Abstract
PURPOSE
Patients with relapsed or refractory primary mediastinal large B-cell lymphoma (rrPMBCL) have a poor prognosis, and their treatment represents an urgent and unmet need. Because PMBCL is associated with genetic aberrations at 9p24 and overexpression of programmed cell death-1 (PD-1) ligands (PD-L1), it is hypothesized to be susceptible to PD-1 blockade.
METHODS
In the phase IB KEYNOTE-013 (ClinicalTrials.gov identifier: NCT01953692) and phase II KEYNOTE-170 (ClinicalTrials.gov identifier: NCT02576990) studies, adults with rrPMBCL received pembrolizumab for up to 2 years or until disease progression or unacceptable toxicity. The primary end points were safety and objective response rate in KEYNOTE-013 and objective response rate in KEYNOTE-170. Secondary end points included duration of response, progression-free survival, overall survival, and safety. Exploratory end points included association between biomarkers and pembrolizumab activity.
RESULTS
The objective response rate was 48% (7 complete responses; 33%) among 21 patients in KEYNOTE-013 and 45% (7 complete responses; 13%) among 53 patients in KEYNOTE-170. After a median follow-up time of 29.1 months in KEYNOTE-013 and 12.5 months in KEYNOTE-170, the median duration of response was not reached in either study. No patient with complete response experienced progression, including 2 patients with complete response for at least 1 year off therapy. Treatment-related adverse events occurred in 24% of patients in KEYNOTE-013 and 23% of patients in KEYNOTE-170. There were no treatment-related deaths. Among 42 evaluable patients, the magnitude of the 9p24 gene abnormality was associated with PD-L1 expression, which was itself significantly associated with progression-free survival.
CONCLUSION
Pembrolizumab is associated with high response rate, durable activity, and a manageable safety profile in patients with rrPMBCL.
INTRODUCTION
Primary mediastinal large B-cell lymphoma (PMBCL) is a rare aggressive B-cell non-Hodgkin lymphoma of thymic origin.1,2 PMBCL diagnosis is based on clinical characteristics and distinct pathologic features. Although a majority of newly diagnosed patients can be cured with multiagent chemoimmunotherapy with or without consolidative radiation,3-5 the outcome for patients with relapsed or refractory PMBCL (rrPMBCL) is poor, especially for patients who are ineligible for or relapse after second-line autologous stem-cell transplantation as a result of the aggressive and chemotherapy-refractory nature of the disease.6-8 Treatment approaches outside of chemotherapy have had mixed success. Axicabtagene ciloleucel is approved in the United States for treatment of rrPMBCL; however, there are no published reports of its outcome specifically in patients with rrPMBCL, because only 8 patients (7%) with rrPMBCL were enrolled in the pivotal study.9 In addition, although most cases of PMBCL are CD30 positive, the anti-CD30 immunoconjugate brentuximab vedotin does not seem to have significant activity in PMBCL.10
Frequent amplification and translocation events occur at 9p24.1 in PMBCL, resulting in tumor expression of the programmed cell death-1 (PD-1) ligands PD-L1 and PD-L2.11-14 This suggests susceptibility of PMBCL to PD-1 blockade. Therefore, patients with PMBCL were included in an expansion cohort of the phase IB KEYNOTE-013 (ClinicalTrials.gov identifier: NCT01953692) study of pembrolizumab, a humanized IgG4 monoclonal antibody targeting PD-1. The phase II KEYNOTE-170 (ClinicalTrials.gov identifier: NCT02576990) study of pembrolizumab in rrPMBCL was launched to confirm preliminary results from KEYNOTE-013 of an objective response rate of 41% and durable remission in responders15 and to allow biomarker analyses. We report results from all 53 patients in KEYNOTE-170 and extended follow-up of 21 patients in KEYNOTE-013. These data were the basis for accelerated approval of pembrolizumab in patients with rrPMBCL by the US Food and Drug Administration in June 2018.
METHODS
Patients
Eligible patients were age 18 years or older with a diagnosis of PMBCL (according to the WHO classification of neoplasms of the hematopoietic and lymphoid tissues).16 Patients had to have experienced relapse after or be ineligible for (or refused) autologous stem-cell transplantation in KEYNOTE-013. Patients ineligible for autologous stem-cell transplantation had to have relapsed or refractory disease after at least 2 prior lines of therapy in KEYNOTE-170. In addition, patients had to have an Eastern Cooperative Oncology Group performance status of 0 or 1 (on a 5-point scale, with higher scores indicating increasing disability) and adequate organ function. Principal exclusion criteria included active CNS involvement, autoimmune disease with systemic treatment within the past 2 years, history of pneumonitis, or prior checkpoint- or costimulatory-directed immunotherapy. Full eligibility criteria are provided in the Protocol.
Trial Design and Treatment
KEYNOTE-013 is an international, phase IB, open-label, multicohort study of pembrolizumab in patients with hematologic malignancies. KEYNOTE-170 is an international, phase II, open-label, multicenter, multicohort study assessing the efficacy and safety of pembrolizumab in patients with relapsed or refractory PMBCL or Richter syndrome. In KEYNOTE-013, the initial 10 patients received pembrolizumab 10 mg/kg every 2 weeks. Per amendment 4, the remaining 11 patients in KEYNOTE-013 and all patients in KEYNOTE-170 received pembrolizumab 200 mg every 3 weeks beginning on day 1 of each treatment cycle. Pembrolizumab was continued for a maximum of 35 cycles or up to 2 years until documented disease progression by investigator, unacceptable toxicity, or patient withdrawal.
