Abstract
PURPOSE
We sought to generate informed and considered opinions regarding acceptable secondary uses of deidentified health information and consent models for oncology learning health care systems.
METHODS
Day-long democratic deliberation sessions included 217 patients with cancer at four geographically and sociodemographically diverse sites. Patients completed three surveys (at baseline, immediately after deliberation, and 1-month follow-up).
RESULTS
Participants were 67.3% female, 21.7% black, and 6.0% Hispanic. The most notable changes in perceptions after deliberation related to use of deidentified medical-record data by insurance companies. After discussion, 72.3% of participants felt comfortable if the purpose was to make sure patients receive recommended care (v 79.5% at baseline; P = .03); 24.9% felt comfortable if the purpose was to determine eligibility for coverage or reimbursement (v 50.9% at baseline; P < .001). The most notable change about secondary research use related to believing it was important that doctors ask patients at least once whether researchers can use deidentified medical-records data for future research. The proportion endorsing high importance decreased from baseline (82.2%) to 68.7% immediately after discussion (P < .001), and remained decreased at 73.1% (P = .01) at follow-up. At follow-up, non-Hispanic whites were more likely to consider it highly important to be able to conduct medical research with deidentified electronic health records (96.8% v 87.7%; P = .01) and less likely to consider it highly important for doctors to get a patient’s permission each time deidentified medical record information is used for research (23.2% v 51.6%; P < .001).
CONCLUSION
This research confirms that most patients wish to be asked before deidentified medical records are used for research. Policies designed to realize the potential benefits of learning health care systems can, and should be, grounded in informed and considered public opinion.
INTRODUCTION
Learning health care systems (LHS) are evolving to leverage routinely collected patient data to inform medical discovery, care, and quality, with traditional distinctions between clinical practice, quality improvement, and research becoming less clear.1-6 Therefore, the ethical and social implications of these developments should be carefully considered.5,7-10
New ethical frameworks proposed for LHS implementation include recognizable obligations from research ethics, including the duty to respect the dignity of the persons contributing data and to avoid imposing nonclinical burdens and risks.7,11 However, the moral presumption shifts in favor of learning, with professionals and institutions obligated to conduct learning activities and patients obligated to contribute their data, laying the groundwork for providing disclosure without necessarily securing explicit consent for participation.7
Empirical research suggests that many patients support the use of medical information for research but also identifies the manner of consent to be important and challenging.12-16 Prior studies suggest that patients with cancer may be more likely to value electronic health information exchange17 and more willing to share sensitive genetic information for research.18 Although we have previously reported perspectives of patients with cancer on the ethical implementation of LHS for oncology care, using standard survey and interview methods,19,20 those findings are limited by respondents’ lack of information and the time necessary to deliberate fully about complex scientific, regulatory, and ethical considerations. Bioethicists have recently begun to embrace deliberative democracy (DD) approaches,21-28 which, unlike standard surveys, can solicit opinions in a manner that is more consistent with normative models of an informed, thoughtful, and community-oriented public. Deliberative procedures emphasize offering reasons and arguments for and against different policies in a cooperative process, rather than simply expressing one's own settled opinions. Participants are encouraged to reconsider their ex ante opinions in light of the interests, perspectives, and arguments of their fellow deliberators. Such approaches have proven to be illuminating in a wide variety of other contexts.29-36
We used a DD approach to generate the informed and considered opinions and recommendations of patients with cancer regarding acceptable secondary uses of their deidentified health information and potential approaches for notification and consent in the context of an LHS, using the example of ASCO’s CancerLinQ.
MATERIALS AND METHODS
Study Design and Recruitment
Deliberative sessions were held at four geographically and sociodemographically diverse sites between June 2017 and May 2018. Participants attended day-long events including educational presentations and facilitated small-group discussions, and completed three surveys. Participants completed survey 1 before the start of any presentations or discussions (baseline), survey 2a immediately after the first small-group discussion about disclosure and consent policies, survey 2b immediately after the second small-group discussion about data use and governance policies, and survey 3 1 month after the session (follow-up).
Patients with cancer who were at least 18 years old were recruited from three sites by oncology providers and staff circulating fliers, and from one site by posting the opportunity on a Website. Eligible patients provided verbal informed consent to participate by telephone and were compensated with $100 for attending the event and $25 for completing the final survey. This study was deemed exempt by the University of Michigan’s Institutional Review Board.
Procedures
Deliberation attendees were randomly assigned to tables of four to eight persons (mean, 6) and one trained facilitator, with eight to 10 tables per deliberation. Each deliberation had two main small-group discussions, each preceded by educational presentations by experts in ethics and LHS, including CancerLinQ, and opportunities for patients to ask questions. Discussions were audio recorded, transcribed, and deidentified.
