Abstract
Objective:
Findings from a large multi-center experience showed that sex influenced the relationship between low nadir hematocrit (Hct) and increased risk of acute kidney injury (AKI) following cardiac surgery. We explored whether sex-related differences persisted among patients undergoing isolated coronary artery bypass grafting (CABG).
Methods:
We undertook a prospective, observational study of 17,363 non-dialysis patients (13,137 male: 75.7%; 4,226 female: 24.3%) undergoing isolated CABG between 2011-2016 across 41 institutions in the Perfusion Measures and Outcomes registry. Odds ratios (OR) between nadir Hct and stage 2 or 3 AKI were calculated and the interaction of sex with nadir Hct was tested. The multivariable generalized linear mixed effect model adjusted for pre- and intra-operative factors and institution.
Results:
Median nadir Hct was 22% among women and 27% among men (p<0.001). Women were administered a greater median net prime volume indexed to body surface area (407 ml/m2 vs. 363 ml/m2) and more red blood cell (RBC) transfusions (55.5% vs. 24.3%), both p<0.001. AKI was higher among women (6.0% vs. 4.3%, p<0.001). There was no effect of sex on the relationship between nadir Hct and AKI (p=0.67). Low nadir Hct was inversely associated with AKI (adjusted OR per 1-unit increase in nadir Hct 0.96, CI 95%: 0.93 to 0.98); this effect was similar across sexes and independent of RBC transfusions.
Conclusions:
We found no sex-related differences in the effect of nadir Hct on AKI following isolated CABG. However, the strong inverse relationship between anemia and AKI across sexes suggests the importance of reducing exposure to low nadir Hct.
Graphical Abstract
Central Picture. Relationship between nadir Hct during bypass and risk of stage 2 or 3 acute kidney injury.
Central Message
Unlike prior studies, we found no interaction by sex in the relationship between low nadir hematocrit and AKI. Given its impact on AKI, anemia during isolated CABG should be avoided.
Introduction
Patients undergoing isolated coronary artery bypass grafting (CABG) surgery are at risk for many morbid events, including acute kidney injury (AKI). Prior reports suggest that upwards of 30% of patients develop a spectrum of AKI, with between 0.4% and 2% of patients requiring post-operative renal replacement therapy [1-4]. Emerging literature has documented the risk of further sequelae attributed to even small rises in creatinine (stage 1 AKI), including an increased risk of mortality (hazard ratio [HR] 1.56, 95% confidence interval [CI] 1.14–2.12), readmission (HR 1.31, CI 1.10–1.57), and a nearly three-fold increased risk of developing end-stage renal disease (ESRD) [HR 2.92, CI 1.87–4.55] [5,6].
Prior studies have documented an inverse relationship between a patient’s intra-operative exposure to low nadir hematocrit (Hct) during cardiopulmonary bypass (CPB) and risk of AKI. While women may be uniquely susceptible to AKI given their increased risk of lower nadir Hct secondary to hemodilution, prior reports have had conflicting results [7-9]. These reports have included a heterogenous population of cardiac procedures and likely cannot be generalizable to isolated CABG procedures. Thus, efforts aimed at reducing a patient’s risk of AKI following isolated CABG requires further investigation into a potential interaction between sex and intra-operative anemia.
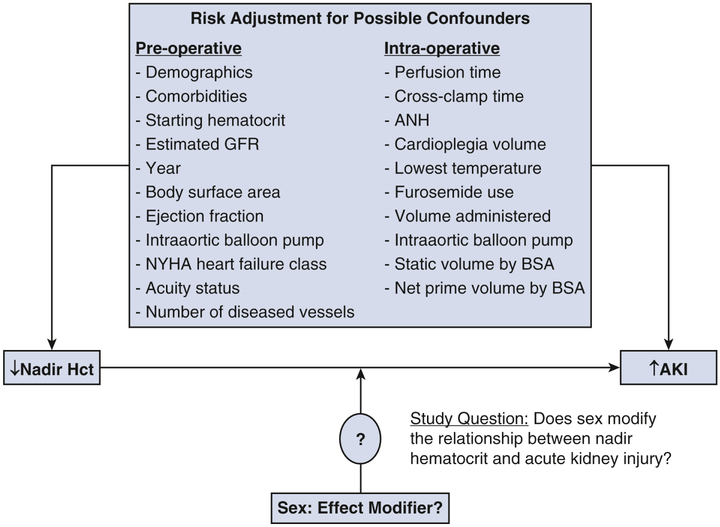
In the present analysis, we identified whether sex modifies the relationship between intra-operative anemia and AKI in the setting of isolated CABG (eFigure). To do so, we conducted an observational study of nondialysis-dependent patients undergoing isolated CABG across 41 hospitals participating in a multi-institutional quality improvement database.
eFigure.
Conceptual model showing the research question of whether sex functions as an effect modifier on the known strong inverse relationship between nadir hematocrit on cardiopulmonary bypass and postoperative acute kidney injury. AKI = acute kidney injury; ANH = acute normovolemic hemodilution; BSA = body surface area; GFR = glomerular filtration rate; Hct = hematocrit; NYHA = New York Heart Association.
Patients and Methods
This study was approved by the Institutional Review Board of the University of Michigan Health System (IRBHUM00117260, notice of determination of “not regulated” status).
