Abstract
Background:
While the close morphological relationship between the exocrine and endocrine pancreas is well established, their functional interaction remains poorly understood. The aim of this study was to investigate the associations between circulating levels of pancreatic proteolytic enzymes and insulin, as well as other pancreatic hormones.
Methods:
Fasting venous blood samples were collected and analyzed for trypsin, chymotrypsin, insulin, glucagon, somatostatin, and pancreatic polypeptide. Linear regression analysis was used in unadjusted and two adjusted (accounting for prediabetes/diabetes, body mass index, smoking, and other covariates) statistical models.
Results:
A total of 93 individuals with a history of acute pancreatitis were included in this cross-sectional study. Chymotrypsin was significantly associated with insulin in the two adjusted models (p = 0.005; p = 0.003) and just missed statistical significance in the unadjusted model (p = 0.066). Chymotrypsin was significantly associated with glucagon in both unadjusted (p = 0.025) and adjusted models (p = 0.014; p = 0.015); as well as with somatostatin - in both unadjusted (p = 0.001) and adjusted models (p = 0.001; p = 0.002). Trypsin was not significantly associated with insulin in any of the models but was significantly associated with glucagon in both unadjusted (p < 0.001) and adjusted models (p < 0.001), and pancreatic polypeptide in both unadjusted (p < 0.001) and adjusted (p < 0.001) models.
Conclusion:
The state of hyperinsulinemia is characterized by a dysfunction of the exocrine pancreas. In particular, chymotrypsin is increased in the state of hyperinsulinemia and trypsin is significantly associated with glucagon and pancreatic polypeptide.
Keywords: Insulin, Insulo-acinar axis, Trypsin, Chymotrypsin, Pancreatic hormones
Introduction
The pancreas is an intricate organ with a dual functionality of both endocrine and exocrine tissues. These parts of the pancreas are linked closely both anatomically and physiologically [1,2], and play an important role in digestion and metabolism. Morphological studies show that the endocrine islet cells are scattered amongst the exocrine tissue [3]. Blood supplied to the islets drains into the surrounding acinar tissue to form islet-acinar portal venous system [4]. As blood leaving the islets flows into the acinar capillaries, the acinar cells are exposed to high concentrations of the islet hormones (such as insulin, glucagon, somatostatin, and pancreatic polypeptide (PP) [5–8]) that regulate pancreatic exocrine function, in particular, the synthesis and secretion of pancreatic enzymes [9]. This has led to the notion of ‘insulo-acinar axis’, explaining the regulatory system based on the interaction between the endocrine and exocrine pancreas [10].
The endocrine islets are made up of five types of cells, with the insulin-producing β cells comprising about 60% of the total cellular population [11–13]. Insulin is known to have a trophic effect on the exocrine pancreas, with high local concentrations of insulin resulting in larger peri-insular acini containing more zymogen granules than the tele-insular acini [14–18]. Other islet hormones are believed to have an inhibitory effect on the function of the exocrine pancreas [19–21]. Relationship between the endocrine and exocrine pancreas is not possible to investigate directly in humans ante-mortem but it could be investigated by studying proxies for the endocrine function (circulating levels of pancreatic hormones - insulin, glucagon, somatostatin, and pancreatic polypeptide) and proxies for the exocrine function (circulating levels of pancreatic proteolytic enzymes (PPE), such as trypsin and chymotrypsin that are, unlike amylase or lipase, unique to the pancreas). To date, studies investigating the association between PPE and pancreatic hormones have been mainly conducted in hypoinsulinemic states [22–27]. To the best of our knowledge, no clinical study has investigated these associations in a hyperinsulinemic state. Hyperinsulinemia has long been recognized as a key pathogenic mechanism associated with obesity [28,29]. More recent data suggest that hyperinsulinemia may also play a causative role in tumorigenesis in general and obesity-associated pancreatic cancer in particular [30,31]. Findings from the DORADO study [32–34] show that insulin levels are also frequently elevated in patients after acute pancreatitis; hence DORADO provides a valuable framework for investigating the association between hyperinsulinemia and circulating levels of PPE.
Until recently, the inability to accurately measure circulating levels of PPE, particularly trypsin, posed a major problem. This was largely because of the use of radio-immunoassays to measure trypsin in blood. However, trypsin in blood is found either as proenzyme trypsinogen [35] or as a complex with the protease inhibitors αl anti-trypsin and α2 macroglobulin [36], making it difficult to measure the exact concentration of trypsin. While radio-immunoassays could measure concentrations of both trypsinogen and trypsin-protease complex in blood [37–40], they could not differentiate between the two [41]. Further, radio-immunoassays-obtained trypsin values are not reproducible [35,37,39]. Development of the new, highly sensitive and specific enzyme linked immunosorbent assays (ELISA) resulted in more accurate measurements of PPE. Enzyme linked immunosorbent assays are quick, inexpensive, do not require handling of radioactive substances, and provide reproducible results [42–44].
The primary aim of this study was to investigate the associations between circulating levels of trypsin and chymotrypsin and insu- linemia. The secondary aim was to investigate the associations between trypsin and chymotrypsin and other pancreatic hormones, as well as their contribution to insulinemia.
Methods
Study protocol
The study design was a cross-sectional study. The study protocol was described in detail elsewhere [33,34]. In brief, individuals with a primary prospectively established diagnosis of acute pancreatitis as per international guidelines [45] were followed up and invited to participate in the study. The study was approved by the Health and Disability Ethics Committee (13/STH/182).
Sample collection and storage
A certified phlebotomist collected fasting venous blood from all patients. The blood samples were then centrifuged for 7.5 min at 4000 g at 4 ° Celsius. The serum separated and stored in Eppendorf tubes at −80 °C until use.
Laboratory assays
Blood tests for insulin, glycated haemoglobin (HbAlc), and fasting blood glucose (FBG) were conducted at LabPlus, an International Accreditation New Zealand (1ANZ) accredited medical laboratory, at Auckland City Hospital. Insulin was measured using a chemiluminescence sandwich immunoassay (Roche products and Roche Diagnostics NZ) while HbAlc was measured using boronate affinity chromatography assay (Trinity Biotech). Fasting blood glucose was measured using enzymatic colourimetric assay (F.Hoffmann-La Roche).
