Abstract
PURPOSE
The R2Pulm trial was conducted to evaluate the effect of busulfan-melphalan high-dose chemotherapy with autologous stem-cell rescue (BuMel) without whole-lung irradiation (WLI) on event-free survival (main end point) and overall survival, compared with standard chemotherapy with WLI in Ewing sarcoma (ES) presenting with pulmonary and/or pleural metastases.
METHODS
From 2000 to 2015, we enrolled patients younger than 50 years of age with newly diagnosed ES and with only pulmonary or pleural metastases. Patients received chemotherapy with six courses of vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) and one course of vincristine, dactinomycin, and ifosfamide (VAI) before either BuMel or seven courses of VAI and WLI (VAI plus WLI) by randomized assignment. The analysis was conducted as intention to treat. The estimates of the hazard ratio (HR), 95% CI, and P value were corrected for the three previous interim analyses by the inverse normal method.
RESULTS
Of 543 potentially eligible patients, 287 were randomly assigned to VAI plus WLI (n = 143) or BuMel (n = 144). Selected patients requiring radiotherapy to an axial primary site were excluded from randomization to avoid excess organ toxicity from interaction between radiotherapy and busulfan. Median follow-up was 8.1 years. We did not observe any significant difference in survival outcomes between treatment groups. Event-free survival was 50.6% versus 56.6% at 3 years and 43.1% versus 52.9% at 8 years, for VAI plus WLI and BuMel patients, respectively, resulting in an HR of 0.79 (95% CI, 0.56 to 1.10; P = .16). For overall survival, the HR was 1.00 (95% CI, 0.70 to 1.44; P = .99). Four patients died as a result of BuMel-related toxicity, and none died after VAI plus WLI. Significantly more patients in the BuMel arm experienced severe acute toxicities than in the VAI plus WLI arm.
CONCLUSION
In ES with pulmonary or pleural metastases, there is no clear benefit from BuMel compared with conventional VAI plus WLI.
INTRODUCTION
Ewing sarcoma (ES) is a rare, malignant sarcoma of the bone and soft tissues that occurs most frequently in adolescents and young adults. Isolated pulmonary metastases are noted in 25% of patients at diagnosis.1 Treatment has been conducted within clinical trials of cooperative groups since the early 1980s.2 Progress in ES therapy has reached a plateau; long-term overall survival (OS) rates remain at less than 30% for patients with extrapulmonary metastases and greater than 75% for patients without clinically overt metastases at diagnosis.3,4 In patients with pulmonary metastases, the outcome remains suboptimal, with 2- to 10-year event-free survival (EFS) of 30% to 36% within the European Intergroup Cooperative Ewing5,6 and Pediatric Oncology Group studies.7 Small series of nonrandomized retrospective analyses have indicated that whole-lung irradiation (WLI) compared with no WLI seems to improve outcome in patients with pulmonary metastases by approximately 20%, with acceptable acute toxicity in both children and adults.5,8-10
The role of high-dose therapy (HDT) with busulfan-melphalan and autologous stem-cell rescue (BuMel) in nonrandomized studies has shown a possible benefit for a subgroup of patients with ES and extrapulmonary metastases.11,12 Furthermore, in patients with relapsed disease and response to standard second- or third-line treatment, a possible benefit from HDT (BuMel or treosulfan melphalan) in nonrandomized studies has also been noted.13 On the basis of these encouraging results in uncontrolled studies, a randomized comparison of HDT against standard chemotherapy was incorporated in two consecutive multinational controlled trials, the European Ewing Tumour Working Initiative of National Groups, 1999 (Euro-E.W.I.N.G. 99) trial and EWING 2008.
The international Ewing 2008 succeeded the Euro-E.W.I.N.G. 99 study in some participating countries and some additional countries. It targeted novel questions for patients with either localized or metastatic disease and continued the R2Pulm arm for patients with isolated pulmonary metastases. The primary objective in R2Pulm was to evaluate whether consolidation with HDT using BuMel improved EFS compared with consolidation with standard chemotherapy (vincristine, dactinomycin, ifosfamide [VAI]) and WLI.
METHODS
Study Design
The R2Pulm trial was an international, randomized, superiority trial comparing two consolidation regimens in a two-parallel-group design: VAI plus WLI and BuMel in patients with ES with pulmonary or pleural metastases (Data Supplement). The R2Pulm randomized trial was a component of the Euro-E.W.I.N.G. 99 study (ClinicalTrials.gov identifier: NCT00020566), recruiting patients with ES at diagnosis, enrolled by five cooperative groups: Children`s Oncology Group, European Organization for Research and Treatment of Cancer, Gesellschaft für Paediatrische Onkologie und Haematologie, French Society of Pediatric Oncology and French Sarcoma Group, and the Children’s Cancer and Leukaemia Group.3,11,14,15 From May 2010, patients were also recruited in the same R2Pulm randomized trial conducted through the EWING 2008 study (ClinicalTrials.gov identifier: NCT00987636). Study protocols were approved by an independent ethics committee and the appropriate institutional review boards. The studies were conducted in accordance with the ethical principles of the Declaration of Helsinki and with Good Clinical Practice guidelines.
Eligibility Criteria
Eligible patients were younger than 50 years of age, enrolled at diagnosis in either the Euro-E.W.I.N.G. 99 or EWING 2008 studies for a newly diagnosed biopsy-proven ES with pulmonary/pleural metastases in the absence of other distant metastases. Pulmonary/pleural metastases were defined as at least one pulmonary nodule larger than 1 cm or more than one nodule larger than 0.5 cm. Multiple nodules of 0.3 to 0.5 cm were defined as questionable; in these patients, biopsy was recommended. Exclusion criteria were medical contraindications to the planned treatments. After two amendments, because of busulfan-related radiosensitivity,16-18 patients who were expected to receive radiotherapy of more than 30 Gy to the spinal cord (Amendment July 2004) or more than 45 Gy to large intestinal volume were no longer eligible (Amendment November 2008). Written informed consent was obtained from all patients and/or their parents/guardians before enrollment.