Assessments
Disease response assessments were performed by positron emission tomography and computed tomography at week 12 and then every 8 weeks (first 10 patients) or at weeks 6 and 12 and then every 9 weeks (subsequent 11 patients) in KEYNOTE-013 or at week 12 and then every 12 weeks in KEYNOTE-170. Disease assessments were performed until disease progression by investigator, new anticancer therapy, withdrawal of consent, or death. Responses were assessed by central review (as a prespecified analysis in KEYNOTE-170 and as a post hoc analysis in KEYNOTE-013) using International Working Group 2007 criteria17 and Lugano criteria.18 Survival was assessed every 12 weeks during follow-up. Adverse events were assessed throughout treatment and for 30 days thereafter (90 days for serious events and events of interest) and were graded according to Common Terminology Criteria for Adverse Events, version 4.03.
Biomarker Analyses
Fluorescence in situ hybridization was performed on formalin-fixed tumor sections to assess copy number changes.19 Malignant cells were identified by means of nuclear morphologic features, and the average number of 9p24.1 (target) signals per cell, average number of pericentric (control) probe signals per cell, and ratio of total number of target signals to total number of control probe signals were calculated from 50 nuclei per sample. Nuclei with a target–to–control probe ratio of at least 3:1 were classified as amplified, and those with a probe ratio of > 1:1 but < 3:1 were classified as having a relative 9p24.1 copy gain. Nuclei with a probe ratio of 1:1 and > 2 copies of each probe were classified as polysomic. Nuclei with a 1:1 ratio and 2 copies of each probe were classified as disomic. Immunohistochemical staining was performed using an automated staining system (BOND-III; Leica Biosystems, Wetzlar, Germany), and a PD-L1 antibody (E1L3N; Cell Signaling Technology, Danvers, MA) according to the recommended protocol. Each sample was examined by 2 pathologists, and a consensus modified H-score ranging from 0-300 was generated, where H-score is defined as the product of the percentage of malignant tumor cells with membranous staining for PD-L1 (0-100) and the intensity of the membranous staining, where 0 indicates no staining, 1 indicates weak staining, 2 indicates moderate staining, and 3 indicates strong staining.19,20 Details on categorizing PD-L1 H-score levels are provided in the Appendix (online only).
Trial Oversight
The study was designed by academic advisors and employees of Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc (Kenilworth, NJ; MSD). The protocol was approved by the appropriate institutional review board or ethics committee at participating institutions. All patients provided voluntary written informed consent. All authors attest that the trial was conducted in accordance with the protocol and all amendments and Good Clinical Practice. All authors had full access to the data and were involved in writing, reviewing, or editing drafts of the article and vouch for the accuracy and completeness of data analyses. Assistance in preparation of the article was provided by a medical writer employed by MSD.
End Points
The primary end points were safety and objective response by investigator assessment in KEYNOTE-013 and objective response by central review in KEYNOTE-170. Key secondary end points included duration of response, progression-free survival, and overall survival in both studies, and safety and tolerability in KEYNOTE-170. Exploratory end points included response to pembrolizumab assessed by Lugano 2014 criteria and the relationship between biomarkers and antitumor activity in KEYNOTE-170.18
Statistical Analyses