Serum from all samples was analyzed for trypsin using the Novateinbio standard sandwich ELISA assay. The standard detection range of the assay was between 0.03 ng/ml −2 ng/ml, with a sensitivity of 0.01 ng/ml, and an intra-assay and inter-assay variation of <10%. Chymotrypsin in serum was analyzed using the Cusabio quantitative sandwich EL1SA assay. The detection range of the assay was between 0.16 ng/ml −10 ng/ml, with a sensitivity of 0.04 ng/ml. The intra- and inter-assay variation for the assay was <8% and <10%, respectively. Somatostatin was measured using the Merck-Millipore EL1SA assay. The results were recorded with the help of a Rayto Microplate Reader (V 2100C, Santa Fe) with an absorbance range of 405–630 nm). All assays were analyzed according to the user’s manuals.
Glucagon and PP were analyzed using M1LL1PEX MAP Human metabolic hormone magnetic bead panel based on Luminex xMAP (Luminex) technology in accordance with the user’s manuals. The results were measured based on fluorescent reporter signals recorded by the Luminex xPONENT software (M1LL1PLEX analyst 5.1).
Definitions
Dysglycemia: was defined as prediabetes (FBG between 5.6 and 6.7 mmol/l and/or HbA1c between 39 and 48 mmol/mol) or diabetes (FBG > 6.7 mmol/l and/or HbA1c > 48 mmol/mol) as per the American Diabetes Association guidelines [46].
Hyperinsulinemia: was defined based on fasting serum insulin levels as the >75th percentile group, in line with previous studies in the field of Diabetology [47–50].
Body Mass 1ndex (BM1) (kg/m2): was measured using a digital scale and stadiometer. Study participants were requested to remove their shoes and any head attire for height measurement (cm). For their weight measurement (kg) participants were asked to empty their pockets and remove their shoes, belt, watch, and jacket.
Physical activity: was recorded as a binary variable, based on whether or not patients exercised for at least 2.5 h per week or 30 min per day [51].
Smoking: was recorded as a binary variable, based on whether or not patients smoked any cigarettes or tobacco-related products.
Chronic alcohol consumption: was deemed to be present if individuals had alcohol etiology of pancreatitis.
Severity of pancreatitis: was defined as per the 2012 determinant-based classification [52].
Recurrence of pancreatitis: was deemed to be present if individuals had one or more episodes from first hospital admission with acute pancreatitis to the time of their participation in the study.
Duration: was defined as the time (months) from individuals’ first hospital admission due to acute pancreatitis to their participation in the study.
Statistical analyses
All statistical analyses were conducted using SPSS for Windows (version 23.0). For all analyses, p-value ≥ 0.05 was accepted as statistically significant.
Data on characteristics of all study participants were presented as either a mean and standard deviation (SD), median and interquartile range, or frequency. The subsequent statistical analyses were conducted in four steps.
First, a multinomial logistic regression analysis was conducted to investigate the associations between insulinemia, trypsin, and chymotrypsin. Insulin was categorized into four quartiles [47–50]: I (<41 pmol/l), II (41–62 pmol/l), III (62–105.50 pmol/l), and IV (≥105.50 pmol/l), using the frequencies function. The Shapiro-Wilk test was used to test for normality. Trypsin and chymotrypsin showed skewed distributions, hence were logarithmically transformed. Outliers were identified using the case-wise diagnostics tool and excluded to derive the most robust and accurate results. Each enzyme was investigated as independent variables in one unadjusted and two adjusted models. Model 1 was an unadjusted model; model 2 was adjusted for patient- and metabolism-related characteristics (age, sex, ethnicity, smoking, physical activity, BMI, and dysglycemia); and model 3, in addition to patient- and metabolism-related characteristics, was also adjusted for pancreatitis-related characteristics (recurrence, severity, and duration). All data were presented as odds ratio (OR) with their corresponding 95% confidence intervals (CI) and p-values.
Second, a linear regression analysis, using generalized linear model, was performed to investigate the associations between pancreatic and trypsin and chymotrypsin. The pancreatic hormones (insulin, glucagon, PP, and somatostatin) and pancreatic enzymes (trypsin and chymotrypsin) did not show a normal distribution and were logarithmically transformed. Each pancreatic hormone was investigated as an independent variable in one unadjusted and two adjusted models, as described above. All data were presented as standardized regression coefficients (β) with their corresponding 95% confidence intervals (CI) and p-values. For all categorical variables, the lowest category was set as the reference.
Third, a sub-group analysis was conducted in which the study cohort was categorized into two groups, based on the presence or absence of chronic alcohol consumption. A linear regression analysis, using a generalized linear model, was performed to investigate the associations between trypsin, chymotrypsin, and insulinemia.
Fourth, a multiple linear regression analysis was performed to investigate the contribution of pancreatic hormones (glucagon, PP, and somatostatin) to hyperinsulinemia. The cutoff value for hyperinsulinemia was determined based on the highest quartile (>75th percentile group) of insulin. All the pancreatic hormones showed a skewed distribution and hence were logarithmically transformed. The variation inflation factor (VIF) score (VIF <10 and tolerance <0.9) was used to detect multicollinearity between the hormones. Each pancreatic hormone (glucagon, PP, and somatostatin) was analyzed independently and in combination with the other two pancreatic hormones in an unadjusted analysis. The resulting constant and β coefficients values were entered into an equation and the corresponding R2 metric obtained.
Results
Study population
A total of 93 individuals were recruited into the study. Of these, 57 (61%) were men. The average age of the entire study cohort was 52 ± 15 years. Sixty-three (67.7%) had a single episode of AP only, 23 (24.7%) had two episodes, and 7 (7.5%) had three or more episodes of AP. Thirty-six patients (38.7%) were enrolled 3–12 months since first attack of AP, 36 – 12–48 months (38.7%), and 21 (22.6%) - more than 48 months. Other characteristics of study participants are presented in Table 1. The median (IQR) trypsin level was 13.11 μg/ml (0.27–27.00 μg/ml) and the median (IQR) chymotrypsin level was 3.11 ng/ml (0.48–5.19 ng/ml) in the study cohort.
Table 1.
