Abstract
PURPOSE
Oral mucositis (OM) remains a common, debilitating toxicity of radiation therapy (RT) for head and neck cancer. The goal of this phase IIb, multi-institutional, randomized, double-blind trial was to compare the efficacy and safety of GC4419, a superoxide dismutase mimetic, with placebo to reduce the duration, incidence, and severity of severe OM (SOM).
PATIENTS AND METHODS
A total of 223 patients (from 44 institutions) with locally advanced oral cavity or oropharynx cancer planned to be treated with definitive or postoperative intensity-modulated RT (IMRT; 60 to 72 Gy [≥ 50 Gy to two or more oral sites]) plus cisplatin (weekly or every 3 weeks) were randomly assigned to receive 30 mg (n = 73) or 90 mg (n = 76) of GC4419 or to receive placebo (n = 74) by 60-minute intravenous administration before each IMRT fraction. WHO grade of OM was assessed biweekly during IMRT and then weekly for up to 8 weeks after IMRT. The primary endpoint was duration of SOM tested for each active dose level versus placebo (intent-to-treat population, two-sided α of .05). The National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, was used for adverse event grading.
RESULTS
Baseline patient and tumor characteristics as well as treatment delivery were balanced. With 90 mg GC4419 versus placebo, SOM duration was significantly reduced (P = .024; median, 1.5 v 19 days). SOM incidence (43% v 65%; P = .009) and severity (grade 4 incidence, 16% v 30%; P = .045) also were improved. Intermediate improvements were seen with the 30-mg dose. Safety was comparable across arms, with no significant GC4419-specific toxicity nor increase of known toxicities of IMRT plus cisplatin. The 2-year follow-up for tumor outcomes is ongoing.
CONCLUSION
GC4419 at a dose of 90 mg produced a significant, clinically meaningful reduction of SOM duration, incidence, and severity with acceptable safety. A phase III trial (ROMAN; ClinicalTrials.gov identifier: NCT03689712) has begun.
INTRODUCTION
Approximately 70% of patients with head and neck cancer (HNC) receiving concurrent cisplatin and radiation experience severe oral mucositis (SOM), defined as grade 3 to 4 by the WHO scale.1-3 SOM causes pain that potentially requires narcotics; adversely affects nutrition (including requiring a feeding tube), hydration, speech, swallowing, and bacteremia risk4,5; leads to radiation treatment breaks, which compromise tumor control6-8; and increases care costs, especially from hospitalization and emergency room use.9,10
Treatment options for SOM are limited to symptom management with topical agents and systemic analgesics.11-19 No drugs are approved to reduce SOM duration, incidence, or severity for patients with solid tumors; palifermin is approved in the United States for patients with hematologic malignancies only.20,21 Consequently, SOM management constitutes an unmet clinical need.
The formation of reactive oxygen species, including superoxide (O2*—), is a critical initiating event in the biologic cascade that results in radiation-induced SOM.22 GC4419 is a superoxide dismutase mimetic that rapidly and specifically converts O2*— to hydrogen peroxide (H2O2),23 arresting the initiation of this cascade. In a phase Ib/IIa trial in patients receiving standard concomitant cisplatin and intensity-modulated radiation therapy (IMRT) for HNC, SOM incidence, duration, and severity (WHO grade 4 oral mucositis [OM] incidence) seemed improved compared with historical data when GC4419 was administered at doses of 30 or 90 mg before each IMRT fraction.24 The safety profile of GC4419 was acceptable. The 1-year tumor outcomes seemed consistent with expectations for IMRT plus cisplatin alone. We now report the efficacy and safety results of a randomized, double-blind trial of 30 mg or 90 mg of GC4419 versus placebo in a similar trial population.
PATIENTS AND METHODS
Trial Design and Oversight
This randomized, double-blind, placebo-controlled phase IIb trial was sponsored and financially supported by Galera Therapeutics. The protocol was approved by each institution’s institutional review board and was registered at ClinicalTrials.gov identifier: NCT02508389). Investigators obtained written informed consent from each participant. Data were anonymized to protect the patients’ identities.
Patients
Eligible patients had stage III to IVb (according to American Joint Committee on Cancer, seventh edition), nonmetastatic, oral cavity or oropharyngeal squamous cell cancer, Eastern Cooperative Oncology Group performance status of 2 or less, and planned treatment with standard fractionation IMRT and concurrent cisplatin (80 to 100 mg/m2 every 3 weeks or 30 to 40 mg/m2 weekly) administered definitively or after surgical resection. IMRT plans had to include at least two oral mucosal sites (upper or lower lip, right or left buccal mucosa, right or left ventral/lateral oral tongue, floor of mouth, or soft palate) within the cumulative 50-Gy isodose line and were centrally reviewed by an independent radiation oncologist to confirm protocol adherence. Adequate marrow, renal, and hepatic functions were required. Prophylactic percutaneous endoscopic gastrostomy tube placement was allowed, but patients were required to be able to eat soft solids at enrollment. Prior induction chemotherapy or concurrent treatment with nitrates was not allowed.
Treatment and Assessments
IMRT was administered in daily 2.0- to 2.2-Gy fractions, Monday through Friday, to a cumulative tumor dose of 60 to 72 Gy. Patients were randomly assigned in a 1:1:1 fashion to receive 30 mg of GC4419, 90 mg of GC4419, or placebo; each was administered intravenously in 250 mL of normal saline over 60 minutes and ended within 60 minutes before each radiation fraction. Enrollment was stratified by cisplatin schedule and tumor human papillomavirus status (p16 staining). Oral rinses—limited to sodium bicarbonate, lidocaine, and antifungal agents—were permitted. Other concurrent available or experimental systemic or topical pharmaceuticals or devices or low-level laser therapy for OM were excluded. Supportive care per ASCO guidelines was encouraged.
OM was assessed by trained investigator-evaluators using WHO criteria: grade 0, no mucositis; grade 1, pain and erythema; grade 2, ulceration, able to eat solid food; grade 3, ulceration, able to eat only liquids; and grade 4, ulceration, inability to eat, requiring tube or parenteral feeding. OM was assessed twice per week with at least 48 hours between assessments during IMRT and weekly thereafter for up to 8 weeks or until the WHO score was less than 2. OM assessment training and quality control were performed by Clinical Assistance Programs (Framingham, MA) to ensure that (1) all oral assessments were performed consistently using standardized questions, oral cavity examination technique and order, and data collection; and (2) WHO grade scoring was correctly assigned per assessment findings for all OM assessments. To reduce the variability in assessing a patient’s diet, investigator-evaluators were trained carefully to elucidate whether dietary compromise was because of oral pain. If not, and if the diet was compromised by confounding factors (eg, dysgeusia, edentulism, nausea, mucous, throat pain, functional dysphagia), the WHO score was determined on the basis of what the patient said they could eat absent these factors. Accuracy and consistency of WHO scores were evaluated within a few days of each assessment. Inconsistent or incomplete mucositis assessments were queried before final WHO scores were entered into the database.
Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Dose reduction of 25% of GC4419 or placebo was required for grade 3 flushing, grade 2 or greater hypotension within 2 hours after the start of GC4419 or placebo infusion, grade 3 or 4 infusion reaction with GC4419 or placebo, or grade 4 vomiting despite optimal antiemetic therapy per current guidelines. Two dose reductions were permitted per patient, and the drug was discontinued if the patient was still unable to tolerate treatment. Tumor progression and survival were assessed every 3 months in the first year, and every 4 months in the second year, after IMRT. Data collected for exploratory assessments included the following: during IMRT, the Oral Mucositis Daily Questionnaire (OMDQ) responses,4 opioid use, insertion of gastrostomy tubes, use and complications of indwelling venous access catheters, and unplanned visits or hospitalizations; and in the 2 years after IMRT, measurement of trismus and visual analog scale of xerostomia. Peripheral blood specimens were collected before and during IMRT for future exploratory assessments of circulating cytokines and gene expression patterns.
Statistical Methods
Efficacy analyses were conducted on the intent-to-treat population (all randomly assigned patients). The primary efficacy endpoint was SOM duration, defined as the number of days from the first to the last occurrence of SOM, including any intervening days with grade 2 or lower OM. Patients with no WHO score greater than 2 had, by definition, an SOM duration of 0 days. Duration in patients with unresolved SOM as of the last evaluation was imputed as the median duration among patients in the same treatment arm with at least that duration. For patients without observed SOM but with incomplete follow-up, duration was imputed as the median duration among patients in the same treatment arm who were free of SOM for at least that length of follow-up. SOM duration in each active arm was compared separately with that of the placebo arm using the nonparametric van Elteren test.25
Secondary endpoints, tested using the Cochran-Mantel-Haenszel test, were SOM incidence at any time during IMRT or through 60 Gy of IMRT and severity (grade 4 incidence) at any time during IMRT. Testing was performed in a conditional, sequential fashion: SOM duration, 90 mg versus placebo; then SOM duration, 30 mg versus placebo; then secondary endpoints, with formal testing proceeding as long as the P value was .05 or less. A sample size of 72 patients per group provided approximately 80% power with a two-sided type I error rate of .05 for each treatment arm’s comparison with placebo. Calculations assumed a median SOM duration of 0 days in the treatment arms compared with 28 days in the placebo arm, with 40% SOM incidence in each treatment arm compared with 65% in the placebo arm. Safety was assessed for all randomly assigned patients who received at least one dose of GC4419 or placebo.
RESULTS
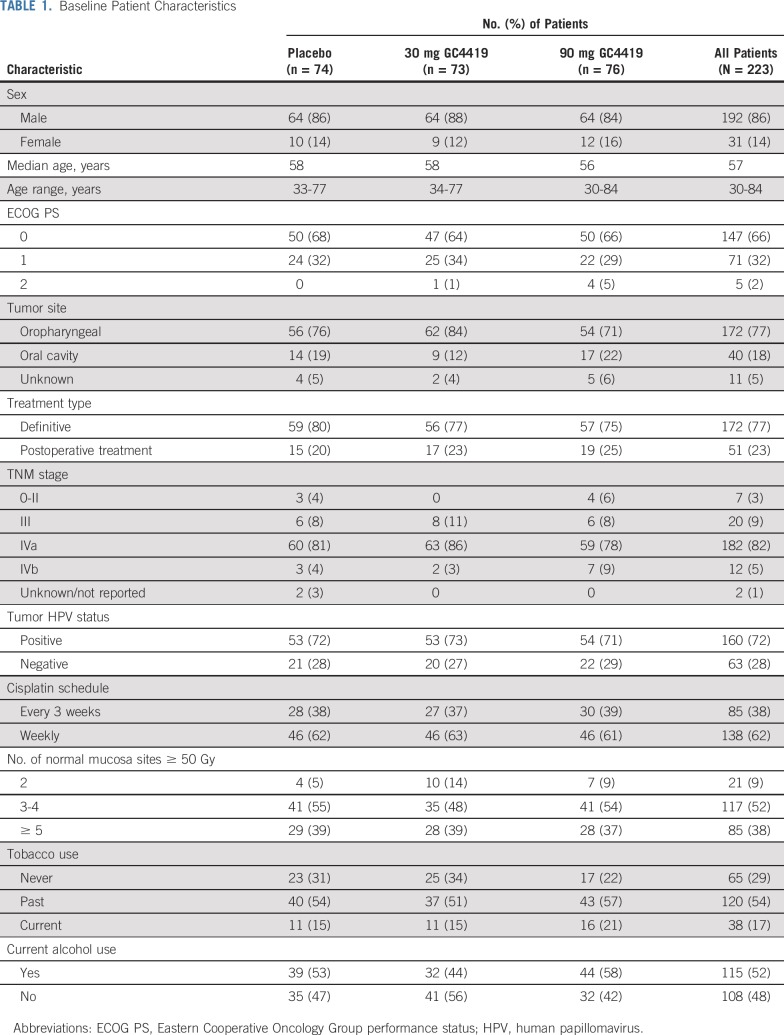
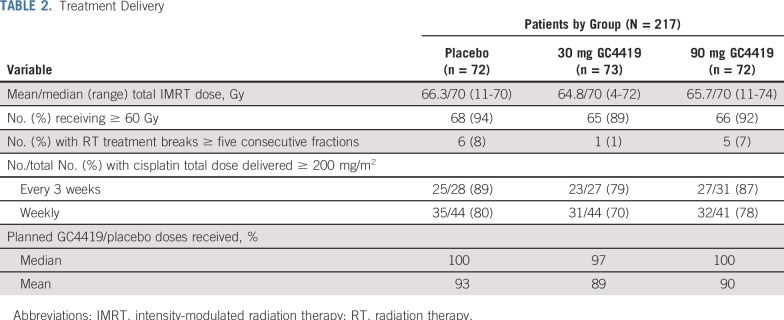
Forty-four US and Canadian sites enrolled 223 patients, 217 of whom received at least one infusion of GC4419 or placebo (CONSORT diagram, Fig 1). Baseline patient characteristics were balanced across the three treatment arms (Table 1), as was delivery of IMRT plus cisplatin, which did not seem to be compromised with the addition of GC4419 (Table 2). Radiation therapy (RT) plan adherence to the protocol was confirmed in all cases. The initially assigned WHO score was correct—that is, consistent with source data—in 3,617 (95.3%) of all 3,794 OM assessments throughout the trial; all errors were queried and corrected to ensure 100% accuracy of scoring. Approximately 80% of patients in each treatment arm had complete OM follow-up; roughly 10% of patients were lost to follow-up before resolution of SOM to a WHO score of 0 or 1, and 10% were lost to follow-up without ever experiencing SOM.
