Surface expression and release of α-tectorin are required for the organization of collagen bundles of the tectorial membrane.
Abstract
The tectorial membrane (TM) is an apical extracellular matrix (ECM) that hovers over the cochlear sensory epithelium and plays an essential role in auditory transduction. The TM forms facing the luminal endolymph-filled space and exhibits complex ultrastructure. Contrary to the current extracellular assembly model, which posits that secreted collagen fibrils and ECM components self-arrange in the extracellular space, we show that surface tethering of α-tectorin (TECTA) via a glycosylphosphatidylinositol anchor is essential to prevent diffusion of secreted TM components. In the absence of surface-tethered TECTA, collagen fibrils aggregate randomly and fail to recruit TM glycoproteins. Conversely, conversion of TECTA into a transmembrane form results in a layer of collagens on the epithelial surface that fails to form a multilayered structure. We propose a three-dimensional printing model for TM morphogenesis: A new layer of ECM is printed on the cell surface concomitant with the release of a preestablished layer to generate the multilayered TM.
INTRODUCTION
The tectorial membrane (TM) is an apical extracellular matrix (ECM) produced by cochlear supporting cells and lies over the organ of Corti. The TM exhibits complex ultrastructure and morphological gradients along the frequency-specific cochlear turn (1–3). As its structure suggests, it plays essential roles in propagation, frequency tuning, and amplification of auditory stimuli (4–6). Abnormalities of the TM structure are frequently observed in a wide range of human hearing disorders (7). Since the TM is an acellular structure, its unique properties arise from a preestablished architecture. Although it is generally accepted that the TM is formed by self-assembly of secreted and released components (8), the molecular mechanism by which this ECM is exquisitely organized in the luminal space is unknown.
The development of the TM needs to overcome the following challenges. (i) The TM exhibits a defined shape and highly ordered ultrastructure (2). (ii) The developing TM forms within a luminal space (the scala media) that is filled with fluid (endolymph) (Fig. 1A). Thus, there must be a mechanism preventing diffusion of secreted TM components during its formation. (iii) Each layer of the TM shows characteristic anisotropy. For example, the major fibers of the top layer (covernet) are oriented along the longitudinal axis (8), while the collagen fibrils of the layer underneath (central body) are radially oriented with a tonotopy-specific, apically directed slant (9). Notably, this apically directed slant is absent when planar cell polarity is disrupted (9), further suggesting the existence of a surface component that signals to the ECM compartment to direct its assembly. (iv) The TM is large, and the growing structure remains attached to the surface of producing cells until maturation is complete (Fig. 1A), at which point it detaches from these cells except in the spiral limbus, and remains associated with the tallest stereocilia of outer hair cells (Fig. 1B). Thus, a preestablished ECM network that formed on the cell surface must be released from the surface membrane during the growth and detachment periods of TM development.
Fig. 1. α-Tectorin is widely expressed in TM-producing cells and tethered to the plasma membrane via a GPI anchorage.

(A and B) Toluidine blue–stained radial sections of the developing mouse cochlea at postnatal day 2 (P2) (A) and P7 (B). The inner sulcus (IS) is formed between P2 and P7, when the TM becomes detached from the surface of columnar supporting cells. IHC, inner hair cell; OHC, outer hair cell; Co, columnar cells; Cu, cuboidal cells; SL, spiral limbus. Scale bar, 50 μm. (C) In situ hybridization of Tecta mRNA in the P2 mouse cochlea. Tecta is expressed in cochlear supporting cells including interdental cells (ID) of the spiral limbus, inner supporting cells of Kölliker’s organ (Ko) including columnar cells, and outer supporting cells including pillar cells (PC), Deiters’ cells (DC), and Hensen’s cells (Hs) but not in inner hair cell and outer hair cell. Scale bar, 50 μm. (D) Schematic of Myc-tagged TECTA structure (top) and cellular localization. Red bars indicate a potential cleavage site of proteolytic sheddases. A blue arrow indicates the cleavage site of bacterial phosphatidylinositol-phospholipase C (PI-PLC) and potential GPI-anchored lipases. N, N terminus; C, C terminus; ER, endoplasmic reticulum; PM, plasma membrane. (E) Myc-TECTA was expressed in human embryonic kidney (HEK) 293T cells, and its localization was determined by Western blots using an anti-Myc antibody. Treatment of TECTA-expressing cells with PI-PLC, which cleaves a GPI anchor, facilitates the release of TECTA into the media (top) and removes surface TECTA as determined by surface biotinylation assay (bottom). (F) Surface expression of TECTA is absent in PI-PLC–treated cells as shown by live cell surface staining of TECTA (green, anti-Myc antibody raised in rabbit), followed by total permeabilized staining (red, anti-Myc antibody raised in mouse). Scale bar, 20 μm.
We asked whether this complex structure can be formed solely by a self-assembly process in the luminal space. The TM is composed of both secreted proteins [collagen type II (Col II), Col V, Col IX, Col XI, otogelin (OTOG), OTOG-like, and carcinoembryonic antigen-related cell adhesion molecule 16 (CEACAM16)] and proteins that are tethered to the membrane via a glycosylphosphatidylinositol (GPI)–anchorage [α-tectorin (TECTA), β-tectorin (TECTB), and otoancorin (OTOA)] (2, 10). We hypothesized that GPI-anchored proteins (GPI-APs) expressed on the surface membrane prevent the diffusion of secreted components into the luminal space and that among GPI-APs, TECTA is best suited for this role. Tecta is highly and broadly expressed in TM-producing cells (Fig. 1C), which include interdental cells in the spiral limbus, inner supporting cells including columnar cells in Kölliker’s organ, and outer supporting cells such as pillar cells, Deiters’ cells, and Hensen’s cells, while Tectb and Otoa show a more restricted expression pattern (11, 12). Consistent with the expression profile, loss of Tecta results in severe disruption of the entire TM (13), while loss of Tectb or Otoa causes malformation of specific ultrastructural features and/or detachment of the TM from the spiral limbus (14, 15). In addition, TECTA contains several ECM-interacting domains, including a nidogen-like domain and von Willebrand factor type D domains, which may allow for binding to collagens and other glycoproteins, and a zona pellucida (ZP) domain for multimerization (16–21). Last, mutations in the TECTA/Tecta gene cause both recessive and dominant nonsyndromic hearing loss in both humans and mice (5, 22), further supporting the essential role of TECTA in TM structure and function.