Characteristics of study participants.
| Characteristic | Study participants (n = 93) |
|---|---|
| Age (years)a | 52 ± 15 |
| Sex | |
| Male | 57 |
| Female | 36 |
| BMI (kg/m2)a | 28.16 ± 5.38 |
| Exercise | |
| Yes | 65 |
| No | 28 |
| Ethnicity | |
| NZ Europeans | 51 |
| Maori | 8 |
| Pacific Islanders | 4 |
| Asian | 11 |
| Other Europeans | 19 |
| Smoking | |
| Yes | 24 |
| No | 69 |
| Chronic alcohol consumption | |
| Yes | 21 |
| No | 72 |
| Severity | |
| Mild | 73 |
| Moderate/Severe/Critical | 20 |
| Recurrence | |
| Yes | 28 |
| No | 65 |
| Dysglycemia | |
| Yes | 40 |
| No | 53 |
| Insulin (pmol/l)b | 62.00 (41.00–105.50) |
| Glucagon (ng/ml)b | 30.73 (8.76–72.34) |
| Pancreatic Polypeptide (ng/ml)b | 39.01 (5.70–114.13) |
| Somatostatin (ng/ml)b | 0.26 (0.08–0.49) |
Abbreviation: BMI- Body Mass Index.
Data are presented as mean ± SD.
Median (interquartile range).
Associations between pancreatic proteolytic enzymes and insulin
Trypsin
The median (IQR) range of trypsin in normoinsulinemic patients was 10.77 μg/ml (0.18–23.26 μg/ml), compared to 19.39 μg/ml (8.67–30.44 μg/ml) in patients with hyperinsulinemia. The difference was not statistically significant in the adjusted and unadjusted models. Compared to the lowest quartile of insulin, OR [95% CI; p-value] in the highest quartile was 1.16 [0.83, 1.63; p = 0.386] in model 3, followed by 1.15 [0.90,1.48; p = 0.253] in model 1, and 1.13 [0.82,1.57; p = 0.444] in model 2.
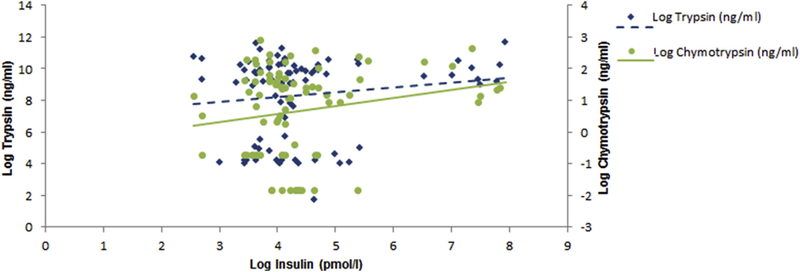
When insulinemia was treated as a continuous variable (Fig. 1), no significant association between trypsin and insulinemia was found in any of the three models.
Fig. 1.
Associations between pancreatic proteolytic enzymes and insulinemia.
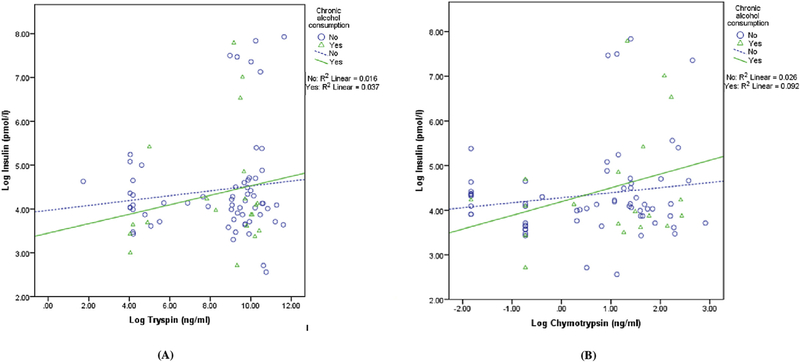
In the sub-group analysis of individuals with chronic alcohol consumption versus those with no chronic alcohol consumption, trypsin was not significantly associated with insulinemia (Fig. 2A).
Fig. 2.
Associations between pancreatic proteolytic enzymes and insulinemia stratified by the presence/absence of chronic alcohol consumption.
Chymotrypsin
The median (IQR) range of chymotrypsin in normoinsulinemic patients was 2.24 ng/ml (0.48–4.97 ng/ml), compared to 3.89 ng/ml (2.70–8.90 ng/ml) in patients with hyperinsulinemia. This difference was not statistically significant in model 1 (p = 0.096) but was statistically significant in the two adjusted models. Compared to the lowest quartile of insulin, OR [95% CI; p-value] in the highest quartile was 3.40 [1.31, 8.81; p = 0.012] in model 3, followed by 2.82 [1.32, 6.00; p = 0.007] in model 2.
When insulinemia was treated as a continuous variable (Fig. 1), the association between chymotrypsin and insulinemia just missed the conventional level of statistical significance in the unadjusted model and was statistically significant in the two adjusted models (Table 2). The most significant association between chymotrypsin and insulin was in model 3 with a β coefficient [95% Cl; p-value] of 1.24 [1.08,1.43; p = 0.003].
Table 2.
Associations between pancreatic proteolytic enzymes and pancreatic hormones.
| Hormone | Model | Trypsin | Chymotrypsin | ||
|---|---|---|---|---|---|
| β (95% C.I) | p | β (95% C.I) | p | ||
| Insulin (pmol/l) | 1 | 1.07 (0.97, 1.18) | 0.187 | 1.17 (1.00, 1.37) | 0.066 |
| 2 | 1.05 (0.97, 1.15) | 0.217 | 1.22 (1.06, 1.40) | 0.005 | |
| 3 | 1.06 (0.97, 1.15) | 0.176 | 1.24(1.08, 1.43) | 0.003 | |
| Glucagon (ng/ml) | 1 | 2.20 (1.54, 3.14) | <0.001 | 1.27 (1.03, 1.55) | 0.025 |
| 2 | 2.50 (1.77, 3.53) | <0.001 | 1.29 (1.05, 1.59) | 0.014 | |
| 3 | 2.58 (1.86, 3.59) | <0.001 | 1.28 (1.05, 1.57) | 0.015 | |
| Pancreatic Polypeptide (ng/ml) | 1 | 1.58 (1.23, 2.04) | <0.001 | 1.08 (0.93, 1.25) | 0.329 |
| 2 | 1.85 (1.40, 2.43) | <0.001 | 1.13 (0.95, 1.33) | 0.166 | |
| 3 | 1.89 (1.44, 2.47) | <0.001 | 1.14 (0.96, 1.35) | 0.123 | |
| Somatostatin (ng/ml) | 1 | 1.28 (0.73, 2.24) | 0.390 | 1.69 (1.24, 2.29) | 0.001 |
| 2 | 1.27 (0.73, 2.21) | 0.405 | 1.70 (1.25, 2.30) | 0.001 | |
| 3 | 1.24 (0.72, 2.14) | 0.442 | 1.60 (1.19, 2.15) | 0.002 | |
Models refer to the unadjusted analysis and adjusted analysis including covariates. Model 1- unadjusted analysis. Model 2- adjusted for age, sex, ethnicity, smoking, exercise, BMI, and dysglycemia. Model 3- adjusted for severity, duration, recurrence and covariates used in model 2.
