Abstract
While pulmonary hypertension (PH) is a potentially life threatening complication of many inflammatory conditions, an association between Aicardi Goutières syndrome (AGS), a rare genetic cause of interferon (IFN) overproduction, and the development of PH has not been characterized to date.
We analyzed the cardiac function of individuals with AGS enrolled in the Myelin Disorders Bioregistry Project using retrospective chart review (n=61). Additional prospective echocardiograms were obtained when possible (n=22). An IFN signature score, a marker of systemic inflammation, was calculated through the measurement of mRNA transcripts of type I IFN-inducible genes (interferon signaling genes or ISG). Pathologic analysis was performed as available from autopsy samples. Within our cohort, four individuals were identified to be affected by PH: three with pathogenic gain-of-function mutations in the IFIH1 gene and one with heterozygous TREX1 mutations. All studied individuals with AGS were noted to have elevated IFN signature scores (Mann-Whitney p<0.001), with the highest levels in individuals with IFIH1 mutations (Mann-Whitney p<0.0001).
We present clinical and histologic evidence of PH in a series of four individuals with AGS, a rare interferonopathy. Importantly, IFIH1 and TREX1 may represent a novel cause of PH. Furthermore, these findings underscore the importance of screening all individuals with AGS for PH.
Keywords: Pulmonary Hypertension, Interferons, Aicardi Goutières Syndrome
Introduction
Pulmonary hypertension (PH) is a rare but devastating disorder characterized by severe vasculopathy and elevated pulmonary artery pressures leading to right-sided heart failure. Recent progress has been made towards understanding the pathophysiology of progressive PH, which encompasses both genetic and inflammatory influences (1–7). In particular, type I interferons (IFN-alpha and IFN-beta) have been established as risk factors for the development of PH (2, 8–11).
A known cause of systemic vasculopathy, Aicardi Goutières Syndrome (AGS) (MIM 615846) is a rare genetic disorder characterized by aberrant type I IFN production and systemic, chronic inflammation (Table 2). Genetic abnormalities in intracellular nucleic acid sensing machinery (ADAR1, IFIH1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and TREX1) trigger an endogenous IFN response resulting in extensive end organ damage injury to the brain, skin, bone marrow, and visceral organs (12–16).
Table 2:
Comparison of genetic interferonopathies with cardiovascular complications
| Disorder | Aicardi Goutières Syndrome (AGS) | Singleton-Merten syndrome (SMS) (40, 51, 60) |
STING-associated vasculopathy with onset in infancy (SAVI) (53, 60, 61) |
Deficiency of adenosine deaminase 2 (DADA2) (61–64) |
|---|---|---|---|---|
| Gene(s) |
ADAR1 IFIH1 SAMHD1 TREX1 RNASEH2A RNASEH2B RNASEH2C |
IFIH1 DDX58 |
TMEM173 | ADA2 |
| Protein(s) | Multiple | Interferon-induced helicase C domain-containing protein 1 Retinoic acid-inducible gene I (RIG-I)-like receptor |
Stimulator of interferon genes (STING) | Deficiency of adenosine deaminase 2 (DADA2) |
| Relationship to IFN activation | Varies by gene, but includes NA metabolism and sensing machinery | Intracellular nucleic acid sensing machinery | DNA sensing machinery (61) | Purine metabolism (64) |
| CV and pulmonary involvement | Peripheral vasculopathy with secondary tissue damage Cardiomyopathy (particularly TREX1) (13) Pericardial effusion (13) HTN (13) PH (this report) Cerebral vasculopathy and infarction (particularly SAMHD1) (39) |
Peripheral vasculopathy with secondary tissue damage (40) Concentric LVH (52) Left atrial and aortic root dilation (52) Aortic valve insufficiency and calcification (52) HTN (52) Pericarditis (65) |
Peripheral vasculopathy with secondary tissue damage (53) Interstitial lung disease (53) Lung fibrosis (53) |
Medium vessel vasculopathy with secondary cerebral infarcts HTN |
| Extra CV/pulm inflammation | Aseptic fevers Cytopenias Glaucoma HSM PN Severe GI discomfort and poor feeding Skin involvement (vasculitic rashes, psoriasis, eczema) Autoantibody production, including autoimmune thyroiditis (66, 67) |
Dental anomalies Glaucoma Psoriasis Skeletal anomalies |
Aseptic fevers Extremity gangrene Skin involvement (vasculitis rashes, ulcerations, vasculitis) |
Aseptic fevers Cytopenias HSM PN Recurrent infections Severe GI discomfort Skin involvement (erythemia nodosum, ulcerations) |
CV: cardiovascular
GI: gastrointestinal
HSM: hepatosplenomegaly
HTN: hypertension
IFN: interferon
LVH: left ventricular hypertrophy
NA: nucleic acid
PH: pulmonary hypertension
PN: peripheral neuropathy
Pulm: pulmonary
Given the potential risk for PH in this disorder of innate immunity, and after PH was incidentally identified in two individuals affected by AGS, we sought to characterize the impact of genetic IFN overexpression on the cardiovascular system within our AGS cohort of the Myelin Disorders Bioregistry Project (MDBP). We compared measures of cardiovascular physiology to systemic IFN scores. IFN scores are based on mRNA expression of IFN signaling genes (ISG) and represent a surrogate marker for autoinflammation in a variety of disorders (17–19). We identified PH in four individuals with molecularly confirmed AGS, underscoring the importance of IFNs in the pathophysiology of PH. As such, PH represents a potentially fatal complication of AGS that should be closely monitored.