Participants heard two original, 45-minute educational presentations introducing the concept of LHS and describing CancerLinQ specifically. The first, entitled “Disclosure and Consent,” summarized LHS benefits and goals, practical and ethical considerations, and policy options for how to notify patients and obtain consent for data inclusion. The second, “Data Protection, Use, and Governance,” covered the potential uses and users of LHS data, ethical issues pertaining to data protection and system oversight, and policy options regarding to whom and for what purpose LHS data should be released.
After each presentation, participants were asked to discuss and vote to choose among prespecified policies corresponding to each of the discussion topics. Voting was intended to enhance discussion by encouraging people to take and defend a position on potential policies. Participants were encouraged to explain the rationale for their votes and to think like citizens who form a community to decide which policy would be the best for society. Qualitative analysis of the discussions requires more extensive description than can be accommodated in this manuscript and is presented separately (Jones et al, manuscript submitted for publication).
Surveys
Survey instruments (Data Supplement) were developed using literature review and input from experts. Some items were drawn from previously validated instruments; others were adapted from prior instruments. We cognitively pretested the full instruments using verbal probing and think-aloud reasoning.37
Measures.
Sociodemographics, clinical features, and health experience–related factors.
Before deliberation, participants self-reported standard sociodemographic and clinical characteristics, including age, sex, race, ethnicity, education, current health, cancer type, and whether cancer was metastatic or incurable, using items from our prior work.21 Satisfaction with health care, familiarity with legal requirements for health information confidentiality, and attitudes about health information privacy were evaluated using previously developed items.12,38
Comfort with secondary uses of health information.
At baseline, after small-group discussions, and 1 month later, we evaluated perceptions in several scenarios involving secondary use of electronic health information, using nine items adapted from prior studies.19,20,39 Participants were asked if they were comfortable (on a 4-point response scale from very uncomfortable to very comfortable, dichotomized for analysis) with various secondary use scenarios, after presenting a question stem describing a system collecting information on patients with cancer from routine clinical care about which patients are notified but not asked for explicit opt-in consent.
Attitudes about importance of secondary research use of data and consent for that use.
At three time points, we also evaluated patients’ perceptions regarding competing considerations of the need for research using secondary data and the need to gain consent for data use. The 5-point response scale was dichotomized for analysis (critically or very important v moderately, somewhat, or not at all important).
Statistical Analysis
We describe baseline sociodemographic, clinical, and health experience–related factors. We then describe comfort and attitudes and how they changed over time. Comparisons of how responses evolved after deliberation were performed by comparing responses at baseline versus after deliberation, and at baseline versus at follow-up, for the same person (restricted to those answering at both time points), using the McNemar test of dependent proportions. Finally, we describe and compare participants’ ultimate considered judgments, as measured by their postdeliberation follow-up comfort and attitudes, by race (dichotomized as non-Hispanic white v other), by educational attainment (dichotomized as some college or less v college graduate or more), and by age (younger than 60 years or 60 years or older). Analyses were conducted using SAS, version 9.4 (Cary, NC). P < .05 was considered significant.
RESULTS
Of 266 patients contacting us, 217 attended a day-long DD-session (82%); 201 completed the follow-up survey 3 (93% of those who attended a deliberation).
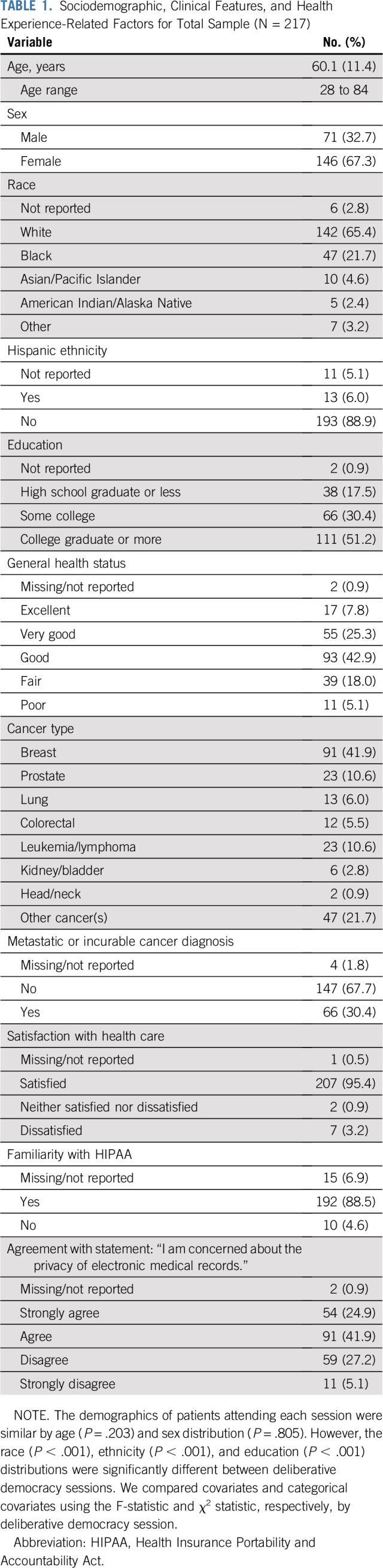
Sociodemographics, Clinical Features, and Health Experience–Related Factors of Participants
The mean age of participants was 60.1 years; 67.3% were women, 21.7% were black; 6.0% were Hispanic; and 17.5% had completed high school or less (Table 1). The most common cancer types were breast (41.9%), prostate (10.6%), and leukemia/lymphoma (10.6%). Nearly one-third (30.4%) reported having been told their cancer was metastatic or incurable. The majority agreed or strongly agreed that they were concerned about the privacy of electronic medical records (66.8%).
