Abstract
Trans-fatty acid (TFA) intake can increase the risk of coronary heart disease (CHD) morbidity and mortality and all-cause mortality. Industrially produced TFAs and ruminant TFAs are the major sources in foods. TFA intake and TFA-attributed CHD mortality vary widely worldwide. Excessive TFA intake is a health threat in high-income countries; however, it is also a threat in low- and middle-income countries (LMICs). Data on TFA intake are scarce in many LMICs and an urgent need exists to monitor TFAs globally. We reviewed global TFA intake and TFA-attributed CHD mortality and current progress toward policy or regulation on elimination of industrially produced TFAs in foods worldwide. Human biological tissues can be used as biomarkers of TFAs because they reflect actual intake from various foods. Measuring blood TFA levels is a direct and reliable method to quantify TFA intake.
Summary.
What is already known on this topic?
Trans-fatty acid (TFA) intake increases the risk of morbidity and mortality due to coronary heart disease and all-cause mortality. Many low- and middle-income countries lack accurate and reliable data on TFA intake in their populations.
What is added by this report?
We found wide variations in global burden of TFA intake. TFA intake and TFA-attributed cardiovascular disease has decreased in high-income countries. An urgent need exists to measure and monitor TFA intake globally. Human biological tissues can be used as biomarkers of TFAs as they reflect actual intake from various foods.
What are the implications for public health practice?
Laboratory testing of TFA levels in plasma or serum is a direct and reliable method for estimating short-term TFA intake from foods. Measuring and monitoring TFA intake in the population is key for making evidence-based policies and evaluating the effectiveness of public health interventions.
Background
The World Health Organization (WHO) calls for elimination of industrially produced (artificial) trans-fatty acids (TFAs) from the global food supply by 2023; in May 2018 WHO launched the REPLACE (review, promote, legislate, assess, create, enforce) action package to provide strategic guidance for all countries to take action toward this goal (1). One of the 6 components of the REPLACE package is to “assess and monitor trans-fats content in the food supply and changes in trans-fat consumption in the population.” Measuring the TFA levels in human biologic tissues can be an objective and reliable method for quantifying total TFA intake from foods (2–4). TFAs are derived from 2 major sources: industrially produced TFAs (iTFAs) and ruminant TFAs (rTFAs). Partially hydrogenated oils are the primary sources of iTFAs and are found in margarines, shortenings, baked goods, some popular processed and frozen foods (microwave popcorn, frozen pizza, snack foods), and fried foods (5). Dairy products and meats of ruminant animals (eg, cattle, sheep, goats) may contain small quantities of rTFAs. Both iTFAs and rTFAs consist of the same positional trans isomers, but they differ in distribution and amount. The number of calories in iTFAs as a proportion of the number of calories in all TFAs in the daily diet varies across countries; this proportion is estimated to range from 20% to 80% (5). In practice, because it is difficult to distinguish between iTFAs and rTFAs in human biologic tissues, total TFAs are quantified.
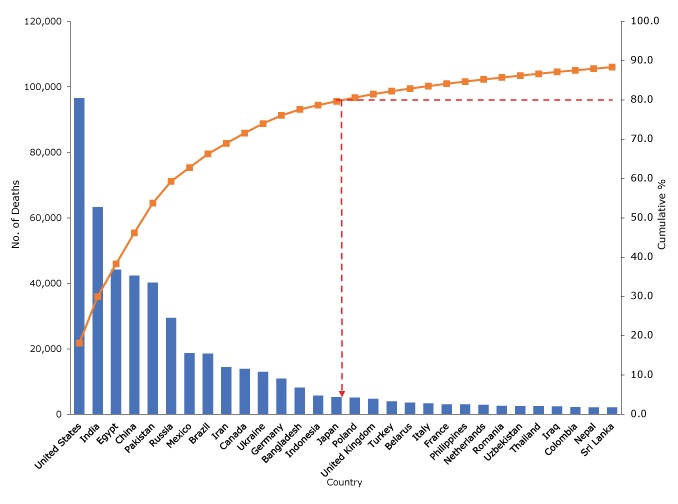
Evidence from experimental studies, dietary trials, and prospective observational studies in which TFAs are measured by biomarkers or dietary records consistently shows that intake of TFAs, from either industrial or natural sources, can increase low-density lipoprotein (“bad”) cholesterol levels, decrease high-density lipoprotein (“good”) cholesterol levels, and is associated with an increased risk of coronary heart disease (CHD) morbidity and mortality and all-cause mortality (6,7). Daily intake of total TFAs varies worldwide; the estimated contribution to total energy intake ranges from 0.2% in Barbados to 6.5% in Egypt. Even small amounts of TFAs can contribute to increased risk of CHD (8). Every year, more than a half-million deaths from CHD worldwide may be attributable to high intake of TFAs (defined by Wang et al [8] as >0.5% of total energy intake); most of these deaths occur in low- and middle-income countries (LMICs) (8). Based on data published by the Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (8) and application of the Pareto principle (9), 15 countries account for approximately 80% of the total number of CHD deaths attributable to high intake of TFAs globally (Figure 1).
