the vaccine company and/or courier, as reported by 86.9% (139) of centers. A majority (86.9%, {139}) of these centers stored their biologics in refrigerators dedicated for vaccines not included in the Expanded Program on Immunization (EPI). The remaining centers reported storing their biologics in refrigerators with EPI vaccines. To monitor the temperature of the biologics 86.3% (138) of centers reported using thermometers, while 5 centers (1 PPMC and 4 DPMCs) reported having trouble maintaining the correct temperature due to power outages.
3.5. Challenges and initiatives
The top three challenges regarding procurement and storage of biologics as reported by 46 centers: delays or shortages from manufacturers, lack of funds to pay for biologics, and the high cost of biologics. Among these respondents, more independent DPMCs (65%) compared to PPMCs (16%) reported these challenges.
When centers were asked what barriers they thought prevented patients from receiving vaccines, 112 centers stated: vaccine cost, distance to a center, the patient’s lack of knowledge about vaccine effectiveness, and the need for multiple visits. When centers were asked what barriers they thought prevented patients from receiving eRIG, 64 centers stated: eRIG cost, distance to a center, and the patient’s lack of knowledge about the effectiveness of eRIG. Regarding wound treatment, 105 centers stated the patient’s lack of knowledge of needing wound care as a barrier. Furthermore, 121 centers reported that hospitals needed additional training regarding wound treatment and rabies PEP options.
When asked whether any initiatives were implemented to address these barriers, 23 centers reported they conducted mass communication campaigns about rabies and rabies treatment options to the community, and 29 centers reported subsidizing rabies vaccine for patients from funds provided by the local government.
4. Discussion
Increasing the availability and use of rabies biologics is a crucial aspect of reducing human rabies deaths. Therefore, it is vital to understand how countries procure, deliver, and forecast rabies biologics. A better understanding of this process will allow for improved global prediction of rabies biologics demand and the fulfillment of biologics requests from endemic countries [11]. In this study, we examined Vietnam’s national procurement and distribution system. While Vietnam has made great strides in making biologics available to patients, we identified on-going challenges to ensuring that biologics are accessible to all patients that need them.
Rabies biologic costs to patients and distribution centers are a major concern in Vietnam and elsewhere [2], [6]. PEP can cost up to 31 days’ wage in Asia and 51 days’ in Africa [2]. In the Philippines, PEP is generally provided for free, but, a full course of PEP can cost up to $37.83 USD to a patient when government supplies are exhausted [15]. In Cambodia, rural patients pay between $8–15 USD per dose of vaccine, compared to subsidized biologics in the capital, Phnom Penh, at $12 USD for a full course and $35 USD for RIG [16]. In our study, we found that PEP could cost up to $118.07 for five doses of pre-qualified vaccine and two vials of imported eRIG. This cost could rise when discounted pricing for biologics is no longer available to Vietnam. (Though Vietnam is no longer a low income country, it has been able to retain the discounted pricing from manufacturers. Furthermore, given its lower middle income status, it is not a Gavi eligible country.)
Based on data and prior experience from Vietnam’s transition from nerve tissue to cell culture vaccines in 2006/2007, we believe the cost of biologics may play a role in decreasing vaccine uptake [17]. During that transition year, surveillance data showed an increase in the number of human rabies deaths 131 in 2007 compared to 82 in 2006) and a decrease in vaccine uptake (446,874 patients vaccinated in 2007 compared to567,173 in 2006) due, in part, because some centers and patients could not afford the cell culture vaccine [17], [18]. While we cannot attribute the deaths to the vaccine transition, we believe the higher vaccine price played a role in the availability and uptake of vaccine.
Biologic shortages and delays are another major concern [11]. An evaluation of bite centers in the Philippines reported that some centers experienced vaccine stock-outs of up to 4 months in 2016 [15]. Our evaluation found that 18%–31% of the centers experienced shortages or delays in biologics procurement in Vietnam. While the scope of our evaluation did not focus on the implications of these shortages and delays on patient care, data from the government’s human rabies surveillance system identified one death linked to the patient’s inability to procure vaccine. In the case investigation form, family members independently reported that the patient had visited a DPMC that had experienced vaccine stock-outs at the time of their initial visit. The patient did not obtain vaccine at another center despite provider recommendation. Given the increased demand for biologics reported by many centers in our study and Vietnam’s inability to secure imported eRIG and additional WHO-prequalified rabies vaccine (since late 2017), additional rabies deaths due to shortages are anticipated in the future.
There are several strategies that could potentially mitigate the impact of increased biologic costs and shortages. One strategy endorsed by the WHO is to provide rabies vaccine using ID administration; a cheaper and dose-sparing alternative to IM administration [2]. Depending on the vial size, one vial could vaccinate five patients via instead of one patient via IM administration [2]. It has been found to be cost-effective, using up to 85% less vaccine [2]. Based on our evaluation, we estimate 25.6% the centers nationwide are already providing ID administration. Expansion of ID administration is feasible given the demand for biologics exists and there is a trained workforce already familiar with ID administration. It is of interest to note that a separate study conducted in Vietnam on vaccine adherence found that 82% of ID patients completed the regimen compared to 41% of IM patients [19]. Furthermore, to address eRIG shortages, Vietnam should consider stricter enforcement of eRIG, offering it to patients with category 3 or higher risk exposure. A recent study found that 51% of patients with a category 1 and 2 wound were given eRIG unnecessarily, thus contributing to a potential shortage [19]. Lastly, our evaluation found that independent DPMCs reported more delays and challenges in paying for biologics than PPMCs. Perhaps encouraging these DPMCs to procure biologics from their PMCs would decrease delays.