TABLE 1.
Sociodemographic, Clinical Features, and Health Experience-Related Factors for Total Sample (N = 217)
Evolution of Perspectives With Deliberation
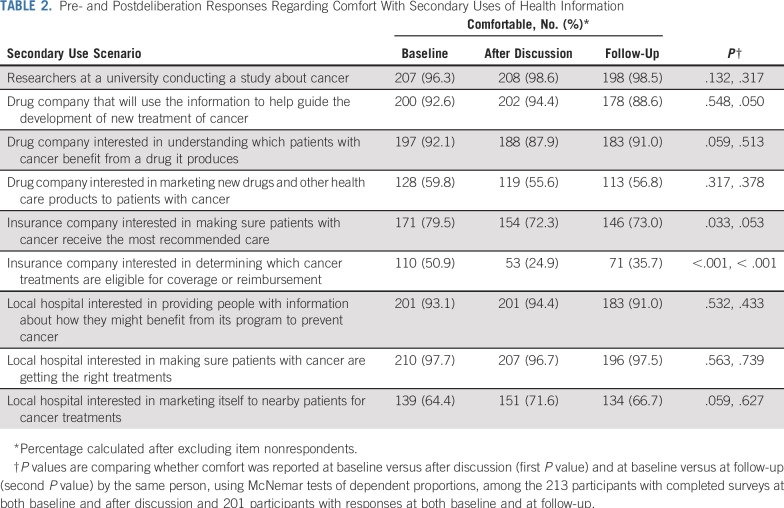
As shown in Table 2, comfort with the secondary use of deidentified medical information varied depending on context and, in some cases, did change after deliberation. At baseline, most participants were comfortable with researchers at a university conducting a study about cancer (96.3%). This did not change with deliberation.
TABLE 2.
Pre- and Postdeliberation Responses Regarding Comfort With Secondary Uses of Health Information
When local hospitals were the users, almost all participants were comfortable if the purpose was making sure patients with cancer were getting the right treatments (97.7%) or providing people with information about how they might benefit from the hospitals’ programs to prevent cancer (93.1%). However, only 64.4% were comfortable at baseline if local hospitals were using the data to market themselves to nearby patients. The proportion of those indicating comfort with these secondary use scenarios remained relatively consistent at follow-up.
When drug companies were the users, at baseline, most participants were comfortable if the information was being used to help develop new treatments (92.6%) or understand which patients benefit from certain drugs (92.1%), but only 59.8% were comfortable if the purpose was marketing. At follow-up, we observed a small but significant (P = .05) decrease in comfort with use of patient information by a drug company to develop new treatments, with 88.6% indicating comfort in this scenario at that time.
When insurance companies were the users, 79.5% of participants felt comfortable if the purpose was to make sure patients receive recommended care, but only 50.9% felt comfortable if the company was using the information to determine eligibility for coverage or reimbursement. Moreover, the most notable changes in perceptions after participating in the deliberation were related to use by insurance companies. After discussion, 72.3% of participants felt comfortable if the purpose was to make sure patients receive recommended care (change from baseline, P = .03), and only 24.9% felt comfortable if the company was using the information to determine eligibility for coverage or reimbursement (change from baseline, P < .001). At follow-up, 73.0% felt comfortable if the purpose was to make sure patients receive recommended care (change from baseline, P = .05), and only 35.7% felt comfortable if the company was using the information to determine eligibility for coverage or reimbursement (change from baseline, P < .001). The latter constituted the single scenario in which a majority of participants were not comfortable at follow-up after having had a chance to reflect on their deliberations.