Figure 1.
Pareto analysis on the estimated number of deaths from coronary heart disease attributable to high intake of trans-fatty acids (TFAs) (defined by Wang et al [8] as >0.5% of total energy intake), top 30 countries. Pareto charts are used to describe the countries in descending order of the total estimated number of CHD deaths worldwide (9). In this chart, bars indicate the estimated number of CHD deaths attributable to high TFA intake, the curved line indicates cumulative percentages, and the dashed line indicates the 15 countries that account for 80% of total CHD deaths attributable to high TFA intake worldwide according to the Pareto principle (the 80/20 rule). Data source: Wang et al (8).
| Country | Annual No. of CHD Deaths Due to High Intake of trans Fatty Acids | Percentage | Cumulative Percentage | Ranking by No. of CHD Deaths |
|---|---|---|---|---|
| United States | 96,611 | 18.11 | 18.11 | 1 |
| India | 63,243 | 11.86 | 29.97 | 2 |
| Egypt | 44,257 | 8.3 | 38.27 | 3 |
| China | 42,356 | 7.94 | 46.21 | 4 |
| Pakistan | 40,239 | 7.54 | 53.75 | 5 |
| Russia | 29,480 | 5.53 | 59.28 | 6 |
| Mexico | 18,732 | 3.51 | 62.79 | 7 |
| Brazil | 18,576 | 3.48 | 66.27 | 8 |
| Iran | 14,398 | 2.70 | 68.97 | 9 |
| Canada | 13,901 | 2.61 | 71.58 | 10 |
| Ukraine | 12,980 | 2.43 | 74.01 | 11 |
| Germany | 10,916 | 2.05 | 76.06 | 12 |
| Bangladesh | 8,137 | 1.53 | 77.59 | 13 |
| Indonesia | 5,760 | 1.08 | 78.67 | 14 |
| Japan | 5,271 | 0.99 | 79.66 | 15 |
| Poland | 5,133 | 0.96 | 80.62 | 16 |
| United Kingdom | 4,712 | 0.88 | 81.50 | 17 |
| Turkey | 3,973 | 0.74 | 82.24 | 18 |
| Belarus | 3,561 | 0.67 | 82.91 | 19 |
| Italy | 3,381 | 0.63 | 83.54 | 20 |
| France | 3,084 | 0.58 | 84.12 | 21 |
| Philippines | 3,067 | 0.57 | 84.69 | 22 |
| Netherlands | 2,881 | 0.54 | 85.23 | 23 |
| Romania | 2,591 | 0.49 | 85.72 | 24 |
| Uzbekistan | 2,535 | 0.48 | 86.20 | 25 |
| Thailand | 2,517 | 0.47 | 86.67 | 26 |
| Iraq | 2,475 | 0.46 | 87.13 | 27 |
| Colombia | 2,215 | 0.42 | 87.55 | 28 |
| Nepal | 2,178 | 0.41 | 87.96 | 29 |
| Sri Lanka | 2,162 | 0.41 | 88.37 | 30 |
Progress Toward Reduction of TFA Intake
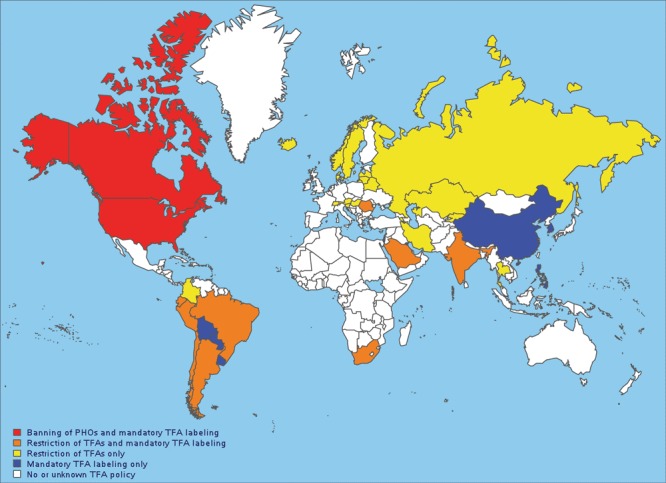
The harmful health effects of TFAs, combined with high levels of iTFA intake, have motivated policy makers in many countries to take action. In the past 15 years, progress has been made toward reducing iTFA intake (10–13). In 2003, Denmark was the first country to enact a law to restrict the iTFA content of all food products and ready-to-eat meals, requiring that no more than 2% of the total fat content could come from iTFAs (10). Canada and the United States, where the daily dietary intakes of TFAs were more than twice the WHO-recommended limit of 1% of energy intake (5), were among the first countries to introduce mandatory labeling of TFAs in packaged foods in 2003; mandatory labeling went into effect in Canada in 2005 and the United States in 2006 (11,12). In the absence of national restrictions, numerous local jurisdictions in the United States, such as New York City, restricted TFAs in food service establishments, including restaurants, caterers, mobile food-vending units, and mobile food commissaries (13). In recent years, both the United States and Canada used existing food additive regulations to issue determinations that partially hydrogenated oils are not generally recognized as safe for any use in human foods, becoming the first countries to effectively ban iTFAs (11,12). To date, some 40 countries, most of which are high-income or upper-middle–income countries, have adopted mandatory restriction of iTFAs, banned the use of partially hydrogenated oils, and/or required mandatory labeling of TFAs on packaged foods (Figure 2).
