Abstract
Background
Mutations in BRCA2 cause a higher risk of early-onset aggressive prostate cancer (PrCa). The IMPACT study is evaluating targeted PrCa screening using prostate-specific-antigen (PSA) in men with germline BRCA1/2 mutations.
Objective
To report the utility of PSA screening, PrCa incidence, positive predictive value of PSA, biopsy, and tumour characteristics after 3 yr of screening, by BRCA status.
Design, setting, and participants
Men aged 40–69 yr with a germline pathogenic BRCA1/2 mutation and male controls testing negative for a familial BRCA1/2 mutation were recruited. Participants underwent PSA screening for 3 yr, and if PSA > 3.0 ng/ml, men were offered prostate biopsy.
Outcome measurements and statistical analysis
PSA levels, PrCa incidence, and tumour characteristics were evaluated. Statistical analyses included Poisson regression offset by person-year follow-up, chi-square tests for proportion t tests for means, and Kruskal-Wallis for medians.
Results and limitations
A total of 3027 patients (2932 unique individuals) were recruited (919 BRCA1 carriers, 709 BRCA1 noncarriers, 902 BRCA2 carriers, and 497 BRCA2 noncarriers). After 3 yr of screening, 527 men had PSA > 3.0 ng/ml, 357 biopsies were performed, and 112 PrCa cases were diagnosed (31 BRCA1 carriers, 19 BRCA1 noncarriers, 47 BRCA2 carriers, and 15 BRCA2 noncarriers). Higher compliance with biopsy was observed in BRCA2 carriers compared with noncarriers (73% vs 60%). Cancer incidence rate per 1000 person years was higher in BRCA2 carriers than in noncarriers (19.4 vs 12.0; p = 0.03); BRCA2 carriers were diagnosed at a younger age (61 vs 64 yr; p = 0.04) and were more likely to have clinically significant disease than BRCA2 noncarriers (77% vs 40%; p = 0.01). No differences in age or tumour characteristics were detected between BRCA1 carriers and BRCA1 noncarriers. The 4 kallikrein marker model discriminated better (area under the curve [AUC] = 0.73) for clinically significant cancer at biopsy than PSA alone (AUC = 0.65).
Conclusions
After 3 yr of screening, compared with noncarriers, BRCA2 mutation carriers were associated with a higher incidence of PrCa, younger age of diagnosis, and clinically significant tumours. Therefore, systematic PSA screening is indicated for men with a BRCA2 mutation. Further follow-up is required to assess the role of screening in BRCA1 mutation carriers.
Patient summary
We demonstrate that after 3 yr of prostate-specific antigen (PSA) testing, we detect more serious prostate cancers in men with BRCA2 mutations than in those without these mutations. We recommend that male BRCA2 carriers are offered systematic PSA screening.
Keywords: BRCA1, BRCA2, Prostate-specific-antigen, Prostate cancer, Targeted prostate screening
Take Home Message
We demonstrate that after 3 yr of prostate-specific-antigen (PSA) testing, we detect more serious prostate cancers in men with BRCA2 mutations than those without these mutations. We recommend that male BRCA2 carriers are offered systematic PSA screening.
1. Introduction
It is well established that BRCA2 gene mutations cause a higher risk of prostate cancer (PrCa), with an estimated relative risk of 2.5–8.6-fold by age 65 yr [1], [2], and are associated with earlier-onset, clinically significant disease. A number of retrospective studies report higher rates of lymph node involvement, distant metastasis at diagnosis, and higher mortality rates in mutation carriers [3], [4], [5], [6]. Germline BRCA2 mutation status is reported to be an independent prognostic factor for poorer outcome [3]. Furthermore, tumours of BRCA2 mutation carriers with localised PrCa have been demonstrated to exhibit genomic instability more typically seen in metastatic castration-resistant PrCa [7].
There is debate about whether there is an increased risk of PrCa for BRCA1 mutation carriers, with an estimated relative risk of 1.8–3.75-fold by age 65 yr [8] and some evidence of more clinically significant disease [3], [9]; however, this warrants further research. It is hypothesised that targeted screening in BRCA1/2 carriers facilitates early detection.
The controversies of using prostate-specific antigen (PSA) screening in the general population are well documented, but PSA remains the most effective PrCa biomarker currently available [10], [11], [12]. Efforts to improve sensitivity and specificity of PSA by incorporating other biological markers, such as the 4 kallikrein (4 K) marker panel [13], [14], PrCa risk calculators [15], [16], magnetic resonance imaging (MRI) [17], [18], and genetic markers [19], [20], into screening algorithms are under evaluation.
The IMPACT study (Identification of Men with a genetic predisposition to ProstAte Cancer: Targeted screening in men at higher genetic risk and controls; http://impact.icr.ac.uk/) is an international, multicentre study evaluating targeted PrCa screening in men with BRCA1/2 mutations. IMPACT aims to evaluate the utility of PSA screening in detecting clinically significant PrCa (defined as intermediate- or high-risk disease using the National Institute for Health and Care Excellence [NICE] guidelines [21]), PrCa incidence, positive predictive value (PPV) of biopsy using a PSA threshold of 3.0 ng/ml, and tumour characteristics in order to establish whether PSA screening detects clinically significant disease in this population compared with the noncarrier control group.
An analysis of the baseline screen for nearly 2500 men enrolled in IMPACT supported the use of targeted PSA screening in BRCA1/2 mutation carriers, suggesting that screening detects a high proportion of clinically significant tumours [22]. Moreover, we have also demonstrated that PSA is more predictive of PrCa in BRCA1/2 carriers than in noncarriers [23]. It has been reported that men with germline BRCA1/ BRCA2 mutations, on active surveillance for low-risk PrCa, are at a higher risk of reclassification to higher-grade PrCa than noncarriers [24].
National Comprehensive Cancer Network guidelines advise PrCa screening to begin at 45 yr for male BRCA2 carriers, consider the same for BRCA1 carriers, and perform routine BRCA1/2 testing for men with high-risk PrCa, family history, or metastatic disease [25], [26].
The aims of this study were to evaluate the utility of PSA screening, by assessing PrCa prevalence/incidence, PPV of biopsy, and tumour characteristics. A secondary aim was to evaluate the addition of 4 K markers to the algorithm predicting biopsy outcome (full details of this analysis can be found in the Supplementary material).
