Climate-smart strategic expansion of protected area networks is essential to protect and reinforce biocultural traditions.
Abstract
New Guinea is the most biologically and linguistically diverse tropical island on Earth, yet the potential impacts of climate change on its biocultural heritage remain unknown. Analyzing 2353 endemic plant species distributions, we find that 63% of species are expected to have smaller geographic ranges by 2070. As a result, ecoregions may have an average of −70 ± 40 fewer species by 2070. Species with future geographic range contractions include 720 endemic plant species that are used by indigenous people, and we find that these will decrease in 80% of New Guinea’s 1030 language areas, with losses of up to 94 species per language area. To mitigate the threats of climate change on the flora, we identify priority sites for protected area expansion that can jointly maximize biodiversity and useful plant conservation.
INTRODUCTION
New Guinea is the largest and most bioculturally diverse island on Earth (1). Biologically, it harbors c. 14,000 native plant species and 9000 endemic species, making it the only island group in Malesia with more endemic than non-endemic plant species (2). Culturally, it is the most linguistically diverse place on Earth with more than 1300 languages (15% of all living languages) in under 1% of the terrestrial land surface (3). Biocultural diversity is a dynamic, place-based aspect of nature arising from links and feedbacks between human cultural diversity and biological diversity (4). Human-driven climate change, however, is expected to become a threat to New Guinea’s biocultural diversity: changes in climate are already affecting species’ distribution patterns elsewhere (5) with negative consequences on ecosystems functioning and human well-being (6). Because most New Guinea cultures are supported by low-income populations that rely on their surrounding natural resources, climate-induced local extinction of wild food, medicine, and ritual plants is likely to diminish indigenous well-being and cultural integrity. Accordingly, understanding the potential impacts of climate change on New Guinea’s biocultural diversity is essential for a strategic expansion of protected area networks and conservation lands in ways that protect and reinforce biocultural traditions and the rights of indigenous peoples (7).
The current protected area system in Indonesian New Guinea is far larger than that of Papua New Guinea, with 20% versus 4% of terrestrial land protected, respectively (8). Protected areas in Indonesian New Guinea were designated between 1975 and 1989 to account for the entire altitudinal spectrum of the region, major centers of endemism, representative cross sections of habitats within each ecoregion, and substantial tracts of lowland rainforests and to protect species with large areas by linking large reserves as far as possible (9). In Papua New Guinea, almost all land and natural resources are under the customary control of communities and landowners (8), but similar large-scale conservation prioritization efforts are missing. In 1997, the Irian Jaya Biodiversity Conservation Priority-Setting Workshop sought to identify priority areas for conservation in Indonesian New Guinea following four criteria: biological importance, human pressures and threats, priority for conservation action, and priority for research (10). Unfortunately, the indicators of biological importance largely ignored two important data sources: herbarium collections and unpublished field data. Moreover, given the paucity of readily available data for plants at the time, two subcriteria were used to identify priority conservation sites: ecological diversity and vegetation types. Since then, no island-wide and data-driven macroecological analysis has addressed protected area selection in the face of climate change or the need to conserve New Guinea’s cultural heritage.
Here, we quantify the potential impacts of climate change on New Guinea’s biodiversity and cultural heritage. Concerning impacts on biodiversity, we first built species distribution models (SDMs) for 2353 endemic species and then assessed how species richness and geographical range size will change between 2000 and 2070 under two climate change scenarios (see Materials and Methods). The first scenario forecasts an increase in temperature of 1.0°C by 2070 on the assumption that CO2 emissions will be based on improved governance (hereafter “RCP 2.6”). A second scenario that lacks climate change mitigation policies (“RCP 8.5”) forecasts a global warming increase of 2.0°C by 2070. Regarding impacts on cultural heritage, we first assessed how species richness and geographical range size will change for 720 endemic plant species that indigenous societies use for food, medicine, construction, and cultural purposes (11, 12). By considering useful plants, we introduce a key element for indigenous livelihoods that had been missing in conservation planning in New Guinea and that relates to indigenous knowledge, practices, and beliefs. Next, we quantified changes in future useful plant richness across 1030 of New Guinea’s indigenous language areas (see Materials and Methods). Last, to inform policy, we identified priority areas for maximizing biocultural conservation gains in the face of climate change.
RESULTS AND DISCUSSION
To determine how climate change will affect New Guinea’s biodiversity, we first quantified current species richness using 2353 New Guinea endemic species with presences in ≥5 grid cells and whose modeled current ranges are significantly different from a bias-corrected null model (see Materials and Methods). We find that mean species richness (at 5–arc min spatial resolution) is 711, with the highest values occurring in the northern half of New Guinea (Fig. 1A). The higher biodiversity of northern New Guinea (including the central mountain range) in comparison with the southern half of the island can partly be explained by undercollecting, especially in the Indonesian part of the craton area (13, 14). However, these results also support previous findings of a significant correlation between numbers of species and plate tectonic activity with its resulting orogenesis (15), as the northern half of New Guinea is the result of an accretion of many microplates (16).
