Abstract
Purpose
Current investigational priorities in the treatment of favorable histology Wilms tumor (FHWT) center on accurate staging and risk-stratification. The extent of lymph node (LN) sampling has not been clearly defined; its importance cannot be overstated as it guides adjuvant therapy. The identification of a minimum LN yield to minimize the risk of harboring occult metastatic disease could help development of surgical guidelines. This study focuses on using the beta-binomial distribution to estimate the risk of occult metastatic disease in patients with FHWT.
Materials & methods
The National Cancer Database was queried for patients with unilateral FHWT from 2004 to 2013. Data were used to characterize nodal positivity for patients who underwent surgery and had ≥1 positive LN and ≥2 LNs examined. The probability of missing a positive LN (i.e., false negative) for a given LN yield was calculated using an empirical estimation and the beta-binomial model. Patients were then stratified by tumor size.
Results
422 patients met study criteria. To limit the chance of missing a positive LN to ≤10%, the empirical estimation and beta-binomialmodel estimated that 6 and 10 LNs needed to be sampled, respectively. Tumor size did not influence the result. Internal validation showed little variation to maintain a false negative rate ≤ 10%.
Conclusions
Using mathematical modeling, it appears that the desired LN yield in FHWT to reduce the risk of false-negative LN sampling to ≤10% is between 6 and 10. The current analysis represents an objective attempt to determine the desired surgical approach to LN sampling to accurately stage patients with FHWT.
Level of evidence
II
Keywords: Wilms tumor, Nephroblastoma, Lymph node, Survival
As survival for patients with Favorable histology (FH) Wilms tumor (WT) has improved dramatically [1], current investigational priorities center on risk-stratification [2]. This involves selection of which patients can avoid treatment-intensification without sacrificing excellent outcomes [3]. Conversely, stratification should accurately select patients who require more intense regimens. The importance of lymph node (LN) sampling in stratifying WT cannot be overstated, as it affects staging and thus adjuvant therapy. However, concerns exist about the accuracy of LN sampling as currently practiced.
This issue is not unique to WT. Previous investigation into patients with thyroid cancer suggests that a limited LN examination is a risk for harboring occult metastatic disease [4]. Using statistical modeling and National Cancer Database (NCDB) data, specific LN yield thresholds were calculated to quantify the probability of missing nodal disease. Similarly, this approach with modeling NCDB data has been used to assess LN thresholds for colon cancer [5]. Given a desire to more accurately risk-stratify patients with FHWT and the background of similar studies for thyroid and colon cancers [4], the objective of the current study is to quantify the risk of missing nodal disease based on LN yields, thus identifying a threshold LN yield that can minimize the false-negative rate of LN sampling. Specifically, how many LNs must be sampled to reduce the chance that a positive LN is missed to below 10%, assuming that a positive LN exists. The potential for a specific nodal yield threshold to change staging, followed by receipt of stage-guided adjuvant therapy and thus potentially outcomes, is the critical importance of this study.
1. Materials & methods
The NCDB was reviewed to identify the study population. All data obtained from the NCDB are deidentified and IRB exemption was obtained. This study was modeled after that of Robinson et al. [4], which focused on the adequacy of LN sampling in patients with thyroid cancer, also utilizing NCDB data.
1.1. Study population
Data on patients with WT between 2004 and 13 (n = 3669) were obtained. Patient records with unknown follow-up, unknown treatment, no LN sampling or unknown LN yield, bilateral disease and unfavourable histology were excluded (n = 1544). Additionally, patients without involved LNs (n = 1369), those with ≪2 LNs sampled (n = 57), or both (n = 277) were excluded. This resulted in a population of 422 patients surgically managed for unilateral FHWT who had ≥2 LNs sampled, had ≥1 LN involved and had detailed count information available about LN sampling. LN yield was defined as the number of LNs surgically obtained and evaluated by pathology.
Specifically, to determine the probability that a positive LN was missed during LN sampling, the analysis was limited to patients with LN involvement. For patients without LN involvement, it was not possible to determine whether there was truly no nodal involvement or whether occult disease may have existed but was missed owing to limited LN sampling. This is the key reasoning behind restricting the analysis to those with positive LNs.