Bold signifies p values ≤ 0.05.
β value refers to the standardized coefficient estimate obtained from linear regression.
In the subgroup analysis of individuals with chronic alcohol consumption versus no chronic alcohol consumption, chymotrypsin was not significantly associated with insulinemia (Fig. 2B).
Associations between pancreatic proteolytic enzymes and other pancreatic hormones
Trypsin
Trypsin was significantly associated with glucagon and PP in all the three models (Table 2). The most significant association between trypsin and glucagon was in model 3 with a β coefficient [95% CI; p-value] of 2.58 [1.86, 3.59; p < 0.001]; and the most significant association between trypsin and PP was, too, in model 3 with a β coefficient of 1.89 [1.44, 2.47; p < 0.001]. Glucagon and PP contributed 18.8% and 13.4%, respectively, to the variance in circulating trypsin.
Trypsin was not significantly associated with somatostatin in any of the three models (Table 2). Somatostatin contributed 0.9% to the variance in circulating trypsin.
Chymotrypsin
Chymotrypsin was significantly associated with glucagon and somatostatin in all the three models (Table 2). The most significant association between chymotrypsin and glucagon was in model 2 with a β coefficient [95% CI; p-value] of 1.29 [1.05,1.59; p < 0.014] whereas the most significant association between chymotrypsin and somatostatin was in model 2 with a β coefficient of 1.70 [1.25, 2.30; p < 0.001]. Glucagon and somatostatin contributed 5.9% and 12.3% respectively to the variance in circulating chymotrypsin.
Chymotrypsin was not significantly associated with PP in any of the three models (Table 2). PP contributed 1.2% to the variance in circulating chymotrypsin.
Contribution of pancreatic hormones to hyperinsulinemia variance
The contribution of the three hormones (glucagon, PP, and somatostatin) to the insulin variance in patients with hyperinsulinemia versus normoinsulinemia was investigated independently and in combination with each other. Glucagon and PP contributed to 15% of insulin variance in individuals with hyperinsulinemia as compared to 1% in individuals with normoinsulinemia. Other comparisons are presented in Table 3.
Table 3.
Contribution of pancreatic hormones to levels of insulin in the studied groups.
| Pancreatic hormones | Normoinsulinemia | Hyperinsulinemia | ||||
|---|---|---|---|---|---|---|
| Glucagon | PP | Somatostatin | Glucagon | PP | Somatostatin | |
| Glucagon | 0.002a | - | 0.059b | 0.069a | - | 0.027b |
| + PP | 0.014d | 0.003a | - | 0.153d | 0.100a | - |
| + Somatostatin | 0.057e | 0.037c | 0.034a | 0.130e | 0.054c | <0.001a |
Abbreviation: PP- pancreatic polypeptide.
R2 metric for unadjusted analysis between individual and clusters of pancreatic hormones and levels of insulin.
No multicollinearity was detected between the pancreatic hormones. VIF scores ranged from 1.00 to 1.49.
Individual contribution of hormones to insulin levels.
Contribution of glucagon + somatostatin to insulin levels.
Contribution of PP + somatostatin to insulin levels.
Contribution of PP + glucagon to insulin levels.
Contribution of glucagon + PP + somatostatin to insulin levels.
Discussion
The key finding of this study is that chymotrypsin levels are significantly elevated in individuals with hyperinsulinemia, after adjustment for diabetes status, BMI, and other covariates. We found that for every 1 pmol/l increase in insulin the chymotrypsin concentration increased by 1.24 ng/ml. Also, pancreatic hormones (glucagon, PP, and somatostatin) exhibited a differential effect on the pancreatic proteolytic enzymes and displayed a differential pattern in hyperinsulinemia versus normoinsulinemia. This suggests that there is a functional interaction between the endocrine and exocrine pancreas in individuals with hyperinsulinemia and this may provide deeper insights into the understanding of metabolic derangements associated with the state of hyperinsulinemia.
Both endocrine and exocrine tissues of the pancreas develop embryologically from endodermal outgrowths of the gut [53]. The endocrine islet cells are scattered within the exocrine tissue and are in close contact with the exocrine acinar cells. Moreover, as there is no distinct membrane/capsule surrounding the acinar cells, this contact between endocrine and exocrine tissues enhances the metabolic activity of the acinar cells [3]. Compared to the exocrine pancreas, the endocrine pancreas has 10 times more [8] dense capillary fenestrations. In addition, the capillaries in the endocrine pancreas issue efferent vessels into the surrounding exocrine tissue forming a microcirculatory pattern called the insulo-acinar portal system. It is well known that the islet capillaries have a wider diameter, are more perfused, and have a higher pressure resulting in an outward, and if required, increased the flow of blood to the exocrine tissue [5]. This predominantly centrifugal flow of blood from the endocrine to exocrine pancreatic tissue exposes the acinar cells to a high titer of islet hormones [4,54,55]. Based on the morphology, hemodynamics, and physiology of the interaction between the endocrine and exocrine tissues, the notion of the ‘insulo-acinar axis’ was proposed [10].
Evidence from numerous functional and morphological studies [11,22,26,27,56,57] shows that the ‘insulo-acinar axis’ provides a conceptual framework for the islet hormones (in particular insulin) to regulate the functions and maintain homeostasis of the exocrine pancreas. To date, most of the clinical studies investigating the relationship between the endocrine and exocrine pancreas were constrained to individuals with hypoinsulinemia – typically type 1 diabetes [22–26], whereas the present study represents the first effort to investigate the intricate relationship between the exocrine and endocrine pancreas in the state of hyperinsulinemia.