Figure 2.
Countries with policies or regulations on industrially produced (artificial) TFAs. Data source: World Health Organization (14). Abbreviation: PHO, partially hydrogenated oil; TFA, trans-fatty acid.
| Country | Policy |
|---|---|
| Afghanistan | No or unknown TFA policy |
| Albania | No or unknown TFA policy |
| Algeria | No or unknown TFA policy |
| American Samoa | No or unknown TFA policy |
| Andorra | No or unknown TFA policy |
| Angola | No or unknown TFA policy |
| Anguilla | No or unknown TFA policy |
| Antarctica | No or unknown TFA policy |
| Antigua and Barbuda | No or unknown TFA policy |
| Argentina | Restriction of TFAs AND mandatory TFA labeling |
| Armenia | Restriction of TFA only |
| Aruba | No or unknown TFA policy |
| Australia | No or unknown TFA policy |
| Austria | Restriction of TFA only |
| Azerbaijan | No or unknown TFA policy |
| Bahamas | No or unknown TFA policy |
| Bahrain | Mandatory TFA labeling only |
| Bangladesh | No or unknown TFA policy |
| Barbados | No or unknown TFA policy |
| Belarus | Restriction of TFA only |
| Belgium | No or unknown TFA policy |
| Belize | No or unknown TFA policy |
| Benin | No or unknown TFA policy |
| Bermuda | No or unknown TFA policy |
| Bhutan | No or unknown TFA policy |
| Bolivia | Mandatory TFA labeling only |
| Bonaire, Saint Eustatius, and Saba | No or unknown TFA policy |
| Bosnia and Herzegovina | No or unknown TFA policy |
| Botswana | No or unknown TFA policy |
| Bouvet Island | No or unknown TFA policy |
| Brazil | Restriction of TFAs AND mandatory TFA labeling |
| British Indian Ocean Territory | No or unknown TFA policy |
| Brunei Darussalam | No or unknown TFA policy |
| Bulgaria | No or unknown TFA policy |
| Burkina Faso | No or unknown TFA policy |
| Burundi | No or unknown TFA policy |
| Cambodia | No or unknown TFA policy |
| Cameroon | No or unknown TFA policy |
| Cameroon/Nigeria | No or unknown TFA policy |
| Canada | Banning of PHOs AND mandatory TFA labeling |
| Cape Verde | No or unknown TFA policy |
| Cayman Islands | No or unknown TFA policy |
| Central African Republic | No or unknown TFA policy |
| Chad | No or unknown TFA policy |
| Chile | Restriction of TFAs AND mandatory TFA labeling |
| China | Mandatory TFA labeling only |
| China/India | No or unknown TFA policy |
| China/Taiwan, Province of China | No or unknown TFA policy |
| Christmas Island | No or unknown TFA policy |
| Cocos (Keeling) Islands | No or unknown TFA policy |
| Colombia | Restriction of TFA only |
| Comoros | No or unknown TFA policy |
| Congo | No or unknown TFA policy |
| Congo, Democratic Republic of the | No or unknown TFA policy |
| Cook Islands | No or unknown TFA policy |
| Costa Rica | No or unknown TFA policy |
| Cote D'Ivoire | No or unknown TFA policy |
| Croatia | No or unknown TFA policy |
| Cuba | No or unknown TFA policy |
| Curacao | No or unknown TFA policy |
| Cyprus | No or unknown TFA policy |
| Czech Republic | No or unknown TFA policy |
| Denmark | Restriction of TFA only |
| Djibouti | No or unknown TFA policy |
| Dominica | No or unknown TFA policy |
| Dominican Republic | No or unknown TFA policy |
| Ecuador | Restriction of TFAs AND mandatory TFA labeling |
| Egypt | No or unknown TFA policy |
| El Salvador | No or unknown TFA policy |
| Equatorial Guinea | No or unknown TFA policy |
| Eritrea | No or unknown TFA policy |
| Estonia | No or unknown TFA policy |
| Ethiopia | No or unknown TFA policy |
| Falkland Islands (Malvinas) | No or unknown TFA policy |
| Faroe Islands | No or unknown TFA policy |
| Fiji | No or unknown TFA policy |
| Finland | No or unknown TFA policy |
| France | No or unknown TFA policy |
| French Guiana | No or unknown TFA policy |
| French Polynesia | No or unknown TFA policy |
| French Southern Territories | No or unknown TFA policy |
| Gabon | No or unknown TFA policy |
| Gambia | No or unknown TFA policy |
| Georgia | No or unknown TFA policy |
| Germany | No or unknown TFA policy |
| Ghana | No or unknown TFA policy |
| Gibraltar | No or unknown TFA policy |
| Golan Heights | No or unknown TFA policy |
| Greece | No or unknown TFA policy |
| Greenland | No or unknown TFA policy |
| Grenada | No or unknown TFA policy |
