Abstract
Background:
Measures of low-density lipoprotein (LDL) subfractions have been proposed as an independent risk factor for cardiovascular disease.
Purpose:
To review published studies that reported relationships between LDL subfractions and cardiovascular outcomes.
Data Sources:
MEDLINE (1950 to 5 January 2009), CAB Abstracts (1973 to 30 June 2008), and Cochrane Central Register of Controlled Trials (2nd quarter of 2008), limited to English-language studies.
Study Selection:
3 reviewers selected longitudinal studies with 10 or more participants that reported an association between LDL subfractions and incidence or severity of cardiovascular disease and in which plasma samples were collected before outcome determination.
Data Extraction:
Data were extracted from 24 studies. The 10 studies that used analytical methods available for clinical use (all of which used nuclear magnetic resonance) had full data extraction, including quality assessment (good, fair, or poor). All studies were extracted by 1 researcher and verified by another.
Data Synthesis:
All 24 studies, and the subset of 10 nuclear magnetic resonance studies, were heterogeneous in terms of the specific tests analyzed, analytical methods used, participants investigated, and outcomes measured. Higher LDL particle number was consistently associated with increased risk for cardiovascular disease, independent of other lipid measurements. Other LDL subfractions were generally not associated with cardiovascular disease after adjustment for cholesterol concentrations. No study evaluated the incremental value of LDL subfractions beyond traditional cardiovascular risk factors or their test performance.
Limitation:
Publication bias was a possibility.
Conclusion:
Higher LDL particle number has been associated with cardiovascular disease incidence, but studies have not determined whether any measures of LDL subfractions add incremental benefit to traditional risk factor assessment. Routine use of clinically available LDL subfraction tests to estimate cardiovascular disease risk is premature.
Acritical component of lowering the cardiovascular disease burden across the population is identification and aggressive treatment of high-risk individuals. The Adult Treatment Panel III of the Expert Panel of the National Cholesterol Education Program (1) has identified a group of risk factors associated with cardiovascular disease, including elevated low-density lipoprotein (LDL) cholesterol concentrations, cigarette smoking, hypertension, reduced high-density lipoprotein (HDL) cholesterol concentrations, family history of premature coronary heart disease, and older age. Current efforts have focused on determining whether additional diagnostic criteria could improve the accuracy of cardiovascular disease risk estimation (2–5). Measures of LDL subfractions have been suggested as a potential risk factor.
Many terms are used to describe the characteristics and distribution of LDL particles; these include LDL subclasses, particles, particle concentration, particle numbers, and various patterns. These terms describe separate, but sometimes overlapping, features of the LDL particle. To simplify matters, we use the generic term subfractions except when describing specific measurements. Despite this simplification, we are not suggesting that the disparate methods for analyzing LDL can be fully subsumed in a single concept. Numerous methods are used to measure or define LDL subfractions. Table 1 lists the principal methods used and the most commonly reported subfraction measures. Only a few of these disparate systems to estimate LDL subfractions are routinely available, and only from selected clinical laboratories.
Table 1.
Commonly Used LDL Subfraction Tests and Terms
| Commonly used tests to measure LDL subfractions |
| Nuclear magnetic resonance: Clinically available test using a mass spectrometer. Measures the signal from the aggregate number of terminal methyl groups in the lipid within the particle. The number of methyl groups is reflected in the amplitude of the methyl NMR signal. The amplitude of each lipoprotein particle signal serves as a measure of the concentration of that lipoprotein. The NMR data are converted into subfraction concentrations by using assumptions in proprietary software. Size and pattern of LDL can be derived through additional calculations. |
| LipoPrint gel electrophoresis*: Clinically available test using a specific gel kit, equipment, and method for defining LDL subfractions. Relatively rapid system compared with most gel electrophoresis methods. Separates particles into 7 subfractions by size and to a lesser extent charge. Lipoprotein subfraction profiles can be classified into type A or B. Use of LipoPrint to determine particle sizes or LDL scores or any other form of classification is not recommended by the manufacturer of the kit but is routinely done by researchers. |
| Berkeley HeartLab segmented gel electrophoresis*: Clinically available test using a specific gel kit together with a proprietary computer algorithm for calculating the number of particles in an LDL subfraction. Separates particles into 7 LDL subfractions (I, IIa, IIb, IIIa, IIIb, IVa, and IVb) on the basis of particle size and shape. |
| Bench gel electrophoresis†: The principal method used in research laboratories to measure LDL subfractions. The LDL subfractions are separated by electrophoresis across a sodium dodecyl sulfate gradient gel. For the majority of specific gel electrophoresis methods, researchers create their own gels and apply nonstandardized techniques to separate the LDL subfractions. Different compounds are used to create the gels, although polyacrylamide is the most common. Different distributions of gel densities are used. Different approaches are used to define and determine the specific LDL subfractions. |
| Ultracentrifugation†: Performed only in research laboratories by using a variety of instruments, specific methods, and definitions of LDL subfractions. The LDL subfractions are separated by ultracentrifugation on the basis of density. A variety of arbitrary density cut-points between subfractions has been reported. |
| Commonly used LDL subfraction terms |
| Small, dense LDL particles: Considered to be most atherogenic. Generally < 197 Å or < 212 Å, but see Table 4 for other definitions. Reported as the plasma concentration. Considered to be the atherogenic subfraction. |
| Medium LDL particles: Particles of a size between small and large. Some reports omit this category. |
| Large LDL particles: Considered to be least atherogenic. Generally > 213 Å, but see Table 4 for other definitions. |
| LDL particle number (or concentration): The concentration of LDL particles, measured in nmol/L. Determined only by NMR as a calculated value. Higher concentrations are considered to be more atherogenic. |
| LDL particle size: Absolute measure of the average or peak size of the LDL particles, reported in Å or nm. Smaller size is considered to be more atherogenic. |
| Pattern A: Predominance of larger, more buoyant LDL particles (“normal”). |
| Pattern B: Predominance of smaller, denser LDL particles (“abnormal”). |
| Pattern AB or intermediate: A pattern either intermediate to or of mixed patterns A and B. Often analyzed together with pattern B. |
| Individual subfractions: Usually divided into 7 subfractions primarily on the basis of density fractions. Various nomenclatures are used, generally with LDL1 (or equivalent) being most buoyant and LDL7 (or equivalent) being most dense. |
| LDL subfraction score: Based on electrophoretic mobility of the particles. Calculated values are based on areas under the curve of subfraction concentration. Can be divided into pattern A (score < 5.5), B (score > 8.5), and intermediate (score of 5.5–8.5). |
LDL low-density lipoprotein; NMR nuclear magnetic resonance.
No studies of incidence or severity of cardiovascular disease used this method.
Studies using these methods are included only in the section “All Methods of Measuring LDL Subfractions.”
If LDL subfractions are predictive of cardiovascular risk and are of incremental value when added to established cardiovascular risk factors, it remains to be determined whether the different characteristics of the LDL subfractions assessed by various methods would result in similar predictive abilities for estimating cardiovascular risk. Lipid researchers have proposed that small, dense LDL particles confer greater atherogenic risk than larger, less dense LDL particles (6, 7). In vitro, small, dense LDL particles are more avidly taken up by macrophages than larger, less dense LDL particles; are more susceptible to oxidative modification, have a greater propensity for transport into the arterial subendothelial space; and have a greater binding potential to arterial wall proteoglycans (8, 9).
The American Diabetes Association and the American College of Cardiology Foundation convened a panel of experts to develop a consensus position for patients with “cardiometabolic risk” (10). They noted that limited data from cross-sectional and prospective studies suggest that LDL particle number may be a better discriminator of cardiometabolic risk than LDL cholesterol concentrations. They pointed out several limitations, including availability and accuracy of the method and consistency of the predictive power across ethnic groups, ages, and conditions that affect lipid metabolism. They concluded that it is yet to be determined whether treatment decisions would be improved if LDL subfraction measurements were added to the current risk factors used to estimate cardiovascular risk.