2. Patients and methods
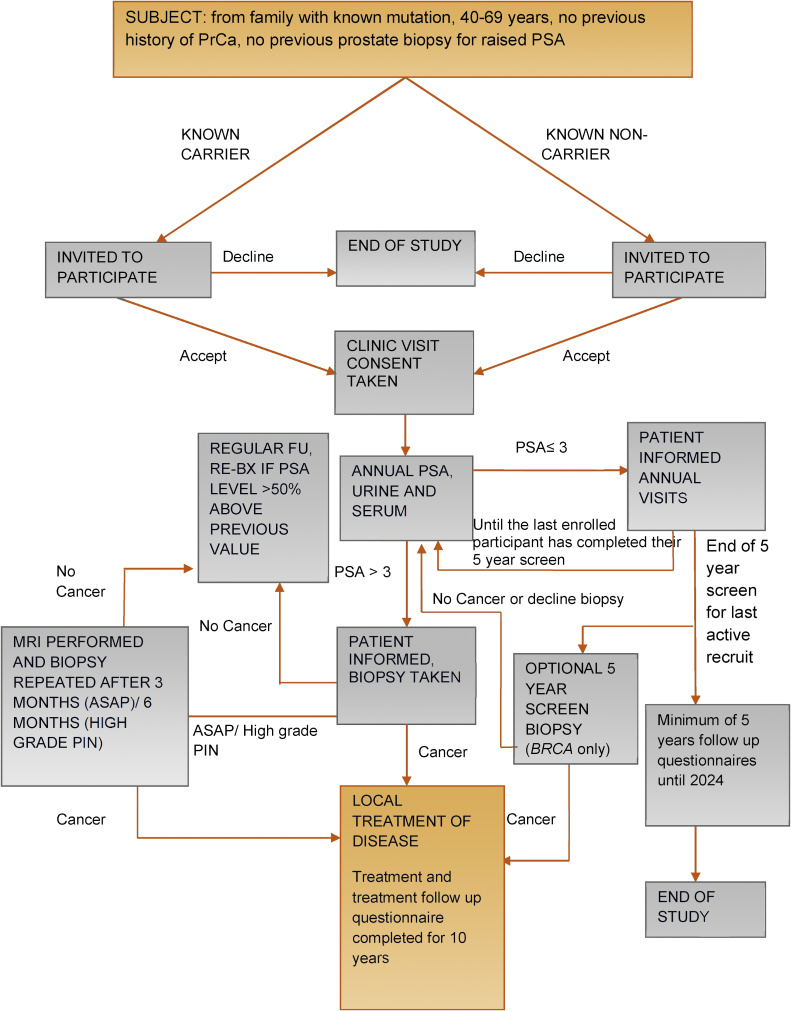
The IMPACT study design has been reported previously [22], [27], [28] and is summarised in Fig. 1. The protocol was approved by the West-Midlands Research and Ethics Committee in the UK (reference: 05/MRE07/25) and subsequently by each participating institution’s local committee. All participants provided written consent, and interim analyses are presented to the Independent Data and Safety Monitoring Committee biannually.
Fig. 1.
Study design algorithm.
ASAP = atypical small acinar proliferation; FU = follow-up; MRI = magnetic resonance imaging; PIN = prostate intraepithelial neoplasia; PrCa = prostate cancer; PSA = prostate-specific-antigen; Re-BX = repeat biopsy.
The target sample is 500 BRCA1 and 350 BRCA2 mutation carriers, and a control group of 850 men who have undergone predictive testing and tested negative for a pathogenic BRCA1/2 mutation known to be present in their family. IMPACT has been powered to detect a two-fold PrCa risk over 5 yr of screening, with 80% power at the p < 0.01 level.
Between October 2005 and February 2013, men aged 45–69 yr in The Netherlands and 40–69 yr in all other countries were recruited from families with known pathogenic BRCA1 or BRCA2 mutations. Further detail of the inclusion criteria were described previously [22], [27], [28]. Participants were screened annually in all centres except for those in The Netherlands, which screened biennially in accordance with local regulations. Recruitment was extended to December 2015, and a subset of 95 BRCA2 noncarriers was sequenced for BRCA1/2 mutations and used as the control group to cover the loss of numbers that resulted in removing The Netherlands cohort from cumulative analyses and include them as BRCA1 noncarriers.
All participants underwent annual PSA testing for four screening rounds. If PSA was >3.0 ng/ml, transrectal ultrasound–guided prostate biopsy (PB) was recommended. Decision to biopsy was based on this single PSA level; PSA was not repeated prior to biopsy unless clinically indicated. Centres were requested to follow a standard biopsy protocol, consisting of 10 and 12 biopsy cores taken from specific locations within the prostate gland. Individuals with a benign PB continued annual PSA follow-up. A repeat PB was recommended if PSA was >50% of the pre-PB PSA [29] (Fig. 1). The local histopathologist at each centre reported the biopsy results to guide treatment in accordance with local guidelines. Cancers were defined using the NICE criteria, and were deemed “clinically significant” if classified as of intermediate or high risk according to these guidelines [21]. Whenever high-grade prostate intraepithelial neoplasia or atypical small acinar proliferation was detected, the biopsy was repeated within 3–6 mo.
The IMPACT results have been compared with the Göteborg cohort of the ERPSC study. This Swedish general population cohort of men aged 50–64 yr was offered biennial PSA screening with further investigations for PSA > 3.0 ng/ml and therefore were the closest general population group available for comparison.
2.1. Statistical analysis
Statistical analyses were undertaken using Stata 14.2 StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP and GraphPad QuickCalcs Web site: https://www.graphpad.com/quickcalcs (accessed August 2019).
PrCa prevalence for individuals with PSA > 3.0 ng/ml at the first PSA test was calculated. The cumulative incidence was calculated at the fourth screening round, stratified by age group, tumour-node-metastasis stage, and Gleason score, and compared by mutation status using Poisson regression offset by person-year follow-up, adjusted for age, ethnicity, and country. Proportions of screen-detected disease and PPV of PB were compared between groups using the chi-square test. Fisher’s exact and Kruskal-Wallis tests were used to compare median age, PSA, and tumour characteristics between groups.
Analyses were performed on the whole cohort and by BRCA status. Secondary analyses were conducted excluding prevalent cancers (cancers diagnosed within <12 mo of enrolment). A p value of <0.05 was considered statistically significant.
