Abstract
HIV-associated neurocognitive disorder (HAND) affects nearly half of all HIV-infected individuals. Synaptodendritic damage correlates with neurocognitive decline in HAND, and many studies have demonstrated that HIV-induced neuronal injury results from excitotoxic and inflammatory mechanisms. The endocannabinoid (eCB) system provides on-demand protection against excitotoxicity and neuroinflammation. Here, we discuss evidence of the neuroprotective and anti-inflammatory properties of the eCB system from in vitro and in vivo studies. We examine the pharmacology of the eCB system and evaluate the therapeutic potential of drugs that modulate eCB signaling to treat HAND. Finally, we provide perspective on the need for additional studies to clarify the role of the eCB system in HIV neurotoxicity and speculate that strategies that enhance eCB signaling might slow cognitive decline in HAND.
Keywords: HIV-1, cannabinoid receptor, anandamide, 2-arachidonoylglycerol, monoacylglycerol lipase, fatty acid amide hydrolase
1. Introduction
Nearly half of all HIV-infected individuals experience cognitive and motor impairments (Heaton et al., 1995; Tozzi et al., 2005); these symptoms are collectively termed HIV-associated neurocognitive disorder (HAND). Combined antiretroviral therapy (cART) has reduced the number of patients that progress to AIDS (Heaton et al., 2010; McArthur, 1993), and increased the lifespan of infected individuals (ATCC, 2008; Dore et al., 2003). However, while cART suppresses viral load, it does not eradicate HIV from the brain (Valcour et al., 2011). Thus, the prevalence of HAND remains high (Cysique et al., 2004; Sacktor et al., 2001; Saylor et al., 2016), with no effective treatment available.
One promising area of therapeutic development is the endocannabinoid (eCB) system. This biological system is composed of endogenous lipid-based signaling molecules that bind to cannabinoid receptors, and the machinery that synthesizes and metabolizes them (Fig. 1). eCB signaling is dependent on neurons interacting with other neurons, astrocytes, and microglia (Ashton and Glass, 2007; Navarrete et al., 2014). The eCB system plays a role in many physiological processes. Here, we review its ability to provide on-demand protection against excitotoxicity and neuroinflammation (Marsicano et al., 2003; Walter and Stella, 2004), two hallmarks of many neurodegenerative disorders including HAND (Amor et al., 2010; Dong et al., 2009; Green et al., 2018).
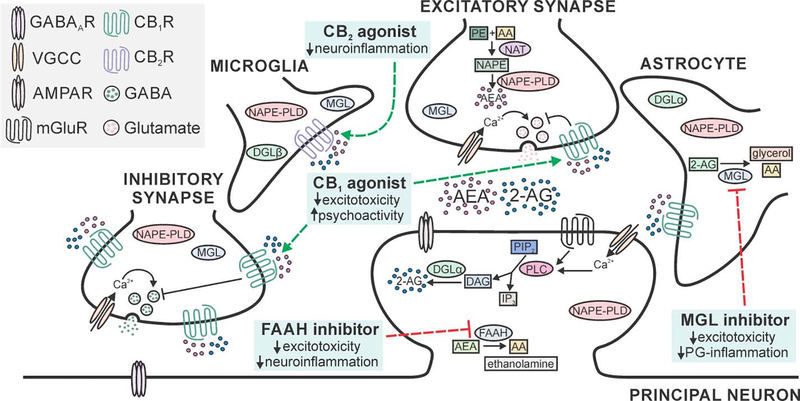
Fig. 1:
Neuroprotective targets in the eCB system. Schematic displays principal neuron receiving excitatory and inhibitory input with neighboring astrocyte and microglial cells. Cannabinoid type 1 receptors (CB1Rs) are present on presynaptic terminals, with high expression on a subset of GABAergic terminals and widespread expression at lower levels on glutamatergic terminals (Marsicano and Lutz, 1999). Cannabinoid type 2 receptors (CB2Rs) are found predominantly on cells of the immune system (microglia shown), with limited expression on neurons (not shown) (Nunez et al., 2004). All four cell types express diacylglycerol lipase (DGL) and N-arachidonoyl phosphatidylethanolamine phospholipase D (NAPE-PLD), the enzymes that synthesize the eCBs 2-arachdionoylglycerol (2-AG) and anandamide (AEA), respectively (Egertova et al., 2008; Ludanyi et al., 2011; Mishra et al., 2016; Viader et al., 2016; Zhang et al., 2011). For clarity, the pathways are described in detail in a single location with high expression. 2-AG is hydrolyzed by monoacylglycerol lipase (MGL) that is expressed in all four cell types, with the highest levels found in astrocytes (Muccioli et al., 2007; Viader et al., 2015). Fatty acid amide hydrolase (FAAH) is predominantly expressed in the somata and dendrites of principal neurons (Egertova et al., 2003; Tsou et al., 1998b). Important neuroprotective drug targets (blue boxes) include agonists for CB1 and CB2Rs (dashed green lines) and inhibitors of MGL and FAAH (dashed red lines). Abbreviations; 2-AG: 2-arachidonoylglycerol; AMPAR: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; AA: arachidonic acid; AEA: arachidonoyl ethanolamine (anandamide); CB1R: cannabinoid type 1 receptor; CB2R: cannabinoid type 2 receptor; DAG: diacylglycerol; DGL: diacylglycerol lipase; FAAH: fatty acid amide hydrolase; GABA: γ-aminobutyric acid; GABAAR: GABA type A receptor; IP3: inositol triphosphate; mGluR: metabotropic glutamate receptor; MGL: monoacylglycerol lipase; NAPE-PLD: N-arachidonoyl phosphatidylethanolamine phospholipase D; NAT: N-acyltransferase; NAPE: N-arachidonoyl phosphatidylethanolamine; PE: phosphatidylethanolamine; PG: prostaglandin; PIP2: phosphatidylinositol 4,5-bisphosphate; PLC: phospholipase C; VGCC: voltage-gated Ca2+ channel.
Drugs can enhance the eCB system by mimicking or potentiating its neuroprotective function. Changes in the eCB system associated with some neurological disorders might impair the neuroprotection that the system affords, exacerbating excitotoxicity. Thus, strategies to prevent loss of eCB signaling may preserve its neuroprotective function, and drugs that mimic or enhance eCB signaling may compensate for its diminished capacity in neurotoxic or inflammatory conditions, such as HAND. Cannabimimetic drugs are relevant to HIV because they are used to treat wasting and nausea in AIDS patients (Plasse et al., 1991), cannabis use among HIV-positive individuals is high (Okafor et al., 2017), and recent efforts to legalize medicinal and recreational marijuana are increasing access (Cerda et al., 2017). Here, we examine the components and pharmacology of the eCB system and discuss evidence of its neuroprotective and anti-inflammatory properties. We then evaluate the therapeutic potential of drugs that modulate the eCB system and speculate that strategies that enhance eCB signaling might slow cognitive decline in HAND.
2. Cannabinoid Receptors
CB1 and CB2 receptors have been studied extensively and are the best understood receptor components of the eCB system. However, eCBs also interact with ion channels and other G protein-coupled receptors (GPCRs). Anandamide activates the transient receptor potential vanilloid 1 (TRPV1) receptor, a calcium permeable, nonselective cation channel (Ross, 2003). Several orphan GPCRs, notably GPR55 and GPR18, have emerged as possible targets of eCBs (for review, see (Cuevas-Olguin et al., 2017)). While eCBs may act on these alternative targets in a manner relevant to pathophysiology, this review will focus on CB1 and CB2 receptors.
2.1. CB1 receptor agonists
Cannabinoid type 1 receptors (CB1Rs) are GPCRs abundantly expressed on neurons and astrocytes (Howlett et al., 2002; Matsuda et al., 1993; Navarrete and Araque, 2008) (Fig. 1). CB1Rs on neurons localize to presynaptic elements, where they modulate neurotransmission. CB1Rs on astrocytes modulate gliotransmission by regulating intracellular calcium, facilitating communication with neurons (Navarrete and Araque, 2008; Navarrete and Araque, 2010; Oliveira da Cruz et al., 2016). Histochemical studies have found CB1R mRNA in the principal (excitatory) neurons of the cerebellum, hypothalamus, thalamus, and lower brain stem (Howlett et al., 2002; Tsou et al., 1998a). CB1Rs are also found in a subset of inhibitory neurons, particularly cholecystokinin (CCK)-positive interneurons of the hippocampus, amygdala, and cerebral cortex (Herkenham et al., 1991; Mackie, 2005), with no expression in parvalbumin-positive interneurons (Marsicano and Lutz, 1999; Matsuda et al., 1993). This distribution corresponds to the physiological role of eCBs in control of motor activity, nociception, learning and memory, food intake, and cognitive function (for review, see (Di Marzo et al., 1998)). Most CB1Rs are coupled to Gi/o (Pertwee et al., 2010), where activation of the Gαi/Gαo subunit reduces cAMP production by inhibiting adenylyl cyclase, while the Gβγ complex targets many physiological effectors, including inwardly rectifying K+ channels, N- and P/Q-type Ca2+ channels, protein kinase signaling cascades, and others (Kano et al., 2009; Smrcka, 2008).
