Abstract
Objectives
To determine Bruton’s tyrosine kinase (BTK) protein and phosphorylation levels in B cell subsets of granulomatosis with polyangiitis (GPA) patients and to investigate the effect of BTK blockade on in vitro B cell cytokine production, subset distribution and (auto)antibody production.
Methods
BTK protein and phosphorylation levels were determined by flow cytometry in peripheral blood B cells of 29 untreated GPA patients [9 active and 20 remission GPA patients (10 ANCA– and 10 ANCA+)], 9 age- and sex-matched healthy controls (HCs) and 9 untreated active RA patients. The effect of BTK blockade on in vitro B cell cytokine production, subset distribution and (auto)antibody production was determined in the same donors in peripheral blood mononuclear cell cultures.
Results
BTK protein levels were significantly increased in transitional and naïve B cells of active GPA and RA patients compared with remission GPA patients and HCs. Both B cell subsets of active patients were more sensitive to B cell receptor stimulation, as BTK and phospholipase Cγ2 phosphorylation were increased in these patients. In vitro BTK blockade had profound effects on B cell cytokine production, plasma cell formation and (auto)antibody production in both GPA patients and HCs. Interestingly, the effect of BTK blockade was less pronounced in active GPA patients, possibly due to increased activation of B cells.
Conclusion
We show that BTK protein and phosphorylation levels are most profoundly increased in newly emerging B cells of active GPA patients compared with remission patients. BTK blockade greatly inhibits in vitro B cell effector functions in GPA patients and HCs. These promising data identify BTK as an interesting novel therapeutic target in the treatment of GPA.
Keywords: vasculitis, Bruton’s tyrosine kinase, B cells, BTK blocker
Rheumatology key messages
Bruton’s tyrosine kinase levels are increased in transitional and naïve B cells of active granulomatosis with polyangiitis patients.
Bruton’s tyrosine kinase blockade inhibits in vitro B cell effector functions in granulomatosis with polyangiitis patients and healthy controls.
Bruton’s tyrosine kinase might be an interesting novel therapeutic target in the treatment of granulomatosis with polyangiitis.
Introduction
Granulomatosis with polyangiitis (GPA) is an autoimmune disease that affects small- to medium-sized blood vessels [1] and is characterized by the presence of ANCA, predominantly directed against PR3. Although progress has been made in the understanding of the disease mechanisms, GPA and its treatment are still associated with high disease burden and mortality [1, 2]. Even with appropriate treatment, ∼50% of patients experience a disease relapse in <4 years, often resulting in irreversible loss of organ function and necessitating toxic immunosuppressive therapy [3]. As precursors of autoantibody-producing cells, B cells are crucially involved in the GPA pathogenesis. In addition, B cells can also present antigen [4] and produce pro- and anti-inflammatory cytokines that have been linked to GPA pathogenesis [5, 6, 7]. GPA patients display shifts in circulating B cell subsets during active disease and remission [8]. This is characterized by increased naïve and decreased memory B cell frequencies compared with healthy controls (HCs) [8]. Additionally, increased circulating plasmablast frequencies during remission were associated with decreased relapse-free survival [9]. Together, this evidence suggests that B cells function not only as precursors of autoantibody-producing cells, but also as important effector cells in GPA pathogenesis. Therefore, modulation of abnormal B cell function might be beneficial in GPA.
It has been demonstrated that aberrancies in Bruton’s tyrosine kinase (BTK) levels may contribute to abnormalities in B cell activity or subset distribution. BTK is a critical mediator of B cell receptor (BCR) signalling and has an important role in B cell growth and differentiation [10]. Upon antigen binding to the BCR, phosphorylated BTK (pBTK) initiates a downstream signalling cascade that eventually leads to activation of extracellular signal–related kinase (ERK), protein kinase B (also known as AKT) and the transcription factor nuclear factor-κB, promoting B cell survival, proliferation and differentiation [10].
Mounting evidence indicates that BTK is an important factor in autoimmune disease pathogenesis, as BTK overexpression in murine B cells is sufficient to induce a spontaneous autoimmune phenotype [11], and BTK inhibition is an effective treatment in many murine autoimmune models [12]. Aberrant BTK activity was also demonstrated in human autoimmune diseases such as primary SS and RA [13, 14]. In untreated SS patients, BTK levels were increased in peripheral B cell subsets, including naïve B cells, compared with HCs [14]. These levels correlated with BTK phosphorylation, serum autoantibodies, circulating T follicular helper (Tfh) cells and infiltrating T cell numbers in salivary glands. Similarly, in ACPA+ RA patients, BTK protein levels were increased compared with ACPA– RA patients and HCs, and correlated with inducible T cell co–stimulator (ICOS) expression on Tfh cells [14]. As B cells and ANCA play an important role in the GPA pathogenesis, it is possible that BTK activity is also dysregulated in GPA patients.
In this study, we aimed to investigate BTK protein levels and phosphorylation in B cell subsets of active and remission GPA patients. Additionally, phosphorylation levels of other proteins up- and downstream of BTK were studied. Also, the effect of BTK blockade on in vitro B cell cytokine production, plasma cell formation and (auto)antibody production was investigated.