We sought to evaluate the association between LDL subfractions and incidence and progression of clinical cardiovascular disease. We focus primarily on the LDL subfraction tests that are available for routine use by clinical laboratories and are thus available to all U.S. clinicians and their patients. We also summarize the potential value of LDL subfraction tests used only in research laboratories. An earlier version of this systematic review was conducted as part of a Technology Assessment for the Centers for Medicare & Medicaid Services (11).
METHODS
Data Sources and Searches
We conducted a comprehensive search of the scientific literature to identify relevant studies in MEDLINE (1950 to 5 January 2009), CAB Abstracts (1973 to 30 June 2008), and the Cochrane Central Register of Controlled Trials (second quarter of 2008). Appendix Table 1 (available at www.annals.org) lists search terms for LDL, particle size or subfractions, and test methodologies. We limited the literature searches to humans and English-language publications. The searches were supplemented by screening reference lists of included studies and selected reviews and requesting more information from domain experts.
Study Selection
Three investigators screened all citations and retrieved articles for eligibility. We included studies of any prospective, longitudinal design that reported an association between any measure of LDL subfractions and either incident cardiovascular disease (cardiac, cerebrovascular, or peripheral vascular disease) or progression of disease severity (for example, coronary atherosclerosis) and had at least 10 adults per study group. Serum (or plasma) samples must have been obtained before determination of outcomes. We evaluated only clinical outcomes or measures of atherosclerosis on which clinical decisions are made (for example, minimum lumen diameter). We placed no further restrictions on study populations and included studies of people with and without cardiovascular disease at baseline. No minimum follow-up duration was required.
Data Extraction
One of the three authors extracted data from each study, and at least 1 additional author reviewed and verified the extractions. Full data extraction, including quality assessment, was performed for studies that used specific methods or kits that are currently available to clinical laboratories (as opposed to research laboratories). From the best information available to us from the Centers for Medicare & Medicaid Services, the U.S. Food and Drug Administration, domain experts, the reviewed studies, internet searches, invited reviewers, and conversations with several laboratories, we limited the full analysis to nuclear magnetic resonance (NMR), the LipoPrint kit (Quantimetrix, Redondo Beach, California) for linear polyacrylamide gel electrophoresis, gradient gel electrophoresis performed at Berkeley HeartLab (LDL-S3 GGE Test, Berkeley HeartLab, Burlingame, California), an ultracentrifugation technique performed at the University of Washington’s Northwest Lipid Research Laboratory, and the Vertical Auto Profile (Atherotech, Birmingham, Alabama). For other laboratory methods, we extracted only limited results data: the type of LDL subfraction measurement (particle size, particle concentration or number, or pattern of LDL subfraction distribution [small, medium, or large LDL, or other subfractions]) and the direction and statistical significance of the association. These other laboratory methods included a range of gel electrophoresis and ultracentrifugation methods that are generally not standardized and are used only in the research setting, high-pressure liquid chromatography, capillary isotachophoresis, and other techniques. We analyzed both unadjusted and adjusted associations between LDL subfractions and clinical cardiovascular outcomes. For the purposes of this review, “adjusted” analyses were multivariable analyses in which the association between LDL subfraction and cardiovascular outcomes were adjusted for LDL cholesterol, HDL cholesterol, non-HDL cholesterol, or triglyceride concentrations; “unadjusted” analyses did not adjust for cholesterol concentrations but may have adjusted for other variables, such as other lipoprotein subfractions, clinical history, demographic characteristics, or blood pressure.
Quality Assessment
We assessed the methodological quality of each fully extracted study on the basis of predefined criteria (12). The primary data extractor determined the study quality, and at least 1 other extractor confirmed it. We used a 3-category grading system to denote the methodological quality of each study. Good-quality studies adhere most closely to the commonly held concepts of high quality, including clear descriptions of the population, setting, LDL subfraction measures, and analytic technique; appropriate measurement of outcomes; appropriate statistical analysis, including multivariable analysis adjusting for lipid measures; no obvious reporting omissions or errors; clear reporting of dropouts; and complete reporting of associations of interest for this systematic review. Fair-quality studies have some deficiencies, but these are unlikely to cause major bias. Poor-quality studies failed to adequately describe the measures, analyses, or results of interest or had substantial flaws in reporting or statistical analyses, such that major bias could not be excluded. The quality assessment was based specifically on the analysis of LDL subfractions and clinical cardiovascular outcomes, regardless of the primary analysis of interest to the original researchers.
Role of the Funding Source
The Agency for Healthcare Research and Quality participated in formulating the study questions but did not participate in the literature search; determination of study eligibility; data analysis or interpretation; or preparation, review, or approval of the manuscript for publication.
RESULTS
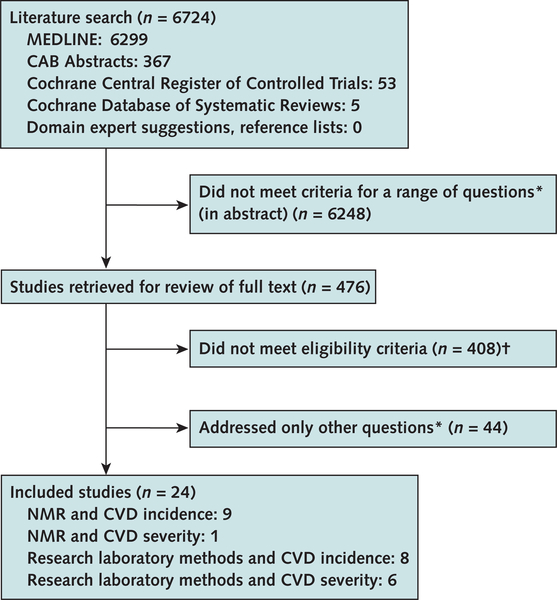
The literature searches yielded 6724 citations (Figure), of which 476 were retrieved for further consideration for this and other research questions of interest. Of these, 24 met eligibility criteria. Ten studies (13–22) used NMR to measure LDL subfractions. Although LipoPrint gel electrophoresis is among the methods more commonly used by clinical laboratories, we identified no study that used this kit to evaluate incidence or progression of cardiovascular disease (at least 6 LipoPrint studies all evaluated prevalent disease). Also, none of the eligible studies used the gradient gel electrophoresis performed at the Berkeley HeartLab or the ultracentrifugation method available at the Northwest Lipid Research Laboratory or the Vertical Auto Profile. An additional 14 studies used other laboratory methods not used by clinical laboratories, including gel electrophoresis (23–32), ultracentrifugation (33–35), and an unreported method (36). Overall, 17 studies evaluated incident cardiovascular outcomes and 7 evaluated progression of existing cardiovascular disease.
Figure. Study flow diagram.
CVD cardiovascular disease; NMR nuclear magnetic resonance.
* Description of methods used to measure low-density lipoprotein (LDL) subfractions, comparison of different methods, variability of measures, link between therapies to alter LDL subfractions and CVD outcomes, and association between LDL subfractions and incident and prevalent CVD.
† No clinical CVD outcome, not LDL subfractions, not an analysis of baseline lipoprotein subfractions, or no original data.
NMR-Measured LDL Subfractions
All 10 studies that examined the relationships between NMR-measured LDL subfractions and cardiovascular outcomes had their samples run by a common group of researchers at LipoScience (Raleigh, North Carolina) or its precursors. The studies included 2 prospective longitudinal studies and 8 nested case–control studies of cardiovascular treatment trials or large epidemiologic studies (Appendix Table 2, available at www.annals.org). Nine studies (with 12 subgroup analyses) evaluated incident cardiovascular disease (Table 2), and 1 study evaluated severity of cardiovascular disease (Table 2). Two of the nested case–control studies fulfilled the criteria of good methodological quality; the other 8 studies were of fair methodological quality. The number of participants in these studies ranged from 118 to 3066 (median, 556). Many of the studies used different definitions of the LDL subfractions (Table 3). The studies evaluated heterogeneous populations, but most included primarily older men with a history of cardiovascular disease. Five studies (13, 14, 16, 18, 19) included healthy populations at baseline. The mean LDL cholesterol concentration across the studies ranged from 2.9 to 4.2 mmol/L (median, 3.3 mmol/L) (113 to 164 mg/dL [median, 130 mg/dL]).
Table 2.