| Guadeloupe | No or unknown TFA policy |
| Guam | No or unknown TFA policy |
| Guatemala | No or unknown TFA policy |
| Guernsey | No or unknown TFA policy |
| Guinea | No or unknown TFA policy |
| Guinea-Bissau | No or unknown TFA policy |
| Guyana | No or unknown TFA policy |
| Haiti | No or unknown TFA policy |
| Heard Island and McDonald Islands | No or unknown TFA policy |
| Holy See (Vatican City State) | No or unknown TFA policy |
| Honduras | No or unknown TFA policy |
| Hungary | Restriction of TFA only |
| Iceland | Restriction of TFA only |
| India | Restriction of TFAs AND mandatory TFA labeling |
| Indonesia | No or unknown TFA policy |
| Iran, Islamic Republic of | Restriction of TFA only |
| Iraq | No or unknown TFA policy |
| Ireland | No or unknown TFA policy |
| Isle of Man | No or unknown TFA policy |
| Israel | Mandatory TFA labeling only |
| Italy | No or unknown TFA policy |
| Jamaica | No or unknown TFA policy |
| Japan | No or unknown TFA policy |
| Jersey | No or unknown TFA policy |
| Jordan | No or unknown TFA policy |
| Kazakhstan | Restriction of TFA only |
| Kenya | No or unknown TFA policy |
| Kiribati | No or unknown TFA policy |
| Korea, Democratic People's Republic of | No or unknown TFA policy |
| Korea, Republic of | Mandatory TFA labeling only |
| Kosovo | No or unknown TFA policy |
| Kuwait | Mandatory TFA labeling only |
| Kyrgyzstan | Restriction of TFA only |
| Lao People's Democratic Republic | No or unknown TFA policy |
| Latvia | Restriction of TFA only |
| Lebanon | No or unknown TFA policy |
| Lesotho | No or unknown TFA policy |
| Liberia | No or unknown TFA policy |
| Libya | No or unknown TFA policy |
| Liechtenstein | No or unknown TFA policy |
| Lithuania | Restriction of TFA only |
| Luxembourg | No or unknown TFA policy |
| Macedonia, the Former Yugoslav Republic of | No or unknown TFA policy |
| Madagascar | No or unknown TFA policy |
| Malawi | No or unknown TFA policy |
| Malaysia | No or unknown TFA policy |
| Maldives | No or unknown TFA policy |
| Mali | No or unknown TFA policy |
| Malta | No or unknown TFA policy |
| Marshall Islands | No or unknown TFA policy |
| Martinique | No or unknown TFA policy |
| Mauritania | No or unknown TFA policy |
| Mauritius | Mandatory TFA labeling only |
| Mayotte | No or unknown TFA policy |
| Mexico | No or unknown TFA policy |
| Micronesia, Federated States of | No or unknown TFA policy |
| Moldova, Republic of | No or unknown TFA policy |
| Monaco | No or unknown TFA policy |
| Mongolia | No or unknown TFA policy |
| Montenegro | No or unknown TFA policy |
| Montserrat | No or unknown TFA policy |
| Morocco | No or unknown TFA policy |
| Mozambique | No or unknown TFA policy |
| Myanmar | No or unknown TFA policy |
| Namibia | No or unknown TFA policy |
| Nauru | No or unknown TFA policy |
| Nepal | No or unknown TFA policy |
| Netherlands | No or unknown TFA policy |
| New Caledonia | No or unknown TFA policy |
| New Zealand | No or unknown TFA policy |
| Nicaragua | No or unknown TFA policy |
| Niger | No or unknown TFA policy |
| Nigeria | No or unknown TFA policy |
| Niue | No or unknown TFA policy |
| Norfolk Island | No or unknown TFA policy |
| Northern Mariana Islands | No or unknown TFA policy |
| Norway | Restriction of TFA only |
| Oman | No or unknown TFA policy |
| Pakistan | No or unknown TFA policy |
| Palau | No or unknown TFA policy |
| Palestinian Territory, Occupied | No or unknown TFA policy |
| Panama | No or unknown TFA policy |
| Papua New Guinea | No or unknown TFA policy |
| Paraguay | Mandatory TFA labeling only |
| Peru | Restriction of TFAs AND mandatory TFA labeling |
| Philippines | Mandatory TFA labeling only |
| Pitcairn | No or unknown TFA policy |
| Poland | No or unknown TFA policy |
| Portugal | No or unknown TFA policy |
| Puerto Rico | No or unknown TFA policy |
| Qatar | No or unknown TFA policy |
| Reunion | No or unknown TFA policy |
| Romania | Restriction of TFAs AND mandatory TFA labeling |
| Russian Federation | Restriction of TFA only |
| Rwanda | No or unknown TFA policy |
| Saint Barthelemy | No or unknown TFA policy |
| Saint Helena | No or unknown TFA policy |
| Saint Kitts and Nevis | No or unknown TFA policy |