METHODS:
Myelin Disorders Bioregistry Project (MDBP):
Design:
Individuals affected by AGS contacting the Myelin Disorders Bioregistry Project (MDBP) were consented and enrolled in the MDBP IRB-approved study protocol (IRB# 14–011236). All individuals with genetically confirmed AGS (ADAR1, IFIH1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and TREX1) and available medical histories were enrolled in our study (n=102). All affected individuals for whom blood samples were available were included in ISG assessments. For the cohort undergoing detailed evaluation for PH, we excluded individuals for whom echocardiography or pulmonary histopathologic data were unavailable (remaining cohort n=61).
Medical Record Data Collection:
All individuals in the cohort underwent a retrospective chart analysis for evidence of cardiovascular disease. As per WHO guidelines, PH was diagnosed by cardiac catheterization was defined as persistently elevated mean pulmonary arterial pressure of ≥25 mmHg and a pulmonary capillary wedge pressure of ≤15 mmHg for precapillary PH (20). In the setting of contraindication to cardiac catheterization (e.g. critical illness), combined echocardiographic and clinical evidence of PH was utilized (21). Echocardiographic evidence of PH in the patient cohort included one or more of the following findings-elevated right ventricular pressure estimate by tricuspid valve regurgitant jet or flattened intraventricular septum during systole with or without right ventricular hypertrophy. Clinical evidence of PH in the patient cohort could consist of examination findings of a single S2, right ventricular heave, hepatomegaly, lower extremity edema, tricuspid valve regurgitant murmur, pulmonary valve insufficiency murmur, and hypoxemia. Official reports and digitized echocardiographic studies were reanalyzed when available by independent pediatric cardiologists. When clinically feasible, prospective echocardiograms were performed (n=22).
Histopathology:
Detailed postmortem examination was conducted on individual 1 with full parental consent. Tissue samples were processed as per standard protocol. Paraffin-embedded, formalin-fixed sections were cut at 5–7 micron thickness and stained with hematoxylin and eosin (HE), periodic acid–Schiff (PAS), and other immunochemical and immunohistochemical staining techniques.
Interferon signature scores:
ISG scores were calculated for all AGS affected individuals in the MDBP registry with available blood samples (180 AGS samples and 104 control samples) as per established methodology (22–25). In brief, copy number of mRNA transcripts of the six type I IFN-inducible genes (IFI27, IFI44L, IFIT1, ISG15, RSAD2, and SIGLEC1) (22) and four housekeeping genes (ALAS1, HPRT1, TBP, and TUBB) are quantified using Nanostring nCounter™ Digital Analyzer. The raw copy number of mRNA transcripts of each type I IFN-inducible gene is standardized (stdGene) using the geometric mean of the four housekeeping genes for each individual. The six-gene IFN signature in each individual is calculated using the median of the Z scores. The IFN signature is considered positive (IFN high) if the value is ≥1.96 (>98centile) (one tail analysis). ISG results were analyzed and compared between affected individuals and controls and also analyzed between genotypes (Prism Graph Pad software) using non-parametric tests.
RESULTS:
MDBP Cohort
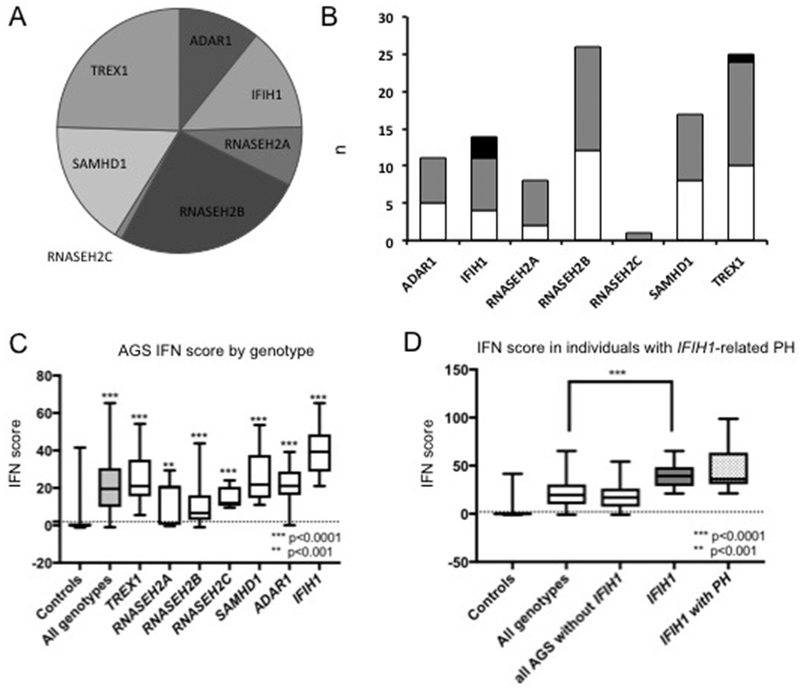
Of the 102 individuals with a clinical and molecular diagnosis of AGS enrolled under the MDBP protocol (Figure 1A), echocardiographic data was available for 61 individuals (60%), with prospective data obtained for 22 individuals. Overall, four individuals had evidence of PH (Figure 1B, Table 1). Of those, three individuals had variants in the IFIH1 gene (n=3/7 total individuals with this genotype) and one in the TREX1 gene (n=1/14 total individuals with this genotype) (Figure 1B). Their clinical histories, relevant to PH, are described below.