Patient Population
The Perfusion Measures and Outcomes (PERForm) registry is organizationally structured within the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS-QC). The MSTCVS-QC began in 2001 as a cardiac surgeon-led quality collaborative embedded in the MSTCVS, and in 2005, it became partially funded by the Blue Cross/Blue Shield of Michigan insurance company. The collaborative meets quarterly to review various processes and outcomes, and to facilitate and evaluate quality improvement studies.
All programs in the MSTCVS-QC utilize The Society of Thoracic Surgeons data collection forms and submit data on a quarterly basis to the MSTCVS-QC data warehouse. The PERForm registry is the official registry of the American Society of ExtraCorporeal Technology (AmSECT), and AmSECT in turn is the PERForm registry’s official societal partner. The PERForm registry contains information related to the care and conduct of cardiovascular perfusion practices. A list of fields and definitions may be found at http://www.performregistry.org. Each center’s surgical record is merged with a perfusion record from the PERForm registry [10]. Participating centers are routinely audited for data validity and accuracy as part of the MSTCVS-QC audit system.
We included patients who underwent isolated CABG with use of cardiopulmonary bypass at any of the 41 centers participating in the PERForm registry between July 11, 2011 and September 30, 2016. We excluded patients undergoing dialysis pre-operatively, cardiopulmonary bypass with circulatory arrest, and those with a ventricular assist device.
Measures
The primary outcome for this analysis was stage 2 or 3 AKI, using the Acute Kidney Injury Network creatinine criteria (defined as a twofold or greater increase in serum creatinine from baseline, creatinine rise to 4.0 mg/dL or more with an acute increase of more than 0.5 mg/dL, or acute dialysis requirement) [11]. We report crude rates of transfusions, hospital operative mortality, reoperation for bleeding, prolonged ventilation time longer than 24 hours, total intensive care unit stay longer than 24 hours, need for ultrafiltration or dialysis, low cardiac output, new onset atrial fibrillation, sepsis, postoperative length of stay, and 30-day readmission.
Statistical Analysis
Categorical variables were presented as percentages and continuous variables as mean (standard deviation) for univariate analyses. Chi-square and Fisher exact tests were used to test statistical significance for categorical variables, while Wilcoxon rank sum tests were used for continuous variables.
Missing values of categorical variables with less than 1% missingness were imputed with their lowest risk values. Missing values of continuous variables were imputed to conditional median by sex. Number of missing observations for variables included in final model and method of imputation are displayed in eTable 1. Variables were selected using a logistic regression model with lasso penalized method. The final model included pre- and intra-operative factors: surgical year, age, sex, body surface area (BSA), diabetes, peripheral vascular disease, chronic lung disease, preoperative hematocrit, estimated glomerular filtration rate (EGFR), WBC, hypertension, congestive heart failure (CHF), New York Heart Association (NYHA) classification, ejection fraction, acuity status, number of diseased vessels, perfusion time, cross clamp time, static volume indexed to BSA, pre- or intra-operative IABP, total volume of fluid in during operation, use of acute normovolemic hemodilution, total cardioplegia volume, lowest temperature during operation, furosemide use, net prime volume indexed to BSA, and lowest hematocrit during the operation (and its interaction with sex). The final generalized linear mixed model used a logit link function and also included centers as the random effect to account for the clustering of patients within each center.
eTable 1.
Missing observations for variables included in the final model, stratified by sex and with imputation methods listed, if applicable.
| Variable | Female missing observations, total n=4,226 (%) |
Male missing observations, total n=13,137 (%) |
Method of imputation, if performed |
|---|---|---|---|
| Nadir hematocrit, median | 47 (1.1) | 95 (0.7) | Median by sex |
| Vascular disease, % | 2 (0.05) | 2 (0.02) | No imputation |
| Moderate to severe COPD, % | 74 (1.8) | 198 (1.5) | No imputation |
| Preoperative hematocrit, median | 28 (0.7) | 62 (0.5) | Median by sex |
| WBC count, mean | 32 (0.8) | 71 (0.5) | Median by sex |
| CPB duration, median | 5 (0.1) | 4 (0.03) | Median by sex |
| Cross-clamp duration, median | 88 (2.1) | 175 (1.3) | Median by sex |
| Static volume, median mL | 75 (1.8) | 180 (1.4) | Median by sex |
| Total fluid administration, median mLs | 315 (7.5) | 904 (6.9) | Median by sex |
| Acute normovolemic hemodiluation, % | 245 (5.8) | 622 (4.7) | Missing imputed as “no” |
| Total cardioplegia volume, median mLs | 187 (4.4) | 462 (3.5) | Median by sex |
| Lowest core temperature, median ℉ | 50 (1.2) | 93 (0.7) | Mode (≥35 ℉) imputed |
| Total prime volume, median mLs | 96 (2.3) | 221 (1.7) | Median by sex |
COPD = chronic obstructive pulmonary disease; CPB = cardiopulmonary bypass; ℉ = degrees Fahrenheit; ICU = intensive care unit; LV = left ventricle; MI = myocardial infarction; mL = milliliter; WBC = white blood cell.
Sensitivity analyses were performed by analyzing the effect of lowest Hct separately by strata of sex. The effect of nadir Hct on AKI is reported as the OR per 1-unit increase in nadir Hct. The predicted probability of AKI, fixing the adjusted covariates at the median of the continuous variables or the mode values of the categorical variables (along with 95% confidence intervals) was plotted separately for men and women. Sensitivity analyses were also performed to assess the effect of sex on the relationship between nadir Hct and AKI when stratified by RBC transfusion status.