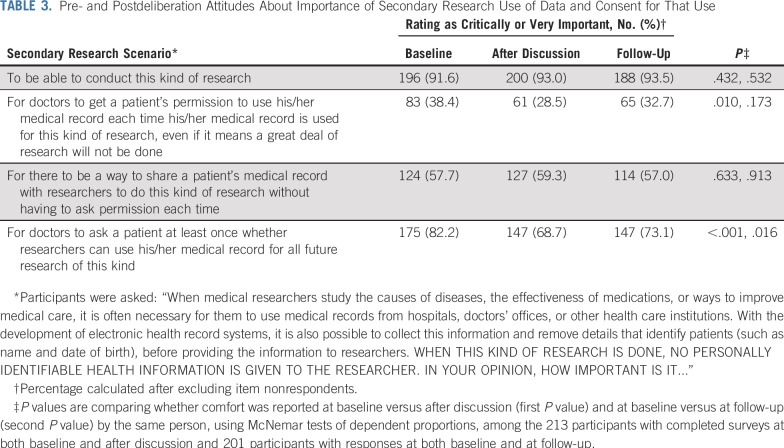
As shown in Table 3, an overwhelming majority of participants thought it was important to be able to conduct medical research using deidentified electronic health records (91.6% at baseline and 93.5% at follow-up). Only a minority expressed that it was important for doctors to get a patient’s permission each time deidentified medical record information is used for research, even if it means that a great deal of research will not be done. The proportion of participants responding this way was 38.4% at baseline, decreased to 28.5% after discussion (P for change = .01), and returned to a level similar (P = .17) to baseline by the time of follow-up (32.7%). A majority indicated that it was important for there to be a way to share a patient’s deidentified medical record for research purposes without having to ask permission each time (57.7% at baseline, 59.3% after discussion, and 57.0% at follow-up).
TABLE 3.
Pre- and Postdeliberation Attitudes About Importance of Secondary Research Use of Data and Consent for That Use
The most notable durable change observed with deliberation about secondary research uses related to the proportion of participants reporting that it was important for doctors to ask patients at least once whether researchers can use deidentified data from their medical records for future research. Although the majority thought this was important at all three time points, the proportion indicating the importance of this item decreased from baseline (82.2%) to 68.7% immediately after discussion (P < .001), and remained decreased at 73.1% (P = .01) at follow-up.
Differences in Considered Judgments by Participant Demographic Features
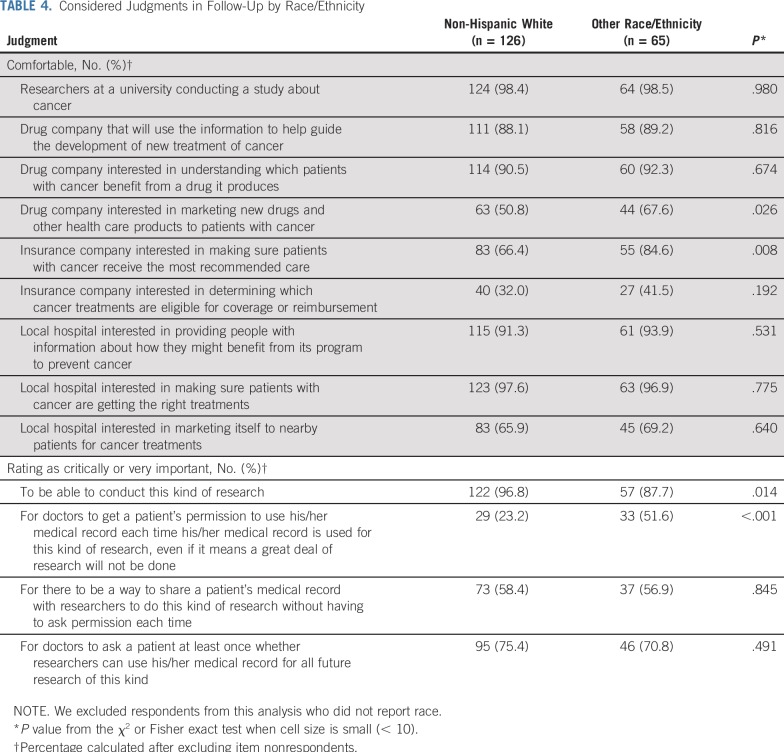
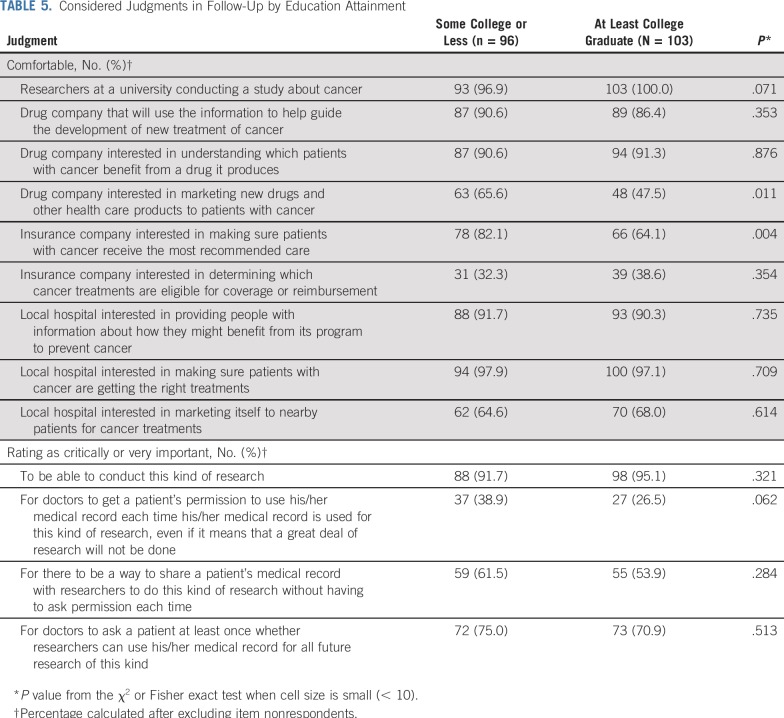
Perspectives differed by race/ethnicity (Table 4) and education (Table 5) for several items. Compared with participants of other races/ethnicities, non-Hispanic whites were less likely to be comfortable with drug companies marketing to patients with cancer (50.8% v 67.6%; P = .03) and insurance companies making sure patients with cancer receive the most recommended care (66.4% v 84.6%; P = .008). In addition, non-Hispanic whites were more likely to think it is highly important to be able to conduct medical research with deidentified electronic health records (96.8% v 87.7%; P = .01) and less likely to think it is highly important for doctors to get a patient’s permission each time deidentified medical record information is used for research (23.2% v 51.6%; P < .001). Compared with participants who were at least college graduates, participants with some college or less were more likely to be comfortable with drug companies marketing to patients with cancer (65.6% v 47.5%; P = .01) and insurance companies making sure patients with cancer receive the most recommended care (82.1% v 64.1%; P = .004). Perspectives on these items did not significantly differ by age.
TABLE 4.
Considered Judgments in Follow-Up by Race/Ethnicity
TABLE 5.
Considered Judgments in Follow-Up by Education Attainment
DISCUSSION