Fig. 1. Endemic plant species richness (number of species per grid cell) in the face of climate change.
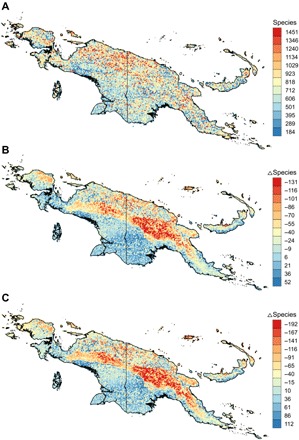
Species richness under current climate (A) and difference in species richness between current climate and 2070 RCP 2.6 (B) and 2070 RCP 8.5 (C).
Projecting SDMs to two future climate change scenarios (Fig. 2 B and C), we find that mean species richness is expected to decrease to 691 under 2070 RCP 8.5 (with similar results of 688 under RCP 2.6). About 63% of species will have smaller geographic ranges, and 37% will have larger ranges under 2070 RCP 8.5. On average, range reductions will be of −19 ± 15 cells per species (range, −1 to −106), and expansions will be of 19 ± 24 cells (range, 1 to 246). Upscaling to the level of ecoregions (17), we find that climate change will result in less diverse ecoregions with an average of −70 ± 40 fewer species per ecoregion (range, −246 to 107 species) under 2070 RCP 8.5 (Fig. 2). Ecoregions with more pronounced reductions in species richness include the Admiralty Islands lowland rain forests, Southern New Guinea lowland rain forests, New Britain–New Ireland montane rain forests, and the Northern New Guinea montane rain forests (table S1). A high reduction in species richness in the lowlands is of special conservation concern because lowland ecoregions are among the least protected and at the same time the most threatened in New Guinea and will require the largest protected area sizes to keep pace with climate change (18).
Fig. 2. Change in species richness across New Guinea’s ecoregions under future climate (2070 RCP 2.6 and RCP 8.5).
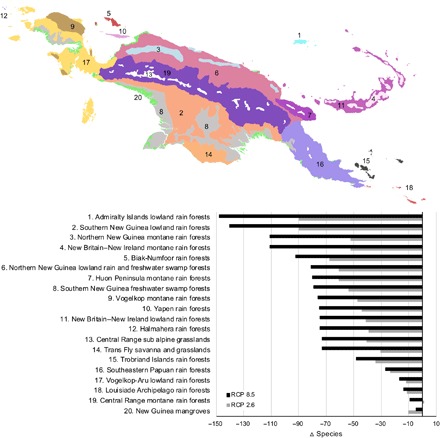
Change in species richness per ecoregion was calculated using stacked SDMs of 2353 endemic species.
Regarding New Guinea’s cultural heritage, we quantified how climate change will affect useful plant species richness across 1030 language areas. To do so, we considered a subset of 720 endemic useful species that indigenous people use for construction, cultural ceremonies, food, and medicine (see Materials and Methods). Overall, we find that 80 to 83% of language areas will experience reductions in useful plant species richness under 2070 RCP 8.5, with similar patterns across all use categories (Fig. 3 and table S2). On average, 16 to 25 useful species will be lost per language area under 2070 RCP 2.6 and RCP 8.5 scenarios, respectively. Climate change by 2070 RCP 8.5 is expected to reduce useful plant species richness in 826 language areas—with maximal losses of 94 species—and to increase richness in 194 language areas—with maximum gains of 79 species (table S3 and fig. S1). These changes in useful species richness will be similar among endangered and nonendangered languages (fig. S2). Last, while there was a net gain in species richness in the southern lowlands and a net loss in the central mountain range in the pixel-level analysis (Fig. 1), the pattern was slightly opposite in the language-level analysis (Fig. 3). This is partly because the pixel-level analyses consider more species (2353 species versus 720 useful species in the language-level analysis) and because the language-level analyses are averaged across larger areas.
Fig. 3. Change in endemic useful plant richness across New Guinea’s language areas by 2070 RCP 8.5.
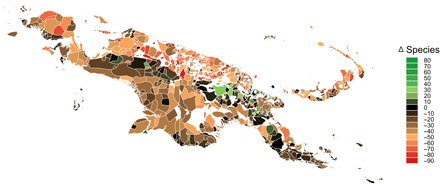
Change in species richness per language area over time was calculated using stacked SDMs of 720 endemic useful species.