Association Between LDL Subfractions and Incident CVD and Severity of CVD
| Author, Year (Reference) |
Cardiovascular Outcome |
Participants | Predictor | Mean Value |
P Value | Other Results | P Value | Adjusted for Lipids? |
Quality | |
|---|---|---|---|---|---|---|---|---|---|---|
| Persons With Event | Persons Without Event |
|||||||||
| Incident CVD | ||||||||||
| Cromwell et al, 2007 (13) | Incident CVD | 1440 men | LDL particle number | 1641 nmol/L | 1509 nmol/L | <0.001 | HR, 1.24 (95% CI, 1.10–1.39) per 1-SD increase in LDL particle number* | <0.001 | No | Fair |
| 1626 women | 1628 | 1344 | <0.001 | HR, 1.33 (95% CI, 1.17–1.50) per 1-SD increase in LDL particle number* | <0.001 | No | ||||
| Event-free survival was higher in participants
with low LDL particle number, after dichotomization by LDL cholesterol level |
Yes | |||||||||
| El Harchaoui et al, 2007 (14) | Fatal or nonfatal CAD |
1003 case patients, 1885 control participants |
Large (212–230 Å) Medium (no data on size) Small (180–212 Å) LDL particle number LDL particle size |
43 nmol/L 568 nmol/L 999 nmol/L 1640 nmol/L 210 Å |
36 nmol/L 572 nmol/L 885 nmol/L 1525 nmol/L 211 Å |
0.003
NS <0.001 <0.001 0.002 |
OR, 1.37 (95% CI, 1.04–1.83)† OR, 0.86 (95% CI, 0.65–1.15)‡ |
0.02 NS |
No No No Yes No |
Good |
| Otvos et al, 2006 (15) | Nonfatal MI or cardiac death | 364 case patients, 697 control participants | Large (212–230 Å) | OR, 1.08 (95% CI, 0.95–1.23) per 1-SD increase§ |
NS | Yes∥ | Fair | |||
| Small (180–212 Å) | OR, 1.11 (95% CI, 0.98–1.27) per 1-SD increase§ |
NS | Yes∥ | |||||||
| LDL particle number | OR, 1.20 (95% CI, 1.05–1.37) per 1-SD increase§ |
<0.05 | Yes∥ | |||||||
| LDL particle size | OR, 0.97 (95% CI, 0.85–1.10) per 1-SD increase§ |
NS | Yes∥ | |||||||
| Kuller et al, 2002 (16) |
Incident MI or angina | Men: 243 case patients, 67 control participants | Large (213–230 Å) Medium (198–212 Å) Small (183–197 Å) LDL particle number LDL particle size |
57.3 mg/dL cholesterol 36 mg/dL cholesterol 25.7 mg/dL cholesterol 1676 nmol/L 209 Å |
58 mg/dL cholesterol 34.5 mg/dL cholesterol 22.7 mg/dL cholesterol 1597 nmol/L 210 Å |
NS NS NS NS NS |
No No No No No |
Fair | ||
| Women: 191 case patients, 182 control participants |
Large (213–230 Å) Medium (198–212 Å) Small (183–197 Å) LDL particle number LDL particle size |
96 mg/dL cholesterol 8.2 mg/dL cholesterol 7.1 mg/dL cholesterol 1680 nmol/L 213 Å |
104 mg/dL cholesterol 6.8 mg/dL cholesterol 0 mg/dL cholesterol 1501 nmol/L 216 Å |
NS NS <0.05 <0.05 <0.05 |
OR, 1.77 (95% CI, 1.12–2.30), presence vs. absence of subfraction OR, 1.11 (95% CI, 1.03–1.09) per 100nmol/L increase¶ OR, 0.68 (95% CI, 0.51–0.91), unit of comparison not reported |
<0.05 <0.05 <0.05 |
No No Unclear Yes Unclear |
|||
| Kuller et al, 2007 (17) | Cardiac death | 214 case patients, 214 control participants | Large (213–230 Å) Small (182–197 Å) LDL particle size |
500.7 nmol/L 1207.1 nmol/L No data |
465.8 nmol/L 1200.2 nmol/L No data |
NS NS NS |
ORs of quartiles and trend (no
data) ORs of quartiles and trend (no data) |
NS NS |
No No No |
Fair |
| Hsia et al, 2008 (18) | ||||||||||
| Substudy 1 | Incident MI, coronary death | 202 case patients, 200 control participants | LDL particle number | 1673 nmol/L | 1513 nmol/L | <0.001** | OR, 1.40 (95% CI, 1.08–1.83) per 1-SD increase†† | 0.01 | No | Fair |
| Substudy 2 | Incident MI, coronary death | 149 case patients, 149 control participants | LDL particle number | 1687 nmol/L | 1525 nmol/L | <0.001 | OR, 1.31 (95% CI, 0.97–1.78) per 1-SD increase | 0.08 | No | Fair |
| Blake et al, 2002 (19) | Cardiac death, incident MI, or stroke | 130 case patients, 130 control paraticipants | Large (213–227 Å) Medium (198–212 Å) Small (183–197 Å) LDL particle number LDL particle size |
886 nmol/L 201 nmol/L 0 1597 nmol/L 215 Å |
1001 nmol/L 126 nmol/L 0 1404 nmol/L 218 Å |
NS 0.008 NS <0.001 0.046 |
RR, 2.90 (95% CI, 1.16–7.30)‡‡ RR, 1.20 (95% CI, 0.51–2.82)‡‡ |
0.02 NS |
No No No Yes Yes |
Good |
| Campbell et al, 2007 (20) | Intracerebral hemorrhage | 41 case patients, 107 control participants | Large (no data on size) Medium (no data on size) Small (no data on size) LDL particle number LDL particle size |
396 nmol/L 473 nmol/L 309 nmol/L 1461 nmol/L 208 Å |
252 nmol/L 473 nmol/L 377 nmol/L 1558 nmol/L |
0.03 NS NS NS |
OR, 1.18 (95% CI, 0.98–1.43) per
100-nmol/L increase§§ OR, 2.15 (95% CI, 0.97–4.78) per 10-Å increase§§ OR, 2.16 (95% CI, 0.97–4.80) per 10-Å increase∥∥ OR, 1.53 (95% CI, 0.55–4.30) per 10-Å increase¶¶ |
0.08 0.06 NS NS |
No No No No No Yes Yes |
Fair |
| Soedamah-Muthu et al., 2003 (21) | Incident CAD | 59 case patients, 59 control participants | Large (213–230 Å) Medium (198–212 Å) Small (183–197 Å) LDL particle size |
603 nmol/L 120 nmol/L 800 nmol/L 206 Å |
688 nmol/L 111 nmol/L 526 nmol/L 210 Å |
NS ≤0.01 ≤0.001 ≤0.01 |
No data*** No data*** No data*** No data*** |
NS NS NS NS |
Yes Yes Yes Yes |
Fair |
| CVD severity | ||||||||||
| Rosenson et al, 2002 (22) | Change in MLD, mm MLD decrease >0.07 mm/y |
241 111 |
Large (213–230 Å) Small (183–197 Å) Large (213–230 Å) Small (183–197 Å) LDL particle number LDL particle size |
– – – – – – |
– – – – – – |
Spearman correlation, 0.03 Spearman correlation, −0.17††† OR, 0.4 (95% CI, 0.1–1.4) for <84 vs. ≥84 mg/dL OR, 9.1 (95% CI, 2.1–39) for <30 vs. ≥30 mg/dL OR, 1.4 (95% CI, 0.3–6.7) for <1825 vs. ≥1825 nmol/L OR, 0.2 (95% CI, 0.1–0.9) for <200 vs. ≥200 Å |
NS <0.01 NS <0.05 NS <0.05 |
Yes Yes Yes Yes Yes Yes |
Fair | |
CAD coronary artery disease; CVD cardiovascular disease; HDL high-density lipoprotein; HR hazard ratio; LDL low-density lipoprotein; MI myocardial infarction; MLD minimum lumen diameter; NMR nuclear magnetic resonance; NS not statistically significant; OR odds ratio; RR risk ratio; Tg triglyceride.
Adjusted for age, systolic and diastolic blood pressure, smoking, and lipid medication use.
