Abstract
Introduction
In veterans, the prevalence of 12-month and lifetime alcohol use disorder (AUD) is 14.8% and 42.2%, respectively. Alcohol use disorder treatment is often plagued by medication discontinuation with relapse rates being as high as 39% in patients who sought treatment. One proposed benefit of long-acting injectable (LAI) medications is improved adherence. The purpose of this trial was to compare the difference in time to relapse between patients on oral and LAI naltrexone.
Methods
This study was a retrospective electronic chart review of patients with AUD who were treated with oral or LAI naltrexone at a Veteran's Affairs Medical Center from August 1, 2016, to July 31, 2018. The primary outcome assessed was time to relapse. Secondary outcomes for this study included medication possession ratio (MPR), comorbid mental health diagnosis, substance use, past pharmacological treatment, liver and kidney function, and enrollment in addiction-focused psychosocial therapy.
Results
Thirty-two patients met inclusion criteria. The median time to relapse was longer for those treated with LAI naltrexone versus oral naltrexone (150.5 days vs 50.5 days, P < .01). The MPR was similar among both groups (P = .47). No significant differences were found between the groups regarding safety outcomes.
Discussion
Results suggest that LAI naltrexone is associated with increased time to relapse and should be considered as a first-line option for patients. Given the retrospective nature and small sample size of this study, larger, randomized, controlled trials comparing LAI and oral naltrexone head to head would help determine most appropriate treatment for these patients.
Keywords: long-acting intramuscular naltrexone, long-acting injectable naltrexone, Vivitrol, oral naltrexone, naltrexone, alcohol use disorder, AUD, substance use disorder, SUD, veterans
Introduction
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition,1 alcohol use disorder (AUD) is a problematic pattern of alcohol use leading to clinically significant impairment or distress, characterized by at least 2 of the listed symptoms within a 12-month period. Symptoms include recurrent drinking resulting in failure to fulfill role obligations; recurrent drinking in hazardous situations; continued drinking despite alcohol-related social or interpersonal problems; evidence of tolerance; evidence of alcohol withdrawal or use of alcohol for relief or avoidance of withdrawal; drinking in larger amounts or over longer periods than intended; persistent desire or unsuccessful attempts to stop or reduce drinking; great deal of time spent obtaining, using, or recovering from alcohol; important activities given up or reduced because of drinking; continued drinking despite knowledge of physical or psychological problems caused by alcohol; and alcohol craving.1
In a study2 done to assess the burden of AUD on veterans, the prevalence of 12-month probable AUD and lifetime AUD was 14.8% and 42.2%, respectively. A study3 following patients over a 4-year time period showed only 39% were in remission, and at the end of the study, 46% still met the Diagnostic and Statistical Manual, 4th edition, criteria for alcohol dependence. Early remission is defined as abstaining from alcohol for 3 to 12 months, and sustained remission is defined as greater than 12 months.1 As age increases, so does the AUD-related mortality.2 The liver, gastrointestinal system, cardiovascular system, and nervous system can all undergo permanent damage from excessive and chronic alcohol intake. Early intervention is critical in managing this disorder. With remission, the body experiences health benefits, such as reversing liver damage, increasing quality of sleep, and reversing the immunocompromising effects of alcohol.4 Substance use disorders and withdrawal are among the most prevalent psychiatric disorders in the United States. Diagnosis of AUD can lead to 1.6 to 3.5 times greater likelihood of co-occurring mood and anxiety disorders.5 The rate of suicidal behavior and completed suicide is increased among those individuals with AUD and can be exacerbated by chronic alcohol use.1,6
Currently, the American Psychiatric Association7 recommends that naltrexone or acamprosate be offered to patients with moderate-to-severe AUD who have a goal of reducing alcohol consumption or achieving abstinence, who prefer pharmacotherapy or have not responded to nonpharmacologic treatments alone, and have no contraindications to the use of these medications. Naltrexone, a μ opioid receptor antagonist, is hypothesized to reduce drinking through modulation of the dopamine reward pathway, therefore, reducing the rewarding effects of alcohol.8 This medication has been associated with a benefit in decreased return to any drinking, decreased return to heavy drinking, decreased frequency of drinking days, and decreased frequency of heavy drinking days.7 Oral naltrexone is usually titrated to the target dose of 50 mg daily, and patients are given 380 mg every 28 days of the long-acting injectable (LAI) naltrexone. A trial period of oral naltrexone is not required for use of LAI naltrexone; however, it is common practice to establish tolerability with oral doses first.8,9 The use of LAI naltrexone may have benefits for adherence when compared with oral naltrexone because nonadherence is common among patients taking medication for alcohol use disorder.7 There have been several trials10,11 that show increased adherence to LAIs for disease states such as schizophrenia, bipolar disorder, and opioid use disorder.