RESULTS
TECTA is a GPI-AP
Tecta encodes a protein with conserved hydrophobic patches at the N and C termini and is predicted to be a GPI-AP (Fig. 1D). To validate the predicted GPI anchorage of TECTA, we expressed Myc-TECTA in human embryonic kidney (HEK) 293T cells and monitored its localization. We detected TECTA in the cell lysate but not in the cell culture medium (Fig. 1E). Treatment of the transfected cells with bacterial phosphatidylinositol-phospholipase C (PI-PLC), which cleaves GPI anchors on the cell surface, released TECTA into the medium. To determine the level of GPI-anchored TECTA on the surface membrane, we performed a surface biotinylation assay, which labels the membrane-associated proteins that are exposed to the extracellular space (10). The surface biotinylation assay showed that PI-PLC treatment completely removed TECTA from the cell surface (Fig. 1E). Consistent with these observations, live cell staining showed that TECTA present on the cell surface was removed by PI-PLC treatment (Fig. 1F).
To further confirm that the GPI anchorage mediates cell surface attachment of TECTA, we generated a secreted form (TECTAS) by introducing a stop codon at the omega site (ω-site), the amino acid to which the GPI anchor is attached (Fig. 2A). Since the C-terminal GPI anchorage signal is removed upon GPI attachment to wild-type TECTA (TECTAWT), TECTAS has the same protein sequence as a mature TECTAWT except that it lacks the ω-site. As expected, TECTAS expressed in HEK293T cells was constitutively secreted into the culture medium and not retained on the cell surface (Fig. 2, B and C). Collectively, these results show that TECTA is tethered to the surface membrane via GPI anchorage.
Fig. 2. GPI anchorage of TECTA is required for the surface localization of TECTA and the organization of the TM.

(A) Schematic of TECTAWT and TECTAS (top) and their cellular localization (bottom). TECTAS is generated by replacing the GPI anchorage site (ω) with a stop codon (X). (B and C) TECTAS is constitutively secreted into the media (B, top) and not retained on the plasma membrane as shown by surface biotinylation (B, bottom), and live cell surface staining (C). Scale bar, 20 μm (C). (D) A mouse line expressing TECTAS was generated by knocking a stop codon into the ω-site of the Tecta gene (fig. S1A). Radial sections of the mature cochlea (P28) stained with Toluidine blue show that the TM is severely disorganized and detached from the organ of Corti in TectaS/S mice. Arrows indicate the TM. Scale bars, 100 μm (D) and 50 μm (inset b). (E and F) Threshold sound pressure level (SPL) for auditory brainstem response (ABR) (E) and distortion product otoacoustic emission (DPOAE) (F) of TectaS/S mice are significantly elevated at all tested frequencies (8, 12, 16, 22, and 32 kHz) in comparison with Tecta+/+ mice at P28 (*P < 0.01, t test; means ± SEM).
GPI anchorage of TECTA is required for the formation of the TM matrix on the cell surface
To test whether GPI anchorage of TECTA is required for normal formation of the TM in vivo, we generated mice expressing a secreted form of TECTA using CRISPR-Cas9 genome editing to substitute the last endogenous ω-site with a stop codon (TectaS allele) (fig. S1A). In these mice, GPI anchorage of TECTA is abrogated, while the mature protein sequence of TECTA is preserved. Genotyping of G0 founders showed efficient biallelic insertion of the donor sequence (fig. S1B). Sanger sequencing of 600 base pairs flanking the targeted site showed the expected insertion of donor sequences with no other mutations (fig. S1C). The expression level (fig. S1D) and spatial distribution (fig. S1E) of TectaS mRNA were comparable to that of TectaWT mRNA, indicating that introduction of a premature termination codon within seven bases upstream of the last exon-exon junction (fig. S1A) does not cause nonsense-mediated decay (NMD). This result is consistent with the observation that a premature termination codon within 50 bases upstream of the 3′ most intron does not trigger NMD (23).
To investigate TM structure of the TectaS/S mice, we examined semithin sections of the cochlea at postnatal day 28 (P28) when the TM is fully matured. As shown in Fig. 2D, the TM (arrows) of TectaS/S mice is severely disorganized and detached from the organ of Corti and appears as an ectopic aggregate that was attached to Reissner’s membrane (Fig. 2D, enlarged in inset a). The cellular architecture of the cochlea appeared grossly normal in TectaS/S mice. Consistent with the observed defects in TM structure, thresholds for auditory brainstem response and distortion product otoacoustic emissions were significantly elevated in TectaS/S mice (Fig. 2, E and F), indicating a reduction in auditory sensitivity that is most likely due to impaired function of the cochlear amplifier as observed in Tecta-null mice (5, 24). Unlike mutant mice with complete hearing loss due to the loss of hair cell function (25, 26), we observed an auditory response in TectaS/S mice to sound stimuli around 80 decibels, suggesting that hair cells can function. Uptake of FM4-64FX dye by inner and outer hair cells of TectaS/S mice ex vivo is normal at P2 (fig. S2), suggesting that mechanotransduction channels operate and further supporting the idea that TectaS/S mice have a specific loss of the TM, while the cellular architecture of the cochlea is preserved.
To determine the role of GPI-anchored TECTA in TM development, we performed ultrastructural analysis of control and TectaS/S cochleae by transmission electron microscopy (TEM) at P2. The wild-type TM grows on the apical surface of supporting cells and is composed of the top covernet layer, the central body, and the bottom border layer (Fig. 3A). The apical surface of inner supporting cells displays densely arrayed microvilli (Fig. 3B, inset b). Collagen fibrils are present in the matrix of the developing TM as previously observed (9). Notably, abutting the border layer of the TM, collagen fibrils are organized into discrete bundles that are associated with the upper surface of the microvilli (Fig. 3B, inset b, arrows), suggesting that the organization and polymerization of collagen bundles occur at the cell surface level. These microvillus-associated collagen bundles are oriented upward from the epithelial surface and lie medially within the radial plane (Fig. 3B). To determine whether TECTA plays a role in the association of collagen bundles with the microvillus surface, we performed immunoelectron microscopy (immuno-EM) with an anti-TECTA antibody. The gold particles are localized proxy to the microvillus surface where collagen bundles are associated (Fig. 3C, arrows) and in between parallel collagen fibrils distal to the apical surface (Fig. 3C, arrowhead). Collagen fibers present in the central body of the TM are evenly spaced throughout the entire TM and are oriented parallel to the radial plane (Fig. 3D). A longitudinal section through the central body layer along the apical-basal axis shows regular spacing of parallel collagen fibrils (Fig. 3D, inset D′). Collagen fibrils located at the apical surface of inner supporting cells and in the body of the TM are associated with thinner fibrillary structures [Fig. 3, B (inset b) and D, arrowheads]. In the TectaS/S cochlea, collagen fibrils are not anchored to the microvillus surface of supporting cells and are not organized into bundles (Fig. 3E). Instead, collagen fibrils are diffusely present in the luminal space above inner supporting cells and are severely disorganized into wavy, nonparallel, and cross-sectioned fibrils (Fig. 3F, arrows). Scanning electron microscopy (SEM) of the wild-type cochlea shows upper layers of the TM: the top covernet layer and the underlying central body (Fig. 3G). Major bundles of the covernet are longitudinally arranged along the apical-basal axis (Fig. 3G, arrows) as observed in the mature TM (8). Branches of the covernet show a reticular configuration (Fig. 3H, arrow) covering the underlying central body layer, where parallel collagen fibrils are radially oriented with an apical-directed slant as previously observed (Fig. 3H, arrowhead) (9). In the TectaS/S cochlea, the covernet and parallel collagen fibers are absent, exposing the smooth microvillar surface of the supporting cells (Fig. 3, I and J). These findings demonstrate that the GPI anchorage of TECTA is essential for the organization of collagen bundles on the apical surface of inner supporting cells during the formation of the TM.