Safety and efficacy analyses were performed in all patients as treated in both studies. The response rate was assessed by point estimate, with associated 95% CIs calculated using the Clopper-Pearson method. Kaplan-Meier estimates were used to assess duration of response, progression-free survival, and overall survival. Summary statistics were provided for safety analyses in both studies. Details on statistical analysis of association between survival and PD-L1 H-score are provided in the Appendix. In KEYNOTE-013, a sample size of 78 patients with non-Hodgkin lymphoma (including rrPMBCL) was planned. This provided approximately 80% power to detect a 14% difference in response under the null hypothesis of a response rate of 25% with 1-sided α of 5% if the true response rate was 39%. In KEYNOTE-170, 53 patients were enrolled to obtain a planned sample size of 50 patients if 6% of enrolled patients would not be treated. This provided approximately 88% power, at a 1-sided nominal α = .025, to detect an objective response rate of 35% or greater with pembrolizumab compared with a fixed control rate of 15% (based on historical data) using the exact binomial test. The full statistical analysis plan is provided in the protocol.
RESULTS
Patients
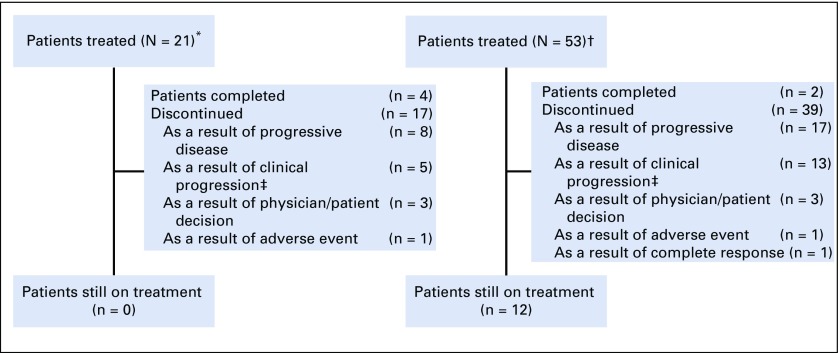
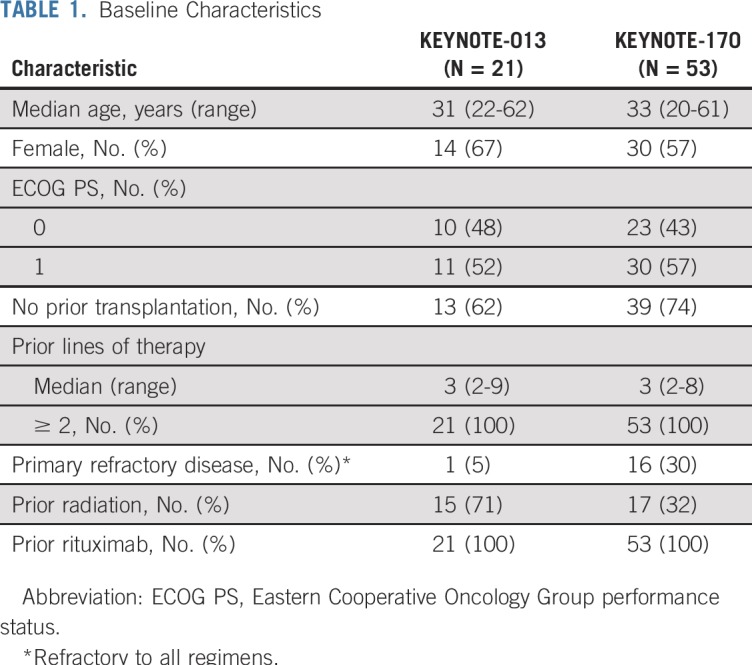
From February 14, 2014, to September 19, 2016, 21 patients with rrPMBCL were enrolled in KEYNOTE-013, and from February 16, 2016, to May 15, 2017, 53 patients with rrPMBCL were enrolled in KEYNOTE-170 (Fig 1). Baseline characteristics were similar between the 2 studies (Table 1). Patients received a median of 3 prior lines of therapy in KEYNOTE-013 (range, 2-9 prior lines) and in KEYNOTE-170 (range, 2-8 prior lines). All patients in both studies received prior rituximab; 15 patients (71%) in KEYNOTE-013 and 17 patients (32%) in KEYNOTE-170 received prior radiation, and 8 patients (38%) in KEYNOTE-013 and 14 patients (26%) in KEYNOTE-170 underwent prior transplantation (Table 1). The median duration of follow-up (defined as the time from random assignment to death or the date of data cutoff for those who were alive) was 29.1 months (range, 0.6-49.6 months) as of April 4, 2018, in KEYNOTE-013 and 12.5 months (range, 0.1-25.6 months) as of April 13, 2018, in KEYNOTE-170. As of April 4, 2018, 17 patients (81%) had discontinued therapy, and 4 patients (19%) had completed at least 2 years of treatment in KEYNOTE-013. As of April 13, 2018, 39 patients (74%) had discontinued treatment, 2 patients (4%) had completed 2 years of treatment, and 12 patients (23%) remained on treatment in KEYNOTE-170. At data cutoff, no patient in KEYNOTE-013 and 12 patients in KEYNOTE-170 remained on treatment (Fig 1).
FIG 1.
Study flow. (*) KEYNOTE-013. (†) KEYNOTE-170. (‡) Clinical progression refers to patients who, based on clinical signs and symptoms or pathologic findings, had malignant neoplasm progression but did not have progression according to the protocol-specified imaging response criteria (International Working Group 2007) or for whom imaging was not able to be performed for assessment.
TABLE 1.
Baseline Characteristics
Efficacy
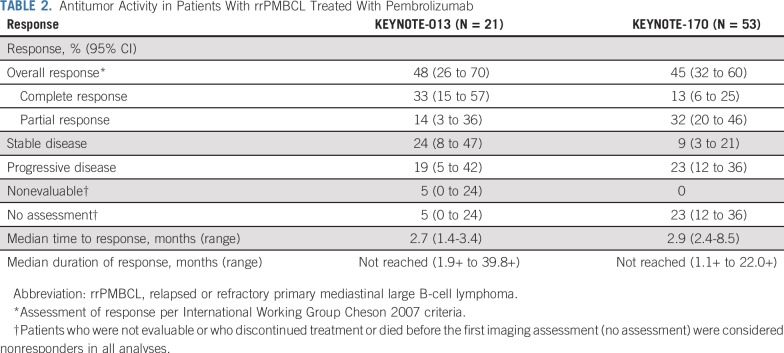
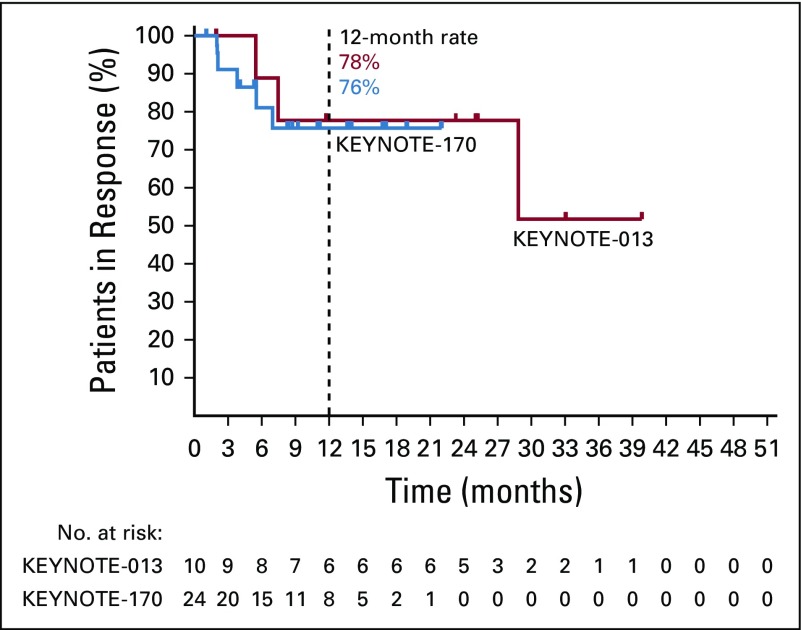
The objective response rate by central review per International Working Group 2007 criteria was 48% (95% CI, 26% to 70%) in KEYNOTE-013 and 45% (95% CI, 32% to 60%) in KEYNOTE-170. Overall, 7 patients (33%) in KEYNOTE-013 and 7 patients (13%) in KEYNOTE-170 achieved a complete response; 11 patients (21%) had complete response by Lugano criteria in KEYNOTE-170. The median time to response was 2.7 months (range, 1.4-3.4 months) in KEYNOTE-013 and 2.9 months (range, 2.4-8.5 months) in KEYNOTE-170. The median duration of response was not reached in KEYNOTE-013 (range, 1.9+ to 39.8+ months), with 78% of patients having response durations of ≥ 12 months (Appendix Fig A1, online only), or in KEYNOTE-170 (range, 1.1+ to 22.0+ months; Table 2), with 76% of patients having response durations of ≥ 12 months (Appendix Fig A1). Thirteen (72%) of 18 evaluable patients in KEYNOTE-013 (Fig 2A) and 30 (75%) of 40 evaluable patients in KEYNOTE-170 (Fig 2B) had an overall reduction from baseline in target lesion size. Two patients in KEYNOTE-013 (Fig 2C) and 1 patient in KEYNOTE-170 (Fig 2D) converted from partial response to complete