FIG 1.
CONSORT diagram: patient random assignment. ITT, intent to treat.
TABLE 1.
Baseline Patient Characteristics
TABLE 2.
Treatment Delivery
Efficacy
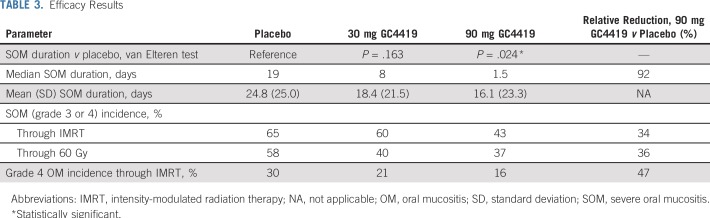
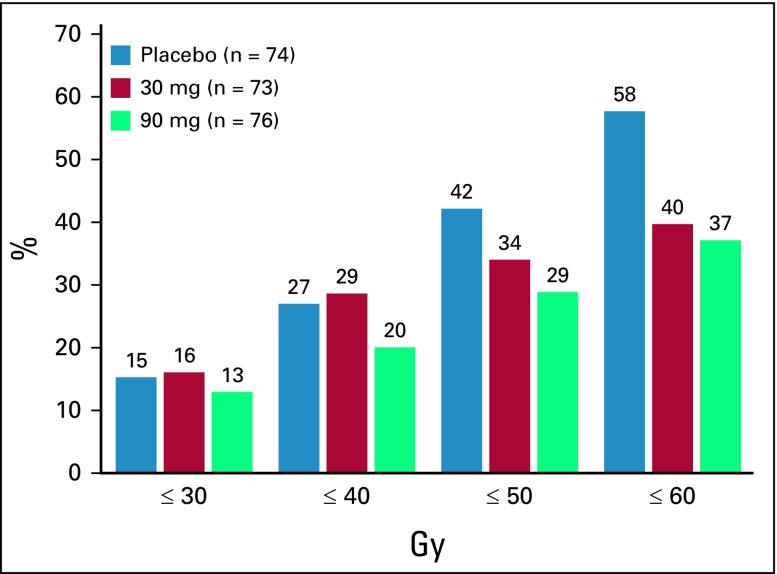
SOM duration was significantly reduced among patients receiving 90 mg of GC4419 compared with those receiving placebo (P < .024 using the van Elteren test; Table 3). The median SOM duration in the 90-mg arm also was reduced versus placebo—1.5 days versus 19 days—which reflected, in part, a 34% relative reduction of SOM incidence at any time during IMRT (43% v 65%; nominal P = .009) and a corresponding increase in the number of patients with an SOM duration of 0 days. Severity (grade 4 OM incidence at any time during IMRT; 16% v 30%; nominal P = .045) and SOM incidence through 60 Gy (37% v 58%; nominal P = .010) also were lower for the 90-mg group than for the placebo group. Cumulative SOM incidence was progressively lower in the 90-mg arm than the placebo arm at serial RT delivery landmarks (30 Gy, 40 Gy, 50 Gy, 60 Gy; Fig 2). Primary and secondary efficacy results for the 30-mg group versus placebo were intermediate (SOM duration, 8 days [P = .163]; SOM incidence thru 60 Gy, 40% [P = .026]).
TABLE 3.
Efficacy Results
FIG 2.
Cumulative severe oral mucositis (WHO grade 3 or 4) incidence (%) at progressive intensity-modulated radiation therapy delivery landmarks (Gray cutoffs).
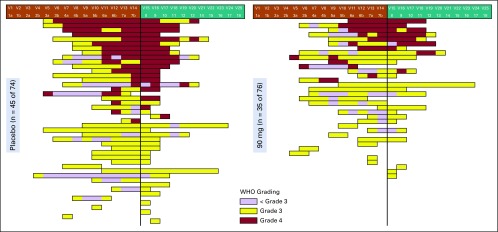
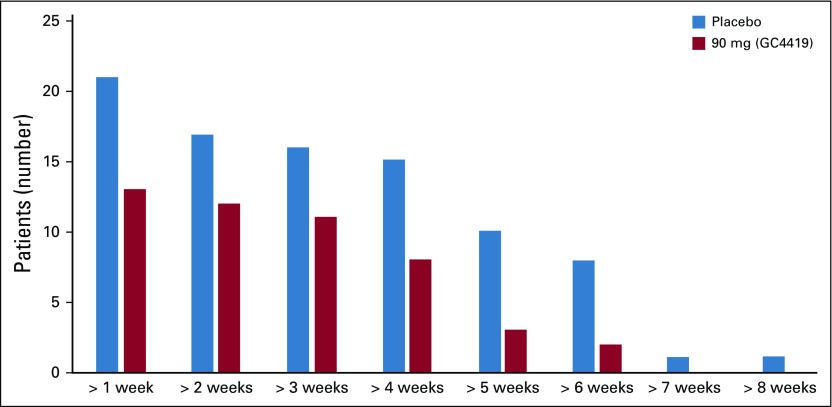
We also asked if GC4419 tempered the course and severity of SOM even if it failed to completely prevent it. In the subgroup of patients who had at least 1 day of SOM, visual inspection of individual patient data (Fig 3) indicated reduction of SOM overall and of grade 4 OM with 90 mg of GC4419 compared with placebo. Consistent with this, the persistence of grade 4 OM was less for 90 mg of GC4419 than for placebo (Appendix Fig A1, online only).
FIG 3.
Swimmer plot of severe oral mucositis scores for the subsets of patients in (left) the placebo arm (n = 45) or (right) the 90-mg arm (n = 35) who had at least one WHO oral mucositis score of grade 3 or 4. Each horizontal lane represents the experience of an individual patient. Time on radiation therapy or after radiation therapy is indicated at the top, for which the vertical line denotes the end of intensity-modulated radiation therapy. Yellow, WHO grade 3; red, WHO grade 4; purple, WHO grade 0 to 2. WHO scoring was done twice per week during radiation therapy, then once per week after radiation therapy in patients who returned for follow-up for up to 8 weeks or until the WHO score was 0 or 1.