Fig. 3. Ultrastructural analysis of the developing TM.

(A to E) TEM of radial sections of the developing mouse cochlea (P2). (A) The wild-type TM grows on the apical surface of supporting cells and is composed of the top covernet layer (CN), the central body, and the bottom border layer. M ↔ L indicates medial-lateral axis of the cochlea. Scale bar, 20 μm. (B, inset b) The apical surface of polarized columnar cells displays densely arrayed microvilli, on which collagen fibril bundles (arrow) are attached. The collagen bundles project up from the epithelial surface and are directed medially in the radial plane. Fine filaments (arrowhead) are intermingled with collagen bundles. Scale bars, 1 μm. (C) Immuno-EM of wild-type cochlea with anti-TECTA antibody shows that TECTA is localized to the microvillus surface of supporting cells where collagen fibril bundles are associated (arrows), as well as in between parallel collagen fibrils distal to the apical surface (arrowhead). Scale bar, 200 nm. (D) The central body of the TM is composed of parallel collagen fibrils oriented along the radial plane (arrow) and intermingled thinner filaments (arrowhead). Scale bar, 1 μm. Inset: (D′) A longitudinal section of the TM shows a regular spacing of collagen fibrils. Scale bar, 0.5 μm. (E) Collagen fibrils are not associated with the apical surface of the inner supporting cells of TectaS/S mice. Scale bar, 1 μm. (F) The collagen fibrils in the luminal space of the scala media that are not associated with the apical surface of the epithelium of TectaS/S mice form wavy and disorganized collagen clusters. The orientation of collagen fibrils is disrupted as shown by the different length of cross-sectioned collagen fibrils (arrows). Scale bar, 1 μm. (G to J) SEM of the developing mouse cochlea (P2). (G) The major bundles of the covernet (arrows) are longitudinally oriented along the apical-basal (A↔B) axis of the cochlea of wild-type mice. GER, greater epithelial ridge. Scale bar, 20 μm. (H) The reticular branches of the covernet (arrow) lie atop parallel collagen fibrils within the central body of the wild-type TM, which are arranged along the radial axis with an apically directed slant (arrowhead). Scale bar, 1 μm. (I and J) The apical surface of the TectaS/S cochlea is smooth due to the lack of collagen fibril bundles associated with microvilli of inner supporting cells. Scale bars, (I) 10 μm and (J) 1 μm.
To examine the role of GPI-anchored TECTA in the distribution and assembly of TM components, we monitored the architecture of the developing TM using high-resolution fluorescence microscopy. Staining of surface and ECM glycoproteins with Pisum sativum agglutinin (PSA), an α-linked mannose-binding lectin (27), revealed that the developing TM is a multilayered structure lying on and above the cochlear supporting cells (Fig. 4A). TECTA and Col II were detected both on the cell surface and in the body of the TM. In the TectaS/S cochlea, however, Col II staining was absent on the apical surface of supporting cells (Fig. 4B, inset a) and instead appeared as a disorganized aggregate in the luminal space of the scala media (Fig. 4B, inset b). These findings demonstrate that surface tethering of TECTA is critical for retention of collagen fibrils at the cell surface. Notably, secreted proteins including OTOG (fig. S6B, inset b) and TECTAS (Fig. 4B, inset b) are weakly associated with the aggregate, indicating that the precise assembly of collagen fibrils is a prerequisite to the recruitment of soluble glycoproteins into the ECM network. To support this, PSA lectin staining, which showed a layered pattern in the wild-type TM, was absent from the collagen aggregate seen in of TectaS/S mice (Fig. 4B, inset b, and fig. S6B).
Fig. 4. Col II is not associated with the surface membrane but aggregated in the luminal space of the scala media in TectaS/S mice.
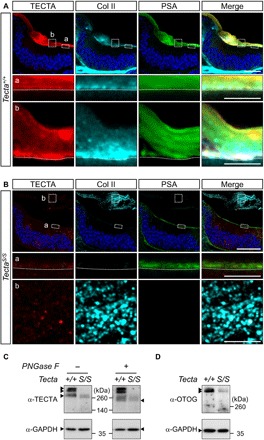
(A and B) Radial sections of P2 Tecta+/+ and TectaS/S cochleae were stained with antibodies against TECTA (red) and Col II (cyan) as well as with PSA lectin (green) and Hoechst (blue) and then analyzed by high-resolution Airyscan fluorescence confocal microscopy. (A) The TM of wild-type mice exhibits a multilayered organization. TECTA, Col II, and PSA staining are detected both immediately adjacent to the apical cell surface of supporting cells (dotted line) and in upper TM layers. Scale bars, 20 μm (A) and 5 μm (insets a and b). (B) Col II does not accumulate on the apical surface of the TectaS/S cochlea. The disorganized collagen located in the luminal space of the scala media is weakly associated with TECTAS protein and lacks PSA staining. Scale bars, 20 μm (B) and 5 μm (insets a and b). (C and D) Western blots of the TectaS/S mouse cochlea (P0) with the littermate control. (C) TECTAWT (arrowheads) appears as a monomer of 260 kDa, as well as higher–molecular weight, oligomeric bands (left). TECTAS protein is present only as a monomer. The total amount of TECTA protein is reduced in the TectaS/S mouse cochlea. The treatment of PNGase F reveals that the high molecular bands of TectaWT are oligomers or hyperglycosylated monomers (right). (D) Steady-state levels of OTOG (arrowheads) protein in the TectaS/S cochlea (P0) are reduced, as compared to the wild-type control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western blot analysis showed that TECTAWT appeared as high–molecular weight bands and a monomeric band near 260 kDa (Fig. 4C). The high–molecular weight bands were present after the removal of the N-linked glycosylation with PNGase F, indicating that they are multimerized forms and are not hyperglycosylated monomers. TECTAS was present only as a monomer, indicating that surface tethering of TECTA is required for protein multimerization. Notably, the ZP domain of ZP-containing proteins including TECTA contains target sequences for endoprotease cleavage, followed by a polymerization-blocking external hydrophobic patch (EHP), which is located proximal to the membrane anchorage site (Fig. 1D) (20, 21, 28). Polymerization of other ZP-containing proteins requires surface expression and subsequent removal of the EHP domain (29, 30). Thus, constitutively secreted TECTAS may not form an oligomeric complex and may remain as a monomer in the luminal space. Not only the level of TECTAS protein but also the level of OTOG protein was reduced in the TectaS/S cochlea (Fig. 4D), suggesting that incorporation of soluble components into the ECM network is critical for protein stability. Note that the total amount of soluble proteins in the endolymph is 4.7- to 6.4-fold lower than the perilymph and more than 100-fold less than the plasma (31). Soluble proteins that are not incorporated into the matrix may be actively cleared from the endolymph, a process that may play a role in maintaining a protective, protease-free environment to preserve the long-term stability of the TM, which persists throughout the life (32).