Treatment
Induction chemotherapy consisted of six chemotherapy courses combining vincristine, ifosfamide, doxorubicin, and etoposide (VIDE).15,19 After one VAI course, allocated consolidation treatment was either seven courses of VAI followed by WLI or one course of high-dose BuMel chemotherapy with autologous stem-cell transplantation. The treatment schedule and chemotherapy are detailed in the Data Supplement.
Local therapy was tailored to patient and tumor characteristics, and included complete surgical removal wherever feasible, radiotherapy, or a combination of both (Data Supplement). Autologous hematopoietic stem-cell harvesting was performed according to local practices, at the earliest after VIDE course 2.
Randomization
Randomization was performed after four to six VIDE courses. It was balanced and stratified according to cooperative group, sex, age (younger than 25 years), and local treatment (resection after chemotherapy alone with or without postoperative radiotherapy v initial surgery v resection after chemotherapy and radiotherapy v radiotherapy only). Centralized randomization software was used in all data centers, ensuring the concealment of the next patient allocation. The Gesellschaft für Paediatrische Onkologie und Haematologie data center used permuted blocks of four. In the European Organization for Research and Treatment of Cancer, Children’s Cancer and Leukaemia Group, and French data centers, randomization was also balanced by the treating center using dynamic allocation of treatment (minimization with a random factor set at 0.8). Blinding to therapy could not be achieved because of the obvious differences between treatments.
End Points and Assessments
The primary end point was EFS defined as the time from randomization to the time of the first event assessed by the investigator, defined as progression, relapse, second malignancy, or death, whatever the cause. Follow-up was planned every 3 months during the first 3 years, every 6 months during years 4 and 5, then yearly, regardless of treatment compliance. Central imaging review of tumor volume and response, and pathologic review were not mandatory. OS from randomization was a secondary efficacy end point, considering all deaths regardless of cause.
Treatment compliance and toxicity were monitored. All chemotherapy doses were recorded, as well as the reasons for dose reduction or treatment delay. Safety and toxicity were secondary end points, and acute toxicity is part of this article. Acute toxicity was assessed after each course, using a list of 22 selected items from the National Cancer Institute Common Terminology Criteria for Adverse Events (version 2.0) and Bearman’s criteria for sinusoidal obstruction syndrome.20 A free text area was available to document other adverse reactions. A modified list was used to evaluate toxicity after radiotherapy, using Radiation Therapy Oncology Group classification for 17 types of toxicity. For each toxicity type, the maximum grade observed over the whole maintenance treatment was computed, including radiotherapy to the primary site. Grade 4 hematologic toxicities and grade 3 or higher for all nonhematologic toxicities were considered severe.
Statistical Considerations
The study was designed to ensure 80% power to detect a 35% reduction in the risk of event in the BuMel arm compared with the VAI plus WLI arm (expected 3-year EFS, 40% v 55%; hazard ratio [HR], 0.65), with a two-sided log-rank test alpha of .05. The initial target sample size was 326 patients (188 events).21 With support from the independent data monitoring committee, recruitment was stopped before reaching this target because of low accrual. This is the final analysis on the basis of the data as of January 2017.
Preplanned efficacy stopping rules were defined using the alpha spending function approach with O'Brien-Fleming boundaries.22-24 These analyses were only disclosed to the independent data monitoring committee. Survival rates (EFS and OS) were estimated using the Kaplan-Meier method with Rothman’s 95% CIs. Median follow-up was estimated using the reverse Kaplan-Meier method. The HR of event (EFS) and the HR of death (OS) were estimated in Cox models. The point estimate of the HR of event, its CI, and the P value were corrected for the three previous interim analyses using the inverse normal method.25 The primary efficacy analysis was performed according to the patients’ randomly assigned treatments (ie, by intention-to-treat population). Post hoc sensitivity analyses were performed (1) adjusted for age (in four categories: < 12, 12 to 17.9, 18 to 24.9, ≥ 25 years), and (2) excluding patients with a major treatment modification (as treated; Data Supplement). The heterogeneity of treatment effect (BuMel v VAI plus WLI) on EFS according to stratification variables and tumor volume, tumor site, and histologic response (post hoc exploratory analysis) was evaluated in multivariable models including interaction terms and illustrated in a forest plot. Because the EFS is a composite end point, a competing risk approach was also used to estimate the effect of treatment on the risk of metastases using subdistribution HRs considered as competing events: local progression/relapse without concomitant metastases, distant metastases, secondary malignancy, and death without prior metastases (post hoc analysis).26,27
Safety analyses were performed on the safety set, excluding patients who did not receive the assigned treatment (as-treated population). For each toxicity category, the relative risk of experiencing a severe toxicity when receiving BuMel versus VAI plus WLI was estimated. No late toxicity was collected as part of this study.
Estimates are provided with 95% CIs. All tests are two sided. The analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC).