The duration and incidence of SOM in the active or placebo arms were not affected by cisplatin schedule (weekly v every 3 weeks), tumor human papillomavirus status, definitive versus postoperative IMRT, or patient-reported smoking status (data not shown). The incidence of ulcerative OM (ie, OM WHO grade 2 or greater) was comparable across the treatment arms.
Safety
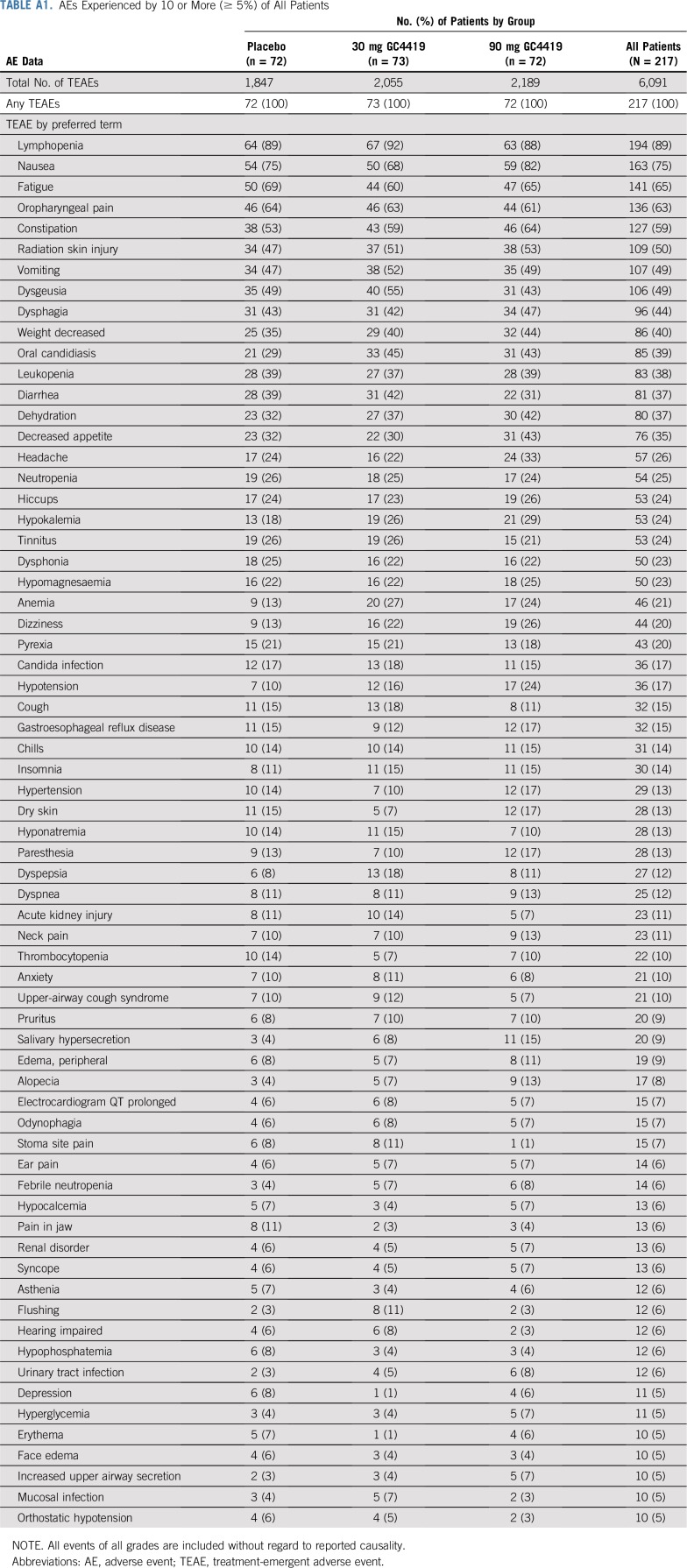
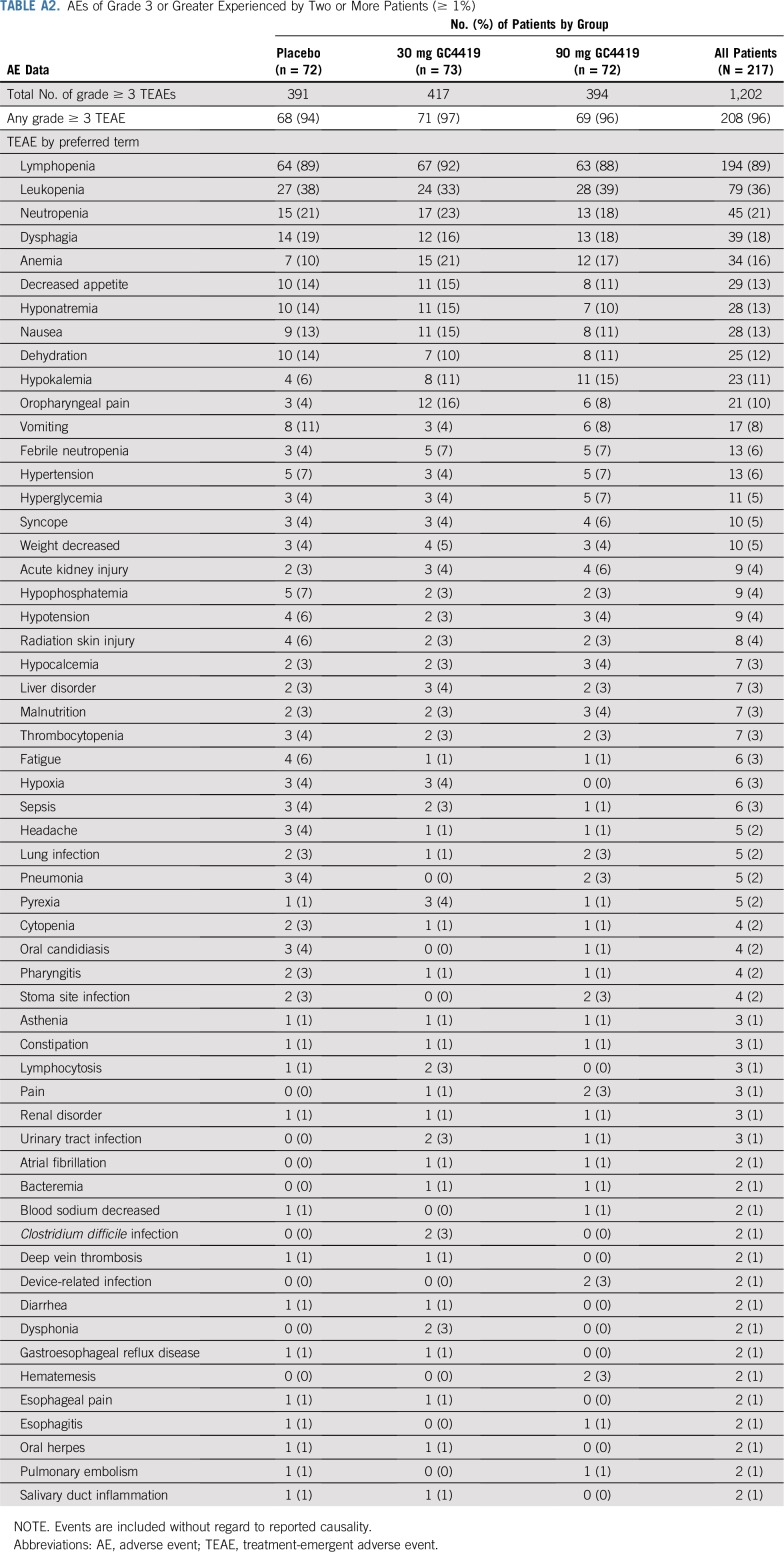
The overall adverse event profile in each of the GC4419 arms was comparable to that in the placebo arm and seemed consistent with the known toxicities of IMRT plus cisplatin when either events of all grades (Appendix Table A1, online only) or grades 3 or greater (Appendix Table A2, online only) were considered. Eight percent of patients, comparably distributed across the three arms, required dose reductions for adverse events.