Several pathways may be involved in the functional interaction between the endocrine and exocrine pancreas in the state of hyperinsulinemia. The first pathway involves insulin, known to have a trophic effect on the acinar cells, playing a significant role in regulating the secretion of PPE. Evidence from earlier clinical studies investigating pancreatic exocrine function showed that serum trypsin levels are decreased in patients with type 1 diabetes [22,26,27]. Findings from a case-control study by Adrian et al. [22], investigating exocrine function in 204 individuals with diabetes, showed an inverse relationship between trypsin levels and insulin dose, as well as the duration of insulin therapy. In contrast, a case-control study by Moles et al. [27], investigating the exocrine function in 302 insulin-dependent diabetes patients, reported no significant correlation between trypsin and the dose of insulin or duration of insulin therapy. The study by Adrian et al. [22] suggested that acinar cell activity may be impaired in patients with hypoinsulinemia. Whether this holds true for individuals with hyperinsulinemia is not known. Our study is the first clinical study to investigate the associations between pancreatic serine proteases (trypsin and chymotrypsin) and pancreatic hormones in individuals with hyperinsulinemia. Based on the findings from this study, it appears that trypsin is not significantly associated with insulin in hyperinsulinemic state. However, the study showed that elevated chymotrypsin levels are significantly associated with hyperinsulinemia. The mechanism underlying this association needs to be investigated in future studies but it is possible that, upon hormonal stimulation, the chymotrypsinogen mRNA rapidly undergoes translation, resulting in an increased rate of enzyme synthesis [58].
The second pathway includes insulin receptors present on the surface of acinar cells [59,60] which are down-regulated by high concentrations of insulin in the insulo-portal system [61,62]. In metabolic disorders, such as non-insulin dependent diabetes and obesity, it is well established that insulin regulates its own receptors on liver cells and adipocytes [63–65]. Early evidence from pre-clinical studies shows insulin stimulates glucose uptake [66] and protein synthesis [58,67] in acinar cells. Hence, by analogy with other chronic metabolic conditions, down-regulation of insulin receptors on acinar cells is likely to reduce glucose uptake by these cells and cause insulin resistance.
The third pathway is based on the islet blood flow (IBF) that influences the effect of islet hormones on the exocrine pancreatic tissue. Pre-clinical studies dating back to late 1980s showed increased IBF in the pancreas of experimentally-induced (either by streptozotocin, continuous glucose transfusions, or surgical reduction of β-cell mass) diabetic rats [68,69]. Furthermore, studies done on both obese [70] and non-obese hyperinsulinemic rats showed that insulin, either secreted endogenously [71] or administered exogenously [72], increases the basal IBF [73]. Based on these findings, it appears that increased insulin levels and IBF may result in acinar cells being exposed to higher concentrations of islet hormones.
Given that pancreatic hormones together form an intra-islet signaling network that plays an important role in maintaining glucose homeostasis and metabolism [74], we hypothesized that the pattern of pancreatic hormones other than insulin is different in individuals with normoinsulinemia versus hyperinsulinemia. Our study demonstrated that glucagon and PP together contributed to more than 15% of variance in insulin in individuals with hyperinsulinemia as opposed to 1% in individuals with normoinsulinemia. The associations between these pancreatic hormones and PPE have never been investigated. Findings from our study showed that glucagon is significantly associated with trypsin and chymotrypsin and contributes 18.8% and 5.9% to the variance of circulating trypsin and chymotrypsin, respectively. The other two studied pancreatic hormones - somatostatin and PP - demonstrated a clear differential effect on the proteolytic enzymes. Somatostatin was significantly associated with chymotrypsin and contributed to 12.3% of the variance of circulating chymotrypsin. By contrast, PP showed a significant association with trypsin and explained 13.4% of the circulating trypsin variance.
This study has several limitations. First, due to the cross-sectional study design, it is not possible to draw inferences as to whether PPE cause hyperinsulinemia or are a consequence of hyperinsulinemia. Further, evidence to date is solely based on studies that investigated the role of PPE in the state of hypo- insulinemia [21,23–26]. Hence, findings from this study are hypothesis-generating and need to be tested in prospective longitudinal studies. Second, the study did not formally have healthy controls. This would artificially enhance the contrast between any biomarker signature of the cases with hyperinsulinemia and the non-affected and ultimately result in a failure to replicate in real life. This problem is known in epidemiology as ‘spectrum bias’ [75,76]. Third, not all the serine proteases were investigated (more specifically, endopeptidases and elastases). However, we measured the most abundant endopeptidases (trypsin and chymotrypsin) [77]. Fourth, while all known isoforms of trypsin (cationic trypsin, anionic trypsin, and mesotrypsin) and chymotrypsin (chymotrypsin B1, chymotrypsin-like protease, and caldecrin) were measured, the specific molecular target and distribution of each isoform in the assays is unknown. Further, the use of ELISA did not allow us to differentiate between trypsin and trypsinogen. Only antibodies raised against trypsin activation peptide would allow to distinguish (indirectly) between trypsin and trypsinogen. Fifth, associations between insulinemia and other key pancreatic enzymes (amylase and lipase) were not studied as the latter are not secreted exclusively by the pancreas. Studying trypsin and chymotrypsin allowed us to focus on the enzymes synthesized specifically in the exocrine pancreas. However, it is acknowledged that elevated lipase activity, within the reference range and in the absence of clinical picture of pancreatitis, may indicate subclinical pancreatic injury in asymptomatic individuals [78]. Associations between insulinemia and blood lipase activity warrants a purposefully designed study. Sixth, we did not measure levels of trypsin and chymotrypsin in the gastrointestinal tract. Whether these levels correlate with pancreatic enzymes in blood is a matter of speculation as evidence to date is controversial [23,25,26,39]. We believe that circulating levels represent poor enzyme processing in the acini and a leak into the circulation — not dissimilar to elevated proinsulin in individuals with diabetes or insulin resistance because of poor insulin processing [79,80]. Seventh, potential effects of diet on enzyme content were not investigated [81,82]. However, this study shows for the first time the associations between PPE and insulin in the absence of food suggesting that other factors may influence the association. This merits purposefully designed studies to determine whether food intake and gut hormonal stimulants affect the associations between PPE and pancreatic hormones. Last, although we investigated the effect of tobacco smoking and alcohol consumption, we did not account for poor personal hygiene (which may be the result of heavy alcohol consumption and smoking). This aspect needs to be investigated in future studies.
In conclusion, the present study has unveiled several previously unknown changes in the state of hyperinsulinemia. Based on the findings from this study, it appears that individuals with hyper- insulinemia have an increased functional activity of the exocrine pancreas. Chymotrypsin is significantly associated with the development of hyperinsulinemia and this association may be affected by other pancreatic hormones (in particular, glucagon and PP). Trypsin is significantly associated with glucagon and PP, which together contribute to more than 15% of the variance of insulin in individuals with hyperinsulinemia. Future studies investigating signaling pathways in individuals with hyperinsulinemia are now warranted.