3. Results
3.1. Study population
A total of 3027 persons (2932 unique individuals) were recruited from 65 centres in 20 countries over 120 mo (Supplementary Table 1): 919 BRCA1 carriers, 709 BRCA1 noncarriers, 902 BRCA2 carriers, and 497 BRCA2 noncarriers. Ninety-five BRCA2 noncarriers sequenced for both BRCA1/2 mutations were included in both control cohorts. The cohorts were overrecruited, as advised by the study’s Independent Data Monitoring Committee. The rationale was that overrecruitment would only strengthen the data and would compensate for any participants who withdrew from the study.
Table 1.
Prostate cancer detection rates after four rounds of screening.
| Total cohorta | Mutation status |
||||
|---|---|---|---|---|---|
| BRCA2+ | BRCA2– | BRCA1+ | BRCA1– | ||
| Baseline (“yr 1”) | |||||
| Unique individuals, n (%) | 2932 | 902 (30) | 497(16) | 919 (30) | 709 (24) |
| Total PSAs taken, n | 2931 | 902 | 497 | 919 | 708 |
| Median PSA (IQR) | 0.9 (0.6-1.5) | 0.9 (0.5-1.5) | 0.9 (0.6-1.4) | 0.9 (0.5-1.4) | 1.0 (0.6-1.7) |
| PSA > 3 ng/ml, n (%) | 228 (7.5) | 68 (7.5) | 29 (5.8) | 73 (7.9) | 61 (8.6) |
| PSA > 3 ng/ml requiring action, n | 228 | 68 | 29 | 73 | 61 |
| Biopsies, n (biopsy rate %) | 180 (79) | 56 (82) | 19 (66) | 57 (78) | 49 (75) |
| Including repeats, n | 195 | 61 | 21 | 62 | 52 |
| Benign, n | 107 | 29 | 12 | 32 | 35 |
| ASAP/HG PIN, n | 13 | 5 | 0 | 6 | 2 |
| Malignant (PrCa incidence), n (%, 95 CI) | 69 (2.4, 1.8-3.0) | 25 (2.8, 1.7-3.8) | 7 (1.4, 3.7-2.4) | 24 (2.6, 1.6-3.6) | 13 (1.8, 0.8-2.8) |
| Diff. in detection rate: BRCA + vs BRCA– (%, 95 CI) | (1.4, -0.1-2.9) | (0.8, -0.6-2.2) | |||
| p value for detection rate: BRCA + vs BRCA– | 0.10 | 0.3 | |||
| Diagnosed within 6 mo of entry, n | 65 | 25 | 6 | 22 | 12 |
| Diagnosed within 12 mo of entry, n | 68 | 25 | 7 | 23 | 13 |
| PPV of biopsy (%, 95 CI) | (35, 29-43) | (41, 29-53) | (33, 13-53) | (39, 27-51) | (25, 13-37) |
| Diff. in PPV, biopsy: BRCA + vs BRCA– (%, 95 CI) | (8, −16-31) | (14, −3-31) | |||
| p value for PPV, biopsy: BRCA + vs BRCA– | 0.5 | 0.12 | |||
| PPV of PSA > 3 ng/ml requiring action (%, 95 CI) | (30, 24-37) | (37, 25-48) | (24, 9-40) | (33, 22-44) | (21, 11-32) |
| Diff. in PPV, PSA > 3: BRCA + vs BRCA– (%, 95 CI) | (13, −7-32) | (12, −3-26) | |||
| p value for PPV, PSA > 3: BRCA + vs BRCA– | 0.2 | 0.14 | |||
| 3-yr follow-up (“yr 4”) | |||||
| Total PSAs taken, n (%) | 9363 | 3108 (32) | 1600 (16) | 2847 (29) | 2183 (22) |
| Median PSA (IQR) | 0.9 (0.6-1.5) | 0.9 (0.6-1.5) | 0.9 (0.6-1.5) | 0.9 (0.5-1.5) | 1.0 (0.6-1.7) |
| PSA > 3 ng/ml, n (%) | 695 (7.4) | 200 (6.4) | 117 (7.3) | 218 (7.7) | 182 (8.3) |
| PSA > 3 ng/ml requiring action, n (%) | 527 (5.6) | 150 (4.8) | 84 (5.3) | 138 (4.8) | 126 (5.8) |
| Biopsies, n (biopsy rate%) | 332 (63) | 110 (73) | 50 (60) | 93 (67) | 89 (71) |
| Including repeats, n | 357 | 122 | 54 | 98 | 95 |
| Benign, n | 208 | 59 | 32 | 60 | 67 |
| ASAP/HG PIN, n | 26 | 10 | 5 | 7 | 6 |
| Malignant (PrCa incidence), n (%, 95 CI) | 112 (3.8, 3.2-4.6) | 47 (5.2, 3.8-6.7) | 15 (3.0, 1.5-4.5) | 31 (3.4, 2.2-4.5) | 19 (2.7, 1.5-3.9) |
| Diff. in detection rate: BRCA + vs BRCA– (%, 95 CI) | (2.2, 0.1-4.2) | (0.7, −0.9-2.3) | |||
| p value for detection rate: BRCA + vs BRCA– | 0.057 | 0.4 | |||
| PPV of biopsy (%, 95 CI) | (31, 27-36) | (39, 30-47) | (28, 16-40) | (32, 22-41) | (20, 12-28) |
| Diff. in PPV, biopsy: BRCA + vs BRCA– (%, 95 CI) | (11, −4-25) | (12, −0.6-24) | |||
| p value for PPV, biopsy: BRCA + vs BRCA– | 0.17 | 0.065 | |||
| PPV of PSA > 3 ng/ml requiring action (%, 95 CI) | (21, 18-25) | (31, 24-39) | (18, 10-26) | (23, 16-29) | (15, 9-21) |
| Diff. in PPV, PSA > 3: BRCA + vs BRCA– (%, 95 CI) | (13, 2-25) | (7, −2-17) | |||
| p value for PPV, PSA > 3: BRCA + vs BRCA– | 0.025 | 0.13 | |||
| Prevalence of PrCa in all PSAs (%, 95 CI) | (1.2, 1-1.4) | (1.5, 1.1-1.9) | (0.9, 0.5-1.4) | (1.1, 0.7-1.5) | (0.9, 0.5-1.2) |
| Diff. in prevalence of PrCa: BRCA + vs BRCA– (%, 95 CI) | (0.6, −0.1-1.2) | (0.2, −0.3-0.8) | |||
| p value for prevalence of PrCa: BRCA + vs BRCA– | 0.10 | 0.4 | |||
| Follow-up time (yr), median (IQR) | |||||
| Noncancers | 3.0 (2.18, 3.12) | 3.0 (2.91, 3.14) | 3.0 (2.17, 3.11) | 3.0 (2.09, 3.12) | 3.0 (2.12, 3.10) |
| Cancers | 0.3 (0.12, 2.05) | 0.4 (0.18, 2.27) | 1.0 (0.12, 2.28) | 0.2(0.10, 1.12) | 0.2 (0.06, 1.43) |
| Total follow-up time, person yrs | |||||
| Noncancers | 7185 | 2371 | 1227 | 2206 | 1674 |
| Cancers | 110 | 57 | 20 | 19 | 14 |
| Cancer incidence rate (per 1000 person yrs) | 15 | 19 | 12 | 14 | 11 |
| Incidence rate ratio (crude), (95 CI) | 1.61 (0.90-2.88) | 1.24 (0.70-2.19) | |||
| IRR, adjusted for age, ethnicity, country (95 CI) (p value) | 1.95 (1.06-3.56) (0.031) | 1.36 (0.75-2.45) (0.3) | |||
ASAP = atypical small acinar proliferation; CI = confidence interval; Diff. = difference; HG PIN = high-grade prostate intraepithelial neoplasia; IQR = interquartile range; IRR = incidence rate ratio; PPV = positive predictive value; PrCa = prostate cancer; PSA = prostate-specific antigen.