response after approximately 1 year. In KEYNOTE-013, 4 patients who completed 2 years of treatment remained in complete response at data cutoff, 2 for > 1 year off treatment (Fig 2C) without any additional therapy. No patient with complete response per central review on either study had experienced progression (Figs 2C and 2D). One of these patients, while on study treatment, had imaging consistent with stable disease per investigator. After demonstration of residual disease by a subsequent biopsy, the patient discontinued study treatment and went on to additional therapy. In addition, the 4 patients with complete response per Lugano 2014 criteria only had not experienced progression either by the data cutoff date. Nine patients from both studies were treated past progression per central review (3 patients on KEYNOTE-013 and 6 patients on KEYNOTE-170) and had at least 1 subsequent imaging assessment. One patient had a partial response, and 8 had continued progressive disease. Nine patients had subsequent stem-cell transplantation, 4 with autografts and 5 with allografts. The median duration of follow-up was 14.4 months for all 9 patients. At data cutoff, all patients with autografts were alive and reported as remaining in complete response. Of patients with allografts, 3 were alive and in remission, 1 was alive with relapsed disease, and 1 died of progressive disease.
TABLE 2.
Antitumor Activity in Patients With rrPMBCL Treated With Pembrolizumab
FIG 2.
Antitumor activity in patients with relapsed or refractory primary mediastinal large B-cell lymphoma (rrPMBCL) treated with pembrolizumab. Percent change from baseline in target lesion size for evaluable patients by central review in (A) KEYNOTE-013 and (B) KEYNOTE-170 and treatment exposure and duration of response in (C) KEYNOTE-013 and (D) KEYNOTE-170. Eighteen patients in KEYNOTE-013 and 40 patients in KEYNOTE-170 were evaluable (had at least 1 postbaseline disease imaging assessment). Patients had stable disease (SD) unless otherwise indicated. CR, complete response; PD, progressive disease; PR, partial response.
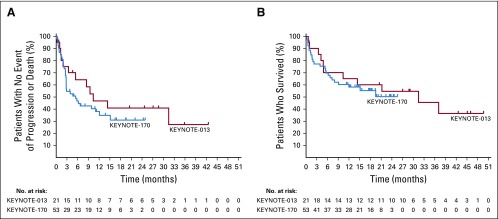
Median progression-free survival was 10.4 months (95% CI, 3.4 months to not reached) in KEYNOTE-013 and 5.5 months (95% CI, 2.8 to 12.1 months) in KEYNOTE-170. Estimated progression-free survival rates at 12 months were 47% and 38% in KEYNOTE-013 and KEYNOTE-170, respectively (Fig 3A). At data cutoff, 11 patients (53%) in KEYNOTE-013 and 24 patients (45%) in KEYNOTE-170 had died. Median overall survival was 31.4 months (95% CI, 4.9 months to not reached) in KEYNOTE-013 and was not reached (95% CI, 7.3 months to not reached) in KEYNOTE-170. The estimated percentages of patients alive at 12 months were 65% and 58% in KEYNOTE-013 and KEYNOTE-170, respectively (Fig 3B). In KEYNOTE-170, among patients who died, 6 died within 30 days and 11 died within 60 days of beginning study treatment. Most had high-risk disease characteristics at study entry (Appendix Table A1, online only).
FIG 3.
Survival in patients with relapsed or refractory primary mediastinal large B-cell lymphoma (rrPMBCL) treated with pembrolizumab. (A) Progression-free survival measured from first dose to first documented disease progression or death from any cause among 21 patients (KEYNOTE-013) and 53 patients (KEYNOTE-170) with rrPMBCL who received pembrolizumab. The estimated rates of patients alive and progression free at 12 months were 47% (KEYNOTE-013) and 38% (KEYNOTE-170), with median progression-free survival times of 10.4 months (95% CI, 3.4 months to not reached) among patients in KEYNOTE-013 and 5.5 months (95% CI, 2.8 to 12.1 months) among patients in KEYNOTE-170. (B) Overall survival measured from first dose to death from any cause. The estimated rates of overall survival at 12 months were 65% (KEYNOTE-013) and 58% (KEYNOTE-170), with median overall survival times of 31.4 months (95% CI, 4.9 months to not reached) among patients in KEYNOTE-013 and not reached (95% CI, 7.3 months to not reached) in KEYNOTE-170.
