Abstract
PURPOSE
In the multicenter, open-label, phase III FOWARC trial, modified infusional fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) plus radiotherapy resulted in a higher pathologic complete response rate than fluorouracil plus radiotherapy in Chinese patients with locally advanced rectal cancer. Here, we report the final results.
METHODS
Adults ages 18 to 75 years with stage II/III rectal cancer were randomly assigned (1:1:1) to five cycles of infusional fluorouracil (leucovorin 400 mg/m2, fluorouracil 400 mg/m2, and fluorouracil 2.4 g/m2 over 48 hours) plus radiotherapy (46.0 to 50.4 Gy delivered in 23 to 25 fractions during cycles 2 to 4) followed by surgery and seven cycles of infusional fluorouracil, the same treatment plus intravenous oxaliplatin 85 mg/m2 on day 1 of each cycle (mFOLFOX6), or four to six cycles of mFOLFOX6 followed by surgery and six to eight cycles of mFOLFOX6. The primary end point was 3-year disease-free survival (DFS).
RESULTS
In total, 495 patients were randomly assigned to treatment. After a median follow-up of 45.2 months, DFS events were reported in 46, 39, and 46 patients in the fluorouracil plus radiotherapy, mFOLFOX6 plus radiotherapy, and mFOLFOX6 arms. In each arm, the probability of 3-year DFS was 72.9%, 77.2%, and 73.5% (P = .709 by the log-rank test), the 3-year probability of local recurrence after R0/1 resection was 8.0%, 7.0%, and 8.3% (P = .873 by the log-rank test), and the 3-year overall survival rate was 91.3%, 89.1%, and 90.7% (P = .971 by log-rank test), respectively.
CONCLUSION
mFOLFOX6, with or without radiation, did not significantly improve 3-year DFS versus fluorouracil with radiation in patients with locally advanced rectal cancer. No significant difference in outcomes was found between mFOLFOX6 without radiotherapy and fluorouracil with radiotherapy, which requires additional investigation of the role of radiotherapy in these regimens.
INTRODUCTION
Multimodal treatment of stage II or III rectal cancer typically involves total mesorectal excision (TME), preoperative concurrent fluoropyrimidine-based chemotherapy with ionizing radiation directed at the pelvis, and postoperative chemotherapy.1 Preoperative chemoradiotherapy has been shown to increase rates of pathologic complete response (pCR) and improve local disease recurrence rates relative to radiotherapy alone2-4; however, neither neoadjuvant radiotherapy nor chemoradiotherapy has been shown to improve disease-free survival (DFS) or overall survival (OS) in patients with locally advanced rectal cancer.5 Therefore, identification of early chemotherapy or total neoadjuvant therapy approaches that can improve survival outcomes is an important current research topic.
Several randomized controlled studies have evaluated the addition of oxaliplatin to a fluoropyridine for neoadjuvant treatment of stage II or III rectal cancer; however, the evidence is inconclusive. An interim analysis of the STAR-01 study showed that the addition of oxaliplatin to fluorouracil-based preoperative chemoradiotherapy significantly increased toxicity without improving the primary tumor response.6 Likewise, the National Surgical Adjuvant Breast and Bowel Project R-04 study found that the addition of oxaliplatin to fluorouracil- or capecitabine-based radiotherapy did not improve locoregional failure rates, DFS, or OS but did increase toxicity.7 In a 5-year follow-up of the ACCORD 12/0405-Prodige 2 study performed in patients with intermediate-risk rectal cancer, local recurrence rates, DFS, and OS were not improved with oxaliplatin, capecitabine, and radiotherapy compared with capecitabine and radiotherapy.8 In contrast, in the German CAO/ARO/AIO-04 trial, the addition of oxaliplatin to a fluorouracil plus radiotherapy regimen increased pCR rates and 3-year DFS rates, although it should be noted that oxaliplatin also was added to the post-surgery chemotherapy regimen in this study.9,10
In addition to evaluating neoadjuvant chemotherapy regimens, consideration of the role of radiotherapy as part of the neoadjuvant treatment of locally advanced rectal cancer is important, particularly given the associated toxicities.11-13 A review of one randomized phase III trial, six single-arm phase II trials, and one retrospective case series study performed in patients with operable locally advanced rectal cancer suggested that neoadjuvant chemotherapy could provide comparable outcomes to chemoradiotherapy.14
The randomized Neoadjuvant FOLFOX6 Chemotherapy With or Without Radiation in Rectal Cancer (FOWARC) study was designed to compare the efficacy and safety of modified infusional fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) with or without radiation and fluorouracil and leucovorin with radiation for the neoadjuvant treatment of locally advanced rectal cancer. Initial results demonstrated that mFOLFOX6-based preoperative chemoradiotherapy produced a higher pCR rate than fluorouracil-based chemoradiotherapy and that perioperative mFOLFOX6 alone produced a lower rate of pCR than either of the chemoradiotherapy regimens evaluated but led to a similar downstaging rate as fluorouracil plus radiotherapy.15 Here, we report the primary end point of the FOWARC study, 3-year DFS, as well as secondary end points, including 3-year local recurrence and OS.
METHODS
Study Design and Patients
The FOWARC study (ClinicalTrials.gov identifier: NCT01211210) was a multicenter, randomized, phase III study conducted at 15 hospitals in China in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was registered at ClinicalTrials.gov and approved by the central ethics committee of The Sixth Affiliated Hospital, Sun Yat-sen University, and local ethics committees of all participating hospitals.