1.2. Data analysis
Statistical analyses were performed using R (v.3.4.1) statistical software package. For the purposes of this study, it was decided a priori that a probability of missing a positive LN of ≤10%, or a false-negative LN sampling rate of ≤10%, was acceptable [4,5]. While the primary focus was to determine the LN yield to keep the probability of missing a positive LN to ≤10%, other false-negative rates (≤5% and ≤ 15%)were examined. Importantly, the false negative ratewas determined by identifying the rate at which 90% of true positive patients would be correctly identified, with the remaining 10% of true positives being missed (false negative).
A binomial distribution could be used to model the number of positive LN for a given LN yield. However, the LN positivity rate is fixed in the binomial model and assumes the same LN positivity rate for all patients, which is unlikely owing to intrinsic heterogeneity of the patient population. Instead of using a fixed LN positivity rate, the beta-binomial distribution could be used instead as this model allows the LN positivity rate to vary across patients (Vector Generalized Linear and Additive Models package in R). The beta-binomial model was ultimately chosen because it allows the LN positivity rate to vary across patients, which is a more realistic assumption. Additionally, this approach has been used in recent studies [4,5] similar to this one.
Empirical calculations were also included based on the binomial model to serve as a sensitivity analysis for the beta-binomial model. For the empirical approach, the rate of LN positivity was averaged based on the entire cohort (i.e. total number of positive LNs divided by the total LN yield). The binomial model was then used to determine the false-negative rate for a given LN yield. Given that the empirical approach uses a fixed rate that is applied to the entire population, it must be noted that the variance will be underestimated and thus results will be biased toward underestimating the yield needed for a given false-negative rate.
Internal validation was conducted on the beta-binomial model where subjects were randomly resampled with replacement using bootstrap resampling 1000 times to assess the variability in the LN yield needed to maintain a false negative rate of ≤10%. The mean and estimated 95% CI (using the Interquartile Range (IQR)) were obtained from the bootstrap samples. In addition, patients were stratified by tumor size to determine if size changed the probability of missing a positiveLN.
2. Results
422 patients met inclusion criteria (Table 1). Tumor size information was missing for some and therefore the sample size was slightly reduced (n=398) when evaluating the effect of size on LN yield threshold. All patients included had positive LNs, thus local stage III, so each one should have received chemotherapy and radiation therapy per protocol. However, as presented in Table 1, it is clear that not all patients were treated per protocol (7.6% without chemotherapy, 10.4% without radiation).
Table 1.
OverView of the NCDB dataset used to assess lymph nodes for patients with FHWT.a
| All Patients (N = 422) | ||
|---|---|---|
| Patient Age, years (median [IQR]) | 3 [2–5] | |
| Sex [N (%)] | ||
| Male | 188 (44.5) | |
| Female | 234 (55.5) | |
| Race [N (%)] | ||
| White | 326 (79.3) | |
| Black | 64 (15.6) | |
| Other | 21 (5.1) | |
| Insurance [N (%)] | ||
| Private | 246 (60.6) | |
| Government | 146 (36.0) | |
| Uninsured | 14 (3.4) | |
| Laterality [N (%)] | ||
| Right | 178 (42.2) | |
| Left | 244 (57.8) | |
| LN Yield (mode) | 2 | |
| LN Yield (median [IQR]) | 6 [3–11] | |
| Number of Positive LNs (median [IQR]) | 2 [1–3] | |
| LN Density (median [IQR]) | 0.33 [0.17–0.53] | |
| Tumor Size, cm (median [IQR]) | 11.55 [9–14] | |
| Overall Tumor Stage [N (%)] | ||
| 3 | 66 (48.5) | |
| 4 | 70 (51.5) | |
| Any Chemotherapy Use [N (%)] | ||
| No | 32 (7.6) | |
| Yes | 390 (92.4) | |
| Any Radiation Use [N (%)] | ||
| No | 44 (10.4) | |
| Yes | 378 (89.6) |
Median LN Yield was 3 (range 0–87, IQR 5) for the NCDB dataset of all patients with FHWT meeting the same study criteria but including LN negative patients [11].