Acknowledgements
This study was part of the Clinical and epidemiological investigations in Metabolism, nutrition, and pancreatic diseases (COSMOS) program. COSMOS is supported in part by the Health and Research Council of New Zealand (grant 15/035 to Dr. Petrov), which played no role in the study design; collection, analysis, or interpretation of data; or writing of the manuscript.
Footnotes
Disclosures
No conflict of interest was declared by the authors.
References
- [1].Ewald N, Raspe A, Kaufmann C, Bretzel R, Kloer H, Hardt P. Determinants of exocrine pancreatic function as measured by fecal elastase-1 concentrations (fec) in patients with diabetes mellitus. Eur J Med Res 2009;14:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Das SL, Kennedy JI, Murphy R, Phillips AR, Windsor JA, Petrov MS. Relationship between the exocrine and endocrine pancreas after acute pancreatitis. WorldJ Gastroenterol 2014;20:17196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Henderson J Why are the islets of Langerhans? Lancet 1969;294:469–70. [DOI] [PubMed] [Google Scholar]
- [4].Murakami T, Fujita T, Taguchi T, Nonaka Y, Orita K. The blood vascular bed of the human pancreas, with special reference to the insulo-acinar portal system. Scanning electron microscopy of corrosion casts. Arch Histol Cytol 1992;55: 381–95. [DOI] [PubMed] [Google Scholar]
- [5].Fraser P, Henderson J. The arrangement of endocrine and exocrine pancreatic microcirculation observed in the living rabbit. QJ Exp Physiol Cogn Med Sci 1980;65:151–8. [DOI] [PubMed] [Google Scholar]
- [6].Henderson J, Daniel P. Portal circulations and their relation to countercurrent systems. Q J Exp Physiol Cogn Med Sci 1978;63:355–69. [DOI] [PubMed] [Google Scholar]
- [7].Henderson J, Daniel P. A comparative study of the portal vessels connecting the endocrine and exocrine pancreas, with a discussion of some functional implications. Q J Exp Physiol Cogn Med Sci 1979;64:267–75. [DOI] [PubMed] [Google Scholar]
- [8].Jansson L, Barbu A, Bodin B, Drott CJ, Espes D, Gao X, et al. Pancreatic islet blood flow and its measurement. Upsala J Med Sci 2016;121:81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beger HG, Buchler M, Kozarek R, Lerch M, Neoptolemos JP, Warshaw A, et al. The pancreas: an integrated textbook of basic science, medicine, and surgery. John Wiley & Sons; 2009. [Google Scholar]
- [10].Williams JA, Goldfine ID. The insulin-pancreatic acinar axis. Diabetes 1985;34: 980–6. [DOI] [PubMed] [Google Scholar]
- [11].Rahier J, Goebbels R, Henquin J. Cellular composition of the human diabetic pancreas. Diabetologia 1983;24:366–71. [DOI] [PubMed] [Google Scholar]
- [12].Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–97. [DOI] [PubMed] [Google Scholar]
- [13].Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren P-O, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U. S. A 2006;103:2334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hellman B, Wallgren A, Petersson B. Cytological characteristics of the exocrine pancreatic cells with regard to their position in relation to the islets of langerhans. Acta Endocrinol 1962;39:465–73. [DOI] [PubMed] [Google Scholar]
- [15].Kramer M, Tan H. The peri-insular acini of the pancreas of the rat. Cell Tissue Res 1968;86:163–70. [DOI] [PubMed] [Google Scholar]
- [16].Malaisse-Lagae F, Ravazzola M, Robberecht P, Vandermeers A, Malaisse W, Orci L. Exocrine pancreas: evidence for topographic partition of secretory function. Science 1975;190:795–7. [DOI] [PubMed] [Google Scholar]
- [17].Bendayan M Pathway of insulin in pancreatic tissue on its release by the β-cell. Am J Physiol Gastrointest Liver Physiol 1993;264:G187–94. [DOI] [PubMed] [Google Scholar]
- [18].Schönfeld J, Goebell H, Mütter MK. The islet-acinar axis of the pancreas. Int J Pancreatol 1994;16:131–40. [DOI] [PubMed] [Google Scholar]
- [19].Nakagawa A, Stagner JI, Ellis S. Suppressive role of the islet-acinar axis in the perfused rat pancreas. Gastroenterology 1993;105:868–75. [DOI] [PubMed] [Google Scholar]
- [20].Dollinger H, Raptis S, Pfeiffer E. Effects of somatostatin on exocrine and endocrine pancreatic function stimulated by intestinal hormones in man. Horm Metab Res 1976;8:74–8. [DOI] [PubMed] [Google Scholar]
- [21].Adrian T, Besterman H, Mallinson C, Greenberg G, Bloom S. Inhibition of secretin stimulated pancreatic secretion by pancreatic polypeptide. Gut 1979;20:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Adrian T, Barnes A, Bloom S. Hypotrypsinaemia in diabetes mellitus. Clin Chim Acta 1979;97:213–6. [DOI] [PubMed] [Google Scholar]
- [23].Frier B, Adrian T, Saunders J, Bloom S. Serum trypsin concentration and pancreatic trypsin secretion in insulin-dependent diabetes mellitus. Clin Chim Acta 1980;105:297–300. [DOI] [PubMed] [Google Scholar]
- [24].Frier B, Saunders J, Wormsley K, Bouchier I. Exocrine pancreatic function in juvenile-onset diabetes mellitus. Gut 1976;17:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lankisch P, Manthey G, Otto J, Koop H, Talaulicar M, Willms B, et al. Exocrine pancreatic function in insulin-dependent diabetes mellitus. Digestion 1982;25:211–6. [DOI] [PubMed] [Google Scholar]
- [26].Dandona P, Freedman D, Foo Y, Perkins J, Katrak A, Mikhailidis D, et al. Exocrine pancreatic function in diabetes mellitus. J Clin Pathol 1984;37: 302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moles K, Kerr J, Armstrong E, Hayes J, Buchanan K. Serum concentrations of trypsin-like immunoreactivity and pancreatic isoamylase in insulin dependent diabetic patients. Pancreas 1988;3:135–9. [DOI] [PubMed] [Google Scholar]