Methods
Study population
We included nine active and 20 remission GPA patients (10 ANCA– GPA patients; Table 1). Also, nine age-matched HCs (44.4% male, median age 56.8 years; range 22–74 years) and nine untreated ACPA+ RA patients, fulfilling ACR/EULAR 2010 classification criteria for RA [15], from the Rotterdam Early Arthritis Cohort [16] were included as active disease controls. GPA was diagnosed according to Chapel Hill Consensus Conference definitions and ACR classification criteria [17, 18]. Active and remission GPA was diagnosed by clinical and laboratory evaluation. Active GPA had to result in start/increase of immunosuppressive medication. Remission was defined as absence of any clinical/laboratory signs of active disease and a BVAS of zero. None of the patients received immunosuppressive treatment. ANCA titres of ⩽1:20 were considered negative. Two patients received rituximab for >3 years before sampling. This study was approved by the medical ethics committee of the University Medical Center Groningen (METc no. 2012/151), informed consent was obtained from all patients and the study complies with the Declaration of Helsinki.
Table 1.
Patient characteristics
| Active GPA patients | Remission GPA patients | Active ACPA+ RA patients | HCs | |
|---|---|---|---|---|
| Subjects, n (% male) | 9 (55.6) | 20 (40) | 9 (11.1) | 9 (44.4) |
| Age years, mean (range) | 57.2 (21.5–78.3) | 59.5 (26–77.8) | 56.3 (35.1–75.8) | 56.8 (22–74) |
| Autoantibody titre, median (range) | 1:160 (1:40–1:640) | 1:80 (0–1:640) | (U/ml) 96 (14–1802) | – |
| Creat µmol/ml, median (range) | 82 (62–151) | 93.5 (57–409) | NA | – |
| CRP mg/ml, median (range) | 43.5 (9–268) | 3.4 (0.5–38) | 5 (1–17) | – |
| BVAS, median (range) | 15 (8–21) | 0 | DAS28: 4.3 (2.0–6.8) | – |
Creat: creatinine; GPA: granulomatosis with polyangiitis; HC: healthy control; NA: not applicable.
Peripheral blood mononuclear cell culture assays
Peripheral blood mononuclear cells (PBMCs) were isolated, frozen and thawed as described previously [19]. For all cultures, 1 × 106 PBMCs/ml were cultured with or without 1000 nM BTK blocker (BMS-986142, kindly provided by Bristol–Myers Squibb, New York, NY, USA [20]). The BTK blocker was pre-incubated for 1 h.
To investigate the effect of BTK inhibition on B cell cytokine production, PBMCs were cultured for 3 days with 1000 nM anti-IgM Fab2 fragments (Jackson ImmunoResearch, West Grove, PA, USA) and restimulated for the last 4.5 h with 50 ng/ml phorbol myristate acetate and 2 mM calcium-ionophore in the presence of 10 μg/ml brefeldin A (Sigma–Aldrich, St. Louis, MO, USA).
To assess the effect of BTK inhibition on plasma cell formation, PBMCs were cultured for 7 days with 1000 nM anti-IgM Fab2 fragments (Jackson ImmunoResearch) and 100 ng/ml B cell activating factor (BAFF; PeproTech Inc., Rocky Hill, NJ, USA).
To determine the effect of the BTK blocker on (auto)antibody production, PBMCs were cultured for 12 days with 1000 nM anti-IgM Fab2 fragments (Jackson ImmunoResearch), 100 ng/ml BAFF and 100 ng/ml IL-21 (ImmunoTools, Friesoythe, Germany) [21].
BTK protein levels and T cell subsets by flow cytometry
BTK protein levels in B– and T cell subset frequencies were assessed ex vivo by flow cytometry as described previously [13]. For antibodies used, see supplementary Table S1, available at Rheumatology online. For gating strategy, see supplementary Figs S1 and S2, available at Rheumatology online. The BTK protein staining was validated using isotype controls and FMOs (fluorescence minus one). The levels of background staining in B and T cells were similar. To account for background in the BTK staining, ratios were calculated by dividing the mean fluorescence intensity (MFI) of BTK protein in B cell subsets by the MFI in T cells within each sample, as T cells do not express BTK (supplementary Fig. S1, available at Rheumatology online). Data were analysed using FlowJo software (Treestar, Ashland, OR, USA).
Phosphorylation of signalling molecules by phosphoflow
To determine ex vivo phosphorylation of intracellular signalling molecules, 4 × 105 PBMCs/sample were kept at 4°C and directly fixed with FoxP3-staining kit Fix/Perm solution (eBioscience, San Diego, CA, USA). Samples were stained for 30 min at 4°C with a staining cocktail to identify B cell subsets, followed by the antibody targeting the phosphorylated signalling molecule (30 min; room temperature), diluted in FoxP3-staining kit Perm/Wash buffer. To determine BCR sensitivity, samples were cultured in Rosswell Park Memorial Institute 1640 medium with 2% fetal calf serum at 37°C for 5 min in the presence/absence of 20 µg/ml anti-IgM (SouthernBiotech, Birmingham, AL, USA) prior to fixation (see supplementary Table S1, available at Rheumatology online, for antibodies). Data were analysed by FlowJo software.