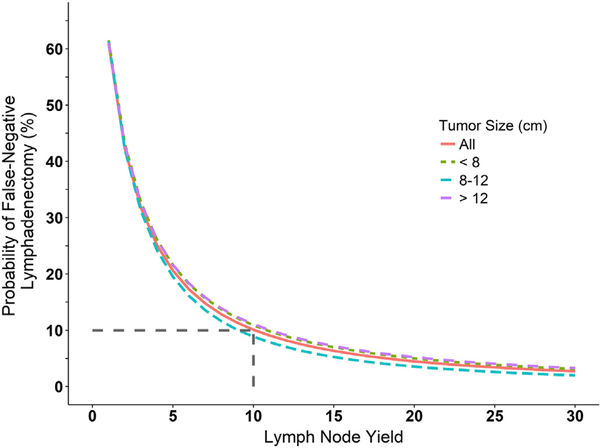
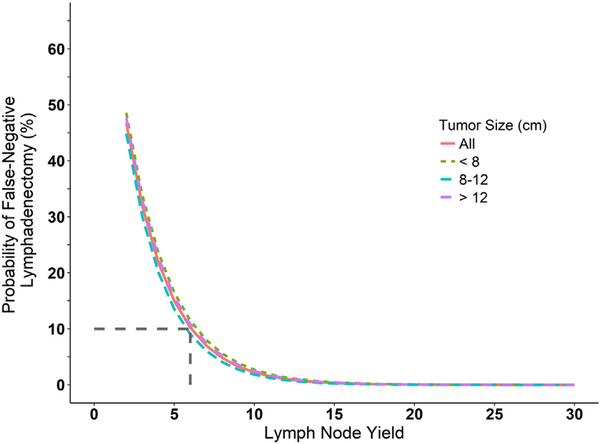
Two model parameters were estimated from the data for the beta-binomial model, μ = 0.39 (IQR 0.36–0.42) and ρ = 0.22 (IQR 0.18–0.26). The interpretation for the estimated mean (μ) of 0.39 is that, on average, for each patient a positive LN was found 39% of the time. This was found to be 33% using the empirical approach. The interpretation of ρ is the correlation between the LNs sampled within an individual. Table 2 summarizes the false negative probability as a function of LN yield for both models. For the beta-binomial model, an LN yield of 10 LNs lowered the probability of missing a positive LN to 10% and for the empirical estimation, this LN yield threshold was 6 LNs. Comparing the most common LN yield of 2 (mode, Table 1) vs. an LN yield of 10, using the beta-binomial model, the rate of false negative sampling is 42% and 10%, respectively (Table 2). This suggests that if the most common LN yield of 2 was increased to 10 it will be possible to reduce the number of incorrectly staged patients by 30%. Comparing the median LN yield of 6 vs. an LN yield of 10, using the beta-binomial model, the rate of false negative sampling is 17% and 10%, respectively (Table 2). This suggests that if the median LN yield of 6 was increased to 10, approximately 7% of patients would have a change in stage. This is presented graphically for the overall population and stratified by tumor size (Fig. 1 and Fig. 2). Stratification by tumor size did not appear to affect the false-negative rate. Furthermore, internal validation (bootstrapping) of the beta-binomial model demonstrated little variation in the LN yield needed to maintain a false negative rate ≤ 10%, with an interquartile range (IQR) of 9–12 LNs.
Table 2.
The beta-binomial and empirical probability of a false-negative LN sampling as a function of LN yield (overall and then stratified by tumor size). Note: The total number of patients (422) is slightly larger than the combined number of patients in the three size categories (398) owing to missing tumor size information.
| False negative probability (%) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Beta-binomial model | Empirical estimation | |||||||
| LN Yield | All Patients (n = 422) | Size ≪8 cm (n = 72) | Size 8–12 cm (n = 159) | Size ≫12 cm (n = 167) | All Patients (n = 422) | Size ≪8 cm (n = 72) | Size 8–12 cm (n = 159) | Size ≫12 cm (n = 167) |
| 2 | 43 | 43 | 42 | 43 | 47 | 49 | 45 | 48 |
| 3 | 32 | 33 | 31 | 33 | 32 | 34 | 30 | 33 |
| 4 | 25 | 26 | 24 | 26 | 22 | 24 | 20 | 23 |
| 5 | 21 | 22 | 20 | 22 | 15 | 16 | 13 | 16 |
| 6 | 17 | 18 | 16 | 18 | 10 | 11 | 9 | 11 |
| 7 | 15 | 16 | 14 | 16 | 7 | 8 | 6 | 7 |
| 8 | 13 | 14 | 12 | 14 | 5 | 6 | 4 | 5 |
| 9 | 11 | 12 | 10 | 12 | 3 | 4 | 3 | 4 |
| 10 | 10 | 11 | 9 | 11 | 2 | 3 | 2 | 2 |
Fig. 1.