To our knowledge, this constitutes the first study to use the innovative approach of DD to generate the informed and considered judgments of a diverse sample of patients with cancer on the ethical implications of LHS implementation, using a real-world example. Growing recognition of the need to respect those whose data are collected motivates ongoing investigation to ensure that policies reflect patient values and preferences. Particularly illuminating is the finding that a majority of patients, albeit a smaller proportion after deliberation than at baseline, believe it is important to obtain consent at least once before embarking on secondary research using data collected. Given that these participants spent many hours in lectures and discussions that ensured they fully understood the promise of LHSs and the importance of complete data, it is noteworthy that many continued to desire at least some level of informed consent beyond notification. Moreover, we found striking the difference in informed and considered attitudes by race/ethnicity, whereby half of our participants whose race and ethnicity was other than non-Hispanic white desired consent to be obtained each time his or her medical record is used, even when clearly aware of the tradeoff that less research might be done.
Our findings regarding the common desire for at least one attempt to be made to obtain consent before secondary research uses of data add meaningfully to prior evidence collected using other techniques.15,16 In a random-digit dial-telephone survey of California consumers, the vast majority preferred to be asked for permission (86.7%) before sharing of unidentified electronic health data for research, with many preferring consent before each research project (44.8%).15 In a qualitative study using focus groups and interviews to examine patient perspectives on LHS, a notable theme was the significance of shared decision-making for informed consent and notification, with multiple participants indicating a strong preference for consent or notification to occur through a conversation directly with a physician.16 As our findings suggest, this desire to maintain a process for obtaining consent persists even after deliberation among patients with cancer specifically and should be viewed as relevant to inform LHS policy.
Our deliberation findings in this population of patients with cancer are comparable to those of our previous survey19 and others40 that suggest racial and ethnic minorities may be particularly concerned about the need to obtain consent in research. These differences likely reflect a number of factors, including the history of racism and legacy of past research violations undermining trust of the black community. Our findings that substantial differences in attitudes persisted even after deliberation are critical to note in ensuring LHSs are designed in ways that address the concerns of communities whose rights have repeatedly been violated in the past. Thus, for LHSs to be successful, communications should clarify the protections in place to ensure respect for these groups and the importance of such systems to gather data to inform care within these groups. Defining how greater individualization of care facilitated by LHSs may directly benefit racial and ethnic minorities should be a central focus of communication efforts.