Here, we focus on the neuroprotective role of CB1Rs that, upon activation, inhibit glutamate release at excitatory synapses (Gerdeman and Lovinger, 2001; Shen et al., 1996). These receptors are part of a retrograde signaling system in which eCBs produced postsynaptically diffuse across the synaptic cleft to act on presynaptic CB1Rs. At glutamatergic synapses, this system provides a form of feedback inhibition. Upon excitation, the postsynaptic cell synthesizes the eCB 2-arachidonoylglycerol (2-AG) that then activates presynaptic CB1Rs to inhibit and reduce glutamate release. Interestingly, a recent study showed that astrocytes produce 2-AG in response to activation of metabotropic glutamate receptors, with subsequent activation of presynaptic CB1R to produce a form of synaptic depression (Smith et al., 2019).
CB1R-mediated presynaptic inhibition provides a basis for the neuroprotective properties of the eCB system, whereby the ability to inhibit glutamate release dampens excitotoxicity caused by excessive activation of glutamatergic pathways. Excitotoxicity is a hallmark of many neurodegenerative disorders (Dong et al., 2009) including HAND (Green et al., 2018), where overstimulation of glutamate receptors leads to a characteristic loss of postsynaptic structures (Dong et al., 2009); thus, preventing it may slow disease progression and attenuate symptoms.
Compelling evidence that CB1Rs protect against excitotoxicity comes from Marsicano et al. (2003), who demonstrated that mice lacking CB1Rs in all principal neurons of the forebrain but not astrocytes experienced more severe seizures in a kainic acid model of excitotoxic epileptiform seizures (Marsicano et al., 2003). Furthermore, Monory et al. (2006) demonstrated that CB1Rs on hippocampal glutamatergic neurons were central mediators of on-demand eCB-dependent protection against kainic acid-induced acute excitotoxic seizures (Monory et al., 2006). Subsequent reports expanded upon these results, demonstrating that viral-mediated overexpression of CB1Rs in the principal neurons of the hippocampus protected against seizure-induced excitotoxicity (Guggenhuber et al., 2010), and that the eCB 2-AG is the key activator of the CB1Rs that ultimately mediate seizure suppression (Sugaya et al., 2016).
In addition to preventing excitotoxicity by suppressing aberrant patterns of glutamatergic activity, CB1R-mediated neuroprotection may also result from activation of cell survival signaling cascades, including the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and the extracellular signal-regulated (ERK) pathway (Dalton and Howlett, 2012; Molina-Holgado et al., 2002). Thus, CB1R agonists may be viable therapeutic options to attenuate changes in network function that contribute to neuronal damage. Indeed, CB1R agonists attenuate excitotoxicity in several models of neurodegenerative disorders. The cannabinoid receptor agonist WIN55,212–2 reduced synaptically-mediated excitotoxicity evoked by reduction of ambient [Mg2+] in primary hippocampal cultures; this effect was blocked by the CB1R inverse agonist rimonabant (SR141716A), indicating a role for CB1Rs (Shen and Thayer, 1998). In rodent models of traumatic brain injury and stroke, pre-treatment with the cannabinoid agonists WIN55,212–2 and 2-AG reduced brain edema and infarct volumes (Nagayama et al., 1999; Panikashvili et al., 2001), and induced faster recovery of motor and behavioral function (Panikashvili et al., 2001). CB1R activation slowed disease progression in a model of multiple sclerosis (de Lago et al., 2012) possibly via suppression of inflammation, although excitotoxic mechanisms also participate in this complex model. In both in vitro and in vivo models of Parkinson’s and Huntington’s diseases, administration of exogenous 2-AG attenuated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)- and mutant huntingtin-induced neurodegeneration, respectively (Mounsey et al., 2015; Scotter et al., 2010). Several studies have also demonstrated that cannabinoid agonists protect against amyloid β-induced alterations in neuronal function and cognitive impairment (for review, see (Aso and Ferrer, 2014)). Notably, Haghani et al. (2012) demonstrated that administration of 2-AG protected against amyloid β-induced changes in intrinsic excitability of CA1 pyramidal neurons and improved retention and recall in a passive avoidance test in mice that were bilaterally injected with the Aβ(1–42) peptide fragment. These effects were blocked by the CB1R antagonist AM251, but not the CB2R antagonist AM630 (Haghani et al., 2012). In the AβPP/PS1 mouse model of Alzheimer’s disease, Aso et al. (2012) demonstrated that chronic administration of the synthetic CB1R agonist arachidonoyl-2’-chloroethylamide (ACEA) protected neurons against Aβ toxicity and improved performance in the two-object recognition test (Aso et al., 2012). Despite these and other promising preclinical studies, results from several clinical trials on the effect of exogenous cannabinoids in relieving dementia and related symptoms were inconclusive (Krishnan et al., 2009).
The protection afforded by CB1R agonists in neurodegeneration models suggests that they may also afford protection in HAND. Indeed, activation of CB1Rs modulates changes in network excitability induced by the HIV protein Tat. Using an in vitro model of HIV neurotoxicity, Xu et al. (2017) demonstrated that the eCBs anandamide and 2-AG reduced Tat-induced increases in intracellular [Ca2+] and promoted neuronal survival; the CB1R inverse agonist rimonabant prevented this eCB-mediated neuroprotection (Xu et al., 2017). While the mechanism of this CB1R-mediated neuroprotection is unknown, this and other studies (discussed in subsequent sections) suggest that enhancing eCB signaling may be beneficial in HAND.
Several important considerations should be noted in regard to targeting CB1Rs to combat HAND and other neurodegenerative disorders. Because CB1R activation largely mediates the psychoactive properties of exogenous cannabinoids (for review, see (Howlett et al., 2002)), the therapeutic potential of CB1R agonists is limited by adverse effects, including changes in feeding behavior (Haney et al., 2005; Wiley et al., 2005), motor slowing (More and Choi, 2015; Prashad and Filbey, 2017), and sedation (Zhornitsky and Potvin, 2012). Cannabinoid-induced cognitive impairment, particularly inhibition of short-term memory (Ranganathan and D’Souza, 2006), has also been demonstrated; this is an important consideration when the therapeutic goal is to prevent cognitive decline. Furthermore, chronic cannabis use can also produce dependence (Lopez-Quintero et al., 2011; Volkow et al., 2014). Thus, while CB1R agonists are neuroprotective in vitro and reduce neuronal damage in animal models of neurodegenerative disease, their shortcomings have spurred interest in other components of the eCB system. Selectively modulating CB2R and slowing eCB metabolism are two promising approaches with reduced psychoactive side effects.
2.2. CB2 receptor agonists
Cannabinoid type 2 receptors (CB2Rs) are Gi/o protein-coupled receptors found mainly in immune cells, including microglia, the resident macrophages of the CNS (Howlett et al., 2002; Nunez et al., 2004) (Fig. 1). Although some reports have described limited CB2R expression in neurons (Gong et al., 2006), activation of CB2Rs is generally thought to affect the immune system without the strong psychoactive effects of CB1R activation. Similar to CB1, CB2Rs regulate signaling cascades that include adenylyl cyclase, MAP kinase, and Ca2+ signaling (Bayewitch et al., 1995; Slipetz et al., 1995). These pathways affect a variety of immune functions, including cytokine release (Cabral and Griffin-Thomas, 2009), cell proliferation (Carrier et al., 2004), migration (Walter et al., 2003), and gene expression (Mecha et al., 2015). CB2Rs are undetectable in unstimulated microglia, and their expression increases in response to inflammatory stimuli (Cabral and Griffin-Thomas, 2009; Nunez et al., 2004); thus, CB2R agonists are preferentially effective during inflammatory states. Because receptor expression is immune cell-specific and state-dependent, CB2Rs can dampen the harmful effects of immune activation in the CNS with few adverse side effects.
Chronic inflammation is implicated in a growing number of neurodegenerative diseases. For example, reactive oxygen species produced by microglia damage neurons in Alzheimer’s and Parkinson’s disease (Javed et al., 2016; Shimohama et al., 2000). Pro-inflammatory cytokines can also damage neurons directly via excitotoxicity (Ye et al., 2013) and synaptodendritic damage (Festa et al., 2015), or indirectly through the recruitment and activation of immune cells (Kozela et al., 2011). In several disease models, CB2R activation counteracts neuroinflammation. Neuroprotection occurs by reducing pro-inflammatory cytokines (Klegeris et al., 2003; Malek et al., 2015) and reactive oxygen species (Javed et al., 2016; Shimohama et al., 2000), increasing anti-inflammatory cytokines (Ehrhart et al., 2005; Malek et al., 2015; Molina-Holgado et al., 2003), inhibiting chemotaxis to a variety of chemokines (Romero-Sandoval et al., 2009), and shifting microglia from a pro-inflammatory M1 phenotype to an alternatively activated reparative M2a phenotype (Mecha et al., 2015). This broad range of effects is well-equipped to combat the main sources of damage during neuroinflammation. Indeed, CB2R agonists reduce neurotoxicity and behavioral impairments in both in vitro and in vivo models of Alzheimer’s disease (Jayant et al., 2016; Ramirez et al., 2005), Parkinson’s disease (Ternianov et al., 2012), Huntington’s disease (Palazuelos et al., 2009), multiple sclerosis (Shao et al., 2014), and amyotrophic lateral sclerosis (Shoemaker et al., 2007). Thus, CB2R agonists hold the potential to treat many neurodegenerative diseases by targeting common neuroinflammatory mechanisms.
