Abstract
Parkinson’s disease (PD), which involves the degeneration of dopaminergic neurons in the basal ganglia, has long been associated with motor deficits. Increasing evidence suggests that language can also be impaired, including aspects of syntactic and lexical processing. However, the exact pattern of these impairments remains somewhat unclear, for several reasons. Few studies have examined and compared syntactic and lexical processing within subjects, so their relative deficits remain to be elucidated. Studies have focused on earlier stages of PD, so syntactic and lexical processing in later stages are less well understood. Research has largely probed English and a handful of other European languages, and it is unclear whether findings generalize more broadly. Finally, few studies have examined links between syntactic/lexical impairments and their neurocognitive substrates, such as measures of basal ganglia degeneration or dopaminergic processes. We addressed these gaps by investigating multiple aspects of Farsi syntactic and lexical processing in 40 Farsi native-speaking moderate-to-severe non-demented PD patients, and 40 healthy controls. Analyses revealed equivalent impairments of syntactic comprehension and syntactic judgment, across different syntactic structures. Lexical processing was impaired only for motor function-related objects (e.g., naming ‘hammer’, but not ‘mountain’), in line with findings of PD deficits at naming action verbs as compared to objects, without the verb/noun confound. In direct comparisons between lexical and syntactic tasks, patients were better at naming words like ‘mountain’ (but not words like ‘hammer’) than at syntactic comprehension and syntactic judgment. Performance at syntactic comprehension correlated with the last levodopa equivalent dose. No other correlations were found between syntactic/lexical processing measures and either levodopa equivalent dose or hypokinesia, which reflects degeneration of basal ganglia motor-related circuits. All critical significant main effects, interactions, and correlations yielded large effect sizes. The findings elucidate the nature of syntactic and lexical processing impairments in PD.
Keywords: Parkinson’s disease, language, syntax, lexicon, dopamine, hypokinesia
1. Introduction
Parkinson’s disease (PD), a progressive disease involving the degeneration of dopaminergic neurons in the basal ganglia, has traditionally been associated with motor deficits. However, research has increasingly shown that aspects of language can also be impaired (Angwin, Chenery, Copland, Murdoch, & Silburn, 2005; Birba et al., 2017; Bocanegra et al., 2015; Cohen, 1998; Gallese & Cuccio, 2018; Grossman, Carvell, Stern, Gollomp, & Hurtig, 1992; Karim Johari et al., 2013; F. M. Lewis, Lapointe, Murdoch, & Chenery, 1998; Lieberman et al., 1992; Ullman et al., 1997). Of interest here, deficits have been found in tasks designed to probe aspects of both syntactic processing (Birba et al., 2017; Gallese & Cuccio, 2018; Grossman, 1999; Hochstadt, Nakano, Lieberman, & Friedman, 2006) and lexical processing (Birba et al., 2017; Gallese & Cuccio, 2018; Ito & Kitagawa, 2006; Rodríguez-Ferreiro, Menéndez, Ribacoba, & Cuetos, 2009), two critical building blocks of language use.
As we will see, although patterns are clearly emerging regarding syntactic and lexical deficits in PD, the nature and extent of these deficits, and their neurocognitive correlates, remain to be fully elucidated. First of all, the relative impairment of (various aspects of) syntactic and lexical processing remains somewhat unclear, in part because most research has probed only syntactic processing or only lexical processing in the same subjects (but see Angwin et al., 2005; Bocanegra et al., 2015; García et al., 2017) – even though a within-subjects approach crucially eliminates contamination from between-subject variability in the comparisons of interest. Further confusing the picture, different studies have tested patients at different PD stages (Grossman et al., 2000; Hochstadt, 2009; Prieto, Radanovic, Schmitt, Barbosa, & Mansur, 2007; Silveri et al., 2012) or have not reported the stage of the disease (Patrice Péran et al., 2003; Terzi, Papapetropoulos, & Kouvelas, 2005), despite evidence that different aspects of language show differential impairments over the course of the disease, including regarding the appearance of cognitive impairments or dementia (Bocanegra et al., 2017; K Johari, Walenski, Reifegerste, Ashrafi, & Ullman, 2019; Ullman et al., 1997). Where studies have focused on particular stages, they have often examined early-stage patients (Birba et al., 2017; Bocanegra et al., 2015; García et al., 2017; Grossman et al., 2000; Ibáñez et al., 2013; Rodríguez-Ferreiro et al., 2009), so less is known about lexical and syntactic deficits in more advanced stages of the disease, including which (if any) aspects of lexical or syntactic processing may remain spared. Additionally, previous research on syntactic and lexical processing in PD has focused on English and (to a lesser extent) some other European languages (Abrevaya et al., 2017; Bocanegra et al., 2017; Bocanegra et al., 2015; Juan F Cardona et al., 2014; García et al., 2018; García et al., 2016; García et al., 2017; Herrera & Cuetos, 2012; Ibáñez et al., 2013; Melloni et al., 2015; Patrice Péran et al., 2009; Patrice Péran et al., 2003; Prieto et al., 2007; Rodríguez-Ferreiro et al., 2009). This leaves open the possibility that findings might not generalize more broadly. Finally, not many studies have examined the neurocognitive substrates of lexical (Abrevaya et al., 2017; Herrera, Rodríguez-Ferreiro, & Cuetos, 2012; Melloni et al., 2015; Patrice Péran et al., 2009) and syntactic (Friederici, Kotz, Werheid, Hein, & von Cramon, 2003; Grossman et al., 2003) deficits in PD, such as the influence of basal ganglia degeneration or dopaminergic processes.
Here we address these gaps. The study examines which aspects of both syntactic (Research Question 1) and lexical (Research Question 2) processing may be affected in moderate-to-severe non-demented PD, as compared to healthy control participants. Different aspects of both syntactic and lexical processing were tested within subjects: syntactic comprehension and syntactic judgment, across different syntactic structures, and different aspects of lexical/semantic processing, in particular naming both manipulated and non-manipulated objects. To widen the literature beyond English and other European languages, the study probed Farsi, in Farsi-native speaking PD patients and healthy control participants in Iran. (Here, we focus on native language, and thus do not discuss PD studies of second language, whose neurocognition seems to differ from that of first language, including in PD (Karim Johari et al., 2013; Ullman, 2014; Zanini et al., 2004).) In Research Question 3, we examine the relative deficits of syntactic versus lexical processing in PD, by directly comparing performance between the syntactic and lexical tasks. In Research Question 4, we test whether the various aspects of syntactic and lexical processing are associated with measures that reflect basal ganglia degeneration (left and right hypokinesia) and dopaminergic processes (levodopa equivalent dose of the last anti-PD medication taken). In the remainder of the Introduction we provide overviews of findings on syntactic and lexical processing in PD to date, including associations between syntactic/lexical processing and measures of neurocognitive substrates (i.e., of basal ganglia degeneration and dopaminergic processes) before introducing the present study. For discussion of neurocognitive accounts of syntactic and lexical deficits in PD, including with respect to the findings from the present study, see Discussion.
1.1. Syntactic processing
Here we briefly review the status of syntactic processing in PD, and point out gaps relevant to the present study. Most studies on syntactic processing in PD have examined syntactic (sentence) comprehension. These have found PD deficits in a variety of relatively complex syntactic structures, including center-embedded constructions (Grossman et al., 2000; Hochstadt, 2009), subject relatives (García et al., 2017; Prieto et al., 2007), and object-gap subordinate clauses (Grossman, Lee, Morris, Stern, & Hurtig, 2002), as well as passive constructions (Hochstadt, 2009). However, the findings have been somewhat inconsistent. For example, Terzi et al. (2005) and Prieto et al. (2007) did not find impairments for passive sentences, and it was not clear whether McNamara et al. (1996) found impairments in the comprehension of arguments and adjuncts.
Surprisingly, given the fact that syntactic judgment has been investigated extensively in language research, we are aware of only one study examining syntactic judgment in PD, which found impairments (McNamara, Krueger, O’quin, Clark, & Durso, 1996). (Two other PD studies probing syntactic judgment focused on first vs. second language, and did not compare syntactic judgment between PD and control subjects, in either the first or second language; Karim Johari et al., 2013; Zanini et al., 2004). Here we do not focus on PD studies of syntax in expressive language tasks (García et al., 2016; Illes, Metter, Hanson, & Iritani, 1988; Murray & Lenz, 2001; Zanini et al., 2003; Zanini et al., 2009).
Few studies have thus far examined links between syntactic processing in PD and likely underlying neurocognitive substrates, in particular measures of basal ganglia degeneration and dopaminergic processes (Birba et al., 2017; Ullman, 2004, 2016; Ullman et al., 1997). We are aware of two neuroimaging studies examining the involvement of the basal ganglia in syntactic processing in PD, which implicated the left and right caudate nucleus in sentence comprehension (Grossman et al., 1993; Grossman et al., 2003). We are not aware of any studies of PD testing associations between syntactic processing and behavioral measures that reflect basal ganglia degeneration, such as hypokinesia (Berardelli, Rothwell, Thompson, & Hallett, 2001; Mazzoni, Shabbott, & Cortés, 2012) For studies examining such associations in morphological processing, see Ullman et al. (1997), Johari et al. (2019), and Reifegerste et al. (under revision); see Discussion.
We know of three studies that have probed the role of levodopa in tasks designed to examine syntax (Grossman et al., 2001; McNamara et al., 1996; Skeel et al., 2001), all of which compared performance on vs. off levodopa. These found positive effects of levodopa on syntactic comprehension but not judgment (McNamara et al., 1996); on syntactic comprehension in more complex (center-embedded) but not simpler sentences (Grossman et al., 2001); or no effect of levodopa at all, in syntactic comprehension of sentences with various levels of complexity (Skeel et al., 2001). We know of no neuroimaging studies testing links between syntactic processing and dopaminergic processes in PD.
In sum, the status of syntactic processing in PD is becoming clear, though various gaps remain. A fair number of studies have reported syntactic comprehension impairments in PD, though the findings are somewhat inconsistent (the reasons for which remain unclear), and only a few types of syntactic structures have been probed. Syntactic judgment has also elicited impairments in PD, though we are aware of only one study using such tasks, despite their importance in language research. Perhaps more importantly, we know of only two studies of syntactic comprehension (and none of syntactic judgment) that have also tested lexical processing within subjects, though neither directly compared syntactic and lexical performance (Bocanegra et al., 2015; García et al., 2017). Thus, relative impairments of syntactic and lexical processing in PD remain unclear. We also know of no studies that have compared syntactic comprehension and judgment within subjects, also leaving their relative deficits uncertain. Most studies have tested patients at mild or mild-to-moderate stages (Grossman et al., 2000; Hochstadt, 2009; Hochstadt et al., 2006). We are not aware of any research examining syntax in PD patients at more advance stages, and thus the pattern of impaired (and spared) syntactic/lexical processing in such patients remains to be elucidated. Most investigations of syntactic comprehension and judgment in PD have focused on English and a few other European languages, leaving open the question of whether patterns generalize more broadly. Finally, little research has thus far examined whether the syntactic impairments in PD are linked to the basal ganglia or dopaminergic processes. Thus, important gaps remain in our understanding of syntactic processing in PD.