To determine whether the GPI anchorage may directly affect protein stability, we measured the degradation rate of TECTAWT or TECTAS proteins in vitro by monitoring their levels over time. Conditioned media containing released TECTAWT or TECTAS in the presence of PI-PLC were kept at 37°C under a cell-free condition. TECTAS was degraded at a faster rate (τ1/2 = 1.8 days) than TECTAWT (τ1/2 = 7.7 days), which was released by PI-PLC and retained the GPI moiety except for the lipid tail (fig. S3, A and B). Thus, GPI anchorage of TECTA may stabilize it in multiple ways: directly by protecting it from the degrading enzymes, presumably carboxyl peptidases, and/or indirectly by incorporating it into the ECM network.
Together, the GPI anchorage of TECTA is required for surface expression, protein stability, and multimerization of TECTA, as well as for attachment of collagen bundles to the microvillar surface of supporting cells, and organization of collagen fibrils throughout the body of the TM. TectaS/S mice resemble Tecta-null mice in many aspects, which include collagen disorganization, impaired incorporation of TM glycoproteins into the matrix, and hearing deficits (13), suggesting that GPI anchorage of TECTA plays a critical role in TECTA function.
These findings could indicate that surface-tethered TECTA directly sequesters collagen fibrils to the microvillus surface of supporting cells. Alternatively, the GPI anchorage of TECTA may be a prerequisite for generating and releasing an active form of TECTA that organizes collagen fibrils in the luminal space. In the latter case, loss of association of collagen fibrils with the microvillus membrane seen in TectaS/S mice can be explained by an assumption that only organized collagen bundles are associated with the microvillus surface via other surface proteins, while individual or disorganized collagen fibrils cannot be associated with the microvillus surface. We observed that TECTA localized to the distal tip of the microvilli where collagen bundles attach (Fig. 3C), suggesting that surface-tethered TECTA plays an active role in sequestering and organizing collagen bundles on the surface. To further elucidate the role of surface-tethered TECTA, we tested whether surface expression of TECTA is sufficient to recruit individual collagen fibrils to the microvillus surface of cochlear supporting cells.
Surface-tethered TECTA recruits collagen fibrils, but GPI-dependent surface tethering of TECTA is required for the TM growth
Is the surface expression of TECTA sufficient to organize collagen ultrastructure within the TM? If so, TECTA tethered to the cell surface by a transmembrane anchor should be functionally indistinguishable from wild-type GPI-anchored TECTA. Alternatively, GPI-dependent targeting of TECTA may play a unique role in TM organization. To test this, we generated a transmembrane form of TECTA (TECTAT) by substituting the C-terminal GPI anchorage signal with CD2 surface antigen, a type I transmembrane protein (fig. S4A). TECTAT was expressed on the surface of HEK293T cells and was not released into the medium of cells in the presence or absence of PI-PLC as determined by Western blotting of cell media (fig. S4B, top), surface biotinylation (fig. S4B, bottom), and live staining (fig. S4C). Next, we generated a TectaT allele in mice using CRISPR-Cas9 genome editing (Fig. 5 and fig. S5). Immunostaining of the TectaT/T cochlea showed that TECTAT protein was expressed on the apical surface of the cochlear supporting cells (Fig. 5A, inset a), indicating that GPI anchorage is dispensable for apical targeting of TECTA. However, unlike TECTAWTor TECTAS protein (Fig. 4), TECTAT is not detected distal to the cell surface (Fig. 5A), indicating that the release of TECTA requires GPI-dependent membrane targeting. GPI-APs are dynamically released from the cell surface by several mechanisms including GPI-anchor lipase, vesicular release, and proteolytic cleavage (33, 34). The GPI anchor of TECTA may be a direct target of GPI-cleaving enzymes and/or vesicular release machinery. Alternatively, GPI anchorage of TECTA may be required for proteolytic cleavage by regulating substrate availability or specificity.
Fig. 5. GPI-dependent release of TECTA is required for the growth of the TM.
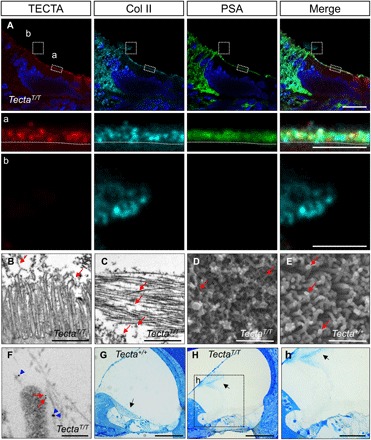
The TectaT allele, encoding a TECTAT, was generated by replacing sequences encoding the TECTA C-terminal GPI anchorage signal with those encoding the transmembrane domain of CD2 (fig. S5A). (A) Airyscan-processed images of the developing TectaT/T cochlea (P2) following TECTA (red), Col II (cyan), PSA (green), and Hoechst (blue) staining. TECTAT protein is expressed on the apical surface of cochlear supporting cells (inset a) (dotted line) and is not detected in the lumen of the cochlear duct (inset b). Coll II accumulates on the cell surface but does not form a multilayered architecture. Excessive Col II not associated with the surface forms an aggregate in the luminal space, which lacks TECTAT and PSA signal. Scale bars, 20 μm (A) and 5 μm (insets a and b). (B and C) TEM of the developing TectaT/T cochlea (P2). Microvilli of supporting cells are associated with electron-dense materials (B) (arrows). Collagen fibrils in the luminal space are disorganized and randomly oriented as shown by the different length of cross-sectioned fibrils (C) (arrows). Scale bars, 1 μm. (D and E) SEM of the apical surface of the developing cochlea of TectaT/T (D) and Tecta+/+ (E) mouse (P2). The TM is removed to reveal the cochlear epithelial cell surface of wild-type mice. Microvilli of TectaT/T mice are covered with electron-dense materials and horizontally orientated fibers (arrows), while those of Tecta+/+ mice show a bare surface except for the top, where ring-shaped electron-dense materials are observed. Scale bars, 1 μm. (F) Double immuno-EM of TECTA (6-nm gold particle; red arrows) and Col II (12 nm-gold particle; blue arrowheads) of the apical surface of polarized columnar cells of the TectaT/T cochlea (P2). Collagen fibrils that were not organized into bundles were associated with the microvillus surface. Scale bar, 200 nm. (G) Radial semithin sections of the mature Tecta+/+ cochlea (P28) stained with toluidine blue. Scale bar,100 μm. (H) (inset h) Radial semithin sections of the mature TectaT/T cochlea (P28) stained with Toluidine blue. In TectaT/T mice, the TM (arrow) is severely disorganized and aggregated in the scala media (arrow) and is not observed over the organ of Corti. Scale bars, 100 μm (H) and 50 μm (inset h).