Safety
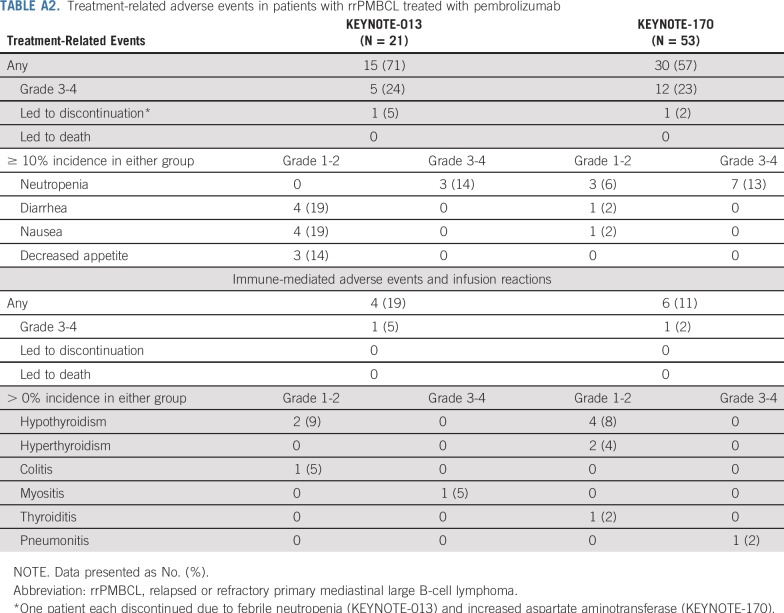
Treatment-related events of any grade occurred in 15 patients (71%) in KEYNOTE-013 and 30 patients (57%) in KEYNOTE-170 (Appendix Table A2, online only). Grade 3 or 4 treatment-related adverse events occurred in 5 patients (24%) and 12 patients (23%) in KEYNOTE-013 and KEYNOTE-170, respectively; the most common adverse event (incidence ≥ 10%) was neutropenia, which occurred in 3 patients (14%) in KEYNOTE-013 and 7 patients (13%) in KEYNOTE-170. One patient each discontinued treatment as a result of treatment-related febrile neutropenia in KEYNOTE-013 and increased AST in KEYNOTE-170 (Appendix Table A2). In KEYNOTE-170, 3 patients (6%) died of adverse events considered not related to treatment by investigator (1 patient each from Aspergillus infection, cardiac tamponade, and myocardial infarction).
Adverse events of interest, based on a list of terms specified by the sponsor and considered regardless of attribution by investigator, occurred in 4 patients (19%) in KEYNOTE-013 and 6 patients (11%) in KEYNOTE-170 (Appendix Table A2). Grade 3 myositis (KEYNOTE-013) and grade 4 pneumonitis (KEYNOTE-170) occurred in 1 patient each. No patient in either study discontinued treatment or died as a result of an adverse event of interest.
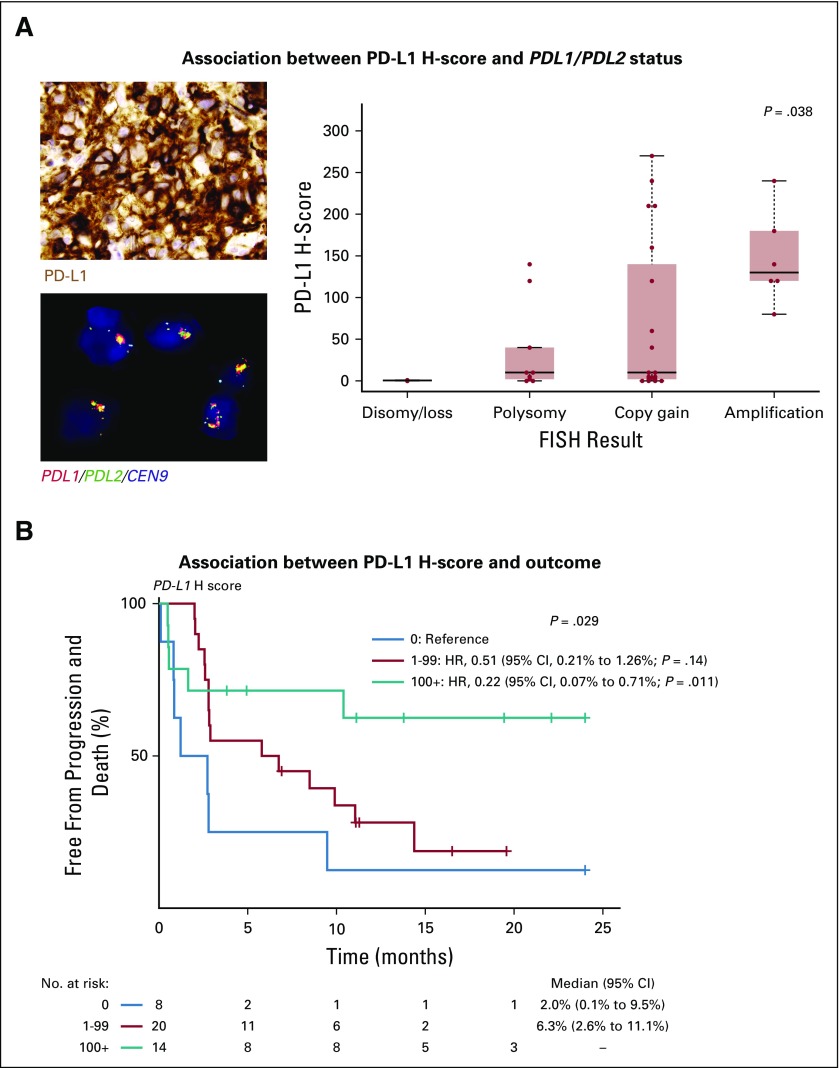
Biomarker
In 42 evaluable samples, PD-L1 expression ranged from an H-score of 0-270 (Fig 4A). In 40 samples evaluable for 9p24.1 status by fluorescence in situ hybridization, 9p24.1 ranged from single copy loss (n = 1; 2%) to disomy (n = 1; 2%), polysomy (n = 9; 22%), copy gain (n = 19; 48%), amplification (n = 6; 15%), or rearrangements (n = 4; 10% [including 3 balanced and 1 unbalanced]) involving PDL1/PDL2. A significant increase in PD-L1 expression was observed with increasing levels of PDL1/PDL2 DNA copy number gain (Fig 4A). The objective response rate was 25% for patients with a PD-L1 H-score of 0, 42% for patients with a PD-L1 H-score of 1-99, and 64% for patients with PD-L1 H-score of ≥ 100 (P = .22). PD-L1 expression level was also significantly associated with progression-free survival (Fig 4B).