Figure 1: Genetics and IFN scores in individuals with AGS.
(A). The distribution of molecular results is shown. (B). Cardiac evaluation within the AGS cohort is shown by genotype (white=ECHO unavailable or not done; grey=no PH detected; black=PH present). (C). IFN signaling gene (ISG) scores were calculated and an IFN signature was derived for all AGS affected individuals in the MDBP registry for whom blood samples were available (180 AGS samples and 104 control samples). Across all genotypes of AGS, ISG scores were significantly increased compared to control samples (by Mann-Whitney all p<0.0001 except for RNASEH2A, p=0.001). (D) Individuals with IFIH1 variants had higher ISGs compared to other AGS genotypes (Mann-Whitney all p<0.0001).
Table 1:
Clinical characteristics of 4 individuals with AGS and PH
| Individual | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| AGS | + | + | + | + | |
| Gene | IFIH1 | IFIH1 | IFIH1 | TREX1 | |
| Variant | c.1009A>G; (p.Arg337Gly) (Het., de novo) | c.2159G>A (p.Arg720Gln) (Het., de novo) | c.2936T>G (p.Leu979Trp) (Het.) | c.506G>A (p.Arg169His) (Het.) | c.581delC (p.Arg194fs) (Het.) |
| gnomAD Variant Allele Frequency | 0.0000 | 0.0000 | 0.0000 | 0.000291 | 0.0000 |
| PolyPhen Score | Probably Damaging (1.0) | Probably Damaging (0.992) | Probably Damaging (1.0) | Probably Damaging (1.0) | N/A |
| SIFT Score | Damaging | Damaging | Damaging | Damaging | N/A |
| CADD Score | 25.3 | 27.4 | 26.6 | 33 | 39 |
| ACMG Classification | Pathogenic | Likely pathogenic | Likely pathogenic | Likely pathogenic | Pathogenic |
| Age of disease presentation | 10 months | Birth | Birth | 2 months | |
| Neonatal complications | − | + | + | + | |
| Presence of PH | + | + | + | + | |
| Age of PH presentation | 16 years | 7 years | 1 month | 2 months | |
| Mechanism of diagnosis of PH | Autopsy | ECHO, cardiac catheterization | ECHO | ECHO | |
| Outcome | Death | Medical management | Death | Death | |
| Signs and symptoms of systemic inflammation | + | + | + | + | |
| CNS perivascular calcifications | + | − | + | + | |
| GI (e.g., hepatitis, poor weight gain, feeding intolerance) | + | + | + | + | |
| Dermatologic (e.g., vasculitic rashes, psoriasis, eczema) | + | + | + | − | |
| Hematologic (e.g., thrombocytopenia, anemia) | − | + | + | UK | |
| Ophthalmologic | UK | − | + | UK | |
| Hypercholesterolemia | + | − | UK | UK | |
AGS: Aicardi Goutières Syndrome; Het.: heterozygous; gnomAD: Genome Aggregation Database; CADD – Combined Annotation Dependent Deletion; ACMG – American College of Medical Genetic; N/A – Not Available; CNS: central nervous system; c/w: consistent with; GI: gastrointestinal; PH: pulmonary hypertension; UK: unknown
Several additional individuals were noted to have non-PH cardiovascular complications as determined by echocardiogram. This included cardiac hypertrophy in three individuals with TREX1 pathogenic variants (n=3/14), as has been previously characterized (26). Aortic dilation was noted in 4 individuals with AGS secondary to changes in ADAR1 (n=1/6), SAMHD1 (n=2/9), and TREX1 (n=1/14).
PH Clinical Case Histories
Individual 1 was a young man with AGS secondary to a pathogenic variant in IFIH1, who died at the age of 16 years from cardiopulmonary arrest (Table 1). In the months prior to his death, his clinical condition declined, with worsening signs of systemic inflammation. One week prior to his death, he was noted to have mild bilateral lower extremity edema with episodes of vomiting. His prior cardiac evaluation was limited to an electrocardiogram (ECG) at the age of 15 which was concerning for possible right ventricular hypertrophy and prolonged QTc. Following his death and postmortem findings of PH (Figure 2), all historical cases in our MDBP registry were reviewed for the presence of PH, and individuals were prospectively screened for PH by echocardiography and clinical findings.
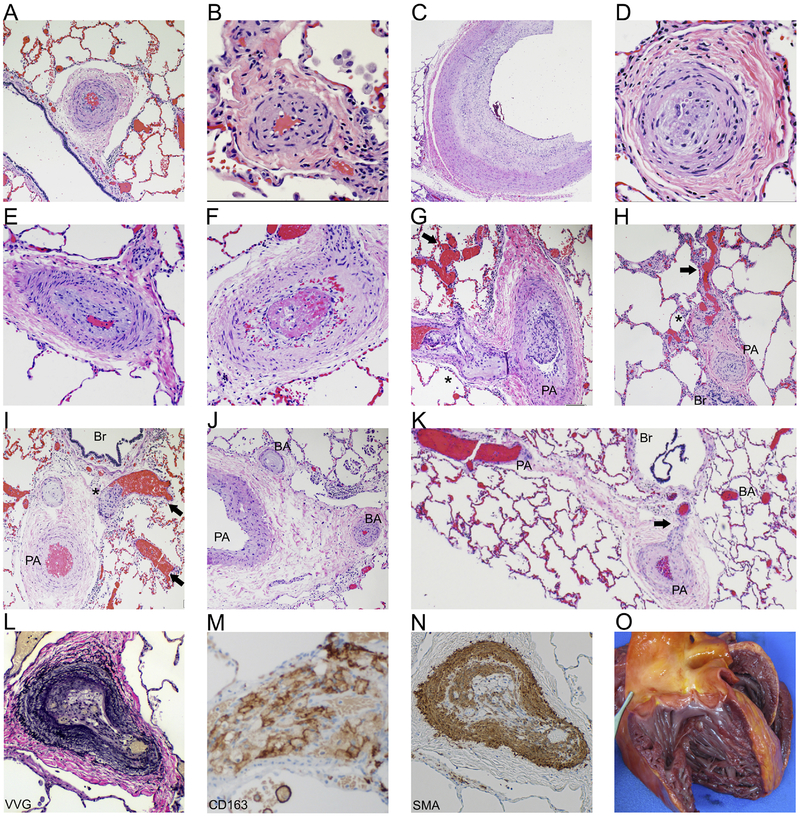
Figure 2: Pathology features consistent with pulmonary hypertension.