Intracellular cytokines by flow cytometry
After restimulation, cultured PBMCs were washed and stained as described previously [19] (supplementary Table S1, available at Rheumatology online, for antibodies). Data were analysed using Kaluza v1.7 (Beckman Coulter, Brea, CA, USA). FACS plots from unstimulated samples were used to define gates (gating example in supplementary Fig. S5, available at Rheumatology online). One HC with <20% viable PBMCs was excluded.
In vitro plasma cell formation by flow cytometry
After 7-day culture, PBMCs were washed twice and stained for 15 min with antibodies (supplementary Table S1, available at Rheumatology online, for antibodies). Data were analysed in Kaluza v1.7. CD27hiCD38hi plasma cell frequencies were determined among CD19+CD22+ B cells (supplementary Fig. S6, available at Rheumatology online).
In vitro total IgG and PR3-ANCA
Total IgG levels (ng/ml) and PR3-ANCA IgG levels (response units) were determined by ELISA and Phadia ImmunoCAP 250 analyser, respectively, in supernatants of anti-IgM Fab2/BAFF/IL-21-stimulated PBMCs after a 12-day culture, as described previously [21].
Statistics
Data were analysed using GraphPad Prism software (GraphPad Prism Inc., La Jolla, CA, USA). Statistical differences between groups were determined by one-way ANOVA with a Tukey’s multiple comparison test or Dunn’s test. Paired data were analysed using the Wilcoxon signed-rank test. Correlations were analysed by Spearman correlation test. P-values <0.05 were considered statistically significant.
Results
Increased BTK protein levels in transitional and naïve B cells from active GPA patients
The frequencies of various B cell subsets were quantified in PBMCs from GPA patients and HCs by flow cytometry (supplementary Fig. S3, available at Rheumatology online). Likely due to low sample numbers, no significant differences were found in B cell subset distribution between HCs and the patient groups, although plasmablasts were slightly increased in active GPA patients.
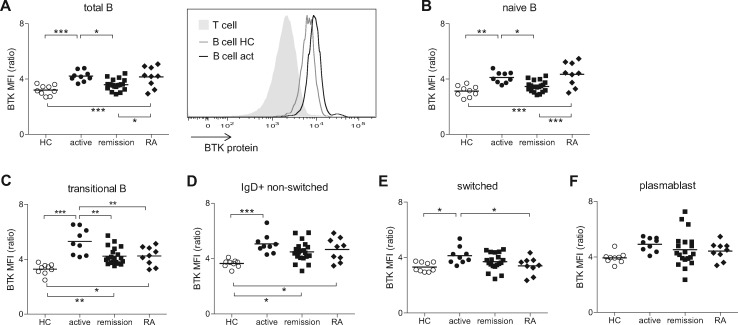
Next, we determined BTK protein levels in different B cell subsets. Compared with HCs, BTK protein levels were increased in total B cells of active GPA patients, which were similar to levels in the active ACPA+ RA patient control group. BTK protein levels were not different in remission GPA patients, and no differences were found between ANCA+ and ANCA– remission patients (Fig. 1A and data not shown). Interestingly, comparable differences were observed in IgD+CD27– naïve B cells and CD38+CD27– transitional B cells from active compared with remission GPA patients (Fig. 1B and C). These differences were not present in switched or non-switched memory B cells or plasmablasts (Fig. 1D–F), indicating that the difference between active and remission patients involves newly emerging B cells rather than antigen-experienced B cells.
Fig. 1.
BTK protein levels are increased in B cells from active GPA patients compared with HCs and remission patients
(A) BTK protein levels in total peripheral B cells from HCs, active GPA, remission GPA and RA patients, analysed by intracellular flow cytometry. Depicted ratio is BTK MFI in B cells/BTK MFI in T cells. Histogram shows an overlay of a representative HC and active GPA patient. (B–F) BTK protein levels in naïve B cells (B), transitional B cells (C), IgD+ non-switched memory B cells (D), switched memory B cells (E) and plasmablasts (F). *P < 0.05; **P < 0.01; ***P < 0.001. act: active; BTK: Bruton’s tyrosine kinase; GPA: granulomatosis with polyangiitis; HCs: healthy controls; MFI: mean fluorescence intensity.