Participants’ comfort with the use of deidentified medical information depended on both the user and the specific use of the data. Consistent with previous studies,19,20, most patients were comfortable with university researchers conducting a study about cancer and were comfortable when hospitals were the users; however, this comfort decreased when the use involved marketing. This did not increase with deliberation. Most participants were also comfortable when drug companies were interested in using deidentified data to develop treatments or to understand which patients with cancer benefit from certain drugs, but again, comfort was considerably lower when the use was marketing and did not increase with deliberation. Finally, the lower comfort among patients with cancer when insurance companies were the users is consistent with prior research in other populations.12 Interestingly, deliberation actually led to decreased comfort with drug company and insurance companies as secondary users of health information collected in LHSs. Because trust is essential to all LHSs, strict scrutiny of requests to use data from drug and insurance companies appears necessary to respect the reasoned and considered judgments of the patients with cancer who served as a citizen’s jury in the current study.
A strength of our study is its inclusion of diverse patients informed by experts and peers in a dynamic process. Limitations include the possibility that patients who chose to participate might have more favorable attitudes toward research and fewer privacy concerns than others. Although session materials were developed on the basis of the diverse viewpoints of practicing clinicians, professional-society staff, ethicists, and patients, it is possible that specific elements of those materials may have unduly influenced patients’ discussions and opinions. Translating findings into policy is challenging. Nevertheless, current and future LHS stakeholders can learn from the reasoned and considered judgments of patients who have engaged in extensive deliberation as representatives of their communities.
This study demonstrates the value of a deliberative approach for obtaining high-quality feedback from patients with cancer on complex ethical and scientific issues like those that arise in the context of LHSs. Particularly noteworthy is the finding that most patients, even after deliberation, endorse the importance of obtaining consent at least once before secondary research use of data. In-depth qualitative analysis of the deliberation discussions will be reported elsewhere and will be valuable to inform whether the current practice in CancerLinQ—notification with an opt-out consent model—sufficiently addresses the preferences and values of patients. Most importantly, this research confirms the need to continue to ground policy in informed and considered public opinion while seeking to realize the potential benefits of these systems. It should also inspire the application of deliberative methods to address other challenging ethical and policy decisions relevant to patients with cancer.
ACKNOWLEDGMENT
We thank all the individuals who participated in this study: the external advisors, the DD session facilitators, and presenters. We also thank Laura Damschroder and Rodney Hayward for contributions to study design.
The ideas and opinions expressed in this article do not represent any position or policy of the National Institutes of Health, the Department of Health and Human Services, or the US government or of the American Society of Clinical Oncology.
Footnotes
Supported by the National Institutes of Health (Grant No. R01 CA201356).
See accompanying Editorial on page 3176
AUTHOR CONTRIBUTIONS
Conception and design: Reshma Jagsi, Rebecca Spence, Raymond De Vries, Sarah T. Hawley, Sage Bolte, Angela R. Bradbury
Financial support: Reshma Jagsi, Sarah T. Hawley
Administrative support: Reshma Jagsi, Chris Krenz, Michele Gornick, Richard L. Schilsky
Provision of study material or patients: Michele Gornick, , Robin Zon, Sage Bolte, Navid Sadeghi, Angela R. Bradbury
Collection and assembly of data: Reshma Jagsi, Rochelle D. Jones, Chris Krenz, Michele Gornick, Rebecca Spence, Raymond De Vries, Sarah T. Hawley, Robin Zon, Sage Bolte
Data analysis and interpretation: Reshma Jagsi, Kent A. Griffith, Chris Krenz, Michele Gornick, Rebecca Spence, Raymond De Vries, Navid Sadeghi, Richard L. Schilsky, Angela R. Bradbury
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of Public Deliberation on Patient Attitudes Regarding Consent and Data Use in a Learning Health Care System for Oncology
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Reshma Jagsi
Employment: University of Michigan
Stock and Other Ownership Interests: Equity Quotient
Consulting or Advisory Role: Amgen, Vizient
Research Funding: AbbVie (Inst)
Travel, Accommodations, Expenses: Amgen
Robin Zon
Consulting or Advisory Role: MedPro Specialty Advisory Board