H+E stained sections of both small and large arteries show marked muscular hypertrophy (A, B), marked cellular intimal proliferation (C) leading to complete luminal occlusion (D). Also noted was concentric intimal fibrosis (E) and intraluminal thrombus formation (F). H+E sections showed histologic features consistent with severe pulmonary hypertension including plexiform (*) and dilation (arrow) lesions associated with markedly remodeled pulmonary arteries (PA) (G, H, I). Open intrapulmonary bronchopulmonary anastomoses (K) that bridge the pulmonary arteries and bronchial arteries (BA), along with marked BA remodeling and occlusions (J) are present (Br: bronchiole). Microscopic examination of the lungs included elastin [Verhoeff-van Gieson (VVG), L] stained sections showing marked pulmonary artery remodeling. Immunohistochemistry on lung sections revealed fibrointimal proliferation with infiltration of macrophages (CD163+, M) and tunica medical thickening [smooth muscle actin (SMA)+, N]. Gross examination of the heart showing dilatation of the right ventricle (O).
Individual 2 is an 8-year-old boy with profound neurologic delay who was diagnosed with AGS secondary to a variant in IFIH1 (Table 1). He demonstrated the classic neonatal complications of AGS, including hepatosplenomegaly and severe thrombocytopenia (27). Signs of pulmonary hypertension on his echocardiogram were first noted prior to his fourth birthday in the setting of acute illness. This was eventually confirmed by cardiac catheterization at the age of 7 years. At 8 years of age, he continues to show echocardiographic evidence of PH, but is clinically stable on ambrisentan, a selective antagonist of the endothelin type A receptor.
Individual 3 was a 4-month-old boy noted to have respiratory distress shortly after birth requiring intubation, identified to have a pathogenic variant in IFIH1 (Table 1). He was diagnosed with PH at 1 month of age. Serial echocardiograms demonstrated significant PH with right ventricular hypertrophy and elevated right ventricular pressure estimate based on tricuspid regurgitant jet. Despite some transient respiratory improvement with the treatment of his PH with sildenafil, he ultimately died at 4 months of age from respiratory complications. An autopsy was not obtained.
Individual 4 presented at 2 months of age with irritability and poor growth, found to have biallelic variants in TREX1 (Table 1). An echocardiogram revealed borderline left ventricular hypertrophy and mild-to-moderately elevated right ventricular pressures with systolic septal flattening. Based on his cardiac testing and clinical history, he was diagnosed with PH, and he died at the age of 12 weeks from worsening respiratory distress. His general autopsy evaluation, which is unavailable for further review, was unremarkable by report.
Genetics
Individuals 1–3 were found to have heterozygous variants in IFIH1 (Table 1). The variants found in individuals 1 and 2 arose de novo, while parental testing was unavailable for individual 3. The p.Arg337Gly change found in individual 1 is located in a conserved residue in the core helicase-1 domain, while the p.Arg720Gln change in individual 2 is found within the helicase-2 domain (28). The p.Leu979Trp change in individual 3 results in a missense variant within helicase C domain 1. All three variants have never previously been reported in population allele frequency databases and are listed in the ClinVar database as pathogenic or likely pathogenic in all cases. The variants are all predicted damaging by a suite of in silico prediction tools. Genetic testing of Individual 4 revealed two variants in TREX1 (p.Arg169His and p.Ala194fs), classified as likely pathogenic and pathogenic respectively, per the American College of Medical Genetics variant classification criteria (29, 30). Parental testing was unavailable for individual 4.
Pathology
Following the unexpected death of individual 1, postmortem examination was conducted. The gross pathology findings in the brain were consistent with the diagnosis of AGS, including calcifications and global atrophy most prominent in the basal ganglia (31). At autopsy, the lungs were grossly unremarkable and within normal weight parameters for body length. Microscopic examination demonstrated a wide range of findings, including features of pulmonary hypertensive arteriopathy affecting pulmonary arteries (PA) of all size (Figure 2). Virtually all PAs demonstrated muscular hypertrophy (Figure 2A–I). Most of the pulmonary arterial vasculature demonstrated neointimal formation and established concentric fibrosis (Figure 2A–K) (32). In some vessels, the extensive fibrosis resulted in luminal occlusion (Figure 2D). Additionally, we noted PA occluded by well-formed thrombi (Figure 2 H–I). The diffuse microthrombi found within his pulmonary vasculature are a known feature of PH and may represent a consequence of the low cardiac output state of the right ventricle and insufficient pulmonary blood flow (33). Highly complex architectural alterations consistent with plexiform and dilation lesions were seen associated with markedly remodeled pulmonary arteries (Figure 2J–K) (33–35). Arterial remodeling was demonstrated by prominent elastin fibers as shown by Verhoeff-van Gieson (VVG) histochemical staining (Figure 2L) (36). Immunohistochemical staining of lung sections revealed fibrointimal proliferation with infiltration of macrophages (CD163+) (Figure 2M) and tunica medical thickening as demonstrated by smooth muscle actin (SMA) staining (Figure 2N), both previously described in PH (33).