The beta-binomial model probability of a false-negative LN sampling as a function of LN yield. Results for the entire patient population (red line) as well as stratification by tumor size are shown. Dashed gray lines indicate the minimum LN yield needed to reach a false-negative rate ≤10%.
Fig. 2.
The empirical probability of a false-negative LN sampling as a function of LN yield. Results for the entire patient population (red line) as well as stratification by tumor size are shown. Dashed gray lines indicate the minimum LN yield needed to reach a false-negative rate ≤10%.
To reduce the probability of missing a positive LN below 5 and 15%, LN yields of 18 and 7 (beta-binomial model) and 8 and 5 (empirical estimation) were identified.
3. Discussion
Lack of LN sampling represents the most frequent surgical protocol deviation inWT [6], and has been observed in numerous studies to impact survival, likely through understaging and inadequate administration of stage-directed adjuvant therapy [7,8]. Factors that result in local stage III designation (LN involvement, tumor spillage, preoperative biopsy, and local residual disease) have all been independently associated with worse survival and thus, such patients are managed with additional adjuvant therapies (radiation and doxorubicin) [2]. While excellent survival can still be achieved, there are increased toxicities associatedwith intensified therapy [9].Not all stage III patients experience the same outcome. LN positivity is associatedwith lower event free survival than the other factors [2,10]. This emphasizes the importance of adequate LN sampling to accurately characterize LN involvement. This study provides the first estimate of potential stage change based on LN yield, specifically that 30% of patients may be identified as having occult LN involvement when comparing an LN yield of 2 (most common LN yield in this cohort; estimated 42% false-negative rate) to 10 (estimated 10% false-negative rate). The clinical implications of this potential understaging are unknown, but this risk may now be quantified and perhaps can be studied in the future as it relates to relapse in stage I/II patients. If these results are extended to patients excluded from the analysis (n= 1703) because the LN sampling was omitted or had≪2 LNs sampled, 86% had an LN yield≪10, and thus a large proportion of patients had a high potential of being inadequately staged.
It is well-reported that LN yield is generally higher in patients with LN positive disease and unfavorable histology, possibly owing to surgeon bias during the nephrectomy [8,11]. In contrast, prior work also demonstrates that surgeons are not reliably able to predict whether LNs are grossly involved [8]. Surgical factors affecting LN yield are important to highlight, as these are modifiable. To determine what drives LN yield, human decision-making should be minimized, and this likely requires a minimal LN yield goal. It could be argued that removing as many LNs as possible is a strategy to accurately determine LN involvement. However, Kieran et al. [8] observed that LN yield in stage I and II WT did not predict event free survival. So while simply increasing the LN yield ad infinitum does not appear to confer any benefit, data do indicate that the ratio of positive LNs to the LN yield may predict survival [12]. The clinical importance of LN sampling lies in its ability to accurately reflect LN involvement, and perhaps could be used to measure surgical standardization.
Based on visual inspection of the histograms of their data alone, Kieran et al. [8] reported that LN positivity increases proportionally with LN yield and that an LN yield ≥7 would be optimal. The methodology used in this prior work was essentially an empirical estimation using a universal LN positivity rate, which was verified by the present study. The current study provides a more robust estimate for an LN yield cutoff since it allows the LN positivity rate to vary across patients, reducing bias when compared to the empirical approach. There are several important differences between the study of Kieran et al. [8] and the one reported herein that warrant discussion. Their study [8] had three primary outcomes; one was to determine if LN yield predicted event-free survival in patients with unilateral, nonmetastatic WT using data from NWTS-4/5. A difference was not observed, but the information gathered was extrapolated to determine a minimal LN yield threshold to ensure “accurate staging”, which was not explicitly defined as it was not a primary study objective. The rate of LN positivity was calculated for various LN yields, with LN positivity reaching a plateau around 28% when 7 LNs are sampled. The correlating false negative rate of 7 LNs was 15% in the present study. This method of identifying a threshold is based on visual inspection of a histogram and is thus very descriptive in nature. The current study’s modeling approach provides a more accurate and objective estimate of the LN yield as it relates to the false negative rate. Additional differences were that the prior study included stage I and II patients, who, by definition do not have LNs involved, and the previously demonstrated relationship of LN yield and LN positivity was not accounted for [8,11]. In the current study, NCDB data were used and analysis was restricted to those who had involved LNs. This method of establishing an LN threshold provides a more objective assessment and accounts for the interaction of LN yield and positivity. The beta-binomial model provides a means to identify the number of LNs that must be sampled to keep the risk of occult nodal disease below a threshold.