Enhanced response to BCR stimulation in newly emerging B cells of active GPA patients
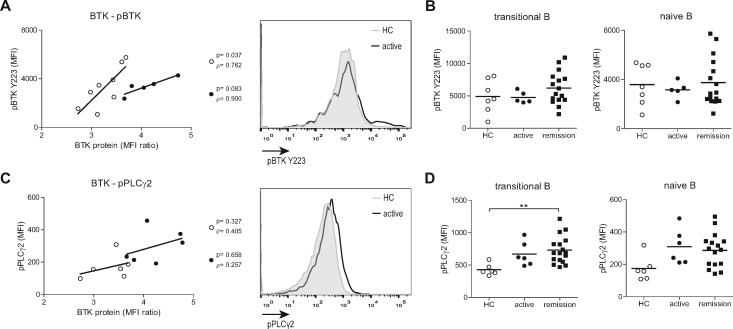
To link BTK protein levels to its activity, we measured pBTK at Y223 [12], as well as phosphorylation of several up- and downstream signalling molecules in a smaller group of patients. As previously shown for RA patients [14], BTK protein levels in total B cells correlated with ex vivo pBTK in HCs and active GPA patients (Fig. 2A). pBTK was not different between HCs and GPA patients ex vivo (Fig. 2B). Phosphorylation of the direct and specific BTK downstream target phospholipase C (PLC) γ2 (Y759), which links BCR stimulation to Ca2+ signalling, appeared increased both in transitional and naïve B cells from active and remission GPA patients, but the increase observed ex vivo only reached significance for the difference between HC and remission patients in the transitional B cell subpopulation (Fig. 2C and D). In contrast, no differences were found in the phosphorylation of the BCR-associated upstream molecule MB1 (CD79a), or the downstream molecules ERK (MAPK pathway) and S6 (AKT pathway) in directly fixed transitional or naïve B cells between active patients and HCs, nor in phosphorylation in memory B cells (ex vivo, data not shown).
Fig. 2.
Increased BCR activity in transitional and naïve B cells of GPA patients ex vivo
(A) Correlations between BTK protein and ex vivo pBTK in total B cells of HCs (open circles) and active GPA patients (closed circles). Histogram overlay shows ex vivo pBTK expression in total B cells. (B) Ex vivo pBTK expression in transitional and naïve B cells of HCs, active and remission GPA patients. (C) Correlations between BTK protein and pPLCγ2 in total B cells of HCs (open circles) and active GPA patients (closed circles). Histogram overlay shows pPLCγ2 expression in total B cells. (D) Ex vivo pPLCγ2 expression in transitional and naïve B cells of HCs, active and remission GPA patients. **P < 0.01. B: B cells; BCR: B cell receptor; BTK: Bruton’s tyrosine kinase; pBTK: phosphorylated BTK; GPA: granulomatosis with polyangiitis; HCs: healthy controls; MFI: mean fluorescence intensity; pPLCγ2: phosphorylated phospholipase Cγ2.
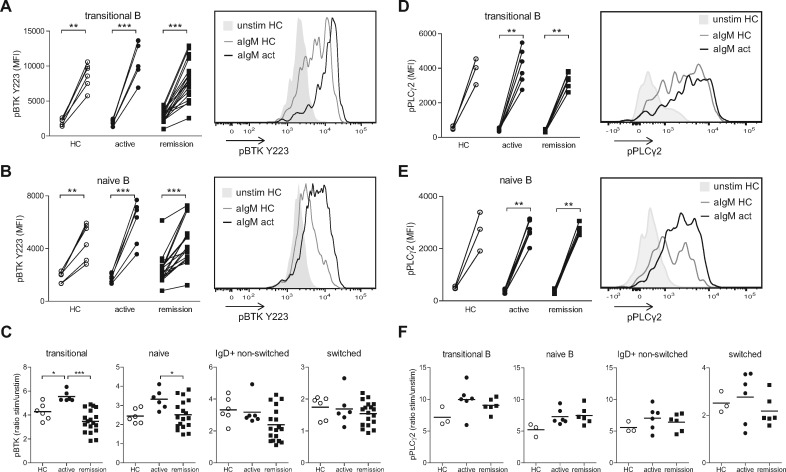
To further determine BCR sensitivity of different B cell subsets, PBMCs from patients and HCs were stimulated in vitro with anti-IgM Fab2 fragments. All groups showed upregulation of pBTK in transitional and naïve B cells upon stimulation (Fig. 3A and B). However, B cell sensitivity, measured by increased pBTK upon anti-IgM stimulation, was significantly increased in these subsets from active compared with remission GPA patients and HCs (Fig. 3C), while sensitivity of memory B cell subsets was not significantly different between the groups. Similar results were obtained for pPLCγ2 expression upon anti-IgM stimulation (Fig. 3D–F). Although the differences between the groups did not reach significance for pPLCγ2, the expressions of pPLCγ2 and pBTK Y223 upon stimulation were significantly correlated (ρ = 0.802; P = 0.007; supplementary Fig. S4, available at Rheumatology online).
Fig. 3.
Increased in vitro BCR responsiveness in transitional and naïve B cells of active GPA patients
pBTK expression on transitional B cells (A) and naïve B cells (B) in vitro, unstimulated (left) and upon stimulation with anti-IgM Fab2 fragments for 5 min (aIgM; right). Histogram shows overlays of pBTK MFI of a representative HC and active GPA patient. (C) Ratio of pBTK MFI on different B cell subsets of anti-IgM stimulated sample/unstimulated samples. (D, E) pPLCγ2 expression on transitional B cells (D) and naïve B cells (E) in vitro, unstimulated (left) and upon stimulation with anti-IgM F(ab)2 fragments for 5 min (right). Histogram shows overlays of pPLCγ2 MFI of a representative HC and active GPA patient. (F) Ratio of pPLCγ2 MFI on different B cell subsets of anti-IgM stimulated sample/unstimulated sample. *P < 0.05; **P < 0.01; ***P < 0.001. act: active; aIgM: anti-IgM; B: B cells; BCR: B cell receptor; GPA: granulomatosis with polyangiitis; HC: healthy control; MFI: mean fluorescence intensity; pBTK: phosphorylated Bruton’s tyrosine kinase; pPLCγ2: phosphorylated phospholipase Cγ2; stim: stimulated; unstim: unstimulated.