FIG 4.
Association between 9p24.1 status, PD-L1 expression, and progression-free survival. (A, left, top) Photomicrograph of a representative sample stained for programmed cell death-1 ligand (PD-L1) protein expression by chromogenic immunohistochemistry (positive staining indicated by brown coloration) and strong PD-L1 expression by the malignant cells (modified H-score, 240). (A, left, bottom) Photomicrograph of the same sample analyzed for PDL1/PDL2 copy number by fluorescence in situ hybridization (FISH; red = PDL1; green = PDL2; yellow = PDL1/PDL2 fused; aqua = pericentromeric chromosome 9) and PDL1/PD2 amplification within nuclei of the malignant cells (classified as amplified). (A, right) Distribution of PD-L1 H-scores across 36 patients (excluding rearrangements) according to PDL1/PDL2 status. P = .038 was determined by the Kruskal-Wallis rank-sum test. (B) Progression-free survival of 42 patients for whom PD-L1 H-score was determined and divided according to those with an H-score of 0 (blue line), H-score of 1-99 (red line), and H-score of ≥ 100 (green line). P = .029 was determined by the log-rank test. Cox regression with hazard ratios (HRs), 95% CIs, and Wald P values are also shown using patients with an H-score of 0 as the reference.
DISCUSSION
Outcomes are poor for patients with PMBCL that has progressed or relapsed after frontline therapy. Conventional chemotherapy is often ineffective in this setting, and the CD30-targeted drug brentuximab vedotin is associated with limited activity.10 In this analysis of 74 patients with rrPMBCL, we establish the clinical benefit and safety of pembrolizumab in this patient population. The safety profile of pembrolizumab was predictable, manageable, and similar to that in other settings. Responses were similar, with almost half of all patients in both studies having an objective response and a third of patients in KEYNOTE-013 achieving a complete response. Although the complete response rate was lower in KEYNOTE-170, it is possible that some responses may deepen with longer follow-up. In addition, responses were durable, with more than three quarters of responding patients remaining in remission for longer than 1 year. Patients with complete response seemed to derive durable benefit from pembrolizumab, with no relapses reported at data cutoff in 17 patients with complete response by International Working Group or Lugano criteria; 2 patients remained in complete response after 2 years of treatment and > 1 year off therapy. In addition, all 4 patients who stopped study treatment with less than a complete response and who went on to autologous stem-cell transplantation remained alive and in complete response, despite all of these patients exhibiting chemotherapy-refractory disease before study enrollment. Although the small numbers allow only a tentative hypothesis, they raise the question of whether PD-1 blockade in this setting might resensitize tumors to chemotherapy, as recently suggested.21-23 If this can be further validated, it could have profound implication for the management of patients with rrPMBCL.
These results validate the hypothesis that PMBCL, with its frequent 9p24.1 aberrations, is dependent on the PD-1 pathway for survival. This is supported by biomarker analyses showing that PD-L1 expression was strongly associated with the magnitude of 9p24 abnormality as determined by fluorescence in situ hybridization and with clinical outcome, as shown with classical Hodgkin lymphoma.20 Additional studies are needed to confirm these biomarker results and to optimize the H-score thresholds.
KEYNOTE-170 also supported the feasibility of dedicated studies in PMBCL, which is important because this disease has a unique biology and may not show the same vulnerabilities as diffuse large B-cell lymphoma, with which it is often grouped in clinical trials. Additional studies will explore the activity of PD-1 blockade in combination with other agents in rrPMBCL and with chemotherapy in frontline treatment. If successful, this latter avenue could increase the cure rate of chemotherapy in PMBCL or allow de-escalation of chemotherapy in some patients. In summary, these data demonstrated that treatment with pembrolizumab is an effective and safe option in patients with rrPMBCL who experience relapse after or are ineligible for autologous stem-cell transplantation.
ACKNOWLEDGMENT
We thank the patients and their families and caregivers for participating in the study, all primary investigators and their site personnel, Luana Atherly-Henderson of Merck Sharp & Dohme (MSD) for medical writing assistance, Patricia Marinello of MSD for clinical study development, and Jonathan Cheng of MSD for critical review.
APPENDIX
Biomarker Analyses
Programmed cell death-1 (PD-L1) H-scores were categorized a priori into 3 groups (prior to analyses of association with outcome): 0, absent expression; 1-99, low expression; and ≥100, high expression, corresponding to ~50% PD-L1–positive cells.
Statistical Analysis
Log-rank tests were used to assess differences in progression-free survival and overall survival for PD-L1 H-score groups. Cox regression was used to estimate hazard ratios, and 95% confidence intervals with Wald P values are reported for model covariates. The association of PD-L1 H-score with FISH 9p24.1 status was assessed using the Kruskal-Wallis rank-sum test, and that of PD-L1 H-score with response using the Fisher’s exact test.
FIG A1.
Kaplan-Meier estimate of response duration by central review in patients with relapsed or refractory primary mediastinal large B-cell lymphoma treated with pembrolizumab.
TABLE A1.
Disease Characteristics of Patients With rrPMBCL Who Died Within 60 Days of Receiving Study Treatment (KEYNOTE-170)

TABLE A2.
Treatment-related adverse events in patients with rrPMBCL treated with pembrolizumab
Footnotes
Presented in part at the 60th Annual Meeting of the American Society of Hematology, San Diego, CA, December 1-4, 2018.