| Saint Lucia | No or unknown TFA policy |
| Saint Martin | No or unknown TFA policy |
| Saint Pierre and Miquelon | No or unknown TFA policy |
| Saint Vincent and the Grenadines | No or unknown TFA policy |
| Samoa | No or unknown TFA policy |
| San Marino | No or unknown TFA policy |
| Sao Tome and Principe | No or unknown TFA policy |
| Saudi Arabia | Restriction of TFAs AND mandatory TFA labeling |
| Senegal | No or unknown TFA policy |
| Serbia | No or unknown TFA policy |
| Seychelles | No or unknown TFA policy |
| Sierra Leone | No or unknown TFA policy |
| Singapore | Restriction of TFAs AND mandatory TFA labeling |
| Sint Maarten (Dutch Part) | No or unknown TFA policy |
| Slovakia | No or unknown TFA policy |
| Slovenia | Restriction of TFA only |
| Solomon Islands | No or unknown TFA policy |
| Somalia | No or unknown TFA policy |
| South Africa | Restriction of TFAs AND mandatory TFA labeling |
| South Georgia and the South Sandwich Islands | No or unknown TFA policy |
| Spain | No or unknown TFA policy |
| Sri Lanka | No or unknown TFA policy |
| Sudan | No or unknown TFA policy |
| Suriname | No or unknown TFA policy |
| Suriname/Guyana | No or unknown TFA policy |
| Svalbard and Jan Mayen | No or unknown TFA policy |
| Swaziland | No or unknown TFA policy |
| Sweden | Restriction of TFA only |
| Switzerland | Restriction of TFA only |
| Syrian Arab Republic | No or unknown TFA policy |
| Taiwan, Province of China | No or unknown TFA policy |
| Tajikistan | No or unknown TFA policy |
| Tanzania, United Republic of | No or unknown TFA policy |
| Thailand | Restriction of TFA only |
| Timor-Leste | No or unknown TFA policy |
| Togo | No or unknown TFA policy |
| Tokelau | No or unknown TFA policy |
| Tonga | No or unknown TFA policy |
| Trinidad and Tobago | No or unknown TFA policy |
| Tunisia | No or unknown TFA policy |
| Turkey | No or unknown TFA policy |
| Turkmenistan | No or unknown TFA policy |
| Turks and Caicos Islands | No or unknown TFA policy |
| Tuvalu | No or unknown TFA policy |
| Uganda | No or unknown TFA policy |
| Ukraine | No or unknown TFA policy |
| United Arab Emirates | No or unknown TFA policy |
| United Kingdom | No or unknown TFA policy |
| United States | Banning of PHOs AND mandatory TFA labeling |
| Uruguay | Mandatory TFA labeling only |
| Uzbekistan | No or unknown TFA policy |
| Vanuatu | No or unknown TFA policy |
| Venezuela | No or unknown TFA policy |
| Viet Nam | No or unknown TFA policy |
| Virgin Islands, British | No or unknown TFA policy |
| Virgin Islands, US | No or unknown TFA policy |
| Wallis and Futuna | No or unknown TFA policy |
| Western Sahara | No or unknown TFA policy |
| Yemen | No or unknown TFA policy |
| Zambia | No or unknown TFA policy |
| Zimbabwe | No or unknown TFA policy |
Reductions in TFA intake, iTFAs in foods, and TFA-attributed CHD mortality have been reported in the United States, Denmark, and Argentina since the implementation of TFA policies (13,15–20). In the United States overall, from 1999–2000 to 2009–2010, plasma TFA levels decreased by 54% in adults aged 20 or older (15); both TFA food labeling and local regulations were enacted during this 10-year period. New York City’s 2007 regulation restricting TFAs in food-service establishments was associated with a significant reduction of 2.4 g in mean TFAs in fast-food purchases (13) and a greater decline in adult serum TFA levels among frequent restaurant diners (61.6%) than among people who rarely dined out (51.1%) (16). These declines show how the effect of the regulation in New York City added to the effect of labeling overall in the United States (16). Cardiovascular disease mortality was also affected, declining by 4.5% in New York City (17) and by 22 deaths per 100,000 person-year in Denmark (18). Restaurant restrictions in 11 counties in New York State were associated with an additional 6.2% decline, compared with 25 counties without TFA restrictions, in the hospital admission rate for myocardial infarction and stroke (19). Similarly, near elimination of iTFAs in Argentina was associated with a 1.3% to 6.3% reduction in CHD events in 2004 and 2014 (20).