A total of 95 individuals contribute to both BRCA1 and BRCA2 controls; therefore, the sum of mutation status will not match the total cohort.
The majority of participants were Caucasian (97%), highly educated, and in work (Supplementary Table 2); median enrolment age was 54 yr; 24% of men reported urinary symptoms and 36% previously had at least one PSA test; and 31% reported a family history of PrCa. No statistically significant differences were observed in sociodemographic variables, symptoms, or previous screening between groups.
Table 2.
Summary of characteristics of men who underwent biopsies in the first four screening rounds of the IMPACT study.
| Total | BRCA1+ | BRCA1– | p value | BRCA2+ | BRCA2– | p value | |
|---|---|---|---|---|---|---|---|
| Total biopsies (n) | 357a | 98 | 95 | 122 | 54 | ||
| Biopsy compliance (%) | 68 | 71 | 75 | 81 | 64 | ||
| Median PSA (ng/ml) to trigger biopsy (IQR) | 4.2 (3.5–5.6) | 4.2 (3.7–5.6) | 4.0 (3.5–4.8) | 0.1 | 4.5 (3.5–5.9) | 4.2 (3.4–6.2) | 0.8 |
| Median age (yr) at biopsy (IQR) | 61 (56–65) | 61 (56–64) | 61 (56–65) | 0.9 | 60 (56–64) | 64 (60–67) | <0.001 |
| Median time difference (d) PSA to biopsy (IQR) | 51 (27–89) | 56 (28–72) | 42 (22–79) | 0.3 | 57 (28–94) | 50 (25–87) | 0.1 |
| Median cores taken, n (IQR) | 10 (8–12) | 10 (9–12) | 10 (8–12) | 0.5 | 10 (8–12) | 10 (8–12) | 1 |
IQR = interquartile range; PSA = prostate-specific antigen.
Twelve biopsies contribute to both BRCA1 and BRCA2 controls, therefore the sum of mutation status will not match total cohort.
3.2. PrCa detection rates after 3 yr of screening and PPV of biopsy
At baseline, 2932 participants had a PSA test, 228 (7.7%) had PSA > 3.0 ng/ml, and 69 (2.4%) had cancers diagnosed from 195 biopsies.
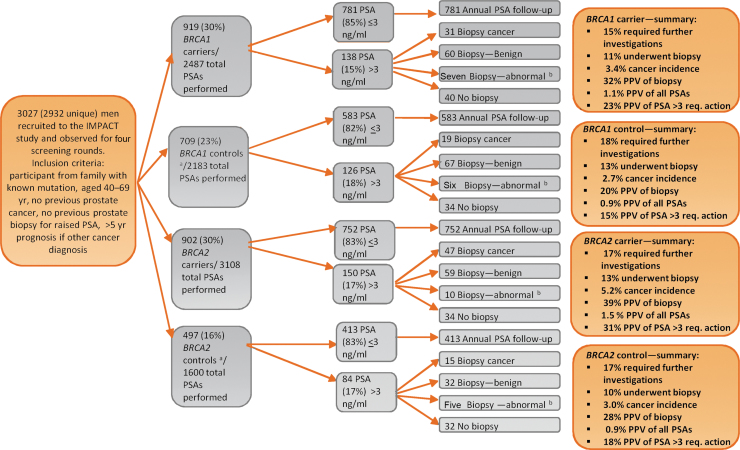
Cumulatively, after four PSA screens, 527 individuals (18%) had PSA > 3.0 ng/ml and 112 cancers diagnosed from 357 biopsies. In the BRCA2 cohort, 47 (5.2%) cancers were diagnosed in carriers and 15 (3.0%) in noncarriers; 31 cancers (3.4%) were diagnosed in BRCA1 carriers compared with 19 (2.7%) in noncarriers (Table 1 and Fig. 2).
Fig. 2.
A consort diagram of the IMPACT study after four screening rounds. ASAP = atypical small acinar proliferation; PIN = prostate intraepithelial neoplasia; PPV = positive predictive value; PSA = prostate-specific-antigen.
aControls were men who had a negative predictive genetic test for the BRCA mutation in their family.
bBiopsy—abnormal refers to high-grade PIN and ASAP.
Overall PrCa detection rate was 3.8% (112/2932), and the cancer incidence rate per 1000 person years was 15. The cancer incidence rates were 19 and 12 in BRCA2 carriers and noncarriers, respectively (incidence rate ratio [IRR] = 1.95, p = 0.031), and 14 and 11 in BRCA1 carriers and noncarriers, respectively (IRR = 1.36, p = 0.3).
Overall, PPV of PB was 31%, with 39% and 28% in BRCA2 carriers and noncarriers, respectively (p = 0.17), and 32% and 20% in BRCA1 carriers and noncarriers, respectively (p = 0.065).
The overall PPV of PSA > 3.0 ng/ml was 21%, with 31% and 18% in BRCA2 carriers and noncarriers, respectively (p = 0.025), and 23% and 15% in BRCA1 carriers and noncarriers, respectively (p = 0.13).
To compare results with the population-based Göteborg cohort [30], we restricted the IMPACT cohort to entry ages 50–64 yr (Supplementary Table 3). The Göteborg study report 2.5% PrCa incidence (confidence interval [CI]: 2.2%, 2.8%) after 4 yr [30], [31] compared with 5.3% (CI: 4.2–6.5) in IMPACT.