Supported by Merck Sharp & Dohme, a subsidiary of Merck & Co, Kenilworth, NJ, and by the Harold and Virginia Lash Foundation (P.A.) and the Leukemia and Lymphoma Society (P.A.), and the Center for Immumo-Oncology of the Dana-Farber Cancer Institute (S.R.).
AUTHOR CONTRIBUTIONS
Conception and design: Philippe Armand, Scott Rodig, Robert Orlowski, Arun Balakumaran, Margaret Shipp, Pier Luigi Zinzani
Provision of study materials or patients: Philippe Armand, Scott Rodig, Vladimir Melnichenko, Catherine Thieblemont, Gayane Tumyan, Muhit Ozcan, Sergio Portino, Laura Fogliatto, Maria D. Caballero, Jan Walewski, Vincent Ribrag, Beth Christian, Guilherme Fleury Perini, Gilles Salles, Jazmine Zain, Robert Orlowski, Arun Balakumaran, Margaret Shipp, Pier Luigi Zinzani
Collection and assembly of data: Philippe Armand, Scott Rodig, Vladimir Melnichenko, Catherine Thieblemont, Gayane Tumyan, Muhit Ozcan, Sergio Portino, Laura Fogliatto, Maria D. Caballero, Jan Walewski, Vincent Ribrag, Beth Christian, Guilherme Fleury Perini, Gilles Salles, Jazmine Zain, Sanjay Patel, Pei-Hsuan Chen, Jing Ouyang, Robert Orlowski, Arun Balakumaran, Margaret Shipp, Pier Luigi Zinzani
Data analysis and interpretation: Philippe Armand, Scott Rodig, Catherine Thieblemont, Kamal Bouabdallah, Gayane Tumyan, Muhit Ozcan, Sergio Portino, Maria D. Caballero, Zafer Gulbas, Vincent Ribrag, Jasmine Zain, Beth Christian, Gilles Salles, Jakub Svoboda, Sanjay Patel, Azra H. Ligon, Jing Ouyang, Donna Neuberg, Robert Redd, Arkendu Chatterjee, Arun Balakumaran, Robert Orlowski, Margaret Shipp, Pier Luigi Zinzani
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Philippe Armand
Honoraria: Merck, Bristol-Myers Squibb
Consulting or Advisory Role: Bristol-Myers Squibb, Merck Sharp & Dohme, Adaptive Biotechnologies, Affimed Therapeutics
Research Funding: Merck Sharp & Dohme, Bristol-Myers Squibb, Tensha Therapeutics, Roche (Inst), Adaptive (Inst), Affimed Therapeutics (Inst), Genentech (Inst), IGM (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck Sharp & Dohme, Sequenta
Scott Rodig
Honoraria: Perkin Elmer, Bristol-Myers Squibb
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb, Merck, Affimed Therapeutics, Kite Pharma
Patents, Royalties, Other Intellectual Property: Patent pending for use of anti-galectin-1 antibodies for diagnostic use
Travel, Accommodations, Expenses: Roche, Bristol-Myers Squibb
Catherine Thieblemont
Honoraria: Celgene, AbbVie, Bayer, Janssen, Roche, Incyte, Novartis, Gilead Sciences
Research Funding: Roche
Kamal Bouabdallah
Honoraria: Roche, Takeda Science Foundation
Consulting or Advisory Role: Roche, Takeda
Travel, Accommodations, Expenses: Roche, Takeda
Muhit Özcan
Honoraria: Takeda, Amgen, Bristol-Myers Squibb
Research Funding: Janssen, Celgene, Takeda, Bayer, Merck, Archigen Biotech, Roche, Pharmacyclics
Travel, Accommodations, Expenses: Takeda, Sanofi, Roche, Bristol-Myers Squibb, Abdi Ibrahim, Amgen, Janssen
Sergio Portino
Consulting or Advisory Role: Grünenthal Group
Travel, Accommodations, Expenses: Janssen Oncology
Jan Walewski
Honoraria: Roche, Takeda, Celgene, Servier, Janssen-Cilag
Consulting or Advisory Role: Roche, Janssen-Cilag, Celgene, Amgen, AbbVie, Takeda/Millennium, Gilead, Novartis
Research Funding: Roche (Inst), GlaxoSmithKline/Novartis (Inst), Takeda (Inst), Janssen-Cilag (Inst)
Travel, Accommodations, Expenses: Roche
Zafer Gulbas
Honoraria: Roche, AbbVie
Research Funding: Gilead
Travel, Accommodations, Expenses: Abbvie, Abdi Ibrahim
Vincent Ribrag
Honoraria: Infinity Pharmaceuticals, Bristol-Myers Squibb, Eisai, PharmaMar, Gilead Sciences, AZD, Epizyme, Incyte, Infinity Pharmaceuticals, Merck Sharp & Dohme, SERVIER
Consulting or Advisory Role: Infinity Pharmaceuticals, Bristol-Myers Squibb, PharmaMar, Gilead Sciences, NanoString Technologies, Incyte, Bristol-Myers Squibb, Merck Sharp & Dohme, Genentech, Epizyme, Immune Design, Roche, Incyte
Research Funding: arGEN-X BVBA, Epizyme (Inst)
Patents, Royalties, Other Intellectual Property: BAY1000394 studies on MCL
Expert Testimony: SERVIER
Travel, Accommodations, Expenses: Roche, Bristol-Myers Squibb, AZD
Beth Christian
Consulting or Advisory Role: Genentech, Seattle Genetics
Research Funding: Seattle Genetics, Immunomedics, Celgene, Genentech, Merck, Acerta Pharma, Janssen, Triphase, Millennium Pharmaceuticals, Cephalon
Guilherme Fleury Perini
Honoraria: Janssen, Takeda
Speakers' Bureau: AbbVie, Janssen
Gilles Salles
Honoraria: Genentech, Amgen, Janssen, Celgene, SERVIER, Gilead Sciences, Novartis, AbbVie, Merck, Takeda, MorphoSys, Autolus
Consulting or Advisory Role: Genentech, Gilead Sciences, Janssen, Celgene, Novartis, Merck, Pfizer, Acerta Pharma, Kite Pharma, SERVIER, MorphoSys, Epizyme
Jakub Svoboda
Consulting or Advisory Role: Seattle Genetics, Bristol-Myers Squibb, AstraZeneca, Pharmacyclics, Kyowa Hakko Kirin
Research Funding: Celgene (Inst), Seattle Genetics (Inst), Pharmacyclics (Inst), Merck (Inst), Bristol-Myers Squibb (Inst), Incyte (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Jasmine Zain