Elimination of iTFAs from the food supply is politically viable, economically favorable, and technically feasible. Successful experiences in high-income countries such as the United States and Denmark can be transferred to LMICs. Some LMICs, such as Argentina, Brazil, Costa Rica, India, and Mexico, have taken steps to enact policies and/or create surveillance systems for monitoring TFA content in cooking oils and foods (21). However, most LMICs have no policies on iTFAs or have not enforced the policies they do have, despite the growing burden of CHD.
Making the case to restrict TFAs through regulation or legislation requires more than just the scientific case against TFAs. Information on the level of iTFAs in foods or on TFA intake are critical to motivate stakeholders and to evaluate the progress of eliminating partially hydrogenated oils in the food supply. To date, however, little is known about TFA intake in most LMICs (5), let alone the potential reduction in CHD burden and health benefits that could result from the elimination of iTFAs. For example, removal of partially hydrogenated oils in the United States was estimated to save $130 billion over 20 years and prevent 3,000 to 7,000 deaths due to CHD annually (12).
Implications for Public Health Practice
Need for objective and accurate measurement of TFA intake worldwide
In light of gaps in data on TFA intake, there is an urgent need to measure and monitor TFA intake globally. The current methods for estimating TFA intake are difficult to implement in LMICs. The primary method for estimating TFA intake is to collect data through food frequency dietary interviews in national health and nutrition surveys. In these surveys, the quantity of food products consumed is examined, and the TFA content is analyzed by consulting nutrition databases. Limitations of this method include incomplete or nonexistent information on TFA content in various food products and the reliance of dietary questionnaires on participant recall. Although it is possible to test popular foods for their TFA content, this testing is complex because TFA content may vary across brand (or vendor) and by geographic region. Furthermore, if countries are not already sampling and testing foods for other nutrients, new surveys would need to be developed and implemented. A second common method for estimating TFA intake is to survey a small sample of people and test duplicate portions of their meals for TFAs; this method is costly and complicated. A fast and effective approach for assessing the effect of policies is to measure TFA levels in blood. Such data are not currently available in many countries. The Centers for Disease Control and Prevention (CDC) pioneered a new technique for measuring TFAs in blood that was used on a subsample of the National Health and Nutrition Examination Survey in 1999–2000 and 2009–2010, allowing the assessment of change in TFA intake over time (15,22).
Types of biological specimens for measuring TFAs
TFAs in the human body are derived from consumption of foods that contain TFAs; they cannot be synthesized by the human body. Therefore, TFA concentrations in human biologic tissues reflect dietary intake (2). Turnover rates or half-lives of fatty acids vary greatly from one part of the human body to another (2,3). Adipose tissue, with a slow turnover (average half-life of approximately 1 or 2 years), can be considered the best choice to assess long-term intake of TFAs. Red blood cells, with a moderate turnover (average half-life of approximately 1–4 months), can be used to assess medium-term intake. Plasma and serum, with a quick turnover (average half-life of approximately 5–10 days), are best used to assess short-term intake. Although adipose tissue might be ideal for assessing long-term intake, in practice, this approach is rarely used in large-scale epidemiologic studies and surveys because of the difficulty in obtaining samples. Specimens of plasma, serum, and red blood cells are widely used because of their accessibility; use of these specimens assumes relatively stable short-term dietary patterns. The standard laboratory protocol developed by CDC can be used to measure TFAs in plasma, serum, and red blood cells (erythrocytes) (22).