HIV causes potentially harmful neuroinflammation (Alonso et al., 1997; Everall et al., 2009; Ubaida-Mohien et al., 2017; Walsh et al., 2014), and CB2Rs are upregulated during HIV and simian immunodeficiency virus (SIV) infection (Benito et al., 2005; Cosenza-Nashat et al., 2011). This makes CB2Rs an attractive target for combating HAND. Indeed, activation of CB2Rs protects against HIV-induced neuroinflammation in vitro, mainly by decreasing inflammatory signaling and suppressing the chemokine-like activity of viral proteins (Kim et al., 2011; Purohit et al., 2014). Kim et al. (2011) demonstrated that the HIV envelope glycoprotein gp120 induced synapse loss in primary hippocampal neuronal/glial cultures. Activating CB2Rs prevented this synapse loss by decreasing production of the pro-inflammatory cytokine IL-1β (Kim et al., 2011; Zhang and Thayer, 2018). Similarly, a higher concentration of gp120 killed neurons in striatal neuronal/glial cultures, and the cannabinoid receptor agonist WIN55,212–2 blocked production of reactive oxygen species and pro-inflammatory cytokines, reduced microglial migration, and increased neuronal survival; these effects were largely CB2R-dependent (Hu et al., 2013).
In addition to preventing the release of neurotoxic cytokines and reactive oxygen species, CB2R activation may actually reduce HIV infection in the CNS. Δ9-tetrahydrocannabinol (Δ9-THC) acts through CB2Rs to reduce expression of the CD4, CXCR4, and CCR5 receptors that are required for HIV infection in monocyte-derived macrophages (Williams et al., 2014). Additionally, CB2R activation prevents productive infection of T-tropic HIV in human CD4+ T-cells by blocking the cytoskeletal rearrangements required for viral fusion (Costantino et al., 2012). CB2R agonists also affect the migration of HIV-infected cells: the HIV protein Tat attracts immune cells by activating chemokine receptors, and cannabinoids prevent migration of microglia-like BV2 cells to Tat (Fraga et al., 2011). Direct activation of CB2Rs also inhibits extracellular matrix adhesion, which monocytes use to cross the blood-brain barrier in response to HIV (Raborn et al., 2014). CB2R activation could, therefore, slow the spread of HIV infection through microglial populations, and reduce recruitment of peripheral immune cells that may contribute to neuroinflammation.
Despite in vitro evidence that activation of CB2Rs affords neuroprotection in the presence of HIV or its proteins, few in vivo studies have used drugs selective for CB2Rs in models of HAND, making it difficult to interpret the contribution of CB2 versus CB1Rs. Nevertheless, the few in vivo studies that have specifically investigated CB2Rs in HAND models suggest that their activation is beneficial. In a humanized mouse model infected with HIV, the CB2R agonist Gp1a reduced levels of the pro-inflammatory cytokine TNFα and microglial activation, as measured by CD11b expression (Gorantla et al., 2010). In gp120 transgenic mice, the selective CB2R agonist AM1241 rescued neurogenesis by increasing proliferation and decreasing apoptosis of neural precursor cells (Avraham et al., 2014). While intriguing, these studies did not investigate neuropathology in mature neurons, or assess functional/behavioral outcomes.
Although these treatment strategies have yet to be translated to humans, there is evidence that cannabinoid agonists are well-tolerated. Many HIV patients use cannabis, either recreationally or medically as an appetite stimulant, without severe adverse effects on overall immune function (Pacek et al., 2018). However, there is little evidence that cannabis users are any less susceptible to HAND than non-users. In fact, Δ9-THC itself can impair cognitive functioning in both controls and HIV-positive individuals (Thames et al., 2016), although this may occur via its psychoactive effects on CB1 rather than a CB2R-mediated effect (Terranova et al., 1995).
Several studies raise concerns about targeting CB2Rs to treat neurodegenerative disorders such as HAND. While CB2R activation is largely associated with anti-inflammatory effects, there is evidence suggesting a pro-inflammatory role. Specifically, CB2R antagonism significantly reduced the release of prostaglandin E2 from primary microglial cells (Saliba et al., 2018), and topical application of 2-AG resulted in dermal inflammation that was blocked by a CB2R antagonist (Oka et al., 2005; Oka et al., 2006). Furthermore, 2-AG enhances the motility of microglia in part via CB2R activation, suggesting that these receptors play a role in the accumulation of microglia at sites of neuroimmune insult (Walter et al., 2003). Thus, further identification of the pro-inflammatory responses induced by CB2R activation will help to define the potentially beneficial role of CB2R agonists in neuroinflammatory disorders.
Because studies in humans focus on Δ9-THC, dronabinol, cannabidiol, or some combination thereof, there is little data on whether a CB2R-selective compound would fare better in humans. The currently available studies investigating medical marijuana in HIV have been limited by small sample size, short duration of study, and difficulty keeping participants blinded to Δ9-THC-containing compounds (Lutge et al., 2013). One CB2R-selective agonist, APD371, is currently in phase 2 clinical trials for the treatment of abdominal pain in Crohn’s disease (ClinicalTrials.gov Identifier: ); however, this compound has low brain penetrance (Han et al., 2017), which makes it better for treating peripheral diseases rather than HAND. To generate high-quality data on the efficacy of CB2R agonists in HAND patients, well-controlled trials using selective CB2R agonists that cross the blood brain barrier are needed.
Despite the aforementioned limitations, selective CB2R activation remains a promising treatment avenue for two main reasons. First, because CB2R agonists primarily target immune cells rather than neurons, they have a lower risk of abuse and fewer psychoactive effects than CB1R agonists or mixed agonists. Second, because CB2R expression is increased during HIV and SIV infection (Benito et al., 2005; Cosenza-Nashat et al., 2011), CB2R agonists are most effective when neuroprotection is most needed.
3. Endogenous Ligands, Synthesis, and Metabolism
Several putative endogenous cannabinoids have been discovered (Hanus et al., 1993; Hanus et al., 2001); here, we focus on the two best-characterized ligands anandamide (AEA) and 2-arachidonoylglycerol (2-AG) (Fig. 1). AEA functions relatively slowly and generates both retrograde and non-retrograde signaling (Ohno-Shosaku and Kano, 2014). 2-AG is best known for its role as a retrograde messenger in the CNS, playing an important role in spike timing-dependent plasticity and long-term depression (Kano, 2014). AEA and 2-AG levels can be increased by inhibiting the metabolic enzymes that degrade them. Numerous selective eCB metabolic inhibitors have been developed and extensively characterized in neuropsychiatric and neurodegenerative models. Unlike broad and sustained CB1 or CB2R activation produced by agonists, boosting eCB tone by inhibiting metabolic enzymes enhances eCB signaling on-demand and in a site-specific manner, resulting in fewer side effects (Farrell and Soltesz, 2018). Metabolic enzyme inhibitors also overcome the issue of rapid degradation of 2-AG or AEA following direct administration (Savinainen et al., 2001). In this section we will focus on the neuroprotective effects of these modulators, discussing common and unique aspects of their uses and mechanisms in neurological disorders, and their therapeutic potential in HAND.
3.1. Inhibition of AEA metabolism
AEA was first isolated in 1992 from porcine brain in a screen for endogenous ligands for the cannabinoid receptor (Devane et al., 1992). AEA behaves as a partial agonist at both CB1 and CB2Rs, and a full agonist at the TRPV1 receptor (Mackie et al., 1993; Sugiura et al., 1999; Sugiura et al., 2000). In the brain, AEA mediates long-term depression (LTD) via the CB1 and TRPV1 receptors (Grueter et al., 2010; Ohno-Shosaku and Kano, 2014). AEA is an arachidonic acid (AA) derivative, generated from its membrane precursor N-arachidonoyl phosphatidyl ethanolamine (NAPE) through cleavage by a phospholipase D (NAPE-PLD) (Di Marzo, 2008; Liu et al., 2006). Hydrolytic metabolism of AEA occurs via fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996), and oxidative metabolism through various lipoxygenases and cyclooxygenase-2 (COX-2) (Burstein et al., 2000). Pharmacological inhibition of FAAH is a promising approach to enhance AEA function.
Selective FAAH inhibitors have been developed and utilized for nearly 20 years. These drugs fall into several families: α-ketoheterocycles (e.g. OL-135), carbamates (e.g. URB597), alkylsufonylfluorides (e.g. AM3506), and aryl ureas (e.g. PF3845) (Blankman and Cravatt, 2013). Inhibition of FAAH activity blocks the degradation of AEA, and thus holds the potential to enhance AEA-mediated synapse-specific neuroprotection (Farrell and Soltesz, 2018).
FAAH inhibitors exhibit robust analgesic and anxiolytic properties. They show particular promise for alleviating neuropathic pain, including in a model of HIV sensory neuropathic pain in which both CB1 and CB2Rs were activated (Chang et al., 2006; Mitchell et al., 2007; Nasirinezhad et al., 2015; Russo et al., 2007). Several novel FAAH inhibitors have recently been developed; these inhibitors are as effective for pain as classical FAAH inhibitors, but with improved metabolic, pharmacokinetic, and brain penetrative properties (Bhuniya et al., 2019). Enhancing AEA levels using FAAH inhibitors also produced anxiolytic effects in a CB1R-dependent manner in rodents, with few adverse effects (Busquets-Garcia et al., 2011; Gomes et al., 2011; Kinsey et al., 2011a; Kinsey et al., 2011b).