Details of the study design and methods have been published previously.15 Eligible patients were ages 18 to 75 years with a confirmed histopathologic diagnosis of adenocarcinoma of the rectum and were considered suitable for curative resection. Tumors were clinically confirmed as stage II (T3 to 4N0) or stage III (T1 to 4N1 to 2) with a positive node defined as 1.0 cm or larger in diameter on imaging and with a distal border located less than 12 cm from the anal verge. Patients also were required to have an Eastern Cooperative Oncology Group performance status of 1 or less and adequate hematologic, liver, and renal function. Key exclusion criteria were metastatic disease, prior radiotherapy or chemotherapy, the presence of other cancers, clinically significant cardiac disease, and known peripheral neuropathy. All participants provided written informed consent.
Randomization and Masking
Patients were enrolled by the study investigators, and random assignment to treatment groups was performed using computer-generated randomization codes (sequential permuted blocks). Patients were randomly assigned (1:1:1) to receive neoadjuvant therapy with fluorouracil plus radiotherapy, mFOLFOX6 plus radiotherapy, or mFOLFOX6 without radiotherapy (mFOLFOX6 group). This was followed by TME and postoperative chemotherapy (fluorouracil or mFOLFOX6 in keeping with neoadjuvant treatment). Random assignment was conducted centrally, and patient assignment was implemented through a fax or Internet interface hosted by the Department of Medical Statistics at Sun Yat-sen University. This was an open-label study.
Study Treatments
Radiotherapy.
Radiotherapy was delivered at 1.8 to 2.0 Gy per day from Monday to Friday for a total of 23 to 25 fractions over 5 to 6 weeks (total dose, 46.0 to 50.4 Gy). Radiation was delivered with a minimum energy of 6-MV photons through a three-field or four-field box technique to the primary tumor and to mesorectal, presacral, and internal iliac lymph nodes.
Chemotherapy.
Patients randomly assigned to the fluorouracil plus radiotherapy group received preoperative treatment with five cycles of fluorouracil (leucovorin 400 mg/m2 intravenously followed by fluorouracil 400 mg/m2 intravenously and fluorouracil 2.4 g/m2 by 48-hour continuous intravenous infusion) with concurrent radiotherapy during cycles 2 to 4 and postoperative chemotherapy with seven cycles of fluorouracil. Patients in the mFOLFOX6 plus radiotherapy group received the same treatment schedule as the fluorouracil plus radiotherapy group with the addition of oxaliplatin 85 mg/m2 intravenously on day 1 of each chemotherapy treatment cycle. Patients randomly assigned to the mFOLFOX6 group received preoperative treatment with four to six cycles of mFOLFOX6 and postoperative chemotherapy with six to eight cycles of mFOLFOX6, with the addition of radiation before or after surgery at the physicians’ discretion.
Study Measurements and End Points
The primary end point was 3-year DFS, defined as the time between random assignment and the occurrence of macroscopically nonradical surgery, locoregional recurrence or metastasis, or death as a result of any cause. Secondary end points were locoregional recurrence, OS, relapse-free survival, and quality of life. Quality-of-life data will be reported in another article. All resection specimens were examined using a standardized protocol that included TNM classification according to the American Joint Committee on Cancer/International Union for Cancer Control (seventh edition). Tumor regression grade after preoperative treatment was evaluated semiquantitatively according to National Comprehensive Cancer Network guidelines.16
Statistical Analysis
Because two hypotheses were tested (mFOLFOX6 or mFOLFOX6 plus radiotherapy v fluorouracil plus radiotherapy), we applied the Bonferroni method for multiple comparisons to adjust the type I error to 2.5% and to control the overall type I error at 5%. A sample size of 165 patients per treatment group was calculated to provide a power of 81% with a type I error of 2.5% (two sided) to detect an estimated improvement in 3-year DFS from 60% in the fluorouracil plus radiotherapy group to 75% in either the mFOLFOX6 or the mFOLFOX6 plus radiotherapy group by log-rank test. This sample size estimation accounted for a 13% dropout rate, a 4-year accrual period, and a minimum follow-up of 3 years. According to the study protocol, the difference in DFS was the only hypothesis to be tested formally, and no formal equivalence margins were specified for secondary end points. The null hypothesis of equal DFS times in each treatment group was tested by a log-rank test. The hazard ratio (HR) and the corresponding 95% CI were estimated with a mixed-effects Cox model using study center as the random effect. The same procedures were used to compare local recurrence and OS. All analyses with regard to survival and local recurrence followed the intention-to-treat (ITT) principle. Prognostic factors for 3-year DFS and local recurrence were investigated in a multivariable analysis that included the variables chemotherapy regimen, patient age, patient sex, cT (cT4 v cT2 to 3), cN (cN1 to 2 v cN0), mesorectal facia, tumor length, distance from anal verge, pretreatment carcinoembryonic antigen (CEA), ypTNM stage, tumor regression grade, tumor deposits, vascular penetration, perineural invasion, and circumferential resection margin. All data for other end points were descriptive and presented as mean (standard deviation [SD]) or median (minimum to maximum). All statistical analyses were conducted using SPSS version 23.0 software (IBM Corporation, Chicago, IL).