Given differences across ecoregions and language areas in future potential reductions in species richness, where should decision-makers focus to maximize conservation gains? An answer to this question depends on which features are selected. This selection, in turn, is subjective and will vary across stakeholders. Accordingly, we considered two viewpoints that span a continuum from biologically driven to locally involved conservation planning. The first view places equal value on all biodiversity features and can be considered typical among conservation planners who value species richness, irrespective of species’ utility. For this view, we modeled conservation priorities for all 2353 endemic species (see Materials and Methods; Fig. 4A). The second view focuses on species that are important for indigenous livelihoods and approximates a socially driven viewpoint in which conservation focuses on livelihoods or on species that provide construction, culture, food, and medicinal services (Fig. 4, B to F). We find that the central mountain range emerges among the top 10% priorities under climate change for all endemic plant species as well as for each use category (red in Fig 4), despite a projected loss of species richness (Fig. 1, B and C). That the central range is identified as a priority while also projected to become unsuitable for a large number of currently extant species is explained by the (i) smaller average range size of species currently present and projected to persist in high elevations, (ii) reductions in range size (and thus an increase in importance) as species shift upslope, and (iii) highlands serving as a “sink” for surrounding low- and mid-elevation species. When comparing both views, we found strong spatial congruence in their top 10% solutions (Fig. 5A). These highly congruent top priority conservation areas occur in 28 administrative units, and some transcend single-administrative, cultural (Fig. 5B), or country boundaries, underscoring the strong need for interadministrative, intercultural, and international transboundary planning.
Fig. 4. Spatial conservation priorities for endemic plants in the face of climate change.
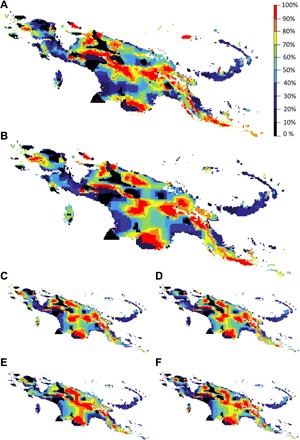
Priority rank map for all endemic plants (n = 2353 species) (A), all endemic useful plants (n = 720 species) (B), useful plants for construction (n = 374) (C), culture (n = 271) (D), food (n = 162) (E), and medicine (n = 187) (F). In each map, each grid cell has a value between 0 and 100: Low values close to 0% were removed first (low conservation value and priority), while high values close to 100% were retained until the end (high priority).
Fig. 5. Congruence in top 10% conservation priorities for 2070 for endemic and useful plants.
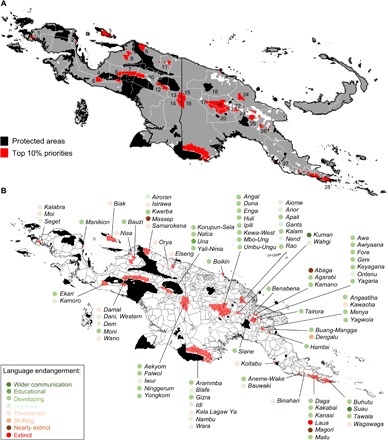
Map of administrative units (A) where numbers indicate administrative units of Indonesian New Guinea and Papua New Guinea containing the top 10% solutions: 1, Sorong; 2, Manokwari; 3, Biak Numfor; 4, Nabire; 5, Mimika; 6, Paniai; 7, Puncak Jaya; 8, Waropen; 9, Sarmi; 10, Jayawijaya; 11, Jayapura; 12, Yahukimo; 13, Pegunungan Bintang; 14, Boven Digoel; 15, Sandaun; 16, Western; 17, Hela; 18, East Sepik; 19, Enga; 20, Southern Highlands; 21, Western Highlands; 22, Jiwaka; 23, Chimbu; 24, Madang; 25, Eastern Highlands; 26, Morobe; 27, Central; 28, Milne Bay. Map of languages (B) that intersect with the top 10% solutions; dot color indicates language endangerment class.
Despite high levels of species richness and endemism, New Guinea remains one of the most botanically undercollected regions in the Asia-Pacific (13, 14). Our study is therefore constrained by low collection densities of generally <25 collections per 100 km2—well under the benchmark minimum of 50 to 100 collections per 100 km2 for adequate floristic inventories (13). In addition, specimen collections are spatially biased in several notable ways: Collection density is lower in western and southwestern New Guinea, is highest in montane ecosystems, and tends to be concentrated around roads, waterways, and major communities. Another limitation of our study is that our SDMs assume that species niches are at equilibrium with their current climate envelope and stable across time. Our modeling approach also does not integrate biotic interactions (19), species-level dispersal information (20), or potential adaptation and evolution (21), which are known to influence species responses to climate change but for which data are lacking for most taxa. Documentation of New Guinea’s useful species is still incipient: Only 19% of the region’s indigenous groups appear in the ethnobotanical literature, and most studies have been fragmentary (11, 12). This translates in that 60% of the 720 modeled endemic useful species were reported in the literature by a single indigenous group and 40% were reported by at least two groups. Thus, our analyses within language areas explore all potentially available endemic useful species, although these may not yet be known locally. Last, we did not include land cost in our analyses, as land use in New Guinea is controlled by a complex mixture of traditional tenure and government land use planning, which varies between countries. Formal acquisition cost is seldom an issue in either setting, but opportunity costs may be substantial.