Hematologic toxicities, particularly lymphopenia, attributable to cisplatin and IMRT were the most prominent toxicities. These were not increased in incidence or severity by the addition of GC4419.
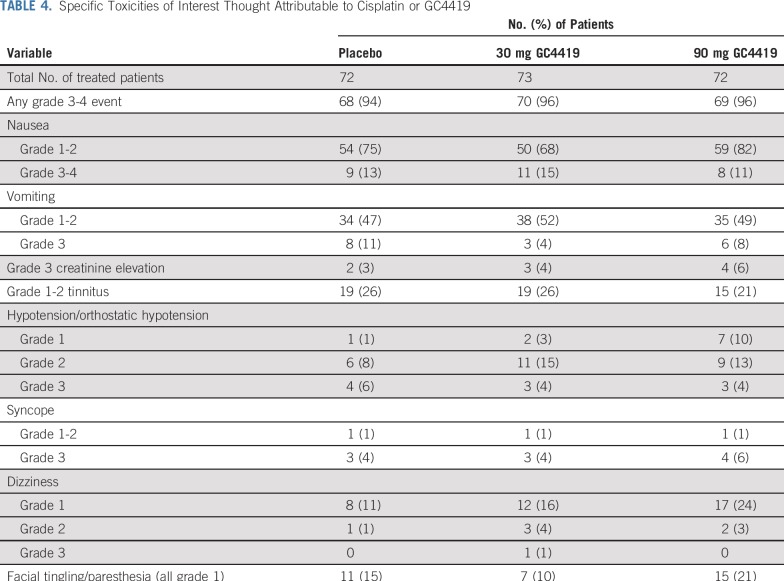
Other specific adverse events attributable to cisplatin—severe nausea or vomiting, increased creatinine, or tinnitus—did not seem more frequent or severe with the addition of GC4419 (Table 4). Transient grade 3 hypokalemia, attributable to cisplatin as well as other factors, such as GI loss and poor oral intake, occurred in 15% of patients in the 90-mg arm versus 11% of patients in the 30-mg arm and 6% in the placebo arm. This adverse event was corrected with supplementation and was otherwise without clinical consequence.
TABLE 4.
Specific Toxicities of Interest Thought Attributable to Cisplatin or GC4419
Because superoxide reacts with nitric oxide, reduction of superoxide by GC4419 is expected to potentiate the activity of nitric oxide. This was reflected (Table 4) in an apparent dose-dependent increase in low-grade hypotension, without frank syncope, and in mild perioral tingling, both of which were transient and resolved promptly after GC4419 infusion (Table 4). One patient in the 90-mg arm who was taking metoprolol for pre-existing hypertension discontinued GC4419 in association with grade 3 orthostatic hypotension. However, overall, grade 3 hypotension was not increased with GC4419 compared with placebo.
An indwelling venous access device was used to facilitate repeated GC4419 or placebo infusion for 196 (90%) of 217 patients; 155 (71%) of the 217 patients had an implantable port. There were 0.09 device-related adverse events per 100 days of use. These results were similar in the three study arms.
Tumor Outcomes
At the time of this report, 2-year post-IMRT follow-up is ongoing for locoregional control, distant metastasis, progression-free survival, and overall survival. In the interim, through 1 year, the numbers of observed deaths or progression events (locoregional failures and distant metastases) were similar in the three arms (data not shown). Full, formal assessment awaits completion of the follow-up period.
Exploratory Evaluations
Among 112 patients who had at least one observation of SOM and who took at least one dose of a narcotic from the start through the end of IMRT, the median total morphine equivalent per patient was 1,410 mg for placebo (n = 40), 1,053 mg for 30 mg of GC4419 (n = 40), and 752 mg for 90 mg of GC4419 (n = 32). Overall, 188 (87%) of the 217 treated patients (a similar percentage in all three arms) took at least one dose of a narcotic. The WHO score was 0 for 116 (62%) of these 188 patients and was less than 3 for 179 (95%) of the 188 patients at the time of first narcotic dose; this result also was comparable across the three arms. The median patient-reported subjective mouth and throat soreness, on the 5-point (0 to 4) OMDQ scale, was 0.5 for placebo and 1.0 for each GC4419 arm at baseline and increased to 2.0 for all three arms by week 4 of treatment.
Gastrostomy tube use varied by institutional practice. Overall, gastrostomy tubes were placed in 145 (67%) of 217 treated patients, and, as expected, gastrostomy use seemed to track with maximum WHO score. Placement was prophylactic (ie, placed before IMRT day 1) for 96 patients (placebo, n = 34; 90-mg arm, n = 28) and emergent (ie, on or after IMRT day 1) for 49 patients (placebo, n = 16; 90-mg arm, n = 13). Gastrostomy tubes were used for feeding at least once in 127 patients (placebo, n = 42; 90-mg arm, n = 36). Weight loss was comparable across the three arms (data not shown). The 2-year follow-up for xerostomia and trismus is in progress.