Adjusted for HDL cholesterol and Tg. Fourth quartile compared with first quartile.
Adjusted for LDL particle number. Fourth quartile compared with first quartile.
Adjusted for treatment group, age, hypertension, smoking, body mass index, and diabetes mellitus status.
Adjustment for LDL cholesterol, HDL cholesterol, and Tg did not “appreciably change these relations.”
Adjusted for age, race, and LDL cholesterol.
Adjusted for treatment group.
Adjusted for treatment group, age, smoking, alcohol use, diabetes, and hypertension.
Adjusted for Tg and total cholesterol/HDL cholesterol. Fourth quartile compared with first quartile.
Adjusted for matching factors.
Adjusted for total cholesterol level.
Adjusted for HDL cholesterol.
( Multivariable analysis with all predictors and Tg and HDL particle number.
Adjusted for LDL cholesterol, HDL cholesterol, Tg, and other factors.
Table 3.
Summary of Associations of LDL Subfractions Measured by Nuclear Magnetic Resonance and Incident Cardiovascular Outcomes
| LDL Sub- fraction |
Definition (Number of Studies) |
Total Participants (Range), n |
Outcomes Analyzed (Number of Studies) |
Study Quality (Number of Studies) |
Significant Difference
in Mean Values* |
Significant Predictor† | |||
|---|---|---|---|---|---|---|---|---|---|
| Studies, n‡ |
Direction of Association§ |
Lipid-Adjusted Studies/Total Studies, n/n‡ |
Unadjusted Studies/Total Studies, n/n‡ |
Direction of Association§ |
|||||
| Small particles | 180–212 Å
(2) <182–197 Å (1) 183–197 Å (4) No data (1) |
5586 (118–2888) | CAD (3) CAD or CAD death (2) CAD death (1) CVD or CVD death (1) Intracerebral hemorrhage (1) |
Good (2) Fair (6) Poor (0) |
3/7 | Higher concentration | 0/2 | 1/2‡ | Higher concentration |
| Large particles | 212–230 Å
(2) 213–227 Å (1) 213–230 Å (5) No data (1) |
5586 (118–2888) | CAD (3) CAD or CAD death (2) CAD death (1) CVD or CVD death (1) Intracerebral hemorrhage (1) |
Good (2) Fair (6) Poor (0) |
2/7 | Higher concentration | 0/2 | 0/2 | |
| Particle number | nmol/L (10), higher level | 8806 (260–3066) | CAD or CAD death (4) CVD or CVD death (3) CAD (2) Intracerebral hemorrhage (1) |
Good (2) Fair (8) Poor (0) |
7/9 | Higher concentration | 4 (or 5)/5 (or 6)∥ | 3/4 | Higher concentration |
| Particle size | Å (8), smaller size | 5586 (118–2888) | CAD (3) CAD or CAD death (2) CAD death (1) CVD or CVD death (1) Intracerebral hemorrhage (1) |
Good (2) Fair (6) Poor (0) |
4/7 1/7 |
Smaller Larger** |
0/4 | 1/3¶ | Smaller |
CAD coronary artery disease; CVD cardiovascular disease (including cerebrovascular and peripheral vascular disease); LDL low-density lipoprotein.
Statistical significance of mean LDL subfraction values in participants with vs. those without cardiovascular events.
Statistical significance of regression analysis (odds ratio, hazard ratio, or risk ratio); significant analyses per total analyses.
Significant analyses per total analyses.
For statistically significant differences.
1 study (13) reported an association (in graphical terms) but did not report statistical significance. 1 study (16) implied, but did not report, a lack of a significant association
Unclear whether the analysis was lipid-adjusted in 1 study (16).
Intracerebral hemorrhage.
For the evaluation of incident cardiovascular disease risk (Tables 2 and 3), 10 analyses evaluated LDL particle number (concentration in nmol/L), 8 evaluated LDL particle size, and 8 evaluated concentrations of different-sized LDL particles (generally small, medium, and large).
LDL Particle Number and Incidence of Cardiovascular Disease
Among the studies evaluating particle number, the 5 that adjusted for cholesterol concentrations (13–16, 19) found associations between higher particle number and increased incidence of cardiovascular disease, although 1 of these studies (13) did not report whether this analysis was statistically significant. In addition, 1 study (16) reported lipid-adjusted analyses in women but not in men (suggesting that the lipid-adjusted analysis in men was not statistically significant). Three of these studies (14, 16, 19) divided participants into quartiles based on LDL particle number and found that those in the highest quartiles were at increased risk for cardiovascular events compared with those in the lowest quartile (2 studies also reported a statistically significant trend across quartiles [14, 19]). The other 2 studies (13, 15) measured increased risk per 1-SD increase in LDL particle number. Two studies (13, 18) found statistically significant associations between higher LDL particle number (without adjustment for cholesterol concentrations) and cardiovascular events in 3 of 4 subgroup analyses. In 6 studies, 7 of 9 subgroup analyses found that participants who developed cardiovascular disease had higher LDL particle numbers at baseline. One of the 2 studies (20) that found no association evaluated intracerebral hemorrhage as an outcome; the other studies all evaluated atherosclerotic cardiovascular disease.
LDL Particle Size and Incidence of Cardiovascular Disease
Seven studies (with 8 subgroup analyses) (14–17, 19–21) evaluated LDL particle size. None of 4 lipid-adjusted analyses found an association with cardiovascular events. Among 3 studies with unadjusted analyses, 1 found no association (14), 1 found that participants in the smallest quartile of LDL particle size had a statistically significantly higher risk for cardiovascular events (although it did not report the unit of analysis or whether the analysis was lipidadjusted) (16), and the study of intracerebral hemorrhage (20) found a trend toward a greater risk with larger particles. In the 7 comparisons of mean baseline LDL particle size, 4 studies found statistically significantly smaller particles among persons with cardiovascular events, whereas 1 study found statistically significantly larger particles in those with intracerebral hemorrhage. However, the mean baseline LDL particle sizes were so similar that the clinical significance of this association is unclear; both means fell within the small LDL category (Table 1). The largest absolute difference in mean particle sizes was 206 versus 210 Å (21).
LDL Subfraction Concentration and Incidence of Cardiovascular Disease
Seven studies (with 8 subgroup analyses) (14–17, 19–21) evaluated small and large LDL particle concentrations as possible predictors of cardiovascular events. Neither study that performed lipid-adjusted analyses (15, 21) found a statistically significant association with either small or large LDL subfractions. Neither lipid-unadjusted analysis of large particles (17, 20) found an association. In an analysis that lacked information on whether lipid adjustment was done (16), participants in the quartile with the highest concentration of the small LDL subfraction were at increased risk for incident coronary artery disease, but the same researchers found no association with cardiac death in a separate cohort (17). Three of 7 analyses found that participants who developed cardiovascular disease had higher concentrations of small LDL particles at baseline. In contrast, the 2 (of 7) statistically significant analyses of mean baseline levels (14, 20) both found that higher concentrations of large LDL particles were associated with intracerebral hemorrhage or coronary artery disease.
LDL Subfractions and Severity of Cardiovascular Disease
One fair-quality study evaluated a measure of coronary artery lumen diameter (Table 2). Rosenson and colleagues (22) reported an association between both smaller LDL particle size and concentration of small LDL particles (183 to 197 Å) and a decrease over time in minimum lumen diameter. The study reported adjusted odds ratios of 0.2 (95% CI, 0.1 to 0.9) for particle size (above vs. below median size) and 9.1 (CI, 2.1 to 39) for concentration of small LDL particles (above vs. below median concentration). Large particle (213 to 230 Å) concentration and LDL particle number were not associated with change in minimum lumen diameter.
All Methods of Measuring LDL Subfractions
Fourteen studies (23–36) used other methods to measure LDL subfractions (not universally available to clinicians). We extracted only basic data from these studies (Appendix Tables 3 and 4, available at www.annals.org) and summarized them together with the fully extracted studies. Table 4 lists the number of studies, separate analyses, and participants included for all methods of measuring LDL subfractions (clinically available and other methods). The data are divided by the outcome type and the LDL subfraction measurement type (size, number, or pattern). We separately counted the results of analyses that were unadjusted or adjusted for cholesterol concentrations. The numbers of studies that reported statistically significant “positive,” “negative,” or nonsignificant associations are summarized. We also enumerated the studies that reported both unadjusted and adjusted analyses.