RESULTS
Patients
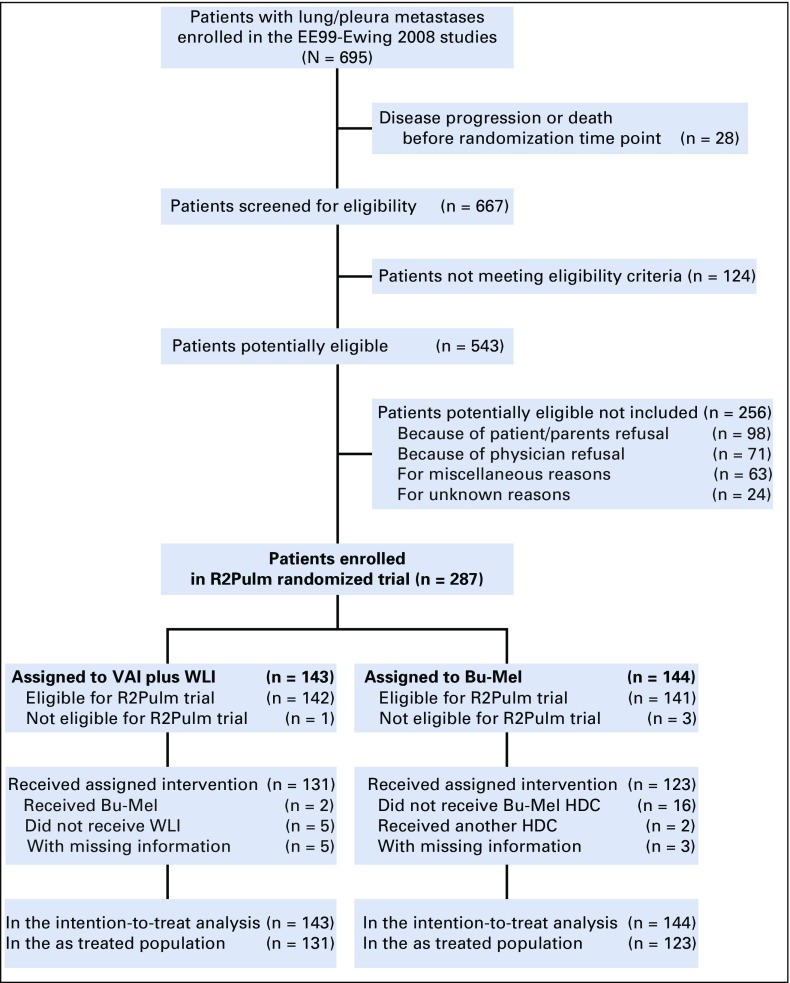
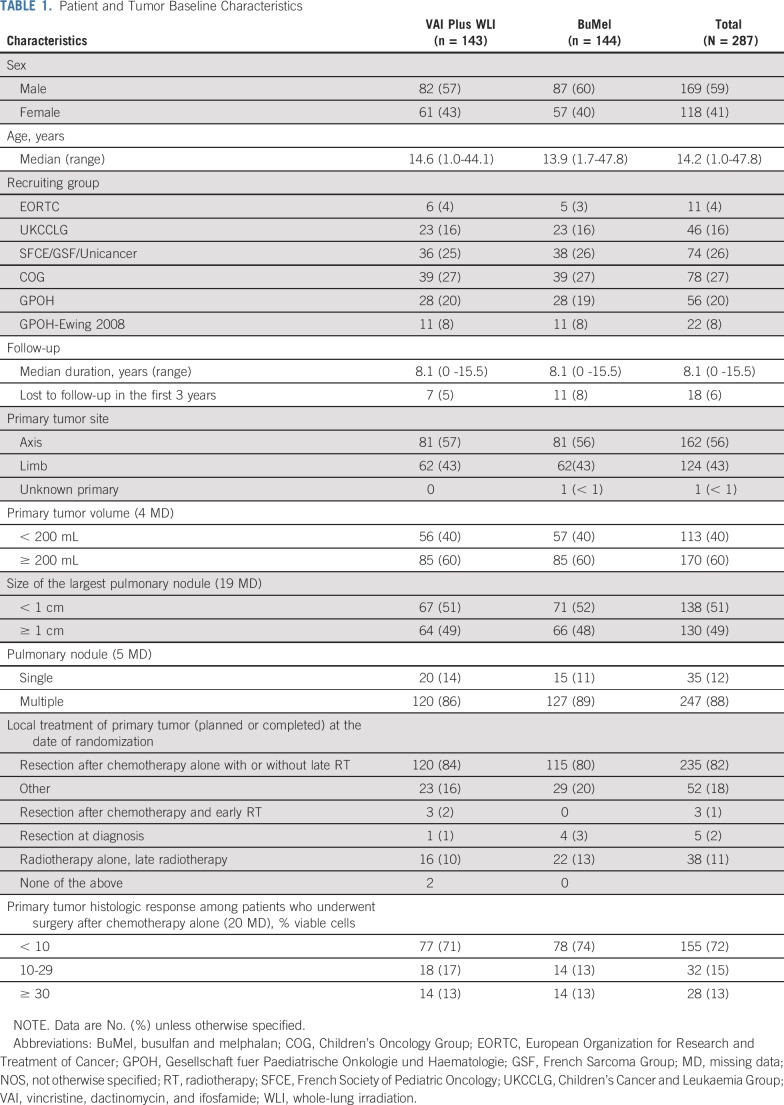
Between February 2000 and December 2015, 667 patients with lung and/or pleural metastases only, enrolled in 144 centers from 14 countries, were assessed for eligibility (Fig 1). Among them, 124 did not meet the eligibility criteria (details listed in the Fig 1 legend). Of 543 potentially eligible patients, 256 were not included in the randomized trial because of patient/parent refusal (n = 98) or physician refusal (n = 71), other reasons (n = 63), and unknown reasons (n = 24). Thus, 287 patients were included in the randomized trial: 143 in the VAI plus WLI arm and 144 in the BuMel arm. The median age was 14.2 years (range, 1.0 to 47.8 years). The baseline characteristics were well balanced between arms (Table 1). Median follow-up was 8.1 years and not significantly different between treatment groups (Data Supplement). Among the 287 enrolled patients, one and three patients, in the VAI plus WLI arm and BuMel arm, respectively, were not eligible (Data Supplement). Of the 143 patients allocated to the VAI plus WLI arm, seven patients did not receive the randomized treatment: two patients received BuMel (one each by patient or physician request), and five did not receive WLI for reasons other than early progression. In the BuMel arm, 16 patients did not receive HDT because of patient refusal (n = 7), medical reason (n = 1), physician decision (n = 3), failure to collect peripheral stem cells (n = 4), and logistic reason (n = 1), and two patients received HDT other than BuMel because of physician decision (Data Supplement). These 25 patients were excluded in the as-treated analyses.
FIG 1.
Trial profile. A total of 124 patients assessed for eligibility for the R2Pulm trial did not meet eligibility criteria because of lung or pleural metastases without metastases at another site; insufficient diagnosis criteria or diagnosis rejected (n = 7); persisting toxicity related to previous treatment and/or contraindication to planned treatment (n = 83), including contraindication to busulfan and melphalan (BuMel) because of planned radiotherapy to an axial site (n = 41); early radiotherapy (n = 18); psychological problems (n = 5); age younger than 4 or older than 50 years (n = 8); and Ewing sarcoma as a second malignancy (n = 3). Sixty-three patients meeting eligibility criteria were not enrolled because of other reasons. EE99, European Ewing Tumour Working Initiative of National Groups, 1999 study; HDC, high dose chemotherapy; WLR, whole-lung irradiation.
TABLE 1.