Honoraria: Seattle Genetics, Celgene, Spectrum Pharmaceuticals
Consulting or Advisory Role: Celgene, Spectrum Pharmaceuticals, Kyocera, Portola
Speakers' Bureau: Celgene, Spectrum Pharmaceuticals, Mallinckrodt, Seattle Genetics
Travel, Accommodations, Expenses: Merck, Mallinckrodt, Seattle Genetics, Spectrum Pharmaceuticals
Azra H. Ligon
Leadership: Travera (I)
Stock and Other Ownership Interests: Travera (I)
Consulting or Advisory Role: Travera (I)
Donna Neuberg
Stock and Other Ownership Interests: Madrigal Pharmaceuticals
Research Funding: Pharmacyclics, Celgene/Jazz
Patents, Royalties, Other Intellectual Property: Targeted sequencing to identify mutations associated with poorer prognosis in patients with myelodysplastic syndromes; targeted sequencing to identify mutations associated with response to hypomethylating agents in myelodysplastic syndromes.
Arun Balakumaran
Employment: Merck, Allogene, Merck (I), Novartis (I)
Stock and Other Ownership Interests: Merck, Allogene, Merck (I), Novartis (I)
Robert Orlowski
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme, Celgene, OncoSec, Nektar, Bluebird Bio
Research Funding: Merck Sharp & Dohme
Margaret Shipp
Honoraria: Bristol-Myers Squibb, AstraZeneca
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb (Inst), Bayer (Inst), Merck (Inst)
Pier Luigi Zinzani
Consulting or Advisory Role: Sanofi, Verastem, Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, SERVIER, Sandoz, Merck Sharp & Dohme, Immune Design, Celgene, Portola Pharmaceuticals, Roche, EUSA Pharma, Kyowa Hakko Kirin
Speakers' Bureau: Verastem, Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, SERVIER, Sandoz, Merck Sharp & Dohme, Immune Design, Celgene, Portola Pharmaceuticals, Roche, EUSA Pharma, Kyowa Hakko Kirin
No other potential conflicts of interest were reported.
REFERENCES
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: Clinical features of the major histologic subtypes—Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 2.Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443–459. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 3.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368:1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rieger M, Osterborg A, Pettengell R, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: Results of the Mabthera International Trial Group study. Ann Oncol. 2011;22:664–670. doi: 10.1093/annonc/mdq418. [DOI] [PubMed] [Google Scholar]
- 5.Zinzani PL, Stefoni V, Finolezzi E, et al. Rituximab combined with MACOP-B or VACOP-B and radiation therapy in primary mediastinal large B-cell lymphoma: A retrospective study. Clin Lymphoma Myeloma. 2009;9:381–385. doi: 10.3816/CLM.2009.n.074. [DOI] [PubMed] [Google Scholar]
- 6.Kuruvilla J, Pintilie M, Tsang R, et al. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma. 2008;49:1329–1336. doi: 10.1080/10428190802108870. [DOI] [PubMed] [Google Scholar]
- 7.Lazzarino M, Orlandi E, Paulli M, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: A multicenter study of 106 patients. J Clin Oncol. 1997;15:1646–1653. doi: 10.1200/JCO.1997.15.4.1646. [DOI] [PubMed] [Google Scholar]
- 8. doi: 10.1016/j.bbmt.2018.06.009. Vardhana S, Hamlin PA, Yang J, et al: Outcomes of relapsed and refractory primary mediastinal (thymic) large B-cell lymphoma treated with second-line therapy and intent to transplant. Biol Blood Marrow Transplant 24:2133-2138, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zinzani PL, Pellegrini C, Chiappella A, et al. Brentuximab vedotin in relapsed primary mediastinal large B-cell lymphoma: Results from a phase 2 clinical trial. Blood. 2017;129:2328–2330. doi: 10.1182/blood-2017-01-764258. [DOI] [PubMed] [Google Scholar]
- 11.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi M, Roemer MG, Chapuy B, et al. Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am J Surg Pathol. 2014;38:1715–1723. doi: 10.1097/PAS.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123:2062–2065. doi: 10.1182/blood-2013-10-535443. [DOI] [PubMed] [Google Scholar]
- 15.Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130:267–270. doi: 10.1182/blood-2016-12-758383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi C, Gilhodes J, Maerevoet M, et al. Efficacy of chemotherapy or chemo-anti-PD-1 combination after failed anti-PD-1 therapy for relapsed and refractory Hodgkin lymphoma: A series from Lysa centers. Am J Hematol. 2018;93:1042–1049. doi: 10.1002/ajh.25154. [DOI] [PubMed] [Google Scholar]
- 22. Carreau NA, Pail O, Armand P, et al: Checkpoint blockade therapy may sensitize Hodgkin lymphoma to subsequent therapy. Blood 132:1626, 2018 (abstr) [Google Scholar]
- 23. Carreau NA, Pail O, Armand P, et al: Checkpoint blockade therapy may sensitize aggressive and indolent non-Hodgkin lymphoma to subsequent therapy. Blood 132:93, 2018 (abstr) [Google Scholar]