RESULTS
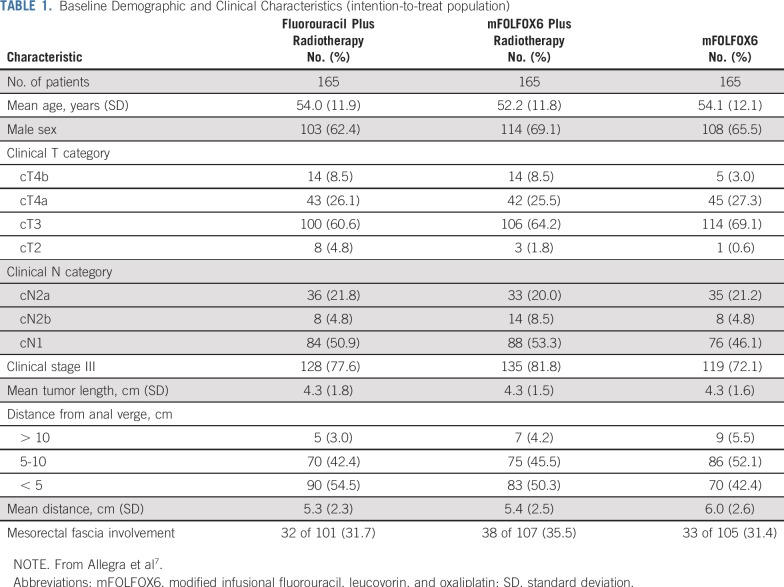
The FOWARC trial profile is shown in Figure 1. Between June 9, 2010, and February 15, 2015, 495 patients were recruited and randomly assigned to treatment (165 patients in each treatment group; ITT population). Baseline demographics and clinical characteristics were well matched among the three treatment groups (Table 1).
FIG 1.
Trial profile. mFOLFOX6, modified infusional fluorouracil, leucovorin, and oxaliplatin; PP, per protocol. (*) excluded from PP analysis. (†) 8 patients received radiation.
TABLE 1.
Baseline Demographic and Clinical Characteristics (intention-to-treat population)
Twelve patients withdrew consent after enrollment, which left 158 patients eligible for preoperative fluorouracil plus radiotherapy, 162 patients eligible for mFOLFOX6 plus radiotherapy, and 163 patients eligible for mFOLFOX6 (modified ITT population). Surgery was performed in 141, 149, and 152 patients in each group, respectively. As previously reported,15 a higher pCR rate was observed in the mFOLFOX6 plus radiotherapy group (27.5%) compared with the fluorouracil plus radiotherapy group (14.0%) or mFOLFOX6 group (6.5%).
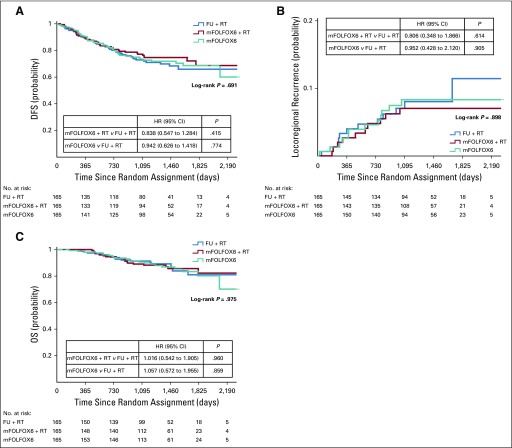
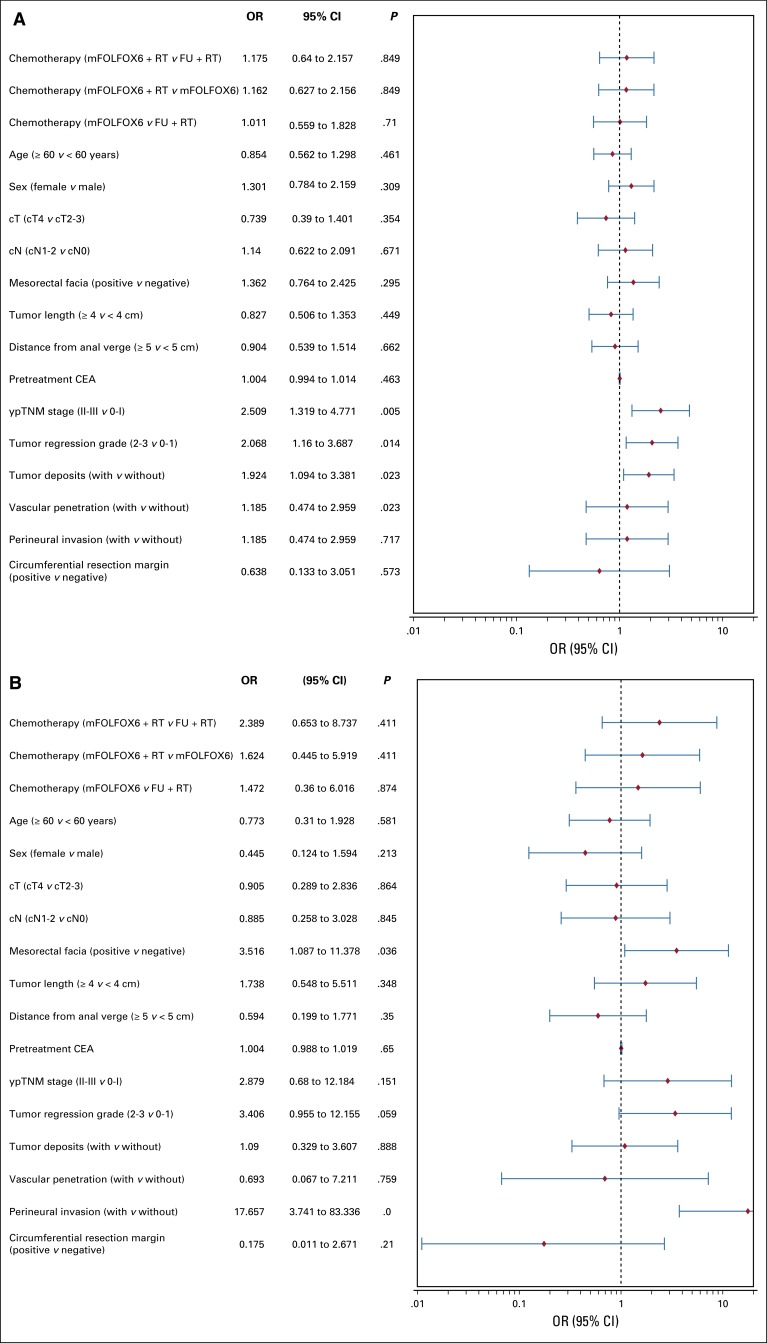
Median follow-up was 45.2 months (range, 1 to 83 months). In the ITT population, macroscopically nonradical surgery, locoregional recurrence or metastasis, or death as a result of any cause was observed in 131 patients (46 events in the fluorouracil plus