The spatial expression pattern of TectaT was not altered as shown by in situ hybridization (fig. S5D). Unexpectedly, unlike TectaS, the total level of TectaT mRNA was reduced as measured by quantitative reverse transcription polymerase chain reaction at P0 (fig. S5E). Since TECTAT protein is not released from the cell surface, this may be due to negative feedback signaling maintaining the abundance of proteins within the cells. However, we cannot exclude the possibility that the engineering of the TectaT allele may affect its transcriptional activity and/or mRNA stability. Western blotting showed the expected size shift of TECTAT protein with reduced overall protein level (fig. S5F).
Unlike TectaS/S mice in which Col II staining was absent from the surface membrane of supporting cells, Col II accumulated on the apical surface of P2 TectaT/T cochlear supporting cells (Fig. 5A, inset a). These data further support the premise that surface-anchored TECTA plays a direct role in recruiting or retaining secreted collagen on the cell surface. Note that although collagens are accumulated on the surface of supporting cells in the TectaT/T cochlea, they did not mature to form a layered architecture (Fig. 5A, inset a), indicating that GPI-dependent surface expression of TECTA is required for collagen organization and TM maturation. Instead, collagens that were not captured on the cell surface diffused away from the surface and formed an aggregate in the luminal space similar to that in the cochlea of TectaS/S mice (Figs. 4B, inset b, and 5A, inset b). The collagen aggregate in the lumen lacked PSA staining (Fig. 5A) and OTOG staining (fig. S6C). The similar disorganization of collagens in the luminal space observed in both TectaS/S and TectaT/T mice indicates that loss of surface association of collagen fibrils in TectaS/S mice is not a consequence of collagen disorganization but due to the absence of surface expression of TECTA, further suggesting the active role of surface TECTA in recruiting collagen fibrils on the surface.
Consistent with the fluorescence imaging, ultrastructural analysis showed that electron-dense materials are associated with microvilli on the apical surface of TectaT/T supporting cells (Fig. 5B, arrows). Unlike wild-type mice where collagen fibrils connected to the microvilli were organized into bundles with a vertical-medial orientation (Fig. 3B), these bundles were absent but disorganized, and disoriented clusters of collagens were detected in the luminal space (Fig. 5C). SEM showed that microvilli of supporting cells of TectaT/T mice were abundantly covered with dense materials and horizontally oriented short fibers (Fig. 5D). Thus, GPI-dependent release of TECTA is required for elongation and bundled organization of collagen fibrils in the TM. In contrast, SEM of the Tecta+/+ cochlea, with the TM removed to reveal the apical surface, showed accumulation of dense materials on the top surface of the microvilli (Fig. 5E, arrows). To identify the electron-dense materials covering the surface of microvilli of the TectaT/T cochlea, we performed double-immuno EM with TECTA (6-nm gold particle; Fig. 5F, red arrows) and Col II (blue arrowheads; 12-nm gold particle) antibodies. Collagen fibrils were associated with the microvillus surface where TECTA locates but were not organized into bundles, further supporting that surface tethering of TECTA is sufficient to recruit individual collagen fibrils to the apical surface. However, GPI-dependent surface expression of TECTA is required for the bundled organization of collagen. Because of the loss of growth of the TM on the surface membrane in TectaT/T mice, the TM was severely disorganized, and the aggregate in the luminal space was attached to the Reissner’s membrane at P28 (Fig. 5H, inset h, arrow). Cellular architecture of the cochlea including hair cells and supporting cells appeared grossly normal in TectaT/T mice (Fig. 5H, inset h), which is further supported by normal uptake of FM4-64FX dye ex vivo (fig. S7).
DISCUSSION
In summary, we show that surface localization and subsequent release of TECTA via a GPI anchorage are critical for morphogenesis of the multilayered ECM that makes up the TM. Surface tethering of TECTA is required for recruiting collagen fibrils to the surface of cochlear supporting cells. GPI-dependent release of TECTA is critical for bundled organization of collagen fibrils and TM growth.
The protein level of TECTA in TectaS/S and TectaT/T mice is reduced compared to that in the wild-type littermates (Fig. 4C and fig. S5F), which may contribute to the severity of structural and functional deficits. However, the structure of the TM is normal in heterozygous Tecta-null mice (13), and inactivating mutations of TECTA are recessive in human (35), suggesting that TECTA function is not sensitive to the dosage. Moreover, TECTAT protein in TectaT/T mice, of which the level is reduced to the similar extent as TECTAS protein in TectaS/S mice, is sufficient to recruit collagen fibrils to the microvilli membrane, indicating that the reduced protein level does not significantly account for the phenotypic defects observed in the mutant mice.
There are multiple routes for the delivery of membrane-associated or secreted proteins to the apical surface, which are regulated by distinct Rab and Rho guanosine triphosphatases, microtubule motors, myosin motors, cytoskeletons, adaptor proteins, and lipid composition of the secretory vesicles (36). The lipid moiety of the GPI anchor and its modification along the secretory pathway make GPI-AP traffic in unique pathways enriched in sphingolipid and cholesterol (37). TECTAT protein in TectaT/T mice is sorted to the apical surface of the cochlear sensory epithelium and recruits collagen fibrils to the microvilli surface (Fig. 5, A and F), suggesting that GPI anchorage of TECTA is dispensable for apical targeting and collagen binding. However, we cannot exclude the possibility that removal or substitution of the GPI anchor of TECTA with a transmembrane domain may result in changes in the trafficking routes to the apical surface, its interaction with binding partners in the secretory pathways, posttranslational modifications of the protein, and microdomain localization of the protein on the apical surface.