P-values of less than 0.05 (2-tailed) were considered statistically significant. All statistical calculations used R version 3.4.0 and SAS 9.4 (SAS Institute, Cary, NC).
Results
Between July 11, 2011 and September 30, 2016, 17,873 patients underwent isolated CABG at the 41 participating centers. After excluding patients presenting on dialysis (n = 443), using a ventricular assist device (n = 2), undergoing intra-operative circulatory arrest (n = 8), undergoing another cardiac procedure (n = 10), and those with missing data for AKI staging (n = 155), the final study population consisted of 17,363 patients (13,137 male and 4,226 female).
Pre-operative patient characteristics are listed in detail in Table 1. Women were slightly older and had higher rates of pre-operative diabetes, vascular disease, hypertension, and CHF than men. Additionally, women had lower baseline Hct and EGFR, while higher procedural acuity. Smoking history and prevalence of significant left main coronary artery disease were similar between women and men.
Table 1.
Pre-operative patient characteristics
| Variable | Female | Male | p-value |
|---|---|---|---|
| Number of procedures | 4,226 | 13,137 | |
| Age, median [IQR] years | 67 [59-74] | 65 [58-72] | <0.001 |
| <60 | 25.5 | 30.2 | |
| 60-69 | 33.8 | 36.7 | |
| 70+ | 40.7 | 33.1 | |
| <0.001 | |||
| Body surface area, m2 | |||
| <1.60 | 10.6 | 0.7 | |
| 1.60-1.79 | 27.1 | 6.1 | |
| 1.80-1.99 | 33.3 | 24.1 | |
| ≥2.00 | 28.9 | 69.2 | |
| <0.001 | |||
| Risk factors | |||
| Diabetes mellitus, % yes | 53.3 | 44.0 | <0.001 |
| Vascular disease, % yes | 16.8 | 14.3 | <0.001 |
| Moderate-severe COPD, % yes | 11.8 | 10.1 | 0.0013 |
| Last preoperative creatinine, mg/dL, median[IQR] | 0.93 [0.70-1.04] | 1.06 [0.88-1.20] | <0.001 |
| Hematocrit, % | |||
| <33 | 20.6 | 8.1 | |
| 33-36 | 30.3 | 14.2 | |
| 37-40 | 31.9 | 28.1 | |
| 41+ | 17.2 | 49.6 | |
| <0.001 | |||
| Hypertension, % yes | 91.8 | 88.8 | <0.001 |
| EGFR <30 mL/min/1.73m2, % yes | 3.1 | 1.3 | <0.001 |
| EGFR, mL/min/1.73m2 | |||
| ≥90 | 26.5 | 30.6 | |
| 60-90 | 42.4 | 50.7 | |
| 30-60 | 28 | 17.4 | |
| 15-30 | 2.9 | 1.1 | |
| <15 | 0.2 | 0.2 | |
| <0.001 | |||
| Last WBC count x 109/L, median [IQR] | 7.8 [6.3-9.5] | 7.5 [6.2-9.2] | <0.001 |
| Current smoker within 30 days, % | 22.6 | 22.7 | 0.949 |
| Cardiac History | |||
| CHF, % yes | 17.4 | 12.8 | <0.001 |
| Prior myocardial infarction, % | 54.4 | 53.2 | 0.15 |
| Prior CABG, % | 1.5 | 2.0 | 0.04 |
| Prior cardiac surgery, % | 2.2 | 2.5 | 0.34 |
| Cardiac anatomy and function | |||
| LVEF, % | |||
| <40 | 12.9 | 15.7 | |
| 40-49 | 13.4 | 15.4 | |
| 50-59 | 32.3 | 34.7 | |
| 60+ | 41.4 | 34.1 | |
| <0.001 | |||
| Left main disease ≥ 50% stenosis, % yes | 34.5 | 33.9 | 0.44 |
| 3+ diseased vessels, % yes | 74.3 | 79.6 | <0.001 |
| Acuity | |||
| Elective | 34.9 | 41.3 | |
| Urgent | 61.1 | 56.2 | |
| Emergent/salvage | 3.9 | 2.5 | |
| <0.001 | |||
| STS mortality and morbidity risk score, median % [IQR] | 14 [10-21] | 10 [7-16] | <0.001 |
Intra-operative characteristics differed between women and men (Table 2). Bypass and cross-clamp times were slightly shorter for women as compared to men, although women returned to bypass more frequently than men (2.6% versus 1.7%, p < 0.001) and received intraaortic balloon pumps more commonly in the intra- or post-operative period (2.2% versus 1.4%, p < 0.001). Women were administered a greater median net prime volume indexed to BSA, greater total fluid, and a lower median volume of ultrafiltration. Acute normovolemic hemodilution, a blood conservation strategy, was used less commonly among women (9.0% versus 15.7%). Median nadir Hct was lower among women (22.1% vs. 26.9%, p < 0.001). Less than half (48.0%) of women reached a nadir Hct less than 21, compared to only 13.3% of men, while women were administered significantly more intra-operative red blood cell transfusions (33.7% versus 8.1%, p < 0.001).
Table 2.