Table 3.
Summary of cancer characteristics of PSA detected cancers using final clinical pathology (ie, if available after prostatectomy)a.
| Genetic status | BRCA2+ (n = 48) | BRCA2– (n = 15) | p value | BRCA1+ (n = 33) | BRCA1– (n = 20) | p value |
|---|---|---|---|---|---|---|
| Median age (yr) at diagnosis | 61 (56, 64) | 64 (60, 66) | 0.044 | 62 (57, 66) | 61 (58, 62) | 0.3 |
| Median PSA (ng/ml) at diagnosis (IQR) | 4.5 (3.6, 5.5) | 4.2 (3.4, 6.1) | 0.9 | 4.4(3.8, 5.9) | 4.4 (3.6, 5.3) | 0.7 |
| Gleason score 6 | 18 (38) | 11 (73) | 0.019b | 18 (55) | 13 (65) | 0.6a |
| Gleason score 7 (3 + 4) | 15 (31) | 1 (7) | 9 (27) | 4 (20) | ||
| Gleason score 7 (4 + 3) | 9 (19) | 2 (13) | 4 (12) | 3 (15) | ||
| Gleason score 8+ | 6 (12) | 1 (7) | 2 (6) | 0 | ||
| T stage—T1/T2a | 16 (35) | 8 (57) | 0.2b | 9 (31) | 8 (40) | 0.6a |
| T Stage—T2b | 2 (4) | 2 (14) | 0 | 1 (5) | ||
| T Stage—T2c/T3 | 28 (61) | 4 (29) | 20 (69) | 11 (55) | ||
| Risk categoryc—low | 11 (23) | 9 (60) | 0.011b | 10 (30) | 4 (20) | 0.5a |
| Risk categoryc—intermediate | 7 (14.5) | 1 (7) | 3 (9) | 6 (30) | ||
| Risk categoryc—high | 30 (62.5) | 5 (33) | 20 (61) | 10 (50) | ||
| Screening round diagnosed—1 | 25 (52) | 7 (47) | 23 (70) | 13 (65) | ||
| Screening round diagnosed—2 | 7 (14.5) | 1 (7) | 3 (9) | 3 (15) | ||
| Screening round diagnosed—3 | 9 (19) | 5 (33) | 6 (18) | 2 (10) | ||
| Screening round diagnosed—4 | 7 (14.5) | 2 (13) | 1 (3) | 2 (10) | ||
| Active surveillance | 8 (17) | 7 (47) | 5 (17) | 6 (30) | ||
| Radical prostatectomy | 32 (70) | 6 (40) | 22 (76) | 12 (60) | ||
| Nonsurgical treatment | 6 (13) | 2 (13) | 2 (7) | 2 (10) |
IQR = interquartile range; NICE = National Institute for Health and Care Excellence; PSA = prostate-specific antigen.
Some pathology information is not available from sites yet.
Values are presented as median (IQR) and n (%).
Note four cancers included in this analysis were diagnosed as a result of additional off-protocol repeat biopsies in men with high PSA.
p values calculated on difference between clinically significant disease and non–clinically significant disease.
Risk category classification system using NICE guidelines [21].
PPV for PB is 30% in the Göteborg cohort and is 26% for PSA (above threshold). PPV for PB is 33% in IMPACT, restricted to the Göteborg age range, and 24% for PSA > 3.0 ng/ml. When comparing PPVs in the Göteborg cohort with IMPACT for clinically significant disease, we see a higher incidence in the BRCA2 carriers for both PSA (p ≤ 0.001) and PB (p ≤ 0.001).
As a sensitivity analysis, analyses were repeated excluding centres in The Netherlands (Supplementary Table 4) that screened patients biennially. No differences in the distributions of cancer incidence, incidence rate, or PPV of PB were observed. To rule out the BRCA2 control group being an outlier, the analyses were repeated combining the control groups, and all significant differences remained.
Analyses were repeated removing cancers diagnosed within <12 mo of study entry (Supplementary Table 5). These analyses show increased PrCa incidence in BRCA2 carriers; however, these analyses are currently underpowered.
During the first four screening rounds, the biopsy compliance rate for raised PSA (>3) was 73% in BRCA2 carriers, 60% in BRCA2 noncarriers, 67% in BRCA1 carriers, and 71% in BRCA1 noncarriers. From the 357 biopsies performed including repeat biopsies, the median age at biopsy of BRCA2 carriers was 60 yr, compared with 64 yr in BRCA2 noncarriers (p ≤ 0.001). No differences were observed in the BRCA1 cohort (Table 2). When comparing by genetic status, no differences were seen in median PSA, which triggered biopsy, time (in days) between PSA test and biopsy, age at biopsy, and number of diagnostic cores taken at biopsy.
3.3. Cancer characteristics
Table 3 and Supplementary Table 6 show the characteristics of all screen-detected PrCa cases diagnosed in patients with a PSA level of >3.0 ng/ml during the first four screening rounds.
The median age at PrCa diagnosis was 61 yr (interquartile range [IQR]: 56, 64) in BRCA2 carriers and 64 yr (IQR: 60, 66) in BRCA2 noncarriers (p = 0.044, Kruskal-Wallis). In the BRCA1 cohort, there was no difference in median age at diagnosis (p = 0.33).
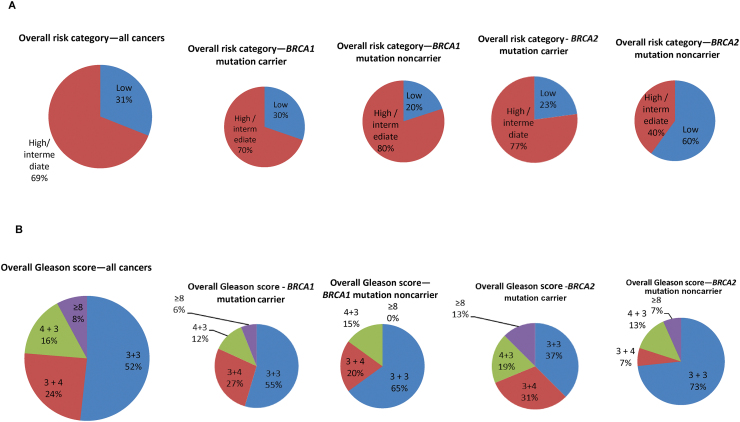
Looking at the overall risk category, 37/48 (77%) BRCA2 carriers had intermediate- or high-risk PrCa (clinically significant disease), compared with six/15 (40%) BRCA2 noncarriers (p = 0.011, Fisher’s exact). There were no statistically significant differences between BRCA1 carriers (70%) and noncarriers (80%; Fig. 3).