Note that TECTA is the major component of both covernet and central body (Fig. 4A) (8), and GPI anchorage of TECTA is required for the organization of both layers (Fig. 3). However, how distinct shape and anisotropy of each layer are determined is unknown. Since surface tethering of TECTA via GPI anchorage plays roles in multiple processes including sequestering collagen fibrils on the surface, releasing, and multimerization of the protein, different regulatory mechanisms of surface-tethered TECTA may generate distinct organization patterns of the matrix. For example, multimerization of TECTA shed via removal of polymerization blocking sequences within a ZP domain may play a role in the formation of dense tectorin fibers in the covernet, while sequestering of collagen fibrils by surface TECTA and subsequent release of collagen-TECTA complex may mediate the organization of the central body.
Our data suggest a three-dimensional (3D) printing model for the organization of complex ECM (Fig. 6). In contrast to the existing extracellular assembly model, in which ECM components including collagens and TECTA are secreted and then self-organized into a complex structure (Fig. 6A), our working model proposes that TECTA functions as a structural organizer on the cell surface to establish layers of the ECM (Fig. 6B). As each successive layer is “printed,” the previously established layer is released through the release of TECTA. TECTA that released together with the established layer may function as a molecular glue, maintaining collagen organization in the extracellular space. This repetitive process establishes the higher-order architecture of the complex ECM of the TM. Further studies of the release mechanism of TECTA and real-time imaging of the dynamic processes that occur during TM formation will reveal the molecular mechanism underlying the morphogenesis of complex ECM.
Fig. 6. Proposed model for the morphogenesis of the TM, a layered ECM structure: 3D printing model.
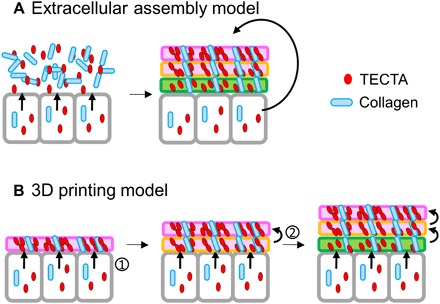
The TM is a multilayered structure: top covernet layer (pink), body layer (orange), and bottom layer (green). (A) An extracellular assembly model. The ECM architecture is formed by self-assembly of secreted and released proteins. (B) A 3D printing model. Printing of a new layer on the surface (1) and the simultaneous release of the preestablished layer (2) builds an organized, multilayered ECM structure.
MATERIALS AND METHODS
Animals and care
All animal care and experiments were conducted in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee (15-03003). C57Bl6/J mouse lines were maintained under the normal housing condition with food and water available ad libitum and a 12:12 light/dark cycle at the University of Utah.
Semithin sections and Toluidine blue staining
After removal of the cochlea from the temporal bone, the oval and round windows were opened. Through a small hole made at the apex, 20 μl of a fixative containing 2.5% glutaraldehyde and 1% tannic acid in washing buffer [0.1 M sodium cacodylate (pH 7.4)] was perfused. The cochlea was then further incubated in the fixative at 4°C overnight. After washing, the cochlea was osmicated (1% osmium tetroxide), dehydrated [ethanol (EtOH) and acetone], and infiltrated with Araldite/TAAB 812 resin kit (E202/1, TAAB Laboratories), according to the manufacturer’s instruction. Briefly, the cochlea was incubated with a 50:50% (v/v) and 70:30% (v/v) resin:acetone mixture sequentially and transferred to 100% resin for 8 hours. Plastic samples were made in specimen embedding capsules (133-P, Ted Pella) at 60°C for 72 hours. Samples were then sectioned in 0.8-μm thickness using Leica UC6 Ultramicrotome at the University of Utah Electron Microscopy Core Laboratory. Each section was stained shortly in a 100-μl drop of 1% (w/v) Toluidine blue solution containing 1% (w/v) sodium borate and air-dried for imaging.
Tissue processing for SEM and TEM
The cochlea tissue preparation for SEM and TEM follows the method shown in semithin sections. For SEM, the fixed cochlea coil was gently removed from the bony shell and subjected to the critical point dry (CPD2, Pelco). Samples were mounted on an aluminum stub with a carbon sticky tab (16084-2, Ted Pella) and coated with gold/palladium on a sputter coater for imaging. For TEM, plastic tissues were sectioned radially with a diamond knife (80- to 99-nm thickness) using Leica UC6 Ultramicrotome at the University of Utah Electron Microscopy Core Laboratory. The sections were mounted on copper grids (200 mesh) and stained sequentially with uranyl acetate (saturated aqueous) and Reynold’s Lead Citrate for imaging.
Immunoelectron microscopy
Dissected cochleae as above were fixed with 4% paraformaldehyde (PFA) for overnight at 4°C and were washed three times with phosphate-buffered saline (PBS). The tissues were incubated in the blocking solution (5% normal goat serum and 5% normal donkey serum in PBS with Tween 20) for 1 hour at room temperature (RT) and were incubated with the primary antibodies at 4°C for 2 days, including α–Col II produced in mouse (1:25; Abcam) and/or α-TECTA produced in rabbit (1:20). After washing with PBST, tissues were incubated with secondary antibodies: 12-nm Colloidal Gold AffiniPure Donkey Anti-Mouse immunoglobulin G (IgG; H + L) (1:20; EM Grade, 715-205-150, Jackson ImmunoResearch) and/or 6-nm Colloidal Gold AffiniPure Goat Anti-Rabbit IgG (H + L) (EM Grade, 111-195-144, Jackson ImmunoResearch) at 4°C for overnight. After washing with PBST, tissues were fixed with 2.5% glutaraldehyde and 1% tannic acid in sucrose (7%)–containing washing buffer [0.1 M sodium cacodylate (pH 7.4)] for 2 hours at RT and were washed three times with sucrose (7%)–containing washing buffer. The samples were postfixed with 1% osmium tetroxide for 1 hour at RT and were rinsed with 0.1 M acetate buffer (pH 5.2). The fixed samples were stained with 1% uranyl acetate in 0.1 M acetate buffer (pH 5.2) for 20 min. After staining, samples were dehydrated (EtOH and acetone) and infiltrated with Araldite/TAAB 812 resin kit as described above. For TEM imaging of immuno-EM samples, staining procedure of thin sections was excluded.