radiotherapy group, 39 in the mFOLFOX6 plus radiotherapy group, and 46 in the mFOLFOX6 group). No significant between-group differences were found in terms of liver or lung metastases. At 3 years, the probability of DFS was 72.9% (SD, 3.6%), 77.2% (SD, 3.4%), and 73.5% (SD, 3.6%) in the fluorouracil plus radiotherapy, mFOLFOX6 plus radiotherapy, and mFOLFOX6 groups, respectively (P = .709 by log-rank test; Fig 2A). Relative to the fluorouracil plus radiotherapy group, the HR for DFS was 0.838 (95% CI, 0.547 to 1.284; P = .415) for the mFOLFOX6 plus radiotherapy group and 0.942 (95% CI, 0.626 to 1.418; P = .774) for the mFOLFOX6 group, and median DFS was not reached. In a multivariable analysis, factors associated with worse DFS were ypTNM stage II or III, tumor regression grade 2 or 3, presence of tumor deposits, and presence of perineural invasion (Fig 3A).
FIG 2.
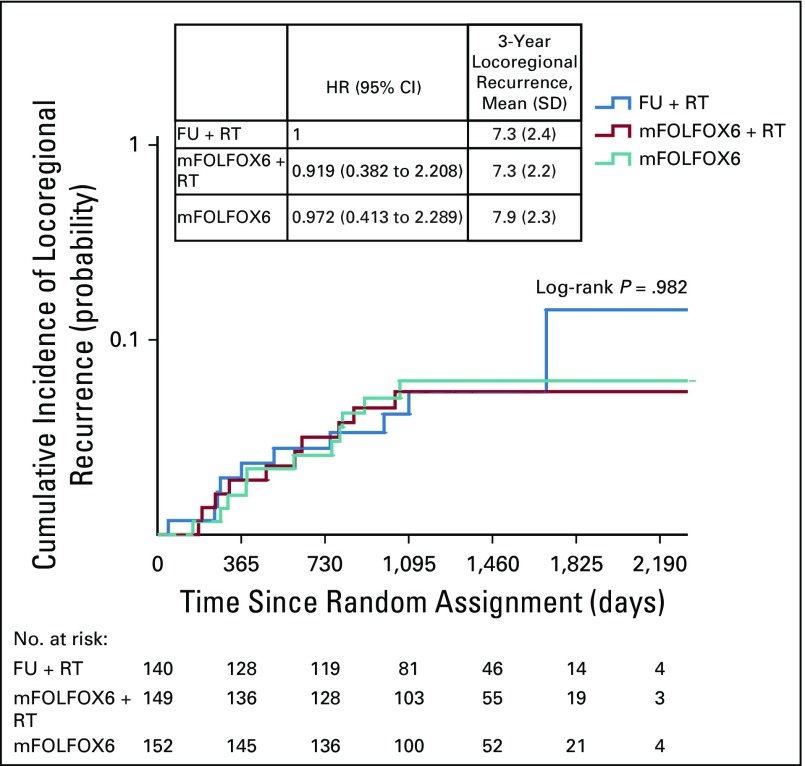
Kaplan-Meier estimates of (A) disease-free survival (DFS), (B) locoregional recurrence after R0/1 resection, and (C) overall survival (OS) in the intention-to-treat population. FU, fluorouracil; HR, hazard ratio; mFOLFOX6, modified infusional fluorouracil, leucovorin, and oxaliplatin; RT, radiotherapy.
FIG 3.
Multivariable analysis of the effects of prognostic factors on (A) 3-year disease-free survival and (B) locoregional recurrence. CEA, carcinoembryonic antigen; FU, fluorouracil; mFOLFOX6, modified infusional fluorouracil, leucovorin, and oxaliplatin; OR, odds ratio; RT, radiotherapy.