Richard L. Schilsky
Research Funding: AstraZeneca (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Roche (Inst), Eli Lilly (Inst), Merck (Inst), Pfizer (Inst), Boehringer Ingelheim (Inst)
Travel, Accommodations, Expenses: Varian
Angela R. Bradbury
Consulting or Advisory Role: AstraZeneca Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Abernethy AP, Etheredge LM, Ganz PA, et al. Rapid-learning system for cancer care. J Clin Oncol. 2010;28:4268–4274. doi: 10.1200/JCO.2010.28.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrager J, Tenenbaum JM. Rapid learning for precision oncology. Nat Rev Clin Oncol. 2014;11:109–118. doi: 10.1038/nrclinonc.2013.244. [DOI] [PubMed] [Google Scholar]
- 3.Sanders JC, Showalter TN. How big data, comparative effectiveness research, and rapid-learning health-care systems can transform patient care in radiation oncology. Front Oncol. 2018;8:155. doi: 10.3389/fonc.2018.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah A, Stewart AK, Kolacevski A, et al. Building a rapid learning health care system for oncology: Why CancerLinQ collects identifiable health information to achieve its vision. J Clin Oncol. 2016;34:756–763. doi: 10.1200/JCO.2015.65.0598. [DOI] [PubMed] [Google Scholar]
- 5.Larson EB. Building trust in the power of “big data” research to serve the public good. JAMA. 2013;309:2443–2444. doi: 10.1001/jama.2013.5914. [DOI] [PubMed] [Google Scholar]
- 6.Chambers DA, Feero WG, Khoury MJ. Convergence of implementation science, precision medicine, and the learning health care system: A new model for biomedical research. JAMA. 2016;315:1941–1942. doi: 10.1001/jama.2016.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faden RR, Kass NE, Goodman SN, et al. An ethics framework for a learning health care system: A departure from traditional research ethics and clinical ethics. Hastings Cent Rep. 2013;43:S16–S27. doi: 10.1002/hast.134. [DOI] [PubMed] [Google Scholar]
- 8.Grady C, Wendler D. Making the transition to a learning health care system. Commentary. Hastings Cent Rep. 2013;43:S32–S33. doi: 10.1002/hast.137. [DOI] [PubMed] [Google Scholar]
- 9.McLennan S, Kahrass H, Wieschowski S, et al. The spectrum of ethical issues in a learning health care system: A systematic qualitative review. Int J Qual Health Care. 2018;30:161–168. doi: 10.1093/intqhc/mzy005. [DOI] [PubMed] [Google Scholar]
- 10.Kass NE, Faden RR, Goodman SN, et al. The research-treatment distinction: A problematic approach for determining which activities should have ethical oversight. Hastings Cent Rep. 2013;43:S4–S15. doi: 10.1002/hast.133. [DOI] [PubMed] [Google Scholar]
- 11.Psek WA, Stametz RA, Bailey-Davis LD, et al. Operationalizing the learning health care system in an integrated delivery system. EGEMS (Wash DC) 2015;3:1122. doi: 10.13063/2327-9214.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damschroder LJ, Pritts JL, Neblo MA, et al. Patients, privacy and trust: Patients’ willingness to allow researchers to access their medical records. Soc Sci Med. 2007;64:223–235. doi: 10.1016/j.socscimed.2006.08.045. [DOI] [PubMed] [Google Scholar]
- 13.Caine K, Hanania R. Patients want granular privacy control over health information in electronic medical records. J Am Med Inform Assoc. 2013;20:7–15. doi: 10.1136/amiajnl-2012-001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell EA, Ohno-Machado L, Grando MA. Sharing my health data: A survey of data sharing preferences of healthy individuals. AMIA Annu Symp Proc. 2014;2014:1699–1708. [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KK, Joseph JG, Ohno-Machado L. Comparison of consumers’ views on electronic data sharing for healthcare and research. J Am Med Inform Assoc. 2015;22:821–830. doi: 10.1093/jamia/ocv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley M, James C, Alessi Kraft S, et al. Patient perspectives on the learning health system: The importance of trust and shared decision making. Am J Bioeth. 2015;15:4–17. doi: 10.1080/15265161.2015.1062163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckjord EB, Rechis R, Nutt S, et al. What do people affected by cancer think about electronic health information exchange? Results from the 2010 LIVESTRONG Electronic Health Information Exchange Survey and the 2008 Health Information National Trends Survey. J Oncol Pract. 2011;7:237–241. doi: 10.1200/JOP.2011.000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grande D, Asch DA, Wan F, et al. Are patients with cancer less willing to share their health information? Privacy, sensitivity, and social purpose. J Oncol Pract. 2015;11:378–383. doi: 10.1200/JOP.2015.004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagsi R, Griffith KA, Sabolch A, et al. Perspectives of patients with cancer on the ethics of rapid-learning health systems. J Clin Oncol. 2017;35:2315–2323. doi: 10.1200/JCO.2016.72.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones RD, Sabolch AN, Aakhus E, et al. Patient perspectives on the ethical implementation of a rapid learning system for oncology care. J Oncol Pract. 2017;13:e163–e175. doi: 10.1200/JOP.2016.016782. [DOI] [PubMed] [Google Scholar]