In an attempt to verify the findings of the beta-binomial model, empirical calculations were also performed. Empirical probability tends to underestimate the LN yield threshold because it does not account for the correlation between the existence of a positive node and the number of nodes that were sampled for an individual. As expected, the threshold using empirical calculations (≥6 LNs) was slightly less than with that of the beta-binomial model (≥10 LNs). However, the lack of external validation is a study limitation. At the very least, the internal validation from bootstrapping suggests that the LN yield thresholds identified are stable as they did not change significantly across 1000 bootstrap samples.
When considering the implication of utilizing such an LN threshold, it is important to balance potential benefit versus harm in applying this threshold. For those with occult LN disease, it potentially reduces the chance of missing the involved LN and the associated stage change. As for harm, there is theoretically an increased risk of chylous ascites. Fortunately, this is an uncommon complication as reported by 2 publications. An NWTS-4 review identified a single case of chylous ascites in 534 randomly sampled patients (0.2%). This is lower than rates of splenic or diaphragmatic or pancreatic injury [12]. There is also a single institution report of 3 cases of chylous ascites in 80 resections for WT (3.75%). When combining these cases with those reported from NWTS-3/4, there were 9 cases, including 1 where LNs were not sampled [13]. Overall, the authors feel that the low incidence of chylous ascites in this population is worth the benefit of increasing the goal LN yield to reduce mis-staging.
Reports from a wide-variety of solid tumors indicate that advanced metrics in assessing LN positivity can serve as useful prognostic markers [11,14–23]. LN density, number of involved LNs/LN yield, specifically has been demonstrated to predict disease recurrence and survival in multiple malignancies [4,15–22], and most relevant to the current study, in FHWT [11]. However, a major limitation of the interpretation and applicability of LN density for WT patients is a lack of standardized guidelines for what constitutes LN sampling. Thus, LN yield, the denominator, can vary widely, making any conclusions involving LN yield limited. Using the findings from the present series, the identification of an LN yield threshold could be used to standardize LN sampling. The extent of LN sampling, as well as the location of LN sampling, must be better defined to further study and understand how this specific element of WT treatment impacts outcomes. This is a major limitation of the NCDB as the extent or location of LNs sampled cannot be determined. However, given the restrictions of funding and general availability of data from NWTS, COG and SIOP cohorts, the NCDB is the next best (and largest) available dataset with patients to study. Even data from NWTS and COG do not record extent or location of LN sampling, so these data may serve as a call to again reemphasize the importance of standardizing LN sampling in all cases. Also, it should encourage prospective study of this issue, likely through surgical templates and/or protocols, to validate the findings of this study.
The present study comes with several other limitations. Data from an administrative dataset come with inherent limitations, such as missing values and reporting and selection bias. It was not designed for this study and allows only secondary analysis. For this study specifically, it is understood that LN yield depends on many factors which may not be able to be accounted for in the NCDB, or any database for that matter. For example, the experience from colorectal cancer has demonstrated that patient, surgeon, and pathologist-specific factors impact nodal yield [24–26]. Additionally, there are data to support that specimen designation (en-bloc resection with the kidney vs. separate specimen) can influence the LN yield [27]. It is possible that surgeons contributing to this database may report higher LN yields owing to his/her performance of more extended LN sampling. Also, in this specific disease, the AJCC staging (captured by NCDB) is slightly different than the COG staging system used clinically and the NCDB does not include timing of chemotherapy or radiation therapy administration (neoadjuvant vs. adjuvant). Additionally, as pointed out by previous studies, several assumptions were made to determine the false-negative rate using the beta-binomial model [4,5]. First is the assumption that there were no false-positives. Second, all LNs sampled have the same probability of being involved. Third, the sensitivity is the same between true positives and false negatives. These assumptions allowed us to generalize the results to all FHWT patients, including node negative individuals. Since the analysis was limited to only individuals that were node positive, our results may be biased toward a more conservative estimate, which may be justified since assessing slightly more LNs than needed likely offers minimal increased patient risk, while the chance of missing metastatic disease may have more patient risk. Lastly, to generalize these results, it is assumed that the patients studied here are representative of the general population with FHWT.