At 3 years, the probability of local recurrence after R0/1 resection was 8.0% (SD, 2.4%), 7.0% (SD, 2.1%), and 8.3% (SD, 2.3%) in the fluorouracil plus radiotherapy, mFOLFOX6 plus radiotherapy, and mFOLFOX6 groups, respectively (log-rank P = .873; Fig 2B). Relative to the fluorouracil plus radiotherapy group, the HR for local recurrence was 0.806 (95% CI, 0.348 to 1.866; P = .614) for the mFOLFOX6 plus radiotherapy group and 0.952 (95% CI, 0.428 to 2.120; P = .905) for the mFOLFOX6 group. In a univariable analysis, mesorectal facial involvement, elevated pretreatment CEA, ypTNM stage II or III, tumor regression grade 2 or 3, presence of tumor deposits, and presence of perineural invasion were prognostic factors for worse DFS (data not shown). However, in multivariable analysis, only clinical mesorectal facial involvement and presence of perineural invasion were significantly associated with worse DFS (Fig 3B).
Overall, 61 patients died during the study: 19, 20, and 22 in the fluorouracil plus radiotherapy, mFOLFOX6 plus radiotherapy, and mFOLFOX6 groups, respectively. The probability of OS at 3 years was 91.3% (SD, 2.3%), 89.1% (SD, 2.6%), and 90.7% (SD, 2.4%) in each group, respectively (intergroup P = .971 by the log-rank test; Fig 2C). Relative to the fluorouracil plus radiotherapy group, the HR for OS was 1.106 (95% CI, 0.542 to 1.905; P = .960) for the mFOLFOX6 plus radiotherapy group and 1.057 (95% CI, 0.572 to 1.955; P = .859) for the mFOLFOX6 group. Similar results were observed for the per-protocol population, which included 445 patients who underwent surgery (Appendix Figs A1, A2, and A3, online only).
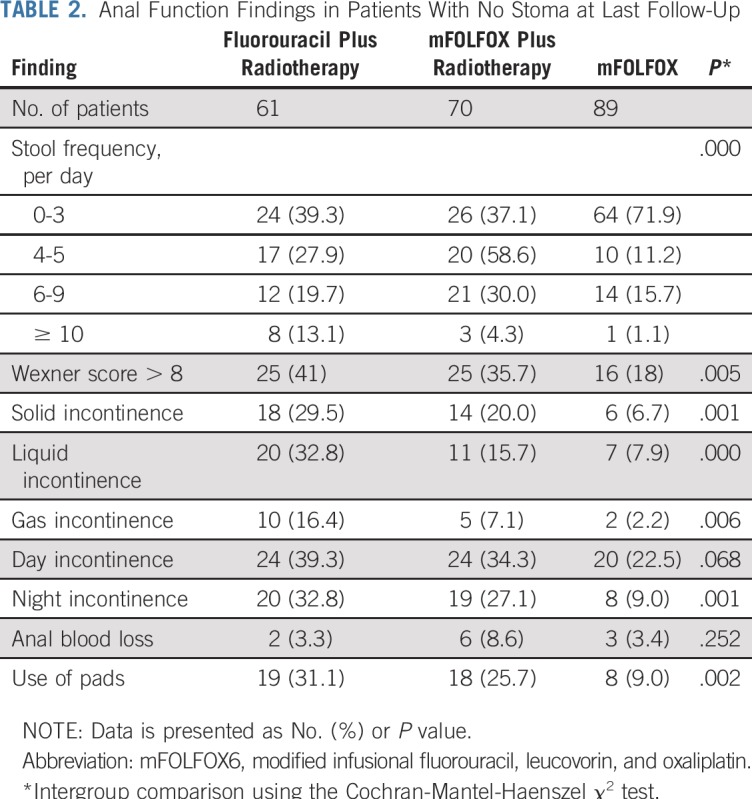
Anal function data were collected for patients with no stoma and no local recurrence who had available data at the most recent follow-up: 60, 67, and 88 patients in the fluorouracil plus radiotherapy, mFOLFOX6 plus radiotherapy, and mFOLFOX6 groups, respectively. Although not representative of the entire patient cohort, in this subpopulation of patients, those who had not received radiotherapy had better anal function in terms of the number of defecations per day, Wexner score, and liquid and nocturnal incontinence (Table 2).
TABLE 2.
Anal Function Findings in Patients With No Stoma at Last Follow-Up
DISCUSSION
Final results from the FOWARC study show that the addition of oxaliplatin to neoadjuvant fluorouracil-based chemoradiotherapy and to adjuvant chemotherapy after TME surgery resulted in a higher pCR rate and a higher proportion of good responses among patients with stage II to III rectal cancer but failed to improve 3-year DFS or reduce local recurrence. The results also show no significant difference in DFS or local recurrence rate with perioperative mFOLFOX6 without radiotherapy and standard fluorouracil-based chemoradiotherapy, which warrants additional investigation to clarify the role of radiotherapy in this treatment setting.