Patient and Tumor Baseline Characteristics
Efficacy
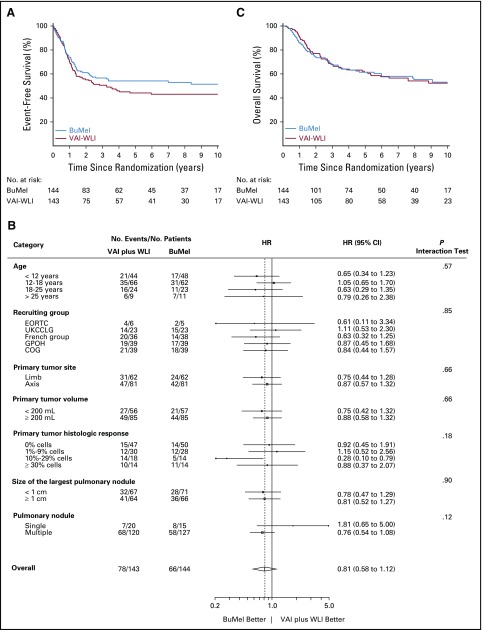
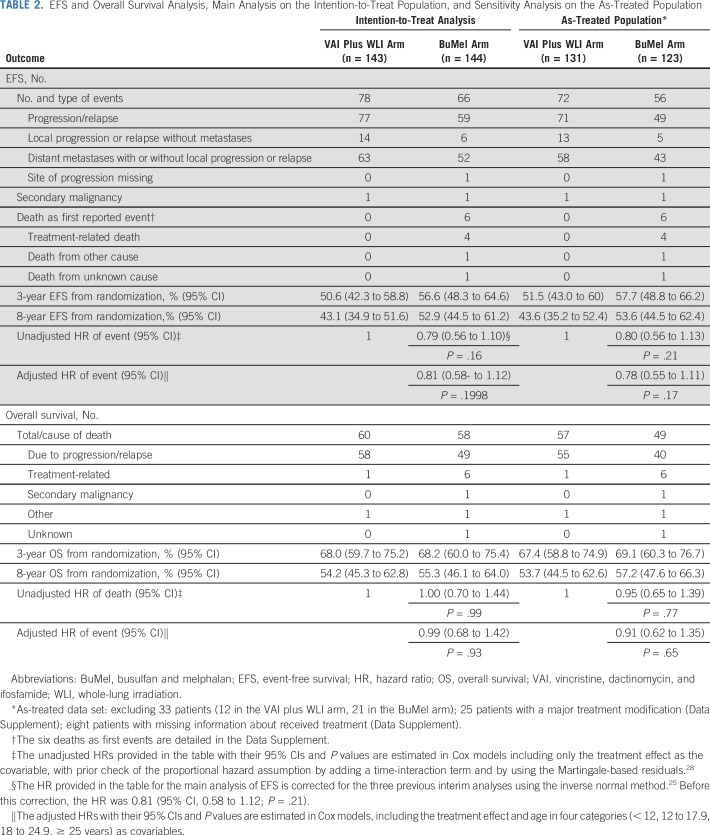
A total of 144 events were reported (78 in the VAI plus WLI arm, 66 in the BuMel arm): 20 incidents of local progression or local relapse, 115 distant metastases (including new lung metastases in 91 patients), one incident of progression without additional detail, two secondary malignancies, and six deaths as first events, including four treatment-related deaths in the BuMel arm (Data Supplement). EFS for all 287 randomly assigned patients was 53.6% (95% CI, 47.7% to 59.4%) at 3 years and 47.9% (95% CI, 42.0% to 53.9%) at 8 years. We observed a nonsignificant benefit of BuMel compared with VAI plus WLI, with an estimated HR of 0.79 (95% CI, 0.56 to 1.10; P = .16), corrected for the three previous interim analyses. EFS rates for VAI plus WLI and BuMel were 50.6% (95% CI, 42.3% to 58.9%) and 56.6% (95% CI, 48.3% to 64.6%) at 3 years, and 43.1% (95% CI, 34.9% to 51.6%) and 52.9% (95% CI, 44.5% to 61.2%) at 8 years, respectively (Fig 2A). The treatment effect estimate seemed to be similar in the sensitivity analyses, when the analysis was adjusted for patient age or when patients with protocol violations were excluded (Table 2). As illustrated in Figure 2B, no significant heterogeneity of the treatment effect was observed according to cooperative group, tumor site, tumor volume, or eligibility criteria for high-risk classification. When considering the different types of events in the competing risk approach (Data Supplement), the subdistribution HR associated with BuMel compared with VAI plus WLI was 0.79 (95% CI, 0.55 to 1.13; P = .20) for the risk of distant metastases.
FIG 2.
(A and B) Event-free survival and (C) overall survival. (A) Kaplan-Meier estimates of event-free survival by treatment group on the intention-to-treat (ITT) population. At the time of this analysis (cutoff date, January 1, 2017), 144 events were reported: 78 in the vincristine, dactinomycin, and ifosfamide (VAI) plus whole-lung irradiation (WLI) group and 66 in the busulfan and melphalan (BuMel) group. (B) Forest plot of event-free survival according to subgroups. The hazard ratio (HR) of events by subgroup were estimated in a Cox model proportional hazard model on the ITT population, including all patients except (1) for the assessment of treatment effect according to primary tumor site: we excluded one patient with an unknown primary; (2) for the assessment of treatment effect according to primary tumor volume: we excluded four patients with missing data; (3) for the assessment of treatment effect according to the size of the largest pulmonary nodule: we excluded 19 patients with missing data; (4) for the assessment of treatment effect according to pulmonary nodule: we excluded five patients with missing data; French group: French Society of Pediatric Oncology/French Sarcoma Group/Unicancer. (C) Kaplan-Meier estimates of overall survival by treatment group on the ITT population. COG, Children’s Oncology Group; EORTC, European Organization for Research and Treatment of Cancer; GPOH, Gesellschaft fuer Paediatrische Onkologie und Haematologie; UKCCLG, Children’s Cancer and Leukaemia Group.
TABLE 2.