Table 4.
Analyses Reporting Unadjusted and Adjusted Associations With Cardiovascular Outcomes, by Outcome and Measurement Type
| Cardiovascular Outcome and Measurement Type |
Unadjusted Analyses* | Lipid-Adjusted Analyses† | Unadjusted Versus Lipid-Adjusted Analyses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies, n‡ |
Participants, n |
Result |
Studies, n‡ |
Participants, n |
Result |
Studies, n‡ |
Participants, n |
Result |
|||||
| Significant Analyses, n§ |
Nonsignificant Analyses, n |
Significant Analyses, n |
Nonsignificant Analyses, n |
Analyses Remained Significant, n |
Significant Analyses Became Nonsignificant, n |
Analyses Remained Nonsignificant, n |
|||||||
| Coronary artery disease, incident | |||||||||||||
| Size | 15 | 16 565∥ | 12 | 6 | 9 | 4579 | 3 | 6 | 8 | 2507 | 2 | 5 | 1 |
| Number | 9 | 6101 | 7 | 2 | 3 | 6754 | 3 | 3 | 6214 | 3 | |||
| Pattern | 11 | 11 511 | 8 | 5 | 5 | 5927 | 3 | 3 | 5 | 5927 | 3 | 2 | 1 |
| Coronary artery disease, progression | |||||||||||||
| Size | 7 | 6531 | 4 | 3 | 4 | 5949 | 1 | 3 | 4 | 5949 | 1 | 1 | 2 |
| Number | 1 | 111 | 1 | 1 | 111 | 1 | 1 | 111 | 1 | ||||
| Pattern | 6 | 6366 | 4 | 2 | 3 | 5752 | 2 | 1 | 3 | 5752 | 2 | 1 | |
| Cerebrovascular disease, incident | |||||||||||||
| Size | 1 | 148 | 1 | 1 | 148 | 1 | 1 | 148 | 1 | ||||
| Number | 1 | 148 | 1 | ||||||||||
| Pattern | 1 | 148 | 1 | ||||||||||
| Incident disease or progression (total of all studies) | |||||||||||||
| Size | 23 | 23 244¶ | 17 | 9 | 14 | 10 675 | 4 | 10 | 13 | 8603 | 3 | 7 | 3 |
| Number | 11 | 6360 | 7 | 4 | 4 | 6865 | 3 | 1 | 4 | 6325 | 3 | 1 | |
| Pattern | 18 | 18 025 | 13 | 7 | 8 | 11 679 | 5 | 4 | 9 | 11 679 | 5 | 2 | 2 |
Associations are not adjusted for other lipids but may be adjusted for other cardiovascular risk factors.
Associations are adjusted for other lipids, including low-density lipoprotein cholesterol or high-density lipoprotein cholesterol levels; ratio of total to high-density lipoprotein cholesterol level; and possibly other cardiovascular risk factors, such as age, weight, and diabetes status.
The number of analyses may exceed the number of studies.
Includes associations that were reported to have been found, but the level of statistical significance was not reported.
The number of participants could also be 17 275 (the number of analyzed participants was unclear in 1 study).
The number of participants could also be 23 954 (the number of analyzed participants was unclear in 1 study).
The designs of the additional studies were similar to those that used NMR or Berkeley HeartLab gel electrophoresis. They evaluated a wide range of populations, including persons with and without baseline cardiovascular disease, with various comorbid conditions and receiving a wide range of medications (although this was generally not explicitly described).
The majority of analyses (37 of 52) found that LDL subfraction size, number, and pattern were statistically significantly associated with cardiovascular outcomes in unadjusted analyses. For each measurement type, the majority of unadjusted analyses found an association with cardiovascular disease.
Compared with the 52 unadjusted analyses, there were only 26 lipid-adjusted analyses. The distribution of statistically significant and nonsignificant associations was more evenly split among the adjusted analyses; 12 of 26 such analyses found statistically significant adjusted associations with incident cardiovascular disease or progression. However, the adjusted analyses differed by measurement type: Only 4 of 14 analyses of LDL size were statistically significantly associated with cardiovascular disease after adjustment, but 8 of 12 LDL number and pattern analyses were. An important caveat about these analyses, however, is that studies used different methods to determine which variables would be adjusted for, including the various lipoprotein cholesterol and triglyceride concentrations, lipid ratios, other cardiovascular risk factors (such as blood pressure), and other variables (such as demographic characteristics). It is impossible to evaluate how the distribution of findings would have changed if all researchers had used similar analytic techniques.
Of note, many of these adjusted analyses were reported without presenting the unadjusted analyses. To understand the impact of adjustment on the findings of statistically significant associations, we evaluated how findings changed within the 26 analyses that reported both unadjusted and lipid-adjusted analyses. Among the analyses that found a statistically significant unadjusted association between LDL subfractions and incident cardiovascular disease or progression, 9 of 20 became statistically nonsignificant after adjusting for lipid and other factors. An additional 6 analyses remained statistically nonsignificant regardless of adjustment. Overall, similar to the separately analyzed unadjusted and lipid-adjusted results, few size analyses remained statistically significant after adjustment (3 of 13), whereas most number and pattern analyses remained statistically significant after adjustment (8 of 13).
DISCUSSION
Many studies have evaluated the association between LDL subfractions and cardiovascular outcomes. However, relatively few of these were performed with 1 commonly used measurement method—NMR—and none with the other clinically available methods. In addition to the variety of measurement methods used among all of the studies and the large number of studies that included methods not in clinical use, the specific subfractions evaluated have been inconsistent. Even among the NMR studies, which mostly evaluated LDL particle number and particle size, different cut-points were used for the various LDL subfractions. Most of the studies were graded fair quality, on the basis of such factors as failure to fully adjust for other risk factors or inadequate descriptions of models used, incomplete reporting of the analyses of interest for this review, small sample size, or incomplete reporting of LDL subfraction test methodology. All of these issues create important limitations in evaluating the comparability of the studies and the applicability of the studies to the question of whether measurement of LDL subfractions is clinically valuable, in terms of helping clinicians and patients to assess both cardiovascular risk and potential need for treatment. Nevertheless, the studies generally found that LDL particle number (an NMR-specific measurement) was associated with incident cardiovascular disease, but LDL particle size and small LDL particle fraction were not as consistently associated with incident disease.
None of the studies reported adequate analyses to determine the relative or incremental value of LDL subfraction measurement as a predictor of cardiovascular disease compared with traditional risk factors (1). No study compared LDL subfraction measurements with cardiovascular risk assessment technologies, by measuring the incremental increase in their diagnostic performance. Also, no study evaluated test performance (for example, sensitivity and specificity) of LDL subfractions to predict cardiovascular disease. Thus, even with evidence that higher LDL particle number may predict incident cardiovascular disease, evidence is lacking to support the clinical usefulness of adding the test to the traditional cardiovascular risk factors, including lipid and blood pressure measurements. This conclusion is consistent with that reached in the American Diabetes Association and the American College of Cardiology Foundation consensus statement (10).
Publication bias against studies that found null or negative outcomes may be an important factor determining the available published evidence (37). All of the articles on clinically available tests and most of the other articles reported data from secondary or post hoc analyses. It is likely that the positive secondary analyses were more often reported because negative results may be considered uninteresting and are thus less likely to be selected for journal publication or included in articles because of space limitations (38). The review process had other limitations beyond the limitations of the evidence itself. We focused on the methods available to clinical laboratories for measuring LDL subfractions. This approach may have put undue emphasis on commercial entities and may have underappreciated unique features of other available tests; however, the generalizability of resource- and time-intensive methods used only by research laboratories is probably limited with respect to clinical practice. It is unclear how the financial interests of the manufacturers of the clinically available methodologies may have affected which sets of samples were analyzed, what analyses were performed, or what results were published. In addition, across studies, we could not adequately judge the nuances of the different methods on the basis of technical details (when reported). We therefore could not evaluate how these issues may have affected the differences in results among the studies. Nonetheless, except for potential publication bias, these issues may be of relatively minor importance compared with the large degree of heterogeneity in test methods and measures, populations evaluated, and outcomes assessed.