Preliminary results from the current study showed a higher pCR rate associated with neoadjuvant FOLFOX6 plus radiotherapy versus fluorouracil plus radiotherapy,15 and this finding is supported by a 2017 meta-analysis in which pooled data from eight clinical trials (including the FOWARC study) showed an improvement in pCR with the addition of oxaliplatin to neoadjuvant chemoradiotherapy (risk ratio, 1.208; 95% CI, 1.070 to 1.364; P = .002).17 Long-term follow-up data, however, revealed no significant difference in 3-year DFS between patients who received FOLFOX6 plus radiotherapy or fluorouracil plus radiotherapy (probability of 3-year DFS, 77.2% v 72.9%, respectively; P = .709). Despite this, the 3-year DFS rates in this study were similar to those reported by the positive A10-04 study (75.9% and 71.2% for neoadjuvant chemoradiotherapy with and without oxaliplatin, respectively).9 In addition, the 3-year DFS rates in the current study are similar to those reported for adjuvant chemotherapy with oxaliplatin plus fluorouracil and leucovorin or fluorouracil and leucovorin in colon cancer (78.2% and 72.9%, respectively).18 Furthermore, after long-term follow-up, we found no difference in rates of 3-year locoregional recurrence among the three treatment groups, and the values observed were within the range of previous reports (7.0% to 8.2% in the current study v 3.0% to 11.8% in previous reports).17,19
The ACCORD 12,20 A10-04,9 and PETACC-0621 trials and a study conducted in China by Jiao et al22 all investigated the addition of oxaliplatin to neoadjuvant chemoradiotherapy, using DFS as the primary end point. In contrast to the findings of the current study, A10-04 and the study by Jiao et al22 reported a longer DFS with the addition of oxaliplatin. Furthermore, a 2017 meta-analysis that combined DFS results from ACCORD 12, A10-04, and PETACC-06 found a significant DFS benefit associated with the addition of oxaliplatin to neoadjuvant chemotherapy (HR, 0.867; 95% CI, 0.741 to 0.992; P = .000), although the authors conceded that additional evidence is required.17 Therefore, combined with the results of the current study, current evidence suggests that the addition of oxaliplatin to neoadjuvant chemoradiotherapy may not be necessary. However, despite the lack of DFS benefit given the higher pCR rate achieved with the addition of oxaliplatin to neoadjuvant chemotherapy, this approach may enable more patients to adopt a watch-and-wait strategy. Under a watch-and-wait strategy, carefully selected patients who achieve a pCR after chemoradiotherapy avoid immediate surgery and are monitored carefully for recurrence, which enables organ preservation, although not enough evidence exists for this to be considered standard practice.23 Finally, it should be noted that the drawing of conclusions about neoadjuvant use of oxaliplatin in rectal cancer is complicated by the wide variety of chemotherapy regimens and doses of oxaliplatin used across published studies. For example, most previous studies, with the exception of A10-04, used only fluorouracil preoperatively in all treatment groups, whereas in the current study, patients who received mFOLFOX6 as neoadjuvant therapy also received mFOLFOX6 after surgery. In addition, there was wide variability in reported safety profiles and treatment compliance among previous studies, which adds difficulty when trying to draw conclusions from the current body of evidence for adding oxaliplatin to neoadjuvant therapy for rectal cancer.
To our knowledge, this randomized controlled study is the first to report a comparison between chemoradiotherapy and chemotherapy alone in the neoadjuvant setting for locally advanced rectal cancer. Although our trial design did not allow for a noninferiority comparison between mFOLFOX6 without radiation and standard chemoradiation, the results showed no significant difference in DFS or locoregional recurrence for patients who received mFOLFOX6 without routine use of radiation and those who received fluorouracil plus radiotherapy. Given these findings, additional investigation of mFOLFOX6 chemotherapy without radiotherapy is required to determine whether this approach can be used as upfront treatment, with perhaps reserving radiation for patients who do not respond to chemotherapy, have mesorectal fascia invasion threatened after chemotherapy, or have lateral lymph node metastasis. Several ongoing studies will provide additional insight into the utility of this strategy, including a single-arm phase II trial that is assessing intensified neoadjuvant chemotherapy with the triplet regimen fluorouracil, oxaliplatin, and irinotecan (ClinicalTrials.gov identifier: NCT02217020) and the randomized phase III PROSPECT trial (Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer; ClinicalTrials.gov identifier: NCT01515787).
Also to our knowledge, this report is the first in patients with locally advanced rectal cancer to show that perineural invasion is a significant independent predictor for local recurrence and DFS. This emphasizes the importance of perineural invasion and suggests that a need may exist for greater surveillance and a more intensive treatment strategy in patients with this risk factor. Pathologic staging after treatment remains the strongest prognostic factor for DFS, with higher staging being an important prognostic factor for poor response to neoadjuvant therapy, especially in patients who receive chemoradiotherapy. This suggests that use of alternative adjuvant chemotherapy in patients with stage III disease after chemoradiotherapy may be worth investigating. Of note, the multivariable analysis found no association between pretreatment CEA level and 3-year DFS or local recurrence. CEA as a prognostic tool in rectal cancer remains controversial, with some studies showing an association between elevated pretreatment CEA and survival and others showing no association.24-26 However, the equivocal findings are likely a result of inconsistent use of CEA level cutoffs among published studies. In addition, more recent studies suggest that the combination of both pre- and post-treatment CEA levels is a more accurate prognostic tool.27
As noted previously,15 several limitations of our trial deserve mention, including a lack of stratification, which may have introduced a staging or center bias, and the proportion of enrolled patients with a protocol violation (approximately 10%) or who were lost to follow-up (approximately 8%). However, it should be noted that the sample size calculation accounted for a dropout rate of 13%.