- [28].Koyama K, Chen G, Lee Y, Unger RH. Tissue triglycerides, insulin resistance, and insulin production: implications for hyperinsulinemia of obesity. Am J Physiol Endocrinol Metabol 1997;273:ME708–13. [DOI] [PubMed] [Google Scholar]
- [29].Singh RG, Pendharkar SA, Plank LD, Petrov MS. Role of human lipocalin proteins in abdominal obesity after acute pancreatitis. Peptides 2017;91:1–7. [DOI] [PubMed] [Google Scholar]
- [30].Eibl G, Cruz-Monserrate Z, Korc M, Petrov MS, Goodarzi MO, Fisher WE, et al. Consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer (CPDPC). Diabetes mellitus and obesity as risk factors for pancreatic cancer. J Acad Nutr Diet 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gillies N, Pendharkar SA, Asrani VM, Mathew J, Windsor JA, Petrov MS. Interleukin-6 is associated with chronic hyperglycemia and insulin resistance in patients after acute pancreatitis. Pancreatology 2016;16:748–55. [DOI] [PubMed] [Google Scholar]
- [33].Pendharkar SA, Asrani VM, Xiao AY, Yoon HD, Murphy R, Windsor JA, et al. Relationship between pancreatic hormones and glucose metabolism: a cross-sectional study in patients after acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2016;311:G50–8. [DOI] [PubMed] [Google Scholar]
- [34].Singh RG, Pendharkar SA, Gillies NA, Miranda-Soberanis V, Plank LD, Petrov MS. Associations between circulating levels of adipocytokines and abdominal adiposity in patients after acute pancreatitis. Clin Exp Med 2017: 1–11. [DOI] [PubMed] [Google Scholar]
- [35].Geokas MC, Largman C, Brodrick JW, Johnson JH. Determination of human pancreatic cationic trypsinogen in serum by radioimmunoassay. Am J Physiol Gastrointest Liver Physiol 1979;236:G77–83. [DOI] [PubMed] [Google Scholar]
- [36].Homer GM, Zipf RE, Hieber TE, Katchman BJ. The trypsin inhibitor capacity of serums in normal and diseased states. Am J Clin Pathol 1960;34:99–107. [DOI] [PubMed] [Google Scholar]
- [37].Temler RS, Felber J-P. Radioimmunoassay of human plasma trypsin. Biochim Biophys Acta (BBA) Enzymol 1976;445:720–8. [DOI] [PubMed] [Google Scholar]
- [38].Borgstrom A, Ohlsson K. Immunoreactive trypsin in serum and peritoneal fluid in acute pancreatitis. Biol Chem 1978;359:677–82. [DOI] [PubMed] [Google Scholar]
- [39].Elias E, Wood T, Redshaw M. Diagnostic importance of changes in circulating concentrations of immunoreactive trypsin. Lancet 1977;310:66–8. [DOI] [PubMed] [Google Scholar]
- [40].Brodrick JW, Geokas MC, Largman C, Fassett M, Johnson JH. Molecular forms of immunoreactive pancreatic cationic trypsin in pancreatitis patient sera. Am J Physiol Endocrinol Metabol 1979;237:E474. [DOI] [PubMed] [Google Scholar]
- [41].O’Connor C, O’Donnell M, McGeeney K. Problems associated with the radioimmunoassay of serum trypsin. Clin Chim Acta 1981;114:29–35. [DOI] [PubMed] [Google Scholar]
- [42].Carrere J, Grataroli R, Marfin J, Ferrua B, Thouvenot J, Figarella C. Noncompetitive enzyme immunoassay of human trypsin 1. J Immunol methods 1983;60:235–42. [DOI] [PubMed] [Google Scholar]
- [43].Kimland M, Russick C, Marks WH, Borgstrom A. Immunoreactive anionic and cationic trypsin in human serum. Clin Chim acta 1989;184:31–46. [DOI] [PubMed] [Google Scholar]
- [44].Steiner JM, Teague SR, Williams DA. Development and analytic validation of an enzyme-linked immunosorbent assay for the measurement of canine pancreatic lipase immunoreactivity in serum. Can J veterinary Res 2003;67: 175–82. [PMC free article] [PubMed] [Google Scholar]
- [45].Poma EM, Olascoaga FZ, Petrov M, Soto SN, Santos CL, Alava FM, et al. SEM- ICYUC 2012. Recommendations for intensive care management of acute pancreatitis. Med Intensiva Engl Ed 2013;37:163–79. [DOI] [PubMed] [Google Scholar]
- [46].Petrov MS. Diabetes of the exocrine pancreas: American Diabetes Association- compliant lexicon. Pancreatology 2017;17:523–6. [DOI] [PubMed] [Google Scholar]
- [47].Park SK, Oh C-M, Jung T, Choi Y-J, Chung JY, Ryoo J-H. Elevated fasting insulin levels increase the risk of abdominal obesity in Korean men. Maturitas 2017;98:1–6. [DOI] [PubMed] [Google Scholar]
- [48].Sung K-CC, Seo M-HH, Rhee E-JJ, Wilson AM. Elevated fasting insulin predicts the future incidence of metabolic syndrome: a 5-year follow-up study. Cardiovasc Diabetol 2011;10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dankner R, Chetrit A, Shanik MH, Raz I, Roth J. Basal-state hyperinsulinemia in healthy normoglycemic adults is predictive of type 2 diabetes over a 24-year follow-up. Diabetes Care 2009;32:1464–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tohidi M, Ghasemi A, Hadaegh F, Derakhshan A, Chary A, Azizi F. Age-and sex- specific reference values for fasting serum insulin levels and insulin resis¬tance/sensitivity indices in healthy iranian adults: Tehran lipid and glucose study. Clin Biochem 2014;47:432–8. [DOI] [PubMed] [Google Scholar]
- [51].American Diabetes Association. The diabetes prevention program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dellinger EP, Forsmark CE, Layer P, Levy P, Maravi-Poma E, Petrov MS, et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg 2012;256:875–80. [DOI] [PubMed] [Google Scholar]
- [53].Orci L Macro-and micro-domains in the endocrine pancreas. Diabetes 1982;31:538–65. [DOI] [PubMed] [Google Scholar]
- [54].Fujita T, Murakami T. Microcirculation of monkey pancreas with special reference to the insulo-acinar portal system. Arch Histol Jpn 1973;35:255–63. [DOI] [PubMed] [Google Scholar]
- [55].Murakami T, Hitomi S, Ohtsuka A, Taguchi T, Fujita T. Pancreatic insulo-acinar portal systems in humans, rats, and some other mammals: scanning electron microscopy of vascular casts. Microsc Res Tech 1997;37:478–88. [DOI] [PubMed] [Google Scholar]