1.2. Lexical processing
Studies have examined various aspects of lexical processing in PD (Buccino et al., 2018; Castner et al., 2007; Cotelli et al., 2007; Friederici et al., 2003; Ito & Kitagawa, 2006; Matison, Mayeux, Rosen, & Fahn, 1982; Rodríguez-Ferreiro et al., 2009). Overall, these have yielded mixed results, with impairments in some studies (Castner et al., 2007; Copland, 2003; Ito & Kitagawa, 2006) but not in others (Angwin et al., 2017; Friederici et al., 2003; York et al., 2014). This mixed pattern may be due to various factors, including the types of tasks (Copland, 2003; York et al., 2014) and the types of items (Buccino et al., 2018; Cotelli et al., 2007; Rodríguez-Ferreiro et al., 2009). For example, production tasks (e.g., picture naming, or word finding in spontaneous speech) may be more difficult than comprehension tasks (Boulenger et al., 2008; Matison et al., 1982; Patrice Péran et al., 2009; Patrice Péran et al., 2003; Rodríguez-Ferreiro et al., 2009; York et al., 2014).
A primary focus of research on lexical processing in PD has been the contrast between naming objects (e.g., house) and naming action verbs (e.g., to throw) (Bocanegra et al., 2017; Bocanegra et al., 2015; Boulenger et al., 2008; Cotelli et al., 2007; Herrera & Cuetos, 2012; Patrice Péran et al., 2009; Patrice Péran et al., 2003; Piatt, Fields, Paolo, & Tröster, 1999; Rodríguez-Ferreiro et al., 2009; Silveri et al., 2012). Since action verbs depend more than many nouns on motor-related circuits (Perani et al., 1999; Raposo, Moss, Stamatakis, & Tyler, 2009), including the basal ganglia (Silveri et al., 2012), PD patients may be expected to have greater difficulty processing the former than the latter (Birba et al., 2017; Gallese, 2008; Gallese & Cuccio, 2018; Ullman, 2004, 2016); also see Discussion. This is in fact the pattern found in PD studies (Bocanegra et al., 2017; Bocanegra et al., 2015; Boulenger et al., 2008; Juan Felipe Cardona et al., 2013; Cotelli et al., 2007; Herrera & Cuetos, 2012; Patrice Péran et al., 2009; Rodríguez-Ferreiro et al., 2009). Consistent with this pattern, action verb processing in PD has been linked to the basal ganglia and motor-related cortical regions in neuroimaging (Abrevaya et al., 2017), as well as to dopaminergic processes (Herrera & Cuetos, 2012; P Péran et al., 2013), though we are not aware of any studies examining associations between action verb processing and hypokinesia in the disorder.
However, in comparisons between naming actions and objects, the motor/non-motor contrast is confounded with the verb/noun contrast, complicating interpretation of results. This confound can be addressed in different ways. For example, actions and objects can be tested with non-verbal tasks, which indeed also yield action/object differences in PD (Bocanegra et al., 2015). Additionally, some studies suggest that action-verb deficits in PD are found even when other verb categories are spared (Fernandino et al., 2013; García et al., 2018), and that particular types of action verbs are impaired, such as action verbs with higher vs. lower motion content (Bocanegra et al., 2017; Herrera et al., 2012; Roberts et al., 2017; Speed, van Dam, Hirath, Vigliocco, & Desai, 2017).
Another way of addressing this problem is to examine lexical processing for commonly or easily manipulated objects vs. less commonly or easily manipulated objects (‘manipulated’ vs. ‘non-manipulated’) (Ullman, 2007; Walenski, Mostofsky, & Ullman, 2007). Indeed, evidence suggests that naming manipulated objects, but not non-manipulated objects, critically depends on motor-related circuits (Chouinard & Goodale, 2010; Damasio, Grabowski, Tranel, Hichwa, & Damasio, 1996; Grafton, Fadiga, Arbib, & Rizzolatti, 1997; Martin, Wiggs, Ungerleider, & Haxby, 1996), including the basal ganglia (Damasio et al., 1996; Walenski et al., 2007). This suggests that PD patients should be more impaired at lexical processing (e.g., in object naming) of manipulated than non-manipulated objects.
We are aware of a small number of studies examining this distinction in PD. In one study, deep brain stimulation of the subthalamic nucleus in early-stage PD patients improved naming manipulated objects but not non-manipulated object (Phillips et al., 2012). This suggests that naming manipulated (but not non-manipulated) objects depends on the basal ganglia, and may be impaired in PD; however, this paper did not report direct comparisons between PD patients and normal control subjects. A second study found that early-stage PD patients without mild cognitive impairment were not impaired at naming either low or high manipulability objects (Bocanegra et al., 2017). A more recent paper reported that whereas healthy control participants were faster at processing pictures or words of non-graspable objects than of graspable objects, mild-to-moderate non-demented PD patients performed similarly on both; though this reveals a particular processing slowdown for non-graspable objects in PD, the authors conclude that the results suggests that “the capacity to process graspable objects and their nouns is impaired” (Buccino et al., 2018). Overall, these studies of (mild to moderate) PD yielded mixed findings regarding deficits of naming (or otherwise processing) manipulated vs. non-manipulated objects, or related distinctions. It remains to be seen whether relative deficits in the lexical processing of manipulated (vs. non-manipulated) objects are observed reliably in more advanced non-demented PD patients (consistent with further degeneration of the basal ganglia), and if so, whether such patients still remain unimpaired on lexical processing of non-manipulated objects, which would suggest that lexical processing itself (independent of motor-related knowledge) remains relatively intact in such patients.
1.3. The present study
The present study examined syntactic and lexical processing in Farsi, in native Farsi-speaking patients with non-demented moderate-to-severe PD, and matched normal control participants. All participants were tested on syntactic comprehension (negative sentences, subject topicalized sentences, object topicalized sentences) and syntactic judgment (various structures), as well as the naming of objects that either are or are not commonly manipulated. The effect of both right-side and left-side hypokinesia on each of these language measures was also examined, as was the effect of the levodopa equivalent dose (LED) (Tomlinson et al., 2010) of the patients’ last anti-PD medication.
Due to the dearth of previous PD studies examining at least some these issues – and none in native speakers of Farsi, or in any language at all probing all of these functions within subjects – we did not have strong predictions. Nevertheless, previous research (see above) suggested a likelihood of: PD impairments in both syntactic comprehension and judgment (Research Question 1); greater deficits at naming manipulated than non-manipulated objects, which might even remain unimpaired (Research Question 2); in direct comparisons between tasks, the possibility of similar impairments at syntactic comprehension, syntactic judgment, and naming manipulated objects, but worse performance at all of these than at naming non-manipulated objects (Research Question 3); and perhaps associations (Research Question 4) between syntactic processing and naming manipulated objects on the one hand, and LED and/or hypokinesia on the other. Note that for hypokinesia, right-side hypokinesia might play a stronger role than left-side hypokinesia given the left lateralization of language, since right-side hypokinesia primarily reflects left basal ganglia degeneration (Berardelli et al., 2001; Mazzoni et al., 2012).
2. Methods
2.1. Participants
We tested 40 PD patients (20 females) and 40 normal control participants (20 females). All participants were right handed (Edinburgh Inventory Total Score > 33; Edinburgh Handedness Inventory) (Oldfield, 1971) monolingual native Farsi speakers. None were demented (all subjects had Farsi MMSE (Mini–Mental State Examination) scores ≥ 25) (Ansari, Naghdi, Hasson, Valizadeh, & Jalaie, 2010) or had any known neurological or psychiatric disorder (other than PD for the PD patients), and none had any known brain injury or surgery. All female participants had completed menopause. All patients were diagnosed with idiopathic PD, and were at stage 3 (moderate stage; bilateral disease) or 4 (advanced stage; severely disabling disease) on the Hoehn and Yahr scale; mean disease stage = 3.32, SD = 0.47 (Hoehn & Yahr, 1998). None had secondary Parkinsonism. Patients were taking levodopa and other anti-PD medication with different individually tailored dosages to maximally reduce motor symptoms. The Levodopa Equivalent Dose (LED) (Tomlinson et al., 2010) of the last anti-PD medication taken by each patient prior to testing was computed from this information; mean LED = 251.25, SD = 41.58. The PD and control participants were matched (Table 1) on age, years of education, handedness (Oldfield, 1971), and (lack of) dementia (Ansari et al., 2010). All study procedures, including recruitment, testing and informed consent, were approved by ethical committee of Shahid Beheshti University of Medical Sciences in Tehran.
Table 1.
Participant demographic and other information
| PD | NC | Comparison | |
|---|---|---|---|
| Age (in years) | 61.50 ± 8.96 | 59.50 ± 5.62 | t(78) = 1.25, P = 0.22 |
| Education (in years) | 10.67 ± 3.27 | 11.57 ± 3.35 | t(78) = 1.21, P = 0.22 |
| Handedness | 71.43 ± 4.40 | 70.01 ± 3.85 | t(78) = 0.40, P = 0.71 |
| MMSE | 27.50 ± 1.1 | 27.62 ± 1.03 | t(78) = 0.52, P = 0.60 |
Note. Means are presented, together with standard deviations. Education reflects years of schooling starting from first grade. Handedness reflects laterality quotients from the Edinburgh Handedness Inventory (Oldfield, 1971), where 100 represents strongly right-handed and −100 represents strongly left-handed. Comparisons reflect results from independent-samples t-tests. PD: patients with Parkinson’s disease; NC: normal controls; MMSE: Mini–Mental State Examination.
A diagnosis of idiopathic Parkinson’s disease was based on clinical examinations performed by a neurologist with a specialty in movement disorders (Ashrafi). Disease stage and severity were assessed by the neurologist on the basis of the Hoehn and Yahr scale (Hoehn & Yahr, 1998) and the UPDRS (Goetz et al., 2008). Left and right upper limb hypokinesia were assessed by the neurologist on the basis of the finger taps, hand movements, and rapid alternating movement of hands items in Part III of the UPDRS; mean left-side hypokinesia = 6.82, SD = 1.73, mean right-side hypokinesia = 8.42, SD = 1.97 (Goetz et al., 2008; Karim Johari et al., 2013; Ullman et al., 1997). The neurologist also recorded the dose of the last anti-Parkinson’s disease medications prior to testing.
2.2. Procedure
All participants completed tasks of syntactic comprehension, syntactic judgment, and object naming. Both task order and item order within each task were counter-balanced across participants. We created two task orders (order A: syntactic comprehension, object naming, syntactic judgment, and order B, which was the reverse order), and two item orders for each task (order 1 and order 2, in which all items were presented in the reverse order to order 1). Half the PD patients and half the controls (and half the females and half the males in each of these groups) were given each combination of task and item orders. The items were pseudo-randomized in each task, so that no more than three consecutive items were of the same type: for syntactic comprehension, no more than 3 sentences of each type (negative, subject topicalized, object topicalized); for syntactic judgment, no more than 3 correct or incorrect sentences; for object naming, no more than 3 of each item type (manipulated, non-manipulated).