Current research has identified a potential association between LDL subfractions and cardiovascular disease (both heterogeneously defined), but the data to support its value as an independent risk factor for general clinical use are currently limited. Future research regarding the putative incremental utility of LDL subfractions to improve estimates of cardiovascular risk will need to focus on uniformly (and universally) defined measures of LDL subfractions. From a clinical perspective (as opposed to a laboratory perspective), it is most important that a given analytical technique can be consistently performed and standardized across laboratories. Thus, standardization of LDL subfractions measures across research and clinical laboratories is needed. Only when clinical laboratories are using the same analytic techniques as researchers can clinicians expect to understand the value of the LDL subfraction tests. Currently, at least 3 LDL subfraction measurement methods are available for clinical use, but the current studies are not adequate to compare their reliability or test performance (39, 40).
The clinical utility of any new test (its value as a new predictor to evaluate cardiovascular risk) is of paramount importance. The observation of an association between risk factor and outcome alone, as was evaluated by the reviewed studies, is only a first step that must be confirmed by trials that attempt to modify the risk factor. This point is illustrated by the case of homocysteine: Strong observational data (41) suggested a positive association between plasma concentrations of homocysteine and cardiovascular outcomes, but randomized trials (42, 43) of treatments that successfully decreased homocysteine concentrations did not result in statistically significant benefit on cardiovascular end points or total mortality. Thus, even if LDL subfraction testing proves to be associated with cardiovascular outcomes, the tests will be of clinical value only if treatments based on the results of the LDL subfraction testing prove to be beneficial (44).
Acknowledgments
Grant Support: By the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services, under contract 29002–0022.
Appendix
Appendix Table 1.
Search Strategy*
| Step | Search Terms |
|---|---|
| 1 | (ldl or ldl-c).mp. or exp Cholesterol, LDL/ or exp Lipoproteins, LDL/ |
| 2 | ldl cholesterol.mp. |
| 3 | or/1–2 |
| 4 | particle size.mp. or exp Particle Size/ |
| 5 | (subfraction$ or subclass$).mp. |
| 6 | particle density.mp. |
| 7 | exp Nuclear Magnetic Resonance, Biomolecular/ or exp Magnetic Resonance Spectroscopy/ |
| 8 | (nuclear magnetic resonance or nmr or magnetic resonance spectroscopy).mp. |
| 9 | exp Chromatography, High Pressure Liquid/ or exp Chromatography/ |
| 10 | (chromatography or hplc or fplc).mp. |
| 11 | ultracentrifugation.mp. or exp Ultracentrifugation/ |
| 12 | centrifugation.mp. or exp Centrifugation/ |
| 13 | exp Electrophoresis/ or electrophoresis.mp. |
| 14 | or/4–13 |
| 15 | 3 and 14 |
| 16 | limit 15 to (humans and english language)† |
Databases used were Ovid MEDLINE, CAB Abstracts, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews.
Limit was not valid in CAB Abstracts, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews; records were retained.
Appendix Table 2.
Characteristics of Studies That Used Clinically Available Methods to Measure LDL Subfractions*
| Author, Year (Reference) |
Cardiovascular Outcome |
Study Design | Follow-up, y |
Participants, n |
Population | Mean Age, y† |
Men, %† |
Diabetic Persons, %† |
Smokers, %† |
Mean or Median LDL-c Level, mmol/L (mg/dL)† |
|---|---|---|---|---|---|---|---|---|---|---|
| Incident CVD | ||||||||||
| Cromwell et al, 2007 (13) | Incident CVD | Prospective longitudinal | 14.8 | 3066 | Framingham offspring without CVD at baseline, Tg <400 mg/dL | 51 | 47 | 4 | 24 | 3.4 (131) |
| El Harchaoui et al, 2007 (14) | Fatal or nonfatal CAD |
Nested case–control | 6 | 2888 | EPIC; healthy at baseline | 65 | 64 | 6 | 16 | 4.2 (164) |
| Otvos et al, 2006 (15) | Nonfatal MI or cardiac death | Nested case–control | 5.1 | 1061 | VA-HIT; established CHD, HDL-c
≤40 mg/dL, LDL-c ≤140 mg/dL, Tg ≤300 mg/dL |
64 | 100 | 37 | 22 | 2.9 (113) |
| Kuller et al, 2002 (16) |
Incident MI or angina | Nested case–control | No data | 683 | Cardiovascular Health Study; age ≥65 y | 73 | 56 | No data | No data | 3.3 (129) |
| Kuller et al, 2007 (17) |
Cardiac death | Nested case–control | 18 | 428 | MRFIT; elevated Framingham score, metabolic syndrome | 48 | 100 | 100‡ | 59 | 4.1 (160) |
| Hsia et al, 2008, substudy 1 (18) | Incident MI, coronary death | Nested case–control | 1 | 404 | Women’s Health Initiative; postmenopausal women (trial of estrogen and progestin vs. placebo) | 66 | 0 | 14 | 21 | 3.9 (151) |
| Hsia et al, 2008, substudy 2 (18) | Incident MI, coronary death | Nested case–control | 1 | 304 | Women’s Health Initiative;
postmenopausal women (trial of estrogen vs. placebo) |
67 | 0 | 24 | 20 | 3.9 (151) |
| Blake et al, 2002 (19) | Cardiac death, incident MI, or stroke | Nested case–control | 3 | 260 | Women’s Health Study, healthy at baseline | 60 | 0 | 11 | 59 | 3.3 (129) |
| Campbell et al, 2007 (20) | Incident stroke | Nested case–control | 3.9 | 158 | PROGRESS; history of stroke or TIA | 64 | 70 | 9 | 17 | 3.1 (118) |
| Soedamah-Muthu et al, 2003 (21) | Incident CAD | Nested case–control | 10 | 118 | Pittsburgh EDC study; type 1
diabetes diagnosed before age 17 y |
35 | 28 | 100 | 31 | 3.3 (126) |
| CVD severity | ||||||||||
| Rosenson et al, 2002 (22) | Change in MLD | Prospective longitudinal | 3 | 241 | PLAC-I trial; CAD, elevated LDL-c, Tg ≤350 mg/dL | 58 | 76 | No data | No data | 4.2 (163) |
CAD coronary artery disease; CHD coronary heart disease; CVD cardiovascular disease; EDC Epidemiology of Diabetes Complications; EPIC European Prospective Investigation into Cancer and Nutrition; HDL-c high-density lipoprotein cholesterol; LDL-c low-density lipoprotein cholesterol; MI myocardial infarction; MLD minimum lumen diameter; MRFIT Multiple Risk Factor Intervention Trial; PLAC-I Pravastatin Limitation of Atherosclerosis in the Coronary Arteries; PROGRESS Perindopril Protection Against Recurrent Stroke Study; Tg triglyceride; TIA transient ischemic attack; VA-HIT Veterans Affairs HDL Intervention Trial.
All studies used nuclear magnetic resonance.
Data at baseline.
These patients had the metabolic syndrome.
Appendix Table 3.