Here, we have shown that climate change will affect both the biological and cultural heritage of New Guinea. Both the Indonesian and Papua New Guinea governments have made strides toward establishing protected area systems as signatories to the Convention on Biological Diversity (22, 23). In 2018, the governors of Indonesia’s two New Guinea Provinces signed the Manokwari Declaration committing to the conservation of 70% of the forest cover for the western half of New Guinea (7). In addition, international initiatives such as the Key Biodiversity Areas (24) and Tropical Important Plant Areas (25) are underway to identify priority sites on the basis of plant species richness, threatened habitats, and socioeconomically important plants. These initiatives, however, emphasize conservation of biodiversity alone, whereas biocultural conservation calls for preserving both habitats and habits (26). The identified network of centers of plant endemism and useful plant diversity represents a first step toward the conservation of New Guinea’s unique biological diversity in the face of climate change, while conserving cultural diversity under climate change rests on understanding and promoting indigenous knowledge systems in relation to their territories. Since transmission of indigenous knowledge is linked to language proficiency, schooling in indigenous languages and culturally relevant school curricula could be a positive policy step towards maintaining cultural diversity (26). Last, conservation programs and the expansion of protected areas have the potential to support local communities by preventing large-scale conversion of lands to industrial logging or plantations or to negatively affect them by reducing access to resources or economic opportunities (27). Thus, conservation actions should strive to work with indigenous communities from the onset to ensure positive impacts (28) and the promotion of indigenous people’s rights.
MATERIALS AND METHODS
Study area
Our study area of “New Guinea” spans a latitudinal range of −0.08° to −10.66°S and a longitudinal range of 129.42° to 150.21°E. It includes the main island of New Guinea and the smaller islands that were connected to mainland New Guinea during the last glacial maximum. We delimited it by selecting areas with a depth of ≥−120 m from the General Bathymetric Chart of the Oceans (www.gebco.net).
Species distributions
Herbarium specimen records were downloaded from the online databases Global Biodiversity Information Facility (www.gbif.org) and Consortium of Pacific Herbaria (https://www.re3data.org/repository/r3d100012011) and supplemented with records from institutional databases of Naturalis Biodiversity Center and Royal Botanic Gardens, Kew. We manually unified headers and standardized entries for the fields “family,” “genus,” “species,” “collector name,” “collector number,” and “date.” All records from outside the study area were removed, and endemic species were identified following Flora Malesiana accounts (www.floramalesiana.org) and taxonomic literature. Collectors’ names were verified using the Cyclopaedia of Malesian collectors (www.nationaalherbarium.nl/FMCollectors). After discarding duplicate records and obvious coordinate errors (i.e., points in the ocean and outside New Guinea), there were 3053 endemic species with at least five occurrences in separate grid cells.
Environmental variables
Current climate information for 19 environmental variables was downloaded from the WorldClim database v 1.4 at 5–arc min spatial resolution (c. 10 × 10 km in the equator) (29). Future climate information was based on a multimodel ensemble mean of available global climate model projections from the Intergovernmental Panel on Climate Change Fifth Assessment Report for two representation concentration pathways: (i) RCP 2.6, which corresponds to a global mean surface temperature change of 1.0°C between 2046 and 2065 and reflects trends of CO2 emissions based on improved governance or a “best-case scenario”; (ii) RCP 8.5, which corresponds to a global mean surface temperature change of 2.0°C between 2046 and 2065 and to the absence of climate change policies or a “worst-case scenario.” Soil information for 20 soil variables was obtained from the SoilGrids database at 1-km resolution and 1-m depth (30).
We performed a principal components analysis on standardized and centered data from 19 current climate variables and 20 soil parameters and retained the variables with the highest vector loadings. The selected climate variables were temperature isothermality (mean diurnal range/temperature annual range; bio 3), minimum temperature of the coldest month (bio 6), annual precipitation (bio 12), precipitation seasonality (bio 15), and precipitation of the warmest quarter (bio 18). The selected soil variables were depth to bedrock (BRICM; R horizon), bulk density (BLFIE; fine earth), cation exchange capacity of soil (CECSOL), soil organic carbon density (OCDENS), pH index measured in KCl solution (PHIKCL), weight percentage of silt particles (SLTPPT; 0.0002 to 0.05 mm), and weight percentage of the sand particles (SNDPPT; 0.05 to 2 mm).