These data indicate that increased activity of BTK in newly emerging B cells is correlated with increased BTK protein levels in active GPA patients, and that this may also affect its downstream target pPLCγ2. These data also suggest that sensitivity of the BCR signalling pathway may be increased in transitional and naïve B cells of active GPA patients.
BTK protein levels correlate with B cell activity and decreased circulating pro-inflammatory CD4+ T cells in GPA patients
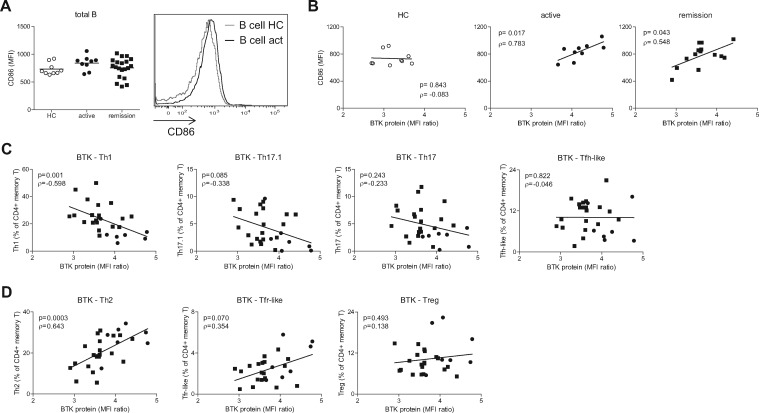
The increased BCR signalling in B cells of active GPA patients suggested increased B cell activation. Surface expression of co-stimulation molecule CD86 on total B cells was moderately, but not significantly, increased in active GPA patients (Fig. 4A). BTK protein levels in GPA patients, but not in HCs, correlated with B cell CD86 expression, indicating that high BTK levels associate with increased B cell activation in GPA patients (Fig. 4B).
Fig. 4.
BTK protein levels of B cells of GPA patients correlate with B cell activation and decreased pro-inflammatory T cell subsets in the circulation
(A) CD86 expression on total peripheral B cells of HCs, active and remission GPA patients. Histogram shows an overlay of a representative HC and active GPA patient. (B–D) Correlation of BTK protein with CD86 expression on total B cells of patients and HCs (B), proportions of circulating pro-inflammatory (C) or regulatory (D) CD4+ T cell subsets in active GPA patients (circles) and remission GPA patients (squares). act: active; B: B cells; BCR: B cell receptor; BTK: Bruton’s tyrosine kinase; GPA: granulomatosis with polyangiitis; HC: healthy control; MFI: mean fluorescence intensity; T: T cells; Tfh-like: T follicular helper-like cell; Tfr-like: T follicular helper regulatory cell.
We previously showed that BTK protein levels were associated with increased pro-inflammatory CD4+ T cell numbers in autoimmune patients [14]. Therefore, the proportions and activation status of different T cell subsets in PBMCs from GPA patients and HCs were quantified. Memory CD4+ T cell proportions were increased in remission but not in active GPA patients compared with HCs (supplementary Fig. S5A, available at Rheumatology online). Within the memory CD4+ T cell pool, pro-inflammatory T cell subsets implicated in autoimmunity were generally decreased in active GPA patients (supplementary Fig. S5B, available at Rheumatology online), whereas anti-inflammatory subsets and non-autoimmune associated Th2 cells were increased (supplementary Fig. S5C, available at Rheumatology online). Interestingly, BTK protein levels were inversely correlated with pro-inflammatory Th1 cells, and a trend was seen for Th17.1, in GPA patients (Fig. 4C). Conversely, BTK levels were positively correlated with the frequencies of Th2 cells, and a trend was seen for regulatory Tfr-like cells (Fig. 4D). ICOS expression on Tfh-like cells in GPA patients was not different from HCs (supplementary Fig. S5D, available at Rheumatology online).
These data show that BTK protein levels in B cells correlate with B cell activity and decreased circulating pro-inflammatory CD4+ T cells in GPA patients.
BTK inhibition decreases in vitro IFNγ+, IL-10+ and IL-6+ B cell frequencies, plasma cell formation and (auto)antibody production
To assess the functional importance of BTK in B cells of GPA patients, PBMCs were stimulated with anti-IgM in the absence or presence of a BTK inhibitor, and in vitro B cell cytokine production was determined. Upon culture without BTK inhibitor, no significant differences in cytokine-positive B cell frequencies were found between groups. In vitro BTK inhibition significantly decreased IFNγ+, IL-10+ and IL-6+ B cell frequencies in all groups (gating strategy is shown in supplementary Fig. S6, available at Rheumatology online). However, the TNFα+ B cell frequency was only decreased in remission GPA patients. Comparing the effect of BTK inhibition between groups showed decreased IFNγ+ B cell frequencies in remission compared with active GPA patients (Fig. 5A).