Association Between LDL Subfraction and Incident Cardiovascular Events (Not Full Extraction)
| Author, Year (Reference) |
Type of Test |
Study Design | Mean Age, y* |
Persons >65 y, %† |
Men, %* | Diabetic Persons, %* |
Smokers, % | Lipid Data |
|---|---|---|---|---|---|---|---|---|
| Howard et al, 2000 (36) | No data | Prospective longitudinal | 56 | ~15 | Not reported | 47 | 35 (current) | LDL-c: 113 mg/dL |
| Arsenault et al, 2007 (23) | GE | Nested case–control | 65 | ~50 | 64 | 6 | 16 (current) | LDL-c: 162 mg/dL |
| St-Pierre et al, 2005 (29) | GE | Prospective longitudinal | 57 | 0 | 100 | 5 | 23 (current) | LDL-c: 148 mg/dL |
| Stampfer et al, 1996 (30) | GE | Nested case–control | 59 | ~25 | 100 | 6 | 56 (ever smoked) | TC–HDL-c ratio: 5.2 |
| Campos et al, 2001 (25) | GE | Nested case–control | 60 | ~30 | 87 | 16 | 17 (current) | LDL-c: 139 mg/dL |
| Austin et al, 2000 (24) | GE | Nested case–control | 68 | ~70 | 100 | 17 | 63 (ever smoked) | LDL-c: 142 mg/dL |
| Mykkänen et al, 1999 (27) | GE | Nested case–control | 69 | 100 | 50 | 33 | 20 (current) | TC–HDL-c ratio: 6.09 |
| Gardner et al, 1996 (26) | GE | Nested case–control | 59 | 33 | 73 | No data | 42 (not defined) | Non–HDL-c: 176 mg/dL |
| LDL Subfraction Data | Sample | Duration of Follow-up, y |
Outcome | Predictor† | Results§ |
|
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| Mean size, 259.1 Å | 3668 or 4378 American Indians | Mean, 4.8 | Fatal and nonfatal CVD event | |||
| Women | Size | + | ||||
| Men | Size | 0 | ||||
| Diabetic persons | Size | |||||
| Mean size, 260 Å; <255 Å, 29% | Men: 660 with CAD event, 1209 control participants | Mean, 7.7 | Fatal or nonfatal CHD Men | |||
| Men | Size | + | ||||
| Pattern | + | + | ||||
| Women: 375 with CAD event, 777 control participants | Women | Size | + | |||
| Pattern | + | + | ||||
| Mean size, 256.9 Å; pattern B, 40% | 2072 healthy persons | Unadjusted, 13; adjusted, 5¶ | Ischemic CAD event | Size Pattern |
0 + |
+ |
| Mean size, 256 Å; pattern B, 47%; indeterminate pattern, 20% | 266 with CAD event, 308 control participants | 7 | Incident MI or CAD death | Size Pattern |
+ + |
0 0 |
| Mean size, 256 Å | Unadjusted analysis: 416 with CAD
event, 421 control participants Adjusted analysis: 242 with CAD event, 218 control participants |
Median, 5 | Confirmed MI or CAD death (on placebo) | Size | + | + |
| Mean size, 260.0 Å | 145 with incident CHD, 296 control participants | 12 | Incident CAD: MI or coronary intervention | Size Pattern |
+ + |
0 |
| Mean size, 268.2 Å; pattern B or indeterminate pattern, 21% | 86 with CAD event, 172 control participants | Mean, 3.5 | Incident MI or CAD death | Size Pattern |
0 0 |
0 |
| Mean size, 261.7 Å; <260 Å, 40%; >274.2 Å, 10% | 124 with CAD event, 124 with no event | Mean, 5 to CAD event | Incident MI or CAD death | Size Pattern+ |
+ +** |
+ |
CAD coronary artery disease; CHD coronary heart disease; GE gel electrophoresis; HDL-c high-density lipoprotein cholesterol; LDL-c low-density lipoprotein cholesterol; MI myocardial infarction; TC total cholesterol.
Of case patients in case–control or cross-sectional studies (when reported separately); data at baseline.
Approximate data were estimated from means (SDs) rounded to the nearest 5%; otherwise, data are the reported values.
“Size” refers to analysis based on actual particle size (regression or comparisons of mean sizes); “pattern” refers to analysis based on distribution across categories of LDL subfractions (small, medium, or large).
+ smaller particles were statistically significantly associated with worse cardiovascular outcomes; 0 no statistically significant association between LDL subfractions and cardiovascular outcomes.
It was unclear how many participants were analyzed.
From reference 44.
The authors reported an association between LDL subfraction and cardiovascular outcomes, but no statistical analysis was performed.
Appendix Table 4.
Association Between LDL Subfraction and Progression of CAD (Not Full Extraction)
| Author, Year (Reference) |
Type of Test |
Study Design | Mean Age, y* |
Persons >65 y, %† |
Men, %* |
Diabetic Persons, %* |
Smokers, % | Lipid Data† |
|---|---|---|---|---|---|---|---|---|
| Zhao et al, 2003 (32) | GE | Prospective
longitudinal RCT |
62 | 40 | 74 | 23 | 64 (ever smoked) | LDL-c: 141 mg/dL |
| Mack et al, 1996 (33) | UC | Prospective
longitudinal RCT |
58 | ~15 | 92 | No data | 79 (ever smoked) | LDL-c: 156 mg/dL |
| Vakkilainen et al, 2003 (31) | GE | Prospective
longitudinal RCT |
56 | 0 | 74 | 100 | No data | LDL-c: 131 mg/dL |
| Miller et al, 1996 (34) |
UC | Prospective
longitudinal RCT |
57 | ~20 | 100 | No data | No data | LDL-c: 156 mg/dL |
| Ruotolo et al, 1998 (28) | GE | Prospective
longitudinal RCT |
42 | 0 | 100 | No data | 24 (current) | LDL-c: 180 mg/dL |
| Watts et al, UC No data 100 No data No
data Total cholesterol: 1993 (35) |
UC | Prospective longitudinal RCT | No data | No data | 100 | No data | No data | Total cholesterol: 280 mg/dL |
| LDL Subfraction Data | Sample | Duration of Follow-up |
Outcome | Predictor | Results‡ |
|
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| Mean size, 266.8 Å; pattern
B, 26%; pattern A, 64%; small (<256 Å), 26% |
278 patients with CAD requiring PTCA or CABG | 3 y | Native CAD progression (angiography) | Size Pattern |
+ + |
|
| Peak flotation rate, Sf: 5.4; IV (Sf 0–3): 14.7 mg/dL | 220 patients with coronary stenosis | 2 y | Change in coronary atherosclerosis (angiography) | Size Pattern |
0 + |
|
| Mean size, 247.6 Å | 207 patients with type 2 diabetes (placebo group) | ≥1 y | Change in coronary atherosclerosis (angiography) | Size | 0 | 0 |
| Small, dense LDL (Sf° 0–5), 44%; pattern B, 41%; indeterminate pattern, 31%; pattern A, 28% | 116 patients with coronary stenosis (usual care group) | 4 y | Change in coronary atherosclerosis (angiography) | Pattern | 0 | |
| Mean size, 230 Å; small (<228 Å), 39% | 92 patients <45 y with MI | 5 y | Change in coronary atherosclerosis (angiography) | Size Pattern |
0 0 |
0 0 |
| LDL2 (density 1.019–1.040 kg/L), 36 mg/dL; LDL3 (density 1.040–1.063 kg/L), 92 mg/dL | 74 patients with angina pectoris not requiring revascularization | 38 mo | Change in coronary atherosclerosis (angiography) | Pattern | + | |
CABG coronary artery bypass graft; CAD coronary artery disease; GE gel electrophoresis; LDL-c low-density lipoprotein cholesterol; MI myocardial infarction; PTCA percutaneous transluminal coronary angioplasty; RCT randomized, controlled trial; UC ultracentrifugation.
Of case patients in case–control or cross-sectional studies (when reported separately); data at baseline.
Approximate data were estimated from means (SDs) rounded to the nearest 5%; otherwise, data are the reported values.
+smaller particles were statistically significantly associated with worse cardiovascular outcomes; 0 no statistically significant association between LDL subfractions and cardiovascular outcomes.
Footnotes
Disclaimer: This report is based on an update of a technology assessment performed as part of the Evidence-based Practice Center program of the Agency for Healthcare Research and Quality. The technology assessment was performed by request of the Centers for Medicare & Medicaid Services (Centers for Medicare & Medicaid Services. Low Density Lipoprotein Subfractions: Systematic Review of Measurement Methods and Association with Cardiovascular Outcomes. Accessed at www1.cms.hhs.gov/mcd/viewtechassess.asp?whereindex&tid56 on 3 February 2009), in response to a public hearing on lipoprotein subfractions held by the U.S. Food and Drug Administration in December 2006 (U.S. Food and Drug Administration. Clinical Chemistry and Clinical Toxicology Devices Panel of the Medical Advisory Committee. Accessed at www.fda.gov/OHRMS/DOCKETS/ac/06/transcripts/2006–4263t1–01t.pdf on 3 February 2009). The Agency for Healthcare Research and Quality participated in determining the key questions, defining the methodology, and reviewing and approving the technology assessment and the manuscript. However, the authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
Potential Financial Conflicts of Interest: None disclosed.