Intra-operative variables
| Variable | Female | Male | p-value |
|---|---|---|---|
| Number of procedures | 4,226 | 13,137 | |
| Number of distal anastomoses | |||
| 1 | 3.2 | 1.6 | |
| 2 | 20.9 | 15.0 | |
| 3 | 44.8 | 41.8 | |
| 4+ | 31.2 | 41.6 | |
| <0.001 | |||
| CPB duration, minutes, median [IQR] | 90 [69-117] | 93 [73-121] | <0.001 |
| Cross-clamp duration, minutes, median [IQR] | 68 [52-89] | 71 [55-93] | <0.001 |
| Returned to bypass, % | 2.6 | 1.7 | <0.001 |
| IABP, % yes | |||
| Pre-operative | 7.3 | 6.7 | |
| Intra-operative | 1.7 | 1.2 | |
| Post-operative | 0.5 | 0.2 | |
| 0.0008 | |||
| Pulsatile perfusion, % yes | 0.8 | 0.8 | 0.80 |
| Strategies to manage hemodilution | |||
| Static prime volume per BSA, mL/m2, median [IQR] | 552 [467-667] | 511 [428-604] | <0.001 |
| Retrograde autologous priming, % yes | 77.9 | 79.2 | 0.051 |
| Retrograde autologous priming, mL, median [IQR] | 550 [400-700] | 600 [400-700] | <0.001 |
| Net prime volumea per BSA, mL/m2, median [IQR] | 407 [282-566] | 363 [251-498] | <0.001 |
| Total fluid administrationb, mL, median [IQR] | 1000 [560-1700] | 938 [518-1600] | <0.001 |
| Blood management strategies | |||
| Conventional ultrafiltration, % yes | 23.1 | 21.8 | 0.073 |
| Volume if performed, mL, median [IQR] | 1200 [700-2050] | 1400 [800-2500] | <0.001 |
| Cell-saving device, % yes | 85.4 | 85.0 | 0.49 |
| Acute normovolemic hemodilution, %yes | 9.0 | 15.7 | <0.001 |
| Volume, mL, median [IQR] | 450 [400-500] | 450 [450-900] | <0.001 |
| Cardiotomy suction, % yes | 61.5 | 59.3 | 0.022 |
| Blood-based cardioplegia, % yes | 75.9 | 74.3 | 0.026 |
| Total cardioplegia volume, mL, median [IQR] | 2000 [1300-2800] | 2136 [1450-3090] | <0.001 |
| Lowest core temperaturec | |||
| Severe hypothermia | 0.3 | 0.3 | |
| Moderate hypothermia | 4.7 | 3.4 | |
| Mild hypothermia | 67.7 | 67.7 | |
| Normothermia | 27.3 | 28.6 | |
| 0.0013 | |||
| Furosemide use, % yes | 4.5 | 5.4 | 0.014 |
| Sodium bicarbonate total dose, mg, median [IQR] | 50 [40-50] | 50 [40-50] | 0.16 |
| Vasopressin, % yes | 9.4 | 9.2 | 0.75 |
| Nadir hematocrit | |||
| <19 | 15.8 | 3.0 | |
| 19-21 | 32.2 | 10.3 | |
| 22-24 | 28.3 | 18.0 | |
| 25-27 | 15.3 | 25.6 | |
| 28+ | 8.4 | 43.2 | |
| <0.001 |
Initial post-operative Hct was 28.0% in women and 32.0% in men, while median post-operative creatinine was 0.9 for women and 1.1 for men (both p < 0.001), Table 3. Significantly more women exhibited stage 2 or 3 AKI than men (6.0% versus 4.3%, p < 0.001). Women had higher rates for prolonged ventilation, post-operative renal replacement therapy, low cardiac output, RBC transfusion, pneumonia, sepsis, and hospital mortality. While men were more likely to have new onset atrial fibrillation, women had longer stays in both the intensive care unit and hospital and were at a higher risk for readmission.
Table 3.
Post-operative outcomes.
| Variable | Female | Male | p-value |
|---|---|---|---|
| Number of procedures | 4,226 | 13,137 | |
| Hematocrit, first in ICU, %, median [IQR] | 28.0 [25.0-31.0] | 32.0 [28.0-35.0] | <0.001 |
| Postoperative creatinine, mg/dL, median [IQR] | 0.9 [0.8-1.3] | 1.1 [0.9-1.4] | <0.001 |
| Outcome, % yes | |||
| Death, in hospital | 3.4 | 1.6 | <0.001 |
| Reoperation for bleeding | 1.4 | 1.7 | 0.17 |
| Prolonged ventilation | 11.1 | 7.1 | <0.001 |
| Need for ultrafiltration | 0.5 | 0.3 | 0.006 |
| Need for dialysis | 1.8 | 1.3 | 0.02 |
| Acute kidney injury, stage 2/3 | 6.0 | 4.3 | <0.001 |
| Low cardiac outputa | 6.3 | 4.6 | <0.001 |
| RBC usage | |||
| None | 44.5 | 75.7 | |
| Intra-operative only | 17.7 | 3.6 | |
| Post-operative only | 21.8 | 16.2 | |
| Intra-operative and post-operative | 16.0 | 4.5 | |
| <0.001 | |||
| Intra-operative RBC units (if transfused), median [IQR] | 2 [1-2] | 1 [0-2] | <0.001 |
| Post-operative RBC units (if transfused), median [IQR] | 2 [1-2] | 2 [1-3] | 0.76 |