Whole blood has been proposed as an alternative specimen, and it could yield results on TFA intake that are similar to the results obtained by examining plasma (4). Moreover, the use of whole blood enables microsampling procedures (fingertip prick or heel prick) to collect dried blood-spot samples, which require low blood volume and simplified procedures for handling, storing, and processing samples. Indeed, dried blood-spot samples have been used to test many biomarkers (eg, hemoglobin, vitamin A, HIV) in the Demographic and Health Surveys Program in more than 50 countries (23). However, the dried blood-spot method is not widely used for fatty acid profiling because of its potential limitations (24). Oxidative degradation and filter paper contamination during sample storage and processing may compromise the accuracy of dried blood-spot assays for analyzing fatty acid composition. In addition, lack of consensus in analytic procedures or standard laboratory guidelines and difficulties in measuring fatty acid concentrations in the dried blood-spot samples may affect the reliability and comparability of laboratory data over time and across different populations.
Practice of measuring TFAs in LMICs
Measuring TFAs as a part of national surveys in LMICs could be the most effective and direct way to understand TFA intake. Many LMICs collect blood samples (plasma or serum) as part of their national health or nutrition surveys, so it would be relatively simple to include TFA biomarkers as an additional test. The World Health Organization’s STEPwise Approach to Surveillance uses blood samples to test for cholesterol and blood glucose levels; this simple, standardized method for collecting, analyzing, and disseminating data has been implemented in more than 100 countries (25). National and regional laboratories would need to establish and build the technical and scientific capacity for measuring TFAs in blood and in foods. These laboratory measurements are complex and require a high-quality system to ensure accuracy and reliability of data over time and across countries and regions. High-quality systems would need to include common and standardized measurement protocols, training of personnel, and external quality assessment conducted within a network of laboratories. The data obtained from these laboratories would lead to the establishment of baseline levels of TFA intake, provide scientific evidence of the effect of public health policy making, and help with directing further actions to eliminate iTFAs in the food supply. With surveys and laboratories adhering to the same standards and using the same high-quality system, data can be compared with global references and across countries. Because laboratory measurements of TFAs also enable the measurement of other fatty acids, building laboratory capacity for measuring TFAs and other biomarkers for cardiovascular disease risk factors in blood enables countries and regions to address public health issues beyond iTFA reduction. Improving cardiovascular health through reduction of iTFA intake is feasible through shared experience, technical assistance, and advanced laboratory technology. It can be achieved by collaborating and partnering with key stakeholders in LMICs, and with public and private partners internationally.
Acknowledgments
This analysis was conducted in conjunction with Resolve to Save Lives, an initiative of Vital Strategies. Resolve to Save Lives is funded by Bloomberg Philanthropies, the Bill & Melinda Gates Foundation, and Gates Philanthropy Partners, which is funded with support from the Chan Zuckerberg Foundation. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. No copyrighted material, surveys, instruments, or tools were used in this article.
Footnotes
The opinions expressed by authors contributing to this journal do not necessarily reflect the opinions of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions.
Suggested citation for this article: Li C, Cobb LK, Vesper HW, Asma S. Global Surveillance of trans-Fatty Acids. Prev Chronic Dis 2019;16:190121. DOI: https://doi.org/10.5888/pcd16.190121.
References
- 1. World Health Organization. REPLACE: trans fat-free by 2023. 2019. https://www.who.int/nutrition/topics/replace-transfat. Accessed August 20, 2019.