Inhibition of FAAH decreases the severity of seizures (Karanian et al., 2007): the FAAH inhibitor URB597 attenuated limbic seizures in a CB1R-dependent manner without impairing short- and long-term plasticity (Colangeli et al., 2017). Activation of CB1Rs is thought to be the primary mechanism of neuroprotection produced by FAAH inhibitors (Schlosburg et al., 2009), although recent studies suggest the TRPV1 receptor may contribute to this protection (Jee Kim et al., 2018; Naziroglu et al., 2018). Repeated seizures are commonly associated with neurodegenerative diseases such as Alzheimer’s disease and traumatic brain injury (Vossel et al., 2017; Webster et al., 2017), and commonly occur in HIV-infected individuals (Ssentongo, 2019). FAAH inhibition is protective in models of Alzheimer’s disease, traumatic brain injury and amyotrophic lateral sclerosis (Bilsland et al., 2006; Tchantchou et al., 2014; Vazquez et al., 2015a). In these disease models, FAAH inhibitors reduce neuroinflammation via cannabinoid receptors, although additional mechanisms may be involved (Vazquez et al., 2015b). Notably, FAAH-mediated degradation of other fatty acid ethanolamide molecules, including palmitoylethanolamide and oleoylethanolamide, alongside AEA may play a role in the potent analgesic and anti-inflammatory effects of FAAH inhibitors (for review see (Alhouayek and Muccioli, 2014)).
Recent clinical evidence indicates several potential safety concerns with FAAH inhibitors. In a phase 1 study, the reversible FAAH inhibitor BIA 10–2474 was orally administered to healthy volunteers to assess safety. Three of four participants developed an acute and rapidly progressive neurologic syndrome, including headache, a cerebellar syndrome, memory impairment, and altered consciousness, and one participant became brain dead (Kerbrat et al., 2016). The underlying mechanism of this unanticipated neurologic disorder remains unknown; however, several off-target effects of BIA 10–2474 were subsequently identified in silico (Molinski et al., 2017) and in vitro (van Esbroeck et al., 2017). The selective FAAH inhibitor PF-04457845 was well-tolerated in humans with no evidence of cannabimimetic adverse events, but lacked efficacy in the primary end point (Huggins et al., 2012). However, this study holds promise that selective FAAH inhibition can be tolerated by humans. Despite the limitations from these and other clinical studies, preclinical data suggest that selective FAAH inhibition may be beneficial in HIV-associated neurodegeneration.
Several recent studies have evaluated inhibition of FAAH in HAND models. Genetic deletion of FAAH rescued neurogenesis in transgenic mice expressing the HIV envelope glycoprotein gp120 (Avraham et al., 2015). The FAAH inhibitor PF3845 also protected against neuronal damage induced by HIV viral proteins via a mechanism involving cannabinoid receptors (Hermes et al., 2018). Thus, FAAH inhibition has therapeutic potential in HAND, although more investigation is needed.
3.2. Inhibition of 2-AG metabolism
2-AG was first isolated as an agonist for cannabinoid receptors in 1995 (Mechoulam et al., 1995). Unlike AEA, 2-AG is present in the brain at high concentrations and is a full agonist for both CB1 and CB2Rs (Buczynski and Parsons, 2010; Gonsiorek et al., 2000; Sugiura et al., 1999; Sugiura et al., 2006). It is derived from AA-containing membrane phospholipids, particularly phosphatidylinositol bisphosphate (PIP2). Phospholipase C (PLC) hydrolyzes the membrane phospholipid into diacylglycerol (DAG), which is cleaved into 2-AG by diacylglycerol lipase (DGL) (Kano et al., 2009; Sugiura et al., 2006). 2-AG production is Ca2+-dependent, and it functions as a retrograde signaling molecule that is released postsynaptically after depolarization-induced Ca2+ influx or activation of Gαq-coupled metabotropic receptors (Ohno-Shosaku et al., 2012). 2-AG diffuses across the synaptic cleft to stimulate presynaptic CB1Rs, suppressing the release of neurotransmitters; 2-AG also mediates CB1R-dependent LTD (Heifets and Castillo, 2009; Ohno-Shosaku and Kano, 2014). Hydrolytic metabolism of 2-AG occurs via monoacylglycerol lipase (MGL) and the serine hydrolases α/β hydrolase domain 6 and 12 (ABHD6 and 12) (Blankman et al., 2007; Di Marzo, 2008). MGL is located in neighboring astrocytes and in the presynaptic terminal, where it hydrolyzes 2-AG into AA and glycerol, thus terminating retrograde signaling. In the brain, 2-AG hydrolysis is the main source of AA for COX-2-mediated production of prostaglandins (Di Marzo et al., 2015; Nomura et al., 2011). Thus, MGL and DGL inhibitors have been explored as an approach to treat neuroinflammation. DGL inhibitors show promise for treating metabolic disorders (Janssen and van der Stelt, 2016), although the psychiatric side effects associated with decreased eCB signaling limit the therapeutic potential of this approach. On the other hand, MGL inhibitors increase 2-AG levels and decrease AA production, thus simultaneously enhancing eCB signaling and decreasing prostaglandin production.
Selective MGL inhibitors were developed slightly later than FAAH inhibitors and include: first-generation MGL inhibitors (e.g. URB602, NAM, and OMDM169), o-aryl carbamates (e.g. JZL184), and o-hexafluorisopropyl carbamates (e.g. KML29) (Blankman and Cravatt, 2013; Granchi et al., 2017). Inhibition of MGL both increases 2-AG levels in the brain, which boosts cannabinoid receptor-mediated neuroprotection and decreases the production of prostaglandins, reducing neuroinflammation (Di Marzo et al., 2015).
Similar to FAAH inhibitors, MGL inhibitors attenuate neuropathic pain and anxiety mainly via CB1R activation (Crowe et al., 2017; Kamimura et al., 2018; Kinsey et al., 2009; Kinsey et al., 2011a; Kinsey et al., 2011b; Sciolino et al., 2011). MGL inhibitors reduce seizures in several epileptic models, although the underlying mechanism remains contentious (Terrone et al., 2018; von Ruden et al., 2015b). Von Ruden et al. (2015) found that the MGL inhibitor JZL184 attenuated kindling progression in wild-type but not CB1R knockout mice, suggesting that CB1Rs suppress epileptogenesis (von Ruden et al., 2015a). However, in a recent study, Terrone et al. (2018) found that JZL184’s therapeutic effect is predominantly anti-inflammatory and not CB1R-dependent, caused by decreased availability of AA and reduced COX-2-mediated prostaglandin production (Terrone et al., 2018). One possible explanation for this difference is that the latter study used a chronic model of epilepsy, suggesting that the CB1R-mediated pathway may play a role initially, but that chronic suppression of inflammation maintains the anti-seizure effect long-term. This transient role for CB1Rs may result from 2-AG overload and desensitization of cannabinoid receptors following excessive inhibition of MGL (Chanda et al., 2010; Schlosburg et al., 2010). Overall, these studies indicate that MGL inhibition is neuroprotective via dual suppression of excitotoxicity and reduced neuroinflammation. A highly potent, orally available CNS-penetrant MGL inhibitor, ABX-1431, is currently in a phase 2 clinical trial for treatment of Tourette Syndrome and a phase 1 trial for neuropathic pain, suggesting therapeutic potential for this class of drugs (Cisar et al., 2018) (ClinicalTrials.gov Identifier: , ).
The effects of MGL inhibition in models of neurocognitive disorders has been extensively studied in recent years, with promising results. MGL inhibitors improved cognition and protected neuronal function in models of Alzheimer’s disease and Down syndrome (Chen et al., 2012; Lysenko et al., 2014). The protection afforded by inhibition of MGL was independent of CB1R activation, and instead involved neuroinflammatory pathways (Chen et al., 2012; Pihlaja et al., 2015; Piro et al., 2012; Yan et al., 2016) where activation of CB2Rs on microglia reduced the production of inflammatory cytokines, and the decreased pool of AA reduces prostaglandin production. These studies further suggest that inhibition of MGL might improve cognition in HAND patients.
Recently, we directly tested MGL inhibition in an in vitro model of HAND and demonstrated that the MGL inhibitor JZL184 protects against neuronal damage and excitatory synapse loss induced by HIV viral proteins (Zhang and Thayer, 2018). The protective mechanism involved activation of cannabinoid receptors, as well as decreased AA production. More studies in HAND models in vivo are needed to further evaluate MGL inhibitors in the treatment of HAND.
While MGL functions as the principle enzyme responsible 2-AG hydrolysis (Savinainen et al., 2012), ABHD6 and ABHD12, two other enzymes that hydrolyze 2-AG, play a smaller but still significant role. These enzymes have been evaluated for their role in eCB-mediated neuroprotection (Savinainen et al., 2012). Recent reports indicate that ABHD6 is the major 2-AG hydrolase in cell types not expressing MGL (Marrs et al.), while ABHD12 is more involved in phospholipid rather than 2-AG metabolism (Grabner et al., 2017). Only a limited number of ABHD6 inhibitors have been developed. These inhibitors include carbamate-based inhibitors WWL70, WWL123 and JZP430, and triazole urea-based inhibitors KT195 and KT20. Many of these drugs have significant off-target effects at high concentrations (Patel et al., 2015; Poursharifi et al., 2017); thus, there is a need for development of more potent and selective ABHD6 inhibitors.