2.3. Syntactic processing tasks
2.3.1. Syntactic (sentence) comprehension
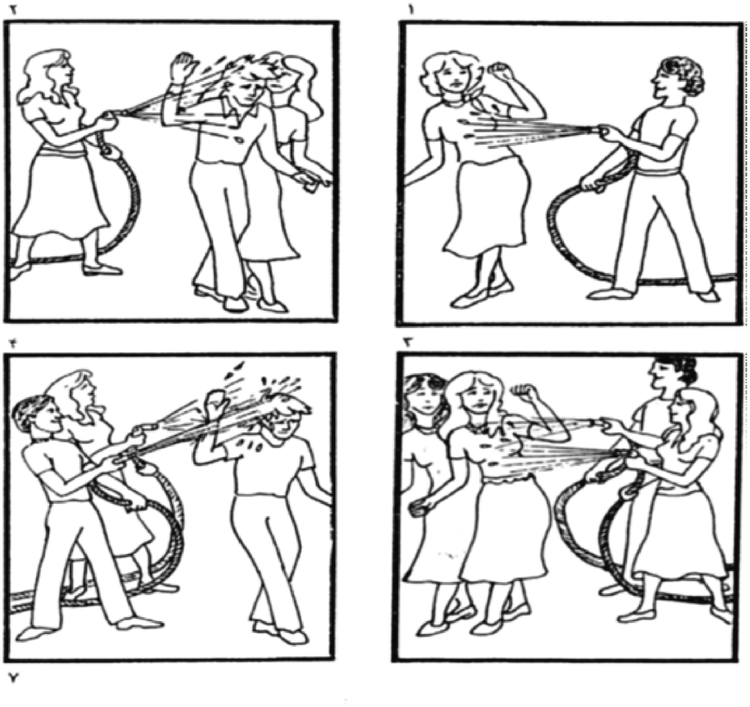
Syntactic comprehension was assessed with items from the syntactic comprehension section of the Farsi version of the Bilingual Aphasia Test (BAT) (Paradis, Paribakht, & Nilipour, 1987). The BAT was used because previous studies suggest that it is sensitive to deficits of syntactic comprehension and judgment in PD in other native languages (Karim Johari et al., 2013; Zanini et al., 2004); moreover, we were (and are) not aware of any other validated test of syntactic processing in Farsi. Items were presented in the pseudo-randomized and counter-balanced orders described above, rather than in the order described in the BAT. The experimenter read each sentence out loud, while the participant looked a set of four pictures (Figure 1); the participant was asked to point to the picture that best corresponded to the sentence.
Figure 1.
Example item from the syntactic comprehension task (Paradis et al., 1987). The item was shown during auditory presentation of the sentence In dokhtar ast ke be anha ab mipashad (‘It is the girl that sprays them’).
Participants were presented with three types of sentences: negative, subject topicalized, and object topicalized. The task comprised 30 sentences, with 10 items for each sentence type. In Farsi, which has a subject-object-verb (SOV) word order, the key structures in these sentence types are constructed as follows. Negatives are created by an inflectional process in which the ne- negative prefix is added to the present tense stem (e.g., mikharad ‘buys’ has as its negative nemikharad ‘does not buy’). Subject topicalized structures are created by a sentence-initial use of the pronoun in preceding the subject, together with a separate clause for the object (e.g., In dokhtar ast ke pesar ra hol midahad. ‘It is the girl that pushes the boy.’). Object topicalized sentences are constructed by switching the subject and object positions, and changing the clause to passive form (e.g., In pesar ast ke dokhtar holash midahad. ‘It is the boy that the girl pushes.’).
2.3.2. Syntactic judgment
Syntactic judgment was assessed with items from the grammatical judgment section of the Farsi version of the BAT (Paradis et al., 1987). Participants were presented with 20 sentences (14 correct and 6 incorrect). The sentences were presented in the pseudo-randomized and counter-balanced orders described above. The experimenter read each sentence out loud. The participant was asked to judge whether the sentence sounded correct or incorrect. Incorrect sentences consisted of three kinds of (morpho-)syntactic errors: an omission or substitution of person/number suffixes; an omission of the accusative morpheme; or the addition of the passive marker suffix in sentences presented in a canonical Farsi word order (subject-object-verb). There were two incorrect sentences of each type, an insufficient number to warrant separate analyses.
2.4. Lexical processing: naming of manipulated and non-manipulated objects
Object naming of manipulated and non-manipulated objects was assessed with 60 pictures of objects. Thirty objects are commonly manipulated (e.g., chakkosh, ‘hammer’) and 30 are not (e.g., kooh, ‘mountain’); see Table 2. The items are a subset of those in the object naming task developed by the Brain and Language lab at Georgetown University (Hedenius, Ullman, Alm, Jennische, & Persson, 2013; Lukács, Kemény, Lum, & Ullman, 2017; Walenski et al., 2007). The items in this subset were selected on the basis of assessments by the first author (Johari), together with other Farsi-speaking Iranians, such that the objects are culturally familiar in Iran (including their pictorial depiction), that they are (or are not) commonly manipulated in Iran, and that their Farsi names are reasonable common. The manipulated and non-manipulated items were matched group-wise on the number of syllables [t(58) = 0.41, P = 0.68], the number of Farsi letters [t(58)= −0.95, P = 0.34], and the natural logarithm-transformed frequencies [t(58) = 0.36,P = 0.71] of the stems (singular forms) of their Farsi object names. Frequency counts were obtained from the Persian Linguistic Database (Assi) (http://pldb.ihcs.ac.ir/).
Table 2.
Manipulated and non-manipulated objects in naming task
| Manipulated | Non-Manipulated | ||
|---|---|---|---|
| Item | Translation | Item | Translation |
| Qashoq | Spoon | Zanboor | Bee |
| Arre | Saw | Halazoon | Snail |
| Medad | Pen | Khers | Bear |
| Dokme | Button | Joqd | Owl |
| Otto | Iron | Khorshid | Sun |
| Soozan | Needle | Sag | Dog |
| Telephon | Telephone | Zarrafe | Giraffe |
| Jaroo | Broom | Shir | Lion |
| Mesvak | Brush | Morche | Ant |
| Tabar | Ax | Goosfand | Sheep |
| Dastgire | Doorknob | Meymoon | Monkey |
| Khodkar | Pencil | Fil | Elephant |
| Toop | Ball | Roobah | Wolf |
| Parch | Pitcher | Sanjab | Squirrel |
| Soot | Whistle | Palang | Panther |
| Eynak | Eyeglass | Tavoos | Peacock |
| Livan | Glass | Kooh | Mountain |
| Zangoole | Bell | Olaq | Donkey |
| Tabl | Drum | Gorbe | Cat |
| Chakkosh | Hammer | Asb | Horse |
| Kelid | Key | Ordak | Duck |
| Gofl | Lock | Mahi | Fish |
| Shane | Comb | Kharghosh | Rabbit |
| Tofang | Gun | Gav | Cow |
| Chatr | Umbrella | Khoroos | Rooster |
| Pakat | Envelope | Moosh | Mouse |
| Chaqoo | Knife | Mar | Snake |
| Qeychi | Scissors | Shotor | Camel |
| Badkonak | Kite | Kangoro | Kangaroo |
| Changal | Fork | Babr | Tiger |
2.5. Data Analysis
Accuracy for each participant on each condition of syntactic comprehension and object naming was computed as the log-odds (logit) transformation (natural log (number correct + 0.5/number incorrect + 0.5), where 0.5 is added to avoid a denominator of zero) (Jaeger, 2008). For syntactic judgment, accuracy was computed as d’ (d-prime), to minimize possible bias (d’ = z(hit rate) -z(false alarm rate), with z-scores computed in Excel with NORMSINV. These transformed accuracy values were entered into analyses performed in SPSS (version 23) on a Windows PC. For all tasks, the transformed accuracy values were submitted to mixed design ANOVAs or independent measures t-tests, with group as the between-subjects factor. Correlations and partial correlations were performed to examine associations between relevant variables (e.g., between right-side hypokinesia and syntactic judgment), with significance assessed using a false discovery rate (FDR) correction (Benjamini & Hochberg, 1995) for multiple comparisons for the number of comparisons at each level of the analysis (e.g., three comparisons for correlations between right-side hypokinesia and each of the three sentence types in the comprehension task). Note that such FDR correction is appropriate for correcting for small numbers of comparisons (Benjamini & Hochberg, 1995). The FDR threshold was set at .05, and significance is expressed as the FDR-corrected Q-value.
3. Results
3.1. Syntactic processing
3.1.1. Syntactic (sentence) comprehension
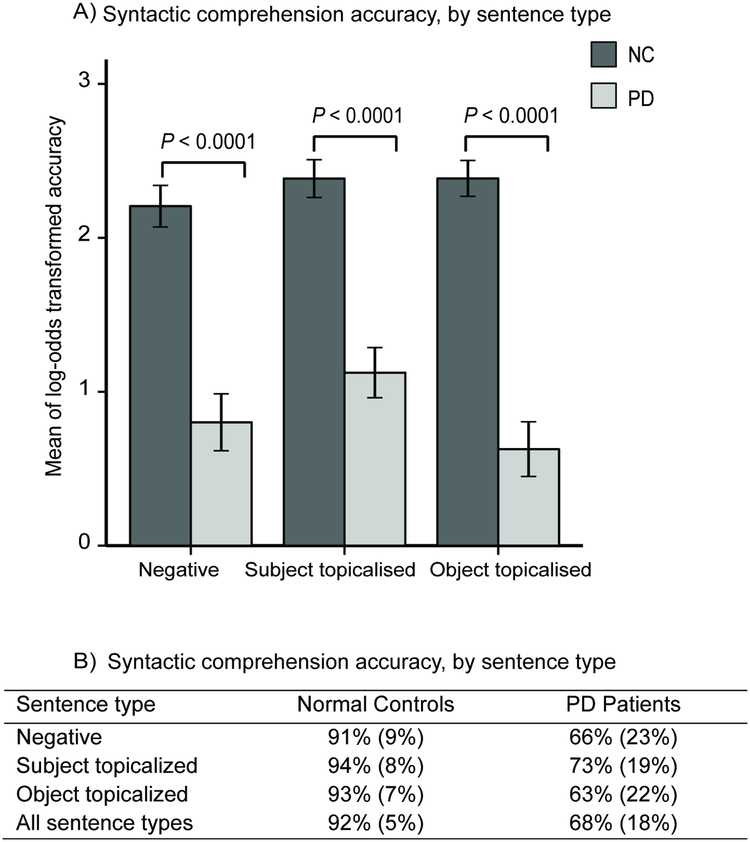
The 2 (group: PD/control) × 3 (sentence type: negative/subject topicalized/object topicalized) ANOVA yielded a statistically significant main effect of group [F(1,78) = 61.45, P < 0.0001; partial η2 = 0.44, a large effect size], with the controls performing better than the PD patients (see Figure 2A and 2B). There were no other main effects or interactions (Ps > 0.18). It might be argued that an MMSE cutoff of ≥ 25 (see section 2.1) is not stringent enough for ruling out individuals with early stages of dementia. We therefore performed this ANOVA again on participants selected with an MMSE cutoff of 27 (i.e., including only participants with MMSE scores ≥ 27), as suggested by an anonymous reviewer. This more stringent cutoff resulted in a sample of 34 PD and 34 NC participants, who again did not differ on age, education, handedness, or MMSE; Ps > 0.44. Analyses on these participants yielded the exact same pattern of significance for all effects in the ANOVA for syntactic comprehension, as well as in all ANOVAs and follow-up analyses below (in sections 3.1.2, 3.2, and 3.3), as the analogous analyses on the full set of 40 PD and 40 NC participants. This suggests that early stages of dementia did not explain the observed results.