Examination of the heart revealed right-sided cardiac dilation, consistent with his prior ECG (Figure 2O) (33). Additional findings included signs of chronic congestive hepatopathy (nutmeg liver and microscopic evidence of significant fibrosis) and systemic vascular changes (lipid laden macrophage infiltration) suggestive of a local vasculopathy (data not shown).
Interferon Scores
IFN signaling gene (ISG) scores based on mRNA expression of ISGs have been used to assign a severity score of autoinflammation in a variety of disorders, including AGS (22) and systemic lupus erythematous (17, 18). We measured ISGs in individuals affected by AGS as per established protocol (22–24). As previously described, all individuals with AGS had elevated IFN levels compared to control samples (Mann-Whitney; all genotypes <0.0001, except for RNASEH2A, p=0.001) (Figure 1C). Of note, not all individuals had significantly increased values. Individuals with IFIH1 variants had higher ISGs compared to other AGS genotypes (Mann-Whitney all p<0.0001). ISG data was available for the three individuals with IFIH1-related PH (individuals 1–3) and did not statistically differ from the total subcohort of IFIH1-affected individuals (Figure 1D). Blood ISGs were not available for individual 4 (TREX1).
Discussion
Due to the widespread, sustained production of type I IFN, individuals with AGS demonstrate extensive inflammatory injury, including systemic vasculopathy (26, 27, 37–41). This report is the first to characterize the association between AGS and PH, a potentially fatal and under-diagnosed complication, in four affected individuals. In these individuals, the PH was diagnosed at variable ages during childhood, suggesting that PH may occur at any stage of AGS. Three individuals in our cohort with molecular confirmation of AGS were found to have IFIH1 pathogenic variants, representing 30% of the total IFIH1 cohort with available cardiac physiologic data (Figure 1B). The fourth identified individual had TREX1-associated AGS, 7% of the TREX1 cohort (Figure 1B).
Regarding the postmortem analysis of PH (Individual 1, Figure 2), undiagnosed, longstanding PH may have contributed to his unexpected death. The plexiform and dilatation lesions seen in his lung vasculature are considered to be a morphologic hallmark of severe pulmonary hypertension, consistent with a Grade 5 category of the histologic Heath-Edwards pulmonary hypertension classification (42). The presence of intimal concentric fibrosis, plexiform and dilatation lesions suggest the pulmonary disease was irreversible and longstanding (43–45). Additionally, the presence of open intrapulmonary bronchopulmonary anastomoses (IBA) shunts in this patient is additional evidence of severe pulmonary hypertension (46, 47). The chronicity of disease in this case is further documented by dilation and hypertrophy of the heart including the right side, as well as chronic congestive hepatopathy. While the connection between IFIH1 and PH was substantiated across multiple individuals and modalities of measure, the confirmation of PH in the individual with TREX1-related AGS was limited by a lack of detailed diagnostic and pathologic data. The significance of the association between TREX1 and PH particularly warrants further validation.
While AGS is known to affect the vascular system, the full impact of this innate inflammatory condition on systemic vasculopathy is currently under-characterized. The skin ulcerations and chilblains found in individuals affected by AGS are hypothesized to be secondary to an underlying vasculopathy (37, 40, 41). Pathologic examination of AGS-associated brain injury often demonstrates a calcifying microangiopathy (48) and occasionally large vessel vasculopathy (38). In addition to classic AGS, TREX1-associated disease can also cause retinal vasculopathy with cerebral leukodystrophy and is a genetic risk factor for the development of lupus (49, 50). Pathogenic variants of IFIH1 are also associated with Singleton–Merten syndrome (SMS) (MIM 182250) (51), a disorder with significant pulmonary and cardiovascular complications, including aortic root dilation and peripheral vasculopathy (Table 2) (52).
This study furthers our understanding of the impact of systemic IFN overexpression on the cardiovascular system. In addition to the novel identification of pulmonary hypertension in a subset of individuals with AGS with IFIH1 and TREX1 variants, we also confirm the presence of hypertrophic cardiomyopathy in patients with AGS (26) and describe aortic dilation in a broader number of genotypes. While the connection between AGS and PH has not been previously described, other monogenic causes of IFN overproduction have been linked with inflammatory pulmonary and cardiac issues (Table 2). In particular, STING-associated vasculopathy with onset in infancy (SAVI) (gain of function variants in the DNA-sensor stimulator of interferon genes (STING) encoded by TMEM173), results in inflammatory interstitial lung disease (53). Other genetic disorders with chronic activation of IFN pathways, including Trisomy 21, can similarly develop PH (54–56). Exogenous type I IFN can facilitate vascular injury and PH specifically (9, 10), including the synthetic type I IFNs employed in chronic inflammatory disorders such as hepatitis and multiple sclerosis (8, 11, 57, 58).