Species distribution models
We modeled species distributions using Maxent 3.3.3k, a maximum entropy algorithm designed for species distribution modeling with presence-only data (https://biodiversityinformatics.amnh.org/open_source/maxent/) (31), which performs well compared to other methods (32, 33). To correct for spatial bias among botanical collections, we used a target-group background sample of all raster cells from which at least one botanical record was made for model training. Hinge, product, and threshold predictor features were excluded from the Maxent algorithm for model training to minimize the chances of overfitting (34). As a measure of SDM accuracy, we used the area under the curve (AUC) of the receiver operating characteristic plot (35). Each SDM was evaluated against a bias-corrected null model built with presence locations randomly selected from the target-group background sample (36). We drew as many random points n from cells where collections were made as number of records used to develop each SDM and repeated this process 100 times. To test for significance, the upper 95% one-sided confidence interval AUC value from the randomization was compared to the AUC in the developed SDM. SDMs with AUC values higher than the 95th percentile of null models were considered to have a significantly stronger relationship between presences and predictors than expected by chance alone and were retained for further analyses. Overall, we retained 2353 significant SDMs (77% of the 3053 SDMs), which represent 26% of New Guinea’s endemic plants. All significant SDMs were projected to the future climatic conditions for two climate change scenarios: RCP 2.6 and RCP 8.5.
Change in species richness and geographic range
To map species richness for current and future conditions, we converted the continuous Maxent predictions to discrete presence/absence values using the “10 percentile presence” threshold. This is a conservative threshold to prevent commission errors (false-positive predictions) and does not rely on absences, which are missing. To map species richness for each of the three climate scenarios (current, RCP 2.6, and RCP 8.5), we stacked the 2353 presence-absence SDMs and summed species presences per raster cell. We then quantified changes in species’ geographic range by subtracting the number of raster cells a species occupied in the current and future climate scenarios. To quantify changes at the ecoregion level, we downloaded ecoregion data from the World Wide Fund for Nature (WWF) (17). For the 390 different polygons—comprising 20 distinct ecoregions of New Guinea—we calculated species richness under current and future climate using the presence-absence stacked SDMs.
Useful plants across New Guinea’s indigenous lands
To select which of the significant SDMs are used by indigenous people, we queried a list of New Guinea useful plants that synthesizes information from 488 references published between 1885 and 2018 (11, 12). There were 720 useful species in our significant SDMs, representing 67% of all known 1070 endemic useful species of New Guinea. The 720 species belong to 100 plant families, the most diverse being Arecaceae (44 species), Rubiaceae (37), Ericaceae (36), Elaeocarpaceae (33), and Myrtaceae (31). Useful species have 3333 different uses (71% of known uses from New Guinea’s endemic species), including food (n = 162 species; 232 uses), medicine (n = 187; 522), culture (n = 271; 415), and construction (n = 374; 824). The 720 useful species are cited by a total of 93 indigenous groups, with 60% of species cited by one indigenous group and 40% cited by at least two indigenous groups. To map useful plant species richness across New Guinea’s indigenous lands, we intersected the stacked SDMs of useful species with 1030 language areas of Ethnologue (www.ethnologue.com) (37). We used language as an indicator of a cultural group because language is the primary medium of cultural transmission (38). Differences in species richness between current and future climate (2070 RCP 2.6, RCP 8.5) were calculated for each language area. To explore the relationship between future changes in species richness and language endangerment, we obtained the language endangerment classification for all indigenous groups in our sample from Ethnologue (37), which uses the Expanded Graded Intergenerational Disruption Scale (39). All SDM analyses were performed in R (40) using commands from the libraries dismo (41), raster (42), and rgdal (43).
Conservation prioritization
We used the Zonation v. 4 software (44) to produce a complementarity-based and balanced ranking of conservation priority over the entire landscape. Zonation iteratively removes the planning units that lead to smallest aggregate loss of conservation value, after accounting for total and remaining distribution of species, species weights, and species-specific connectivity. Because Zonation applies successive range-size normalization for all species (44), and species with a small range receive higher priority, we did not assign higher weights to species with a small range size. To model conservation value in the context of climate change, we selected interaction as the connectivity method linking present distributions to future distributions (45). As this method links present distributions to future distributions within a dispersal distance, areas with greater overlap of present and future ranges will emerge as priorities. These areas will tend to be in regions with more available niche space within dispersal distance—and therefore potentially attainable by the species as the climate changes. We set a 2000 to 2070 dispersal parameter of 100 km that resembles the 16.9–km decade−1 median shift to higher latitudes exhibited by terrestrial taxa in response to changing climate (5). For the cell-removal rule, we used the core area zonation (CAZ) rule, which bases ranking on the most important occurrence of a species in a cell (rarity), so that even species-poor cells may be identified as priorities. To avoid identifying as priorities those cells with low habitat intactness, we removed cells with a human footprint score greater than 6 [i.e., areas of higher human modification (46)]. Human footprint data at a 1-km resolution were downloaded from (46, 47) and aggregated to a 10-km resolution. We omitted protected areas from solutions, since these areas are already protected. Protected area polygons were downloaded from the World Database on Protected Areas dataset of the Protected Planet website (https://protectedplanet.net) (48), and only “designated” areas that belong to International Union for Conservation of Nature protected area categories I to VI were selected.