Figure 2.
NC (normal control) and PD (Parkinson’s disease) performance at the Farsi syntactic comprehension task. A) Comparisons between NC and PD participants for the three sentence types, showing log-odds transformed accuracy means and standard errors (error bars), with p values from independent measures t-tests. B) Mean untransformed accuracy scores (and standard errors) for each group, for each sentence type.
Neither right- nor left-side hypokinesia correlated with any of the three sentence types, even with MMSE scores and opposite-side hypokinesia partialed out (Qs > 0.53). The LED of the patients’ last anti-PD medication correlated with all three sentence types (large effect sizes), even when partialing out dementia and right-side hypokinesia (all Qs ≤ 0.05). Note that even when variables do no show substantial variability (e.g., dementia), including them as covariates, e.g., in partial correlations, can reduce the error term, and thus should lead to more accurate results.
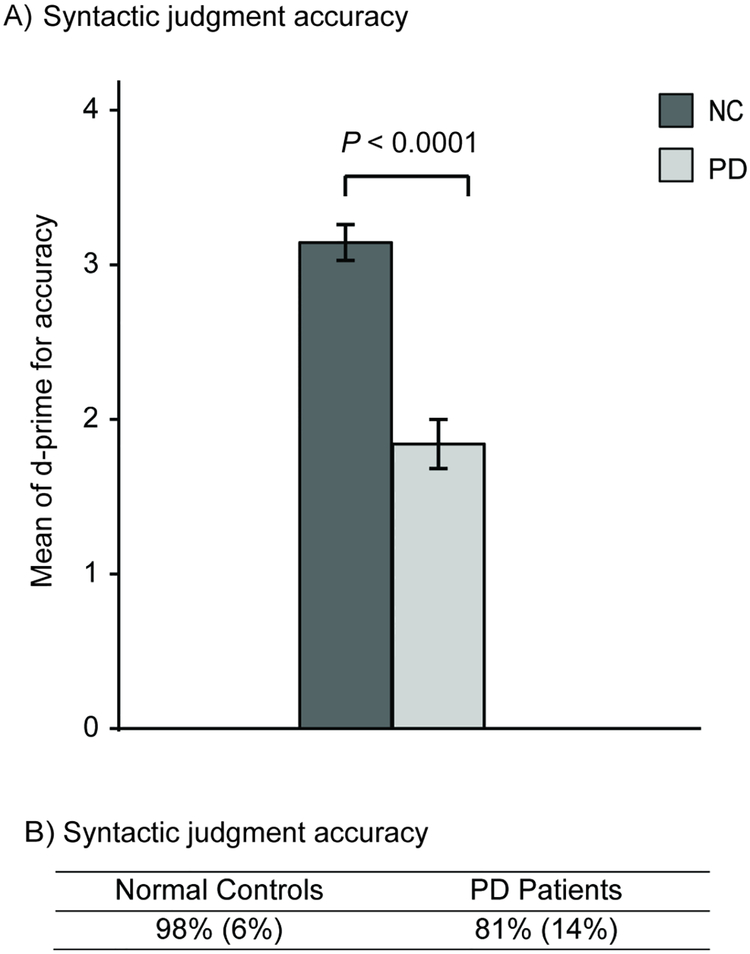
3.1.2. Syntactic judgment
The PD patients showed lower accuracy than the control subjects on the syntactic judgment task [t(78) = 7.55, P < 0.0001]; see Figure 3A and 3B. Neither right- nor left-side hypokinesia correlated with accuracy at syntactic judgment, even with dementia and opposite-side hypokinesia partialed out (Ps > 0.25). Similarly, the LED of the patients’ last medication did not correlate with syntactic judgment, even when partialing out MMSE scores and right-side hypokinesia (Ps > 0.53).
Figure 3.
NC (normal control) and PD (Parkinson’s disease) performance at the Farsi syntactic judgment task. A) Comparisons between NC and PD participants, showing d-prime means and standard errors (error bars), with p value from an independent measures t-test. B) Accuracy results as a percentage (over both correct and incorrect sentences), with mean accuracy scores (and standard errors) shown for each group.
3.2. Lexical processing: naming manipulated and non-manipulated objects
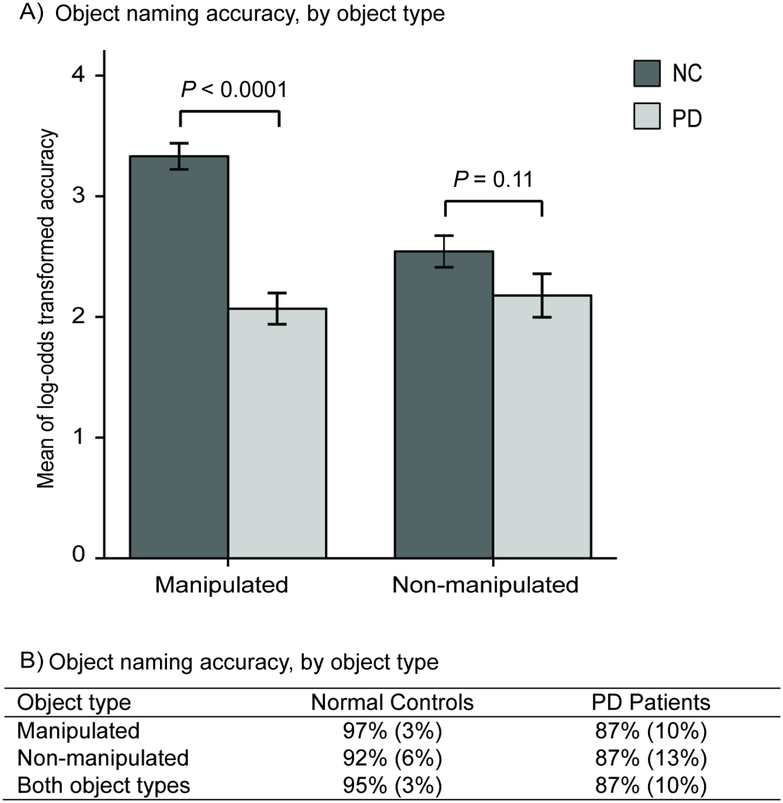
The 2 (group: PD/control) ×2 (object type: manipulated/non-manipulated) ANOVA yielded main effects of group [F(1,78) = 22.63, P < 0.0001; partial η2 = 0.23] and object type [F(1,78) = 11.88, P = 0.001; partial η2 = 0.13], with overall worse performance by the PD patients than controls, and for the non-manipulated than manipulated objects. See Figure 4A and 4B. However, the main effects of group and object type were qualified by a group by object type interaction [F(1,78) = 20.68, P < 0.0001; partial η2 = 0.21, a large effect size]. Follow-up t-tests revealed that the PD patients were less accurate than the controls at naming manipulated objects [t(78) = −7.43, P <0.0001], whereas there was no group difference for naming non-manipulated objects [t(78) =−1.64, P = 0.11]. Moreover, whereas the controls were worse at naming non-manipulated than manipulated objects [t(39) =5.59, P < 0.0001], no such difference was found for the PD patients [t(39) =0.78, P = 0.43]. Neither right- nor left-side hypokinesia correlated with accuracy at naming either object type, even with dementia and opposite-side hypokinesia partialed out (Qs > 0.47). Similarly, the LED of the patients’ last medication did not correlate with either object type, even when partialing out MMSE scores and right-side hypokinesia (Qs > 0.10).
Figure 4.
NC (normal control) and PD (Parkinson’s disease) performance at the Farsi object naming task. A) Comparisons between NC and PD participants for the two object types, showing log-odds transformed accuracy means and standard errors (error bars), with p values from independent measures t-tests. B) Mean untransformed accuracy scores (and standard errors) for each group, for each object type.
3.3. Comparisons between tasks
We also directly compared performance between tasks, within subjects. First, we contrasted syntactic comprehension with naming non-manipulated objects (30 items in both tasks; log-odds transformed accuracy as the dependent measure for both). The 2 (group: PD/control) × 2 (task: syntactic comprehension/object naming) ANOVA on non-manipulated objects yielded not only a main effect of group [F(1,78) = 29.96, P < 0.0001, partial η2 = 0.28], with worse performance by the PD than control group (over both tasks), as well as of task [F(1,78) = 20.19, P < 0.0001, partial η2 = 0.21], with worse performance (over both groups) at syntactic comprehension, but crucially also an interaction between group and task [F(1,78) = 20.32, P < 0.0001, partial η2 = 0.21, a large effect size]. Follow up analyses indicated not only that syntactic comprehension but not naming non-manipulated objects was impaired in PD patients as compared to controls (reported in sections 3.1.1 and 3.2 above, respectively), but also that the PD patients were worse at syntactic comprehension than naming non-manipulated objects [t(39) = 4.79, P < 0.0001], with no such difference for the controls [t(39) = 0.011, P = 0.98]. In contrast, the equivalent ANOVA on manipulated objects (30 items) yielded the expected main effect of group [F(1,78) = 75.42, P < 0.0001, partial η2 = 0.49, a large effect size], as well as a main effect of task [F(1,78) = 49.86, P < 0.0001, partial η2 = 0.39], indicating worse performance at syntactic comprehension than naming manipulated objects, but, crucially, no interaction. Thus the PD patients were 1) more impaired at syntactic comprehension than lexical processing (object naming) of non-manipulated objects (which indeed were unimpaired; section 3.2); and 2) equally impaired at syntactic comprehension and lexical processing of manipulated objects.