EFS and Overall Survival Analysis, Main Analysis on the Intention-to-Treat Population, and Sensitivity Analysis on the As-Treated Population
We did not observe any benefit of BuMel compared with VAI plus WLI in terms of OS: 118 deaths were reported (60 in the VAI-WLI arm, 58 in the BuMel arm), leading to an HR of 1.00 (95% CI, 0.70 to 1.44; P = .99). OS rates for VAI plus WLI and BuMel were 68.0% (95% CI, 59.7% to 75.2%) versus 68.2% (95% CI, 60.0% to 75.4%) at 3 years, and 54.2% (95% CI, 45.3% to 62.8%) versus 55.3% (95% CI, 46.1% to 64.0%) at 8 years, respectively (Fig 2C; Table 2).
Safety
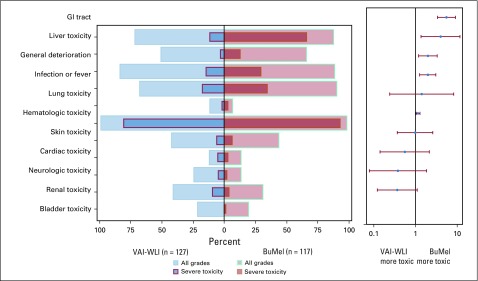
Significantly more patients taking BuMel experienced severe acute toxicities, in particular, GI and liver toxicity, hematologic toxicity, and infection, as well as general condition deterioration (Fig 3; Data Supplement). However, toxicity arose from the single HDT course versus multiple VAI courses. Four patients died as a result of BuMel-related toxicity (Data Supplement), and none died after standard chemotherapy.
FIG 3.
Adverse events. The panel on the left is a butterfly plot showing the proportion of patients experiencing an adverse event, whatever the grade (light red for the busulfan and melphalan [BuMel] arm and light blue for the vincristine, dactinomycin, and ifosfamide [VAI] plus whole-lung irradiation [WLI] arm) and a severe adverse event (dark red for the BuMel arm and dark blue for VAI plus WLI arm) according to the randomization group. The panel on the right displays the relative risk of a severe adverse event in patients with BuMel relative to patients with VAI plus WLI, with 95% CIs for a 2 × 2 table. The acute toxicity related to chemotherapy was assessed after each course, using a list of 22 selected items from the National Cancer Institute Common Terminology Criteria for Adverse Events (version 2.0). A modified list of items was used to evaluate toxicity after radiotherapy, using Radiation Therapy Oncology Group classification for eight types of specific toxicities. A free text area was available to document other adverse reactions. The toxicity items were then pooled by category: bladder toxicity, cardiac toxicity, GI toxicity, general deterioration, hematologic toxicity, infection, liver toxicity, lung toxicity, neurologic toxicity (including mood alteration), renal toxicity, and skin toxicity. The respiratory tract toxicity (larynx, pharynx, salivary gland) reported after radiotherapy was pooled within the category of GI toxicity because of small numbers and because they were usually associated. Details are provided in the Data Supplement. For each adverse event type, the analysis was based on the maximum grade observed over the whole maintenance treatment duration. A grade 4 hematologic toxicity and a grade 3 or higher nonhematologic toxicity were classified as severe toxicities. The categories of adverse events was ordered by decreasing value of the relative risk of severe toxicity. This analysis was performed on the safety set (127 patients taking VAI plus WLI and 117 patients taking BuMel), excluding patients who did not receive the treatment allocated by randomization (as-treated population), as well as patients with missing data for toxicity assessment. The number of chemotherapy courses followed by toxicity over the whole maintenance treatment duration is detailed in the Data Supplement.
DISCUSSION
We investigated whether BuMel followed by autologous hematopoietic stem-cell rescue in patients with ES with isolated lung metastases was more effective than the standard consolidation chemotherapy of VAI plus WLI. Compared with historical results, EFS and OS were improved in both the experimental and the standard arms of chemotherapy plus WLI.5 The reasons for this improved outcome compared with historic expectation remain speculative and should be the focus of future detailed analyses. We did not observe a significant improvement in EFS or OS for BuMel over VAI plus WLI consolidation. However, the relatively small sample size limited our power to detect a smaller treatment effect than prospectively anticipated. To our knowledge, this is the only randomized study that addresses the role of BuMel in patients with ES with isolated pulmonary metastases. The conduct of this study was made possible through the cooperation of multiple European countries and centers, as well as Children’s Oncology Group centers predominately in North America. Given the rarity of ES, international cooperation is important for future trials. The trial required 16 years to complete; the prolonged study duration was mainly as a result of low randomization success. Only 53% of the eligible patients were randomly assigned, predominantly because of patient/parent refusal. We acknowledge that this limits the external validity of our findings. Given the large number of participating institutions, central review of imaging was not part of the protocol, a factor that may also limit interpretation of results. Because we detected a major switch after randomization in the BuMel arm of 11%, it is possible that BuMel is not acceptable to all patients and clinicians. Contraindications to BuMel, such as central axis irradiation, may also explain why a sizable proportion of those with pulmonary metastases were not eligible, also excluding a significant portion of patients with large-volume tumors in the chest, pelvis, or paraspinal sites.16,17
Toxicity from BuMel, as expected, was greater than with the standard arm of VAI and WLI for GI tract, liver, and infection.11,12 There was no increased incidence of second malignancies in the experimental arm, but there were four treatment-related deaths. Although we have limited late-effects data from this study, we expect infertility in both female and male patients who received BuMel and male patients who received VAI and WLI.29-31 It is likely that female patients may have normal fertility after standard chemotherapy and WLI.29,30 A possible reduction in lung function in both arms is also to be expected, although this may be worse in those who received VAI and WLI after major chest wall surgery.10,32 Additional late effects are currently being evaluated and updated in the Pan-European Networks (www.pancare.eu; www.pancarelife.eu) and will be reported later.29,31 Because EFS and OS were not significantly different in the two arms, patients and physicians might pick one over the other on the basis of late effects in the future.
Over the last 20 years, the role of BuMel in ES has been unclear. Nonrandomized clinical trials have suggested a possible benefit in patients with widely metastatic disease.11,12,33,34 Our results give clarity to the role of BuMel in selected patients with pulmonary metastatic disease only and cannot be extrapolated to ES in other clinical settings. The other randomized study from our Euro-E.W.I.N.G. 99 and EWING 2008 study groups, concerning patients with localized ES, poor histologic response to induction chemotherapy, and/or large tumor volume, Euro-E.W.I.N.G. 99 R2Loc, demonstrated a positive effect of BuMel versus standard VAI consolidation chemotherapy in a selected group of 40% of eligible patients.14 Grunewald35,37 However, these results also cannot be directly extrapolated to ES beyond those with poor-risk localized disease.