Supplementary Material
Acknowledgments
We thank the collectors, herbarium and database curators, and colleagues at our institutions for support and discussions. Funding: The authors acknowledge support from the Royal Society International Exchanges (grant IE 170241 to R.C.-L. and P.C.v.W. “Building the New Guinea Research Team”) and the Global Environment Facility [grant GEF-5810 “Spatial Planning for Area Conservation in Response to Climate Change” (SPARC)]. Author contributions: R.C.-L., L.H., P.R., and N.R. designed the study. N.R. and R.C.-L. ran species distribution models. R.C.-L. and P.R. ran Zonation analyses. R.C.-L., N.R., P.R., L.H., Y.D.-F., C.D.H., L.R., A.S., and P.v-W. contributed to the discussion of results. R.C.-L. wrote the manuscript with input from N.R., P.R., L.H., Y.D.-F., C.D.H., L.R, A.S., and P.v-W. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the corresponding author.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/11/eaaz1455/DC1
Fig. S1. Predicted net gains and losses of useful endemic species across New Guinea’s languages by 2070 RCP 8.5.
Fig. S2. Predicted difference in useful endemic species richness between current climate and 2070 RCP 8.5 for endangered (red) and nonendangered languages (green).
Table S1. Predicted means (and ranges) of endemic plant species richness in 20 WWF ecoregions of New Guinea under current and future climate.
Table S2. Predicted means (and ranges) of endemic useful plant richness in 1030 language areas under current and future climate in New Guinea.
Table S3. Potential change in endemic useful plant richness across New Guinea’s languages from current to future climate.
REFERENCES AND NOTES
- 1.Loh J., Harmon D., A global index of biocultural diversity. Ecol. Indic. 5, 231–241 (2005). [Google Scholar]
- 2.Roos M. C., Keßler P. J. A., Gradstein S. R., Baas P., Species diversity and endemism of five major Malesian islands: Diversity–area relationships. J. Biogeogr. 31, 1893–1908 (2004). [Google Scholar]
- 3.B. Palmer, Language families of the New Guinea Area, in The Languages and Linguistics of the New Guinea Area: A Comprehensive Guide, B. Palmer, Ed. (De Gruyter Mouton, 2018), pp. 1–19. [Google Scholar]
- 4.Bridgewater P., Rotherham I. D., A critical perspective on the concept of biocultural diversity and its emerging role in nature and heritage conservation. People Nat. 1, 291–304 (2019). [Google Scholar]
- 5.Chen I.-C., Hill J. K., Ohlemüller R., Roy D. B., Thomas C. D., Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Pecl G. T., Araújo M. B., Bell J. D., Blanchard J., Bonebrake T. C., Chen I.-C., Clark T. D., Colwell R. K., Danielsen F., Evengård B., Falconi L., Ferrier S., Frusher S., Garcia R. A., Griffis R. B., Hobday A. J., Janion-Scheepers C., Jarzyna M. A., Jennings S., Lenoir J., Linnetved H. I., Martin V. Y., McCormack P. C., McDonald J., Mitchell N. J., Mustonen T., Pandolfi J. M., Pettorelli N., Popova E., Robinson S. A., Scheffers B. R., Shaw J. D., Sorte C. J. B., Strugnell J. M., Sunday J. M., Tuanmu M.-N., Vergés A., Villanueva C., Wernberg T., Wapstra E., Williams S. E., Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355, eaai9214 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Cámara-Leret R., Schuiteman A., Utteridge T., Bramley G., Deverell R., Fisher L. A., McLeod J., Hannah L., Roehrdanz P., Laman T. G., Scholes E., de Fretes Y., Heatubun C., The Manokwari Declaration: Challenges ahead in conserving 70% of Tanah Papua’s forests. Forest Soc. 3, 148–151 (2019). [Google Scholar]
- 8.Y. de Fretes, The protected area system in Papua, in The Ecology of Papua, Part Two, A. Marshall, B. Beehler, Eds. (Periplus, 2007), pp. 1251–1275. [Google Scholar]
- 9.R. G. Petocz, Conservation and Development in Irian Jaya: A Strategy for Rational Resource Utilization (Brill, 1989). [Google Scholar]