Immunohistochemistry
Mice were euthanized after anesthetized with isoflurane, and the cochlea was dissected from the temporal bone. A small hole at the cochlea apex was made, and the oval and round windows were opened. Twenty microliters of 4% PFA in PBS was gently injected through the hole, and the cochlea was incubated with 4% PFA at 4°C overnight. The cochlea was rinsed with PBS, incubated in 50% optimal cutting temperature compound (27050, Ted Pella), mixed with 30% sucrose solution in PBS (v/v) at RT for 1 hour, transferred to embedding mold (18646A-1, Polysciences), and frozen on dry ice. The cochlea was sectioned in 14-μm thickness and attached to the slide glass (22-037-246, Thermo Fisher Scientific). All tissue sections were blocked with the blocking solution [10% normal goat serum in PBST (0.1% Trition X-100)] for 1 hour and incubated with the primary antibodies at 4°C overnight, including α-TECTA produced in rabbit (1:200) and α–Col II produced in mouse (1:100; ab150771, Abcam). After washing, tissues were treated with secondary antibodies, such as Cy3 AffiniPure Donkey α-Rabbit IgG (H + L) (1:500) and Cy5 Goat α-Mouse IgG (H + L) (1:500), and fluorescein isothiocyanate–PSA (20 μg/ml; L0770, Sigma-Aldrich) with Hoechst 33342 (1:20,000) at RT for 1 hour. After washing, tissues were treated with fluoromount-G and coverslipped for imaging.
Imaging
Images for immunocytochemistry and immunohistochemistry were acquired using a Nikon A1R confocal microscope with a NIS-Elements multiplatform acquisition software or a Zeiss 880 Airyscan confocal microscope with a Zen Black software at the Fluorescence Microscopy Core Facility of the University of Utah. Images for SEM and TEM were taken by the FEI Quanta 600 field emission gun at the University of Utah Nanofab and FEI Tecnai 12 transmission electron microscope at the University of Utah Electron Microscopy Core Laboratory, respectively. All images were processed and analyzed with the Fiji open source software (https://fiji.sc/). Images for semithin sections and in situ hybridization were obtained by a Leica DM2500 optical microscope with Leica Las software V3.8.
Supplementary Material
Acknowledgment
We thank G. Richardson for plasmids and antibodies; A. Park and J. Skyler from the Hearing Research Facility (University of Utah); Cell Imaging Core and Electron Microscopy Core (University of Utah); G. Richardson, J. Christian, S. Mansour, R. Dorsky, and M. Vetter for comments on the manuscript; and lab members for discussion. Funding: This work was funded by the National Institute of Neurological Disorders and Stroke (R01NS102444) and the National Institute on Deafness and Other Communication Disorders (R21DC016750). Author contributions: D.-K.K., J.A.K., and S.P. designed the study. D.-K.K., J.A.K, J.P., A.N., and A.A. performed experiments. D.-K.K., and S.P wrote the first draft of the manuscript. All authors discussed the results and contributed to the final manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/11/eaay6300/DC1
Supplementary Materials and Methods
Fig. S1. Generation and characterization of TectaS mice.
Fig. S2. The function of the hair cells is normal in TectaS/S mice.
Fig. S3. The GPI anchorage of TECTA plays roles in multimerization and stability of the protein.
Fig. S4. TECTAT is expressed on the cell surface but is not released by PI-PLC.
Fig. S5. Generation and characterization of TectaT mice.
Fig. S6. Incorporation of OTOG, a secreted TM component, into the disorganized collagen network is impaired in TectaS/S and TectaT/T mice.
Fig. S7. The function of the hair cells is unaffected in TectaT/T mice.
REFERENCES AND NOTES
- 1.Lim D. J., Development of the tectorial membrane. Hear. Res. 28, 9–21 (1987). [DOI] [PubMed] [Google Scholar]
- 2.Richardson G. P., Lukashkin A. N., Russell I. J., The tectorial membrane: One slice of a complex cochlear sandwich. Curr. Opin. Otolaryngol. Head Neck Surg. 16, 458–464 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodyear R. J., Richardson G. P., Structure, function, and development of the tectorial membrane: An extracellular matrix essential for hearing. Curr. Top. Dev. Biol. 130, 217–244 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Lukashkin A. N., Richardson G. P., Russell I. J., Multiple roles for the tectorial membrane in the active cochlea. Hear. Res. 266, 26–35 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Legan P. K., Goodyear R. J., Morín M., Mencia A., Pollard H., Olavarrieta L., Korchagina J., Modamio-Hoybjor S., Mayo F., Moreno F., Moreno-Pelayo M.-A., Richardson G. P., Three deaf mice: Mouse models for TECTA-based human hereditary deafness reveal domain-specific structural phenotypes in the tectorial membrane. Hum. Mol. Genet. 23, 2551–2568 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H. Y., Raphael P. D., Park J., Ellerbee A. K., Applegate B. E., Oghalai J. S., Noninvasive in vivo imaging reveals differences between tectorial membrane and basilar membrane traveling waves in the mouse cochlea. Proc. Natl. Acad. Sci. U.S.A. 112, 3128–3133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishai R., Kamakura T., Nadol J. B. Jr., Abnormal tectorial membranes in sensorineural hearing loss: A human temporal bone study. Otol. Neurotol. 40, e732–e738 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Andrade L. R., Salles F. T., Grati M., Manor U., Kachar B., Tectorins crosslink type II collagen fibrils and connect the tectorial membrane to the spiral limbus. J. Struct. Biol. 194, 139–146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodyear R. J., Lu X., Deans M. R., Richardson G. P., A tectorin-based matrix and planar cell polarity genes are required for normal collagen-fibril orientation in the developing tectorial membrane. Development 144, 3978–3989 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim B. J., Kim D.-K., Han J. H., Oh J., Kim A. R., Lee C., Kim N. K. D., Park H.-R., Kim M. Y., Lee S., Lee S., Oh D. Y., Park W.-Y., Park S., Choi B. Y., Clarification of glycosylphosphatidylinositol anchorage of OTOANCORIN and human OTOA variants associated with deafness. Hum. Mutat. 40, 525–531 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rau A., Legan P. K., Richardson G. P., Tectorin mRNA expression is spatially and temporally restricted during mouse inner ear development. J. Comp. Neurol. 405, 271–280 (1999). [PubMed] [Google Scholar]