DISCUSSION
Oxidative stress plays a critical initiating role in the pathogenesis of OM. RT causes rapid production of large amounts of O2*—, which overwhelms the capacity of native superoxide dismutase enzymes to convert the O2*— to H2O2 and so triggers signaling pathways in the submucosa, which results in apoptosis of epithelial stem cells, consequent loss of epithelial renewal, atrophy, and mucosal ulceration.22 Unlike palliative measures, the dismutase mimetic GC4419 is hypothesized to arrest this process at its initiation. The results of this trial extend prior nonclinical and clinical observations, which suggest that GC4419 protects normal oral mucosa from damage associated with RT for locally advanced HNC. In a phase Ib/IIa trial, SOM incidence, duration, and severity (WHO grade 4 OM incidence) seemed reduced compared with historical expectations with either the 30-mg or 90-mg doses of GC4419, indicating that additional evaluation of both doses was warranted.24
In this trial, GC4419 at the 90-mg dose reduced SOM duration compared with placebo. SOM incidence and severity (grade 4 OM incidence) were reduced by 34% and 47%, respectively. SOM results in the placebo arm were consistent with historical data, whereas results for 90 mg of GC4419 seem clinically significant and favorable in the context of historical data.1,2 Results for the 30-mg arm were intermediate between those for the 90-mg and placebo arms, consistent with a dose-response relationship for efficacy.
The WHO OM score has the advantage of combining anatomic, functional, and symptomatic elements of OM and, when assessed by trained investigator-evaluators after a formal training process, is a well-defined, consistently and systematically applied measure of SOM that has become widely accepted for use in clinical trials of interventions intended to reduce SOM. As such, confirmation of reduced SOM by the WHO score in appropriately designed clinical trials may be considered strong evidence of efficacy.
GC4419 did not seem to increase the known toxicity of IMRT plus cisplatin at either dose. Toxicity attributable specifically to GC4419 was modest and transient, resolved with cessation or dose reduction of GC4419 infusion, and did not compromise treatment with GC4419 or chemoradiation. These effects likely are the result of potentiation of nitric oxide by GC4419, as previously reported with this class of compounds.26
Our exploratory data suggest that GC4419 may be associated with a decreased need for narcotic use. This requires more assessment, however; patients took narcotics for reasons other than OM, and no requirements or guidelines for narcotic prescribing and administration were used. Other exploratory assessments (gastrostomy tube use, OMDQ) are subject to similar limitations, such as different institutional practices, multiple clinical factors (eg, mucous production, dysgeusia, xerostomia, swallowing dysfunction) affecting outcomes, or broad effects of the multimodality disease management for this patient population.
It is critical that any supportive cancer care intervention not antagonize anticancer efficacy, and 1-year tumor outcomes from the prior phase Ib/IIa trial did not suggest compromise with GC4419.24 The 2-year follow-up results for this phase IIb trial are still pending.
Mechanistically, GC4419 is expected not to antagonize tumor response to chemoradiotherapy and may, under certain conditions, enhance it. Normal and cancer cells respond to O2*— and H2O2 differently. Specifically, normal cells tend to be more sensitive to elevations in superoxide, whereas moderate elevations in superoxide may serve to promote tumor growth. Conversely, significant increases in hydrogen peroxide flux are less well tolerated by cancer cells than by normal cells.27 Thus, GC4419, by converting RT-induced O2*— into H2O2, should reduce normal tissue toxicity while maintaining anticancer efficacy. Nonclinical data have demonstrated anticancer synergy between GC4419 and higher dose-fraction RT regimens, as used in stereotactic body RT, with that synergy reversible in an inducible-catalase (which efficiently removes H2O2) tumor model.28 These data form the basis for an ongoing phase I/II clinical trial of GC4419 plus stereotactic body RT for patients with locally advanced pancreatic cancer (ClinicalTrials.gov identifier: NCT03340974).
SOM remains a devastating toxicity, inadequately described by warning patients of mouth sores that may complicate their therapy, for which treatment options are limited. Our results demonstrate the potential for GC4419, an agent intended to interrupt SOM pathogenesis, to reduce the duration, incidence, and severity of radiation-induced SOM and thereby become an important new tool in the management of this adverse event in at-risk patients. Accordingly, a phase III confirmatory trial, the ROMAN trial, has been initiated (ClinicalTrials.gov identifier: NCT03689712).
ACKNOWLEDGMENT
We thank Chelsea Eckman, Sheryl Kelley, Chris Rao, Beth Rayworth, Amy Thorndike, and the rest of the Alira Health Clinical team for their study management; Robert Beardsley, Mel Sorensen, and Art Fratamico of Galera Therapeutics for helpful comments on the manuscript; and the investigators and institutions who enrolled patients to the study (as listed in the Appendix).
Appendix
The following researchers and institutions provided patients for this study:
Sanjiv S. Agarwala, MD, St. Luke's Cancer Center and Temple University, Easton, PA; Carryn Anderson, MD, University of Iowa, Iowa City, IA; Roberto Arevalo-Araujo, MD, Pasco Pinellas Cancer Center, Holiday, FL; Voichita Bar Ad, MD, Thomas-Jefferson University Hospital, Philadelphia, PA; Maura Barry, MD, The University of Vermont Cancer Center, Burlington, VT; Ariel Birnbaum, MD, Rhode Island Hospital, Providence, RI; Dukagjin Blakaj, MD, PhD, Ohio State University, Columbus, OH; Marcelo Bonomi, MD, Wake Forest Baptist Health, Winston-Salem, NC; Leander Cannick III, MD, Anmed Health Cancer Center, Anderson, SC; Daniel Clayburgh, MD, PhD, VA Portland Health Care, System, Portland, OR; Patrick Cobb, MD, FACP, St Vincent Frontier Cancer Center, Billings, MT; Kevin Collins, MD, JD, Fowler Family Center for Cancer Care, Jonesboro, AR; Kyle Colvett, MD, Mountain States Health Alliance Research Department, Johnson City, TN; Amy Curtis, MD, Spartanburg Medical Center, Spartanburg, SC; Bianca de Souza, MD, Henry Ford Allegiance Health, Jackson, MI; Neal Dunlap, MD, University of Louisville, Louisville, KY; Elizabeth Feldman, MS, DMD, UF Health Cancer Center at Orlando