Current author addresses are available at www.annals.org.
References
- 1.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal RS, Michos ED, Nasir K. Further improvements in CHD risk prediction for women [Editorial]. JAMA. 2007;297:641–3. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 5.Ware JH. The limitations of risk factors as prognostic tools. N Engl J Med. 2006;355:2615–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 6.Krauss RM. Dense low density lipoproteins and coronary artery disease. Am J Cardiol. 1995;75:53B–57B. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 7.Krauss RM. Dietary and genetic effects on low-density lipoprotein heterogeneity. Annu Rev Nutr. 2001;21:283–95. [PMID: 8] [DOI] [PubMed] [Google Scholar]
- 8.Chapman MJ, Guérin M, Bruckert E. Atherogenic, dense low-density lipoproteins. Pathophysiology and new therapeutic approaches. Eur Heart J. 1998;19 Suppl A:A24–30. [PMID: ] [PubMed] [Google Scholar]
- 9.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 10.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, et al. ; American Diabetes Association. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31:811–22. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 11.Balk E, Ip S, Chung M, Lau J, Lichtenstein A. Low Density Lipoprotein Subfractions: Systematic Review of Measurement Methods and Association with Cardiovascular Outcomes Evidence Report/Technology Assessment (Prepared by Tufts Medical Center Evidence-based Practice Center, under contract No. 290–02-0022). Rockville, MD: Agency for Healthcare Research and Quality; 2008. Accessed at www1.cms.hhs.gov/mcd/viewtechassess.asp?from2viewtechassess.asp&whereindex&tid56&on3February2009. [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality. Methods Reference Guide for Effectiveness and Comparative Effectiveness Reviews, Version 1.0 [Draft posted Oct. 2007] Rockville, MD: Agency for Healthcare Research and Quality; 2007. Accessed at effectivehealthcare.ahrq.gov/repFiles/2007_10DraftMethodsGuide.pdfon3February2009. [Google Scholar]
- 13.Cromwell WC, Otvos JD, Keyes MJ, Pencina MJ, Sullivan L, Vasan RS, et al. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study—Implications for LDL management. J Clin Lipidol. 2007;1:583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Harchaoui K, van der Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:54753 [PMID: ] [DOI] [PubMed] [Google Scholar]
- 15.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006; 113:1556–63. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 16.Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22: 1175–80. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 17.Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R; Multiple Risk Factor Intervention Trial Research Group. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195:122–8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsia J, Otvos JD, Rossouw JE, Wu L, Wassertheil-Smoller S, Hendrix SL, et al. ; Women’s Health Initiative Research Group. Lipoprotein particle concentrations may explain the absence of coronary protection in the women’s health initiative hormone trials. Arterioscler Thromb Vasc Biol. 2008;28:1666–71. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 20.Campbell DJ, Neal BC, Chalmers JP, Colman SA, Jenkins AJ, Kemp BE, et al. Low-density lipoprotein particles and risk of intracerebral haemorrhage in subjects with cerebrovascular disease. Eur J Cardiovasc Prev Rehabil. 2007;14: 413–8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 21.Soedamah-Muthu SS, Chang YF, Otvos J, Evans RW, Orchard TJ; Pittsburgh Epidemiology of Diabetes Complications Study. Lipoprotein subclass measurements by nuclear magnetic resonance spectroscopy improve the prediction of coronary artery disease in type 1 diabetes. A prospective report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2003; 46:674–82. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 22.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002;90:89–94. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 23.Arsenault BJ, Lemieux I, Després JP, Wareham NJ, Luben R, Kastelein JJ, et al. Cholesterol levels in small LDL particles predict the risk of coronary heart disease in the EPIC-Norfolk prospective population study. Eur Heart J. 2007;28: 2770–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 24.Austin MA, Rodriguez BL, McKnight B, McNeely MJ, Edwards KL, Curb JD, et al. Low-density lipoprotein particle size, triglycerides, and high-density lipoprotein cholesterol as risk factors for coronary heart disease in older Japanese-American men. Am J Cardiol. 2000;86:412–6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 25.Campos H, Moye LA, Glasser SP, Stampfer MJ, Sacks FM. Low-density lipoprotein size, pravastatin treatment, and coronary events. JAMA. 2001;286: 1468–74. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 26.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–81. [PMID: ] [PubMed] [Google Scholar]
- 27.Mykkänen L, Kuusisto J, Haffner SM, Laakso M, Austin MA. LDL size and risk of coronary heart disease in elderly men and women. Arterioscler Thromb Vasc Biol. 1999;19:2742–8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 28.Ruotolo G, Ericsson CG, Tettamanti C, Karpe F, Grip L, Svane B, et al. Treatment effects on serum lipoprotein lipids, apolipoproteins and low density lipoprotein particle size and relationships of lipoprotein variables to progression of coronary artery disease in the Bezafibrate Coronary Atherosclerosis Intervention Trial (BECAIT). J Am Coll Cardiol. 1998;32:1648–56. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 29.St-Pierre AC, Cantin B, Dagenais GR, Mauriège P, Bernard PM, Després JP, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Québec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25:553–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 30.Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–8. [PMID: ] [PubMed] [Google Scholar]
- 31.Vakkilainen J, Steiner G, Ansquer JC, Aubin F, Rattier S, Foucher C, et al. ; DAIS Group. Relationships between low-density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: the Diabetes Atherosclerosis Intervention Study (DAIS). Circulation. 2003;107:1733–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 32.Zhao XQ, Kosinski AS, Barnhart HX, Superko HR, King SB 3rd. Prediction of native coronary artery disease progression following PTCA or CABG in the Emory Angioplasty Versus Surgery Trial. Med Sci Monit. 2003;9:CR48–54. [PMID: ] [PubMed] [Google Scholar]
- 33.Mack WJ, Krauss RM, Hodis HN. Lipoprotein subclasses in the Monitored Atherosclerosis Regression Study (MARS). Treatment effects and relation to coronary angiographic progression. Arterioscler Thromb Vasc Biol. 1996;16:697–704. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 34.Miller BD, Alderman EL, Haskell WL, Fair JM, Krauss RM. Predominance of dense low-density lipoprotein particles predicts angiographic benefit of therapy in the Stanford Coronary Risk Intervention Project. Circulation. 1996;94:2146–53. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 35.Watts GF, Mandalia S, Brunt JN, Slavin BM, Coltart DJ, Lewis B. Independent associations between plasma lipoprotein subfraction levels and the course of coronary artery disease in the St. Thomas’ Atherosclerosis Regression Study (STARS). Metabolism. 1993;42:1461–7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 36.Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The Strong Heart Study. Arterioscler Thromb Vasc Biol. 2000;20:830–5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 37.Davey Smith G, Ebrahim S. Folate supplementation and cardiovascular disease. Lancet. 2005;366:1679–81. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 38.Chan AW, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ. 2005;330: 753 [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung M, Lichtenstein AH, Ip S, Lau J, Balk EM. Comparability of methods for LDL subfraction determination: A systematic review. Atherosclerosis. 2009. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 40.Ensign W, Hill N, Heward CB. Disparate LDL phenotypic classification among 4 different methods assessing LDL particle characteristics. Clin Chem. 2006;52:1722–7. [PMID: 1] [DOI] [PubMed] [Google Scholar]
- 41.De Bree A, Verschuren WM, Kromhout D, Kluijtmans LA, Blom HJ. Homocysteine determinants and the evidence to what extent homocysteine determines the risk of coronary heart disease. Pharmacol Rev. 2002;54:599–618. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 42.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296:2720–6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 43.Bleys J, Miller ER 3rd, Pastor-Barriuso R, Appel LJ, Guallar E. Vitaminmineral supplementation and the progression of atherosclerosis: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;84:880–7; quiz 954–5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- 44.Lamarche B, St-Pierre AC, Ruel IL, Cantin B, Dagenais GR, Després JP. A prospective, population-based study of low density lipoprotein particle size as a risk factor for ischemic heart disease in men. Can J Cardiol. 2001;17:859–65. [PMID: ] [PubMed] [Google Scholar]