Historically, medications for the treatment of AUD were not widely used with previous studies12,13 showing that counselor attitudes toward medication, physician-perceived treatment barriers, and patient willingness play a role in the decreased use of pharmacotherapy in AUD. In a study6 conducted in 2014, of the Veterans Health Administration patients with AUD, 3.1% received any form of naltrexone and only 0.24% of the patients received LAI naltrexone. Although about 40% of veterans have suffered from some sort of alcohol abuse during their lifetime, few are accessing appropriate treatment. This same study found that those patients who received the intramuscular injection utilized more outpatient and inpatient mental health services. Combined pharmacologic treatment and use of mental health resources have improved outcomes for patients struggling with AUD.6 These mental health resources include substance use monitoring, individual drug counseling, care management, or intensive outpatient treatment.9
According to the Centers for Disease Control,14 in 2010, the United States spent $28 billion on health care–related costs caused by AUD. Another $221 billion spent on AUD was related to decreased workplace productivity, collisions, and criminal justice–associated costs.14 AUD can cause significant morbidity and mortality, but treatment is often plagued by medication discontinuation and relapse. Currently, there are few published studies that directly compare outcomes of oral naltrexone versus LAI naltrexone. A study15 that reviewed 90-day alcohol-related hospital admissions per patient found that LAI naltrexone may not provide additional benefit that justifies the cost of the long-acting dosage form. If it is found that the use of LAI naltrexone can decrease time to relapse or improve adherence to medication treatment, its use may become more feasible. This study was designed to compare the effectiveness, defined by time to relapse, of LAI naltrexone versus oral naltrexone.
Methods
This study was approved by the Indiana University/Veterans Affairs Medical Center (VAMC) System Institutional Review Board. This study was a retrospective electronic chart review of patients with AUD who were treated as an outpatient with oral naltrexone or LAI naltrexone at a VAMC. Medication fill records from August 1, 2016, through July 31, 2018, were reviewed, and the patient list was generated from the computerized patient record system. All data was obtained using the patients' electronic medical records and data was deidentified.
Subjects prescribed either oral or LAI naltrexone were included if they were greater than 21 years of age, had a diagnosis of AUD or alcohol dependence based on ICD-9 and ICD-10 (International Classification of Diseases) codes, received treatment in the Substance Use Disorder Recovery Program (SUDRP) at the VAMC main campus, and were abstaining from alcohol use at medication initiation. Participants were excluded if they were prescribed naltrexone for any indication other than AUD, pregnant, initiated on either dosage form during hospital admission, discontinued LAI naltrexone after 1 dose, or relapse was not documented. Those prescribed oral naltrexone were randomized to match LAI naltrexone subjects at a 1:1 ratio. The objective of this study was to compare the use of LAI naltrexone versus oral naltrexone in patients with AUD. The primary end point of time to relapse was defined as patient, family, or friend volunteered report; hospitalization for alcohol intoxication; positive ethyl glucuronide; or elevated blood alcohol concentration. Secondary outcomes included medication possession ratio (MPR), other substance use, past pharmacologic treatment for alcohol use disorder with either acamprosate or disulfiram, liver and kidney function, and enrollment in addiction-focused psychosocial therapy. For oral naltrexone, MPR was defined as number of tablets dispensed during a time period divided by days in that time period. For LAI naltrexone, MPR was defined as number of injections given multiplied by 28 days divided by the number of days in the time period.