As changes in the AGS-related gene TREX1 are a risk factor for the development of SLE, and the two disorders share several pathophysiologic features, we hypothesize that the mechanism of injury may be similar as well (49, 50). In SLE, inflammatory neutrophils result in IFN-dependent vascular injury (59). Furthermore, elevation of IFNs and downstream markers of interferonopathies have been documented in individuals with systemic sclerosis-associated PH, and mouse models of PH implicate a role for interferonopathies in the development of PH (3).
As the breadth of vascular injury associated with excessive IFN production is fully appreciated, we can better characterize the multisystem involvement found in individuals with AGS and other genetic interferonopathies. In particular, we identified that AGS may represent a novel cause of PH, a potentially fatal complication of IFN over-activation. As such, we would recommend screening all individuals with known AGS for PH. Given the extent of neurologic involvement, there are unique challenges to the recognition of PH in the AGS population. Accordingly, at the time of diagnosis, individuals should be referred to a pediatric cardiologist for evaluation by echocardiogram and ECG. If there is concern for right ventricular dysfunction, a brain-type natriuretic peptide (BNP) or NT-pro-BNP should be obtained (21). The required frequency of longitudinal monitoring has yet to be established as the rate of disease progression is unknown.
With any clinical concerns, individuals should be urgently evaluated as PH is a potentially fatal complication. Signs and symptoms of PH may include cyanosis or hypoxemia, increasing fatigue, lower extremity edema, frequent dizziness or near-syncope and syncope, and respiratory symptoms of shortness of breath, dyspnea on exertion, and increased work of breathing. Furthermore, as therapeutic trials to treat interferonopathies such as AGS commence, inclusion of patients with a co-diagnosis of PH may be warranted, and the effect of drug treatment on progression of PH could be a clinically important outcome measure. This study describes a novel connection between AGS and PH, emphasizing the need for further study in this population as well as the characterization of IFIH1 and TREX1 in idiopathic PH of childhood.
Supplementary Material
Acknowledgement
We thank the families and patient advocacy groups involved in MDBP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
LA, DBF, AG, AT, NU, ZC, CG, GH, UK, SK, DS, OS, BH: No conflicts to disclose
AV: receives support from Gilead Sciences Inc, Eli Lily and Company, Shire, and Ilumina Inc.
AC: consultant for Bracket Global, Inc and Ultragenyx Pharma, Inc.
References
- 1.Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circulation research. 2014;115(1):189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George PM, Oliver E, Dorfmuller P, Dubois OD, Reed DM, Kirkby NS, et al. Evidence for the involvement of type I interferon in pulmonary arterial hypertension. Circulation research. 2014;114(4):677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma L, Chung WK. The role of genetics in pulmonary arterial hypertension. The Journal of pathology. 2017;241(2):273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huertas A, Perros F, Tu L, Cohen-Kaminsky S, Montani D, Dorfmuller P, et al. Immune dysregulation and endothelial dysfunction in pulmonary arterial hypertension: a complex interplay. Circulation. 2014;129(12):1332–40. [DOI] [PubMed] [Google Scholar]
- 5.Bazan IS, Mensah KA, Rudkovskaia AA, Adonteng-Boateng PK, Herzog EL, Buckley L, et al. Pulmonary arterial hypertension in the setting of scleroderma is different than in the setting of lupus: A review. Respiratory medicine. 2018;134:42–6. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez-Sanchez J, Harlow L, Church C, Gaine S, Knightbridge E, Bunclark K, et al. Clinical trial protocol for TRANSFORM-UK: A therapeutic open-label study of tocilizumab in the treatment of pulmonary arterial hypertension. Pulmonary circulation. 2018;8(1):2045893217735820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato M, Atsumi T. Pulmonary arterial hypertension associated with connective tissue diseases: A review focusing on distinctive clinical aspects. European journal of clinical investigation. 2017. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons E, Promislow S, Davies RA, Chandy G, Stewart DJ, Vladamir CD, et al. Reversible pulmonary arterial hypertension associated with interferon-beta treatment for multiple sclerosis. Canadian respiratory journal. 2015;22(5):263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savale L, Chaumais MC, O’Connell C, Humbert M, Sitbon O. Interferon-induced pulmonary hypertension: an update. Current opinion in pulmonary medicine. 2016;22(5):415–20. [DOI] [PubMed] [Google Scholar]
- 10.Savale L, Chaumais MC, Sitbon O, Humbert M. Pulmonary arterial hypertension in patients treated with interferon. The European respiratory journal. 2015;46(6):1851–3. [DOI] [PubMed] [Google Scholar]
- 11.Tsuchiya H, Kioka H, Ozu K, Ohtani T, Yamaguchi O, Yazaki Y, et al. Interferon Therapy Exacerbated Pulmonary Hypertension in a Patient with Hepatitis C Virus Infection: Pathogenic Interplay among Multiple Risk Factors. Internal medicine (Tokyo, Japan). 2017;56(9):1061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice GI, Bond J, Asipu A, Brunette RL, Manfield IW, Carr IM, et al. Mutations involved in Aicardi-Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nature genetics. 2009;41(7):829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice GI, del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G, et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nature genetics. 2014;46(5):503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, et al. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nature genetics. 2012;44(11):1243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, et al. Mutations in the gene encoding the 3′−5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nature genetics. 2006;38(8):917–20. [DOI] [PubMed] [Google Scholar]