- 10.J. Supriatna, Y. de Fretes, A. Mack, C. P. Yeager, S. Olivieri, J. B. Burnett, I. Wijayanto, S. Suryadi, A. Suhandi, The Irian Jaya Biodiversity Conservation Priority-Setting Workshop (Conservation International, 1999). [Google Scholar]
- 11.Cámara-Leret R., Dennehy Z., Information gaps in indigenous and local knowledge for science-policy assessments. Nat. Sustain. 2, 736–741 (2019). [Google Scholar]
- 12.Cámara-Leret R., Dennehy Z., Indigenous knowledge of New Guinea’s useful plants: A review. Econ. Bot. , 1–11 (2019). [Google Scholar]
- 13.B. J. Conn, Documentation of the flora of New Guinea, in Biodiversity and Terrestrial Ecosystems, C.-I. Peng, C. H. Chouu, Eds. (Institute of Botany, Academia Sinica, 1994), pp. 123–156. [Google Scholar]
- 14.D. Frodin, Biological exploration of New Guinea, in The Ecology of Papua, Part One, A. Marshall, B. Beehler, Eds. (Periplus, 2007), pp. 87–130. [Google Scholar]
- 15.P. C. van Welzen, Increased speciation in New Guinea: Tectonic causes?, in Plant Diversity in Malesia III, J. Dransfield, M. J. E. Coode, D. A. Simpson, Eds. (Royal Botanic Gardens, 1997), pp. 363–387. [Google Scholar]
- 16.Pigram C. J., Davies H. L., Terranes and the accretion history of the New Guinea orogen. BMR J. Aust. Geol. Geophys. 10, 193–211 (1987). [Google Scholar]
- 17.Olson D. M., Dinerstein E., Wikramanayake E. D., Burgess N. D., Powell G. V. N., Underwood E. C., D'Amico J. A., Itoua I., Strand H. E., Morrison J. C., Loucks C. J., Allnutt T. F., Ricketts T. H., Kura Y., Lamoreux J. F., Wettengel W. W., Hedao P., Kassem K. R., Terrestrial ecoregions of the world: A new map of life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience 51, 933–938 (2001). [Google Scholar]
- 18.Loarie S. R., Duffy P. B., Hamilton H., Asner G. P., Field C. B., Ackerly D. D., The velocity of climate change. Nature 462, 1052–1055 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Bascompte J., García M. B., Ortega R., Rezende E. L., Pironon S., Mutualistic interactions reshuffle the effects of climate change on plants across the tree of life. Sci. Adv. 5, eaav2539 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guisan A., Thuiller W., Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 8, 993–1009 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann A. A., Sgrò C. M., Climate change and evolutionary adaptation. Nature 470, 479–485 (2011). [DOI] [PubMed] [Google Scholar]
- 22.BAPPENAS, Indonesian Biodiversity Strategy and Action Plan (IBSAP) 2015–2020 (The National Development Planning Agency BAPPENAS, Jakarta, 2016).
- 23.Papua New Guinea Department of Conservation, Papua New Guinea’s Fifth National Report to the Convention on Biological Diversity (Port Moresby, 2017).
- 24.IUCN, A Global Standard for the Identification of Key Biodiversity Areas, version 1.0 (IUCN, Gland, 2016).
- 25.Darbyshire I., Anderson S., Asatryan A., Byfield A., Cheek M., Clubbe C., Ghrabi Z., Harris T., Heatubun C. D., Kalema J., Magassouba S., McCarthy B., Milliken W., de Montmollin B., Lughadha E. N., Onana J.-M., Saïdou D., Sârbu A., Shrestha K., Radford E. A., Important plant areas: Revised selection criteria for a global approach to plant conservation. Biodivers. Conserv. 26, 1767–1800 (2017). [Google Scholar]
- 26.R. Rozzi, Biocultural ethics: From biocultural homogenization toward biocultural conservation, in Linking Ecology and Ethics for a Changing World: Values, Philosophy, and Action, R. Rozzi, S. T. A. Pickett, C. Palmer, J. J. Armesto, J. B. Callicott, Eds. (Springer, 2013), pp. 9–32. [Google Scholar]
- 27.Adams W. M., Aveling R., Brockington D., Dickson B., Elliott J., Hutton J., Roe D., Vira B., Wolmer W., Biodiversity conservation and the eradication of poverty. Science 306, 1146–1149 (2004). [DOI] [PubMed] [Google Scholar]
- 28.P. Franks, R. Small, Understanding the Social Impacts of Protected Areas: A Community Perspective. IIED Research Report (IIED, 2016).