The Italian and Scandinavian sarcoma groups conducted a contemporary, nonrandomized study with a design similar to the BuMel arm in patients mainly with isolated pulmonary metastases, demonstrating that it was feasible to add WLI after BuMel and primary site radiation before HDT.36 Overall outcome, with an EFS of 43%, was poorer compared with our study; however, their series included a small cohort of patients with single bony metastases.36 Whether the addition of WLI to BuMel would have affected our results is speculative.
This study did not support a change in the standard of care for patients with ES with isolated pulmonary metastases. The overall results were better than anticipated on the basis of historic series. However, the benefit associated with BuMel compared with VAI to reduce the risk of relapse was smaller than what we hoped to achieve. The length of the study and the need to improve the outcome of patients in this study demonstrate the need for large cooperative studies to answer questions in a timely manner, perhaps exploring targeted agents specific to ES in addition to standard backbone chemotherapy and, currently, WLI.
Footnotes
Presented in part at the 2016 ASCO Annual Meeting, Chicago, IL, June 3 to 7, 2016; and at the Connective Tissue Oncology Meeting, Lisbon, Portugal, November 9, 2016.
Supported by FP7-EURO EWING Consortium; Association Enfants et Santé, Société Française de Lutte Contre les Cancers et les Leucémies de l’Enfant et de l’Adolescent, Unicancer; Cancer Research UK (CRUK/02/014), National Institute for Health Research, University College London Hospitals, Biomedical Research Centre; Deutsche Krebshilfe (70-2551-Jue3 and 108128), and Bundesministerium für Bildung und Forschung (BMBF 01GM0869; BMBF/Era-Net 01KT1310); National Cancer Institute, Bethesda, MD (U10CA098413, U10CA098543, U10CA180886, and U10CA180899); and The Swedish Childhood Cancer Fund.
Clinical trial information: NCT00020566, NCT00987636.
See accompanying Editorial on page 3173
AUTHOR CONTRIBUTIONS
Conception and design: Uta Dirksen, Bernadette Brennan, Marie-Cécile Le Deley, Henk van den Berg, Alan Craft, Heribert Juergens, Ian Judson, Ruth Ladenstein, Ian Lewis, Bruce Morland, Michael Paulussen, Andreas Ranft, Gwénaël Le Teuff, Odile Oberlin
Administrative support: Uta Dirksen
Provision of study materials or patients: Uta Dirksen, Bernadette Brennan, Henk van den Berg, Bénédicte Brichard, Alan Craft, Natalie Gaspar, Hans Gelderblom, Jean-Marc Guinbretiere, Peter Hauser, Heribert Juergens, Robert Goldby, Holocombe E. Grier, Perrine Marec Berard, Odile Oberlin, Thomas Kuehne, Ruth Ladenstein, Stephen L. Lessnick, Ian Lewis, Claude Linassier, Neyssa Marina, Bruce Morland, Hélène Pacquement, Michael Paulussen, Jeremy Whelan, Richard Womer, Douglas S. Hawkins
Collection and assembly of data: Uta Dirksen, Bernadette Brennan, Marie-Cécile Le Deley, Nathalie Cozic, Henk van den Berg, Vivek Bhadri, Bénédicte Brichard, Line Claude, Alan Craft, Richard Gorlick, Jean-Marc Guinbretiere, Peter Hauser, Lars Hjorth, Katherine Janeway, Natalie Gaspar, Hans Gelderblom, Holcombe E. Grier, Robert Goldsby, Thomas Kuehne, Ruth Ladenstein, Ian Lewis, Odile Oberlin, R. Lor Randall, Robert Goldby, Hans Gelderblom, Neyssa Marina, Heribert Juergens, Ian Judson, Jarmila Kruseova, Ruth Ladenstein, Cyril Lervat, Stephen L. Lessnick, Claude Linassier, Perrine Marec-Berard, Hélène Pacquement, Michael Paulussen, Andreas Ranft, Gwénaël Le Teuff, Jeremy Whelan, Richard Womer, Douglas S. Hawkins
Data analysis and interpretation: Uta Dirksen, Marie-Cécile Le Deley, Nathalie Cozic, Susanne Amler, Mark Krailo, Andreas Ranft, Gwénaël Le Teuff, Keith Wheatley, Douglas S. Hawkins
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
High-Dose Chemotherapy Compared With Standard Chemotherapy and Lung Radiation in Ewing Sarcoma With Pulmonary Metastases: Results of the European Ewing Tumour Working Initiative of National Groups, 99 Trial and EWING 2008
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Uta Dirksen
Consulting or Advisory Role: Eli Lilly (Inst), Ipsen
Vivek Bhadri
Consulting or Advisory Role: Eisai, Eli Lilly
Nathalie Gaspar
Consulting or Advisory Role: EISAI
Travel, Accommodations, Expenses: EISAI
Hans Gelderblom
Patents, Royalties, Other Intellectual Property: Amgen (Inst), Boehringer Ingelheim (Inst), Pfizer (Inst), Novartis (Inst), Pharmamar (Inst), Daiichi (Inst), Five Prime (Inst)
Robert Goldsby
Stock and Other Ownership Interests: Pfizer
Richard Gorlick
Research Funding: Eisai (Inst)
Jean-Marc Guinbretiere
Expert Testimony: Genomic Health
Lars Hjorth
Stock and Other Ownership Interests: Bioinvent
Katherine Janeway
Consulting or Advisory Role: Bayer
Patents, Royalties, Other Intellectual Property: Amgen (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Bayer
Ian Judson
Honoraria: Eli Lilly
Consulting or Advisory Role: Eli Lilly
Patents, Royalties, Other Intellectual Property: Rewards to inventors relating to patent for abiraterone acetate
Travel, Accommodations, Expenses: Eli Lilly
Mark Krailo
Consulting or Advisory Role: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Thomas Kuehne
Consulting or Advisory Role: Novartis, Amgen, UCB, Sobi
Research Funding: Amgen (Inst)
Expert Testimony: Amgen
Travel, Accommodations, Expenses: CSL Behring, Bayer, Novartis, Sobi, Novo Nordisk