Fig. 3.
(A) Pie charts showing the overall prostate cancer risk category (as defined by the NICE guidelines—www.nice.org.uk: low: PSA < 10 and Gleason ≤6 and T1/T2a; intermediate: PSA 10–20 or Gleason 7 or T2b; and high: PSA > 20 or Gleason 8–10 or ≥ T2c), for all study PSA-detected cancers in screening rounds 1–4 and broken down by genetic status. (B) Pie charts showing the overall Gleason score, for all study PSA-detected cancers in screening rounds 1–4 and broken down by genetic status.
NICE = National Institute for Health and Care Excellence; PSA = prostate-specific-antigen.
Limiting to incident cases, 48 cancers were diagnosed: 23 in BRCA2 carriers, eight in BRCA2 noncarriers, 10 in BRCA1 carriers, and seven in BRCA1 noncarriers; there is no significant difference by carrier status. No significant difference was also seen when limiting to incident cases and excluding men who had had a previous benign biopsy (n = 9).
After four screening rounds, no deaths from PrCa were reported in the IMPACT study participants.
4. Discussion
The IMPACT study is the only international prospective PrCa screening study conducted exclusively in families with BRCA1/2 mutations. IMPACT will screen all but the Dutch patients for a total of five screening rounds, and collect cancer incidence and mortality data for a further 5 yr.
Controversy about PSA level that used to trigger PB continues, and we have demonstrated that using a PSA level of >3.0 ng/ml, after four screening rounds, 13% of the total cohort was recommended to have a PB with a 3.8% cancer detection rate. The IMPACT study continues to collect screening data, and a further component of the protocol is to offer men the option of undergoing PB after the completion of five screening rounds, irrespective of the PSA level. This will provide the opportunity to evaluate the number of clinically significant cancers missed in carriers and noncarriers when using a PSA threshold of 3.0 ng/ml.
We have demonstrated that the trends reported after the baseline screen are strengthened after 3 yr of follow-up. The PPV of PSA > 3.0 ng/ml was significantly higher in BRCA2 mutation carriers (31%) compared with noncarriers (18%; p = 0.025). When compared with the Göteborg cohort, the PPV of PB in BRCA2 carriers was 41% compared with 30%, therefore biopsying fewer men unnecessarily. As previously reported, no significant differences were detected between BRCA1 carriers and noncarriers [19].
After four screening rounds, BRCA2 carriers have a statistically significantly higher cancer incidence rate (19) per 1000 person years compared with noncarriers (12; p = 0.03). With a higher number of cancers detected than reported previously [22], we have confirmed that BRCA2 carriers are diagnosed at a younger age (p = 0.044) than BRCA2 noncarriers and that a significantly greater proportion of cancers were intermediate- or high-risk disease (p = 0.011). Overall, 77% of cancers diagnosed in BRCA2 carriers were clinically significant, compared with 49% in the general population [11]. The youngest age of diagnosis of clinically significant disease was 41 yr for BRCA2 carriers and 43 yr for BRCA1 carriers, which may suggest screening from an early age. Regarding the number of men needed to screen to detect one clinically significant PrCa after four screening rounds, screening 60 BRCA2 carriers aged 40–54 yr and 13 carriers aged 55–69 yr will detect one clinically significant PrCa, respectively. Eventually, long-term follow-up data on the clinical benefit of early detection are needed to determine the best starting age.
Analyses of the cancer detection rates, PPV, and characteristics were repeated excluding prevalent cancers (PrCa diagnosed within 12 mo of baseline PSA). It was found that whilst not statistically significant, there was greater PrCa incidence in BRCA2 carriers than in noncarriers (9.1 vs 6.4), and substantially higher numbers of intermediate- and high-risk cancers were detected in BRCA2 carriers than in noncarriers (p = 0.074). Owing to the relatively small number of cancers diagnosed in the BRCA2 noncarriers, we also re-ran these analyses combining the two noncarrier control groups, and statistically significant differences in tumour characteristics remained. In addition, clinically significant cancers were diagnosed at every screening round, supporting the use of systematic PSA screening. After 3 yr of follow-up, it is possible that disease was present, but not detectable by PSA, at study entry. A further aim of the IMPACT study is to offer PB to all men after five screening rounds, to evaluate the utility of a baseline biopsy irrespective of the PSA level with respect to cancer prevalence and tumour characteristics. However, it is reassuring to see from the data presented that using a cut-off of 3 ng/ml, the majority of the tumours detected were at an early stage.
No statistically significant differences were detected in age of onset or cancer characteristics between BRCA1 carriers and noncarriers. Further follow-up is required to conclude the clinical management of BRCA1 carriers.
Similar to our report for the IMPACT baseline screen [22], the ProtecT score using the 4 K panel (Supplementary material) was able to predict PB outcome, with a discrimination of 0.73 for high-grade disease. This adds further evidence to support the use of additional biological markers, such as the 4 K panel in improving the detection of clinically significant PrCa.
4.1. Limitations
A limitation of IMPACT is that not all men comply with the study protocol, and therefore cancers may be missed either in men who refuse PB, or in men who are advised locally to have MRI or repeat PSA instead of a PB. Genetic status may play a role in protocol compliance with fewer noncarriers, particularly BRCA2 noncarriers, proceeding with a PB (73% vs 60%). This differential biopsy rate is likely to have underestimated the PrCa incidence in both BRCA2 carriers and noncarriers. Complete data would be expected to strengthen the power to detect the difference in clinically significant disease between these groups. As follow-up will continue for a further 5 yr, these data will become available as part of future analyses. The higher observed biopsy rate within BRCA2 carriers could represent variation in how health professionals counsel men with high PSA levels, with a bias towards encouraging biopsy in BRCA2 carriers. Of note, no variation was seen between the number of cores taken at biopsy and mutation status. Variation was observed between sites and across the course of the study as the protocol increased from 10 to 12 biopsy cores as standard practice changed.
Given the rarity of BRCA1/2 mutations, it was not possible to restrict the protocol to those with no prior urinary symptoms or PSA testing. Those with a prior PB were excluded. There was no difference in cancer incidence rates in those with symptoms or prior PSA testing.