Next we performed analogous comparisons between syntactic judgment and naming non-manipulated or manipulated objects. Because the dependent measures (and number of items; 20 items in syntactic judgment) on these tasks differed, we first computed z-scores 1) for the syntactic judgment d’ values (that is, over both the PD and control subjects; thus a z-score of 0 represents the mean d’ value over both groups); 2) similarly, for the log-odds transformed accuracy values for naming manipulated objects; and 3) again, for non-manipulated objects. The 2 (group: PD/control) × 2 (task: syntactic judgment/object naming) ANOVA on non-manipulated objects yielded a main effect of group [F(1,78) = 28.39, P <0.0001, partial η2 = 0.28], with worse performance by the PD than control group, no effect of task [F(1,78) = 0, P =1, partial η2 = 0; as expected, given that the analysis was performed on z-scores computed separately for each task], but crucially an interaction between group and syntactic judgment/object naming [F(1,78) = 14.39, P <0.0001, partial η2 = 0.16, a large effect size]. Follow-up analyses revealed not only PD impairments at syntactic judgment but not at naming non-manipulated objects, as compared to controls (reported in sections 3.1.2 and 3.2 above), but also that the PD patients were worse at syntactic judgment than naming non-manipulated objects [t(39) = −2.39, P = 0.02], whereas controls showed the opposite pattern on the z-scores [t(39) = 3.10, P = 0.004]. In contrast, the equivalent ANOVA on manipulated objects yielded the predicted main effect of group [F(1,78) = 81.67, P <0.0001, partial η2 = 0.51, a large effect size], no effect of task [F(1,78) = 0, P =1, partial η2 = 0; again, as expected], and, crucially, no interaction [F(1,78) = 0.001, P =0.97, partial η2 < 0.0001]. Thus, the same pattern was found in comparisons between syntactic judgment and naming non-manipulated/manipulated objects as for the comparisons between syntactic comprehension and naming non-manipulated/manipulated objects.
Finally, we examined whether syntactic comprehension or syntactic judgment might be differentially or similarly impaired, based on z-scores computed separately from both tasks. The 2 (group: PD/control) × 2 (task: syntactic comprehension/syntactic judgment) ANOVA yielded only a main effect of group [F(1,78) = 60.35, P <0.0001, partial η2 = 0.52, a large effect size], with no effect of Task [F(1,78) = 0, P =1, partial η2 = 0; as expected] and no interaction [F(1,78) = 0.31, P =0.58, partial η2 = 0.004]. Thus, syntactic comprehension and syntactic judgment showed similar levels of impairment in the PD patients.
4. Discussion
This study examined syntactic and lexical processing within subjects, in moderate-to-severe non-demented PD patients and healthy controls. All participants were native speakers of Farsi and were tested on Farsi. PD patients were impaired at both syntactic comprehension (equivalently across negative, subject topicalized, and object topicalized sentences) and syntactic judgment (various structures), and at naming manipulated objects but not at naming non-manipulated objects. In direct comparisons between tasks, the PD patients were equivalently impaired at syntactic comprehension, syntactic judgment, and naming manipulated objects, while both syntactic comprehension and syntactic judgment were more impaired than naming non-manipulated objects. Levodopa equivalent dose correlated with syntactic comprehension (for all three sentence types) but not with syntactic judgment or naming either object type. Neither right-nor left-side hypokinesia correlated with any syntactic or object naming measure. All critical significant main effects, interactions, and correlations yielded large effect sizes.
The results do not appear to be explained by a number of potentially confounding subject- or item-level factors. The PD and control participants were matched on age, education, handedness, and (lack of) dementia, and the manipulated and non-manipulated items in the object naming task were matched on phonological length, orthographic length, and frequency. Moreover, the same pattern of results was found with more stringent MMSE cutoffs, or when partialling out MMSE scores.
In the next two sections (4.1 and 4.2) we discuss our results in relation to previous findings in order to draw conclusions about broader patterns regarding the status in PD of syntactic processing (Research Question 1), lexical processing (Research Question 2), relative deficits of syntactic vs. lexical processing (Research Question 3), and associations between syntactic/lexical processing and measures of likely neurocognitive substrates (Research Question 4). In the following section (4.3) we discuss these patterns in relation to previously posited neurocognitive accounts of syntactic and lexical deficits in PD. Finally, after a brief discussion of limitations, we summarize the study and conclude.
4.1. Syntactic vs. lexical processing (Research Questions 1, 2, and 3)
The findings suggest that non-demented moderate-to-severe PD patients show reliable syntactic processing deficits. Impairments were found across syntactic comprehension and syntactic judgment, and across different syntactic structures. The syntactic comprehension deficits observed here are consistent with previous observations of deficits in most studies of syntactic comprehension in PD (all in earlier stage PD), but extend them to Farsi as well as to syntactic structures that have previously been unstudied in PD. It is unclear why two previous PD studies found spared syntactic comprehension (Prieto et al., 2007; Terzi et al., 2005), though their findings might be attributable to the fact that most of the patients in these studies were at earlier stages, and/or that the constructions examined (passives) are not as complex as those in studies that have reported deficits. Our findings complement the one previous PD study of syntactic judgment we are aware of (McNamara et al., 1996), which also found impairments. Moreover, our between-task analysis indicates that syntactic comprehension and syntactic judgment are equivalently impaired. Overall, the data from the present study and previous research suggests that 1) across languages and language families, syntactic comprehension is largely impaired in PD, perhaps especially (but not only; e.g., García et al., 2017) at more advanced stages of the disease and for more complex syntactic structures; 2) syntactic judgment may be more consistently impaired in PD, though further studies are clearly needed; 3) at least in non-demented moderate-to-severe PD, patients show equivalent impairments at syntactic comprehension and judgment, across syntactic structures.
The findings from the object naming task suggest that moderate-to-severe non-demented PD is associated with impairments at naming manipulated objects but not at naming non-manipulated objects. Thus, these patients show deficits at lexical processing (at least with object naming) for manipulated objects, even though such impairments are not clearly observed in earlier stages of PD (Bocanegra et al., 2017; Buccino et al., 2018). Moreover, the findings support the view that PD deficits that have been observed for action naming as compared to object naming are at least partly due to differences in their relations to motor functions, and not simply a result of verb/noun difference (Birba et al., 2017; Bocanegra et al., 2017; Bocanegra et al., 2015; Buccino et al., 2018; Fernandino et al., 2013; Gallese, 2008; Gallese & Cuccio, 2018; García et al., 2018; Herrera et al., 2012; Roberts et al., 2017; Speed et al., 2017).
The direct comparisons between the syntactic processing tasks and object naming revealed that both syntactic comprehension and syntactic judgment yielded significantly worse performance in the PD patients than naming non-manipulated objects, but not than naming manipulated objects. Indeed, PD performance did not differ among syntactic comprehension, syntactic judgment, and naming manipulated objects. We are not aware of any prior studies reporting such direct comparisons within subjects. The task comparisons suggest that non-demented PD patients can show 1) equivalent impairments at quite different tasks of syntactic processing and lexical processing, as long as the latter involve motor-related words, and 2) spared lexical processing of non-motor related words, even when syntactic processing and lexical processing of motor-related words are impaired.
The fact that naming reasonably common non-manipulated objects was not significantly impaired suggests that lexical retrieval itself may be relatively spared in non-demented PD, even at moderate-to-severe stages. Previous studies reporting greater PD deficits at naming action than object words have often found impairments in naming objects as well as actions (Cotelli et al., 2007; Crescentini, Mondolo, Biasutti, & Shallice, 2008; Patrice Péran et al., 2003; Rodríguez-Ferreiro et al., 2009). However, the nature of the objects in at least some of these studies has been unspecified (Cotelli et al., 2007; Rodríguez-Ferreiro et al., 2009), leaving open the possibility that many manipulated objects were included. Indeed, one study, which specified that half the objects were “natural” (which seem unlikely to be commonly manipulated), did not find a PD impairment in their object naming task (Silveri et al., 2012). In addition, another study found that lexical processing of non-motor-related words was impaired in PD patients with mild cognitive impairment, but not in patients without mild cognitive impairment (Bocanegra et al., 2017). Together, the data suggest that lexical retrieval can remain relatively spared in PD, even at more advanced stages (Silveri et al., 2012), at least for words not related to motor functions, and in patients without dementia or cognitive impairment.
We emphasize that it does not appear to be the case that there are no impairments at all in lexical retrieval in PD. Indeed, such impairments have long been reported (Matison et al., 1982), and are consistent with evidence suggesting that certain frontal/basal ganglia circuits may subserve lexical recall (Ullman, 2006). Rather, the presence of lexical deficits in PD may depend on a number of factors, including not only the semantics of the word (in particular, impairments may be found on items with motor skill associations), but also the type of task (cued recall such as object naming may be easier than free recall, and comprehension may be easiest of all; York et al., 2014), disease stage (more advanced stages may be more likely to affect circuits underlying lexical recall), and dementia (which can affect declarative memory (Piatt, Fields, Paolo, Koller, & Tröster, 1999), which in turn seems to underlie lexical memory (Ullman, 2016)). Nevertheless, we suggest that even in the presence of such aggravating factors, let alone in their absence, lexical retrieval tends to remain relatively spared in PD patients as compared to grammar, as was observed in the present study.
4.2. Associations between syntactic/lexical processing and hypokinesia/LED (Research Question 4)
The absence of correlations between performance at the syntactic tasks and hypokinesia suggests that the syntactic impairments in PD are not linked closely to the neural correlates of either right- or left-side hypokinesia, which primarily reflect degeneration of contralateral basal ganglia circuits related to motor function (Chesselet & Delfs, 1996; Desmurget, Grafton, Vindras, Grea, & Turner, 2004; Penney & Young, 1983). As mentioned in the Introduction, we are not aware of any prior studies examining such associations between syntactic processing and hypokinesia. However, studies have reported correlations between right-side (but not left-side) hypokinesia and the processing of regular inflectional morphological forms, which, like syntactic structures, are thought to undergo rule-governed (de)composition (K Johari et al., 2019; Reifegerste et al., under revision; Ullman et al., 1997). Indeed, this correlational pattern was found for Farsi regular inflectional morphology in the same patients that were examined here (K Johari et al., 2019). In contrast, these studies did not observe correlations between hypokinesia and the processing of irregular inflectional morphological forms, which are thought to be retrieved from lexical memory. These contrasting findings from the present study and other research suggest that the neurocognitive basis of syntactic comprehension and judgment impairments in PD differs from that of impairments of regular morphology in the disorder, despite the fact that both involve rule-governed grammatical processes (see below for explanatory accounts).
The finding that syntactic comprehension but not syntactic judgment correlated with LED links the former but not the latter to dopaminergic processes in PD. The fact that there were equivalent impairments in syntactic comprehension and judgment suggests this discrepancy was not due to differences in subject variability between the tasks. The results are consistent with findings from on/off levodopa studies reporting positive effects of levodopa on syntactic comprehension but not judgment (McNamara et al., 1996), and on syntactic comprehension in more complex sentences but not simpler sentences (Grossman et al., 2001). Despite one prior study that did not find any such effects, in syntactic comprehension of sentences with various levels of complexity (Skeel et al., 2001), the bulk of the evidence thus suggests that syntactic comprehension is linked to dopaminergic processes, whereas this does not appear to be the case for syntactic judgment. Note that the same PD participants that were tested here also showed correlations between LED and Farsi regular (but not irregular) inflectional morphology. Together, the findings suggest that even though syntactic comprehension and regular morphology differ in their links to motor-related basal ganglia circuits, they are both modulated by dopaminergic processes (see below).