Fig. 5.
BTK blockade inhibits in vitro B cell cytokine production, plasma cell formation and (auto)antibody production
(A) The frequencies of IFNγ+ (top left), TNFα+ (top right), IL-10+ (bottom left) and IL-6+ (bottom right) B cells are given in samples stimulated with anti-IgM only (open circle) and samples stimulated with anti-IgM + BTK blocker (open squares) for HCs, active and remission GPA patients. (B) For HCs, active and remission GPA patients, the frequencies of memory B cells (left) and plasma cells (right) within CD19+CD22+ B cells are depicted. Open circles represent samples stimulated with anti-IgM/BAFF, open squares represent samples stimulated with anti-IgM/BAFF in the presence of the BTK blocker. (C) PR3–ANCA levels (left) for anti–IgM/BAFF/IL–21 and anti–IgM/BAFF/IL–21 + BTK blocker stimulated samples for active (open circles), ANCA– (open squares), and ANCA+ (open diamonds) remission GPA patients, and total IgG concentrations (right) in anti–IgM/BAFF/IL–21 (open circles) and anti–IgM/BAFF/IL–21 + BTK blocker (open squares) stimulated samples for HCs, active and remission GPA patients. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. BAFF: B cell activating factor; BTK: Bruton’s tyrosine kinase; GPA: granulomatosis with polyangiitis; HCs: healthy controls; RU: respone units.
The sensitivity of IL-10+ B cells to BTK inhibition was decreased in active GPA patients compared with HCs, as the ratio of anti-IgM/anti-IgM + BTK inhibitor was decreased in active patients (supplementary Fig. S8A, available at Rheumatology online). Together, cytokine-positive B cell frequencies upon BTK inhibition are decreased, and the effect is possibly lower in active GPA patients.
To determine whether in vitro plasma cell formation is affected by BTK inhibition, PBMCs were stimulated with anti-IgM and BAFF in the absence or presence of a BTK inhibitor. Upon culture without BTK inhibitor, no differences were found in memory B cell formation between groups; however, plasma cell formation was increased in active compared with remission GPA patients (for gating strategy, see supplementary Fig. S7, available at Rheumatology online). Upon in vitro BTK inhibition, plasma cell formation was decreased in remission GPA patients, but could not be decreased in active GPA patients and HCs (Fig. 5B). Also, memory B cell formation was decreased upon BTK inhibition in remission GPA patients and HCs, but not in active GPA patients. Interestingly, plasma cell frequencies were increased upon BTK inhibition in active compared with remission GPA patients (Fig. 5B). Importantly, transitional B cell frequencies were not different between groups upon BTK inhibition (supplementary Fig. S8B, available at Rheumatology online). Naïve B cell frequencies in the absence and presence of BTK inhibition were increased in remission compared with active GPA patients and HCs (supplementary Fig. S8B, available at Rheumatology online). This indicates that the efficacy of BTK inhibition is lower in active GPA patients.
To determine the effect of BTK inhibition on (auto)antibody secretion, PBMCs were cultured with anti-IgM, BAFF and IL-21 in the absence or presence of the BTK inhibitor for 12 days, and PR3-ANCA and total IgG concentrations were determined in supernatants. In cultures of eight GPA patients, including five active patients, PR3-ANCA were secreted and BTK inhibition appeared to decrease the PR3-ANCA levels (Fig. 5C). However, no significant differences were found, possibly due to large variation between samples. Total in vitro IgG production was decreased in HCs and remission patients upon BTK inhibition, whereas a trend towards a decrease was seen in active GPA patients (Fig. 5C).
Together, these data show that BTK inhibition may decrease in vitro B cell differentiation towards memory B cells and antibody-producing plasma cells in HCs and remission GPA patients, but seemed less effective in active GPA patients. Furthermore, BTK inhibition diminished in vitro (auto)antibody production in remission and active GPA patients.
Discussion
GPA is characterized by a high morbidity and is fatal if left untreated. Although disease remission can be established by immunosuppressive therapy, these treatments are not directed specifically at pathogenic processes and can lead to severe side-effects [22]. New therapies that specifically target pathogenic pathways and cells involved in GPA are therefore needed. Here we assessed BTK expression and the effect of BTK blockade on B cell function as a novel treatment target in GPA.
Although GPA patients commonly receive medication at the time of diagnosis, we were able to include nine untreated active GPA patients. Despite this low number, we found clear differences in the BTK protein levels compared with remission GPA patients and HCs. These protein levels correlated with BCR sensitivity and circulating pro-inflammatory T cells. Furthermore, clear effects of in vitro BTK inhibition on B cell effector functions were observed in remission GPA patients and HCs, but were less pronounced in active GPA patients, showing that with the current sample size, active and remission patients can be distinguished.