The neuroprotective effects of ABHD6 inhibition have been reported in several models of neurological disorders, including neuropathic pain, seizure, traumatic brain injury, and multiple sclerosis. The mechanisms underlying neuroprotection by inhibition of ABHD6 are varied. In a model of neuropathic pain, treatment with the selective ABHD6 inhibitor WWL70 attenuated thermal hyperalgesia and mechanical allodynia by reducing the production of prostaglandin E2 and expression of COX-2, indicating an anti-inflammatory effect (Wen et al., 2018). In a model of multiple sclerosis, ABHD6 inhibition reduced neuroinflammation by activating CB2Rs (Manterola et al., 2018; Wen et al., 2015). A mouse model of traumatic brain injury reported that WWL70 protects through activation of CB1Rs (Tchantchou and Zhang, 2013). In contrast to these studies, in a pentylenetetrazole-induced seizure model, ABHD6 inhibition alleviated epileptic symptoms through a cannabinoid receptor-independent pathway that involved GABAA receptors (Naydenov et al., 2014). Thus, the specific neuroprotective mechanisms of ABHD6 inhibition varies across different disease models and requires further investigation. Inhibition of ABHD6 provides a novel strategy to evaluate in HAND and avoids side effects associated with desensitization of cannabinoid receptors.
3.3. Dual inhibition of AEA and 2-AG metabolism
Dual FAAH and MGL inhibitors attenuate the neuroinflammation and excitotoxicity associated with neurological disorders, such as chronic pain and seizure (Naidoo et al., 2012; Sakin et al., 2015; Sakin et al., 2018; Woodhams et al., 2017). JZL195 is the most commonly used dual inhibitor; other dual inhibitors include organophosphates (e.g. isopropyldodecylfluorophosphonate), and o-hydroxyacetamide carbamates (e.g. SA-57) (Blankman and Cravatt, 2013). The ability of these dual inhibitors to enhance eCB-mediated neuroprotection is unclear. In rodent models of pain, these dual inhibitors usually display higher efficacy than selective MGL or FAAH inhibitors alone, and a wider therapeutic window than cannabinoid receptor agonists (Adamson Barnes et al., 2016; Anderson et al., 2014). However, in an anxiety model, JZL195 did not reduce anxiety, in contrast to selective MGL or FAAH inhibitors (Bedse et al., 2018). Recent reports show that dual inhibitors produce more cannabinoid-related side effects compared to the selective MGL or FAAH inhibitors (Seillier et al., 2014; Wise et al., 2012). Thus, the relative risks and benefits of FAAH-MGL dual inhibitors must be evaluated further in neurological disease models, including HAND.
4. Pathological Impairment of the Endocannabinoid System
One important determinant of clinical outcome is the degree to which the target biological system is functional. Studies in vitro and in vivo have shown that exposure to excitotoxic stimuli alters the eCB system (Chen et al., 2003; Feng et al., 2016; Li et al., 2012) with increased CB1R-mediated inhibition of GABA release changing the balance of network excitability. Currently, it is not known if eCB signaling is altered in the presence of HIV. In the context of another neurodegenerative disorder, Westlake et al. (1994) found reduced CB1R agonist binding in the hippocampus and caudate of Alzheimer’s patients using in vitro receptor autoradiography. Using in situ hybridization histochemistry, they also found regionally discrete losses of mRNA expression in Alzheimer’s relative to normal brains (Westlake et al., 1994). Similarly, Ramirez et al. (2005) determined that cannabinoid receptors couple less efficiently to their G-proteins in Alzheimer’s brains (Ramirez et al., 2005). These and other studies suggest that impaired CB1R-mediated signaling might diminish the neuroprotective capacity of the eCB system, reducing the efficacy of exogenous CB1R agonists.
Several reports indicate changes in CB1 and CB2Rs during SIV and HIV infection. Benito et al. (2005) identified increases in CB2R immunoreactivity in cortical tissue from rhesus macaques with SIV-induced encephalitis (Benito 2005). In this same study, these SIV-infected macaques also showed increased FAAH immunoreactivity in brain cortical samples. These results agree with previous observations in Alzheimer’s disease brains (Benito et al., 2003), indicating a common pattern of response despite different primary inflammatory insults. Indeed, Consenza-Nashat et al. (2011) found CB1 and CB2R upregulation in macrophages and microglia in tissue from patients with HIV encephalitis (HIVE). The functional consequences of these changes are unknown; thus, it is important to determine how eCB signaling changes during exposure to HIV to evaluate the feasibility of targeting components of the eCB system and, if necessary, devise strategies to protect or enhance signaling.
5. Conclusions and Perspectives
The unique pathophysiology of HAND may be particularly amenable to modulation by the eCB system. The excitotoxic component of HAND is attenuated by activating presynaptic CB1Rs, either directly by agonists or indirectly through inhibition of eCB metabolism. The chronic neuroinflammation associated with HAND can be attenuated by activating CB2Rs on immune cells or inhibiting MGL-mediated AA production. Furthermore, expression of cannabinoid receptors is increased in HAND, potentially enhancing the sensitivity of the eCB system in the presence of HIV.
There are drawbacks to targeting certain elements of the eCB system. Of particular concern are the psychoactive effects resulting from CB1R activation. Motor impairment and reduced short term memory limit the utility of CB1R agonists. However, it may be possible to avoid the desensitization of cannabinoid receptors that accompanies chronic activation by enhancing endogenously produced agonists with MGL and FAAH inhibitors. Because HAND patients will likely not be treated prophylactically, it will be essential to understand the time course of the disease, particularly changes in neuroinflammation, to understand the relative importance of excitotoxic and neuroinflammatory targets.
In summary, we have discussed several promising targets in the eCB system for suppressing the neuronal damage that underlies HAND (Fig. 1). CB2R agonists suppress neuroinflammation without the psychoactive side effects of non-selective drugs that also activate CB1Rs. MGL inhibitors are promising because they potentiate endogenously produced 2-AG to inhibit glutamate release via CB1Rs and suppress immune function via CB2Rs, while also decreasing the pool of AA needed for the synthesis of prostaglandins in the CNS. Both CB2R-selective agonists and MGL inhibitors are well-tolerated and are currently in clinical trials for indications other than HAND. The work reviewed here suggests that these agents may slow the progression of HAND in individuals living with HIV.
Highlights.
Nearly half of all HIV-infected individuals experience cognitive and motor deficits.
HIV-induced neuronal injury results from excitotoxic and inflammatory mechanisms.
The endocannabinoid (eCB) system provides on-demand protection against excitotoxicity and neuroinflammation.
We discuss the potential of drugs that modulate eCB signaling to treat HIV-associated neurocognitive disorder.
Acknowledgements
This work was supported by National Institutes of Health Grants DA007304 and DA044809 to ST. MW was supported by NIH training grant T32 DA007097.
Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- 2-AG
2-arachidonoylglycerol
- AA
arachidonic acid
- AEA
arachidonoyl ethanolamine (anandamide)
- AIDS
acquired immunodeficiency syndrome
- CB1R
cannabinoid type 1 receptor
- CB2R
cannabinoid type 2 receptor
- DAG
diacylglycerol
- DGL
diacylglycerol lipase
- FAAH
fatty acid amide hydrolase
- GPCR
G protein-coupled receptor
- HIV
human immunodeficiency virus
- IP3
inositol triphosphate
- MGL
monoacylglycerol lipase
- NAPE-PLD
N-arachidonoyl phosphatidylethanolamine phospholipase D
- NAT
N-acyltransferase
- NAPE
N-arachidonoyl phosphatidylethanolamine
- PE
phosphatidylethanolamine
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PLC
phospholipase C
- SIV
simian immunodeficiency virus
- VGCC
voltage-gated Ca2+ channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson Barnes NS, et al. , 2016. Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine neuropathic pain model. Br J Pharmacol. 173, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhouayek M, Muccioli GG, 2014. Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discov Today. 19, 1632–9. [DOI] [PubMed] [Google Scholar]
- Alonso K, et al. , 1997. Cytokine patterns in adults with AIDS. Immunol Invest. 26, 341–50. [DOI] [PubMed] [Google Scholar]
- Amor S, et al. , 2010. Inflammation in neurodegenerative diseases. Immunology. 129, 154–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WB, et al. , 2014. Actions of the dual FAAH/MAGL inhibitor JZL195 in a murine inflammatory pain model. Neuropharmacology. 81, 224–30. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Glass M, 2007. The Cannabinoid CB2 Receptor as a Target for Inflammation-Dependent Neurodegeneration. Curr Neuropharmacol. 5, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso E, et al. , 2012. CB1 agonist ACEA protects neurons and reduces the cognitive impairment of AbetaPP/PS1 mice. J Alzheimers Dis. 30, 439–59. [DOI] [PubMed] [Google Scholar]
- Aso E, Ferrer I, 2014. Cannabinoids for treatment of Alzheimer’s disease: moving toward the clinic. Front Pharmacol. 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATCC, 2008. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 372, 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham HK, et al. , 2014. The cannabinoid CB(2) receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis. Br J Pharmacol. 171, 468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham HK, et al. , 2015. Impaired neurogenesis by HIV-1-Gp120 is rescued by genetic deletion of fatty acid amide hydrolase enzyme. Br J Pharmacol. 172, 4603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayewitch M, et al. , 1995. The peripheral cannabinoid receptor - adenylate cyclase inhibition and g protein coupling. Febs Letters. 375, 143–147. [DOI] [PubMed] [Google Scholar]
- Bedse G, et al. , 2018. Therapeutic endocannabinoid augmentation for mood and anxiety disorders: comparative profiling of FAAH, MAGL and dual inhibitors. Transl Psychiatry. 8, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, et al. , 2003. Cannabinoid CB2 Receptors and Fatty Acid Amide Hydrolase Are Selectively Overexpressed in Neuritic Plaque-Associated Glia in Alzheimer’s Disease Brains. J. Neurosci. 23, 11136–11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C, et al. , 2005. A Glial Endogenous Cannabinoid System Is Upregulated in the Brains of Macaques with Simian Immunodeficiency Virus-Induced Encephalitis. J. Neurosci. 25, 2530–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuniya D, et al. , 2019. Discovery and evaluation of novel FAAH inhibitors in neuropathic pain model. Bioorg Med Chem Lett. 29, 238–243. [DOI] [PubMed] [Google Scholar]