| Pneumonia | 3.0 | 2.4 | 0.02 |
| New onset atrial fibrillation | 23.1 | 27.1 | <0.001 |
| Sepsis | 1.3 | 0.8 | 0.01 |
| Intensive care unit, days | |||
| <1 | 22.6 | 28.2 | |
| 1-2 | 23.1 | 25.9 | |
| 2-3 | 19.4 | 20.0 | |
| 3-4 | 12.6 | 10.2 | |
| 4+ | 22.3 | 15.8 | |
| <0.001 | |||
| Postoperative hospital days, median [IQR] | 6 [5-8] | 6 [5-7] | <0.001 |
| Readmission, % | 5.7 | 3.6 | <0.001 |
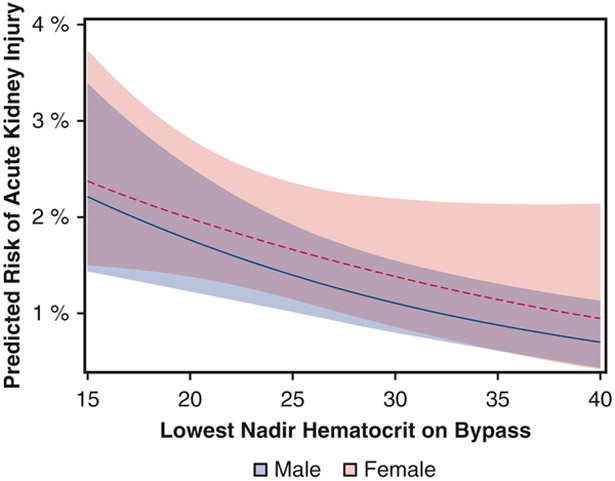
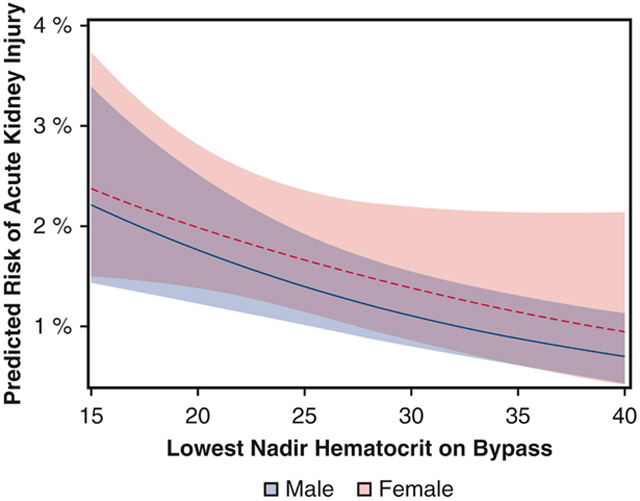
After adjustment, sex did not modify the relationship between nadir Hct and the risk of AKI (p = 0.67, full multivariable table in eTable 2). Lower nadir Hct was inversely associated with AKI (adjusted OR per 1-unit increase in nadir Hct 0.96, 95% confidence interval [CI]: 0.93 to 0.98), and this adjusted effect was similar across women (adjusted OR 0.96, 95% CI: 0.94 to 99) and men (adjusted OR 0.96, 95% CI: 0.93 to 0.98) [Figure 1]. In sensitivity analyses, the interaction between nadir Hct and sex remained nonsignificant both for patients who received intra-operative RBC transfusion (p = 0.88) and for those who did not (p = 0.32).
eTable 2.
Multivariable generalized linear mixed effect model output.
| Variable | Estimate | Standard Error |
t Value | p-value |
|---|---|---|---|---|
| Year | ||||
| 2011 | 0.2877 | 0.1945 | 1.48 | 0.1391 |
| 2012 | 0.1769 | 0.146 | 1.21 | 0.2258 |
| 2013 | −0.00635 | 0.1366 | −0.05 | 0.9629 |
| 2014 | −0.00303 | 0.1244 | −0.02 | 0.9806 |
| 2015 | 0.2209 | 0.1136 | 1.94 | 0.0518 |
| 2016 | 0 | . | . | . |
| Age, median years | 0.01823 | 0.004384 | 4.16 | <.0001 |
| Body surface area (m2) | 1.0744 | 0.2017 | 5.33 | <.0001 |
| Diabetes | 0.293 | 0.08165 | 3.59 | 0.0003 |
| Peripheral vascular disease | 0.1552 | 0.09572 | 1.62 | 0.105 |
| Chronic lung disease | 0.4771 | 0.1018 | 4.69 | <.0001 |
| Preoperative hematocrit, median % | −0.04097 | 0.008797 | −4.66 | <.0001 |
| Hypertension | 0.1487 | 0.1533 | 0.97 | 0.3322 |
| Estimated GFR, mL/min/1.73m2 | ||||
| ≥90 | −2.5981 | 0.3843 | −6.76 | <.0001 |
| 60-90 | −2.9167 | 0.3806 | −7.66 | <.0001 |
| 30-60 | −2.5684 | 0.3816 | −6.73 | <.0001 |
| 15-30 | −1.1354 | 0.4031 | −2.82 | 0.0049 |
| <15 | 0 | |||
| Last white blood cell count x 109/L, median | ||||
| <4.5 | −0.2796 | 0.2253 | −1.24 | 0.2146 |
| ≥4.5 and ≤10 | −0.1732 | 0.09652 | −1.79 | 0.0728 |
| >10 | 0 | . | . | . |
| Congestive heart failure | 0.278 | 0.1507 | 1.84 | 0.0652 |
| NYHA class III-IV | 0.09068 | 0.165 | 0.55 | 0.5826 |
| Left ventricle ejection fraction, % | ||||
| <40 | 0.3685 | 0.1192 | 3.09 | 0.002 |
| 40-49 | 0.2886 | 0.1172 | 2.46 | 0.0138 |
| 50-59 | 0.07976 | 0.1003 | 0.8 | 0.4264 |
| ≥60 | 0 | . | . | . |
| Acuity | ||||
| Elective | −0.4597 | 0.2011 | −2.29 | 0.0223 |
| Urgent | −0.4052 | 0.1881 | −2.15 | 0.0312 |
| Emergent/salvage | 0 | . | . | . |
| 3+ diseased vessels | 0.03741 | 0.1004 | 0.37 | 0.7096 |
| Perfusion time, minutes, median | 0.009287 | 0.001678 | 5.53 | <.0001 |
| Cross-clamp time, minutes, median | −0.00627 | 0.002316 | −2.71 | 0.0068 |
| Static volume per body surface area, mL/m2, median | 0.0002 | 0.000498 | 0.4 | 0.6878 |
| Intraaortic balloon pump | 0.4528 | 0.1199 | 3.78 | 0.0002 |
| Total fluid administration, mL, median | −0.00006 | 0.000037 | −1.64 | 0.1015 |
| Acute normovolemic hemodilution, % yes | −0.1429 | 0.1573 | −0.91 | 0.3637 |
| Total cardioplegia volume, mL, median | 0.000033 | 0.000022 | 1.53 | 0.1257 |
| Lowest temperature on bypass, °C | ||||
| Severe hypothermia (<28) | 0.7353 | 0.4883 | 1.51 | 0.1321 |
| Moderate hypothermia (≥28 and <32) | 0.06801 | 0.2085 | 0.33 | 0.7443 |
| Mild hypothermia (≥32 and <35) | −0.04016 | 0.09781 | −0.41 | 0.6814 |
| Normothermia (≥35) | 0 | . | . | . |
| Furosemide use | 0.5675 | 0.1623 | 3.5 | 0.0005 |
| Net prime volume by body surface are, mL/m2, median | 0.000285 | 0.000244 | 1.17 | 0.2427 |