- 2. Arab L. Biomarkers of fat and fatty acid intake. J Nutr 2003;133(Suppl 3):925S–32S. 10.1093/jn/133.3.925S [DOI] [PubMed] [Google Scholar]
- 3. Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 1997;38(10):2012–22. [PubMed] [Google Scholar]
- 4. Baylin A, Kim MK, Donovan-Palmer A, Siles X, Dougherty L, Tocco P, et al. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol 2005;162(4):373–81. 10.1093/aje/kwi213 [DOI] [PubMed] [Google Scholar]
- 5. Wanders AJ, Zock PL, Brouwer IA. Trans fat intake and its dietary sources in general populations worldwide: a systematic review. Nutrients 2017;9(8):E840. 10.3390/nu9080840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354(15):1601–13. 10.1056/NEJMra054035 [DOI] [PubMed] [Google Scholar]
- 7. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all-cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 2015;351:h3978. 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Q, Afshin A, Yakoob MY, Singh GM, Rehm CD, Khatibzadeh S, et al. Impact of nonoptimal intakes of saturated, polyunsaturated, and trans fat on global burdens of coronary heart disease. J Am Heart Assoc 2016;5(1):e002891. 10.1161/JAHA.115.002891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harvey HB, Sotardi ST. The Pareto principle. J Am Coll Radiol 2018;15(6):931. 10.1016/j.jacr.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 10. Stender S, Dyerberg J, Bysted A, Leth T, Astrup A. A trans world journey. Atheroscler Suppl 2006;7(2):47–52. 10.1016/j.atherosclerosissup.2006.04.011 [DOI] [PubMed] [Google Scholar]
- 11. Government of Canada. Regulations amending certain regulations made under the food and drugs act (nutrition symbols, other labelling provisions, partially hydrogenated oils and vitamin d). Canada Gazette 2018;152(6):279–369.
- 12. Food and Drug Administration. Final determination regarding partially hydrogenated oils. Fed Regist 2015;80(116):34650–70. https://www.govinfo.gov/content/pkg/FR-2015-06-17/pdf/2015-14883.pdf. [PubMed] [Google Scholar]
- 13. Angell SY, Cobb LK, Curtis CJ, Konty KJ, Silver LD. Change in trans fatty acid content of fast-food purchases associated with New York City’s restaurant regulation: a pre-post study. Ann Intern Med 2012;157(2):81–6. 10.7326/0003-4819-157-2-201207170-00004 [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. WHO Global database on the Implementation of Nutrition Action (GINA). 2019. https://extranet.who.int/nutrition/gina. Accessed August 20, 2019.
- 15. Vesper HW, Caudill SP, Kuiper HC, Yang Q, Ahluwalia N, Lacher DA, et al. Plasma trans-fatty acid concentrations in fasting adults declined from NHANES 1999–2000 to 2009–2010. Am J Clin Nutr 2017;105(5):1063–9. 10.3945/ajcn.116.141622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wright M, McKelvey W, Curtis CJ, Thorpe LE, Vesper HW, Kuiper HC, et al. Impact of a municipal policy restricting trans fatty acid use in New York City restaurants on serum trans fatty acid levels in adults. Am J Public Health 2019;109(4):634–6. 10.2105/AJPH.2018.304930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Restrepo BJ, Rieger M. Trans fat and cardiovascular disease mortality: evidence from bans in restaurants in New York. J Health Econ 2016;45:176–96. 10.1016/j.jhealeco.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 18. Restrepo BJ, Rieger M. Denmark’s policy on artificial trans fat and cardiovascular disease. Am J Prev Med 2016;50(1):69–76. 10.1016/j.amepre.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 19. Brandt EJ, Myerson R, Perraillon MC, Polonsky TS. Hospital admissions for myocardial infarction and stroke before and after the trans-fatty acid restrictions in New York. JAMA Cardiol 2017;2(6):627–34. 10.1001/jamacardio.2017.0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubinstein A, Elorriaga N, Garay OU, Poggio R, Caporale J, Matta MG, et al. Eliminating artificial trans fatty acids in Argentina: estimated effects on the burden of coronary heart disease and costs. Bull World Health Organ 2015;93(9):614–22. 10.2471/BLT.14.150516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monge-Rojas R, Colón-Ramos U, Jacoby E, Alfaro T, Tavares do Carmo MDG, Villalpando S, et al. Progress towards elimination of trans-fatty acids in foods commonly consumed in four Latin American cities. Public Health Nutr 2017;20(13):2440–9. 10.1017/S1368980017001227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuiper HC, Wei N, McGunigale SL, Vesper HW. Quantitation of trans-fatty acids in human blood via isotope dilution-gas chromatography-negative chemical ionization-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2018;1076:35–43. 10.1016/j.jchromb.2017.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The DHS Program at ICF. Demographic and Health Surveys. Using biomarkers to collect health data. https://www.dhsprogram.com/What-We-Do/Biomarkers.cfm. Accessed August 20, 2019.
- 24. Gunash J, Aristizabal-Henao JJ, Stark KD. Quantitating fatty acids in dried blood spots on a common collection card versus a novel wicking sampling device. Prostaglandins Leukot Essent Fatty Acids 2019;145:1–6. 10.1016/j.plefa.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization. Noncommunicable diseases and their risk factors: STEPS country reports. 2019. https://www.who.int/ncds/surveillance/steps/reports/en/. Accessed August 20, 2019.