- Bilsland LG, et al. , 2006. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. Faseb J. [DOI] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF, 2007. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 14, 1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Cravatt BF, 2013. Chemical probes of endocannabinoid metabolism. Pharmacol Rev. 65, 849–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczynski MW, Parsons LH, 2010. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. Br J Pharmacol. 160, 423–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein SH, et al. , 2000. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 61, 29–41. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A, et al. , 2011. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 70, 479–86. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L, 2009. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 11, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier EJ, et al. , 2004. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 65, 999–1007. [DOI] [PubMed] [Google Scholar]
- Cerda M, et al. , 2017. Association of State Recreational Marijuana Laws With Adolescent Marijuana Use. JAMA Pediatr. 171, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda PK, et al. , 2010. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 78, 996–1003. [DOI] [PubMed] [Google Scholar]
- Chang L, et al. , 2006. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br J Pharmacol. 148, 102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, et al. , 2006. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature. 440, 1208–12. [DOI] [PubMed] [Google Scholar]
- Chen K, et al. , 2003. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 39, 599–611. [DOI] [PubMed] [Google Scholar]
- Chen R, et al. , 2012. Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell Rep. 2, 1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar JS, et al. , 2018. Identification of ABX-1431, a Selective Inhibitor of Monoacylglycerol Lipase and Clinical Candidate for Treatment of Neurological Disorders. J Med Chem. 61, 9062–9084. [DOI] [PubMed] [Google Scholar]
- Colangeli R, et al. , 2017. The FAAH inhibitor URB597 suppresses hippocampal maximal dentate afterdischarges and restores seizure-induced impairment of short and long-term synaptic plasticity. Sci Rep. 7, 11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza-Nashat MA, et al. , 2011. Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities. Neuropathol Appl Neurobiol. 37, 464–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino CM, et al. , 2012. Cannabinoid receptor 2-mediated attenuation of CXCR4-tropic HIV infection in primary CD4+ T cells. PLoS One. 7, e33961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, et al. , 1996. Molecular Characterization of an Enzyme That Degrades Neuromodulatory Fatty-Acid Amides. Nature. 384, 83–87. [DOI] [PubMed] [Google Scholar]
- Crowe MS, et al. , 2017. The monoacylglycerol lipase inhibitor KML29 with gabapentin synergistically produces analgesia in mice. Br J Pharmacol. 174, 4523–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Olguin R, et al. , 2017. Interleukin 6 trans-signaling regulates basal synaptic transmission and sensitivity to pentylenetetrazole-induced seizures in mice. Synapse. 71. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ, 2004. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus–infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: A combined study of two cohorts. J Neurovirol. 10, 350–357. [DOI] [PubMed] [Google Scholar]
- Dalton GD, Howlett AC, 2012. Cannabinoid CB1 receptors transactivate multiple receptor tyrosine kinases and regulate serine/threonine kinases to activate ERK in neuronal cells. Br J Pharmacol. 165, 2497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lago E, et al. , 2012. Cannabinoids ameliorate disease progression in a model of multiple sclerosis in mice, acting preferentially through CB1 receptor-mediated anti-inflammatory effects. Neuropharmacology. 62, 2299–308. [DOI] [PubMed] [Google Scholar]
- Devane WA, et al. , 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 258, 1946–1949. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, et al. , 1998. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action [Review]. Trends in Neurosciences. 21, 521–528. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, 2008. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol. 160, 1–24. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Stella N, Zimmer A, 2015. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 16, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XX, Wang Y, Qin ZH, 2009. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 30, 379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore GJ, et al. , 2003. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 17, 1539–45. [DOI] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, Elphick MR, 2003. Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 119, 481–96. [DOI] [PubMed] [Google Scholar]
- Egertova M, et al. , 2008. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: A new perspective on N-acylethanolamines as neural signaling molecules. J Comp Neurol. 506, 604–15. [DOI] [PubMed] [Google Scholar]
- Ehrhart J, et al. , 2005. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall I, et al. , 2009. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 15, 360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell JS, Soltesz I, 2018. Plants come to mind: phytocannabinoids, endocannabinoids and the control of seizures. Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, et al. , 2016. Transient increase of interleukin-1beta after prolonged febrile seizures promotes adult epileptogenesis through long-lasting upregulating endocannabinoid signaling. Sci Rep. 6, 21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa L, et al. , 2015. Induction of Interleukin-1beta by Human Immunodeficiency Virus-1 Viral Proteins Leads to Increased Levels of Neuronal Ferritin Heavy Chain, Synaptic Injury, and Deficits in Flexible Attention. J Neurosci. 35, 10550–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga D, et al. , 2011. Cannabinoids inhibit migration of microglial-like cells to the HIV protein Tat. J Neuroimmune Pharmacol. 6, 566–77. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM, 2001. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. Journal of Neurophysiology. 85, 468–471. [DOI] [PubMed] [Google Scholar]
- Gomes FV, et al. , 2011. Facilitation of CB1 receptor-mediated neurotransmission decreases marble burying behavior in mice. Prog Neuropsychopharmacol Biol Psychiatry. 35, 434–8. [DOI] [PubMed] [Google Scholar]
- Gong JP, et al. , 2006. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 1071, 10–23. [DOI] [PubMed] [Google Scholar]
- Gonsiorek W, et al. , 2000. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: Antagonism by anandamide. Molecular Pharmacology. 57, 1045–1050. [PubMed] [Google Scholar]
- Gorantla S, et al. , 2010. Immunoregulation of a CB2 Receptor Agonist in a Murine Model of NeuroAIDS. Journal of Neuroimmune Pharmacology. 5, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner GF, et al. , 2017. Monoglyceride lipase as a drug target: At the crossroads of arachidonic acid metabolism and endocannabinoid signaling. Pharmacol Ther. 175, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granchi C, et al. , 2017. A patent review of Monoacylglycerol Lipase (MAGL) inhibitors (2013–2017). Expert Opin Ther Pat. 27, 1341–1351. [DOI] [PubMed] [Google Scholar]
- Green MV, et al. , 2018. Scaling Synapses in the Presence of HIV. Neurochem Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC, 2010. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 13, 1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenhuber S, et al. , 2010. AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PLoS One. 5, e15707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghani M, et al. , 2012. CB1 cannabinoid receptor activation rescues amyloid beta-induced alterations in behaviour and intrinsic electrophysiological properties of rat hippocampal CA1 pyramidal neurones. Cell Physiol Biochem. 29, 391–406. [DOI] [PubMed] [Google Scholar]
- Han S, et al. , 2017. Discovery of APD371: Identification of a Highly Potent and Selective CB2 Agonist for the Treatment of Chronic Pain. ACS Med Chem Lett. 8, 1309–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, et al. , 2005. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Berl). 181, 170–8. [DOI] [PubMed] [Google Scholar]
- Hanus L, et al. , 1993. Two new unsaturated fatty acid ethanolamides in brain that bind to the cannabinoid receptor. J Med Chem. 36, 3032–4. [DOI] [PubMed] [Google Scholar]
- Hanus L, et al. , 2001. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proceedings of the National Academy of Sciences of the United States of America. 98, 3662–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. , 1995. The HNRC 500--neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J Int Neuropsychol Soc. 1, 231–51. [DOI] [PubMed] [Google Scholar]
- Heaton RK, et al. , 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 75, 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE, 2009. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 71, 283–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, et al. , 1991. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 11, 563–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes DJ, et al. , 2018. Neuroprotective effects of fatty acid amide hydrolase catabolic enzyme inhibition in a HIV-1 Tat model of neuroAIDS. Neuropharmacology. 141, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, et al. , 2002. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors [Review]. Pharmacological Reviews. 54, 161–202. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Rock RB, 2013. CB2 receptor agonists protect human dopaminergic neurons against damage from HIV-1 gp120. PLoS One. 8, e77577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins JP, et al. , 2012. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 153, 1837–46. [DOI] [PubMed] [Google Scholar]
- Janssen FJ, van der Stelt M, 2016. Inhibitors of diacylglycerol lipases in neurodegenerative and metabolic disorders. Bioorg Med Chem Lett. 26, 3831–7. [DOI] [PubMed] [Google Scholar]
- Javed H, et al. , 2016. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson’s Disease. Front Neurosci. 10, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayant S, et al. , 2016. Pharmacological benefits of selective modulation of cannabinoid receptor type 2 (CB2) in experimental Alzheimer’s disease. Pharmacol Biochem Behav. 140, 39–50. [DOI] [PubMed] [Google Scholar]
- Jee Kim M, et al. , 2018. Analgesic effects of FAAH inhibitor in the insular cortex of nerve-injured rats. Mol Pain. 14, 1744806918814345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura R, et al. , 2018. Inhibition of 2-arachydonoylgycerol degradation attenuates orofacial neuropathic pain in trigeminal nerve-injured mice. J Oral Sci. 60, 37–44. [DOI] [PubMed] [Google Scholar]
- Kano M, et al. , 2009. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 89, 309–80. [DOI] [PubMed] [Google Scholar]
- Kano M, 2014. Control of synaptic function by endocannabinoid-mediated retrograde signaling. Proc Jpn Acad Ser B Phys Biol Sci. 90, 235–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanian DA, et al. , 2007. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J Pharmacol Exp Ther. 322, 1059–66. [DOI] [PubMed] [Google Scholar]