| Nadir hematocrit | −0.03727 | 0.02163 | −1.72 | 0.0848 |
| Male sex | 0.06987 | 0.5031 | 0.14 | 0.8896 |
| Nadir hematocrit and sex interaction term | −0.00944 | 0.02217 | −0.43 | 0.6704 |
| Nadir hematocrit given female | −0.0327 | 0.967829 | ||
| Nadir hematocrit given male | −0.04671 | 0.954364 | ||
| Model intercept | −2.2147 | 0.9288 | −2.38 | 0.0221 |
°C = degrees Celsius; GFR = glomerular filtration rate; L = liters; m2 = meters squared; mL = milliliter; NYHA = New York Heart Association
Figure 1.
Relationship between nadir hematocrit during cardiopulmonary bypass and risk of stage 2 or 3 acute kidney injury (AKI). The predicted probability of AKI is plotted for male patients (solid line) and female patients (dashed line), along with 95% confidence intervals (shaded areas). The model adjusted for pre- and intra-operative factors as well as hospital variation.
Comment
We used our multi-institutional quality improvement collaborative to investigate whether sex modified the relationship between nadir Hct on CPB and AKI among isolated CABG patients. Relative to men, women were more likely to be exposed to a lower Hct, receive RBC transfusions, and develop post-operative AKI. Women were also at higher risk for nearly every other post-operative complication as compared to men, except for new onset atrial fibrillation. As expected, nadir Hct was inversely associated with AKI. However, in contrast to the prior series evaluating CABG and/or valve operations [9], sex did not modify the relationship between nadir Hct and AKI for patients undergoing isolated CABG – a finding independent of intra-operative RBC transfusions.
We acknowledge several limitations to our present study. First, while we adjusted for commonly reported risk factors, we cannot rule out the effect of unmeasured confounding. Second, we acknowledge that a number of potential mechanisms may contribute to the development of AKI (e.g. hypotension, pre-operative volume status, nephrotoxins); nonetheless, we evaluated the role of a number of factors likely contributing to hemodilution (e.g., administration of priming fluid, cardioplegia, total fluid administration). Third, while other studies have evaluated AKI incidence and staging using urine output, we report previously established creatinine-based criteria. Fourth, due to the large size of our dataset, we recognize that some statistically significant differences in sex across many characteristics (e.g., prime volume per BSA, retrograde autologous priming volume) are small in absolute magnitude and thus unlikely to be clinically relevant.
Multiple single-center studies have demonstrated increased risk of AKI associated with a lower intra-operative Hct, including cohorts consisting of cardiac surgery utilizing CPB [8,12] as well as those limited to isolated CABG [13-14]. One multicenter study of isolated CABG patients not only found nadir Hct to be independently associated with post-operative renal failure and low cardiac output syndrome, but also found female sex to be associated with increased mortality on multivariable analysis [15]. Few prior studies have assessed the effect of sex on the well-established relationship between nadir Hct and AKI. The studies exploring the relationship between nadir Hct and AKI have divided patients into two of three cohorts and established various threshold Hcts which conferred harm, e.g. concluding those with Hct < 21% or > 25% have increased risk of dialysis [12], hemodilution to Hct < 24% associated with increased likelihood of renal injury [14], and Hct > 21% deemed safe for renal function [16] in three separate analyses. In contrast to these studies, we evaluate Hct as a continuous variable across the entire range of nadir values, while allows assessment of AKI risk across all Hct and not as a binary variable.