4. Conclusion
This is an objective attempt to determine the desired LN yield to accurately stage patients with FHWT, and it is suggested from these data that by standardizing LN sampling patterns and emphasizing the clinical importance of appropriate LN sampling, the risk of a false-negative LN sampling can be reduced in order to more accurately risk-stratify these patients. These data may be used to standardize future surgical guidelines.
Acknowledgments
Funding: Etkin Family Fund of the Aspen Community Foundation and Colorado Clinical and Translational Sciences Institute Research Grant (NIH/NCATS Colorado CTSA Grant Number KL2 TR001080) (NGC).
Footnotes
Conflict of Interest: none.
Potential Reviewers:Todd Heaton, MDRodrigo Romao, MDAndrew Davidoff, MDElisabeth Tracey, MDKathleen Kieran, MD.
Ethical Approval: IRB exemption was obtained.
References
- [1].Cotton CA, Peterson S, Norkool PA, et al. Early and late mortality after diagnosis of Wilms tumor. J Clin Oncol 2009;27:1304–9. 10.1200/JCO.2008.18.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ehrlich PF, Anderson JR, Ritchey ML, et al. Clinicopathologic findings predictive of relapse in children with stage III favorable-histology Wilms tumor. J Clin Oncol 2013; 31:1196–201. 10.1200/JCO.2011.41.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fernandez CV, Perlman EJ, Mullen EA, et al. Clinical outcome and biological predictors of relapse after nephrectomy only for very low-risk Wilms tumor: a report from Childrenʼs Oncology Group AREN0532. Ann Surg 2016:1 10.1097/SLA.0000000000001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Robinson TJ, Thomas S, Dinan MA, et al. How many lymph nodes are enough? Assessing the adequacy of lymph node yield for papillary thyroid cancer. J Clin Oncol 2016;34:3434–9. 10.1200/JCO.2016.67.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gönen M, Schrag D, Weiser MR. Nodal staging score: a tool to assess adequate staging of node-negative colon cancer. J Clin Oncol 2009;27:6166–71. 10.1200/JCO.2009.23.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ehrlich PF, Ritchey ML, Hamilton TE, et al. Quality assessment for Wilms’ tumor: a report from the National Wilms’ Tumor Study-5. J Pediatr Surg 2005;40:208–13. 10.1016/j.jpedsurg.2004.09.044. [DOI] [PubMed] [Google Scholar]
- [7].Honeyman JN, Rich BS, McEvoy MP, et al. Factors associated with relapse and survival in Wilms tumor: a multivariate analysis. J Pediatr Surg 2012;47:1228–33. [DOI] [PubMed] [Google Scholar]
- [8].Kieran K, Anderson JR, Dome JS, et al. Lymph node involvement in Wilms tumor: results from National Wilms Tumor Studies 4 and 5. J Pediatr Surg 2012;47:700–6. 10.1016/j.jpedsurg.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Green DM. The treatment of stages I–IV favorable histology Wilms’ tumor. J Clin Oncol 2004;22:1366–72. 10.1200/JCO.2004.08.008. [DOI] [PubMed] [Google Scholar]
- [10].Graf N, Furtwängler R. Preoperative chemotherapy and local stage III in nephroblastoma. Transl Pediatr 2014;3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saltzman AF, Carrasco A, Amini A, et al. Patterns of lymph node sampling and the impact of lymph node density in favorable histology Wilms tumor: an analysis of the national cancer database. J Pediatr Urol 2017. 10.1016/j.jpurol.2017.09.025. [DOI] [PubMed] [Google Scholar]
- [12].Ritchey ML, Shamberger RC, Haase G, et al. Surgical complications after primary nephrectomy for Wilms’ tumor: report from the National Wilms’ Tumor Study Group. J Am Coll Surg 2001;192:63–8. [DOI] [PubMed] [Google Scholar]