The absence of correlations between naming manipulated (and non-manipulated) objects with either hypokinesia or LED suggests that the deficit at naming manipulated objects depends on somewhat different circuits than (left- or right-side) hypokinesia, despite the strong motor component of both, and is not modulated by dopamine. As mentioned in the Introduction, action naming in PD has been linked to motor related circuits, including the basal ganglia, as well as to dopaminergic processes (Herrera & Cuetos, 2012; P Péran et al., 2013), though we are not aware of any other studies examining associations between hypokinesia and naming action verbs or manipulated objects. The findings in the present study thus seem somewhat inconsistent with prior research, though the reasons for this discrepancy remain unclear. We suggest that one possibility is that the non-motor knowledge associated with manipulated objects (e.g., what a hammer looks like, who uses it) is relied on heavily by PD patients in naming such objects, whose motor skill knowledge may be degraded or difficult to access.
4.3. Explanatory neurocognitive accounts
Although this study was not designed to test any particular theoretical framework, it may at least partially elucidate neurocognitive accounts of both syntactic and lexical processing in PD. Syntactic and other grammatical impairments have been explained by (at least) three neurocognitive accounts. First, in the context of the declarative/procedural model of language, such impairments have been explained by the dysfunction of procedural memory, which is rooted in frontal/basal ganglia circuits and is affected in the disorder (K Johari et al., 2019; Ullman, 2004; Ullman et al., 1997). Second, and related, it has been posited that the syntactic deficits in PD may be explained by the ‘disrupted motor grounding hypothesis’, which conceptualizes the deficits as disruptions of embodied mechanisms rooted in fronto-striatal circuits (Birba et al. 2017). Third, syntactic comprehension deficits in PD have been linked to executive deficits, in particular working memory, which indeed is impaired in the disorder (Birba et al., 2017; Bocanegra et al., 2015; Grossman et al., 2000).
The lack of correlations between hypokinesia and either syntactic comprehension or syntactic judgment suggests that neither type of syntactic processing deficit in PD is linked closely to the degeneration of basal ganglia motor-related circuits. This argues against such processing deficits – at least in the current study – being explained by a strong version of the disrupted motor grounding hypothesis. Similarly, it argues against the notion that procedural memory deficits underlie such syntactic impairments – at least such deficits that are closely tied to motor circuits (Ullman, 2004, 2016; Ullman & Pierpont, 2005). This contrasts with findings from regular morphology, including from the same patients that were examined here, which show strong correlations with right-side hypokinesia (K Johari et al., 2019; Reifegerste et al., under revision; Ullman et al., 1997). Thus, even if such accounts may not explain significant syntactic processing variability in PD, they appear to successfully explain grammatical problems in rule-governed morphological processing. Finally, it is possible that either or both of these views (procedural memory deficits and the disrupted motor grounding hypothesis) may explain at least some of the observed syntactic processing impairments, but other factors not directly examined in the present study (e.g., working memory) account for most of the variability. For example, increasing evidence suggests that declarative memory can compensate for at least some grammatical (and other) deficits in PD (Ullman & Pullman, 2015).
Indeed, the syntactic comprehension and judgment impairments observed here and in other studies of PD may be at least partly explained by deficits of working memory or other executive functions. Such deficits have been observed in PD (Birba et al., 2017; Gabrieli, Singh, Stebbins, & Goetz, 1996), consistent with links between executive function and working memory and both the basal ganglia and frontal structures (Ullman, 2006; Voytek & Knight, 2010). In fact, it has been suggested that resource limitations, including problems with working memory, contribute to syntactic comprehension difficulties in the disorder (Grossman et al., 2000). The view that working memory or other executive deficits may help explain syntactic processing impairments in PD is bolstered by evidence suggesting that within the striatum of the basal ganglia, motor function, as reflected in hypokinesia, is strongly linked to the putamen (Doyon et al., 2009; Sapir, Kaplan, He, & Corbetta, 2007), whereas working memory and other executive functions depends more heavily on (anterior portions of) the caudate nucleus (Draganski et al., 2008; S. J. Lewis, Dove, Robbins, Barker, & Owen, 2004; Postuma & Dagher, 2005; Seger, Peterson, Cincotta, Lopez-Paniagua, & Anderson, 2010). Thus, the absence of correlations between syntactic processing abilities in PD and hypokinesia suggest that putamenal degeneration might not play an important explanatory role in syntactic deficits in PD. This in turn increases the likelihood that degeneration of the caudate nucleus (the other structure in the striatum) underlies these deficits, thereby underscoring a possible role for executive functions, including working memory. This view is further strengthened by previous imaging work linking syntactic comprehension deficits in PD to the caudate nucleus (Grossman et al., 1993). Moreover, the evidence from this and previous studies that syntactic comprehension is modulated by dopaminergic processes is consistent with working memory deficits, since evidence suggests that levodopa modulates working memory deficits in PD (S. J. Lewis, Slabosz, Robbins, Barker, & Owen, 2005). Indeed, syntactic comprehension may depend particularly on working memory (King & Just, 1991; Miyake, Carpenter, & Just, 1994; Zurif, Swinney, Prather, Wingfield, & Brownell, 1995), consistent with the correlation between LED and syntactic comprehension but not syntactic judgment. Thus, overall, the evidence seems to be in line with the view that syntactic comprehension deficits in PD may be modulated primarily by deficits of working memory (and perhaps other executive functions), whereas this is less clear for the syntactic judgment deficits. In contrast neither type of deficit seems to be closely tied to the degeneration of basal ganglia motor-related circuits – even if those play a more important role in the processing of simpler rule-governed grammatical forms such as in regular morphology.
The observed impairments in naming manipulated (but not non-manipulated) objects seem to be more easily explained. In particular, these results, together with a large literature of prior findings of deficits in action verbs and related results (see Introduction), may be largely explained by accounts linking difficulties in processing words with motor-related functions to deficits of motor-related circuitry in PD. These accounts include posited deficits in procedural memory (Ullman, 2004, 2016), the disrupted motor grounding hypothesis (Birba et al., 2017), and the ‘neural exploitation hypothesis’ (Gallese, 2008; Gallese & Cuccio, 2018). Such views are consistent with the embodied cognition framework (Birba et al., 2017; Bocanegra et al., 2015; Gallese, 2008; Gallese & Cuccio, 2018). The absence of correlations between naming manipulated objects and hypokinesia is somewhat puzzling in this context, though, as we suggested above, it is possible that the non-motor semantic knowledge of manipulated objects is relied on especially in such patients during lexical processing. That is, such declarative memory-based knowledge may play a compensatory role, as it also appears to for other aspects of language and non-language functions in the PD (see above, and Ullman and Pullman, 2015). Future studies may elucidate this issue.
4.4. Limitations and future studies
This study has limitations and suggests future lines of research. First, measures of working memory and other executive functions were not tested, and thus we could not examine whether deficits in these functions were associated with the observed syntactic (and lexical) processing deficits. Other functions such as procedural and declarative memory that may have elucidated the nature of the impairments were also not examined. Second, the study could have also probed the processing of action verbs, to test whether they too showed impairments, and whether such impairments were linked to naming manipulated (but not non-manipulated objects), and perhaps to hypokinesia and LED. Third, the study focused on later stages of PD, and moreover non-demented patients. Future studies with a similar design could also examine patients at earlier stages of the disease, and both with and without dementia or cognitive impairment.
4.5. Conclusion
In sum, the present study, which examined multiple aspects of syntactic and lexical processing in Farsi within subjects in non-demented PD patients at moderate-to-severe stages, yielded the following key findings. First, syntactic processing was reliably and similarly impaired across syntactic comprehension and syntactic judgment and across different syntactic structures. Second, lexical processing, as tested in object naming, was impaired for nouns linked to motor functions (manipulated objects such as ‘hammer’), but not for nouns that are not associated with motor functions (non-manipulated objects such as ‘mountain’). Third, in direct comparisons, syntactic comprehension, syntactic judgment, and naming motor-related words showed equivalent impairments, and all of these were more impaired than naming non-motor related words. Fourth, syntactic comprehension correlated with LED but not hypokinesia; neither syntactic judgment nor naming either manipulated or non-manipulated objects correlated with either LED or hypokinesia.
Together with findings from previous studies, the results suggest the following. Syntactic comprehension is impaired in PD, across languages and language families, perhaps especially for more complex syntactic structures and at more advanced stages of the disease. Syntactic judgment is also impaired in PD, with equivalent impairments as syntactic comprehension, though more studies are needed. Lexical processing of words linked to motor functions is impaired, particularly at more advanced stages of PD. In contrast, the processing of words not linked to motor functions can remain unimpaired, even in (non-demented) moderate-to-severe PD. Syntactic comprehension and syntactic judgment show similar impairments as lexical processing of motor-related words, but are more impaired than lexical processing of non-motor-related words, even in more advanced (non-demented) patients. Dopaminergic processes appear to modulate deficits of syntactic comprehension but perhaps not (or less so) deficits of syntactic judgment or lexical processing. PD deficits at both types of syntactic tasks, though perhaps especially syntactic comprehension, may be best explained by executive function impairments, in particular of working memory. Overall, the findings elucidate the nature of syntactic and lexical deficits in PD.
Highlights:
We probed syntactic and lexical processing in Farsi-speaking Parkinson’s patients
Syntax showed clear deficits: in judgment and comprehension, across structures
Lexical processing was unimpaired, except for motor-related words (e.g., hammer)
Syntactic comprehension was modulated by patients’ last levodopa equivalent dose
The study elucidates language in Parkinson’s, moreover in an understudied language
Acknowledgments
The authors are grateful to the patients and healthy controls for their participation, and to the Functional Neurosurgery Research Center and the Shohadaye Tajrish Hospital in Tehran for accommodating this research.