- [56].Goda K, Sasaki E, Nagata K, Fukai M, Ohsawa N, Hahafusa T. Pancreatic volume in type 1 and type 2 diabetes mellitus. Acta diabetol 2001;38:145–9. [DOI] [PubMed] [Google Scholar]
- [57].Keller J, Aghdassi AA, Lerch MM, Mayerle JV, Layer P. Tests of pancreatic exocrine function—clinical significance in pancreatic and non-pancreatic disorders. Best Pract Res Clin Gastroenterol 2009;23:425–39. [DOI] [PubMed] [Google Scholar]
- [58].Lahaie R Translational control of protein synthesis in isolated pancreatic acini: role of cck8 carbachol, and insulin. Pancreas 1986;1:403–10. [DOI] [PubMed] [Google Scholar]
- [59].Korc M, Sankaran H, Wong K, Williams JA, Goldfine ID. Insulin receptors in isolated mouse pancreatic acini. Biochem Biophys Res Commun 1978;84: 293–9. [DOI] [PubMed] [Google Scholar]
- [60].Mössner J, Logsdon CD, Williams JA, Goldfine ID. Insulin, via its own receptor, regulates growth and amylase synthesis in pancreatic acinar ar42j cells. Diabetes 1985;34:891–7. [DOI] [PubMed] [Google Scholar]
- [61].Mossner J, Logsdon C, Goldfine I, Williams J. Regulation of pancreatic acinar cell insulin receptors by insulin. Am J Physiol Gastrointest Liver Physiol 1984;247:G155–60. [DOI] [PubMed] [Google Scholar]
- [62].Okabayashi Y, Maddux BA, McDonald AR, Logsdon CD, Williams JA, Goldfine ID. Mechanisms of insulin-induced insulin-receptor downregulation: decrease of receptor biosynthesis and mrna levels. Diabetes 1989;38:182–7. [DOI] [PubMed] [Google Scholar]
- [63].Kolterman O, Gray R, Griffin J, Burstein P, Insel J, Scarlett J, et al. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin¬dependent diabetes mellitus. J Clin Investig 1981;68:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Martin C, Desai K, Steiner G. Receptor and postreceptor insulin resistance induced by in vivo hyperinsuiinemia. Can J Physiol Pharmacol 1983;61: 802–7. [DOI] [PubMed] [Google Scholar]
- [65].Garvey WT, Olefsky J, Marshall S. Insulin receptor down-regulation is linked to an insulin-induced postreceptor defect in the glucose transport system in rat adipocytes. J Clin Investig 1985;76:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Williams JA, Bailey AC, Preissler M, Goldfine ID. Insulin regulation of sugar transport in isolated pancreatic acini from diabetic mice. Diabetes 1982;31: 674–82. [DOI] [PubMed] [Google Scholar]
- [67].Korc M, Iwamoto Y, Sankaran H, Williams JA, Goldfine I. Insulin action in pancreatic acini from streptozotocin-treated rats. I. Stimulation of protein synthesis. Am J Physiol Gastrointest Liver Physiol 1981;240:G56–62. [DOI] [PubMed] [Google Scholar]
- [68].Styrud J, Eriksson U, Jansson L. A continuous 48-hour glucose infusion in rats causes both an acute and a persistent redistribution of the blood flow within the pancreas. Endocrinology 1992;130:2692–6. [DOI] [PubMed] [Google Scholar]
- [69].Jansson L, Sandler S. Pancreatic and islet blood flow in the regenerating pancreas after a partial pancreatectomy in adult rats. Surgery 1989;106: 861–6. [PubMed] [Google Scholar]
- [70].Atef N, Ktorza A, Picon L, Penicaud L. Increased islet blood flow in obese rats: role of the autonomic nervous system. Am J Physiol Endocrinol Metabol 1992;262:E736–40. [DOI] [PubMed] [Google Scholar]
- [71].Vetterlein F, Senske D, Bornkessel C, Schmidt G. Effects of tolbutamide on blood flow in islets and exocrine tissue of the rat pancreas. Eur J Pharmacol 1985;113:395–8. [DOI] [PubMed] [Google Scholar]
- [72].Sparrow R, Beckingham I. Islet blood flow following insulin administration. J Anat 1989;163:75. [PMC free article] [PubMed] [Google Scholar]
- [73].Oöstenson C-G, Khan A, Abdel-Halim S, Guenifi A, Suzuki K, Goto Y, et al. Abnormal insulin secretion and glucose metabolism in pancreatic islets from the spontaneously diabetic gk rat. Diabetologia 1993;36:3–8. [DOI] [PubMed] [Google Scholar]
- [74].Brereton MF, Vergari E, Zhang Q, Clark A. Alpha-, delta-and pp-cells: are they the architectural cornerstones of islet structure and co-ordination? J Histochem Cytochem 2015;63:575–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N. Engl J Med 1978;299:926–30. [DOI] [PubMed] [Google Scholar]
- [76].Pepe MS, Fan J, Seymour CW, Li C, Huang Y, Feng Z. Biases introduced by choosing controls to match risk factors of cases in biomarker research. Clin Chem 2012;58:1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci 2007;52:1–17. [DOI] [PubMed] [Google Scholar]
- [78].Weiss FU, Schurmann C, Guenther A, Ernst F, Teumer A, Mayerle J, et al. Fucosyltransferase 2 (FUT2) non-secretor status and blood group B are associated with elevated serum lipase activity in asymptomatic subjects, and an increased risk for chronic pancreatitis: a genetic association study. Gut 2015;64:646–56. [DOI] [PubMed] [Google Scholar]
- [79].Rhodes CJ, Alarcon C. What β-cell defect could lead to hyperproinsulinemia in niddm?: Some clues from recent advances made in understanding the proinsulin-processing mechanism. Diabetes 1994;43:511–7. [DOI] [PubMed] [Google Scholar]
- [80].Ward W, LaCava E, Paquette T, Beard J, Wallum B, Porte D. Disproportionate elevation of immunoreactive proinsulin in type 2 (non-insulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia 1987;30:698–702. [DOI] [PubMed] [Google Scholar]
- [81].Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut 2005;54:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Perkins PS, Rutherford RE, Pandol SJ. Effect of chronic ethanol feeding on digestive enzyme synthesis and mrna content in rat pancreas. Pancreas 1995;10:14–21. [DOI] [PubMed] [Google Scholar]