Descriptive statistics were used to characterize the study subjects. For continuous data, outcomes were compared using the Mann-Whitney U test for independent samples. For nominal data, chi-square was used to analyze data. Alpha was set at 0.05. In order to achieve 80% power, this study required an 80-day difference between oral and LAI groups with 15 patients enrolled in each study arm. Data was extracted and analyzed utilizing Microsoft Excel (Redmond, WA) and PSPP 1.2.0 (GNU Scientific Library, Boston, MA).
Results
A total of 410 patients were identified as having a prescription for either dosage form of naltrexone in the study period. Of these 410 patients, 361 were excluded. Reasons for exclusion include not enrolled in SUDRP (n = 129), naltrexone prescribed for treatment of something other than AUD (n = 75), not abstaining from alcohol at medication initiation (n = 63), initiated in the inpatient setting (n = 35), relapse not documented (n = 18), or did not initiate or continue therapy due to unforeseen circumstances such as incarceration or death (n = 41). Of the remaining 49 patients, 33 were prescribed oral naltrexone and 16 were prescribed LAI naltrexone. The 33 oral naltrexone patients were randomized and matched in a 1:1 ratio to the LAI naltrexone patients, leading to oral naltrexone (n = 16) and LAI (n = 16) naltrexone groups for analysis.
Baseline characteristics were similar in both groups (Table 1). Most patients were white males. The patients in the oral naltrexone group were approximately 7 years younger than the LAI naltrexone group. Most patients had a comorbid mental health diagnosis with depressive disorders being the most prominent diagnosis in both groups.
TABLE 1.
Baseline demographics of the study population
| Oral Naltrexone (n =16) |
Long-Acting Injectable Naltrexone (n = 16) |
P Value |
|
| Sex, n (%) | .626 | ||
| Male | 14 (87.5) | 13 (81.3) | |
| Female | 2 (12.5) | 3 (18.7) | |
| Age (mean ± SD), y | 49.25 ± 13.12 | 56.81 ± 12.93 | .165 |
| Race, n (%) | .465 | ||
| White | 11 (68.8) | 9 (56.3) | |
| African American | 5 (31.2) | 7 (43.7) | |
| Other | 0 (0) | 0 (0) | |
| Co-occurring mental health diagnosis, n (%) | |||
| Depressive | 10 (62.5) | 9 (56.3) | .719 |
| Psychotic | 0 (0) | 1 (6.3) | 1.0 |
| Mood | 2 (12.5) | 3 (18.7) | .626 |
| Posttraumatic stress disorder | 3 (18.7) | 5 (31.3) | .414 |
| Other | 2 (12.5) | 3 (18.7) | .626 |
| None | 4 (25.0) | 3 (18.7) | .669 |
The median time to relapse for the LAI naltrexone group was found to be significantly longer compared to the oral naltrexone group (150.5 days vs 50.5 days, P .01; Figure). No statistically significant differences were found between the 2 groups and their secondary outcomes (Table 2). The MPR was similar among both groups at 76.22% for the oral naltrexone group and 81.00% for the LAI naltrexone group (P = .47). Most patients in both treatment arms used some other substance with a majority of patients utilizing tobacco. Most patients had not had past pharmacologic treatment before the initiation of naltrexone. Our inclusion criteria required patients to be enrolled SUDRP, an addiction-focused psychosocial therapy at the VAMC, but a few patients in each treatment arm also participated in Alcoholics Anonymous in addition to VAMC treatment. No statistically significant differences were found in safety parameters between the 2 groups, including serum creatinine, aspartate transaminase, or alanine transaminase greater than 3 times the upper limit of normal.
FIGURE.