- Kerbrat A, et al. , 2016. Acute Neurologic Disorder from an Inhibitor of Fatty Acid Amide Hydrolase. N Engl J Med. 375, 1717–1725. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Shin AH, Thayer SA, 2011. Activation of cannabinoid type 2 receptors inhibits HIV-1 envelope glycoprotein gp120-induced synapse loss. Mol Pharmacol. 80, 357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, et al. , 2009. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 330, 902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, et al. , 2011a. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol Biochem Behav. 99, 718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey SG, et al. , 2011b. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol Biochem Behav. 98, 21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, Bissonnette CJ, McGeer PL, 2003. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. British Journal of Pharmacology. 139, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E, et al. , 2011. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br J Pharmacol. 163, 1507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Cairns R, Howard R, 2009. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev. CD007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Krogh KA, Thayer SA, 2012. Epileptic stimulus increases Homer 1a expression to modulate endocannabinoid signaling in cultured hippocampal neurons. Neuropharmacol. 63, 1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. , 2006. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 103, 13345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Quintero C, et al. , 2011. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 115, 120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludanyi A, et al. , 2011. Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience. 174, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutge EE, Gray A, Siegfried N, 2013. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev. 4, CD005175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysenko LV, et al. , 2014. Monoacylglycerol lipase inhibitor JZL184 improves behavior and neural properties in Ts65Dn mice, a model of down syndrome. PLoS One. 9, e114521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Devane WA, Hille B, 1993. Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Molecular Pharmacology. 44, 498–503. [PubMed] [Google Scholar]
- Mackie K, 2005. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System In: Cannabinoids. Vol., Pertwee RG, ed.êds. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 299–325. [DOI] [PubMed] [Google Scholar]
- Malek N, et al. , 2015. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015, 130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manterola A, et al. , 2018. Re-examining the potential of targeting ABHD6 in multiple sclerosis: Efficacy of systemic and peripherally restricted inhibitors in experimental autoimmune encephalomyelitis. Neuropharmacology. 141, 181–191. [DOI] [PubMed] [Google Scholar]
- Marrs WR, et al. , The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 13, 951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B, 1999. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. European Journal of Neuroscience. 11, 4213–4225. [DOI] [PubMed] [Google Scholar]
- Marsicano G, et al. , 2003. CB1 Cannabinoid Receptors and On-Demand Defense Against Excitotoxicity. Science. 302, 84–88. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TL, Lolait SJ, 1993. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 327, 535–550. [DOI] [PubMed] [Google Scholar]
- McArthur JC, 1993. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 43, 2230–2237. [DOI] [PubMed] [Google Scholar]
- Mecha M, et al. , 2015. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. 49, 233–45. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, et al. , 1995. Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds to Cannabinoid Receptors. Biochemical Pharmacology. 50, 83–90. [DOI] [PubMed] [Google Scholar]
- Mishra A, et al. , 2016. Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat Neurosci. 19, 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell VA, et al. , 2007. Actions of the endocannabinoid transport inhibitor AM404 in neuropathic and inflammatory pain models. Clin Exp Pharmacol Physiol. 34, 1186–90. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, et al. , 2002. Cannabinoids Promote Oligodendrocyte Progenitor Survival: Involvement of Cannabinoid Receptors and Phosphatidylinositol-3 Kinase/Akt Signaling. J. Neurosci 22, 9742–9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado F, et al. , 2003. Endogenous Interleukin-1 Receptor Antagonist Mediates Anti-Inflammatory and Neuroprotective Actions of Cannabinoids in Neurons and Glia. J. Neurosci 23, 6470–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinski SV, et al. , 2017. Computational proteome-wide screening predicts neurotoxic drug-protein interactome for the investigational analgesic BIA 10–2474. Biochem Biophys Res Commun. 483, 502–508. [DOI] [PubMed] [Google Scholar]
- Monory K, et al. , 2006. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 51, 455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SV, Choi DK, 2015. Promising cannabinoid-based therapies for Parkinson’s disease: motor symptoms to neuroprotection. Mol Neurodegener. 10, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounsey RB, et al. , 2015. Increasing levels of the endocannabinoid 2-AG is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Exp Neurol. 273, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muccioli GG, et al. , 2007. Identification of a Novel Endocannabinoid-Hydrolyzing Enzyme Expressed by Microglial Cells. J. Neurosci 27, 2883–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama T, et al. , 1999. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J Neurosci 19, 2987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo V, et al. , 2012. Equipotent inhibition of fatty acid amide hydrolase and monoacylglycerol lipase - dual targets of the endocannabinoid system to protect against seizure pathology. Neurotherapeutics. 9, 801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasirinezhad F, et al. , 2015. Attenuation of persistent pain-related behavior by fatty acid amide hydrolase (FAAH) inhibitors in a rat model of HIV sensory neuropathy. Neuropharmacology. 95, 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Araque A, 2008. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 57, 883–93. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A, 2010. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 68, 113–26. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Diez A, Araque A, 2014. Astrocytes in endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 369, 20130599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naydenov AV, et al. , 2014. ABHD6 blockade exerts antiepileptic activity in PTZ-induced seizures and in spontaneous seizures in R6/2 mice. Neuron. 83, 361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naziroglu M, et al. , 2018. Inhibitions of anandamide transport and FAAH synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1 channel in an in vitro seizure model. Mol Cell Biochem. [DOI] [PubMed] [Google Scholar]
- Nomura DK, et al. , 2011. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 334, 809–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez E, et al. , 2004. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse. 53, 208–13. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, et al. , 2012. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist. 18, 119–32. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Kano M, 2014. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol. 29C, 1–8. [DOI] [PubMed] [Google Scholar]
- Oka S, et al. , 2005. Evidence for the involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in 12-O-tetradecanoylphorbol-13-acetate-induced acute inflammation in mouse ear. J Biol Chem. 280, 18488–97. [DOI] [PubMed] [Google Scholar]
- Oka S, et al. , 2006. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol. 177, 8796–805. [DOI] [PubMed] [Google Scholar]
- Okafor CN, et al. , 2017. Prevalence and correlates of marijuana use among HIV-seropositive and seronegative men in the Multicenter AIDS Cohort Study (MACS), 1984–2013. Am J Drug Alcohol Abuse. 43, 556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira da Cruz JF, et al. , 2016. Astroglial type-1 cannabinoid receptor (CB1): A new player in the tripartite synapse. Neuroscience. 323, 35–42. [DOI] [PubMed] [Google Scholar]
- Pacek LR, et al. , 2018. Frequency of Cannabis Use and Medical Cannabis Use Among Persons Living With HIV in the United States: Findings From a Nationally Representative Sample. AIDS Educ Prev. 30, 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J, et al. , 2009. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, et al. , 2001. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 413, 527–31. [DOI] [PubMed] [Google Scholar]
- Patel JZ, et al. , 2015. Optimization of 1,2,5-thiadiazole carbamates as potent and selective ABHD6 inhibitors. ChemMedChem. 10, 253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, et al. , 2010. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol Rev. 62, 588–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlaja R, et al. , 2015. Monoacylglycerol lipase inhibitor JZL184 reduces neuroinflammatory response in APdE9 mice and in adult mouse glial cells. J Neuroinflammation. 12, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piro JR, et al. , 2012. A dysregulated endocannabinoid-eicosanoid network supports pathogenesis in a mouse model of Alzheimer’s disease. Cell Rep. 1, 617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasse TF, et al. , 1991. Recent clinical experience with dronabinol. Pharmacology, Biochemistry & Behavior. 40, 695–700. [DOI] [PubMed] [Google Scholar]
- Poursharifi P, Madiraju SRM, Prentki M, 2017. Monoacylglycerol signalling and ABHD6 in health and disease. Diabetes Obes Metab. 19 Suppl 1, 76–89. [DOI] [PubMed] [Google Scholar]
- Prashad S, Filbey FM, 2017. Cognitive motor deficits in cannabis users. Curr Opin Behav Sci. 13, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Rapaka RS, Rutter J, 2014. Cannabinoid receptor-2 and HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol. 9, 447–53. [DOI] [PubMed] [Google Scholar]
- Raborn ES, et al. , 2014. Cannabinoid inhibits HIV-1 Tat-stimulated adhesion of human monocyte-like cells to extracellular matrix proteins. Life Sci. 104, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez BG, et al. , 2005. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 25, 1904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC, 2006. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl). 188, 425–44. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval EA, et al. , 2009. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol Pain. 5, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, 2003. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 140, 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, et al. , 2007. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3’-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J Pharmacol Exp Ther. 322, 236–42. [DOI] [PubMed] [Google Scholar]