Additional data from our evaluation found several important findings that warrant further investigation. First, despite MoH guidelines requiring vaccine to be available at every district, we identified 51 districts that did not follow these guidelines. Second, a majority (75.6%, {121}) of centers reported that wound treatment training and treatment options are needed in the curative health system. Given that patients present to the curative health system for wound treatment, we need to understand and address what these issues are. Informal discussions with survey respondents suggested that the curative systems provide incorrect rabies biologics recommendations (such as telling patients to get eRIG and/or vaccinated) and treated the wound incorrectly (such as suturing the wound without allowed space for eRIG to be infiltrated). Finally, five centers reported having trouble maintaining the correct temperature due to power outages. Assistance is needed to help facilities correct these issues.
Given the scope of our evaluation, there are several limitations to note. Ideally, we would have surveyed all DPMCs that ordered biologics. Secondly, while we attempted to survey all the independent DPMCs, 15% of our subset did not respond. However, further analysis showed no geographic difference (urban, rural, or peri-urban districts, and by region location) of the non-respondents. Lastly, our survey did not include questions about ID vaccine cost to the patient because at the beginning of this study we did not know how many centers provided vaccine via ID administration.
5. Conclusions
While Vietnam is among the few canine endemic rabies countries with biologics being widely available, more work is needed to provide affordable and reliable supply of biologics to patients. This includes the expansion of ID regimen use throughout the country to lower vaccine demand and decrease the costs to centers and patients. Furthermore, a more coordinated effort to share biologics among centers, possibly through a more centralized system at the provincial level may alleviate delays and shortages.
6. What is known on this subject
Appropriate use of rabies Post-Exposure Prophylaxis (PEP) can prevent rabies deaths. Canine-mediated rabies deaths typically occur in poor and rural populations that have limited access to this life-saving treatment. To address this issue, the World Health Organization (WHO) is exploring strategies to make PEP more accessible to these populations. To make PEP more accessible, procurement and forecasting data from rabies endemic countries are needed.
7. Contributors’ statement page
Huong TT. Nguyen: Conceptualized and designed the study, interpreted the data, obtained funding to acquire the data, contributed to revising of the manuscript for intellectual content, and approved the final manuscript as submitted.
Nhan DT. Le: Analyzed and interpreted the data, drafted the initial manuscript, and approved the final manuscript as submitted.
Thach N. Pham: Acquired data, contributed to revising of the manuscript for intellectual content, and approved the final manuscript as submitted.
Maho Urabe: Conceptualized and designed the study, obtained funding to acquire the data, contributed to revising of the manuscript for intellectual content, and approved the final manuscript as submitted.
Satoko Otsu: Obtained funding to acquire the data, contributed to revising of the manuscript for intellectual content, and approved the final manuscript as submitted.
Doris Afriyie: Contributed to revising of the manuscript for intellectual content, and approved the final manuscript as submitted.
Duong N. Tran: Helped acquired data, contributed to revising of the manuscript for intellectual content, and approved the final manuscript as submitted.
Huong GT. Tran: Helped acquired data, contributed to revising of the manuscript for intellectual content, and approved the final manuscript as submitted.
Hoang V. Nguyen: Conceptualized and designed the study, contributed to revising of the manuscript for intellectual content, and approved the final manuscript as submitted.
Ha T. Le: Helped acquired data, contributed to revising of the manuscript for intellectual content, and approved the final manuscript as submitted.
Cuc H. Tran: Conceptualized and designed the study, obtained funding to acquire the data, analyzed and interpreted the data, drafted the initial manuscript, and approved the final manuscript as submitted.
Funding source
This funding was supported through the Gavi Learning Agenda, Switzerland via the WHO Neglected Tropical Diseases Department, Switzerland.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
The authors have no conflicts of interest relevant to this article to disclose.
Acknowledgement
We would like to thank the Ministry of Health, Vietnam staffs for participating in our survey. We also recognize National Institute of Hygiene and Epidemiology, Vietnam, General Department of Preventive Medicine, Vietnam, and Field Epidemiology Training Program for their support of this project. We wish to thank Drs. Matthew Moore, Hien Do, Masaya Kato, Anthony Mounts, Bernadette Abela-Ridder, Lea Knopf, Miriam Shiferaw, Nandini Sreenivasan, Mary Reynolds, Brett Petersen, and others we may have missed.