Funding
This work was supported in part by NSF BCS 1439290, NIH R21 HD 087088, and the Mabel H. Flory Trust to Michael Ullman, and by the Tabriz University of Medical Sciences and the Shahid Beheshti University of Medical Sciences to Karim Johari. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrevaya S, Sedeño L, Fitipaldi S, Pineda D, Lopera F, Buritica O, … Trujillo N (2017). The road less traveled: alternative pathways for action-verb processing in Parkinson’s disease. Journal of Alzheimer’s Disease, 55(4), 1429–1435. [DOI] [PubMed] [Google Scholar]
- Angwin AJ, Chenery HJ, Copland DA, Murdoch BE, & Silburn PA (2005). Summation of semantic priming and complex sentence comprehension in Parkinson’s disease. Cognitive Brain Research, 25(1), 78–89. [DOI] [PubMed] [Google Scholar]
- Angwin AJ, Dissanayaka NN, Moorcroft A, McMahon KL, Silburn PA, & Copland DA (2017). A neurophysiological study of semantic processing in Parkinson’s disease. Journal of the International Neuropsychological Society, 23(1), 78–89. [DOI] [PubMed] [Google Scholar]
- Ansari NN, Naghdi S, Hasson S, Valizadeh L, & Jalaie S (2010). Validation of a Mini-Mental State Examination (MMSE) for the Persian population: a pilot study. Applied neuropsychology, 17(3), 190–195. [DOI] [PubMed] [Google Scholar]
- Assi SM. Persian Linguistic Database [Internet] Retrieved from http://pldb.ihcs.ac.ir/. from Institute for Humanities and Cultural Studies http://pldb.ihcs.ac.ir/
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the royal statistical society. Series B (Methodological), 289–300. [Google Scholar]
- Berardelli A, Rothwell J, Thompson P, & Hallett M (2001). Pathophysiology of bradykinesia in Parkinson’s disease. Brain, 124(11), 2131–2146. [DOI] [PubMed] [Google Scholar]
- Birba A, García-Cordero I, Kozono G, Legaz A, Ibáñez A, Sedeño L, & García AM (2017). Losing ground: frontostriatal atrophy disrupts language embodiment in Parkinson’s and Huntington’s disease. Neuroscience & Biobehavioral Reviews, 80, 673–687. [DOI] [PubMed] [Google Scholar]
- Bocanegra Y, García AM, Lopera F, Pineda D, Baena A, Ospina P, … Ibáñez A (2017). Unspeakable motion: Selective action-verb impairments in Parkinson’s disease patients without mild cognitive impairment. Brain and language, 168, 37–46. [DOI] [PubMed] [Google Scholar]
- Bocanegra Y, García AM, Pineda D, Buriticá O, Villegas A, Lopera F, … Trujillo N (2015). Syntax, action verbs, action semantics, and object semantics in Parkinson’s disease: Dissociability, progression, and executive influences. Cortex, 69, 237–254. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Mechtouff L, Thobois S, Broussolle E, Jeannerod M, & Nazir TA (2008). Word processing in Parkinson’s disease is impaired for action verbs but not for concrete nouns. Neuropsychologia, 46(2), 743–756. [DOI] [PubMed] [Google Scholar]
- Buccino G, Dalla Volta R, Arabia G, Morelli M, Chiriaco C, Lupo A, … Quattrone A (2018). Processing graspable object images and their nouns is impaired in Parkinson’s disease patients. Cortex, 100, 32–39. [DOI] [PubMed] [Google Scholar]
- Cardona JF, Gershanik O, Gelormini-Lezama C, Houck AL, Cardona S, Kargieman L, … Manes F (2013). Action-verb processing in Parkinson’s disease: new pathways for motor–language coupling. Brain Structure and Function, 218(6), 1355–1373. [DOI] [PubMed] [Google Scholar]
- Cardona JF, Kargieman L, Sinay V, Gershanik O, Gelormini C, Amoruso L, … Michon M (2014). How embodied is action language? Neurological evidence from motor diseases. Cognition, 131(2), 311–322. [DOI] [PubMed] [Google Scholar]
- Castner JE, Chenery HJ, Copland DA, Coyne TJ, Sinclair F, & Silburn PA (2007). Semantic and affective priming as a function of stimulation of the subthalamic nucleus in Parkinson’s disease. Brain, 130(5), 1395–1407. [DOI] [PubMed] [Google Scholar]
- Chesselet M-F, & Delfs JM (1996). Basal ganglia and movement disorders: an update. Trends in neurosciences, 19(10), 417–422. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, & Goodale MA (2010). Category-specific neural processing for naming pictures of animals and naming pictures of tools: An ALE meta-analysis. Neuropsychologia, 48(2), 409–418. [DOI] [PubMed] [Google Scholar]
- Cohen H (1998). Language impairment in Parkinson’s Disease Handbook of neurolinguistics (pp. 475–483): Elsevier. [Google Scholar]
- Copland D (2003). The basal ganglia and semantic engagement: potential insights from semantic priming in individuals with subcortical vascular lesions, Parkinson’s disease, and cortical lesions. Journal of the International Neuropsychological Society, 9(7), 1041–1052. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Borroni B, Manenti R, Zanetti M, Arévalo A, Cappa S, & Padovani A (2007). Action and object naming in Parkinson’s disease without dementia. European Journal of Neurology, 14(6), 632–637. [DOI] [PubMed] [Google Scholar]
- Crescentini C, Mondolo F, Biasutti E, & Shallice T (2008). Supervisory and routine processes in noun and verb generation in nondemented patients with Parkinson’s disease. Neuropsychologia, 46(2), 434–447. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, & Damasio AR (1996). A neural basis for lexical retrieval. Nature, 380(6574), 499. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S, Vindras P, Grea H, & Turner RS (2004). The basal ganglia network mediates the planning of movement amplitude. European Journal of Neuroscience, 19(10), 2871–2880. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, … Benali H (2009). Contributions of the basal ganglia and functionally related brain structures to motor learning. Behavioural brain research, 199(1), 61–75. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJ, … Frackowiak RS (2008). Evidence for segregated and integrative connectivity patterns in the human basal ganglia. Journal of Neuroscience, 28(28), 7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandino L, Conant LL, Binder JR, Blindauer K, Hiner B, Spangler K, & Desai RH (2013). Parkinson’s disease disrupts both automatic and controlled processing of action verbs. Brain and language, 127(1), 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA, Werheid K, Hein G, & von Cramon DY (2003). Syntactic comprehension in Parkinson’s disease: Investigating early automatic and late integrational processes using event-related brain potentials. Neuropsychology, 17(1), 133. [PubMed] [Google Scholar]
- Gabrieli JD, Singh J, Stebbins GT, & Goetz CG (1996). Reduced working memory span in Parkinson’s disease: Evidence for the role of frontostriatal system in working and strategic memory. Neuropsychology, 10(3), 322. [Google Scholar]
- Gallese V (2008). Mirror neurons and the social nature of language: The neural exploitation hypothesis. Social neuroscience, 3(3–4), 317–333. [DOI] [PubMed] [Google Scholar]
- Gallese V, & Cuccio V (2018). The neural exploitation hypothesis and its implications for an embodied approach to language and cognition: insights from the study of action verbs processing and motor disorders in Parkinson’s disease. Cortex, 100, 215–225. [DOI] [PubMed] [Google Scholar]
- García AM, Bocanegra Y, Herrera E, Moreno L, Carmona J, Baena A, … Legaz A (2018). Parkinson’s disease compromises the appraisal of action meanings evoked by naturalistic texts. Cortex, 100, 111–126. [DOI] [PubMed] [Google Scholar]
- García AM, Carrillo F, Orozco-Arroyave JR, Trujillo N, Bonilla JFV, Fittipaldi S, … Slezak DF (2016). How language flows when movements don’t: an automated analysis of spontaneous discourse in Parkinson’s disease. Brain and language, 162, 19–28. [DOI] [PubMed] [Google Scholar]
- García AM, Sedeño L, Trujillo N, Bocanegra Y, Gomez D, Pineda D, … Ibáñez A (2017). Language deficits as a preclinical window into Parkinson’s disease: evidence from asymptomatic parkin and dardarin mutation carriers. Journal of the International Neuropsychological Society, 23(2), 150–158. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez‐Martin P, … Dodel R (2008). Movement Disorder Society‐sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS‐UPDRS): Scale presentation and clinimetric testing results. Movement disorders, 23(15), 2129–2170. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fadiga L, Arbib MA, & Rizzolatti G (1997). Premotor cortex activation during observation and naming of familiar tools. Neuroimage, 6(4), 231–236. [DOI] [PubMed] [Google Scholar]
- Grossman M (1999). Sentence processing in Parkinson’s disease. Brain and Cognition, 40(2), 387–413. [DOI] [PubMed] [Google Scholar]
- Grossman M, Carvell S, Gollomp S, Stern MB, Reivich M, Morrison D, … Hurtig HI (1993). Cognitive and physiological substrates of impaired sentence processing in Parkinson’s disease. Journal of Cognitive Neuroscience, 5(4), 480–498. [DOI] [PubMed] [Google Scholar]
- Grossman M, Carvell S, Stern MB, Gollomp S, & Hurtig HI (1992). Sentence comprehension in Parkinson’s disease: The role of attention and memory. Brain and language, 42(4), 347–384. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Lee C, Alsop D, Detre J. e., … Hurtig H (2003). Grammatical and resource components of sentence processing in Parkinson’s disease: An fMRI study. Neurology, 60(5), 775–781. [DOI] [PubMed] [Google Scholar]
- Grossman M, Glosser G, Kalmanson J, Morris J, Stern MB, & Hurtig HI (2001). Dopamine supports sentence comprehension in Parkinson’s disease. Journal of the Neurological Sciences, 184(2), 123–130. [DOI] [PubMed] [Google Scholar]
- Grossman M, Kalmanson J, Bernhardt N, Morris J, Stern MB, & Hurtig HI (2000). Cognitive resource limitations during sentence comprehension in Parkinson’s disease. Brain and language, 73(1), 1–16. [DOI] [PubMed] [Google Scholar]
- Grossman M, Lee C, Morris J, Stern MB, & Hurtig HI (2002). Assessing resource demands during sentence processing in Parkinson’s disease. Brain and language, 80(3), 603–616. [DOI] [PubMed] [Google Scholar]
- Hedenius M, Ullman MT, Alm P, Jennische M, & Persson J (2013). Enhanced recognition memory after incidental encoding in children with developmental dyslexia. PloS one, 8(5), e63998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, & Cuetos F (2012). Action naming in Parkinson’s disease patients on/off dopamine. Neuroscience letters, 513(2), 219–222. [DOI] [PubMed] [Google Scholar]
- Herrera E, Rodríguez-Ferreiro J, & Cuetos F (2012). The effect of motion content in action naming by Parkinson’s disease patients. Cortex, 48(7), 900–904. [DOI] [PubMed] [Google Scholar]
- Hochstadt J (2009). Set-shifting and the on-line processing of relative clauses in Parkinson’s disease: Results from a novel eye-tracking method. Cortex, 45(8), 991–1011. [DOI] [PubMed] [Google Scholar]
- Hochstadt J, Nakano H, Lieberman P, & Friedman J (2006). The roles of sequencing and verbal working memory in sentence comprehension deficits in Parkinson’s disease. Brain and language, 97(3), 243–257. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, & Yahr MD (1998). Parkinsonism: onset, progression, and mortality. Neurology, 50(2), 318–318. [DOI] [PubMed] [Google Scholar]
- Ibáñez A, Cardona JF, Dos Santos YV, Blenkmann A, Aravena P, Roca M, … Gómez-Arévalo G (2013). Motor-language coupling: direct evidence from early Parkinson’s disease and intracranial cortical recordings. Cortex, 49(4), 968–984. [DOI] [PubMed] [Google Scholar]