Health, Orlando, FL; Madhu Garg, MD, Montefiore Medical Center, Bronx, NY; Sharon M. Gordon, DDS, MPH, PhD, School of Dental Medicine at East Carolina University, Greenville, NC; Alan Gowan, DO, Scott & White Memorial Hospital and Clinic, Temple, TX; Charles Holladay, MD, Charleston Cancer Center, Charleston, SC; Anshu K. Jain, MD, Ashland-Bellefonte Cancer Center, Ashland, KY and Yale School of Medicine; Joseph Kelley, MD, PhD, University of Tennessee Medical Center, Knoxville, TN; Vernon King, MD, St Mary's Regional Cancer Center, Grand Junction, CO; Clint Daniel Kingsley, MD, Ellis Fischel Cancer Center - University of Missouri, Columbia, MO; Philip Kovoor, MD, Texas Oncology, Plano West, Plano, TX; Ganesh Kudva, Henry Ford Allegiance Health, Jackson, MI; Arielle Lee, MD, Tyler Hematology-Oncology, Tyler, TX; Christopher Lee, MD, Cancer Care Northwest, Spokane, WA; Steve P. Lee, MD, PhD, Long Beach Veteran Affairs Healthcare System, Long Beach, CA; Ronald Maggiore, MD, VA Portland Health Care System, Portland, OR; Maria Matsangou, MD, Northwestern University, Chicago, IL; Douglas Miller, MD, Jersey Shore University Medical Center, Neptune, NJ; Rex Mowat, MD, Toledo Clinic Cancer Center, Toledo, OH; Rupali Nabar, MD, UC Irvine Medical Center, Orange, CA; Chaitali Nangia, MD, UC Irvine Medical Center, Orange, CA; Jorge Nieva, MD, USC Norris Cancer Center, Los Angeles, CA; Celine Ord, MD, University of Arizona Cancer Center at Dignity Health St Joseph's, Phoenix, AZ; Shymal Patel, MD, University of Arizona Cancer Center at Dignity Health St Joseph's, Phoenix, AZ; Abhinand Peddada, MD, Renown Regional Medical Center, Reno, NV; Mercedes Porosnicu, MD, Wake Forest Baptist Health, Winston-Salem, NC; Waqas Rehman, Hunterdon Hematology Oncology, LLC, Flemington, NJ; Deborah P. Saunders, DND, B.Sc, Northeast Cancer Centre of Health Sciences North Northern Ontario School of Medicine, Sudbury, ON, Canada; Khalil Sultanem, MD, Jewish General Hospital, Montreal, QC, Canada; Michael Trendle, MD, Ellis Fischel Cancer Center - University of Missouri, Columbia, MO; Madhavi Venigalla, MD, Lakeland Regional Cancer Center, Lakeland, FL; Francois Vincent, MD, Centre Intégré Universitaire de Santé et de Services Sociaux de la Mauricie et du Centre du Québec Trois-Rivieres, QC, Canada; James Wheeler, MD, Goshen Center for Cancer Care, Goshen, IN; William Wisbeck, MD, Providence Regional Medical Center, Everett, WA; Francis Worden, MD, University of Michigan, Ann Arbor, MI.
FIG A1.
Numbers of patients with grade 4 oral mucositis of progressively greater length, 90 mg v placebo.
TABLE A1.
AEs Experienced by 10 or More (≥ 5%) of All Patients
TABLE A2.
AEs of Grade 3 or Greater Experienced by Two or More Patients (≥ 1%)
Footnotes
Presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5, 2018; Multinational Association of Supportive Care in Cancer Annual Meeting, Vienna, Austria, June 28-30, 2018; and American Society of Radiation Oncology Annual Meeting, San Antonio, TX, October 21-24, 2018.
Supported by Galera Therapeutics, Malvern, PA.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase IIb, Randomized, Double-Blind Trial of GC4419 Versus Placebo to Reduce Severe Oral Mucositis Due to Concurrent Radiotherapy and Cisplatin For Head and Neck Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carryn M. Anderson
Employment: University of Iowa Hospitals and Clinics, University of Iowa Hospitals and Clinics (I)
Research Funding: Galera Therapeutics (Inst)
Travel, Accommodations, Expenses: Elekta
Christopher M. Lee
Employment: Cancer Care Northwest
Leadership: Cancer Care Northwest, Gamma Knife of Spokane
Stock and Other Ownership Interests: Cancer Care Northwest, Gamma Knife of Spokane
Honoraria: Lilly Pharmaceutical, Bayer Pharmaceutical, Merck, Bristol-Myers Squibb
Consulting or Advisory Role: Elekta
Speakers' Bureau: Bayer, Merck, Lilly, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Kobold Medical, Axcend
Travel, Accommodations, Expenses: Bayer, Lilly, Merck, Bristol-Myers Squibb, Elekta
Deborah P. Saunders
Honoraria: Amgen, Pfizer
Consulting or Advisory Role: Amgen
Research Funding: Amgen
Travel, Accommodations, Expenses: Amgen
Amarinthia Curtis
Travel, Accommodations, Expenses: Galera Therapeutics
Neal Dunlap
Speakers' Bureau: AstraZeneca
Chaitali Nangia
Consulting or Advisory Role: Novartis
Speakers' Bureau: Merck, Novartis
Travel, Accommodations, Expenses: Novartis
Sharon M. Gordon
Research Funding: Galera Therapeutics (Inst)
Philip Kovoor
Stock and Other Ownership Interests: Gridalis
James Wheeler
Stock and Other Ownership Interests: Stryker, Syndax Pharmaceuticals, Koninklijke Phillips ADR, United Health Group, Zimmer BioMet, Novanta, Care.com, Hologic, Ionis Pharmaceuticals, Siemens AG, McKesson, United Guardian, Addus Homecare, CVS Health, HealthEquity, Walgreens Boots Alliance, Zoetis, Gilead Sciences (I), Stryker (I)
Sanjiv S. Agarwala
Consulting or Advisory Role: MSD
Travel, Accommodations, Expenses: MSD, Bristol-Myers Squibb
Madhur Garg
Speakers' Bureau: Varian Medical Systems
Francis Worden
Honoraria: Merck Sharp & Dohme, Eisai, Bristol-Myers Squibb, Loxo, Bayer, Cue, Fusion Pharmaceuticals
Consulting or Advisory Role: Merck, Loxo, Bristol-Myers Squibb, Eisai, Bayer, Cue Biopharma, Fusion Pharmaceuticals
Research Funding: Pfizer (Inst), Merck (Inst), Eisai (Inst), Bristol-Myers Squibb, Loxo, Oragenics
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bayer
Jon Holmlund
Employment: Galera Therapeutics
Leadership: Galera Therapeutics
Stock and Other Ownership Interests: Galera Therapeutics
Consulting or Advisory Role: Prometheus Laboratories, Aspire IRB/WIRB-Copernicus, OncoNano, Geistlich Pharma
Jeffrey M. Brill
Employment: Galera Therapeutics, Incyte Pharmaceuticals (I), Array BioPharma (I)
Stock and Other Ownership Interests: Galera Therapeutics, Incyte Pharmaceuticals (I), Array BioPharma (I)
Matt Downs
Consulting or Advisory Role: Statistics Collaborative (Inst)
Travel, Accommodations, Expenses: The Medicines Company, Seattle Genetics
Stephen T. Sonis
Employment: Biomodels, Primary Endpoint Solutions
Leadership: Biomodels, Primary Endpoint Solutions
Stock and Other Ownership Interests: Inform Genomics, Immunity Health, BioInsight Diagnostics
Patents, Royalties, Other Intellectual Property: Pending patent No. 15/953,544
Sanford Katz
Consulting or Advisory Role: Primary Endpoint Solutions, PRA Health Sciences, Alira Health, Galera Therapeutics, Second Genome
Travel, Accommodations, Expenses: Galera
No other potential conflicts of interest were reported.