One large single-center study by Mehta et al. [8] examined the effect of sex on the relationship between nadir Hct and AKI among 13,734 cardiac surgical patients. The authors found that nadir Hct was significantly associated with AKI in both men and women, albeit women had a lower nadir Hct during CPB. The authors further concluded that exposure to Hct ≤ 22% during CPB was associated with a nonsignificant increased rate of AKI among men, albeit this relationship did not persist when stratified by transfusion status. Ellis used the PERForm registry to assess the relationship between nadir Hct and AKI among patients undergoing a variety of cardiac surgical procedures [9]. The authors concluded that lower nadir Hct was associated with an increased risk of AKI among both men and women. Additionally, sex modified the relationship between nadir Hct and AKI – the odds of AKI was significantly lower in women than men at a given nadir Hct. In contrast to Mehta et al., this relationship persisted after adjusting for RBC transfusion. Nonetheless, the majority of men in the Ellis series underwent isolated CABG (57.1% versus 41.3%), while far fewer men underwent isolated valve procedures (22.6% versus 38.1%). The fundamental pathophysiology of coronary disease and valvular dysfunction vastly differ, which may confound the apparent relationship between nadir Hct during CPB and AKI for both men and women. The present analysis furthers our understanding of the relationship between nadir Hct and AKI by assessing whether the demonstrated interaction by sex persists among patients presenting for treatment of their coronary disease. Notably, we conclude that the aforementioned interaction by sex was not significant, and that the inverse relationship between nadir Hct and AKI persisted across men and women, and independent of RBC transfusions.
A number of reasons might help explain why our present findings differ from those reported by Ellis. The difference in outcome in the effect of sex on the relationship between nadir Hct and AKI may be due to the difference in surgical procedures, involved centers, and time period. Specifically, Ellis et al. included 15,221 patients across 26 centers who underwent a variety of surgical procedures (i.e., CABG, valve, or CABG/valve) between 2010 and 2014, while the present analysis examined 17,363 isolated CABG patients across 41 centers between 2011 and 2016. Additionally, the effect demonstrated in the Ellis et al. analysis did not persist among those patients who did not receive intra-operative RBC transfusion (p = 0.16). The effect of sex in our analysis was nonsignificant for both patients who received intra-operative RBC transfusion (p = 0.88) and those who did not (p = 0.32).
While one may expect women to reach a lower nadir Hct intra-operatively since they began with lower Hct pre-operatively, our data showing increased risk of AKI with lower nadir Hct across sexes should prompt enhanced fluid restrictive management strategies for women, as they did not appear to have sex-related renal protective mechanisms during CABG and may enter the operation at increased risk for post-operative AKI. In contrast, the administered net prime volume adjusted for BSA was higher for women than men (407 versus 363 mLd/m2, p < 0.001). Additionally, only 9% of women underwent acute normovolemic hemodilution, despite approximately 80% of women presenting with a hematocrit greater than 33%. Our data show that teams were less willing to attempt blood conservation techniques and more likely to transfuse RBCs in women as compared to men. The pre-operative risk factors carried by women were compounded by intra-operative management strategies and resulted in a higher rate of AKI in women (6.0% versus 4.3%, p < 0.001).
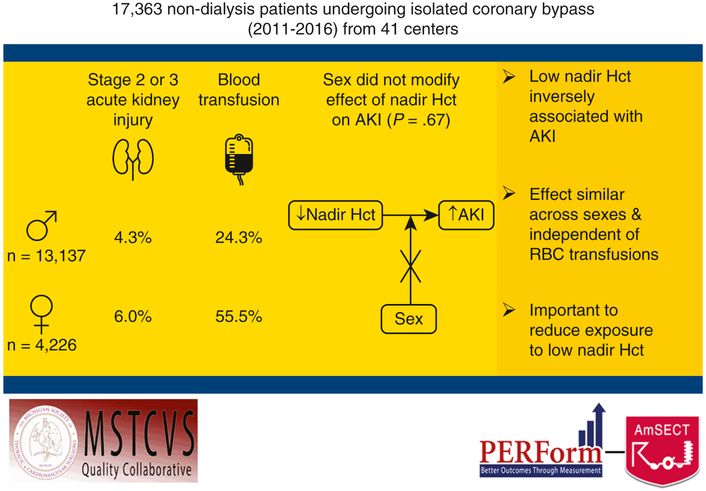
This multicenter observational study reinforces the potential harmful associated sequelae attributed to low nadir Hct during CPB. We found that women were administered more net prime volume per BSA intra-operatively, reached lower nadir Hct, and had higher rates of stage 2 and 3 AKI post-operatively, in addition to being at higher risk for nearly every post-operative complication. In conclusion, we report that in an isolated CABG population, no effect of sex was found on the established inverse relationship between nadir Hct and AKI – a finding that persisted after adjustment for RBC transfusions (Figure 2). These data indicate that the same deleterious effects of low Hct persist across sexes and reinforce the importance of reducing exposure to low nadir Hct on CPB.
Figure 2.
Visual abstract showing rates of stage 2 or 3 acute kidney injury and blood transfusions by sex, interaction of sex on the relationship between nadir Hct and acute kidney injury, and study conclusions (right). AKI, acute kidney injury; Hct, hematocrit; RBC, red blood cell.