- 12.Zwaenepoel I., Mustapha M., Leibovici M., Verpy E., Goodyear R., Liu X. Z., Nouaille S., Nance W. E., Kanaan M., Avraham K. B., Tekaia F., Loiselet J., Lathrop M., Richardson G., Petit C., Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc. Natl. Acad. Sci. U.S.A. 99, 6240–6245 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legan P. K., Lukashkina V. A., Goodyear R. J., Kössl M., Russell I. J., Richardson G. P., A targeted deletion in α-tectorin reveals that the tectorial membrane is required for the gain and timing of cochlear feedback. Neuron 28, 273–285 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Russell I. J., Legan P. K., Lukashkina V. A., Lukashkin A. N., Goodyear R. J., Richardson G. P., Sharpened cochlear tuning in a mouse with a genetically modified tectorial membrane. Nat. Neurosci. 10, 215–223 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukashkin A. N., Legan P. K., Weddell T. D., Lukashkina V. A., Goodyear R. J., Welstead L. J., Petit C., Russell I. J., Richardson G. P., A mouse model for human deafness DFNB22 reveals that hearing impairment is due to a loss of inner hair cell stimulation. Proc. Natl. Acad. Sci. U.S.A. 109, 19351–19356 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legan P. K., Rau A., Keen J. N., Richardson G. P., The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J. Biol. Chem. 272, 8791–8801 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Zheng J., Miller K. K., Yang T., Hildebrand M. S., Shearer A. E., DeLuca A. P., Scheetz T. E., Drummond J., Scherer S. E., Legan P. K., Goodyear R. J., Richardson G. P., Cheatham M. A., Smith R. J., Dallos P., Carcinoembryonic antigen-related cell adhesion molecule 16 interacts with α-tectorin and is mutated in autosomal dominant hearing loss (DFNA4). Proc. Natl. Acad. Sci. U.S.A. 108, 4218–4223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hohenester E., Engel J., Domain structure and organisation in extracellular matrix proteins. Matrix Biol. 21, 115–128 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Springer T. A., von Willebrand factor, Jedi knight of the bloodstream. Blood 124, 1412–1425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin S. J., Hu Y., Zhu J., Woodruff T. K., Jardetzky T. S., Structure of betaglycan zona pellucida (ZP)-C domain provides insights into ZP-mediated protein polymerization and TGF-β binding. Proc. Natl. Acad. Sci. U.S.A. 108, 5232–5236 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokhove M., Nishimura K., Brunati M., Han L., de Sanctis D., Rampoldi L., Jovine L., A structured interdomain linker directs self-polymerization of human uromodulin. Proc. Natl. Acad. Sci. U.S.A. 113, 1552–1557 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhoeven K., Laer L. V., Kirschhofer K., Legan P. K., Hughes D. C., Schatteman I., Verstreken M., Hauwe P. V., Coucke P., Chen A., Smith R. J. H., Somers T., Offeciers F. E., Heyning P. V., Richardson G. P., Wachtler F., Kimberling W. J., Willems P. J., Govaerts P. J., Camp G. V., Mutations in the human α-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nat. Genet. 19, 60–62 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Nagy E., Maquat L. E., A rule for termination-codon position within intron-containing genes: When nonsense affects RNA abundance. Trends Biochem. Sci. 23, 198–199 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Xia A., Gao S. S., Yuan T., Osborn A., Bress A., Pfister M., Maricich S. M., Pereira F. A., Oghalai J. S., Deficient forward transduction and enhanced reverse transduction in the alpha tectorin C1509G human hearing loss mutation. Dis. Model. Mech. 3, 209–223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawashima Y., Géléoc G. S. G., Kurima K., Labay V., Lelli A., Asai Y., Makishima T., Wu D. K., Della Santina C. C., Holt J. R., Griffith A. J., Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J. Clin. Invest. 121, 4796–4809 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giese A. P. J., Tang Y.-Q., Sinha G. P., Bowl M. R., Goldring A. C., Parker A., Freeman M. J., Brown S. D. M., Riazuddin S., Fettiplace R., Schafer W. R., Frolenkov G. I., Ahmed Z. M., CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat. Commun. 8, 43 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rueda J., Cantos R., Lim D. J., Distribution of glycoconjugates during cochlea development in mice: Light microscopic lectin study. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 274, 923–933 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Jovine L., Darie C. C., Litscher E. S., Wassarman P. M., Zona pellucida domain proteins. Annu. Rev. Biochem. 74, 83–114 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Jovine L., Qi H., Williams Z., Litscher E. S., Wassarman P. M., A duplicated motif controls assembly of zona pellucida domain proteins. Proc. Natl. Acad. Sci. U.S.A. 101, 5922–5927 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaeffer C., Santambrogio S., Perucca S., Casari G., Rampoldi L., Analysis of uromodulin polymerization provides new insights into the mechanisms regulating ZP domain-mediated protein assembly. Mol. Biol. Cell 20, 589–599 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wangemann P., Supporting sensory transduction: Cochlear fluid homeostasis and the endocochlear potential. J. Physiol. 576, 11–21 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi H., Schrott-Fischer A., Glueckert R., Liu W., Salvenmoser W., Santi P., Rask-Andersen H., Molecular organization and fine structure of the human tectorial membrane: Is it replenished? Cell Tissue Res. 362, 513–527 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Park S., Lee C., Sabharwal P., Zhang M., Meyers C. L. F., Sockanathan S., GDE2 promotes neurogenesis by glycosylphosphatidylinositol-anchor cleavage of RECK. Science 339, 324–328 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Müller G. A., The release of glycosylphosphatidylinositol-anchored proteins from the cell surface. Arch. Biochem. Biophys. 656, 1–18 (2018). [DOI] [PubMed] [Google Scholar]
- 35.Moreno-Pelayo M. Á., Goodyear R. J., Mencía A., Modamio-Høybjør S., Legan P. K., Olavarrieta L., Moreno F., Richardson G. P., Characterization of a spontaneous, recessive, missense mutation arising in the Tecta gene. J. Assoc. Res. Otolaryngol. 9, 202–214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisz O. A., Rodriguez-Boulan E., Apical trafficking in epithelial cells: Signals, clusters and motors. J. Cell Sci. 122, 4253–4266 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muniz M., Zurzolo C., Sorting of GPI-anchored proteins from yeast to mammals–common pathways at different sites? J. Cell Sci. 127, 2793–2801 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/11/eaay6300/DC1
Supplementary Materials and Methods
Fig. S1. Generation and characterization of TectaS mice.
Fig. S2. The function of the hair cells is normal in TectaS/S mice.
Fig. S3. The GPI anchorage of TECTA plays roles in multimerization and stability of the protein.
Fig. S4. TECTAT is expressed on the cell surface but is not released by PI-PLC.
Fig. S5. Generation and characterization of TectaT mice.
Fig. S6. Incorporation of OTOG, a secreted TM component, into the disorganized collagen network is impaired in TectaS/S and TectaT/T mice.
Fig. S7. The function of the hair cells is unaffected in TectaT/T mice.