- Illes J, Metter E, Hanson W, & Iritani S (1988). Language production in Parkinson’s disease: Acoustic and linguistic considerations. Brain and language, 33(1), 146–160. [DOI] [PubMed] [Google Scholar]
- Ito J, & Kitagawa J (2006). Performance monitoring and error processing during a lexical decision task in patients with Parkinson’s disease. Journal of Geriatric Psychiatry and Neurology, 19(1), 46–54. [DOI] [PubMed] [Google Scholar]
- Jaeger TF (2008). Categorical data analysis: Away from ANOVAs (transformation or not) and towards logit mixed models. Journal of memory and language, 59(4), 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johari K, Ashrafi F, Zali A, Ashayeri H, Fabbro F, & Zanini S (2013). Grammatical deficits in bilingual Azari–Farsi patients with Parkinson’s disease. Journal of Neurolinguistics, 26(1), 22–30. [Google Scholar]
- Johari K, Walenski M, Reifegerste J, Ashrafi F, & Ullman M (2019). Sex, dopamine, and hypokinesia: A study of inflectional morphology in Parkinson’s disease. Neuropsychology. [DOI] [PubMed] [Google Scholar]
- King J, & Just MA (1991). Individual differences in syntactic processing: The role of working memory. Journal of memory and language, 30(5), 580–602. [Google Scholar]
- Lewis FM, Lapointe LL, Murdoch BE, & Chenery HJ (1998). Language impairment in Parkinson’s disease. Aphasiology, 12(3), 193–206. [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, & Owen AM (2004). Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. European Journal of Neuroscience, 19(3), 755–760. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Slabosz A, Robbins TW, Barker RA, & Owen AM (2005). Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson’s disease. Neuropsychologia, 43(6), 823–832. [DOI] [PubMed] [Google Scholar]
- Lieberman P, Kako E, Friedman J, Tajchman G, Feldman LS, & Jiminez EB (1992). Speech production, syntax comprehension, and cognitive deficits in Parkinson’s disease. Brain and language, 43(2), 169–189. [DOI] [PubMed] [Google Scholar]
- Lukács Á, Kemény F, Lum JA, & Ullman MT (2017). Learning and overnight retention in declarative memory in specific language impairment. PloS one, 12(1), e0169474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, & Haxby JV (1996). Neural correlates of category-specific knowledge. Nature, 379(6566), 649. [DOI] [PubMed] [Google Scholar]
- Matison R, Mayeux R, Rosen J, & Fahn S (1982). “Tip‐of‐the‐tongue” phenomenon in Parkinson disease. Neurology, 32(5), 567–567. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Shabbott B, & Cortés JC (2012). Motor control abnormalities in Parkinson’s disease. Cold Spring Harbor perspectives in medicine, 2(6), a009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P, Krueger M, O’quin K, Clark J, & Durso R (1996). Grammaticality judgments and sentence comprehension in Parkinson’s disease: A comparison with Broca’s aphasia. International Journal of Neuroscience, 86(1–2), 151–166. [DOI] [PubMed] [Google Scholar]
- Melloni M, Sedeño L, Hesse E, García-Cordero I, Mikulan E, Plastino A, … Lopera F (2015). Cortical dynamics and subcortical signatures of motor-language coupling in Parkinson’s disease. Scientific reports, 5, 11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Carpenter PA, & Just MA (1994). A capacity approach to syntactic comprehension disorders: Making normal adults perform like aphasic patients. Cognitive neuropsychology, 11(6), 671–717. [Google Scholar]
- Murray LL, & Lenz LP (2001). Productive syntax abilities in Huntington’s and Parkinson’s diseases. Brain and Cognition, 46(1–2), 213–219. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Paradis M, Paribakht T, & Nilipour R (1987). Bilingual aphasia test (Farsi version): Lawrence Erlbaum Associates New Jersey. [Google Scholar]
- Penney J, & Young A (1983). Speculations on the functional anatomy of basal ganglia disorders. Annual review of neuroscience, 6(1), 73–94. [DOI] [PubMed] [Google Scholar]
- Péran P, Cardebat D, Cherubini A, Piras F, Luccichenti G, Peppe A, … Sabatini U (2009). Object naming and action-verb generation in Parkinson’s disease: A fMRI study. Cortex, 45(8), 960–971. [DOI] [PubMed] [Google Scholar]
- Péran P, Nemmi F, Méligne D, Cardebat D, Peppe A, Rascol O, … Sabatini U (2013). Effect of levodopa on both verbal and motor representations of action in Parkinson’s disease: a fMRI study. Brain and language, 125(3), 324–329. [DOI] [PubMed] [Google Scholar]
- Péran P, Rascol O, Démonet JF, Celsis P, Nespoulous JL, Dubois B, & Cardebat D (2003). Deficit of verb generation in nondemented patients with Parkinson’s disease. Movement Disorders, 18(2), 150–156. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, & Fazio F (1999). The neural correlates of verb and noun processing: A PET study. Brain, 122(12), 2337–2344. [DOI] [PubMed] [Google Scholar]
- Phillips L, Litcofsky KA, Pelster M, Gelfand M, Ullman MT, & Charles PD (2012). Subthalamic nucleus deep brain stimulation impacts language in early Parkinson’s disease. PloS one, 7(8), e42829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, Koller WC, & Tröster AI (1999). Lexical, semantic, and action verbal fluency in Parkinson’s disease with and without dementia. Journal of Clinical and Experimental Neuropsychology, 21(4), 435–443. [DOI] [PubMed] [Google Scholar]
- Piatt AL, Fields JA, Paolo AM, & Tröster AI (1999). Action (verb naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia, 37(13), 1499–1503. [DOI] [PubMed] [Google Scholar]
- Postuma RB, & Dagher A (2005). Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral cortex, 16(10), 1508–1521. [DOI] [PubMed] [Google Scholar]
- Prieto F, Radanovic M, Schmitt C, Barbosa ER, & Mansur LL (2007). Sentence comprehension in Parkinson’s disease. Dementia & Neuropsychologia, 1(4), 386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, Moss HE, Stamatakis EA, & Tyler LK (2009). Modulation of motor and premotor cortices by actions, action words and action sentences. Neuropsychologia, 47(2), 388–396. [DOI] [PubMed] [Google Scholar]
- Reifegerste J, Estabrooke IV, Maloof CJ, Johari K, & Ullman MT (under revision). Can sex influence the neurocognition of language? Evidence from Parkinson’s disease. Neuropsychologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Nguyen P, Orange JB, Jog M, Nisbet KA, & McRae K (2017). Differential impairments of upper and lower limb movements influence action verb processing in Parkinson disease. Cortex, 97, 49–59. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ferreiro J, Menéndez M, Ribacoba R, & Cuetos F (2009). Action naming is impaired in Parkinson disease patients. Neuropsychologia, 47(14), 3271–3274. [DOI] [PubMed] [Google Scholar]
- Sapir A, Kaplan JB, He BJ, & Corbetta M (2007). Anatomical correlates of directional hypokinesia in patients with hemispatial neglect. Journal of Neuroscience, 27(15), 4045–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Peterson EJ, Cincotta CM, Lopez-Paniagua D, & Anderson CW (2010). Dissociating the contributions of independent corticostriatal systems to visual categorization learning through the use of reinforcement learning modeling and Granger causality modeling. Neuroimage, 50(2), 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MC, Ciccarelli N, Baldonero E, Piano C, Zinno M, Soleti F, … Daniele A (2012). Effects of stimulation of the subthalamic nucleus on naming and reading nouns and verbs in Parkinson’s disease. Neuropsychologia, 50(8), 1980–1989. [DOI] [PubMed] [Google Scholar]
- Skeel RL, Crosson B, Nadeau SE, Algina J, Bauer RM, & Fennell EB (2001). Basal ganglia dysfunction, working memory, and sentence comprehension in patients with Parkinson’s disease. Neuropsychologia, 39(9), 962–971. [DOI] [PubMed] [Google Scholar]
- Speed LJ, van Dam WO, Hirath P, Vigliocco G, & Desai RH (2017). Impaired comprehension of speed verbs in Parkinson’s disease. Journal of the International Neuropsychological Society, 23(5), 412–420. [DOI] [PubMed] [Google Scholar]
- Terzi A, Papapetropoulos S, & Kouvelas ED (2005). Past tense formation and comprehension of passive sentences in Parkinson’s disease: Evidence from Greek. Brain and language, 94(3), 297–303. [DOI] [PubMed] [Google Scholar]
- Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, & Clarke CE (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement disorders, 25(15), 2649–2653. [DOI] [PubMed] [Google Scholar]
- Ullman MT (2004). Contributions of memory circuits to language: The declarative/procedural model. Cognition, 92(1–2), 231–270. [DOI] [PubMed] [Google Scholar]
- Ullman MT (2006). Is Broca’s area part of a basal ganglia thalamocortical circuit? Cortex, 42(4), 480–485. [DOI] [PubMed] [Google Scholar]
- Ullman MT (2007). The biocognition of the mental lexicon. The Oxford handbook of psycholinguistics, 267–286. [Google Scholar]
- Ullman MT (2014). The declarative/procedural model In VanPatten B & Williams J (Eds.), Theories in Second Language Acquisition: An Introduction: Routledge. [Google Scholar]
- Ullman MT (2016). The declarative/procedural model: a neurobiological model of language learning, knowledge, and use Neurobiology of language (pp. 953–968): Elsevier. [Google Scholar]
- Ullman MT, Corkin S, Coppola M, Hickok G, Growdon JH, Koroshetz WJ, & Pinker S (1997). A neural dissociation within language: Evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of cognitive neuroscience, 9(2), 266–276. [DOI] [PubMed] [Google Scholar]
- Ullman MT, & Pierpont EI (2005). Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex, 41(3), 399–433. [DOI] [PubMed] [Google Scholar]
- Ullman MT, & Pullman MY (2015). A compensatory role for declarative memory in neurodevelopmental disorders. Neuroscience & Biobehavioral Reviews, 51, 205–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, & Knight RT (2010). Prefrontal cortex and basal ganglia contributions to visual working memory. Proceedings of the National Academy of Sciences, 107(42), 18167–18172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walenski M, Mostofsky SH, & Ullman MT (2007). Speeded processing of grammar and tool knowledge in Tourette’s syndrome. Neuropsychologia, 45(11), 2447–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York C, Olm C, Boller A, McCluskey L, Elman L, Haley J, … Rascovsky K (2014). Action verb comprehension in amyotrophic lateral sclerosis and Parkinson’s disease. Journal of neurology, 261(6), 1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini S, Melatini A, Capus L, Gioulis M, Vassallo A, & Bava A (2003). Language recovery following subthalamic nucleus stimulation in Parkinson’s disease. Neuroreport, 14(3), 511–516. [DOI] [PubMed] [Google Scholar]
- Zanini S, Moschella V, Stefani A, Peppe A, Pierantozzi M, Galati S, … Stanzione P (2009). Grammar improvement following deep brain stimulation of the subthalamic and the pedunculopontine nuclei in advanced Parkinson’s disease: A pilot study. Parkinsonism & related disorders, 15(8), 606–609. [DOI] [PubMed] [Google Scholar]
- Zanini S, Tavano A, Vorano L, Schiavo F, Gigli GL, Aglioti S, & Fabbro F (2004). Greater syntactic impairments in native language in bilingual Parkinsonian patients. Journal of Neurology, Neurosurgery & Psychiatry, 75(12), 1678–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurif E, Swinney D, Prather P, Wingfield A, & Brownell H (1995). The allocation of memory resources during sentence comprehension: Evidence from the elderly. Journal of Psycholinguistic Research, 24(3), 165–182. [DOI] [PubMed] [Google Scholar]