- 16.Crow YJ, Leitch A, Hayward BE, Garner A, Parmar R, Griffith E, et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutieres syndrome and mimic congenital viral brain infection. Nature genetics. 2006;38(8):910–6. [DOI] [PubMed] [Google Scholar]
- 17.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice GI, Forte GM, Szynkiewicz M, Chase DS, Aeby A, Abdel-Hamid MS, et al. Assessment of interferon-related biomarkers in Aicardi-Goutieres syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. The Lancet Neurology. 2013;12(12):1159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank DB, Crystal MA, Morales DL, Gerald K, Hanna BD, Mallory GB Jr., et al. Trends in pediatric pulmonary hypertension-related hospitalizations in the United States from 2000–2009. Pulmonary circulation. 2015;5(2):339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037–99. [DOI] [PubMed] [Google Scholar]
- 22.Rice GI, Forte GM, Szynkiewicz M, Chase DS, Aeby A, Abdel-Hamid MS, et al. Assessment of interferon-related biomarkers in Aicardi-Goutieres syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case-control study. The Lancet Neurology. 2013;12(12):1159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice GI, Melki I, Fremond ML, Briggs TA, Rodero MP, Kitabayashi N, et al. Assessment of Type I Interferon Signaling in Pediatric Inflammatory Disease. J Clin Immunol. 2017;37(2):123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armangue T, Orsini JJ, Takanohashi A, Gavazzi F, Conant A, Ulrick N, et al. Neonatal detection of Aicardi Goutieres Syndrome by increased C26:0 lysophosphatidylcholine and interferon signature on newborn screening blood spots. Molecular genetics and metabolism. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, de Jesus AA, Brooks SR, Liu Y, Huang Y, VanTries R, et al. Development of a Validated Interferon Score Using NanoString Technology. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2018;38(4):171–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crow YJ, Chase DS, Lowenstein Schmidt J, Szynkiewicz M, Forte GM, Gornall HL, et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. American journal of medical genetics Part A. 2015;167a(2):296–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice G, Patrick T, Parmar R, Taylor CF, Aeby A, Aicardi J, et al. Clinical and molecular phenotype of Aicardi-Goutieres syndrome. Am J Hum Genet. 2007;81(4):713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice GI, Del Toro Duany Y, Jenkinson EM, Forte GM, Anderson BH, Ariaudo G, et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nature genetics. 2014;46(5):503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orebaugh CD, Fye JM, Harvey S, Hollis T, Perrino FW. The TREX1 exonuclease R114H mutation in Aicardi-Goutieres syndrome and lupus reveals dimeric structure requirements for DNA degradation activity. The Journal of biological chemistry. 2011;286(46):40246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Piana R, Uggetti C, Roncarolo F, Vanderver A, Olivieri I, Tonduti D, et al. Neuroradiologic patterns and novel imaging findings in Aicardi-Goutieres syndrome. Neurology. 2016;86(1):28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arbustini E, Morbini P, D’Armini AM, Repetto A, Minzioni G, Piovella F, et al. Plaque composition in plexogenic and thromboembolic pulmonary hypertension: the critical role of thrombotic material in pultaceous core formation. Heart (British Cardiac Society). 2002;88(2):177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pogoriler JE, Rich S, Archer SL, Husain AN. Persistence of complex vascular lesions despite prolonged prostacyclin therapy of pulmonary arterial hypertension. Histopathology. 2012;61(4):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, et al. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121(25):2747–54. [DOI] [PubMed] [Google Scholar]
- 35.Fishman AP. Changing concepts of the pulmonary plexiform lesion. Physiological research. 2000;49(5):485–92. [PubMed] [Google Scholar]
- 36.Percival KR, Radi ZA. A modified Verhoeff’s elastin histochemical stain to enable pulmonary arterial hypertension model characterization. European journal of histochemistry: EJH. 2016;60(1):2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kisla Ekinci RM, Balci S, Bisgin A, Altintas DU, Yilmaz M. A homozygote TREX1 mutation in two siblings with different phenotypes: Chilblains and cerebral vasculitis. European journal of medical genetics. 2017;60(12):690–4. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh V, Bernardi B, Stafa A, Garone C, Franzoni E, Abinun M, et al. Intracerebral large artery disease in Aicardi-Goutieres syndrome implicates SAMHD1 in vascular homeostasis. Developmental medicine and child neurology. 2010;52(8):725–32. [DOI] [PubMed] [Google Scholar]
- 39.Xin B, Jones S, Puffenberger EG, Hinze C, Bright A, Tan H, et al. Homozygous mutation in SAMHD1 gene causes cerebral vasculopathy and early onset stroke. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(13):5372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bursztejn AC, Briggs TA, del Toro Duany Y, Anderson BH, O’Sullivan J, Williams SG, et al. Unusual cutaneous features associated with a heterozygous gain-of-function mutation in IFIH1: overlap between Aicardi-Goutieres and Singleton-Merten syndromes. The British journal of dermatology. 2015;173(6):1505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolivras A, Aeby A, Crow YJ, Rice GI, Sass U, Andre J. Cutaneous histopathological findings of Aicardi-Goutieres syndrome, overlap with chilblain lupus. Journal of cutaneous pathology. 2008;35(8):774–8. [DOI] [PubMed] [Google Scholar]