Perspective Statement.
While sex does not modify the relationship between nadir hematocrit and acute kidney injury (AKI) in an isolated CABG population, nadir hematocrit continues to confer an increased risk of AKI. These findings persisted after adjusting for baseline risk and red blood cell transfusions and reinforce the importance of reducing exposure to low nadir hematocrit on cardiopulmonary bypass.
Acknowledgments
Conflict of Interest Statement and Sources of Funding:
Dr. Brescia is supported by the National Research Service Award postdoctoral fellowship (No. 5T32HL076123). Dr. Likosky is supported in part by the Agency for Healthcare Research and Quality (AHRQ) under grants R01HS02600301A1 and R01HS022535. The opinions expressed in this document are those of the authors and do not reflect the official position of the AHRQ or the U.S. Department of Health and Human Services.
Support for the Michigan Society of Thoracic and Cardiovascular Surgeons (MSTCVS) Quality Collaborative is provided by the Blue Cross and Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program.
Although BCBSM and MSTVS-QC work collaboratively, the opinions, beliefs and viewpoints expressed by the author do not necessarily reflect the opinions, beliefs and viewpoints of BCBSM or any of its employees.
Dr. Patel is a consultant for W.L. Gore, Medtronic, and Terumo.
Glossary
- AKI
acute kidney injury
- AmSECT
American Society of ExtraCorporeal Technology
- BSA
body surface area
- CABG
coronary artery bypass grafting
- CHF
congestive heart failure
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CPB
cardiopulmonary bypass
- EGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- Hct
hematocrit
- IABP
intra-aortic balloon pump
- ICU
intensive care unit
- LVEF
left ventricular ejection fraction
- MSTCVS-QC
Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative
- NYHA
New York Heart Association
- OR
odds ratio
- PERForm
Perfusion Measures and Outcomes
- RBC
red blood cell
- STS
Society of Thoracic Surgeons
- WBC
white blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006; 1(1):19–32. [DOI] [PubMed] [Google Scholar]
- 2.Ryden L, Ahnve S, Bell M, Hammar N, Ivert Tm Sartipy U, et al. Acute kidney injury after coronary artery bypass grafting and long-term risk of myocardial infarction and death. Int J. Cardiol. 2014. March 1;172(1): 190–5. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher S, Jones DA, Lovell MJ, Hassan S, Wragg A, Kapur A, et al. The impact of acute kidney injury on midterm outcomes after coronary artery bypass graft surgery: a matched propensity score analysis. J Thorac Cardiovasc Surg. 2014. March;147(3):989–95. [DOI] [PubMed] [Google Scholar]
- 4.Dardashti A, Ederoth P, Algotsson L, Bronden B, Bjursten H. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg. 2014. February;147(2):800–7. [DOI] [PubMed] [Google Scholar]
- 5.Brown JR, Hisey WM, Marshall EJ, Likosky DS, Nichols EL, Everett AD, et al. Acute Kidney Injury Severity and Long-Term Readmission and Mortality After Cardiac Surgery. Ann Thorac Surg 2016. November;102(5):1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation. 2014. December 2;130(23):2005–11. [DOI] [PubMed] [Google Scholar]
- 7.Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth 2012;109(Suppl 1):29–38. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RH, Castelvecchio S, Ballotta A, Frigiola A, Bossone E, Ranucci M. Association of gender and lowest hematocrit on cardiopulmonary bypass with acute kidney injury and operative mortality in patients undergoing cardiac surgery. Ann Thorac Surg 2013;96:133–40. [DOI] [PubMed] [Google Scholar]
- 9.Ellis MC, Paugh TA, Dickinson TA, Fuller J, Chores J, Paone G, et al. Nadir Hematocrit on Bypass and Rates of Acute Kidney Injury: Does Sex Matter? Ann Thorac Surg 2015;100:1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paugh TA, Dickinson TA, Theurer PF, Bell GF, Shann KG, Baker RA, et al. Validation of a perfusion registry: methodological approach and initial findings. J Extra Corpor Technol 2012;44:104–15. [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11 :R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karkouti K, Beattie WS, Wijeysundera DN, Rao V, Chan C, Dattilo KM, et al. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg 2005;129:391–400. [DOI] [PubMed] [Google Scholar]
- 13.Swaminathan M, Phillips-Bute BG, Conlon PJ, Smith PK, Newman MF, Stafford-Smith M. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg 2003;76:784–92. [DOI] [PubMed] [Google Scholar]
- 14.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Engoren M, Durham SJ, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med 2005;33:1749–56. [DOI] [PubMed] [Google Scholar]
- 15.Ranucci M, Biagioli B, Scolletta S, Grillone G, Cazzaniga A, Cattabriga I, et al. Lowest Hematocrit on Cardiopulmonary Bypass Impairs the Outcome in Coronary Surgery. Tex Heart Inst J 2006;33(3): 300–305. [PMC free article] [PubMed] [Google Scholar]
- 16.Ariturk C, Ozgen ZS, Kilercik M, Ulugol H, Okten EM, Aksu U, et al. Comparative effects of hemodilutional anemia and transfusion during cardiopulmonary bypass on acute kidney injury: a prospective randomized study. Heart Surg Forum. 2015. August 30;18(4):E154–60. [DOI] [PubMed] [Google Scholar]