- 21.Gornick MC, Scherer AM, Sutton EJ, et al. Effect of public deliberation on attitudes toward return of secondary results in genomic sequencing. J Genet Couns. 2017;26:122–132. doi: 10.1007/s10897-016-9987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomlinson T, De Vries RG, Kim HM, et al. Effect of deliberation on the public’s attitudes toward consent policies for biobank research. Eur J Hum Genet. 2018;26:176–185. doi: 10.1038/s41431-017-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutmann A, Thompson D. Deliberating about bioethics. Hastings Cent Rep. 1997;27:38–41. [PubMed] [Google Scholar]
- 24.Kim SY, Wall IF, Stanczyk A, et al. Assessing the public’s views in research ethics controversies: Deliberative democracy and bioethics as natural allies. J Empir Res Hum Res Ethics. 2009;4:3–16. doi: 10.1525/jer.2009.4.4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. doi: 10.1177/1049732318791826. De Vries RG, Ryan KA, Gordon L, et al: Biobanks and the moral concerns of donors: A democratic deliberation. Qual Health Res . [epub ahead of print on August 10, 2018] [DOI] [PubMed] [Google Scholar]
- 26.De Vries R, Stanczyk AE, Ryan KA, et al. A framework for assessing the quality of democratic deliberation: Enhancing deliberation as a tool for bioethics. J Empir Res Hum Res Ethics. 2011;6:3–17. doi: 10.1525/jer.2011.6.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vries R, Stanczyk A, Wall IF, et al. Assessing the quality of democratic deliberation: A case study of public deliberation on the ethics of surrogate consent for research. Soc Sci Med. 2010;70:1896–1903. doi: 10.1016/j.socscimed.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carman K, Maurer M, Mallery C, et al: Community Forum Deliberative Methods Demonstration: Evaluating Effectiveness and Eliciting Public Views on Use of Evidence - Executive Summary. (Prepared by the American Institutes for Research under Contract No. 290-2010-00005). Rockville, MD, Agency for Healthcare Research and Quality, 2013.
- 29.Kim SY, Uhlmann RA, Appelbaum PS, et al. Deliberative assessment of surrogate consent in dementia research. Alzheimers Dement. 2010;6:342–350. doi: 10.1016/j.jalz.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim SY, Kim HM, Knopman DS, et al. Effect of public deliberation on attitudes toward surrogate consent for dementia research. Neurology. 2011;77:2097–2104. doi: 10.1212/WNL.0b013e31823648cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maxwell J, Rosell S, Forest PG. Giving citizens a voice in healthcare policy in Canada. BMJ. 2003;326:1031–1033. doi: 10.1136/bmj.326.7397.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosconi P, Castellani C, Villani W, et al. Cystic fibrosis: Tto screen or not to screen? Involving a citizens’ jury in decisions on screening carrier. Health Expect. 2015;18:1956–1967. doi: 10.1111/hex.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkin L, Paul C. Public good, personal privacy: A citizens’ deliberation about using medical information for pharmacoepidemiological research. J Epidemiol Community Health. 2011;65:150–156. doi: 10.1136/jech.2009.097436. [DOI] [PubMed] [Google Scholar]
- 34.Paul C, Nicholls R, Priest P, et al. Making policy decisions about population screening for breast cancer: the role of citizens’ deliberation. Health Policy. 2008;85:314–320. doi: 10.1016/j.healthpol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Rychetnik L, Carter SM, Abelson J, et al. Enhancing citizen engagement in cancer screening through deliberative democracy. J Natl Cancer Inst. 2013;105:380–386. doi: 10.1093/jnci/djs649. [DOI] [PubMed] [Google Scholar]
- 36.Street J, Duszynski K, Krawczyk S, et al. The use of citizens’ juries in health policy decision-making: A systematic review. Soc Sci Med. 2014;109:1–9. doi: 10.1016/j.socscimed.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Willis G. Cognitive interviewing as a tool for improving the informed consent process. J Empir Res Hum Res Ethics. 2006;1:9–24. doi: 10.1525/jer.2006.1.1.9. [DOI] [PubMed] [Google Scholar]
- 38.The California Healthcare Foundation Medical Privacy and Confidentiality Survey. Final Topline. 1999 https://www.chcf.org/wp-content/uploads/2017/12/PDF-topline.pdf
- 39.Grande D, Mitra N, Shah A, et al. Public preferences about secondary uses of electronic health information. JAMA Intern Med. 2013;173:1798–1806. doi: 10.1001/jamainternmed.2013.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garrison NA, Sathe NA, Antommaria AH, et al. A systematic literature review of individuals’ perspectives on broad consent and data sharing in the United States. Genet Med. 2016;18:663–671. doi: 10.1038/gim.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]