In summary, the final results from the FOWARC study show that mFOLFOX6 with or without radiation did not significantly improve 3-year DFS relative to fluorouracil plus radiation in patients with locally advanced rectal cancer. However, although the study was not set up to detect noninferiority among treatment arms, no difference in outcomes was found between patients who received mFOLFOX6 without radiotherapy and those who received standard fluorouracil plus radiotherapy, which warrants additional investigation to clarify the role of radiotherapy in neoadjuvant treatment of locally advanced rectal cancer. Long-term follow-up also is required to establish any differences in OS.
ACKNOWLEDGMENT
Writing support for this manuscript was paid for by Sanofi and provided by Jake Burrell at Rude Health Consulting. All forest plots were generated using Forest Plot Viewer (https://ntp.niehs.nih.gov/ntp/ohat/forestplot/forest_plotviewermanual_v1_1_508.pdf).
Appendix
FIG A1.
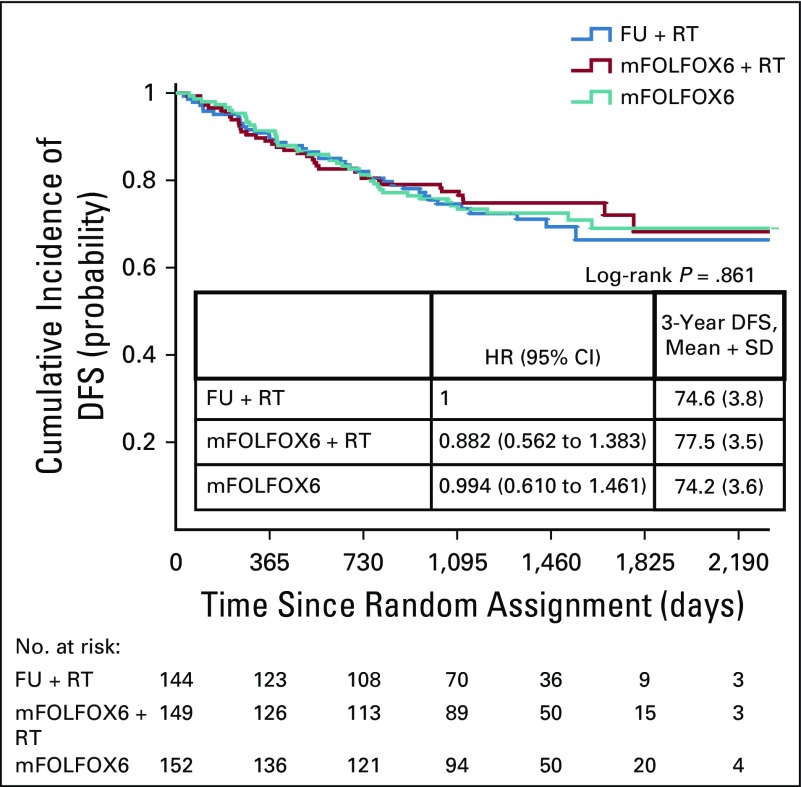
Kaplan-Meier estimates of disease-free survival (DFS) in the per-protocol population, which included 445 patients who underwent surgery. FU, fluorouracil; HR, hazard ratio; mFOLFOX6, modified infusional fluorouracil, leucovorin, and oxaliplatin; RT, radiotherapy; SD, standard deviation.
FIG A2.
Kaplan-Meier estimates of locoregional recurrence in the per-protocol population, which included 445 patients who underwent surgery. FU, fluorouracil; HR, hazard ratio; mFOLFOX6, modified infusional fluorouracil, leucovorin, and oxaliplatin; RT, radiotherapy; SD, standard deviation.
FIG A3.
Kaplan-Meier estimates of overall survival (OS) in the per-protocol population, which included 445 patients who underwent surgery. FU, fluorouracil; HR, hazard ratio; mFOLFOX6, modified infusional fluorouracil, leucovorin, and oxaliplatin; RT, radiotherapy; SD, standard deviation.
Footnotes
Presented at the 2018 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018.
Supported by National Key Clinical Discipline, China National Natural Science Foundation, grant numbers 81172040, 81372566, and 81472249.
Clinical trials information: NCT01211210.
AUTHOR CONTRIBUTIONS
Conception and design: Yanhong Deng, Pan Chi, Ping Lan, Lei Wang, Jianping Wang
Financial support: Jianping Wang, Yanhong Deng
Administrative support: Jianping Wang, Pan Chi, Ping Lan
Provision of study material or patients: Yanhong Deng, Lei Wang, Long Cui, Jie Cao, Hongbo Wei, Xiang Peng, Guanfu Cai, Ren Zhao, Zhongcheng Huang, Liang Kang
Collection and assembly of data: Yanhong Deng, Pan Chi, Ping Lan, Lei Wang, Long Cui, Daoda Chen, Jie Cao, Hongbo Wei, Xiang Peng, Zonghai Huang, Ren Zhao, Zhongcheng Huang, Lin Xu, Hongfeng Zhou, Yisheng Wei, Hao Zhang, Jian Zheng, Zhiyang Zhou, Yue Cai, Liang Kang, Meijin Huang, Xiaojian Wu, Junsheng Peng, Donglin Ren, Jianping Wang
Data analysis and interpretation: Yanhong Deng, Ping Lan, Weiqing Chen, Jianping Wang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
No potential conflicts of interest were reported.