References
- 1.Hampson K., Coudeville L., Lembo T., Sambo M., Kieffer A., Attlan M. Estimating the global burden of endemic canine rabies. PLoS NeglTrop Dis. 2015;9:e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization WHO Expert Consultation on Rabies. Third Report. World Health Organ Tech Rep Ser. 2018 http://www.who.int/rabies/resources/who_trs_1012/en/ [Google Scholar]
- 3.Hampson K., Dobson A., Kaare M., Dushoff J., Magoto M., Sindoya E. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS NeglTrop Dis. 2008;2:e339. doi: 10.1371/journal.pntd.0000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilde H., Lumlertdacha B., Meslin F.X., Ghai S., Hemachudha T. Worldwide rabies deaths prevention–a focus on the current inadequacies in postexposure prophylaxis of animal bite victims. Vaccine. 2016;34:187–189. doi: 10.1016/j.vaccine.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Dimaano E.M., Scholand S.J., Alera M.T., Belandres D.B. Clinical and epidemiological features of human rabies cases in the Philippines: a review from 1987 to 2006. Int J Infect Dis: IJID: Offic Public Int Soc Infect Dis. 2011;15:e495–e499. doi: 10.1016/j.ijid.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Sreenivasan N, Li A, Shiferaw M, Tran CH, Wallace RM, Blanton JD, et al. Overview of rabies post-exposure prophylaxis access, procurement and distribution in selected countries in Asia and Africa 2017-2018 Vaccine. 2018; In Press. [DOI] [PMC free article] [PubMed]
- 7.Tran C.H., Kligerman M., Lesly A.L., Etheart M.D., Adrien P., Blanton J.D. Rabies vaccine initiation and adherence among animal-bite patients in Haiti 2015. PLoS Neglect Trop Dis. 2018 doi: 10.1371/journal.pntd.0006955. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarantola A., Blanchi S., Cappelle J., Ly S., Chan M., In S. Rabies postexposure prophylaxis noncompletion after dog bites: estimating the unseen to meet the needs of the underserved. Am J Epidemiol. 2018;187:306–315. doi: 10.1093/aje/kwx234. [DOI] [PubMed] [Google Scholar]
- 9.Gavi. History of Vaccine Investment Strategy; 2013. https://www.gavi.org/about/strategy/vaccine-investment-strategy/.
- 10.Minghui R., Stone M., Semedo M.H., Nel L. New global strategic plan to eliminate dog-mediated rabies by 2030. Lancet Global Health. 2018 doi: 10.1016/S2214-109X(18)30302-4. [DOI] [PubMed] [Google Scholar]
- 11.Abela-Ridder B., Knopf L., Martin S., Taylor L., Torres G., De Balogh K. 2016: the beginning of the end of rabies? Lancet Global Health. 2016;4:e780–e781. doi: 10.1016/S2214-109X(16)30245-5. [DOI] [PubMed] [Google Scholar]
- 12.Ministry of Agriculture and Rural Development and Ministry of Health., National Program for Rabies Control and Elimination in Viet Nam in the period from 2017-2021. http://nihe.org.vn/farm/nihe/2018/02/27/35aa38a1-57c0-437d-96fc-add336992325.pdf. 2016; 2016.
- 13.Ministry of Health. Guidelines on human rabies surveillance and prevention. Hanoi: Socialist Republic of Vietnam. http://nihe.org.vn/en/news-events/guidelines-on-human-rabies-surveillance-and-prevention-in-vietnam-c12499i17959.htm?fbclid=IwAR2xYGH-7efHVhR2XNGnX9a4T1yVzg8805yX_g5lD6wSor_pg0i4t3mNUtM; 2014.
- 14.World Health Organization. Prequalified Vaccines. https://extranet.who.int/gavi/PQ_Web/; 2018.
- 15.Amparo A.C.B., Jayme S.I., Roces M.C.R., Quizon M.C.L., Mercado M.L.L., Dela Cruz M.P.Z. The evaluation of animal bite treatment centers in the Philippines from a patient perspective. PLoS ONE. 2018;13:e0200873. doi: 10.1371/journal.pone.0200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li A.J., Sreenivasan N., Siddiqi U.R., Tahmina S., Penjor K., Sovann L. Descriptive assessment of rabies post-exposure prophylaxis procurement, distribution, monitoring, and reporting in four Asian countries: Bangladesh, Bhutan, Cambodia, and Sri Lanka, 2017–2018. Vaccine. 2019;37(S1):A14–A19. doi: 10.1016/j.vaccine.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen H.T.T., Afriyie D.O., Tran C.H., Dang T.Q., Otsu S., Urabe M.I. Towards rabies control and elimation in Vietnam. OIE Scient Tech Rev. 2018;38:1. doi: 10.20506/rst.38.1.2953. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Agriculture and Rural Development (MARD), Ministry of Health (MoH). National Program for Rabies Control and Elimination in Vietnam: 2017-2021. Hanoi: MoH; 2016.
- 19.Tran C.H., Afriyie D.O., Pham T.N., Otsu S., Urabe M., Dang A.D. Rabies Post-Exposure Prophylaxis Initation and Adherence among Patients in Vietnam 2014-2016. Vaccine. 2019;37(S1):A54–A63. doi: 10.1016/j.vaccine.2019.01.030. [DOI] [PubMed] [Google Scholar]