BTK levels were increased in some memory B cell subsets in remission patients, but—most importantly—we observed a striking difference between active disease and remission in newly formed naïve and transitional B cells. Hereby only active GPA patients show increased BTK protein levels compared with HCs. Transitional and naïve B cells from these patients show increased BCR signalling ex vivo, as pPLCγ2 levels were increased, as well as increased BCR sensitivity in vitro, inferred from the enhanced pBTK levels upon BCR stimulation. These observations parallel our previous findings of increased BTK protein levels in naïve B cells of untreated active RA and SS patients [14]. In these patients, BTK levels correlated with autoantibodies. We did not find a correlation with ANCA titres, possibly due to low sample numbers. Increased BTK protein levels in transitional and naïve B cells do correlate with a more activated B cell phenotype in GPA patients, and a decrease in pro-inflammatory CD4+ T cell proportions in the circulation. We therefore propose that pathogenic B–T cell interaction occurs already at an early stage of B cell differentiation, and that newly emerging B cells may be actively involved in autoimmune disease pathology.
We previously demonstrated correlations between BTK levels and T cell activity in RA and SS patients and showed that inhibition of B–T cell or dendritic cell–T cell co-stimulation by abatacept was sufficient to reduce BTK protein in naïve B cells to HC levels [14]. In SS, B cell BTK protein levels correlated with infiltrating T cell numbers in parotid glands. Although target organs in GPA patients were not studied here, it is conceivable that the numbers of activated T cells involved in disease pathogenesis are higher in target organs of active GPA patients than in remission patients. Normalized BTK expression in remission GPA patients and abatacept-treated SS patients both indicate that BTK is not intrinsically dysregulated in autoimmune patients, but that a pro-inflammatory micro-environment including activated T cells is responsible for increased BTK expression and BCR activity.
B cells play an essential role in GPA pathogenesis as ANCA-producing cells. As expected, BTK blockade inhibited in vitro plasma cell formation, PR3-ANCA and total IgG production upon BCR stimulation. GPA patients show increased B cell activation and activated effector B cells may be an important source of pro-inflammatory cytokines, although the contribution of these cytokines to GPA pathogenesis is unclear [19]. Importantly, in vitro BTK blockade resulted in inhibition of B cell cytokine production (IFNγ, IL-10, IL-6) in active and remission GPA patients and HCs. Thus, BTK inhibition affects several B cell effector functions in GPA B cells.
We also show a clear effect of in vitro BTK blockade on plasma cell formation in remission patients, accompanied by a decrease in memory B cells, whereas no such effect was seen in active GPA patients. Altogether, in our in vitro cultures of B cells from active GPA patients, the proportions of differentiated plasma cells were increased compared with remission patients and HCs. Although in vitro effects on BCR signalling in Ramos cells were shown with lower doses of the BTK inhibitor [20], it is possible that the dose used in our study was insufficient to inhibit in vivo activated B cells of active patients, or that activated B cells in these patients no longer depend on BTK-mediated survival signals. In vitro BTK inhibition significantly reduced IgG production in remission GPA patients and HCs, but not in active GPA patients. This suggests that B cells in patients with active disease are more activated and may require a higher dose of BTK inhibition to significantly reduce IgG secretion. In the five samples from active patients that produced PR3-ANCA in vitro, we found reduced levels upon BTK inhibition, indicating that BTK kinase activity may play an important role in autoantibody production.
Taken together, BTK protein levels were increased in most B cell subsets of active GPA patients, including newly emerging transitional and naïve B cells, but only in a limited number of memory B cell subsets in remission GPA patients. These levels are correlated with enhanced BCR activity and sensitivity, and an increased expression of the co-stimulatory molecule CD86 on B cells. The correlation of B cell BTK levels with decreased numbers of pro-inflammatory Th1 and T17.1 cells in the circulation would be consistent with migration of these pro-inflammatory T cells to target organs during active disease. This might be supported by our previous finding in SS patients that BTK protein levels in circulating B cells correlated with T cell infiltration in the salivary glands [14].
Furthermore, we found that BTK inhibition in vitro can inhibit B cell cytokine production, plasma cell formation and total IgG (auto)antibody secretion. Although B cells from active GPA patients respond less to BTK inhibition in vitro than healthy B cells, further studies in larger patient cohorts may identify BTK as an interesting novel therapeutic target in GPA patients.
Supplementary Material
Acknowledgements
We thank Theo Bijma and Minke Huitema for the experimental help. J.S.S. is supported by personal grants from the Dutch Kidney Foundation (grant number 13OKJ39) and the Dutch Organization for Scientific Research (Clinical Fellow grant number 907-14-542). W.H.A. and P.H. are supported by a grant from the European Union’s Horizon-2020 research and innovation programme project RELENT (grant number 668036), and O.B.J.C. is supported by a grant from the Dutch Arthritis Foundation (Reumafonds, grant number 13-2-301).