REFERENCES
- 1.Benson AB, III, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:874–901. doi: 10.6004/jnccn.2018.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 3.De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev. 2013;(2):CD006041. doi: 10.1002/14651858.CD006041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy K, Pearson K, Fulton R, et al. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev. 2012;12:CD008368. doi: 10.1002/14651858.CD008368.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Rahbari NN, Elbers H, Askoxylakis V, et al. Neoadjuvant radiotherapy for rectal cancer: Meta-analysis of randomized controlled trials. Ann Surg Oncol. 2013;20:4169–4182. doi: 10.1245/s10434-013-3198-9. [DOI] [PubMed] [Google Scholar]
- 6.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: Pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–2780. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 7.Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: A phase III randomized clinical trial. J Natl Cancer Inst. 2015;107:djv248. doi: 10.1093/jnci/djv248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azria D, Doyen J, Jarlier M, et al. Late toxicities and clinical outcome at 5 years of the ACCORD 12/0405-PRODIGE 02 trial comparing two neoadjuvant chemoradiotherapy regimens for intermediate-risk rectal cancer. Ann Oncol. 2017;28:2436–2442. doi: 10.1093/annonc/mdx351. [DOI] [PubMed] [Google Scholar]
- 9.Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–989. doi: 10.1016/S1470-2045(15)00159-X. [DOI] [PubMed] [Google Scholar]
- 10.Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: Initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–687. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 11.Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: Increased bowel dysfunction in irradiated patients–a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 12.Lange MM, den Dulk M, Bossema ER, et al. Risk factors for faecal incontinence after rectal cancer treatment. Br J Surg. 2007;94:1278–1284. doi: 10.1002/bjs.5819. [DOI] [PubMed] [Google Scholar]
- 13.Bruheim K, Guren MG, Skovlund E, et al. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76:1005–1011. doi: 10.1016/j.ijrobp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Jalil O, Claydon L, Arulampalam T. Review of neoadjuvant chemotherapy alone in locally advanced rectal cancer. J Gastrointest Cancer. 2015;46:219–236. doi: 10.1007/s12029-015-9739-7. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: Initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. 2016;34:3300–3307. doi: 10.1200/JCO.2016.66.6198. [DOI] [PubMed] [Google Scholar]
- 16.Ryan R, Gibbons D, Hyland JM, et al. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141–146. doi: 10.1111/j.1365-2559.2005.02176.x. [DOI] [PubMed] [Google Scholar]
- 17.Zheng J, Feng X, Hu W, et al. Systematic review and meta-analysis of preoperative chemoradiotherapy with or without oxaliplatin in locally advanced rectal cancer. Medicine (Baltimore) 2017;96:e6487. doi: 10.1097/MD.0000000000006487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 19. doi: 10.1097/SLA.0000000000003530. Lefevre JH, Mineur L, Cachanado M, et al: Does a longer waiting period after neoadjuvant radiochemotherapy improve the oncological prognosis of rectal cancer?: Three-year follow-up results of the GRECCAR-6 randomized multicenter trial. J Clin Oncol 37, 2019 (suppl; abstr 483) [DOI] [PubMed] [Google Scholar]
- 20.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–1644. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 21. Schmoll H-J, Haustermans K, Price T, et al: Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine and oxaliplatin versus capecitabine alone in locally advanced rectal cancer: Disease-free survival results at interim analysis. Proc Am Soc Clin Oncol 32:3501, 2014. [Google Scholar]
- 22.Jiao D, Zhang R, Gong Z, et al. Fluorouracil-based preoperative chemoradiotherapy with or without oxaliplatin for stage II/III rectal cancer: A 3-year follow-up study. Chin J Cancer Res. 2015;27:588–596. doi: 10.3978/j.issn.1000-9604.2015.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernier L, Balyasnikova S, Tait D, et al. Watch-and-wait as a therapeutic strategy in rectal cancer. Curr Colorectal Cancer Rep. 2018;14:37–55. doi: 10.1007/s11888-018-0398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim CH, Lee SY, Kim HR, et al. Prognostic effect of pretreatment serum carcinoembryonic antigen level: A useful tool for prediction of distant metastasis in locally advanced rectal cancer following neoadjuvant chemoradiotherapy and total mesorectal excision. Medicine (Baltimore) 2015;94:e1291. doi: 10.1097/MD.0000000000001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Kim SH, Jang HS, et al. Preoperative elevation of carcinoembryonic antigen predicts poor tumor response and frequent distant recurrence for patients with rectal cancer who receive preoperative chemoradiotherapy and total mesorectal excision: A multi-institutional analysis in an Asian population. Int J Colorectal Dis. 2013;28:511–517. doi: 10.1007/s00384-012-1584-6. [DOI] [PubMed] [Google Scholar]
- 26.Yang KL, Yang SH, Liang WY, et al. Carcinoembryonic antigen (CEA) level, CEA ratio, and treatment outcome of rectal cancer patients receiving pre-operative chemoradiation and surgery. Radiat Oncol. 2013;8:43. doi: 10.1186/1748-717X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung S, Son SH, Kay CS, et al. Prognosis can be predicted more accurately using pre- and postchemoradiotherapy carcinoembryonic antigen levels compared to only prechemoradiotherapy carcinoembryonic antigen level in locally advanced rectal cancer patients who received neoadjuvant chemoradiotherapy. Medicine (Baltimore) 2016;95:e2965. doi: 10.1097/MD.0000000000002965. [DOI] [PMC free article] [PubMed] [Google Scholar]