- Sacktor N, et al. , 2001. CSF antiretroviral drug penetrance and the treatment of HIV-associated psychomotor slowing. Neurology. 57, 542–4. [DOI] [PubMed] [Google Scholar]
- Sakin YS, et al. , 2015. The effect of FAAH, MAGL, and Dual FAAH/MAGL inhibition on inflammatory and colorectal distension-induced visceral pain models in Rodents. Neurogastroenterol Motil. 27, 936–44. [DOI] [PubMed] [Google Scholar]
- Sakin YS, Tanoglu A, Gulsen M, 2018. Dual FAAH and MAGL inhibition might play a key role in visceral pain. Turk J Gastroenterol. 29, 625–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba SW, et al. , 2018. Anti-neuroinflammatory effects of GPR55 antagonists in LPS-activated primary microglial cells. J Neuroinflammation. 15, 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savinainen JR, et al. , 2001. Despite substantial degradation, 2-arachidonoylglycerol is a potent full efficacy agonist mediating CB1 receptor-dependent G-protein activation in rat cerebellar membranes. British Journal of Pharmacology. 134, 664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, Laitinen JT, 2012. The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol (Oxf). 204, 267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, et al. , 2016. HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol. 12, 234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Kinsey SG, Lichtman AH, 2009. Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation. Aaps J. 11, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, et al. , 2010. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 13, 1113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG, 2011. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res. 64, 226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotter EL, et al. , 2010. Neuroprotective potential of CB1 receptor agonists in an in vitro model of Huntington’s disease. Br J Pharmacol. 160, 747–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillier A, Dominguez Aguilar D, Giuffrida A, 2014. The dual FAAH/MAGL inhibitor JZL195 has enhanced effects on endocannabinoid transmission and motor behavior in rats as compared to those of the MAGL inhibitor JZL184. Pharmacol Biochem Behav. 124, 153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao BZ, et al. , 2014. Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis via activation of autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci Ther. 20, 1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, et al. , 1996. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 16, 4322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Thayer SA, 1998. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Molec Pharmacol. 54, 459–462. [DOI] [PubMed] [Google Scholar]
- Shimohama S, et al. , 2000. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun. 273, 5–9. [DOI] [PubMed] [Google Scholar]
- Shoemaker JL, et al. , 2007. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J Neurochem. 101, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slipetz DM, et al. , 1995. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Molecular Pharmacology. 48, 352–361. [PubMed] [Google Scholar]
- Smith NA, Bekar LK, Nedergaard M, 2019. Astrocytic Endocannabinoids Mediate Hippocampal Transient Heterosynaptic Depression. Neurochem Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka AV, 2008. G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 65, 2191–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssentongo P, 2019. Prevalence and incidence of new-onset seizures and epilepsy in patients with human immunodeficiency virus (HIV): Systematic review and meta-analysis. Epilepsy Behav. 93, 49–55. [DOI] [PubMed] [Google Scholar]
- Sugaya Y, et al. , 2016. Crucial Roles of the Endocannabinoid 2-Arachidonoylglycerol in the Suppression of Epileptic Seizures. Cell Rep. [DOI] [PubMed] [Google Scholar]
- Sugiura T, et al. , 1999. Evidence that the cannabinoid CB1 receptor is a 2arachidonoylglycerol receptor. Structure-activity relationship of 2-arachidonoylglycerol, ether-linked analogues, and related compounds. J Biol Chem. 274, 2794–801. [DOI] [PubMed] [Google Scholar]
- Sugiura T, et al. , 2000. Evidence that 2-arachidonoylglycerol but not N-palmitoylethanolamine or anandamide is the physiological ligand for the cannabinoid CB2 receptor - Comparison of the agonistic activities of various cannabinoid receptor ligands in HL-60 cells. Journal of Biological Chemistry. 275, 605–612. [DOI] [PubMed] [Google Scholar]
- Sugiura T, et al. , 2006. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. [DOI] [PubMed] [Google Scholar]
- Tchantchou F, Zhang Y, 2013. Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J Neurotrauma. 30, 565–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchantchou F, et al. , 2014. The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology. 85, 427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternianov A, et al. , 2012. Overexpression of CB2 cannabinoid receptors results in neuroprotection against behavioral and neurochemical alterations induced by intracaudate administration of 6-hydroxydopamine. Neurobiol Aging. 33, 421 e1–16. [DOI] [PubMed] [Google Scholar]
- Terranova JP, et al. , 1995. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and win55212–2 - reversal by sr141716 a, a selective antagonist of cb1 cannabinoid receptors. Naunyn Schmiedebergs Archives of Pharmacology. 352, 576–579. [DOI] [PubMed] [Google Scholar]
- Terrone G, et al. , 2018. Inhibition of monoacylglycerol lipase terminates diazepam-resistant status epilepticus in mice and its effects are potentiated by a ketogenic diet. Epilepsia. 59, 79–91. [DOI] [PubMed] [Google Scholar]
- Thames AD, et al. , 2016. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care. 28, 628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, et al. , 2005. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses. 21, 706–13. [DOI] [PubMed] [Google Scholar]
- Tsou K, et al. , 1998a. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 83, 393–411. [DOI] [PubMed] [Google Scholar]
- Tsou K, et al. , 1998b. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci Lett. 254, 137–40. [DOI] [PubMed] [Google Scholar]
- Ubaida-Mohien C, et al. , 2017. Modifications in acute phase and complement systems predict shifts in cognitive status of HIV-infected patients. AIDS. 31, 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, et al. , 2011. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 8, 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Esbroeck ACM, et al. , 2017. Activity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10–2474. Science. 356, 1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez C, et al. , 2015a. Endocannabinoid regulation of amyloid-induced neuroinflammation. Neurobiol Aging. 36, 3008–3019. [DOI] [PubMed] [Google Scholar]
- Vazquez C, et al. , 2015b. Endocannabinoids regulate the activity of astrocytic hemichannels and the microglial response against an injury: In vivo studies. Neurobiol Dis. 79, 41–50. [DOI] [PubMed] [Google Scholar]
- Viader A, et al. , 2015. Metabolic Interplay between Astrocytes and Neurons Regulates Endocannabinoid Action. Cell Rep. 12, 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viader A, et al. , 2016. A chemical proteomic atlas of brain serine hydrolases identifies cell type-specific pathways regulating neuroinflammation. Elife. 5, e12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, et al. , 2014. Adverse health effects of marijuana use. N Engl J Med. 370, 2219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ruden EL, et al. , 2015a. Inhibition of monoacylglycerol lipase mediates a cannabinoid 1-receptor dependent delay of kindling progression in mice. Neurobiol Dis. 77, 238–45. [DOI] [PubMed] [Google Scholar]
- von Ruden EL, et al. , 2015b. Analysis in conditional cannabinoid 1 receptor-knockout mice reveals neuronal subpopulation-specific effects on epileptogenesis in the kindling paradigm. Neurobiol Dis. 73, 334–47. [DOI] [PubMed] [Google Scholar]
- Vossel KA, et al. , 2017. Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol. 16, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JG, et al. , 2014. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology. 11, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, et al. , 2003. Nonpsychotropic Cannabinoid Receptors Regulate Microglial Cell Migration. J. Neurosci. 23, 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Stella N, 2004. Cannabinoids and neuroinflammation [Review]. British Journal of Pharmacology. 141, 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KM, et al. , 2017. Inflammation in epileptogenesis after traumatic brain injury. J Neuroinflammation. 14, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J, et al. , 2015. Activation of CB2 receptor is required for the therapeutic effect of ABHD6 inhibition in experimental autoimmune encephalomyelitis. Neuropharmacology. 99, 196–209. [DOI] [PubMed] [Google Scholar]
- Wen J, et al. , 2018. WWL70 protects against chronic constriction injury-induced neuropathic pain in mice by cannabinoid receptor-independent mechanisms. J Neuroinflammation. 15, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake TM, et al. , 1994. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience. 63, 637–52. [DOI] [PubMed] [Google Scholar]
- Wiley JL, et al. , 2005. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Br J Pharmacol. 145, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JC, et al. , 2014. Delta(9)-Tetrahydrocannabinol treatment during human monocyte differentiation reduces macrophage susceptibility to HIV-1 infection. J Neuroimmune Pharmacol. 9, 369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, et al. , 2012. Dual fatty acid amide hydrolase and monoacylglycerol lipase blockade produces THC-like Morris water maze deficits in mice. ACS Chem Neurosci. 3, 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams SG, et al. , 2017. The cannabinoid system and pain. Neuropharmacology. 124, 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, et al. , 2017. Endocannabinoids exert CB1 receptor-mediated neuroprotective effects in models of neuronal damage induced by HIV-1 Tat protein. Mol Cell Neurosci. 83, 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, et al. , 2016. NO2 inhalation promotes Alzheimer’s disease-like progression: cyclooxygenase-2-derived prostaglandin E2 modulation and monoacylglycerol lipase inhibition-targeted medication. Sci Rep. 6, 22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, et al. , 2013. IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem. 125, 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, et al. , 2011. Cannabinoid receptor and N-acyl phosphatidylethanolamine phospholipase D--evidence for altered expression in multiple sclerosis. Brain Pathol. 21, 544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Thayer SA, 2018. Monoacylglycerol lipase inhibitor JZL184 prevents HIV-1 gp120-induced synapse loss by altering endocannabinoid signaling. Neuropharmacology. 128, 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, Potvin S, 2012. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 5, 529–52. [DOI] [PMC free article] [PubMed] [Google Scholar]