Funding: This work was supported by Bristol-Myers Squibb, the Dutch Organization for Scientific Research (grant number 907-14-542), the Jan-Kornelis de Cock foundation and the Dutch Arthritis Foundation (Reumafonds, grant number 13-2-301).
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Jennette J, Falk R.. Small-vessel vasculitis. N Engl J Med 1997;337:1512–23. [DOI] [PubMed] [Google Scholar]
- 2. de Joode A.A.E., Sanders JSF, Rutgers A, Stegeman CA.. Maintenance therapy in antineutrophil cytoplasmic antibody-associated vasculitis: who needs what and for how long? Nephrol Dial Transplant 2015;30:i150–8. [DOI] [PubMed] [Google Scholar]
- 3. Puéchal X, Pagnoux C, Perrodeau E. et al. Long-term outcomes among participants in the WEGENT trial of remission-maintenance therapy for granulomatosis with polyangiitis (Wegener’s) or microscopic polyangiitis. Arthritis Rheumatol 2016;68:690–701. [DOI] [PubMed] [Google Scholar]
- 4. Rodríguez-Pinto D. B cells as antigen presenting cells. Cell Immunol 2005;238:67–75. [DOI] [PubMed] [Google Scholar]
- 5. Stone JH, Merkel PA, Spiera R. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010;363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones RB, Furuta S, Cohen Tervaert JW. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis 2015;74:1178–82. [DOI] [PubMed] [Google Scholar]
- 7. von Borstel A, Sanders JS, Rutgers A, Stegeman CA, Heeringa P, Abdulahad WH. Cellular immune regulation in the pathogenesis of ANCA-associated vasculitides. Autoimmunity Reviews 2018;17:413–421. [DOI] [PubMed] [Google Scholar]
- 8. Lepse N, Abdulahad WH, Rutgers A. et al. Altered B cell balance, but unaffected B cell capacity to limit monocyte activation in anti-neutrophil cytoplasmic antibody-associated vasculitis in remission. Rheumatology (Oxford) 2014;53:1683–92. [DOI] [PubMed] [Google Scholar]
- 9. von Borstel A, Abdulahad WH, Rutgers A. et al. Increased CD38hiCD27+ plasmablast frequency in remission predicts relapsing disease in granulomatosis with polyangiitis patients. Arthr Rheumatol 2017;69:suppl. 10. https://acrabstracts.org/abstract/increased–cd38hicd27–plasmablast–frequency–in–remission–predicts–relapsing–disease–in–granulomatosis–with–polyangiitis–patients/ (27 May 2019, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corneth OBJ, Klein Wolterink RGJ, Hendriks RW.. BTK signaling in B cell differentiation and autoimmunity. Curr Top Microbiol Immunol 2016;393:67–105. [DOI] [PubMed] [Google Scholar]
- 11. Kil LP, Bruijn MJ, Nimwegen MV. et al. Btk levels set the threshold for B–cell activation and negative selection of autoreactive B cells in mice. Blood 2012;119:3744–56. [DOI] [PubMed] [Google Scholar]
- 12. Rip J, van der Ploeg EK, Hendriks RW, Corneth O.. The role of Bruton’s tyrosine kinase in immune cell signaling and systemic autoimmunity. Crit Rev Immunol 2018;38:17–62. [DOI] [PubMed] [Google Scholar]
- 13. Hartkamp LM, Fine JS, van Es IE. et al. Btk inhibition suppresses agonist-induced human macrophage activation and inflammatory gene expression in RA synovial tissue explants. Ann Rheum Dis 2015;74:1603–11. [DOI] [PubMed] [Google Scholar]
- 14. Corneth OBJ, Verstappen GM, Paulissen SMJ. et al. Enhanced Bruton’s tyrosine kinase activity in peripheral blood B lymphocytes from patients with autoimmune disease. Arthritis Rheumatol 2017;69:1313–24. [DOI] [PubMed] [Google Scholar]
- 15. Aletaha D, Neogi T, Silman AJ. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 16. Paulissen SMJ, van Hamburg JP, Davelaar N. et al. CCR6+Th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis. Arthritis Res Ther 2015;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leavitt RY, Fauci A, Bloch DA. et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum 2010;33:1101–7. [DOI] [PubMed] [Google Scholar]
- 18. Jennette JC, Falk RJ, Bacon PA. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 19. Land J, Abdulahad WH, Sanders JSF. et al. Regulatory and effector B cell cytokine production in patients with relapsing granulomatosis with polyangiitis. Arthritis Res Ther 2016;18:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gillooly KM, Pulicicchio C, Pattoli MA. et al. Bruton’s tyrosine kinase inhibitor BMS-986142 in experimental models of rheumatoid arthritis enhances efficacy of agents representing clinical standard-of-care. PLoS One 2017;12:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lepse N, Land J, Rutgers A. et al. Toll-like receptor 9 activation enhances B cell activating factor and interleukin-21 induced anti-proteinase 3 autoantibody production in vitro. Rheumatology (Oxford) 2016;55:162–72. [DOI] [PubMed] [Google Scholar]
- 22. Little MA, Nightingale P, Verburgh CA. et al. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis. Ann Rheum Dis 2010;69:1036–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.