- 42.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 1958;18(4 Part 1):533–47. [DOI] [PubMed] [Google Scholar]
- 43.Wagenvoort CA. Morphological substrate for the reversibility and irreversibility of pulmonary hypertension. European heart journal. 1988;9 Suppl J:7–12. [DOI] [PubMed] [Google Scholar]
- 44.Wagenvoort CA, Wagenvoort N, Draulans-Noe Y. Reversibility of plexogenic pulmonary arteriopathy following banding of the pulmonary artery. The Journal of thoracic and cardiovascular surgery. 1984;87(6):876–86. [PubMed] [Google Scholar]
- 45.Sakao S, Voelkel NF, Tanabe N, Tatsumi K. Determinants of an elevated pulmonary arterial pressure in patients with pulmonary arterial hypertension. Respiratory research. 2015;16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galambos C, Sims-Lucas S, Abman SH, Cool CD. Intrapulmonary Bronchopulmonary Anastomoses and Plexiform Lesions in Idiopathic Pulmonary Arterial Hypertension. American journal of respiratory and critical care medicine. 2016;193(5):574–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galambos C, Sims-Lucas S, Ali N, Gien J, Dishop MK, Abman SH. Intrapulmonary vascular shunt pathways in alveolar capillary dysplasia with misalignment of pulmonary veins. Thorax. 2015;70(1):84–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klok MD, Bakels HS, Postma NL, van Spaendonk RM, van der Knaap MS, Bugiani M. Interferon-alpha and the calcifying microangiopathy in Aicardi-Goutieres syndrome. Annals of clinical and translational neurology. 2015;2(7):774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rice GI, Rodero MP, Crow YJ. Human disease phenotypes associated with mutations in TREX1. Journal of clinical immunology. 2015;35(3):235–43. [DOI] [PubMed] [Google Scholar]
- 50.Kavanagh D, Spitzer D, Kothari PH, Shaikh A, Liszewski MK, Richards A, et al. New roles for the major human 3’−5’ exonuclease TREX1 in human disease. Cell cycle (Georgetown, Tex). 2008;7(12):1718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu C, MacDougall M. RIG-I-Like Receptor Signaling in Singleton-Merten Syndrome. Frontiers in genetics. 2017;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Carvalho LM, Ngoumou G, Park JW, Ehmke N, Deigendesch N, Kitabayashi N, et al. Musculoskeletal Disease in MDA5-Related Type I Interferonopathy: A Mendelian Mimic of Jaccoud’s Arthropathy. Arthritis & rheumatology (Hoboken, NJ). 2017;69(10):2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Sanchez GAM, et al. Activated STING in a vascular and pulmonary syndrome. The New England journal of medicine. 2014;371(6):507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bush D, Abman SH, Galambos C. Prominent Intrapulmonary Bronchopulmonary Anastomoses and Abnormal Lung Development in Infants and Children with Down Syndrome. The Journal of pediatrics. 2017;180:156–62.e1. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan KD, Evans D, Pandey A, Hraha TH, Smith KP, Markham N, et al. Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Scientific reports. 2017;7(1):14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sullivan KD, Lewis HC, Hill AA, Pandey A, Jackson LP, Cabral JM, et al. Trisomy 21 consistently activates the interferon response. eLife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beuthien W, Mellinghoff HU, Kempis J. Vasculitic complications of interferon-alpha treatment for chronic hepatitis C virus infection: case report and review of the literature. Clinical rheumatology. 2005;24(5):507–15. [DOI] [PubMed] [Google Scholar]
- 58.Szilasiova J, Gdovinova Z, Jautova J, Baloghova J, Ficova M, Bohus P. Cutaneous vasculitis associated with interferon beta-1b treatment for multiple sclerosis. Clinical neuropharmacology. 2009;32(5):301–3. [DOI] [PubMed] [Google Scholar]
- 59.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. Journal of immunology (Baltimore, Md: 1950). 2010;184(6):3284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Volpi S, Picco P, Caorsi R, Candotti F, Gattorno M. Type I interferonopathies in pediatric rheumatology. Pediatric rheumatology online journal. 2016;14(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain A, Misra DP, Sharma A, Wakhlu A, Agarwal V, Negi VS. Vasculitis and vasculitis-like manifestations in monogenic autoinflammatory syndromes. Rheumatology international. 2018;38(1):13–24. [DOI] [PubMed] [Google Scholar]
- 62.Caorsi R, Penco F, Grossi A, Insalaco A, Omenetti A, Alessio M, et al. ADA2 deficiency (DADA2) as an unrecognised cause of early onset polyarteritis nodosa and stroke: a multicentre national study. Annals of the rheumatic diseases. 2017;76(10):1648–56. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Zavialov AV, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. The New England journal of medicine. 2014;370(10):911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meyts I, Aksentijevich I. Deficiency of Adenosine Deaminase 2 (DADA2): Updates on the Phenotype, Genetics, Pathogenesis, and Treatment. Journal of clinical immunology. 2018;38(5):569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pettersson M, Bergendal B, Norderyd J, Nilsson D, Anderlid BM, Nordgren A, et al. Further evidence for specific IFIH1 mutation as a cause of Singleton-Merten syndrome with phenotypic heterogeneity. American journal of medical genetics Part A. 2017;173(5):1396–9. [DOI] [PubMed] [Google Scholar]
- 66.Cattalini M, Galli J, Andreoli L, Olivieri I, Ariaudo G, Fredi M, et al. Exploring Autoimmunity in a Cohort of Children with Genetically Confirmed Aicardi-Goutieres Syndrome. Journal of clinical immunology. 2016;36(7):693–9. [DOI] [PubMed] [Google Scholar]
- 67.Rasmussen M, Skullerud K, Bakke SJ, Lebon P, Jahnsen FL. Cerebral thrombotic microangiopathy and antiphospholipid antibodies in Aicardi-Goutieres syndrome--report of two sisters. Neuropediatrics. 2005;36(1):40–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.