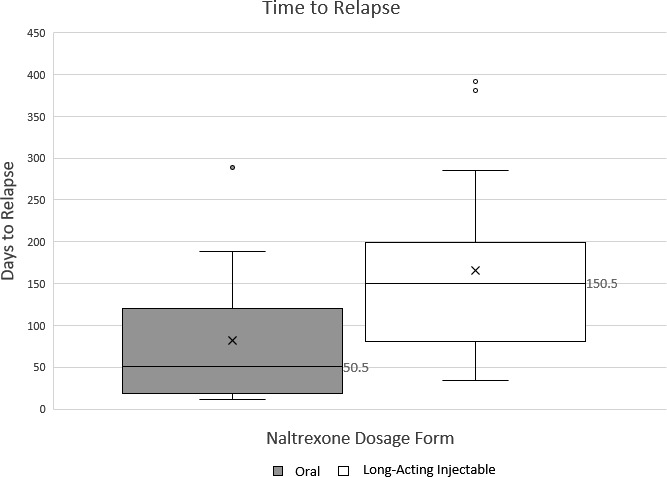
Long-acting injectable and oral naltrexone time to relapse; box and whisker plot showing difference between patients on oral naltrexone therapy versus long-acting injectable naltrexone therapy (mid-box line is indicative of the median, x represents average; 75% of patients fall within the boxes, the other 25% is represented by the whiskers)
TABLE 2.
Primary and secondary outcomes associated with oral naltrexone use versus long-acting injectable naltrexone use
| Oral Naltrexone (n = 16) |
Long-Acting Injectable Naltrexone (n = 16) |
P Value |
|
| Primary outcome | |||
| Time to relapse (median), d | 50.5 ± 78.8 | 150.5 ± 108.9 | .009 |
| Secondary outcomes | |||
| Medication possession ratio (%) | 81.0 | 76.2 | .478 |
| Other substance use, n (%) | 13 (81.3) | 14 (87.5) | .626 |
| Past pharmacological treatment, n (%) | 4 (25.0) | 3 (18.7) | .669 |
| Engagement in alcoholics anonymous, n (%) | 3 (18.7) | 1 (6.2) | .285 |
Discussion
This study evaluated the efficacy of LAI naltrexone compared to oral naltrexone and time to relapse for AUD. The results showed a statistically significant difference in time to relapse but did not show differences in secondary outcomes, such as MPR or other substance use. There were no differences found between the 2 groups regarding safety outcomes. At the time of the study, there were no published studies comparing time to relapse between oral naltrexone and LAI naltrexone in this population. Based on the significant increase in time to relapse while using LAI naltrexone, this medication should be considered as a first line option for veterans.
Limitations of this study include its retrospective nature, small sample size, single practice center, difference in patient age, and relapse assessed via volunteered report. Most patients were excluded due to not being enrolled in the studied treatment programming. There was a 7-year difference in age between both groups, which could be a confounding variable. The date for time to relapse was recorded as the day they reported the relapse, but the patient could have relapsed days to weeks prior. Although patient self-report increased variability, this factor was consistent for both the oral naltrexone and LAI naltrexone groups in this study.
This study emphasizes the need for further studies that assess the potential benefits of LAI naltrexone specifically with regards to adherence, cost, and clinical outcomes. This study shows a benefit in clinical outcomes, relating to time to relapse, but this could be due to the small sample size and single-facility study. More data is needed to further define the benefits of LAI naltrexone before identifying this medication as standard of care treatment. Further studies with a larger sample size and variety of patient populations would help support or reject this initial data. Large, randomized, controlled trials in the general population comparing the two medications head to head would benefit in determining place in treatment of oral versus LAI naltrexone.