REFERENCES
- 1.Henke M, Alfonsi M, Foa P, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: A randomized, placebo-controlled trial. J Clin Oncol. 2011;29:2815–2820. doi: 10.1200/JCO.2010.32.4103. [DOI] [PubMed] [Google Scholar]
- 2.Le QT, Kim HE, Schneider CJ, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: A randomized, placebo-controlled study. J Clin Oncol. 2011;29:2808–2814. doi: 10.1200/JCO.2010.32.4095. [DOI] [PubMed] [Google Scholar]
- 3.Levy A, Blanchard P, Bellefqih S, et al. Concurrent use of cisplatin or cetuximab with definitive radiotherapy for locally advanced head and neck squamous cell carcinomas. Strahlenther Onkol. 2014;190:823–831. doi: 10.1007/s00066-014-0626-0. [DOI] [PubMed] [Google Scholar]
- 4.Elting LS, Keefe DM, Sonis ST, et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: Demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer. 2008;113:2704–2713. doi: 10.1002/cncr.23898. [DOI] [PubMed] [Google Scholar]
- 5.Traynor AM, Richards GM, Hartig GK, et al. Comprehensive IMRT plus weekly cisplatin for advanced head and neck cancer: The University of Wisconsin experience. Head Neck. 2010;32:599–606. doi: 10.1002/hed.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HSM, Rutter CE, Lester-Coll NH, et al. Facility case volume and outcomes for intensity modulated radiation therapy in head and neck cancer. Int J Radiat Oncol Biol Phys. 2016;96:E325. [Google Scholar]
- 7.Russo G, Haddad R, Posner M, et al. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13:886–898. doi: 10.1634/theoncologist.2008-0024. [DOI] [PubMed] [Google Scholar]
- 8.Vera-Llonch M, Oster G, Hagiwara M, et al. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006;106:329–336. doi: 10.1002/cncr.21622. [DOI] [PubMed] [Google Scholar]
- 9.Elting LS, Cooksley CD, Chambers MS, et al. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68:1110–1120. doi: 10.1016/j.ijrobp.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 10.Nonzee NJ, Dandade NA, Patel U, et al. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: Results from a Northwestern University Costs of Cancer Program pilot study with head and neck and non–small-cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer. 2008;113:1446–1452. doi: 10.1002/cncr.23714. [DOI] [PubMed] [Google Scholar]
- 11.Allison RR, Ambrad AA, Arshoun Y, et al. Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer. 2014;120:1433–1440. doi: 10.1002/cncr.28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber C, Powell R, Ellis A, et al. Comparing pain control and ability to eat and drink with standard therapy vs Gelclair: A preliminary, double centre, randomised controlled trial on patients with radiotherapy-induced oral mucositis. Support Care Cancer. 2007;15:427–440. doi: 10.1007/s00520-006-0171-1. [DOI] [PubMed] [Google Scholar]
- 13.Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120:1453–1461. doi: 10.1002/cncr.28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambrecht M, Mercier C, Geussens Y, et al. The effect of a supersaturated calcium phosphate mouth rinse on the development of oral mucositis in head and neck cancer patients treated with (chemo)radiation: A single-center, randomized, prospective study of a calcium phosphate mouth rinse + standard of care versus standard of care. Support Care Cancer. 2013;21:2663–2670. doi: 10.1007/s00520-013-1829-0. [DOI] [PubMed] [Google Scholar]
- 15.Leenstra JL, Miller RC, Qin R, et al. Doxepin rinse versus placebo in the treatment of acute oral mucositis pain in patients receiving head and neck radiotherapy with or without chemotherapy: A phase III, randomized, double-blind trial (NCCTG-N09C6 [Alliance]) J Clin Oncol. 2014;32:1571–1577. doi: 10.1200/JCO.2013.53.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao NG, Trotti A, Kim J, et al. Phase II multicenter trial of Caphosol for the reduction of mucositis in patients receiving radiation therapy for head and neck cancer. Oral Oncol. 2014;50:765–769. doi: 10.1016/j.oraloncology.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarvizadeh M, Hemati S, Meidani M, et al. Morphine mouthwash for the management of oral mucositis in patients with head and neck cancer. Adv Biomed Res. 2015;4:44. doi: 10.4103/2277-9175.151254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong KH, Kuciejewska A, Sharabiani MTA, et al. A randomised controlled trial of Caphosol mouthwash in management of radiation-induced mucositis in head and neck cancer. Radiother Oncol. 2017;122:207–211. doi: 10.1016/j.radonc.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sio TT, Le-Rademacher JG, Leenstra JL, et al. Effect of doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash vs placebo on radiotherapy-related oral mucositis pain: The Alliance A221304 randomized clinical trial. JAMA. 2019;321:1481–1490. doi: 10.1001/jama.2019.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Food and Drug Administration http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/125103s0120lbl.pdf Palifermin (Kepivance) prescribing information.
- 21.Spielberger R, Stiff P, Bensinger W, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 22.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 23.Riley DP, Schall OF. Structure-activity studies and the design of synthetic superoxide dismutase (SOD) mimetics as therapeutics, in van Eldik R, Bowman-James K (eds): Advances in Inorganic Chemistry. XXXX, Academic Press, 2006, pp 233-263. [Google Scholar]
- 24.Anderson CM, Sonis ST, Lee CM, et al. Phase 1b/2a trial of the superoxide dismutase mimetic GC4419 to reduce chemoradiotherapy-induced oral mucositis in patients with oral cavity or oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2018;100:427–435. doi: 10.1016/j.ijrobp.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Elteren P. On the combination of independent two-sample tests of Wilcoxon. Bulletin of the International Statistical Institute. 1960;37:351–361. [Google Scholar]
- 26. https://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=57718 US Food and Drug Administration: Nitrolingual: nitroglycerin spray, metered.
- 27.Buettner GR, Ng CF, Wang M, et al. A new paradigm: Manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med. 2006;41:1338–1350. doi: 10.1016/j.freeradbiomed.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Story MD, Sishc BJ, Polsdofer EM, et al. The radioprotector GC4419 ameliorates radiation induced lung fibrosis while enhancing the response of non–small-cell lung cancer tumors to high dose per fraction radiation exposures. Int J Radiat Oncol Biol Phys. 2018;102:S187. [Google Scholar]