- [13].Weiser AC, Lindgren BW, Ritchey ML, et al. Chylous ascites following surgical treatment for Wilms tumor. J Urol 2003;170:1667–9. 10.1097/01.ju.0000085655.48806.87. [DOI] [PubMed] [Google Scholar]
- [14].Stein J, Cai J, Groshen S, et al. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: concept of lymph node density. J Urol 2003;170:35–41. [DOI] [PubMed] [Google Scholar]
- [15].Gil Z, Carlson DL, Boyle JO, et al. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer 2009;115:5700–10. 10.1002/cncr.24631. [DOI] [PubMed] [Google Scholar]
- [16].Kassouf W, Svatek RS, Shariat SF, et al. Critical analysis and validation of lymph node density as prognostic variable in urothelial carcinoma of bladder. Urol Oncol Semin Orig Investig 2013;31:480–6. 10.1016/j.urolonc.2011.02.011. [DOI] [PubMed] [Google Scholar]
- [17].Kim RY, Ward BB, Brockhoff HC 2nd, et al. Correlation of lymph node density with negative outcome predictors in oral and maxillofacial squamous cell carcinoma. J Oral Maxillofac Surg 2016;74(10):2081–4. 10.1016/j.joms.2016.03.023. [DOI] [PubMed] [Google Scholar]
- [18].Ku JH, Kang M, Kim HS, et al. Lymph node density as a prognostic variable in node-positive bladder cancer: a meta-analysis. BMC Cancer 2015;15 10.1186/s12885-015-1448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li Z-S, Yao K, Chen P, et al. Development of a new classification method for penile squamous cell carcinoma based on lymph node density and standard pathological risk factors: the ND staging system. J Cancer 2016;7:262–7. 10.7150/jca.13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ooki A, Yamashita K, Kobayashi N, et al. Lymph node metastasis density and growth pattern as independent prognostic factors in advanced esophageal squamous cell carcinoma. World J Surg 2007;31:2184–91. 10.1007/s00268-007-9198-9. [DOI] [PubMed] [Google Scholar]
- [21].Passoni N, Abdollah F, Suardi N, et al. Head-to-head comparison of lymph node density and number of positive lymph nodes in stratifying the outcome of patients with lymph node-positive prostate cancer submitted to radical prostatectomy and extended lymph node dissection. Urol Oncol Semin Orig Investig 2014;32:29e21–28. [DOI] [PubMed] [Google Scholar]
- [22].Patel SG, Amit M, Yen TC, et al. Lymph node density in oral cavity cancer: results of the international consortium for outcomes research. Br J Cancer 2013;109:2087–95. 10.1038/bjc.2013.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tarin T, Power N, Ehdaie B, et al. Lymph node-positive bladder cancer treated with radical cystectomy and lymphadenectomy: effect of the level of node positivity. Eur Urol 2012;61:1025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morris EJA, Maughan NJ, Forman D, et al. Identifying stage III colorectal cancer patients: the influence of the patient, surgeon, and pathologist. J Clin Oncol 2007;25: 2573–9. 10.1200/JCO.2007.11.0445. [DOI] [PubMed] [Google Scholar]
- [25].Marijnen CA, van Krieken JH, Mekenkamp LJ. Lymph node retrieval in rectal cancer is dependent on many factors—the role of the tumor, the patient, the surgeon, the radiotherapist, and the pathologist; 2009. [DOI] [PubMed] [Google Scholar]
- [26].Storli K, Lindboe CF, Kristoffersen C, et al. Lymph node harvest in colon cancer specimens depends on tumour factors, patients and doctors, but foremost on specimen handling: lymph node harvest and pathology quality control in colon cancer. APMIS 2011;119:127–34. 10.1111/j.1600-0463.2010.02702.x. [DOI] [PubMed] [Google Scholar]
- [27].Stewart CL, Bruny JL. Maximizing lymph node retrieval during surgical resection of Wilms tumor. Eur J Pediatr Surg 2015;25:109–12. [DOI] [PubMed] [Google Scholar]