In conclusion, based on the results of this study, LAI naltrexone is associated with increased time to relapse compared to oral naltrexone at this VAMC. This study showed no difference in patient adherence or safety outcomes. Moving forward, LAI naltrexone should be considered as a first-line agent for veterans diagnosed with alcohol use disorder.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington (VA): American Psychiatric Publishing;; 2013. [Google Scholar]
- 2.Fuehrlein BS, Mota N, Arias AJ, Trevisan LA, Kachadourian LK, Krystal JH, et al. The burden of alcohol use disorders in US military veterans: results from the National Health and Resilience in Veterans Study. Addiction. 2016;111(10):1786–94. doi: 10.1111/add.13423. DOI: 10.1111/add.13423 PubMed PMID: 27061707. [DOI] [PubMed] [Google Scholar]
- 3.Hasin DS, Grant B, Endicott J. The natural history of alcohol abuse: implications for definitions of alcohol use disorders. Am J Psychiatry. 1990;147(11):1537–41. doi: 10.1176/ajp.147.11.1537. DOI: 10.1176/ajp.147.11.1537 PubMed PMID: 2221170. [DOI] [PubMed] [Google Scholar]
- 4.Vice [Internet] This is what happens to your body when you stop drinking. 2019 [cited. Jun 3]. Available from: https://www.vice.com/en_us/article/ev39wk/this-is-what-happens-to-your-body-when-you-stop-drinking.
- 5.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61(8):807–16. doi: 10.1001/archpsyc.61.8.807. DOI: 10.1001/archpsyc.61.8.807 PubMed PMID: 15289279. [DOI] [PubMed] [Google Scholar]
- 6.Marienfeld C, Iheanacho T, Issa M, Rosenheck RA. Long-acting injectable depot naltrexone use in the Veterans' Health Administration: a national study. Addict Behav. 2014;39(2):434–8. doi: 10.1016/j.addbeh.2013.05.006. DOI: 10.1016/j.addbeh.2013.05.006 PubMed PMID: 23790742. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Washington: American Psychiatric Association;; 1969. [Google Scholar]
- 8.Alkermes, Inc. Vivitrol (naltrexone) injection. DailyMed. 1984 [rev. December 2018; cited 2019 March 7] In. [Internet] Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=cd11c435-b0f0-4bb9-ae78-60f101f3703f.
- 9.Aletraris L, Shelton JS, Roman PM. Counselor attitudes toward contingency management for substance use disorder: effectiveness, acceptability, and endorsement of incentives for treatment attendance and abstinence. J Subst Abus Treat. 2015;57:41–8. doi: 10.1016/j.jsat.2015.04.012. DOI: 10.1016/j.jsat.2015.04.012 PubMed PMID: 26001821 PubMed Central PMCID: PMC4561006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene M, Yan T, Chang E, Hartry A, Touya M, Broder MS. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2017;21(2):127–34. doi: 10.1080/13696998.2017.1379412. DOI: 10.1080/13696998.2017.1379412 PubMed PMID: 28895758. [DOI] [PubMed] [Google Scholar]
- 11.Kjome KL, Moeller FG. Long-acting injectable naltrexone for the management of patients with opioid dependence. Subst Abuse. 2011;5:1–9. doi: 10.4137/SART.S5452. DOI: 10.4137/SART.S5452 PubMed PMID: 22879745 PubMed Central PMCID: PMC3411517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris AHS, Ellerbe L, Reeder RN, Bowe T, Gordon AJ, Hagedorn H, et al. Pharmacotherapy for alcohol dependence: perceived treatment barriers and action strategies among Veterans Health Administration service providers. Psychol Serv. 2013;10(4):410–9. doi: 10.1037/a0030949. DOI: 10.1037/a0030949 PubMed PMID: 23356858. [DOI] [PubMed] [Google Scholar]
- 13.VA Pharmacy Benefits Management Services; Medical Advisory Panel; VISN Pharmacists Executives. Naltrexone extended-release injectable suspension criteria for use in alcohol use disorder and opioid use disorder. Washington: Veterans Affairs;; 2017. [Google Scholar]
- 14.Centers for Disease Control and Prevention [Internet] Alcohol use costs increase. 2019 [cited. Mar 4]. Available from: https://www.cdc.gov/features/costsofdrinking/index.html.
- 15.Beatty A, Stock C. Efficacy of long-acting, injectable versus oral naltrexone for preventing admissions for alcohol use disorder. Ment Health Clin [Internet] 2017;7(3):106–10. doi: 10.9740/mhc.2017.05.106. DOI: 10.9740/mhc.2017.05.106 PubMed PMID: 29955507 PubMed Central PMCID: PMC6007564. [DOI] [PMC free article] [PubMed] [Google Scholar]