- 29.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 30.Hengl T., de Jesus J. M., MacMillan R. A., Batjes N. H., Heuvelink G. B. M., Ribeiro E., Samuel-Rosa A., Kempen B., Leenaars J. G. B., Walsh M. G., Gonzalez M. R., SoilGrids1km—Global soil information based on automated mapping. PLOS ONE 9, e105992 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips S. J., Maximum entropy modeling of species geographic distributions. Ecol. Model. 190, 231–259 (2006). [Google Scholar]
- 32.Elith J., Graham C. H., Anderson R. P., Dudík M., Ferrier S., Guisan A., Hijmans R. J., Huettmann F., Leathwick J. R., Lehmann A., Li J., Lohmann L. G., Loiselle B. A., Manion G., Moritz C., Nakamura M., Nakazawa Y., Overton J. M., Townsend Peterson A., Phillips S. J., Richardson K., Scachetti-Pereira R., Schapire R. E., Soberón J., Williams S., Wisz M. S., Zimmermann N. E., Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29, 129–151 (2006). [Google Scholar]
- 33.Aguirre-Gutiérrez J., Carvalheiro L. G., Polce C., van Loon E. E., Raes N., Reemer M., Biesmeijer J. C., Fit-for-purpose: Species distribution model performance depends on evaluation criteria—Dutch hoverflies as a case study. PLOS ONE 8, e63708 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elith J., Phillips S. J., Hastie T., Dudik M., Chee Y. E., Yates C. J., A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 17, 43–57 (2011). [Google Scholar]
- 35.Fielding A. H., Bell J. F., A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24, 38–49 (1997). [Google Scholar]
- 36.Raes N., ter Steege H., A null-model for significance testing of presence-only species distribution models. Ecography 30, 727–736 (2007). [Google Scholar]
- 37.G. F. Simons, C. D. Fennig, Ethnologue: Languages of the World 21st Edition (SIL International, 2018); http://www.ethnologue.com.
- 38.Raven P. H., Berlin B., Breedlove D. E., The origins of taxonomy. Science 174, 1210–1213 (1971). [DOI] [PubMed] [Google Scholar]
- 39.Lewis M. P., Simons G. F., Assessing endangerment: Expanding fishman’s GIDS. Rev. Roum. Linguist. 55, 103–120 (2010). [Google Scholar]
- 40.R Core Team A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2019).
- 41.R. Hijmans, S. Phillips, J. Leathwick, J. Elith, dismo: Species Distribution Modeling. R Package version 1.1–4 (2017).
- 42.R. Hijmans, J. van Etten, raster: Geographic Data Analysis and Modeling. R Package version 2.8–19 (2019).
- 43.R. Bivand, T. Keitt, B. Rowlingson, E Pebesma, rgdal: Bindings for the ‘Geospatial’ Data Abstraction Library. R Package version 1.4–3 (2019).
- 44.Moilanen A., Franco A. M. A., Early R. I., Fox R., Wintle B., Thomas C. D., Prioritizing multiple-use landscapes for conservation: Methods for large multi-species planning problems. Proc. R. Soc. Lond. B Biol. Sci. 272, 1885–1891 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carroll C., Dunk J. R., Moilanen A., Optimizing resiliency of reserve networks to climate change: Multispecies conservation planning in the Pacific northwest, USA. Glob. Chang. Biol. 16, 891–904 (2010). [Google Scholar]
- 46.Venter O., Sanderson E. W., Magrach A., Allan J. R., Beher J., Jones K. R., Possingham H. P., Laurance W. F., Wood P., Fekete B. M., Levy M. A., Watson J. E. M., Global terrestrial Human Footprint maps for 1993 and 2009. Sci. Data 3, 160067 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O. Venter, E. W. Sanderson, A. Magrach, J. R. Allan, J. Beher, K. R. Jones, H. P. Possingham, W. F. Laurance, P. Wood, B. M. Fekete, M. A. Levy, J. E. Watson, Last of the Wild Project, Version 3 (LWP-3): 2009 Human Footprint, 2018 Release. Palisades, NY: NASA Socioeconomic Data and Applications Center (SEDAC) (2018); 10.7927/H46T0JQ4. [DOI]
- 48.UNEP-WCMC and IUCN (2019), Protected Planet: The World Database on Protected Areas (WDPA) [On-line], [August 2018], Cambridge, UK:UNEP-WCMC and IUCN; www.protectedplanet.net.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/11/eaaz1455/DC1
Fig. S1. Predicted net gains and losses of useful endemic species across New Guinea’s languages by 2070 RCP 8.5.
Fig. S2. Predicted difference in useful endemic species richness between current climate and 2070 RCP 8.5 for endangered (red) and nonendangered languages (green).
Table S1. Predicted means (and ranges) of endemic plant species richness in 20 WWF ecoregions of New Guinea under current and future climate.
Table S2. Predicted means (and ranges) of endemic useful plant richness in 1030 language areas under current and future climate in New Guinea.
Table S3. Potential change in endemic useful plant richness across New Guinea’s languages from current to future climate.