Ruth Ladenstein
Honoraria: Apeiron Biologics, Boehringer Ingelheim, EUSA Pharma
Consulting or Advisory Role: Apeiron Biologics, Boehringer Ingelheim, EUSA Pharma (Inst)
Research Funding: Apeiron Biologics (Inst), EUSA Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Apeiron Biologics (Inst), EUSA Pharma (Inst)
Expert Testimony: Apeiron Biologics, EUSA Pharma
Travel, Accommodations, Expenses: Apeiron Biologics, EUSA Pharma
Stephen L. Lessnick
Leadership: Salarius Pharmaceuticals
Stock and Other Ownership Interests: Salarius Pharmaceuticals
Consulting or Advisory Role: Salarius Pharmaceuticals
Patents, Royalties, Other Intellectual Property: (1) United States Patent No. US 7,939,253 B2, “Methods and compositions for the diagnosis and treatment of Ewing’s Sarcoma,” Stephen L. Lessnick, Assigned to The University of Utah Research Foundation, Salt Lake City, UT, filed on May 9, 2007, awarded May 10, 2011. (2) United States Patent No. US 8,557,532, “Diagnosis and treatment of drug-resistant Ewing’s sarcoma,” Stephen L. Lessnick and Wen Luo, Assigned to The University of Utah Research Foundation, Salt Lake City, UT, filed on August 30, 2010, awarded October 15, 2013
Other Relationship: Multiple pharmaceutical, biotech, device, and similar companies, multiple pharmaceutical, biotech, device, and similar companies (I)
Claude Linassier
Employment: Bayer (I)
Honoraria: Astellas Pharma
Consulting or Advisory Role: Astellas Pharma
Travel, Accommodations, Expenses: Astellas Pharma, Pfizer, Janssen
Neyssa Marina
Employment: Five Prime Therapeutics, Genentech (I)
Stock and Other Ownership Interests: Five Prime Therapeutics, Genentech (I)
Travel, Accommodations, Expenses: Five Prime Therapeutics
Other Relationship: Novartis, Macrogenics, Bristol-Myers Squibb, GlaxoSmithKline
Bruce Morland
Speakers' Bureau: Clinigen
R. Lor Randall
Honoraria: Biomet, Daiichi Sankyo
Travel, Accommodations, Expenses: Biomet, Daiichi Sankyo/Eli Lilly
Richard Womer
Consulting or Advisory Role: Loxo
Douglas S. Hawkins
Research Funding: Loxo (Inst)
Research Funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Bayer (Inst), Eli Lilly (Inst), Eisai (Inst)
Travel, Accommodations, Expenses: Loxo Bayer, Bristol-Myers Squibb, Celgene
No other potential conflicts of interest were reported.
REFERENCES
- 1. Hawkins DS, Brennan B, Bölling T, et al: Ewing sarcoma, in Pizzo PA, Poplack DG (eds): Principles and Practice of Pediatric Oncology (ed 7). Philadelphia, PA, Wolters-Kluwer, 2015, pp. 855-872. [Google Scholar]
- 2.Gaspar N, Hawkins DS, Dirksen U, et al. Ewing sarcoma: Current management and future approaches through collaboration. J Clin Oncol. 2015;33:3036–3046. doi: 10.1200/JCO.2014.59.5256. [DOI] [PubMed] [Google Scholar]
- 3.Le Deley MC, Paulussen M, Lewis I, et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: Results of the randomized noninferiority Euro-EWING99-R1 trial. J Clin Oncol. 2014;32:2440–2448. doi: 10.1200/JCO.2013.54.4833. [DOI] [PubMed] [Google Scholar]
- 4. doi: 10.1200/JCO.2011.41.5703. Womer RB, West DC, Krailo MD, et al: Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J Clin Oncol 30:4148-4154, 2012 [Erratum: J Clin Oncol 33:814, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulussen M, Ahrens S, Craft AW, et al. Ewing’s tumors with primary lung metastases: Survival analysis of 114 (European Intergroup) Cooperative Ewing’s Sarcoma Studies patients. J Clin Oncol. 1998;16:3044–3052. doi: 10.1200/JCO.1998.16.9.3044. [DOI] [PubMed] [Google Scholar]
- 6.Paulussen M, Craft AW, Lewis I, et al. Results of the EICESS-92 study: Two randomized trials of Ewing’s sarcoma treatment--cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol. 2008;26:4385–4393. doi: 10.1200/JCO.2008.16.5720. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein ML, Devidas M, Lafreniere D, et al. Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children’s Cancer Group Phase II Study 9457--A report from the Children’s Oncology Group. J Clin Oncol. 2006;24:152–159. doi: 10.1200/JCO.2005.02.1717. [DOI] [PubMed] [Google Scholar]
- 8.Tanguturi SK, George S, Marcus KJ, et al. Whole lung irradiation in adults with metastatic Ewing sarcoma: Practice patterns and implications for treatment. Sarcoma. 2015;2015:591698. doi: 10.1155/2015/591698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey DL, Alektiar KM, Gerber NK, et al. Whole lung irradiation for adults with pulmonary metastases from Ewing sarcoma. Int J Radiat Oncol Biol Phys. 2014;89:1069–1075. doi: 10.1016/j.ijrobp.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 10.Bölling T, Schuck A, Paulussen M, et al. Whole lung irradiation in patients with exclusively pulmonary metastases of Ewing tumors. Toxicity analysis and treatment results of the EICESS-92 trial [in German] Strahlenther Onkol. 2008;184:193–197. doi: 10.1007/s00066-008-1810-x. [DOI] [PubMed] [Google Scholar]
- 11.Ladenstein R, Pötschger U, Le Deley MC, et al. Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J Clin Oncol. 2010;28:3284–3291. doi: 10.1200/JCO.2009.22.9864. [DOI] [PubMed] [Google Scholar]
- 12.Oberlin O, Rey A, Desfachelles AS, et al. Impact of high-dose busulfan plus melphalan as consolidation in metastatic Ewing tumors: A study by the Société Française des Cancers de l’Enfant. J Clin Oncol. 2006;24:3997–4002. doi: 10.1200/JCO.2006.05.7059. [DOI] [PubMed] [Google Scholar]