In comparing with the Göteborg cohort, we acknowledge that this general population cohort is not stratified for BRCA status; however, the population frequency of BRCA1/2 mutations is low. This study also used biennial rather than yearly PSA and was restricted to sextant PB, and therefore cancer detection rates at PB may be lower than that in IMPACT.
IMPACT started in 2005, prior to the implementation of multiparametric MRI in PrCa screening [17], [18]. Without a systematic evaluation of the use of MRI in men at genetically high risk, it is difficult to extrapolate general population data to this setting and needs further research.
The Dutch protocol, as outlined above, screened men every 2 yr, and also included digital rectal examination and PCA3 in the algorithm of whether to proceed to biopsy or not. Therefore, some men with PSA < 3.0 ng/ml were biopsied, some of whom were diagnosed with cancer. However, despite this differing protocol in this cohort, sensitivity analyses excluding the Dutch data demonstrate that this approach did not affect the overall results.
A challenge of a longitudinal study such as IMPACT is in balancing the standardisation of procedures and changes in practice. For example, there have been changes in PB during the course of this study; the protocol has been updated to increase the number of diagnostic cores from 10 to 12 during the study’s duration. Some centres have used the transperineal approach in line with local practice guidelines.
5. Conclusions
We demonstrate that, after four annual PSA screening rounds, BRCA2 mutation carriers have a higher incidence of PrCa, are diagnosed at a younger age, and present with more clinically significant tumours than BRCA2 noncarriers. Further follow-up is required to assess the role of screening in BRCA1 mutation carriers. Therefore, these data support the use of systematic PSA screening in male BRCA2 carriers.
Author contributions: Rosalind A. Eeles had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Aaronson, Ardern-Jones, Bancroft, Bangma, Castro, Dearnaley, Eccles, Evans, Eyfjord, Falconer, Foster, Gronberg, Hamdy, Johannsson, Khoo, Kote-Jarai, Lilja, Lindeman, Lubinski, Mahle, Mikropoulos, Mitra, Moynihan, Page, Rennert, Suri.
Acquisition of data: All authors.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Page, Bancroft, Brook, Assel, Vickers, Lilja.
Obtaining funding: Eeles and all IMPACT collaborating sites obtained their own funding for running the study at their site.
Administrative, technical, or material support: All authors.
Supervision: Eeles.
Other: None.
Financial disclosures: Rosalind A. Eeles certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Hans Lilja holds patents for intact PSA assays, and is named, along with Andrew J. Vickers, on a patent application for a statistical method to detect prostate cancer. The patents have been licensed and commercialised as the 4 Kscore by OPKO Health. Drs. Vickers and Lilja receive royalties from sales of this test. Additionally, Dr. Lilja owns stock and Dr. Vickers owns stock options in OPKO. Professor Rosalind Eeles: Royal Marsden Hospital—Nov 2017; support from Janssen; honorarium as speaker £1100; University of Chicago invited talk May 2018; honorarium as speaker $1000. The remaining authors have no other conflict of interest to declare.
Funding/Support and role of the sponsor: This research is coordinated by the Institute of Cancer Research, London, UK, and is supported by grants from Cancer Research UK (grant references C5047/A21332, C5047/A13232, and C5047/A17528) and the Ronald and Rita McAulay Foundation. Judith Offman is supported by Cancer Research UK Programme Grant reference C8161/A16892. Mr. and Mrs. Jack Baker are acknowledged for supporting the study in NorthShore University HealthSystem, Evanston, IL, USA and Myriad Genetics Laboratory, Salt Lake City, UT, USA, for providing research BRCA testing rates for NorthShore University HealthSystem patients. We acknowledge funding from the National Institute for Health Research (NIHR) to the Biomedical Research Center at the Institute of Cancer Research and the Royal Marsden NHS Foundation Trust, at Manchester University Foundation Trust (IS-BRC-1215-20007), the Oxford Biomedical Research Centre Program, and the Cambridge Clinical Research Centre, NIHR Cambridge Biomedical Research Centre. We acknowledge that in Australia, this project was cofunded by Cancer Council Tasmania and Cancer Australia (grant number 1006349 [2011–2013]), Prostate Cancer Foundation of Australia (grant number PCFA PRO4 [2008]), Cancer Councils of Victoria and South Australia (grant number 400048 [2006–2008]), the Victorian Cancer Agency Clinical Trial Capacity CTCB08_14, Cancer Australia and Prostate Cancer Foundation of Australia (2014–2016; grant number 1059423), and Translational grants EOI09_50. The Association of International Cancer Research funded data collection in The Netherlands (AICR 10-0596). We acknowledge funding from the Basser Center for BRCA (to Susan Domchek). This work was supported in part by the National Institutes of Health/National Cancer Institute (NIH/NCI) with a Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748), a SPORE grant in Prostate Cancer to Dr. H. Scher (P50-CA92629), the Sidney Kimmel Center for Prostate and Urologic Cancers, David H. Koch through the Prostate Cancer Foundation. This work was also supported in part by the NIHR Oxford Biomedical Research Centre Program in UK, the Swedish Cancer Society (CAN 2017/559), the Swedish Research Council (VR-MH project no. 2016-02974), and General Hospital in Malmö Foundation for Combating Cancer. We acknowledge funding from the Slovenian Research Agency, Research programme P3-0352. We thank CERCA Program/Generalitat de Catalunya for their institutional support. Elena Castro acknowledges funding from Prostate Cancer Foundation. We acknowledge the support of the Asociación Española Contra el Cáncer (AECC), the Instituto de Salud Carlos III (organismo adscrito al Ministerio de Economía y Competitividad), “Fondo Europeo de Desarrollo Regional (FEDER), una manera de hacer Europa” (PI10/01422, PI13/00285, PIE13/00022, PI16/00563, JR18/00011 and CIBERONC), and the Institut Català de la Salut and Autonomous Government of Catalonia (2009SGR290, 2014SGR338 and PERIS Project MedPerCan). We acknowledge funding support from Fundação para a Ciência e a Tecnologia to the IPO Porto study (project grant PTDC/DTP-PIC/1308/2014 to Manuel R. Teixeira and fellowship grant SFRH/BD/116557/2016 to Marta Cardoso).
Acknowledgements
We are indebted to all the men who took part in this study. We are grateful to the members of the Data and Safety Monitoring Committee: S. Duffy (Chair), P. White (UK NEQAS representative), and J. McGrath (BAUS representative), and past member R. Pocock. We acknowledge the contribution of past members of the IMPACT Steering Committee: S. Moss, J. Melia, G. Mitchell, D. Easton, S. Peock, F. Schroder, R. Sharifi, and P. Sibley.