- 13.Rasper M, Jabar S, Ranft A, et al. The value of high-dose chemotherapy in patients with first relapsed Ewing sarcoma. Pediatr Blood Cancer. 2014;61:1382–1386. doi: 10.1002/pbc.25042. [DOI] [PubMed] [Google Scholar]
- 14. doi: 10.1200/JCO.2018.78.2516. Whelan J, Le Deley MC, Dirksen U, et al: High-dose chemotherapy and blood autologous stem-cell rescue compared with standard chemotherapy in localized high-risk Ewing sarcoma: Results of Euro-E.W.I.N.G.99 and Ewing-2008. J Clin Oncol 36:3110-3119, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juergens C, Weston C, Lewis I, et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer. 2006;47:22–29. doi: 10.1002/pbc.20820. [DOI] [PubMed] [Google Scholar]
- 16.Bölling T, Dirksen U, Ranft A, et al. Radiation toxicity following busulfan/melphalan high-dose chemotherapy in the EURO-EWING-99-trial: Review of GPOH data. Strahlenther Onkol. 2009;185(suppl 2):21–22. doi: 10.1007/s00066-009-1009-9. [DOI] [PubMed] [Google Scholar]
- 17.Claude L, Carrie C, Alapetite C, et al. Toxicity of high-dose chemotherapy (busulfan-melphalan) followed by radiation therapy (RT) in Ewing’s axial tumours: Results of the French study. Pediatr Blood Cancer. 2006;49:555. [Google Scholar]
- 18. Carrie C, Le Deley M, Claude L, et al: The radiosensitization effect and toxicity of busulfan containing chemotherapy before radiotherapy for Ewing’s sarcomas, Strahlentherapie Und Onkologie, Urban & Vogel Neumarkter Strasse 43, D-81673 Munich. Germany 2009, pp 31. [Google Scholar]
- 19.Strauss SJ, McTiernan A, Driver D, et al. Single center experience of a new intensive induction therapy for Ewing’s family of tumors: Feasibility, toxicity, and stem cell mobilization properties. J Clin Oncol. 2003;21:2974–2981. doi: 10.1200/JCO.2003.04.106. [DOI] [PubMed] [Google Scholar]
- 20.Bearman SI, Anderson GL, Mori M, et al. Venoocclusive disease of the liver: Development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729–1736. doi: 10.1200/JCO.1993.11.9.1729. [DOI] [PubMed] [Google Scholar]
- 21.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1:121–129. doi: 10.1002/sim.4780010204. [DOI] [PubMed] [Google Scholar]
- 22.Gordon Lan KK, Demets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 23.Gordon MS, Robert F, Matei D, et al. An open-label phase Ib dose-escalation study of TRC105 (anti-endoglin antibody) with bevacizumab in patients with advanced cancer. Clin Cancer Res. 2014;20:5918–5926. doi: 10.1158/1078-0432.CCR-14-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 25.Wassmer G. Planning and analyzing adaptive group sequential survival trials. Biom J. 2006;48:714–729. doi: 10.1002/bimj.200510190. [DOI] [PubMed] [Google Scholar]
- 26. Lakbfleisch R, Prentics R: The Statistical Analysis of Failure Time Data (ed 2). New York, NY, Wiley, 2002. [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 28.Lin DY Wei LJ, Ying Z: Checking the Cox model with cumulative sums of Martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 29.Thomas-Teinturier C, Allodji RS, Svetlova E, et al. Ovarian reserve after treatment with alkylating agents during childhood. Hum Reprod. 2015;30:1437–1446. doi: 10.1093/humrep/dev060. [DOI] [PubMed] [Google Scholar]
- 30.van Dorp W, Mulder RL, Kremer LC, et al. Recommendations for premature ovarian insufficiency surveillance for female survivors of childhood, adolescent, and young adult cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. J Clin Oncol. 2016;34:3440–3450. doi: 10.1200/JCO.2015.64.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skinner R, Mulder RL, Kremer LC, et al. Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol. 2017;18:e75–e90. doi: 10.1016/S1470-2045(17)30026-8. [DOI] [PubMed] [Google Scholar]
- 32.Motosue MS, Zhu L, Srivastava K, et al. Pulmonary function after whole lung irradiation in pediatric patients with solid malignancies. Cancer. 2012;118:1450–1456. doi: 10.1002/cncr.26371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ladenstein R, Lasset C, Pinkerton R, et al: Impact of megatherapy in children with high-risk Ewing’s tumours in complete remission: A report from the EBMT Solid Tumour Registry. Bone Marrow Transplant 15:697-705, 1995 [Erratum: Bone Marrow Transplant 18:675, 1996] [PubMed] [Google Scholar]
- 34.Ferrari S, Sundby Hall K, Luksch R, et al. Nonmetastatic Ewing family tumors: High-dose chemotherapy with stem cell rescue in poor responder patients. Results of the Italian Sarcoma Group/Scandinavian Sarcoma Group III protocol. Ann Oncol. 2011;22:1221–1227. doi: 10.1093/annonc/mdq573. [DOI] [PubMed] [Google Scholar]
- 35.Pappo AS, Dirksen U. Rhabdomyosarcoma, Ewing sarcoma, and other round cell sarcomas. J Clin Oncol. 2018;36:168–179. doi: 10.1200/JCO.2017.74.7402. [DOI] [PubMed] [Google Scholar]
- 36.Luksch R, Tienghi A, Hall KS, et al. Primary metastatic Ewing’s family tumors: Results of the Italian Sarcoma Group and Scandinavian Sarcoma Group ISG/SSG IV Study including myeloablative chemotherapy and total-lung irradiation. Ann Oncol. 2012;23:2970–2976. doi: 10.1093/annonc/mds117. [DOI] [PubMed] [Google Scholar]
- 37.Grünewald TGP, Cidre-Aranaz F, Surdez D, et al. Ewing Sarcoma. Nat Rev Dis Primers. 2018;(1):5. doi: 10.1038/s41572-018-0003-x. [DOI] [PubMed] [Google Scholar]