Associate Editor: T. Morgan
Statistical Editor: Emily Zabor
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.eururo.2019.08.019.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Breast Cancer Linkage Consortium Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 2.Kote-Jarai Z., Leongamornlert D., Saunders E. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer. 2011;105:1230–1234. doi: 10.1038/bjc.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro E., Goh C., Olmos D. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitra A., Fisher C., Foster C.S. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer. 2008;98:502–507. doi: 10.1038/sj.bjc.6604132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorne H., Willems A.J., Niedermayr E. Decreased prostate cancer-specific survival of men with BRCA2 mutations from multiple breast cancer families. Cancer Prev Res (Phila) 2011;4:1002–1010. doi: 10.1158/1940-6207.CAPR-10-0397. [DOI] [PubMed] [Google Scholar]
- 6.Tryggvadottir L., Vidarsdottir L., Thorgeirsson T. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99:929–935. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 7.Taylor R.A., Fraser M., Livingstone J. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat Commun. 2017;8:13671. doi: 10.1038/ncomms13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leongamornlert D., Mahmud N., Tymrakiewicz M. Germline BRCA1 mutations increase prostate cancer risk. Br J Cancer. 2012;106:1697–1701. doi: 10.1038/bjc.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giusti R.M., Rutter J.L., Duray P.H. A twofold increase in BRCA mutation related prostate cancer among Ashkenazi Israelis is not associated with distinctive histopathology. J Med Genet. 2003;40:787–792. doi: 10.1136/jmg.40.10.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinsky P.F., Yu K., Kramer B.S. Extended mortality results for ovarian cancer screening in the PLCO trial with median 15 years follow-up. Gynecol Oncol. 2016;143:270–275. doi: 10.1016/j.ygyno.2016.08.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroder F.H., Hugosson J., Roobol M.J. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–2035. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin R.M., Donovan J.L., Turner E.L. Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319:883–895. doi: 10.1001/jama.2018.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assel M., Sjoblom L., Murtola T.J. A four-kallikrein panel and beta-microseminoprotein in predicting high-grade prostate cancer on biopsy: an independent replication from the Finnish section of the European Randomized Study of Screening for Prostate Cancer. Eur Urol Focus. 2017 doi: 10.1016/j.euf.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E.H., Andriole G.L., Crawford E.D. Detection of high grade prostate cancer among PLCO participants using a prespecified 4-kallikrein marker panel. J Urol. 2017;197:1041–1047. doi: 10.1016/j.juro.2016.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poyet C., Nieboer D., Bhindi B. Prostate cancer risk prediction using the novel versions of the European Randomised Study for Screening of Prostate Cancer (ERSPC) and Prostate Cancer Prevention Trial (PCPT) risk calculators: independent validation and comparison in a contemporary European cohort. BJU Int. 2016;117:401–408. doi: 10.1111/bju.13314. [DOI] [PubMed] [Google Scholar]
- 16.Verbeek J.F.M., Bangma C.H., Kweldam C.F. Reducing unnecessary biopsies while detecting clinically significant prostate cancer including cribriform growth with the ERSPC Rotterdam risk calculator and 4Kscore. Urol Oncol. 2019;37:138–144. doi: 10.1016/j.urolonc.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Brown L.C., Ahmed H.U., Faria R. Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound-guided prostate biopsy alone: the PROMIS study. Health Technol Assess. 2018;22:1–176. doi: 10.3310/hta22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston E., Pye H., Bonet-Carne E. INNOVATE: a prospective cohort study combining serum and urinary biomarkers with novel diffusion-weighted magnetic resonance imaging for the prediction and characterization of prostate cancer. BMC Cancer. 2016;16:816. doi: 10.1186/s12885-016-2856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eklund M., Nordstrom T., Aly M. The Stockholm-3 (STHLM3) model can improve prostate cancer diagnostics in men aged 50–69 yr compared with current prostate cancer testing. Eur Urol Focus. 2018;4:707–710. doi: 10.1016/j.euf.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Lecarpentier J., Silvestri V., Kuchenbaecker K.B. Prediction of breast and prostate cancer risks in male BRCA1 and BRCA2 mutation carriers using polygenic risk scores. J Clin Oncol. 2017;35:2240–2250. doi: 10.1200/JCO.2016.69.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management, NICE guideline, NG. 131. https://www.nice.org.uk/guidance/ng131. [PubMed]
- 22.Bancroft E.K., Page E.C., Castro E. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol. 2014;66:489–499. doi: 10.1016/j.eururo.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikropoulos C., Hutten Selkirk C.G., Saya S. Prostate-specific antigen velocity in a prospective prostate cancer screening study of men with genetic predisposition. Br J Cancer. 2018;118:e17. doi: 10.1038/bjc.2017.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter H.B., Helfand B., Mamawala M. Germline mutations in ATM and BRCA1/2 are associated with grade reclassification in men on active surveillance for prostate cancer. Eur Urol. 2019;75:743–749. doi: 10.1016/j.eururo.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein J.I., Egevad L., Amin M.B. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 26.Daly M.B., Pilarski R., Berry M. NCCN guidelines insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9–20. doi: 10.6004/jnccn.2017.0003. [DOI] [PubMed] [Google Scholar]
- 27.Mitra A.V., Bancroft E.K., Barbachano Y. Targeted prostate cancer screening in men with mutations in BRCA1 and BRCA2 detects aggressive prostate cancer: preliminary analysis of the results of the IMPACT study. BJU Int. 2011;107:28–39. doi: 10.1111/j.1464-410X.2010.09648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitra A.V., Bancroft E.K., Eeles R.A. A review of targeted screening for prostate cancer: introducing the IMPACT study. BJU Int. 2007;99:1350–1355. doi: 10.1111/j.1464-410X.2007.06759.x. [DOI] [PubMed] [Google Scholar]
- 29.Ehdaie B., Poon B.Y., Sjoberg D.D. Variation in serum prostate-specific antigen levels in men with prostate cancer managed with active surveillance. BJU Int. 2016;118:535–540. doi: 10.1111/bju.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hugosson J., Godtman R.A., Carlsson S.V. Eighteen-year follow-up of the Goteborg Randomized Population-based Prostate Cancer Screening Trial: effect of sociodemographic variables on participation, prostate cancer incidence and mortality. Scand J Urol. 2018;52:27–37. doi: 10.1080/21681805.2017.1411392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hugosson J., Carlsson S., Aus G. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.